Introduction
Rheumatoid arthritis (RA) is a systemic autoimmune
disease that is characterized by chronic joint inflammation,
synovial hyperplasia and the progressive destruction of cartilage
and bone (1,2). It has been revealed that a primary
manifestation in RA is the excessive proliferation of RA
fibroblast-like synoviocytes (RAFLS) (3,4),
which may be closely associated with impaired RAFLS apoptosis
(5,6), which is believed to be a result of
overexpression of the antiapoptotic gene B cell lymphoma 2 (Bcl2)
(7,8). Therefore, the downregulation of Bcl2
expression to increase RAFLS apoptosis may be a promising
therapeutic option for the treatment of RA (9).
Daphnetin, an active ingredient extracted from
Daphne odora var. marginata, possesses many
biological and medical properties, including antiparasitic,
anti-inflammatory and analgesic properties (10). Daphnetin may also kill aphids,
inhibit protein kinases and improve abnormal blood rheology of type
2 diabetic rats (11). Results
from our preliminary study performed in 2011 revealed that
daphnetin may have therapeutic and immunomodulatory effects in rats
with collagen II-induced arthritis (CIA) (12). In 2012, it was demonstrated that
the apoptosis rate of RAFLS was elevated upon treatment with
daphnetin (13).
RNA interference (RNAi) is a gene silencing
technology. RNAi has been widely used for the anti-inflammatory
treatment of RA in a number of studies worldwide (14–18).
The results of these studies have revealed that RNAi is a promising
therapeutic option for RA. However, RNAi targeting of antiapoptotic
genes has not yet been reported in RA.
In the present study, Bcl2-targeted small
interfering (si)RNA (si-Bcl2) was synthesized and transfected into
RAFLS using a liposome-mediated method. si-Bcl2 treatment was
demonstrated to successfully interfere with the expression of Bcl2.
Subsequently, RAFLS were treated with daphnetin, and the apoptotic
rate was measured, along with the mRNA expression levels of Bcl2
and signal transducer and activator of transcription 3 (STAT3). In
addition, the possible underlying mechanism by which daphnetin
combined with si-Bcl2 affects antiapoptotic gene expression in the
RAFLS of CIA rats was investigated. The present study is expected
to make a new contribution to the treatment of RA.
Materials and methods
Materials
FLS from CIA rats and healthy rats were purchased
from Shanghai Institute of Health Industry Co., Ltd. (Shanghai,
China). Daphnetin, extracted from Daphne odora var.
marginata by high-speed counter-current chromatography, was
purchased from Shanghai Yuanye Biotechnology Co., Ltd. (Shanghai,
China). Other reagents were purchased as follows:
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA); siRNAs (Ribobio Co., Ltd.,
Guangzhou, China); Opti-MEM I (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA); reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
primers (Sangon Biotech Co., Ltd., Shanghai, China); ReverTra Ace
qPCR RT kit (Toyobo Life Science, Osaka, Japan); SYBR Green Real
Time PCR Master mix (Toyobo Life Science); rabbit anti-Bcl2
antibody conjugated to fluorescein isothiocyanate (FITC; bs0032r-F;
Beijing Biosynthesis Biotechnology Co., Ltd., Beijing, China);
annexin V/propidium iodide (PI) kit (KeyGen Biotechology Co. Ltd.,
Nanjing, China); diethyl pyrocarbonate (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany); and TransZol (Beijing TransGen Biotech Co.,
Ltd., Beijing, China).
Cultured FLS from CIA rats
FLS were cultured and passaged in Dulbecco's
Modified Eagle's medium (DMEM) supplemented with penicillin (100
U/ml), streptomycin (100 µg/ml) and 10% foetal bovine serum (FBS,
Tianjin Haoyang Biological Manufacture Co., Ltd., Tianjin, China).
