Introduction
Polycystic ovary syndrome (PCOS) is a common
endocrine disorder that affects 5–10% of 20–37 years old women
(1). PCOS is characterized by
anovulation, hyperandrogenemia and polycystic ovaries. Up to 60% of
all patients with PCOS are overweight or obese (2); recent studies have demonstrated the
negative effects of increased body weight on reproductive outcomes
(3,4). In the past few decades, advances in
assisted reproductive techniques allowed for the selection of high
quality embryos, but the implantation rates for patients with PCOS
remained low in clinical settings (5,6).
Poor reproductive outcomes in patients with PCOS are probably
associated with decreased endometrial receptivity (7). Therefore, it is important to improve
the endometrial receptivity of women with PCOS.
During the window for embryo implantation, a rich
vascular network is necessary to supply nutrients and oxygen for
cell proliferation and implantation of the blastocyst (8). At this time, the endometrium
microenvironment is considered to be hypoxic (9). Hypoxic conditions activate
hypoxia-inducible factor (HIF)-1, which is an oxygen-sensitive
transcription factor. HIF-1 is a heterodimer, which comprises α and
β subunits (10). The HIF1-β
subunit is stably expressed, whereas the expression of HIF-1α is
regulated by oxygen concentration (11,12).
HIF-1α is rapidly degraded through the ubiquitin-proteasome pathway
under physiological conditions. Under hypoxic conditions, HIF-1α is
stabilized and is involved in the regulation of cellular processes,
including angiogenesis, glucose metabolism and cell differentiation
(13,14). Previous studies have reported that
HIF-1α expression in the endometrium occurs exclusively during the
secretory and menstrual phase, which may be associated with the
physiological changes associated with menstruation (15).
Vascular endothelial growth factor (VEGF) is an
important mediator of angiogenesis (16). Glucose transporter protein (GLUT)
is responsible for glucose uptake and storage in the endometrium
(17–19). VEGF and GLUT are targets of HIF-1α
and their expression is necessary for endometrial receptivity
(20). The implantation window is
in the mid-secretory phase of human endometrium, that is, 7–10 days
following luteinizing hormone (LH) surge (21). The role of HIF-1α in the regulation
of endometrial receptivity is yet to be confirmed. In the present
study, the expressions of HIF-1α and its target genes VEGF, GLUT1
and GLUT4 were investigated in the endometrium of women with and
without PCOS during the implantation window, and the effects of
body weight on their expression levels were also examined.
Materials and methods
Subjects
The present study recruited 40 women with PCOS and
41 women with tubal blockage who served as controls. Women with
PCOS were divided into overweight (OW)-PCOS (n=22) and
normal-weight (NW)-PCOS (n=18) subgroups. Women in the control
group were also divided into OW-Control (n=21) and NW-Control
(n=20) subgroups. All participants were referred to the
Reproductive Medicine Center, Yantai Yuhuangding Hospital (Yantai,
China) for in vitro fertilization pre-embryo transfer. The
patients were 20–37 years old with normal basal serum follicle
stimulating hormone levels and antral follicle counts (AFC). None
of the patients had taken oral contraceptives or other medicines in
the preceding 3 months. Hysteroscopic examination was performed to
exclude endometrial diseases in all participants. Patients with
endometriosis, intrauterine adhesions, endometrial polyps,
recurrent history of miscarriage and those with a recurrent
implantation failure were excluded from the present study. PCOS was
diagnosed according to the 2003 Rotterdam Consensus (22). Overweight and/or obesity were
defined according to the 2000 World Health Organization and The
International Obesity Task Force diagnostic criteria for the
Asia-Pacific population (23).
Clinical data, including age, duration of infertility, body weight,
height, waist-to-hip ratio (WHR), basal serum follicle-stimulating
hormone, LH, total testosterone (TT), fasting glucose (mmol/l),
fasting insulin (mIU/l), estradiol (E2) and progesterone (P) levels
following human chorionic gonadotropin (HCG) administration during
controlled ovarian hyperstimulation (COH), number of oocytes,
endometrial thickness and high-quality embryo rate were recorded.
Body mass index (BMI) was calculated as weight/height2
(kg/m2) and the homeostatic model assessment-insulin
resistance (HOMA-IR) was calculated as [(fasting basal blood
glucose)x(fasting basal insulin)]/22.5 (24). This study was approved by The
Ethics Committee of Yantai Yuhuangding Hospital (Yantai, China),
and written informed consent was received from each patient prior
to enrolment in the study.
Ovarian stimulation
The gonadotropin-releasing hormone antagonist
(GnRH-ant) protocol was used for COH (25). On day 3 of menstruation, 125–225
IU/day of recombinant FSH (Puregon; Merck Sharp &
Dohme-Hoddesdon, UK) was used for ovarian stimulation, according to
the patient's age, BMI, anti-Müllerian hormone, infertility
duration and AFC. A 0.25 mg dose of GnRH antagonist (Orgalutran;
Merck Sharp & Dohme-Hoddesdon) was administered daily when the
follicle diameter reached 12 mm until the day of HCG (Beijing
Saisheng Pharmaceutical Co, Ltd., Beijing, China) administration.
Highly purified human menopausal gonadotropin (Menopur; Ferring
Pharmaceuticals, Saint-Prex, Switzerland) was administered at the
late follicle phase. HCG (6,000 IU) was administered for final
oocyte maturation when at least two leading follicles were ≥18 mm
in size, and oocytes were retrieved following 34–36 h. All patients
received 600 mg/day progestogen (Utrogestan; Besins Manufacturing
Belgium, France) for luteal phase support.
