Introduction
Prostate cancer (PCa) is one of the most common
types of cancer, and the leading cause of cancer-associated
mortality in men worldwide (1,2). The
molecular mechanisms that underlie the tumorigenesis, progression
and metastasis of PCa remain unclear, regardless of a large number
of research studies. Noncoding RNAs have been reported to serve
pivotal roles in the pathogenesis of numerous types of cancer via
regulating the diversity of biological processes. Therefore,
identifying the noncoding RNAs associated with PCa may provide a
novel insight into the mechanisms underlying PCa carcinogenesis
(3–6).
MicroRNAs (miRNAs/miRs) are small
post-transcriptional regulatory noncoding RNAs, 20–22 nt in length.
Previous studies have reported the aberrant expression of miRNAs in
numerous types of human malignancy, including breast and lung
cancer, and PCa (7–11). In PCa, numerous dysregulated
miRNAs, inducing miR-27a (12,13),
miR-135a (14), miR-186 (15), miR-4638-5p (16), miR-124 (17–19)
and miR-320 (20,21), have been reported to regulate cell
growth, apoptosis, migration and invasion. These findings indicated
that dysregulation of miRNA expression may be associated with
carcinogenesis of PCa.
miR-512-3p has been reported to act as a tumor
suppressor in hepatocellular carcinoma (22) and lung adenocarcinoma (23). In addition, miR-512-3p has also
been revealed to be upregulated in non-small cell lung cancer
(NSCLC) A549 cells following retinoic acid (RA) treatment, and has
been demonstrated to inhibit the adhesion, migration and invasion
of NSCLC cells (23). However, the
role of miR-512-3p in PCa remains poorly understood and therefore
requires further investigation.
The present study identified differentially
expressed miRNAs in PCa, by analyzing two publicly available gene
expression datasets, GSE14857 (24) and GSE21036 (25). Furthermore, the molecular functions
of miR-512-3p in PCa were investigated, in order to identify their
potential roles in the carcinogenesis of PCa.
Materials and methods
miRNA profile data collection
miRNA profile datasets (GSE14857 and GSE21036) were
collected from the Gene Expression Omnibus database (www.ncbi.nlm.nih.gov/gds). Comparison of the
miRNA profiles between PCa samples and normal tissue samples was
performed with limma package in R software using raw microarray
data. Significantly differentially expressed miRNAs were identified
with thresholds of |logFC|>1.0 and P<0.05.
Cell culture
LNCaP cells were purchased from the American Type
Culture Collection (Manassas, VA, USA). PC-3 and 22RV1 cells, and
the noncancerous prostatic cell line WPMY-1, were obtained from the
Cell Bank of Chinese Academy of Sciences (Shanghai, China). All
cell lines were confirmed by short tandem repeat analysis. The four
cell lines were cultured in Ham's F12K media (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10%
(vol/vol) fetal bovine serum (cat. no. 10099141M; Gibco; Thermo
Fisher Scientific, Inc.), at 37°C in a humidified atmosphere
containing 5% CO2.
Cell transfection
The synthetic miR-512-3p mimics and a scrambled
control miRNA [miR-negative control (NC)] were purchased from
Shanghai GenePharma Co., Ltd. (Shanghai, China). The sequences were
as follows: miR-512-3p mimics, 5′-AAGUGCUGUCAUAGCUGAGGUC-3′ (sense)
and 5′-CCUCAGCUAUGACAGCACUUUU-3′ (antisense); NC mimics,
5′-UUCUCCGAACGUGUCACGUTT-3′ (sense) and 5′-ACGUGACACGUUCGGAGAATT-3′
(antisense). PCa cells were seeded at 3×105 cells/wells
in 6-well plates and were incubated at 37°C in a humidified
atmosphere containing 5% CO2 overnight. Subsequently,
transfection with the miR-512-3p mimic or miR-NC was performed
using Lipofectamine 2000 transfection reagent (Invitrogen; Thermo
Fisher Scientific, Inc.). The cells were transfected with 300 nmol
miRNA according to the manufacturer's protocol. Total RNA was
extracted from the cells 48 h post-transfection and western
blotting was also performed.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA, which was used for RT-qPCR analysis, was
extracted from the cells using TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol.
RT was performed using the PrimeScript™ RT reagent kit (Takara Bio,
Inc., Otsu, Japan) according to the manufacturer's protocol. The
sequence of the miR-512-3p-specific RT primer was
5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACGACCTC-3′. To
analyze miRNA expression, RT-qPCR was performed using SYBR-Green
Reagents (Bio-Rad Laboratories, Inc., Hercules, CA, USA) on a
LightCycler 480 system (Roche Diagnostics, Basel, Switzerland). The
expression levels of miR-512-3p were normalized to U6. The PCR
primers for mature miR-512-3p, Rho family GTPase 3 (RND3), MX
dynamin like GTPase 1 (MXI1), mitofusin 2 (MFN2), forkhead box O1
(FOXO1), RNA binding motif protein38 (RBM38), transforming
coiled-coil containing protein 1 (TACC1) and U6 were as follows:
miR-512-3p forward, 5′-CGGCGGCACTCAGCCTTGAGGG-3′ and reverse,
5′-GTGCAGGGTCCGAGGT-3′; RND3 forward, 5′-AAAAACTGCGCTGCTCCAT-3′ and
reverse, 5′-TCAAAACTGGCCGTGTAATTC-3′; MXI1 forward,
5′-CATGGAGCGGGTGAAGAT-3′ and reverse, 5′-ATGAAGAGGCGTAGCCATGT-3′;
MFN2 forward, 5′-TGCCTCAGAGCCCGAGTA-3′ and reverse,
5′-CTGGTACAACGCTCCATGTG-3′; FOXO1 forward,
5′-AAGGGTGACAGCAACAGCTC-3′ and reverse, 5′-TTCCTTCATTCTGCACACGA-3′;
RBM38 forward, 5′-TTGATCCAGCGGACTTACG-3′ and reverse,
5′-AATGTAGGGCGAGGACAGC-3′; TACC1 forward, 5′-GCGAAATGGACGTGGTCT-3′
and reverse, 5′-CACCTTACAGCCACTCCTGAA-3′; and U6 forward,
5′-CGCTTCGGCAGCACATATACTAA-3′ and reverse
5′-TATGGAACGCTTCACGAATTTGC-3′. The results were normalized to those
of β-actin or U6 as the internal control to estimate the different
expression of genes. Relative mRNA and miRNA expression was
calculated using the 2−ΔΔCq method (26). Each sample was assayed in
triplicate to ensure quantitative accuracy.