All cultures were incubated at 37°C in 5% humidified
CO2. Following 2 days incubation, FLS were digested
using 0.25% trypsin prior to centrifugation at low speed (112 × g
for 10 min at 25°C). Nonadherent granulocytes were removed, and FLS
were cultured in 24-or 6-well plates (5×104 or
1×105 cells/ml) in 500 µl or 2 ml DMEM, respectively,
containing 10% FBS without penicillin and streptomycin for 24 h
following treatment with siRNA (various concentrations) or
daphnetin (40 µg/ml, 37°C).
siRNA synthesis and transfection
All siRNA sequences were purchased from Ribobio Co.,
Ltd. (Guangzhou, China). A total of three siRNA sequences specific
to Bcl2 were selected: i) si-Bcl2.1, GCC UCC GAC CCU ACG GAA A; ii)
siBcl2.2, CCU ACU GAC UCA UGG ACU U; and iii) siBcl2.3, CCU CUU GUC
CCA UAC UAU U. siRNAs were chemically synthesised and annealed by
Nanjing Anbo Ruila Biotechnology Co., Ltd. Three targeting siRNA
was respectively used to silence the Bcl2 gene in FLS of CIA rats.
siRNAs labelled with fluorescent cyanine 3 (Cy3; siN05815122149;
Ribobio Co., Ltd.) were used to test whether the transfection was
successful. An siRNA specific to an invalid gene (siN05815122147;
Ribobio Co., Ltd.) was used as a negative silencing control.
Experimental groups were used to determine the optimal siRNA
concentration, including siRNA transfection negative control group
(NC group), si-Bcl2.1-transfection group, si-Bcl2.2-transfected
group and si-Bcl2.3-transfection group.
Transfection was conducted according to the
manufacturer's protocol. Briefly, 50 µl Opti-Mem I (serum-free,
penicillin-free and streptomycin-free) was used in 24-well plates
(or 250 µl in 6-well plates) to dilute 5, 2.5, 1.25, 0.75, 0.5 and
0.25 µl annealed siRNA (or 20, 10, 5, 3, 2 and 1 µl siRNA in 6-well
plates). Another 50 µl Opti-Mem I was used in 24-well plates (or
250 µl in 6-well plates) to dilute 1 µl Lipofectamine 2000 (or 5 µl
in 6-well plates). Subsequently, the diluted siRNA-Lipofectamine
2000 complex was incubated at room temperature for 20 min. This
mixture was then added to 400 µl FLS cell culture in 24-well plates
(5×104 cells/well), or 1,500 µl in 6-well plates
(1×105 cells/well). A concentration gradient of the
siRNA was generated as follows: 200, 100, 50, 30, 20 and 10 nM.
Following 6 h incubation at 37°C, an equal volume of DMEM
supplemented with 10% FBS was added to the cells. When combined
with siRNA, daphnetin (final concentration 40 µg/ml) was added to
the FLS cell culture as appropriate for each of the treatment
groups. Following 24 or 48 h transfection at 37°C, Cy3 fluorescence
was observed under a fluorescent microscope to confirm that the
transfection was successful. The transfected RAFLS were washed with
DMEM and then immediately used for subsequent experiments.
Combined treatment of CIA rat FLS
cells with daphnetin and si-Bcl2
Experimental groups were: i) FLS of untreated
healthy Wistar rats (healthy group); ii) untreated FLS of CIA rats
(CIA group); iii) daphnetin (40 µg/ml)-treated group (E003 group);
iv) si-Bcl2-treated group; v) daphnetin (40 µg/ml) plus si-Bcl2
combined treatment group (E003-B group). Each group of cells was
treated by the method of siRNA transfection as described above.
Following treatment (5×104/1×105 cells/well
at 37°C in 5% humidified CO2), FLS were cultured for 24,
48 and 72 h, and total RNA was extracted to measure the relative
mRNA expression levels of Bcl2 and STAT3. In addition, resuspended
FLS were used to measure Bcl2 protein expression levels and to
assess apoptosis by flow cytometry.
Total RNA extraction and reverse
transcription
Total RNA was isolated from FLS (5×104
cells) following gene silencing using TRIzol (All-in gold
Biological Co., Ltd.), according to the manufacturer's protocol.
cDNA was synthesized using the ReverTra Ace qPCR RT kit (Toyobo
Life Science, Osaka, Japan) was used. Briefly, extracted RNA was
heat denatured at 65°C for 5 min and immediately cooled on ice.
Subsequently, 1 µl RT Enzyme mix, 1 µl Primer mix and 4 µl 5X RT
buffer were incubated with 1 µg DNA-free total RNA for 15 min at
37°C. The mixture was incubated for 5 min at 98°C to inactivate the
RT enzyme, and the first-strand cDNA was stored at 4°C until use in
qPCR.