Specimens
Endometrial tissues were collected from patients
with a curette on day 5 following the oocyte pick-up, certain
tissues were fixed in 4% formaldehyde for 12 h at room temperature
for paraffin embedding; the other tissues were washed in
phosphate-buffered saline and stored immediately at −70°C for RNA
isolation.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from tissues using an RNeasy
Mini kit (Qiagen, Inc., Valencia, CA, USA), as described previously
(26). A BioWaveII
spectrophotometer (Biochrom, Ltd., Cambridge, UK) was used to
evaluate the concentration and purity of the RNA (A260:A280 ratio).
cDNA was produced by reverse transcription using a PrimeScript RT
Reagent kit (Takara Bio, Inc., Otsu, Japan), according to the
manufacturer's protocol. qPCR was performed in 20 µl reactions
using 1 µl of cDNA, 10 µM of each primer and 2X Platinum SYBR Green
qPCR Supermix-UDG (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), following the manufacturer's protocol. The
following gene-specific primers were purchased from Invitrogen
(Thermo Fisher Scientific, Inc.) (27): HIF-1α, forward
5′-CATCAGCTATTTGCGTGTGAGGA-3′, reverse
5′-AGCAATTCATCTGTGCTTTCATGTC-3′; VEGF, forward
5′-CCTGGTGGACATCTTCCAGGAGTACC-3′, reverse 5′-GAAGC-TCA TCT CTC CTA
TGT GCT GGC-3′; GLUT1, forward 5′-CCAGCTGCCATTGCCGTT-3′, reverse
5′-GACGTAGGGACCACACAGTTGC-3′; GLUT4, forward
5′-CTGGGCCTCACAGTGCTAC-3′, reverse, 5′-GTCAGGCGCTTCAGACTCTT-3′;
GAPDH, forward 5′-GGGAAACTGTGGCGTGAT-3′, reverse
5′-GAGTGGGTGTCGCTGTTGA-3′. PCR thermocycling was performed at 95°C
for 30 sec, followed by 45 cycles at 95°C for 5 sec and at 60°C for
60 sec.
Specificity of the PCR reactions was assessed by
melting curve analysis. Correct melting temperatures were obtained
for all products. The relative gene expression levels in each
sample were normalized to the expression levels of GAPDH, which was
used as the housekeeping gene, and were analyzed using the
2−ΔΔCq method (28,29).
This normalization could account for the inherent variability in
the efficiency of the reverse transcription reactions. Each set of
RT-qPCR reactions was repeated three times.
Hematoxylin and eosin (H&E) and
immunohistochemical staining of endometrial sections
H&E staining was used for examination of the
morphological characteristics of the endometrium and for
demonstration of tissue integrity (30). Briefly, the tissues were hydrated
in H2O for 30 sec. Then the slides were immersed in
hematoxylin (Beyotime Institute of Biotechnology, Shanghai, China;
cat. no. C0105-1) for 30 sec and rinsed in H2O for 1
min. Subsequently, the slides were stained with 1% eosin Y solution
(Beyotime Institute of Biotechnology; cat. no. C0105-2) for 30 sec
and dehydrated in 95 and 100% alcohol.
Immunohistochemical staining of 4 µm thick sections
of the formaldehyde-fixed and paraffin-embedded endometrial tissue
was performed, as described previously (31). Briefly, the tissue sections were
deparaffinized in xylene and rehydrated in a graded ethanol series.
Endogenous peroxidase activity was blocked by incubation of
sections in 3% hydrogen peroxide in methanol for 10 min at room
temperature. Following three washes in PBS, the sections were
placed in a blocking solution sheep heat-inactivated serum
(Beyotime Institute of Biotechnology; cat. no. C0265) for 30 min at
oom temperature to block nonspecific binding sites. Subsequently,
sections were incubated with the following primary antibodies:
Rabbit polyclonal anti-HIF-1α (1:700; Abcam, Cambridge, UK, cat.
no. ab85886), rabbit polyclonal anti-VEGF (1:200; Abcam; cat. no.
ab46154), rabbit monoclonal anti-GLUT1 (1:150; Abcam; cat. no.
ab150299) and rabbit polyclonal anti-GLUT4 (1:400; Abcam; cat. no.
ab654), overnight at 4°C. The sections were washed three times in
PBS and were incubated with horseradish peroxidase-conjugated goat
anti-rabbit immunoglobulin G (1:50, Beyotime Institute of
Biotechnology; cat. no. A0208) at 37°C for 1 h. 3,3-diaminobenzidin
was used as the chromogen and counterstaining was done with
hematoxylin.
Sections were examined with an Olympus CX31-LV320
light microscope (Olympus Corporation, Tokyo, Japan) at ×20
magnification. Reactions demonstrating the specificity of primary
antibodies were also carried out by omission of the antibodies in
the incubating medium; no immunoreactivity was observed in these
sections (data not shown). Experiments were repeated at least three
times. The immunostaining density of HIF-1α, VEGF, GLUT1 and GLUT4
was evaluated using a semi-quantitative method, as previously
described (32). The Image-Pro
Plus 6.0 software (Media Cybernetics company, USA) was used to
calculate the integrated optical density (IOD) and areas, three
fields were examined in per slide. Data are presented as mean
density (IOD/area) to indicate the relative expression (33).
Statistical analysis
Data analysis was performed using SPSS 19.0 (IBM
Corp., Armonk, NY, USA). Data were expressed as the mean ± standard
deviation (or standard error of the mean for the RT-qPCR
experiments). Inter-group differences with respect to normally
distributed variables were assessed with Student's t-test;
differences pertaining to non-normally distributed variables were
assessed with the Mann-Whitney U test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Clinical and COH characteristics of
patients
Serum levels of LH, TT, fasting insulin, fasting
glucose and HOMA-IR in patients with PCOS were significantly higher
than those in the controls (P<0.05; Table I). No significant inter-group
differences were observed with respect to E2, P level on HCG day
and high-quality embryo rate. OW-PCOS patients exhibited higher
BMI, WHR, fasting insulin and HOMAR-IR compared with NW-PCOS
patients. In the control group, only BMI and WHR were significantly
different between the OW-Control and NW-Control groups (P<0.05;
Table II).
 | Table I.Clinical characteristics and
laboratory parameters after controlled ovarian hyperstimulation in
PCOS and controls. |
Table I.