Western blot analysis
Cells were lysed in radioimmunoprecipitation assay
buffer (Boston Bioproducts, Inc., Ashland, MA, USA) supplemented
with cOmplete™, EDTA-free Protease Inhibitors (Roche Diagnostics)
and phenylmethylsulfonyl fluoride (Calbiochem; EMD Millipore,
Billerica, MA, USA). The protein concentration was determined using
the Pierce™ Bicinchoninic acid Protein Assay (cat. no. 23222;
Thermo Fisher Scientific, Inc.), in accordance with the
manufacturer's instructions. A total of 30 µg/lane protein was
loaded and separated by 12% SDS-PAGE, which was then transferred to
PVDF membranes. Membranes were blocked in Tris buffered saline with
0.05% Tween-20 containing 5% non-fat dry milk at room temperature
for 1 h. Immunoblots were incubated overnight at 4°C with the
following primary antibodies: Anti-p21 (1:1,000; cat. no. ab109520;
Abcam, Cambridge, MA, USA) and anti-β-actin (1:3,000; cat. no.
A1978; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany).
Subsequently, the blots were incubated at room temperature for 1 h
with goat anti-mouse immunoglobulin (Ig)G-horseradish peroxidase
(HRP)-conjugated and goat anti-rabbit IgG-HRP-conjugated secondary
antibodies (1:4,000; cat. nos. A4416 and A6154, respectively;
Sigma-Aldrich; Merck KGaA). An Electrochemiluminescence Plus kit
(cat. no. RPN2132; GE Healthcare Life Sciences, Uppsala, Sweden)
was used for visualization.
Cell proliferation assay
Cells were seeded into 96-well plates at 2,000
cells/well 6 h post-transfection. The Cell Counting kit-8 (CCK-8;
Dojindo Molecular Technologies, Inc., Kumamoto, Japan) was used to
detect relative cell proliferation for 4 days. Briefly, 10 µl/well
CCK-8 agent was added to the cells, which were incubated for 2 h at
37°C; subsequently, absorbance was measured at 450 nm using an
ELx808 microplate reader (BioTek Instruments, Inc., Winooski, VT,
USA).
Cell cycle analysis
Transfected LNCaP, 22Rv1 and PC-3 cells in the log
phase of growth were collected and fixed in 0.03% Triton X-100 and
propidium iodide (PI; 50 ng/ml) at room temperature for 15 min, 48
h post-transfection. For cell cycle analysis, the transfected cells
were examined using a FACSCalibur flow cytometer (BD Biosciences,
San Jose, CA, USA) and were analyzed with ModFit version 4.1
software (Verity Software House, ME, USA). Each test was performed
in triplicate.
Gene Ontology (GO) and Kyoto
Encyclopedia of Genes and Genomes (KEGG) pathway analysis
The Molecule Annotation System (MAS; version 3.0),
provided by CapitalBio Corporation (Beijing, China; bioinfo.capitalbio.com/mas3/) was used to
determine the biological roles of differentially expressed mRNAs.
Gene functions were classified in to three subgroups: Biological
process, cellular component and molecular function. The enriched GO
terms were presented by enrichment scores. KEGG pathway analysis
was carried out to determine the involvement of differentially
expressed mRNAs in different biological pathways. The recommended
hypergeometric-P-value used as the cut-off was P<0.05.
Statistical analysis
Numerical data were presented as the mean ± standard
deviation of at least three determinations. Statistical comparisons
between groups of normalized data were performed using an unpaired
Student's t-test and SPSS v13.0 software (SPSS, Inc., Chicago, IL,
USA) or a Mann-Whitney U-test according to the test condition.
Statistical comparisons among multiple groups of normalized data
were performed using one-way analysis of variance followed by a
Dunnett's post hoc test. P<0.05 was considered to indicate a
statistically significant difference with a 95% confidence
level.