RT-qPCR
For qPCR, reactions were performed in a volume of 20
µl SYBR−Green Real Time PCR Master mix (Toyobo Life
Science). The primers used in this study were as follows: Bcl2,
forward 5′-GGGATGCCTTTGTGGAACTAT-3, reverse
5′-AGGTATGCACCCAGAGTGATG-3′ (124 bp); STAT3, forward
5′-GGGCACAAACACAAAAGTGAT-3′, reverse 5′-CAGTCACAATCAGGGAAGCAT-3′
(140 bp); β-actin, forward 5′-TGACAGGATGCAGAAGGAGA-3′, reverse
5′-TAGAGCCACCAATCCACACA-3′ (106 bp). Thermocycling conditions
included initial denaturation at 95°C for 1 min, followed by 40
cycles of denaturation at 95°C for 15 sec, annealing at 60°C for 15
sec and extension at 72°C for 45 sec. qPCR was performed on an ABI
7500 PCR instrument. A relative quantitative assay was adopted
(19), and the melting curve
increased following amplification. The reference gene β-actin, and
Bcl2 and STAT3 were simultaneously amplified in the samples of each
experimental group. Each sample was tested in triplicate. Samples
were obtained from ≥3 independent experiments to calculate the mean
and standard deviation. The formula 2−ΔΔCq was used to
calculate the relative expression of Bcl-2, STAT3mRNA (19).
Flow cytometry
Bcl2 protein expression analysis and the apoptotic
rate of FLS from CIA rats were performed on a FACScan flow
cytometer (BD Biosciences, Franklin Lakes, NJ, USA). For the Bcl2
protein, a FITC-conjugated rabbit anti-rat Bcl2 antibody
(Biosynthesis Corporation, Beijing, China) was used. FLS cells
(1×105 cells/well) were treated as aforementioned,
collected by trypsinization following 72 h incubation, fixed by 1%
paraformaldehyde at room temperature for 15 min, and resuspended
with 100 µl or 500 µl PBS. Following collection, cells were stained
with (FITC)-labeled rabbit anti-rat Bcl2 antibody (1:100) and 70%
ethanol added in the dark for 15 min at room temperature for
permeabilisation. Bcl2 protein expression was assessed by
determining the average fluorescence intensity. Apoptosis of FLS
was examined using a FITC-labeled Annexin V/propidium iodide (PI)
Apoptosis Detection kit (Nanjing KeyGen Biotechology Co. Ltd.,
Nanjing, China) according to the manufacture's protocol. Cells
(1×105 cells/well) treated as aforementioned for 72 h
were collected by trypsinization, fixed by 70% ethanol in the dark
for 15 min at 4°C and washed twice with PBS. The cells were
resuspended in 500 µl binding buffer. Then 5 µl Annexin-V-FITC and
5 µl PI-FITC were added and incubated in the dark for 30 min at
room temperature. Data acquisition and analysis were performed in a
Becton Dickinson FACSCalibur flow cytometer using Cell Quest
software version 6.1.2 (BD Biosciences, Franklin Lakes, NJ,
USA).
Statistical analysis
All data are expressed as the mean ± standard
deviation, and were analysed by one-way analysis of variance
followed by LSD post hoc multiple comparison test using SPSS
version 19.0 software (IBM Corp., Armonk, NY, USA). A Student's
t-test was used to determine the significances of differences in
multiple comparisons. P<0.05 was considered to indicate a
statistically significant difference.