Clinical characteristics and
laboratory parameters after controlled ovarian hyperstimulation in
PCOS and controls.
| Variable | PCOS (n=40) | Controls
(n=41) |
|---|
| Age (years) |
31.10±2.62 |
31.12±2.43 |
| Infertility
duration (years) |
3.96±2.69 |
3.74±2.76 |
| BMI
(kg/m2) |
26.12±3.63 |
25.05±3.67 |
| WHR |
0.87±0.05 |
0.85±0.03 |
|
Follicle-stimulating hormone (mIU/ml) |
5.78±2.15 |
6.48±1.51 |
| LH (mIU/ml) |
9.48±5.54 |
5.67±2.16a |
| TT (ng/ml) |
0.43±0.20 |
0.30±0.18a |
| Fasting glucose
(mg/dl) |
5.2±0.19 |
4.65±0.68a |
| Fasting insulin
(mIU/ml) |
14.45±6.24 |
7.93±2.33a |
| HOMA-IR |
3.38±1.54 |
1.66±0.54a |
| E2 levels on the
day of HCG (pg/ml) |
3,468.64±1,935.33 |
3,124.83±1,862.69 |
| P levels on the day
of HCG administration (ng/ml) |
0.93±0.38 |
0.88±0.34 |
| Endometrial
thickness on HCG day (mm) |
10.43±2.09 |
10.59±1.64 |
| Number of
oocytes |
11.00±5.64 |
9.
95±4.28 |
| High-quality embryo
rate (%) |
65.37±25.96 |
70.68±21.76 |
 | Table II.Clinical characteristics of
overweight and normal-weight subgroups of the PCOS and
controls. |
Table II.
Clinical characteristics of
overweight and normal-weight subgroups of the PCOS and
controls.
| Variable | NW-PCOS (n=18) | OW-PCOS (n=22) | NW-Control
(n=20) | OW-Control
(n=21) |
|---|
| Age (years) |
30.94±2.65 |
31.18±2.75 |
30.65±2.94 |
31.57±1.77 |
| Infertility
duration (year) |
3.25±1.68 |
4.48±3.17 |
4.60±3.22 |
2.93±2.01 |
| BMI
(kg/m2) |
22.64±1.73 |
28.66±2.27a |
22.0±1.97 |
27.94±2.27b |
| WHR |
0.82±0.01 |
0.93±0.04a |
0.83±0.01 |
