Introduction
As a group of neurological diseases, epilepsy is
characterized by epileptic seizures (1,2).
Worldwide, ~1% (65,000,000 individuals) of the population suffer
with epilepsy (3), with almost 80%
of new cases occurring in developing countries (4). In 2013, the mortality rate was
~116,000, which was an increase from 112,000 in 1990 (5).
Astrocytes initiate, regulate and amplify
immune-mediated mechanisms, and are associated with several
diseases of the human central nervous system, including epilepsy
(6,7). In vitro studies have
documented the ability of astrocytes, particularly active
astrocytes, to produce cytokines, including interleukin (IL)-1β and
tumor necrosis factor (TNF)-α, which are expressed at high levels
in experimental and human epileptogenic brain tissues (8,9).
As a common antibiotic, penicillin functions as a
chemical convulsant for the establishment of an experimental
epilepsy model (10,11). At least 100–5,000 µM penicillin is
required to inhibit GABA (12).
It is understood that astragaloside IV
(3-o-β-d-xylopyranosyl-6-o-β-d-glucopyranosyl-cycloastragenol;
AS-IV), the primary pure saponin isolated from the root of
Astragalus membranaceus, is an effective compound with
distinct pharmacological effects, including anti-inflammatory
effects (13,14). To the best of our knowledge, the
protective effects of AS-IV on epilepsy remain to be elucidated,
therefore, the present study aimed to investigate the effect of
AS-IV in a primary astrocyte model of penicillin-induced
epilepsy.
Materials and methods
Cell culture
Primary astrocytes were derived from 1–5 day
postnatal Sprague-Dawley male rats (n=10). Neonatal rats were
purchased from the Model Animal Research Center of Nanjing
University. Briefly, neonatal rats were anesthetized and sacrificed
by alcohol immersion. Following removal of the meninges and blood
vessels, the cerebral cortices were collected and minced in 20
µg/ml DNase and 0.3% bovine serum albumin (BSA)-containing medium
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). The animal
protocol was approved by Wuxi Laboratory Animal Management and the
Animal Ethics Committee. All animal experiments were carried out in
accordance with the ethical guidelines of the Ethics Committee of
Jiangnan University (Wuxi, China).
The tissues were digested in 0.25% trypsin/EDTA
solution for 30 min at 37°C. The tissues were centrifuged (300 × g,
5 min at room temperature) and digested in 0.25% trypsin/EDTA
solution for 30 min at 37°C. The suspension was filtered through a
70 µm nylon filter, pelleted by centrifugation (600 × g, 5
min at room temperature) to remove trypsin, and then suspended in
10% (v/v) fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) in Dulbecco's modified Eagle's medium/F12
(DMEM/F12; Gibco; Thermo Fisher Scientific, Inc.) containing a
penicillin and streptomycin antibiotic mixture. The mixture was
transferred to flasks and incubated under conditions of 37°C, 5%
CO2 and 90% relative humidity. When the cells reached
confluence, the flasks were gently shaken to remove microglia and
oligodendrocytes. Following shaking, the astrocytes were rinsed
with phosphate-buffered saline (PBS) three times and trypsinized,
followed by physical loosening of the astrocytes. The medium was
removed and the astrocytes were placed in new flasks for culture in
medium (DMEM/F12, 15% FBS, L-glutamine and 500 ng/ml insulin) until
they were confluent. The cells were trypsinized for subsequent
experiments.
For primary astrocyte culture, 2,500 µM (500 IU) of
penicillin was used (15).
Different concentrations of AST-IV (20, 40, 80 and 160 µmol/l) were
administrated 2 h prior to penicillin treatment. At 12 h following
treatment with penicillin, the primary astrocytes were used to
perform a series of experiments.