Results
miR-512-3p is overexpressed in
PCa
To identify the differentially expressed miRNAs in
PCa, two publicly available gene expression datasets, GSE14857 and
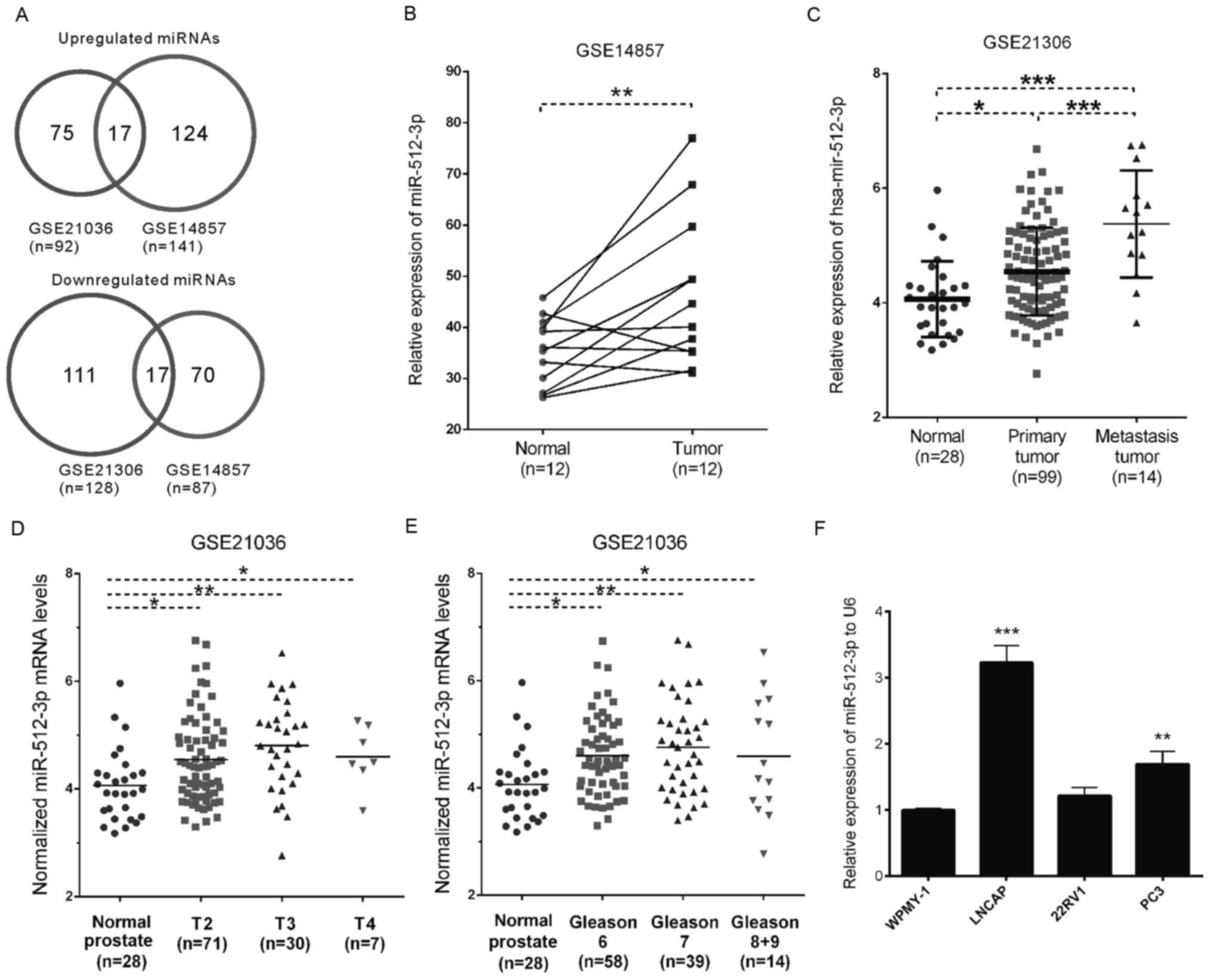
GSE21036, were analyzed (Fig. 1A).
A total of 17 miRNAs were downregulated and 17 miRNAs were
overexpressed in PCa in both databases. The present study primarily
focused on the 17 upregulated miRNAs (miR-106b, miR-93, miR-148a,
miR-25, miR-375, miR-130b, miR-512-3p, miR-18a, miR-518c*, miR-7,
miR-95, miR-96, miR-32, miR-663, miR-182, miR-183 and miR-153) as
putative biomarkers. The majority of these miRNAs have been
reported to be involved in the carcinogenesis of PCa (27–37);
however, the molecular functions of miR-512-3p and miR-518c* in PCa
remained unclear.
Analysis of the GSE14857 and GSE21036 datasets
indicated that miR-512-3p expression was significantly upregulated
in tumor samples compared with in normal samples (P<0.01;
Fig. 1B), and was overexpressed in
metastatic samples compared with in primary tumor tissues
(P<0.001; Fig. 1C). A clinical
significance analysis of GSE21036 demonstrated that miR-512-3p
expression levels were overexpressed in patients with T2
(P<0.05), T3 (P<0.01) and T4 (P<0.05) PCa compared with
the normal controls (Fig. 1D).
Subsequently, the patients in GSE21036 were categorized based on
Gleason grades, and the results demonstrated that tissues from
patients with Gleason grades 6, 7, 8 and 9 PCa exhibited
significantly higher levels of miR-512-3p compared with the matched
normal tissues (Fig. 1E).
The present study also detected miR-512-3p
expression in PCa cell lines. RT-qPCR was conducted to detect the
expression levels of miR-512-3p in PCa cell lines LNCaP, 22Rv1 and
PC-3, and in the noncancerous prostatic cells WPMY-1 cell line. The
results demonstrated that miR-512-3p was upregulated in PCa cells
(including LNCaP and PC-3; Fig.
1F). However, no significant upregulation of miR-512-3p was
observed in 22Rv1 cells. These results were consistent with the
previous findings in PCa and normal tissues.