0.89±0.02b |
| FSH (mIU/ml) |
5.47±1.68 |
6.00±2.45 |
6.67±1.33 |
6.31±1.69 |
| LH (mIU/ml) |
10.89±6.64 |
8.46±4.48 |
5.85±1.78 |
5.49±2.51 |
| TT (ng/ml) |
0.44±0.25 |
0.43±0.18 |
0.29±0.20 |
0.30±0.15 |
| Fasting glucose
(mg/dl) |
5.11±0.43 |
5.11±0.21 |
4.75±0.77 |
4.70±0.67 |
| Fasting insulin
(mIU/ml) |
9.72±3.99 |
16.78±5.91a |
7.73±2.83 |
8.25±2.12 |
| HOMA-IR |
2.22±0.95 |
3.84±1.41a |
1.63±0.64 |
1.73±0.52 |
| Endometrium
thickness on the day of HCG administration (mm) |
10.31±2.30 |
10.52±1.99 |
10.60±1.53 |
10.57±1.77 |
| Number of
oocytes |
11.00±5.52 |
11.00±5.85 |
11.00±4.07 |
8.95±4.34 |
| High-quality embryo
rate (%) |
70.5±24.4 |
61.4±20.42 |
73.62±20.70 |
68.0±22.85 |
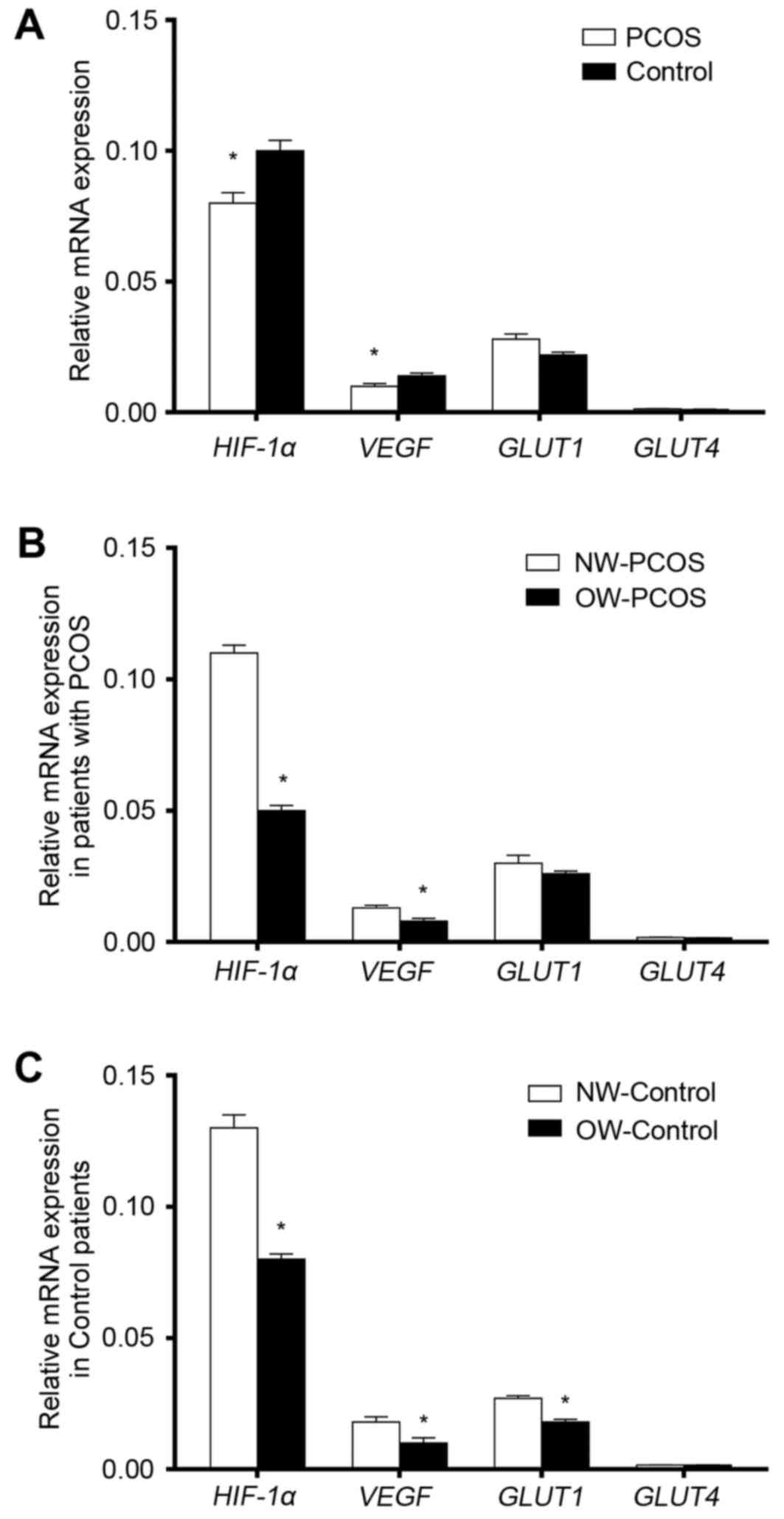
Endometrial mRNA expression levels of
HIF-1α, VEGF, GLUT1 and GLUT4 during the implantation window
In both the PCOS and the Control groups, mRNA
expression levels of HIF-1α were notably higher compared
with the mRNA expression levels of of VEGF, GLUT1 and
GLUT4. mRNA expression levels of HIF-1α and
VEGF in patients with PCOS were significantly lower compared
with the respective expression levels in the Control patients
(P<0.05; Fig. 1A). GLUT4
mRNA expression levels were very low in both groups and there was
no statistically significant inter-group differences with respect
to GLUT1 and GLUT4 expression levels (P>0.05;
Fig. 1A).
Association between HIF-1a, VEGF,
GLUT1 and GLUT4 mRNA expression levels with body weight
mRNA expression levels of HIF-1α and
VEGF in OW patients were significantly lower than in the NW