Results
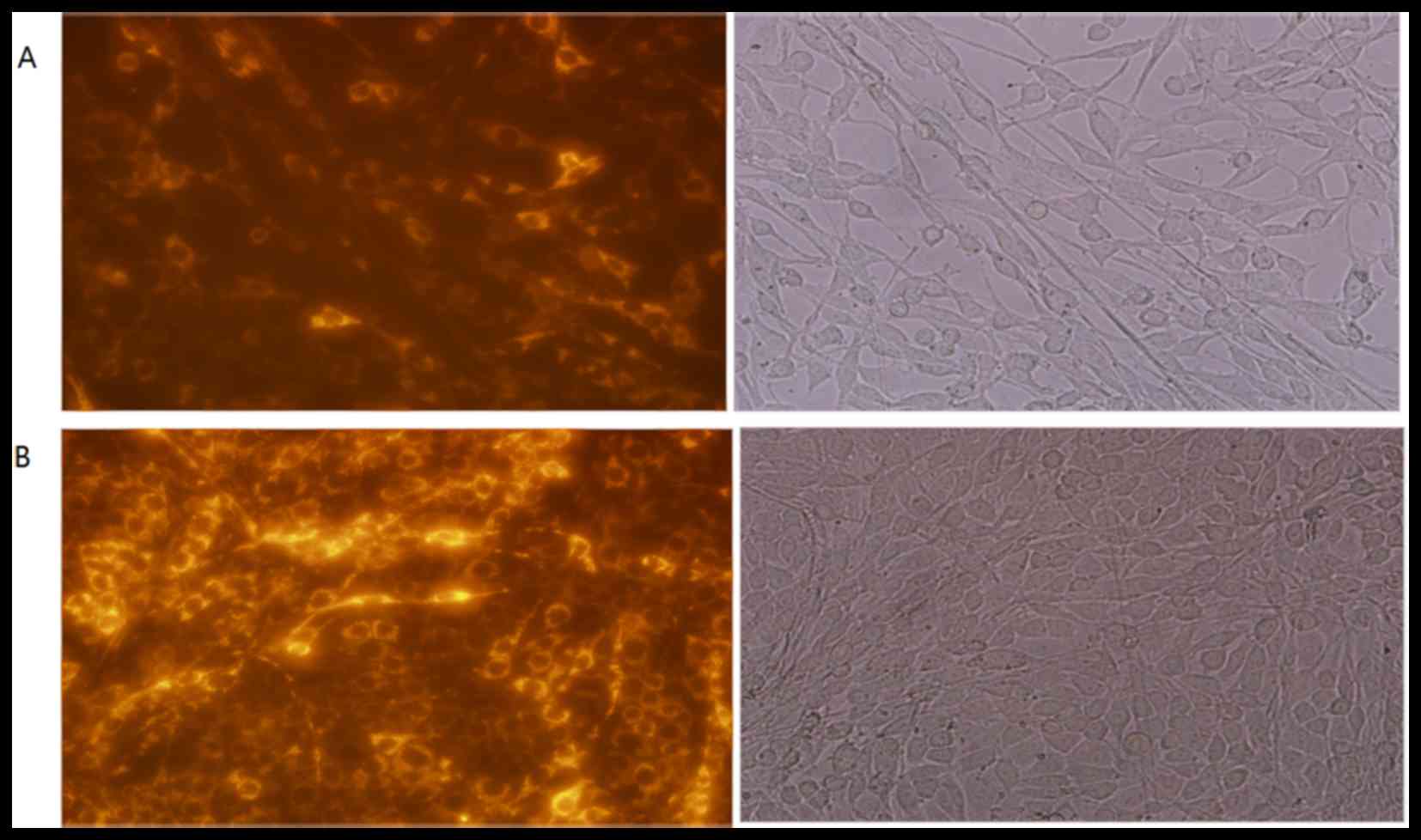
Cy3-labeled siRNA transfection
efficiency of FLS
Cy3-labeled si-Bcl2 was transfected into FLS. To
determine whether the transfection was successful, the fluorescence
was observed under a microscope at 24 and 48 h post-transfection
(Fig. 1A and B, respectively).
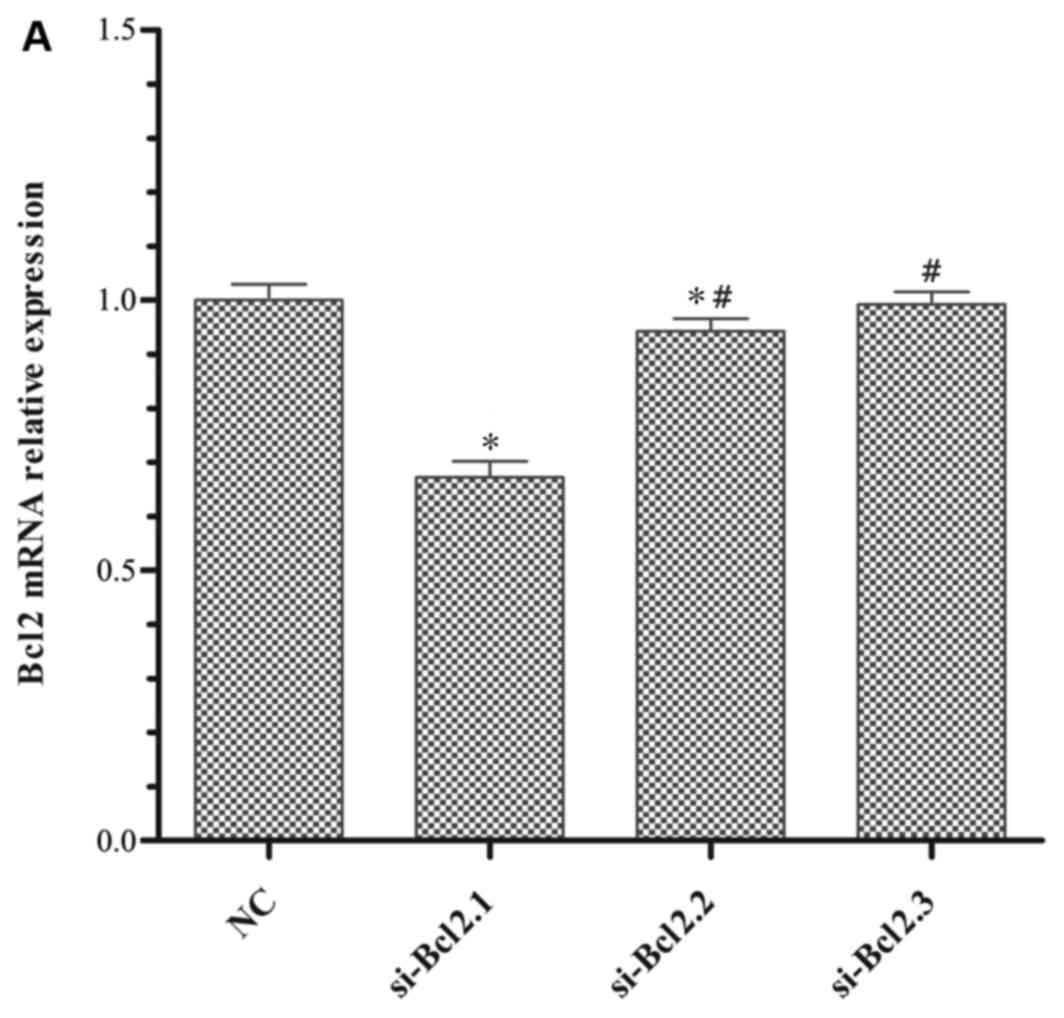
Optimisation of experimental
conditions
Identification of the optimal siRNA concentration
and incubation time was examined, as well as determining the
optimal treatment time for daphnetin. All experimental conditions
were designed to assess the mRNA expression levels of the
antiapoptotic gene Bcl2, which was determined using RT-qPCR. The
results revealed that Bcl2 mRNA expression in the si-Bcl2.1 group
was significantly lower compared with the NC, si-Bcl2.2, and
si-Bcl2.3 groups (Fig. 2A). As
demonstrated in Fig. 2B, 100 nM
si-Bcl2.1 inhibited Bcl2 mRNA expression most following
transfection for 48 h compared with the NC group (Fig. 2B). In a previous study, it was
determined that 40 µg/ml daphnetin significantly promoted FLS
apoptosis (13). Therefore, the
present study focused on determining the most suitable length of
time required to downregulate Bcl2 mRNA expression. The results
demonstrated that Bcl2 mRNA expression in FLS cells following
daphnetin treatment for 48 or 72 h was significantly lower compared
with cells treated for 24 h and untreated CIA FLS cells (Fig. 2C). However, daphnetin treatment for
48 h was not statistically significant (P>0.05) compared with