Western blot analysis
To determine temporal expression profiles of β-actin
(1:1,000, #3700, Cell Signaling Technology, Inc., Danvers, MA,
USA), IL-1β (1:1,000, #5204, Cell Signaling Technology, Inc.),
TNF-α (1:1,000, #11948, Cell Signaling Technology) and
phosphorylated extracellular signal-regulated kinase (p-ERK,
1:1,000, #8544, Cell Signaling Technology, Inc.)/c-Jun N-terminal
kinase (p-JNK, 1:2,000, #9255, Cell Signaling Technology,
Inc.)/p-P38 (1:1,000, #4511, Cell Signaling Technology, Inc.)
mitogen-activated protein kinases (MAPKs), the astrocyte extract
lysates were collected and analyzed. Briefly, the samples were
washed with PBS rapidly and homogenized in RIPA lysis buffer
containing a cocktail of protease inhibitors and phosphatase
inhibitors (Roche Diagnostics, Nanjing, China). Protein
concentration was determined and quantified using the bicinchoninic
acid (Sigma-Aldrich) method. The sample lysates (10 µg) were
transferred and collected carefully for separation by 8% SDS-PAGE,
following which they were electrotransferred onto polyvinylidene
fluoride membranes (EMD Millipore, Bedford, MA, USA). The membranes
were blocked in 5% BSA for 1 h at room temperature, and incubated
overnight at 4°C with primary antibodies, respectively. Following
washing with TBST, the membranes were incubated with corresponding
HRP-conjugated secondary antibodies (RPN4301, GE Healthcare Life
Sciences, Little Chalfont, UK) for 1 h at room temperature. β-actin
was used as a loading control.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted by Trizol (Roche
Diagnostics) method. RNA was reverse transcribed into cDNA using
reverse transcriptase, in accordance with the manufacturer's
protocol (Takara Biotechnology, Co., Ltd., Dalian, China). The
sample used for RT-qPCR analysis comprised 2 µl cDNA, 5 µl 2X mix
(Bio-Rad Laboratories, Inc., Hercules, CA, USA), 0.5 µl forward
primer, 0.5 µl reverse primer and 2 µl nanopure water to a final
volume of 10 µl. The conditions for amplification were as follows:
10 sec at 95°C for denaturation, 60 sec at 60°C for annealing and
extension, and 40 cycles for all primers. Experiments were
performed in replicate. GAPDH was used as an endogenous control to
normalize differences in the quantity of total RNA from each
sample. The primer sequences were as follows: IL-1β, forward
5′-TGAAATGCCACCTTTTGACAG-3′ and reverse
5′-CCACAGCCACAATGAGTGATAC-3′; TNF-α, forward
5′-CCTGTCTCTTCCTACCCAACC-3′ and reverse 5′-GCAGGAGTGTCCGTGTCTTC-3′;
GAPDH, forward 5′-CTTTGGTATCGTGGAAGGACTC-3′ and reverse
5′-GTAGAGGCAGGGATGATGTTCT-3′. The comparative Cq
(2−∆∆Cq) method was used to analyze the relative
expression levels (16).
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide
(MTT) assay
Cell viability was measured using an MTT assay. The
cells (~200 µl) at a concentration of 1×104/ml were
seeded into 96-well plates. Following incubation for 24 h, 20 µl of
5 mg/ml MTT solution was added to each well and the plate was
incubated at 37°C for 4 h. Subsequently, the medium was aspirated,
the wells were washed with PBS and allowed to dry for ~4 h,
following which 150 µl DMSO was added to each well. The microtitre
plate was placed on a shaker in order to dissolve the dye and
absorbance was read at 450 nm using a Bio-Rad iMark plate reader
(Bio-Rad Laboratories, Inc.).
Cell Counting Kit-8 (CCK-8) assay
The numbers of cells were measured using a CCK-8 kit
(Dojindo Molecular Technologies, Inc., Kumamoto, Japan). The cells
(~5×103) cells were seeded into 96-well plates. Into
each well, 10 µl CCK-8 solution was added and the cells were
incubated at 37°C for 2 h. The absorbance was read at 450 nm using
a Bio-Rad iMark plate reader.
Statistical analysis
Differences among groups were analyzed using two-way
analysis of variance followed by Bonferroni post hoc tests. Data
were analyzed by SPSS software version 19.0 (IBM SPSS, Armonk, NY,
USA) and presented as mean ± standard error of the mean. P<0.05
was considered to indicate a statistically significant
difference.