patients in both the PCOS and Control groups (P<0.05; Fig. 1B and C, respectively). GLUT1
mRNA expression levels were significantly lower in OW-Control
patients compared with expression in the NW-Controls (Fig. 1C; P<0.05); no statistically
significant differences in GLUT4 expression levels were
identified between OW and NW patients with our without PCOS
(P>0.05; Fig. 1B and C,
respectively).
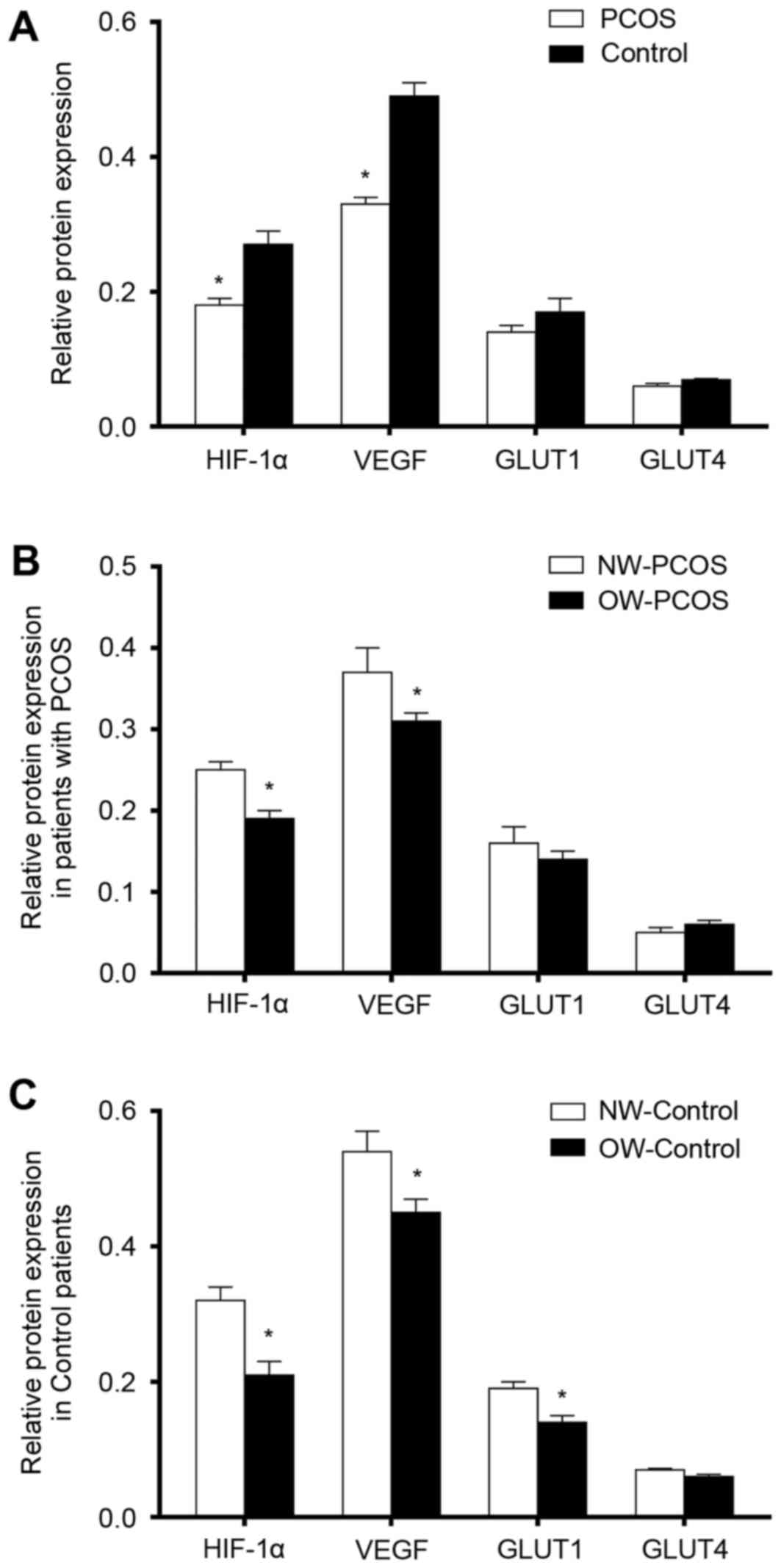
Endometrial histology and protein
expression levels of HIF-1α, VEGF, GLUT1 and GLUT4 during
implantation window
The histological stage of endometrium in PCOS and
control groups was in secretory phase according to the Noyes
standard (Fig. 2) (34). In the secretory endometrium, strong
brown immunostaining for HIF-1α and VEGF were observed in the
nuclei of epithelial and stroma cells in the PCOS and control
groups. Positive cytoplasmic and nuclear immunostaining for GLUT1
and GLUT4 was detected in the epithelial cells of OW-PCOS and
NW-PCOS patients. However, these were localized mainly in the cell
membrane of cells in the OW-control and NW-control group (Fig. 2).
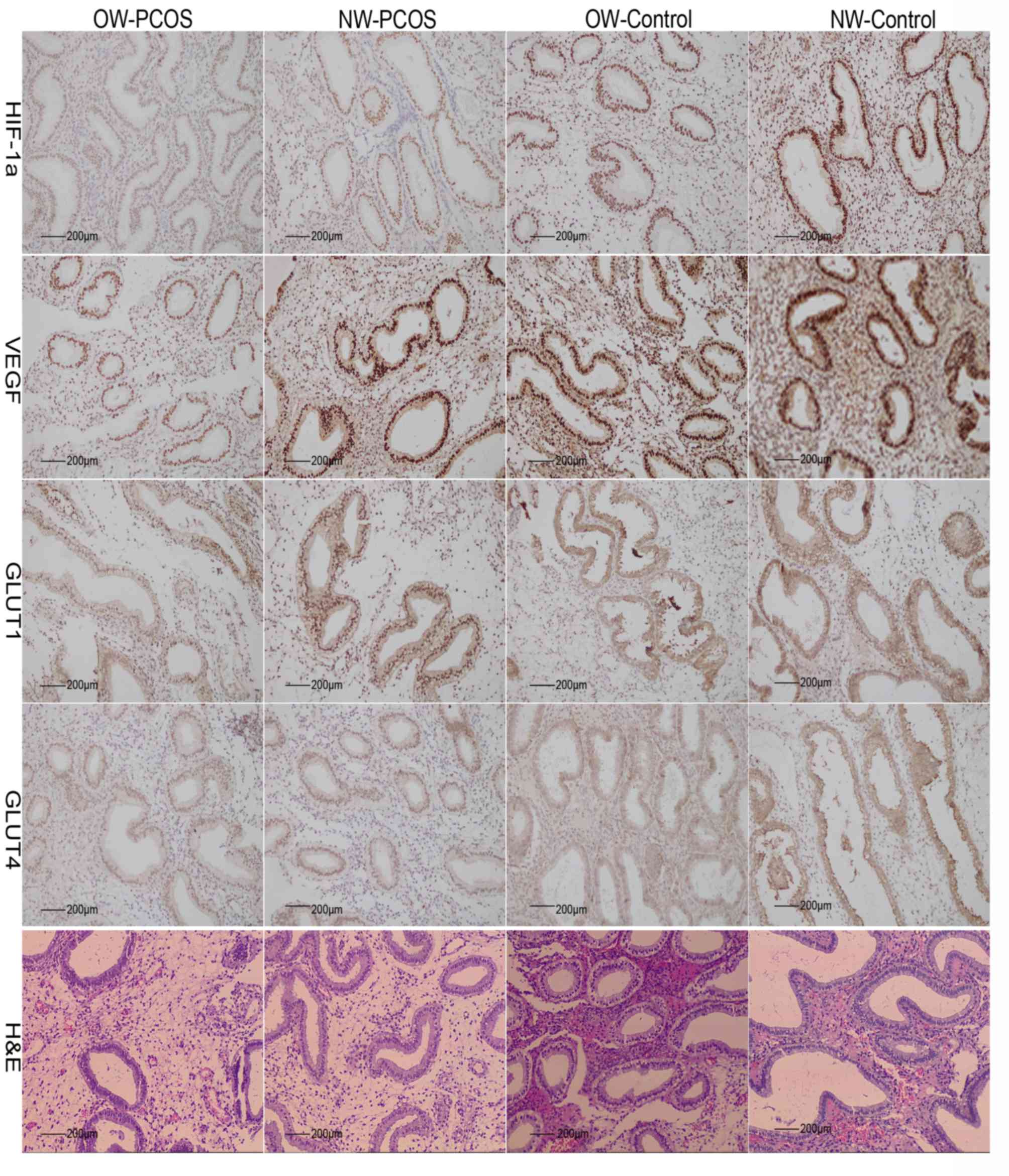 | Figure 2.Immunohistochemical and H&E
staining of endometrial tissues. Immunohistochemical detection of
HIF-1α, VEGF, GLUT1 and GLUT4 proteins in the endometrium of
OW-PCOS, NW-PCOS, OW-Control and NW-Control patients. Brown
positive immunostaining was detected in epithelial and stroma cells
of endometria for all antigens. H&E, hematoxylin and eosin.