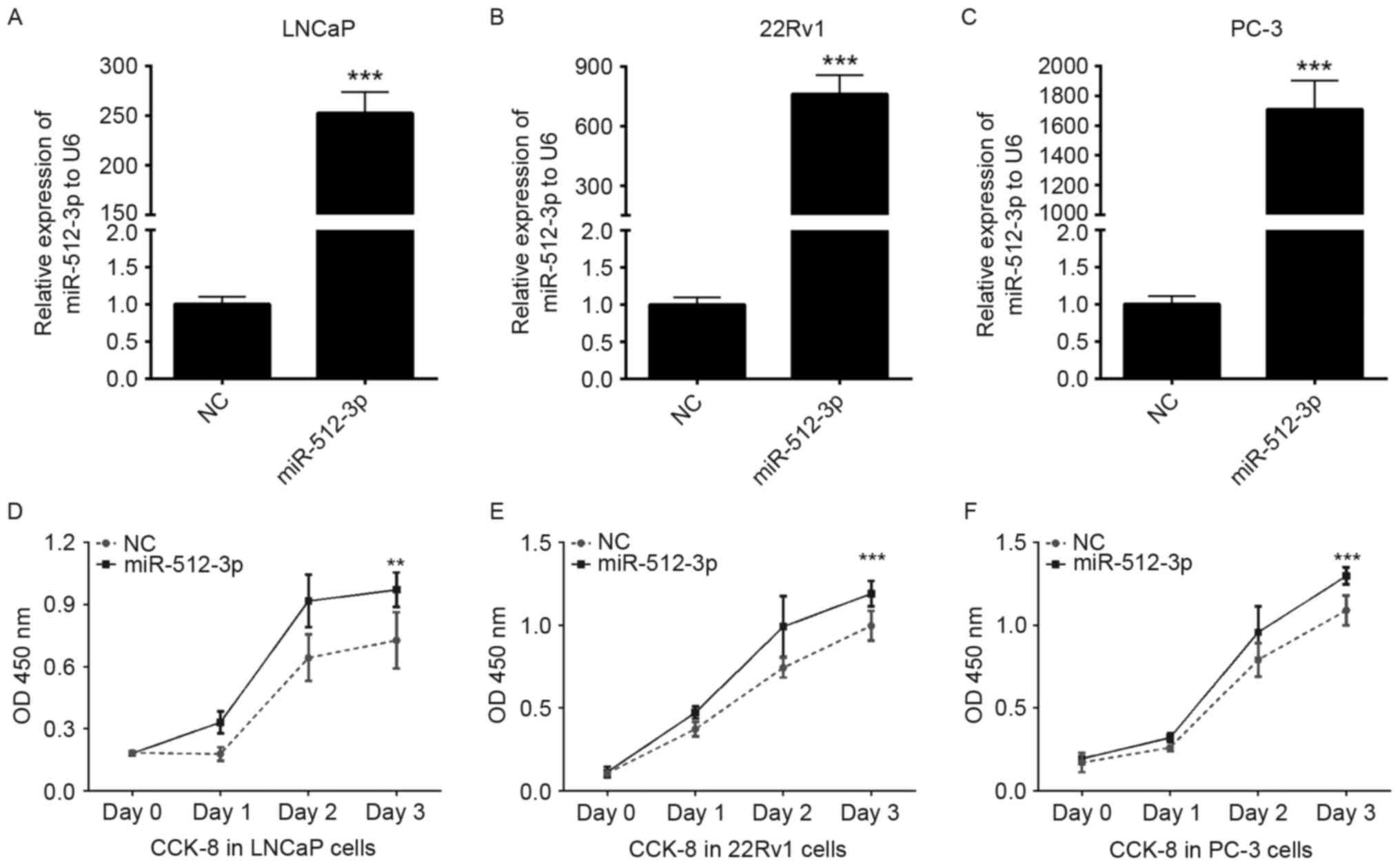
Overexpression of miR-512-3p promotes
PCa cell proliferation
The present study aimed to explore the potential
effects of miR-512-3p on the proliferation of PCa cells. Initially,
the effects of a miR-512-3p mimic were determined on the expression
of miR-512-3p. LNCaP, 22Rv1 and PC-3 cells were transfected with NC
or miR-512-3p mimics. A total of 48 h post-transfection, the
expression levels of miR-512-3p were significantly increased in the
miR-512-3p mimic group compared with the NC group (P<0.001;
Fig. 2A-C). Subsequently, cell
proliferation was investigated using a CCK-8 assay, overexpression
of miR-512-3p significantly promoted the proliferation of LNCaP,
22Rv1and PC-3 cells (P<0.01 and P<0.001; Fig. 2D-F).
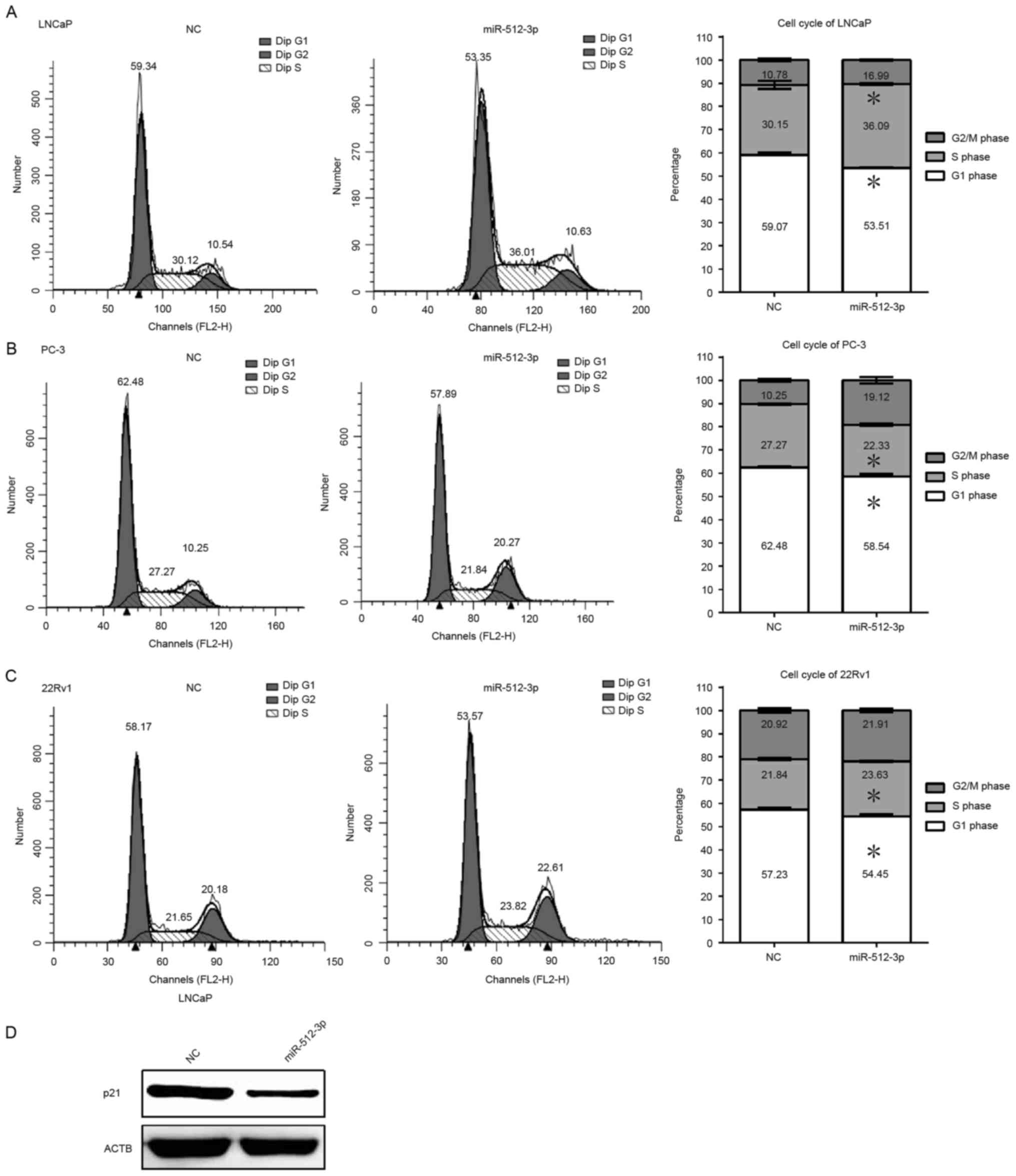
Overexpression of miR-512-3p prevents
G1 phase cell cycle arrest in vitro
The present study assessed the function of
miR-512-3p on cell cycle progression in LNCaP, 22Rv1 and PC-3
cells. Flow cytometric analysis revealed that overexpression of
miR-512-3p in LNCaP and 22RV1 cells resulted in a significant
increase in the proportion of cells in S phase and a decrease in
the proportion of cells in G1 phase. However, overexpression of
miR-512-3p in PC-3 cells decreased the proportion of cells in S
phase and increased the proportion of cells in the G2/M phase
(P<0.05; Fig. 3A-C). In
addition, a decrease in the protein expression levels of cell cycle
inhibitor p21 was detected in cells overexpressing miR-512-3p
(Fig. 3D). Downregulation of p21
promotes cell cycle progression, thus these results suggest that
the overexpression of miR-512-3p may promote cell cycle progression
by inhibiting p21 (38,39).
GO category and KEGG pathway
analyses
To obtain valuable insights into the potential
mechanisms of miR-512-3p, a bioinformatics analysis was performed
to identify the target genes of miR-512-3p using starBase (40). starBase is a database that combines
data from six prediction programs TargetScan (41), PicTar (www.pictar.org/), miRanda (www.microrna.org/microrna/home.do), PITA (https://genie.weizmann.ac.il/), RNA22 (https://cm.jefferson.edu/rna22/) and CLIP-Seq
(www.starbase.sysu.edu.cn/). A total of
663 targets of miR-512-3p were used to perform the KEGG pathway
(www.genome.jp/kegg/) and GO category
(www.geneontology.org/) analyses using
MAS 3.0 system (http://bioinfo.capitalbio.com/mas3/). The results
revealed that miR-512-3p may affect numerous biological processes,