Results
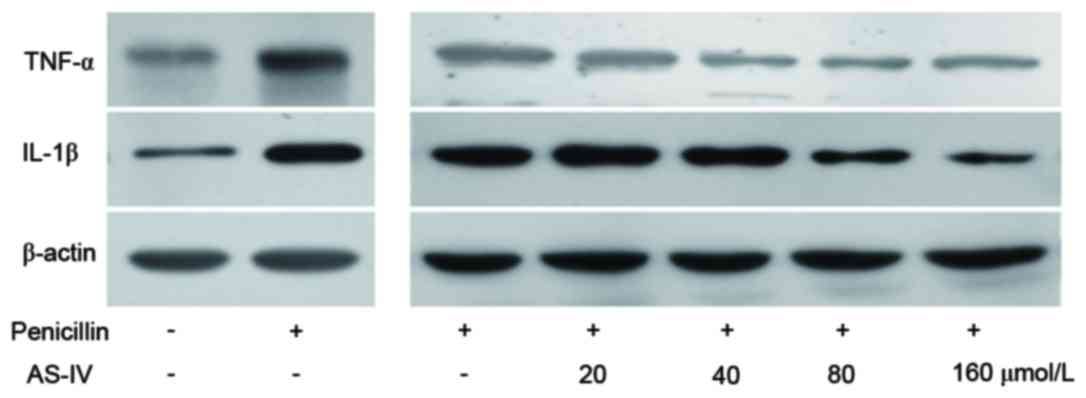
AS-IV significantly suppresses the
translation of penicillin-induced inflammatory factors of primary
astrocytes in a dose-dependent manner
Penicillin upregulated the protein levels of TNF-α
and IL-1β, whereas AS-IV (20, 40, 80 and 160 µmol/l)
dose-dependently suppressed the penicillin-induced upregulation in
inflammatory factors (Fig. 1).
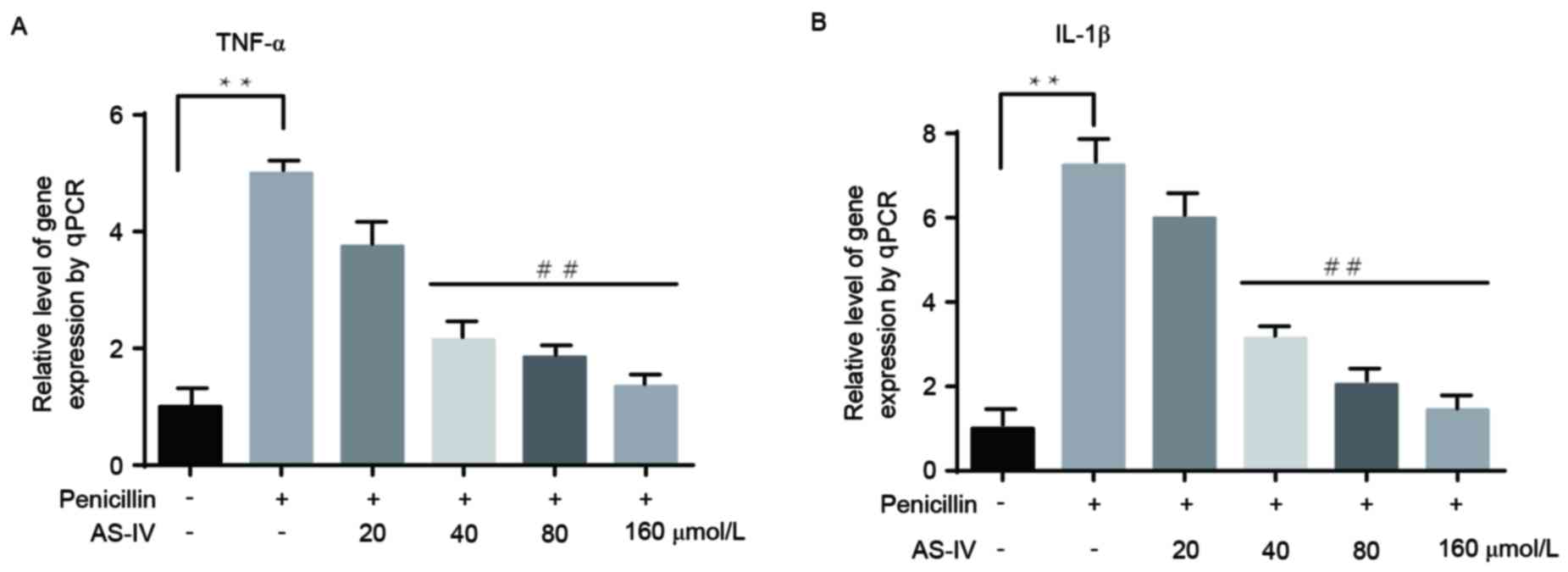
AS-IV significantly suppresses the
transcription of penicillin-induced inflammatory factors of primary
astrocytes in a dose-dependent manner
The changes in the transcription of inflammatory
factors were consistent with those at the translational level. Only
the higher doses of AS-IV (40, 80 and 160 µmol/l) notably reduced
the penicillin-induced upregulation of inflammatory factors
(Fig. 2A and B).