for 72 h, so cells were treated for 48 and 72 h to observe Bcl2
mRNA expression in the later combined experiment.
Influence of daphnetin combined with
si-Bcl2 on mRNA and protein expression of Bcl2 and mRNA expression
of STAT3 in RAFLS derived from CIA rats
In the optimal conditions, si-Bcl2.1 was transfected
into FLS and daphnetin was added to FLS cultures 6 h following gene
silencing. Bcl2 and STAT3 mRNA expression levels were determined by
RT-qPCR (Fig. 3A and B,
respectively). Bcl2 protein expression was assessed by determining
the average fluorescence intensity using flow cytometry (Fig. 3C). The results demonstrated that
Bcl2 mRNA expression in the E003-B group was significantly lower
than that in the other treatment groups. In addition, Bcl2 mRNA
expression in the E003-B group treated for 48 h was significantly
lower compared with that in the same group treated for 72 h
(P<0.05), and no significant difference was observed in
expression between the E003-B group treated for 48 h and the
healthy group treated for 48 h (P>0.05; Fig. 3A). It suggested that daphnetin
combined with si-Bcl2 for the treatment of RA was effective,
particularly as in the treatment for 48 h, Bcl2 mRNA was
downregulated most. Consistent with Bcl2 mRNA expression, STAT3
mRNA expression in the E003-B group was also decreased compared
with the other treatment groups (P<0.05; Fig. 3B). Bcl2 protein expression in the
E003-B group was significantly lower compared with expression in
the other treatment groups and the CIA group (P<0.05; Fig. 3C).