HIF-1α, hypoxia-inducible factor-1α; GLUT, glucose transporter
protein; NW, normal weight; OW, overweight; POCS, polycystic ovary
syndrome; VEGF, vascular endothelial growth factor. |
Semi-quantitative IOD protein expression levels of
HIF-1α and VEGF in PCOS samples were significantly lower compared
with expression levels in the controls (P<0.05; Fig. 3A). No statistically significant
inter-group differences were identified with respect to GLUT1 and
GLUT4 immunostaining (P>0.05; Fig.
3A).
Association between body weight and
protein expression levels of HIF-1α, VEGF, GLUT1 and GLUT4
Semi-quantitative protein expression levels of
HIF-1α and VEGF in OW patients were significantly lower compared
with expression levels in the NW patients in both groups
(P<0.05; Fig. 3B and C).
Protein expression levels of GLUT1 were significantly lower in
OW-Control patients compared with expression levels in the
NW-Control group (Fig. 3C;
P<0.05). No statistically significant differences in GLUT4
protein expression levels were identified between the two subgroups
of patients with or without PCOS (P>0.05; Fig. 3B and C, respectively).
Discussion
Human embryogenesis takes place in a hypoxic
environment. At the time of embryo implantation, cell proliferation
and implantation of the blastocyst requires an increasing supply of
nutrients and oxygen, which promotes the establishment of the
vascular network at the implantation site. Hypoxia-induced
synthesis of HIF-1α modifies the endometrial microenvironment and
contributes to an improvement in uterine receptivity (35,36).
In the present study, quantitative mRNA and semi-quantitative
protein expression levels of HIF-1α were determined in the
endometrium during the implantation window. To the best of our
knowledge, this is the first study to investigate the role of
HIF-1α in determining endometrial receptivity for embryo
implantation.
Previous reports have demonstrated that HIF-1α
expression peaks during the endometrial secretory phase, suggesting
its involvement in endometrium repair (15). It was also observed that HIF-1α
expression in endometrium peaked at the implantation window at the
mRNA but not at the protein levels, suggesting stabilization of
HIF-1α in the hypoxic endometrial microenvironment and that HIF-1α
may serve an important role in the establishment of uterine
receptivity. This may be due to protein turnover rates affecting
protein concentrations. In biological systems, there is a high
protein turnover in proliferative and dividing cells; however, a
steady state may be reached following certain stimuli (37). Therefore, the inconsistency between
mRNA and protein expression levels of HIF-1α may reflect rapid
protein degradation that occurs with alleviation of hypoxia during
the time window for embryo implantation.
It is well known that HIF-1α may participate in the
establishment of endometrial receptivity during the time window for
embryo implantation (38).
However, in the present study, both mRNA and protein expression
levels of HIF-1α were significantly decreased in patients with
PCOS, which suggested that the hypoxic endometrial microenvironment
in patients with PCOS may be impaired. A recent study reported
significant changes in uterine receptivity in overweight or obese
women (4). The decreased HIF-1α
expression in OW-PCOS patients may be a cause of endometrial
dysfunction in these patients.
HIF-1α is a co-activator of estrogen-dependent VEGF
synthesis (39,40). The expression of VEGF is associated
with vascular density. A number of previous studies have suggested
that the vascularization of endometrium is a critical factor for
successful implantation. VEGF is an important mediator of
angiogenesis in female genital organs (41). VEGF expression was significantly
reduced in women with unexplained infertility and recurrent
spontaneous abortions (42,43),
which indicated it was important to the endometrial receptivity and
embryo development. VEGF expression levels in the endometrium of
patients with PCOS was significantly decreased during the
implantation window, which might reduce the vascular density of
endometrium and cause implantation failure.
HIF-1α is known to activate the expression of
hypoxic-sensitive genes GLUT1 and GLUT4. During the
window for embryo implantation, large amounts of energy are
required to fulfill the endometrial function and glucose is the
main energy source of endometrial cells (38). GLUT1 is a trans-membrane
glycoprotein that is responsible for the uptake and storage of
glucose in the endometrium, whereas GLUT4 is an insulin-dependent
glucose transporter that regulates fast glucose uptake (44). GLUT4 expression levels in the
present study were the lowest among those factors. This observation
was consistent with a previous study in which GLUT1, but not GLUT4,
was demonstrated to be essential for the decidualization of the
secretory endometrium (45). There
were no statistically significant differences in GLUT1 and GLUT4
expression levels between the POCS and Control groups; however,
immunohistochemistry demonstrated differences in their
intracellular localization. In Control patients, these proteins
were localized in the cell membrane of epithelial cells, while in
patients with PCOS, they were observed mainly in the cytoplasm and
nucleus. Under basal conditions, GLUT1 and GLUT4 are localized into
intracellular vesicles and glucose uptake is completed when the
vesicles translocate to the cell surface (46). Reduced translocation of GLUT1
vesicles to the cell membrane of cells in patients with PCOS was
observed, which probably impaired glucose uptake by the cells and
affect the decidualization of the endometrium. Therefore, the
impaired endometrial receptivity in PCOS patients may be associated
with impaired GLUT1 functions.