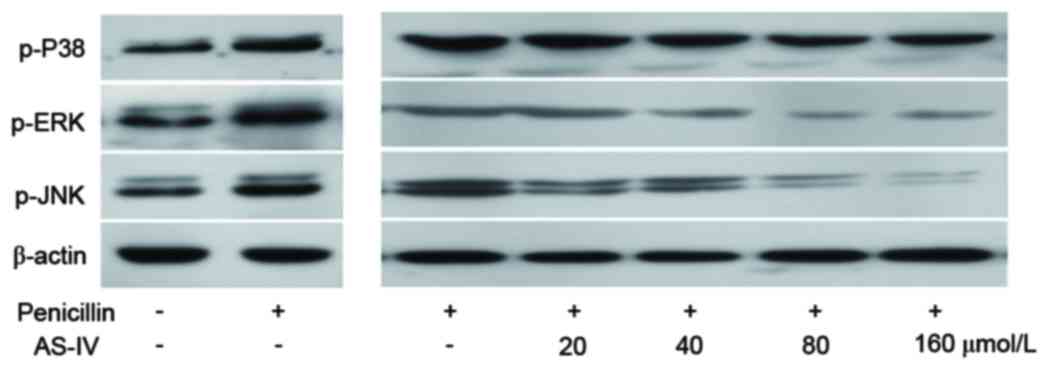
Penicillin-induced protein levels of
the p-MAPK family are decreased by AS-IV
AS-IV selectively reduced the penicillin-induced
p-JNK and p-p38 MAPKs, but not p-ERK (Fig. 3).
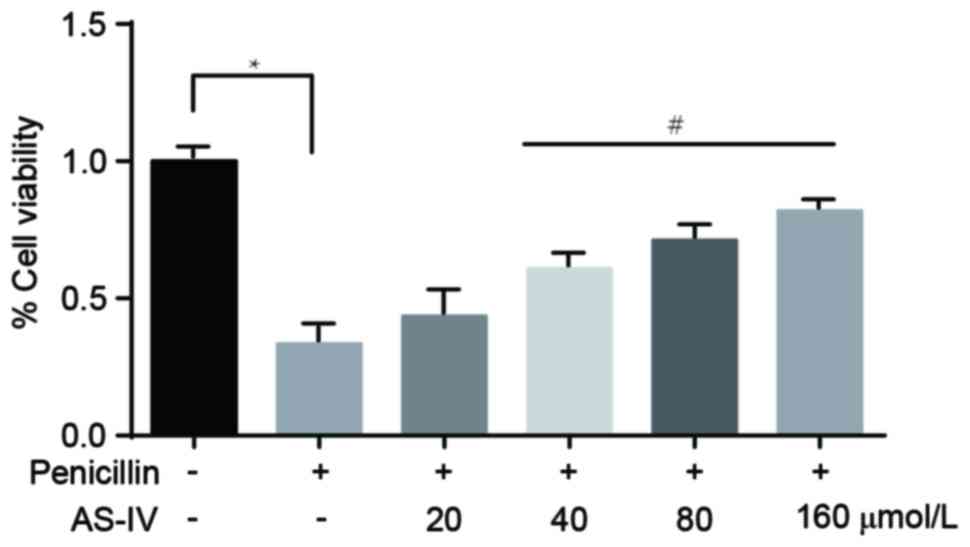
Penicillin-induced downregulation of
primary astrocyte viability is significantly increased following
administration of AS-IV
The MTT assay revealed that, compared with the
control group, the viability of the primary astrocytes in the
penicillin-induced group was markedly downregulated. In addition,
administration with the higher doses of AS-IV (40, 80 and 160
µmol/l) significantly increased cell viability (Fig. 4).