including cell adhesion, cell proliferation, cell cycle and
apoptosis (Fig. 4A). Pathway
enrichment analysis demonstrated that miR-512-3p predominantly
participated in the mitogen-activated protein kinase (MAPK)
signaling pathway, focal adhesion, cell cycle and transforming
growth factor (TGF)-β pathway (Fig.
4B). To further validate these findings the present study
detected the expression of numerous pathway-associated genes using
RT-qPCR. LNCaP cell lines retain characteristics associated with
early androgen-dependent molecular biology and tumor cytology
(42), and LNCaP is one of the
most commonly used cell lines in the PCa research field (43). Thus, LNCaP cell selected for
further validation. Overexpression of miR-512-3p was able to
significantly reduce the expression levels of Rho family GTPase 3,
MAX interactor 1, dimerization protein, MFN2 and forkhead box O1
(Fig. 4C).
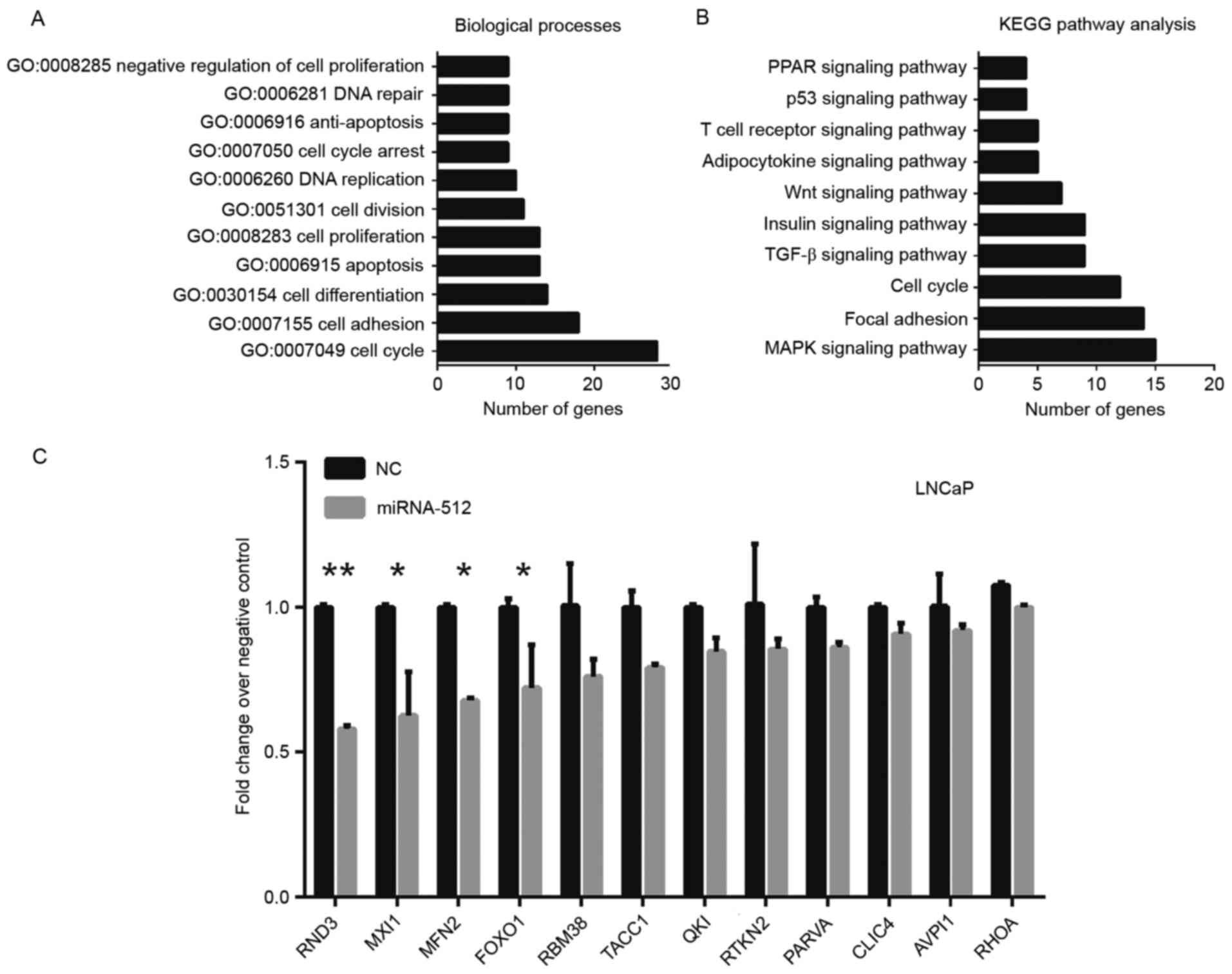 | Figure 4.(A) Biological processes and (B) KEGG
pathway analyses of miR-512-3p. (C) mRNA expression levels of
numerous pathway-related genes following overexpression of
miR-512-3p. *P<0.05 and **P<0.01 vs. NC. AVPI1, arginine
vasopressin induced 1; CLIC4, chloride intracellular channel 4;
FOXO1, forkhead box O1; GO, Gene Ontology; KEGG, Kyoto Encyclopedia
of Genes and Genomes; MAPK, mitogen-activated protein kinase; miR,
microRNA; MXI1, MX dynamin like GTPase 1; NC, negative control;
PARVA, parvin α; PPAR, peroxisome proliferator-activated receptor;
QKI, QKI, KH domain containing RNA binding; RBM38, RNA binding
motif protein 38; RND3, Rho family GTPase 3; TACC1, transforming
coiled-coil containing protein 1; TGF, transforming growth factor;
MFN2, mitofusin 2; RHOA, Ras homolog family member A; RTKN2,
rhotekin 2. |
Discussion
PCa is a leading cause of cancer-associated
mortality in men worldwide; however, the precise molecular
mechanisms underlying the progression of PCa remain unclear.
Numerous studies have revealed that miRNAs regulate several
biological processes in PCa, including proliferation, cell cycle
progression and metastasis (44–47).
miRNA expression profiles provide valuable insights into the
molecular mechanisms of PCa, and may be used to identify novel
biomarkers of PCa. The present study analyzed two publicly
available gene expression datasets and screened differentially
expressed miRNAs in PCa.
A total of 17 miRNAs were downregulated and 17
miRNAs were overexpressed in PCa samples compared with normal