In conclusion, the present study indicated that
HIF-1α may be involved in the molecular mechanisms underlying
endometrial dysfunction in patients with PCOS, particularly in
those who are overweight. Further functional studies need to be
conducted in these patients to confirm these findings.
References
|
1
|
Duncan WC: A guide to understanding
polycystic ovary syndrome (PCOS). J Fam Plann Reprod Health Care.
40:217–225. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lim SS, Davies MJ, Norman RJ and Moran LJ:
Overweight, obesity and central obesity in women with polycystic
ovary syndrome: A systematic review and meta-analysis. Hum Reprod
Update. 18:618–637. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bellver J, Mifsud A, Grau N, Privitera L
and Meseguer M: Similar morphokinetic patterns in embryos derived
from obese and normoweight infertile women: A time-lapse study. Hum
Reprod. 28:794–800. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Provost MP, Acharya KS, Acharya CR, Yeh
JS, Steward RG, Eaton JL, Goldfarb JM and Muasher SJ: Pregnancy
outcomes decline with increasing body mass index: Analysis of
239,127 fresh autologous in vitro fertilization cycles from the
2008–2010 Society for Assisted Reproductive Technology registry.
Fertil Steril. 105:663–669. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lopes IM, Baracat MC, Mde Simoes J, Simões
RS, Baracat EC and Soares JM Jr: Endometrium in women with
polycystic ovary syndrome during the window of implantation. Rev
Assoc Med Bras (1992). 57:702–709. 2011.(In English, Portuguese).
PubMed/NCBI
|
|
6
|
Savaris RF, Groll JM, Young SL, DeMayo FJ,
Jeong JW, Hamilton AE, Giudice LC and Lessey BA: Progesterone
resistance in PCOS endometrium: A microarray analysis in clomiphene
citrate-treated and artificial menstrual cycles. J Clin Endocrinol
Metab. 96:1737–1746. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lam P, Johnson I and Raine-Fenning N:
Endometrial blood flow is impaired in women with polycystic ovarian
syndrome who are clinically hyperandrogenic. Ultrasound Obstet
Gynecol. 34:326–334. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Torry DS, Leavenworth J, Chang M,
Maheshwari V, Groesch K, Ball ER and Torry RJ: Angiogenesis in
implantation. J Assist Reprod Genet. 24:303–315. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kingdom JC and Kaufmann P: Oxygen and
placental vascular development. Adv Exp Med Biol. 474:259–275.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schofield CJ and Ratcliffe PJ: Oxygen
sensing by HIF hydroxylases. Nat Rev Mol Cell Biol. 5:343–354.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kaelin WG Jr and Ratcliffe PJ: Oxygen
sensing by metazoans: The central role of the HIF hydroxylase
pathway. Mol Cell. 30:393–402. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Majmundar AJ, Wong WJ and Simon MC:
Hypoxia-inducible factors and the response to hypoxic stress. Mol
Cell. 40:294–309. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Han ZB, Ren H, Zhao H, Chi Y, Chen K, Zhou
B, Liu YJ, Zhang L, Xu B, Liu B, et al: Hypoxia-inducible factor
(HIF)-1 alpha directly enhances the transcriptional activity of
stem cell factor (SCF) in response to hypoxia and epidermal growth
factor (EGF). Carcinogenesis. 29:1853–1861. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gómez-Raposo C, Mendiola M, Barriuso J,
Casado E, Hardisson D and Redondo A: Angiogenesis and ovarian
cancer. Clin Transl Oncol. 11:564–571. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Critchley HO, Osei J, Henderson TA,
Boswell L, Sales KJ, Jabbour HN and Hirani N: Hypoxia-inducible
factor-1alpha expression in human endometrium and its regulation by
prostaglandin E-series prostanoid receptor 2 (EP2). Endocrinology.
147:744–753. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shibuya M: Vascular endothelial growth
factor and its receptor system: Physiological functions in
angiogenesis and pathological roles in various diseases. J Biochem.
153:13–19. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Binder NK, Evans J, Gardner DK, Salamonsen
LA and Hannan NJ: Endometrial signals improve embryo outcome:
Functional role of vascular endothelial growth factor isoforms on
embryo development and implantation in mice. Hum Reprod.
29:2278–2286. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hayashi M, Sakata M, Takeda T, Yamamoto T,
Okamoto Y, Sawada K, Kimura A, Minekawa R, Tahara M, Tasaka K and
Murata Y: Induction of glucose transporter 1 expression through
hypoxia-inducible factor 1alpha under hypoxic conditions in
trophoblast-derived cells. J Endocrinol. 183:145–154. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhai J, Liu CX, Tian ZR, Jiang QH and Sun
YP: Effects of metformin on the expression of GLUT4 in endometrium
of obese women with polycystic ovary syndrome. Biol Reprod.
87:292012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Goteri G, Lucarini G, Montik N, Zizzi A,
Stramazzotti D, Fabris G, Tranquilli AL and Ciavattini A:
Expression of vascular endothelial growth factor (VEGF), hypoxia
inducible factor-1alpha (HIF-1alpha), and microvessel density in
endometrial tissue in women with adenomyosis. Int J Gynecol Pathol.