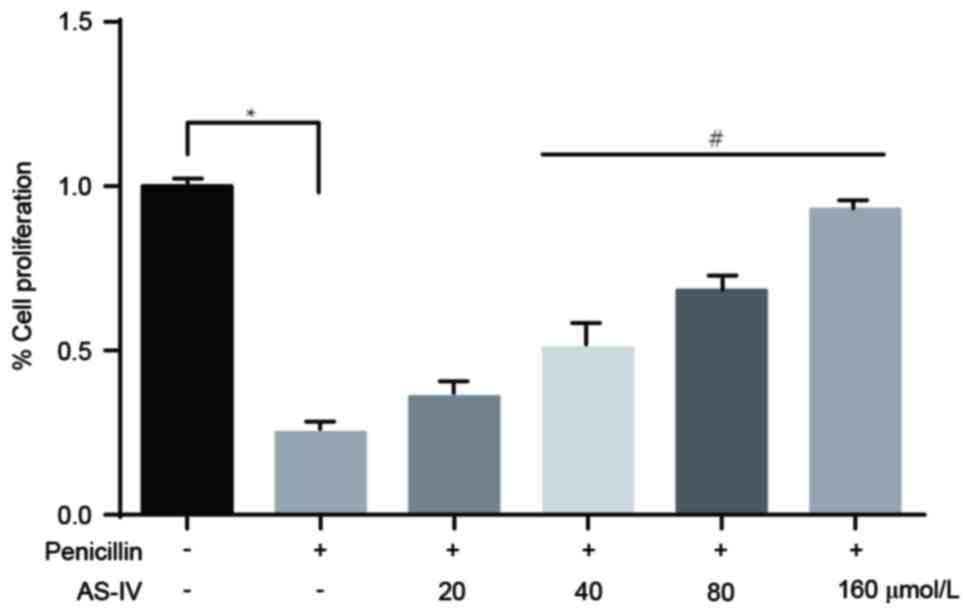
Penicillin-induced downregulation of
primary astrocyte numbers is significantly elevated following
administration of AS-IV
The results of the CCK8 assay revealed that,
compared with the control group, the number of primary astrocytes
in the penicillin-induced group was markedly reduced. The
administration of higher doses of AS-IV (40, 80 and 160 µmol/l)
significantly reversed the penicillin-induced reduction in cell
number (Fig. 5).
Discussion
As one of the most common serious neurological
disorders (17), epilepsy is
characterized by epileptic seizures (1,2) and
becomes more common as people age (18,19).
However, the exact mechanism underlying epilepsy remains to be
elucidated (19). In normal
conditions, brain electrical activity is modulated by various
factors within and around neurons. Factors within neurons include
the type, number and distribution of ion channels, and changes in
receptor and gene expression (21). Factors around the neurons include
ion concentrations, synaptic plasticity and the regulation of
transmitter breakdown by glial cells (21,22).
Astrocytes initiate, regulate and amplify
immune-mediated mechanisms associated with epilepsy (6,7).
In vitro studies have confirmed the ability of active
astrocytes to produce cytokines, including IL-1β and TNF-α, which
are expressed at high levels in experimental and human
epileptogenic brain tissues (8,9).
Reactive astrogliosis was found to be a pathological hallmark of
medically refractory epilepsy (23). Increasing evidence supports the
hypothesis that activation of the innate immune response is
involved in experimental and human epilepsy, and in the critical
association of the inflammatory process in the etiopathogenesis of
seizures (8,24). Vezzani et al further
examined the role of inflammation in epilepsy (25). The aim of the present study was to
identify a novel method to attenuate epilepsy via inhibiting the
inflammatory pathway.
AS-IV is an effective compound with distinct
pharmacological anti-inflammatory effects (13,14),
however, the role of AS-IV in epilepsy remains to be fully
elucidated. Penicillin has been demonstrated to function as a
convulsant for the establishment of experimental epilepsy models
(10,11). The present study examined the
effect of AS-IV against penicillin-induced epilepsy in primary
astrocytes.
In the present study, penicillin was used to induce
epilepsy in Primary astrocytes from SD rats, and the protein and
mRNA levels of TNF-α and IL-1β were examined in different groups to
investigate whether the model was successfully established. The
results revealed that penicillin upregulated the protein and mRNA
levels of TNF-α and IL-1β, which were consistent with the results
of previous studies showing the upregulation of pro-inflammatory
cytokines (25,26). The present study then examined
whether AS-IV (20, 40, 80 and 160 µmol/l) had effects on the levels
of inflammatory factors using western blot and RT-qPCR analyses.