controls in both datasets. The present study primarily focused on
the 17 upregulated miRNAs (miR-106b, miR-93, miR-148a, miR-25,
miR-375, miR-130b, miR-512-3p, miR-18a, miR-518c*, miR-7, miR-95,
miR-96, miR-32, miR-663, miR-182, miR-183 and miR-153) as putative
biomarkers. The majority of these miRNAs have been reported to be
involved in the carcinogenesis of PCa (27–37).
The present study revealed that miR-512-3p was
significantly upregulated in PCa compared with in normal tissues;
however, to the best of our knowledge, the molecular functions of
miR-512-3p in PCa have yet to be reported in PCa. In lung
adenocarcinoma, miR-512-3p has been reported to act as a tumor
suppressor that inhibits cell adhesion, migration and invasion of
NSCLC cells (23). However, the
roles of miR-512-3p in PCa remain unclear. The present study
demonstrated that overexpression of miR-512-3p promoted PCa cell
proliferation and reduced G1 phase cell cycle arrest in
PCa. The results indicated that miR-512-3p may act as an oncogene
in PCa and may serve varying roles in different types of
cancers.
To obtain valuable insights into the potential
mechanisms of miR-512-3p, a bioinformatics analysis was conducted
to identify miR-512-3p target genes using starBase (40). A total of 663 targets of miR-512-3p
were used to perform KEGG pathway and GO category analyses. The
results revealed that miR-512-3p may affect numerous biological
processes, including cell adhesion, cell proliferation, cell cycle
and apoptosis. Pathway enrichment analyses demonstrated that
miR-512-3p was associated with the MAPK signaling pathway, focal
adhesion, cell cycle and TGF-β pathway. Further validation revealed
that overexpression of miR-512-3p significantly reduced the
expression levels of RND3, MXI1, MFN2 and FOXO1. These results
suggest that miR-512-3p may serve an important role in the
regulation of PCa progression by regulating several genes,
including RND3, MXI1, MFN2 and FOXO1.
In conclusion, the present study analyzed two
publicly available gene expression datasets, and screened
differentially expressed miRNAs in PCa. The results demonstrated
that miR-512-3p may promote PCa cell proliferation and cell cycle
progression, thus suggesting that miR-512-3p may be considered a
potential diagnostic and therapeutic target of PCa.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang D, Ding L, Wang L, Zhao Y, Sun Z,
Karnes RJ, Zhang J and Huang H: LncRNA MALAT1 enhances oncogenic
activities of EZH2 in castration-resistant prostate cancer.