28:157–163. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Giudice LC: Application of functional
genomics to primate endometrium: Insights into biological
processes. Reprod Biol Endocrinol. 4 Suppl 1:S42006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rotterdam ESHRE/ASRM-Sponsored PCOS
consensus workshop group: Revised 2003 consensus on diagnostic
criteria and long-term health risks related to polycystic ovary
syndrome (PCOS). Hum Reprod. 19:41–47. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cole TJ, Bellizzi MC, Flegal KM and Dietz
WH: Establishing a standard definition for child overweight and
obesity worldwide: International survey. BMJ. 320:1240–1243. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xing XY, Yang WY and Yang ZJ: The
diagnostic significance of homeostasis model assessment of insulin
resistance in metabolic syndrome among subjects with different
glucose tolerance. Chin J Diabetes. 12:182–186. 2004.(In
Chinese).
|
|
25
|
Bellver J, Pellicer A, Garcia-Velasco JA,
Ballesteros A, Remohí J and Meseguer M: Obesity reduces uterine
receptivity: Clinical experience from 9,587 first cycles of ovum
donation with normal weight donors. Fertil Steril. 100:1050–1058.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu Z, Hao C, Song D, Zhang N, Bao H and
Qu Q: Androgen receptor coregulator CTBP1-AS is associated with
polycystic ovary syndrome in chinese women: A preliminary study.
Reprod Sci. 22:829–837. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fang Y, Yu S, Ma Y, Sun P, Ma D, Ji C and
Kong B: Association of Dll4/notch and HIF-1α-VEGF signaling in the
angiogenesis of missed abortion. PLoS One. 8:e706672013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pfaffl MW: A new mathematical model for
relative quantification in real-time RT-PCR. Nucleic Acids Res.
29:e452001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fischer AH, Jacobson KA, Rose J and Zeller
R: Hematoxylin and eosin staining of tissue and cell sections. CSH
Protoc. 2008:pdb prot4986. 2008.
|
|
31
|
Dubicke A, Fransson E, Centini G,
Andersson E, Byström B, Malmström A, Petraglia F,
Sverremark-Ekström E and Ekman-Ordeberg G: Pro-inflammatory and
anti-inflammatory cytokines in human preterm and term cervical
ripening. J Reprod Immunol. 84:176–185. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rivero R, Garin CA, Ormazabal P, Silva A,
Carvajal R, Gabler F, Romero C and Vega M: Protein expression of
PKCZ (Protein Kinase C Zeta), Munc18c and Syntaxin-4 in the insulin
pathway in endometria of patients with polycystic ovary syndrome
(PCOS). Reprod Biol Endocrinol. 10:172012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
García V, Oróstica L, Poblete C, Rosas C,
Astorga I, Romero C and Vega M: Endometria from obese PCOS women
with hyperinsulinemia exhibit altered adiponectin signaling. Horm
Metab Res. 47:901–909. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Noyes RW, Hertig AT and Rock J: Dating the
endometrial biopsy. Am J Obstet Gynecol. 122:262–263. 1975.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bianju Xu and Hongmei Li: The progress of
HIF-1a in the embryo development. Chinese J Trauma and Disab Med.
20:208–210. 2011.(In Chinese).
|
|
36
|
Tsuzuki T, Okada H, Cho H, Tsuji S,
Nishigaki A, Yasuda K and Kanzaki H: Hypoxic stress stimultaneously
stimulates vascular endothelial growth factor via hypoxia-inducible
factor-1α and inhibits stromal cell-derived factor-1 in human
endometrial stromal cells. Hum Reprod. 27:523–530. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
De Sousa Abreu R, Penalva LO, Marcotte EM
and Vogel C: Global signatures of protein and mRNA expression
levels. Mol Biosyst. 5:1512–1526. 2009.PubMed/NCBI
|
|
38
|
Xu BF, Sun XX, Feng Y, Zhang AJ and Cheng
LN: Mechanism of hypoxia inducing factor-1α in low endometrial
receptivity. Zhonghua Fu Chan Ke Za Zhi. 46:355–359. 2011.(In
Chinese). PubMed/NCBI
|
|
39
|
Koos RD, Kazi AA, Roberson MS and Jones
JM: New insight into the transcriptional regulation of vascular
endothelial growth factor expression in the endometrium by estrogen
and relaxin. Ann N Y Acad Sci. 1041:233–247. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Okada H, Tsutsumi A, Imai M, Nakajima T,
Yasuda K and Kanzaki H: Estrogen and selective estrogen receptor
modulators regulate vascular endothelial growth factor and soluble
vascular endothelial growth factor receptor 1 in human endometrial
stromal cells. Fertil Steril. 93:2680–2686. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shimizu T, Hoshino Y, Miyazaki H and Sato
E: Angiogenesis and microvasculature in the female reproductive
organs: Physiological and pathological implications. Curr Pharm
Des. 18:303–309. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hannan NJ, Paiva P, Meehan KL, Rombauts
LJ, Gardner DK and Salamonsen LA: Analysis of fertility-related
soluble mediators in human uterine fluid identifies VEGF as a key
regulator of embryo implantation. Endocrinology. 152:4948–4956.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lash GE, Innes BA, Drury JA, Robson SC,
Quenby S and Bulmer JN: Localization of angiogenic growth factors
and their receptors in the human endometrium throughout the
menstrual cycle and in recurrent miscarriage. Hum Reprod.
27:183–195. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mueckler M: Family of glucose-transporter
genes. Implications for glucose homeostasis and diabetes. Diabetes.
39:6–11. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Frolova A, Flessner L, Chi M, Kim ST,
Foyouzi-Yousefi N and Moley KH: Facilitative glucose transporter
type 1 is differentially regulated by progesterone and estrogen in
murine and human endometrial stromal cells. Endocrinology.
150:1512–1520. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Diamanti-Kandarakis E and Dunaif A:
Insulin resistance and the polycystic ovary syndrome revisited: An
update on mechanisms and implications. Endocr Rev. 33:981–1030.
2012. View Article : Google Scholar : PubMed/NCBI
|