The results revealed that AS-IV dose-dependently suppressed the
penicillin-induced increase in inflammatory factors. These results
suggested that the effects of AS-IV on ameliorating epilepsy were
dependent on the reduced release of inflammatory factors from the
cultured cells. These findings were consistent with previous
studies. For example, it has been reported that transgenic mice
with low-moderate overexpression of TNF-α in astrocytes exhibit
reduced susceptibility to seizures (27), and mice lacking caspase-1, which is
the biosynthetic enzyme of IL-1β, are unable to release the
biologically active form of IL-1β and also exhibit reduced seizure
susceptibility (28). However,
which proteins are involved in this process remain to be
elucidated, and the present study performed further experiments to
investigate this.
TNF-α is a cytokine released from activated
astrocytes and microglia, and is closely associated with IL-1β. In
the hippocampus, IL-1β affects synaptic transmission, and inhibits
long-term potentiation via the activation of JNK and p38 MAPK
(29,30). Therefore, the present study
performed western blot analysis to examine the levels of p-MAPK in
different groups. The results revealed that AS-IV decreased the
penicillin-induced upregulation of p-p38 and p-JNK, which was in
line with the results of previous studies (29,30).
Taken together, the above results led to the
conclusion that AS-IV suppressed penicillin-induced inflammatory
factor release, and suppressed activation of the p-p38 and p-JNK
MAPK signaling pathway.
It has been reported that cytokines have the ability
to contribute to excitotoxic and apoptotic neuronal death (31), indicating the possibility of
generating seizure-mediated neuronal damage. Therefore, the present
study examined the effects of AS-IV on Primary astrocytes from SD
rats. An MTT assay was performed, which revealed that the higher
doses of AS-IV improved the viability of the primary astrocytes
from SD rats. The effects of AS-IV on the proliferation of primary
astrocytes were also determined using a CCK8 assay, the results of
which were consistent with those of cell viability. Taken together,
the results of the present study demonstrated that AS-IV suppressed
the penicillin-induced release of inflammatory factors and
suppressed activation of the MAPK signaling pathway, ultimately
attenuating epilepsy. These findings provide a basis for further
investigations of the therapeutic role of AS-IV in epilepsy via
targeting astrocytes.
References
|
1
|
Chang BS and Lowenstein DH: Epilepsy. N
Engl J Med. 349:1257–1266. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fisher RS, Acevedo C, Arzimanoglou A,
Bogacz A, Cross JH, Elger CE, Engel J Jr, Forsgren L, French JA,
Glynn M, et al: ILAE Official Report: A practical clinical
definition of epilepsy. Epilepsia. 55:475–482. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Thurman DJ, Beghi E, Begley CE, Berg AT,
Buchhalter JR, Ding D, Hesdorffer DC, Hauser WA, Kazis L, Kobau R,
et al: Standards for epidemiologic studies and surveillance of
epilepsy. Epilepsia. 52 Suppl 7:S2–S26. 2011. View Article : Google Scholar
|
|
4
|
EpilepsyFact Sheets. World Health
Organization; 2012
|
|
5
|
GBD 2013 Mortality and Causes of Death
Collaborators: regional, and national age-sex specific all-cause
and cause-specific mortality for 240 causes of death, 1990–2013: A
systematic analysis for the Global Burden of Disease Study 2013.
Lancet. 385:117–171. 2013.
|
|
6
|
Farina C, Aloisi F and Meinl E: Astrocytes
are active players in cerebral innate immunity. Trends Immunol.
28:138–145. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Seifert G, Carmignoto G and Steinhäuser C:
Astrocyte dysfunction in epilepsy. Brain Res Rev. 63:212–221. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Aronica E and Crino PB: Inflammation in
epilepsy: Clinical observations. Epilepsia. 52 Suppl 3:S26–S32.
2011. View Article : Google Scholar
|
|
9
|
Vezzani A, Ravizza T, Balosso S and
Aronica E: Glia as a source of cytokines: Implications for neuronal
excitability and survival. Epilepsia. 49 Suppl 2:S24–S32. 2008.
View Article : Google Scholar
|
|
10
|
Bizière K and Chambon JP: Animal models of
epilepsy and experimental seizures. Rev Neurol (Paris).