Oncotarget. 6:41045–41055. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Misawa A, Takayama K, Urano T and Inoue S:
Androgen-induced long noncoding RNA (lncRNA) SOCS2-AS1 promotes
cell growth and inhibits apoptosis in prostate Cancer Cells. J Biol
Chem. 291:17861–17880. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Işın M, Uysaler E, Özgür E, Köseoğlu H,
Şanlı Ö, Yücel ÖB, Gezer U and Dalay N: Exosomal IncRNA-p21 levels
may help to distinguish prostate cancer from benign disease. Front
Genet. 6:1682015.PubMed/NCBI
|
|
6
|
Zhu M, Chen Q, Liu X, Sun Q, Zhao X, Deng
R, Wang Y, Huang J, Xu M, Yan J and Yu J: lncRNA H19/miR-675 axis
represses prostate cancer metastasis by targeting TGFBI. FEBS J.
281:3766–3775. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang JX, Zhai JF, Yang XT and Wang J:
MicroRNA-132 inhibits migration, invasion and
epithelial-mesenchymal transition by regulating TGFβ1/Smad2 in
human non-small cell lung cancer. Eur Rev Med Pharmacol Sci.
20:3793–3801. 2016.PubMed/NCBI
|
|
8
|
Zhou Q, Zhu Y, Wei X, Zhou J, Chang L, Sui
H, Han Y, Piao D, Sha R and Bai Y: MiR-590-5p inhibits colorectal
cancer angiogenesis and metastasis by regulating nuclear factor
90/vascular endothelial growth factor A axis. Cell Death Dis.
7:e24132016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cui Z and Hu Y: MicroRNA-124 suppresses
Slug-mediated lung cancer metastasis. Eur Rev Med Pharmacol Sci.
20:3802–3811. 2016.PubMed/NCBI
|
|
10
|
Zhi Y, Pan J, Shen W, He P, Zheng J, Zhou
X, Lu G, Chen Z and Zhou Z: Ginkgolide B inhibits human bladder
cancer cell migration and invasion through MicroRNA-223-3p. Cell
Physiol Biochem. 39:1787–1794. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zuo J, Wang D, Shen H, Liu F, Han J and
Zhang X: MicroRNA-153 inhibits tumor progression in esophageal
squamous cell carcinoma by targeting SNAI1. Tumour Biol. Oct
13–2016.(Epub ahead of print). View Article : Google Scholar :
|
|
12
|
Mo W, Zhang J, Li X, Meng D, Gao Y, Yang
S, Wan X, Zhou C, Guo F, Huang Y, et al: Identification of novel
AR-targeted microRNAs mediating androgen signalling through
critical pathways to regulate cell viability in prostate cancer.
PLoS One. 8:e565922013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fletcher CE, Dart DA, Sita-Lumsden A,
Cheng H, Rennie PS and Bevan CL: Androgen-regulated processing of
the oncomir miR-27a, which targets prohibitin in prostate cancer.
Hum Mol Genet. 21:3112–3127. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kroiss A, Vincent S, Decaussin-Petrucci M,
Meugnier E, Viallet J, Ruffion A, Chalmel F, Samarut J and Allioli
N: Androgen-regulated microRNA-135a decreases prostate cancer cell
migration and invasion through downregulating ROCK1 and ROCK2.
Oncogene. 34:2846–2855. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hua X, Xiao Y, Pan W, Li M, Huang X, Liao
Z, Xian Q and Yu L: miR-186 inhibits cell proliferation of prostate
cancer by targeting GOLPH3. Am J Cancer Res. 6:1650–1660.
2016.PubMed/NCBI
|
|
16
|
Wang Y, Shao N, Mao X, Zhu M, Fan W, Shen
Z, Xiao R, Wang C, Bao W, Xu X, et al: MiR-4638-5p inhibits
castration resistance of prostate cancer through repressing
Kidins220 expression and PI3K/AKT pathway activity. Oncotarget.
7:47444–47464. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shi XB, Ma AH, Xue L, Li M, Nguyen HG,
Yang JC, Tepper CG, Gandour-Edwards R, Evans CP, Kung HJ and deVere
White RW: miR-124 and androgen receptor signaling inhibitors
repress prostate cancer growth by downregulating androgen receptor
splice variants, EZH2, and Src. Cancer Res. 75:5309–5317. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qin W, Pan Y, Zheng X, Li D, Bu J, Xu C,
Tang J, Cui R, Lin P and Yu X: MicroRNA-124 regulates TGF-α-induced
epithelial-mesenchymal transition in human prostate cancer cells.
Int J Oncol. 45:1225–1231. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lü L, Yuan JD, Cao ZL, Huang T, Zhang CH,
Wang L and Zeng FQ: MiR-124 suppresses the proliferation of human
prostate cancer PC3 cells by targeting PKM2. Zhonghua Nan Ke Xue.
20:495–499. 2014.(In Chinese). PubMed/NCBI
|
|
20
|
Sato S, Katsushima K, Shinjo K, Hatanaka
A, Ohka F, Suzuki S, Naiki-Ito A, Soga N, Takahashi S and Kondo Y:
Histone deacetylase inhibition in prostate cancer triggers
miR-320-mediated suppression of the androgen receptor. Cancer Res.
76:4192–4204. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hsieh IS, Chang KC, Tsai YT, Ke JY, Lu PJ,
Lee KH, Yeh SD, Hong TM and Chen YL: MicroRNA-320 suppresses the
stem cell-like characteristics of prostate cancer cells by
downregulating the Wnt/beta-catenin signaling pathway.
Carcinogenesis. 34:530–538. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen F, Zhu HH, Zhou LF, Wu SS, Wang J and
Chen Z: Inhibition of c-FLIP expression by miR-512-3p contributes
to Taxol-induced apoptosis in hepatocellular carcinoma cells. Oncol
Rep. 23:1457–1462. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu X, Gao G, Chu K, Yang X, Ren S, Li Y,
Wu H, Huang Y and Zhou C: Inhibition of RAC1-GEF DOCK3 by
miR-512-3p contributes to suppression of metastasis in non-small
cell lung cancer. Int J Biochem Cell Biol. 61:103–114. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schaefer A, Jung M, Mollenkopf HJ, Wagner
I, Stephan C, Jentzmik F, Miller K, Lein M, Kristiansen G and Jung
K: Diagnostic and prognostic implications of microRNA profiling in
prostate carcinoma. Int J Cancer. 126:1166–1176. 2010.PubMed/NCBI
|
|
25
|
Taylor BS, Schultz N, Hieronymus H,
Gopalan A, Xiao Y, Carver BS, Arora VK, Kaushik P, Cerami E, Reva
B, et al: Integrative genomic profiling of human prostate cancer.