143:329–340. 1987.(In French). PubMed/NCBI
|
|
11
|
Fisher RS: Animal models of the
epilepsies. Brain Res Brain Res Rev. 14:245–278. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Twyman RE, Green RM and MacDonald RL:
Kinetics of open channel block by penicillin of single GABAA
receptor channels from mouse spinal cord neurons in culture. J
Physiol. 445:97–127. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qiu YY, Zhu JX, Bian T, Gao F, Qian XF, Du
Q, Yuan MY, Sun h, Shi LZ and Yu H: Protective effects of
Astragaloside IV against ovalbumin-induced lung inflammation are
regulated/mediated by T-bet/GATA-3. Pharmacol. 94:51–59. 2014.
View Article : Google Scholar
|
|
14
|
Qiu L, Yin G, Cheng L, Fan Y, Xiao W, Yu
G, Xing M, Jia R, Sun R, Ma X, et al: Astragaloside IV ameliorates
acute pancreatitis in rats by inhibiting the activation of nuclear
factor-κB. Int J Mol Med. 35:625–636. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Özdemir MB, Akça H, Erdoğan Ç, Tokgün O,
Demiray A, Semin F and Becerir C: Protective effect of insulin and
glucose at different concentrations on penicillin-induced astrocyte
death on the primer astroglial cell line. Neural Regen Res.
7:1895–1899. 2012.PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hirtz D, Thurman DJ, Gwinn-Hardy K,
Mohamed M, Chaudhuri AR and Zalutsk R: How common are the ‘common’
neurologic disorders? Neurol. 68:326–337. 2007. View Article : Google Scholar
|
|
18
|
Brodie MJ, Elder AT and Kwan P: Epilepsy
in later life. Lancet Neurol. 8:1019–1030. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Holmes, Thomas R. Browne and Gregory L:
Handbook of epilepsy. 4th edition. Philadelphia: Lippincott
Williams & Wilkins; pp. 7ISBN 978-0-7817-7397-3. 2008
|
|
20
|
Noebels JL, Avoli M, Rogawski MA, et al:
Jasper's Basic Mechanisms of the Epilepsies. Oxford University
Press; pp. 466–470. 2012
|
|
21
|
Bromfield EB, Cavazos JE and Sirven JI: An
introduction to epilepsy. American Epilepsy Society. 2006.
|
|
22
|
Blumenfeld H: Cellular and network
mechanisms of spike-wave seizures. Epilepsia. 46:21–33. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sofroniew MV and Vinters HV: Astrocytes:
Biology and pathology. Acta Neuropathol. 119:7–35. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vezzani A, Maroso M, Balosso S, Sanchez MA
and Bartfai T: IL-1 receptor/Toll-like receptor signaling in
infection, inflammation, stress and neurodegeneration couples
hyperexcitability and seizures. Brain Behav Immun. 25:1281–1289.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vezzani A, French J, Bartfai T and Baram
TZ: The role of inflammation in epilepsy. Nat Rev Neurol. 7:31–40.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vezzani A, Balosso S and Ravizza T: The
role of cytokines in the pathophysiology of epilepsy. Brain Behav
Immun. 22:797–803. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Balosso S, Ravizza T, Perego C, Peschon J,
Campbell IL, De Simoni MG and Vezzani A: Tumor necrosis
factor-alpha inhibits seizures in mice via p75 receptors. Ann
Neurol. 57:804–812. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ravizza T, Lucas SM, Balosso S, Bernardino
L, Ku G, Noé F, Malva J, Randle JC, Allan S and Vezzani A:
Inactivation of caspase-1 in rodent brain: A novel anticonvulsive
strategy. Epilepsia. 47:1160–1168. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bellinger FP, Madamba S and Siggins GR:
Interleukin 1 beta inhibits synaptic strength and long-term
potentiation in the rat CA1 hippocampus. Brain Res. 628:227–234.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schneider H, Pitossi F, Balschun D, Wagner
A, del Rey A and Besedovsky H: A neuromodulatory role of
interleukin-1beta in the hippocampus. Ann NY Acad Sci.
95:7778–7783. 1998. View Article : Google Scholar
|
|
31
|
Allan SM, Tyrrell PJ and Rothwell NJ:
Interleukin-1 and neuronal injury. Nat Rev Immunol. 5:629–640.
2005. View
Article : Google Scholar : PubMed/NCBI
|