Cancer Cell. 18:11–22. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Choi N, Park J, Lee JS, Yoe J, Park GY,
Kim E, Jeon H, Cho YM, Roh TY and Lee Y:
miR-93/miR-106b/miR-375-CIC-CRABP1: A novel regulatory axis in
prostate cancer progression. Oncotarget. 6:23533–23547. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang Y, Lieberman R, Pan J, Zhang Q, Du M,
Zhang P, Nevalainen M, Kohli M, Shenoy NK, Meng H, et al: miR-375
induces docetaxel resistance in prostate cancer by targeting SEC23A
and YAP1. Mol Cancer. 15:702016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu L, Zhong J, Guo B, Zhu Q, Liang H, Wen
N, Yun W and Zhang L: miR-96 promotes the growth of prostate
carcinoma cells by suppressing MTSS1. Tumour Biol. 37:12023–12032.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jalava SE, Urbanucci A, Latonen L,
Waltering KK, Sahu B, Jänne OA, Seppälä J, Lähdesmäki H, Tammela TL
and Visakorpi T: Androgen-regulated miR-32 targets BTG2 and is
overexpressed in castration-resistant prostate cancer. Oncogene.
31:4460–4471. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jiao L, Deng Z, Xu C, Yu Y, Li Y, Yang C,
Chen J, Liu Z, Huang G, Li LC and Sun Y: miR-663 induces
castration-resistant prostate cancer transformation and predicts
clinical recurrence. J Cell Physiol. 229:834–844. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wallis CJ, Gordanpour A, Bendavid JS,
Sugar L, Nam RK and Seth A: MiR-182 is associated with growth,
migration and invasion in prostate cancer via suppression of FOXO1.
J Cancer. 6:1295–1305. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Larne O, Östling P, Haflidadóttir BS,
Hagman Z, Aakula A, Kohonen P, Kallioniemi O, Edsjö A, Bjartell A,
Lilja H, et al: miR-183 in prostate cancer cells positively
regulates synthesis and serum levels of prostate-specific antigen.
Eur Urol. 68:581–588. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu Z, He B, He J and Mao X: Upregulation
of miR-153 promotes cell proliferation via downregulation of the
PTEN tumor suppressor gene in human prostate cancer. Prostate.
73:596–604. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Murata T, Takayama K, Katayama S, Urano T,
Horie-Inoue K, Ikeda K, Takahashi S, Kawazu C, Hasegawa A, Ouchi Y,
et al: miR-148a is an androgen-responsive microRNA that promotes
LNCaP prostate cell growth by repressing its target CAND1
expression. Prostate Cancer Prostatic Dis. 13:356–361. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen Q, Zhao X, Zhang H, Yuan H, Zhu M,
Sun Q, Lai X, Wang Y, Huang J, Yan J and Yu J: MiR-130b suppresses
prostate cancer metastasis through down-regulation of MMP2. Mol
Carcinog. 54:1292–1300. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ottman R, Levy J, Grizzle WE and
Chakrabarti R: The other face of miR-17-92a cluster, exhibiting
tumor suppressor effects in prostate cancer. Oncotarget.
7:73739–73753. 2016.PubMed/NCBI
|
|
38
|
Yanagi T, Nagai K, Shimizu H and Matsuzawa
SI: Melanoma antigen A12 regulates cell cycle via tumor suppressor
p21 expression. Oncotarget. 8:68448–68459. 2017.PubMed/NCBI
|
|
39
|
Prasad R and Katiyar SK: Down-regulation
of miRNA-106b inhibits growth of melanoma cells by promoting
G1-phase cell cycle arrest and reactivation of p21/WAF1/Cip1
protein. Oncotarget. 5:10636–10649. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li JH, Liu S, Zhou H, Qu LH and Yang JH:
starBase v2.0: Decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA
interaction networks from large-scale CLIP-Seq data. Nucleic Acids
Res. 42:(Database Issue). D92–D97. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Horoszewicz JS, Leong SS, Kawinski E, Karr
JP, Rosenthal H, Chu TM, Mirand EA and Murphy GP: LNCaP model of
human prostatic carcinoma. Cancer Res. 43:1809–1818.
1983.PubMed/NCBI
|
|
43
|
Seim I, Jeffery PL, Thomas PB, Nelson CC
and Chopin LK: Whole-genome sequence of the metastatic PC3 and
LNCaP human prostate cancer cell lines. G3 (Bethesda). 7:1731–1741.
2017.PubMed/NCBI
|
|
44
|
Chen W, Liu Y, Chen H, Ning H and Ding K:
Loss of miR-449a-caused PrLZ overexpression promotes prostate
cancer metastasis. Int J Oncol. 51:435–444. 2017.PubMed/NCBI
|
|
45
|
Zhou Y, Ji Z, Yan W, Zhou Z and Li H: The
biological functions and mechanism of miR-212 in prostate cancer
proliferation, migration and invasion via targeting Engrailed-2.
Oncol Rep. 38:1411–1419. 2017.PubMed/NCBI
|
|
46
|
Lu S, Wang MS, Chen PJ, Ren Q and Bai P:
miRNA-186 inhibits prostate cancer cell proliferation and tumor
growth by targeting YY1 and CDK6. Exp Ther Med. 13:3309–3314. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Huang YQ, Ling XH, Yuan RQ, Chen ZY, Yang
SB, Huang HX, Zhong WD and Qiu SP: miR-30c suppresses prostate
cancer survival by targeting the ASF/SF2 splicing factor
oncoprotein. Mol Med Rep. 16:2431–2438. 2017.PubMed/NCBI
|