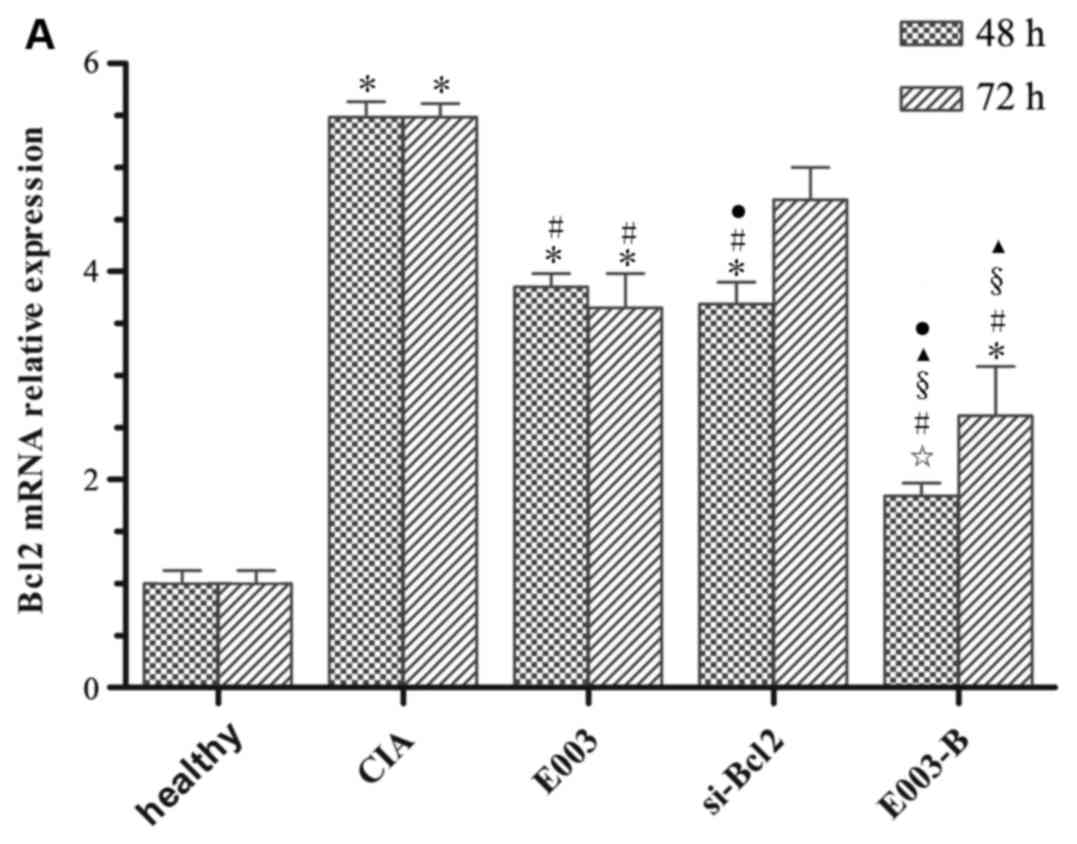 | Figure 3.Influence of E300 combined with
si-Bcl2 on the gene encoding antiapoptotic Bcl2 and the signalling
protein STAT3. (A) mRNA expression levels of Bcl2 in healthy FLS,
CIA FLS or CIA FLS treated with E003, si-Bcl2 or E003-B. 100 nM
si-Bcl2.1 was transfected into FLS for 48 h and 40 µg/ml E003 was
cultured together with FLS for 72 h. Owing to the different periods
of time required for si-Bcl2 and E003 to affect Bcl2 mRNA
expression in FLS, mRNA expression of Bcl2 was measured after the
cells had been incubated with both agents for 48 and 72 h. (B)
STAT3 mRNA expression was determined following treatment of FLS for
72 h. (C) Bcl2 protein expression was detected by flow cytometry,
also following treatment of FLS for 72 h. Cells in all groups were
treated as in section 2 for 72 h. ✩P>0.05 vs. healthy
group; *P<0.05 vs. healthy group #P<0.05 vs. CIA
group; §P<0.05 vs. E003 group; ▲P<0.05
vs. si-Bcl2 group; ●P<0.05 vs. 72 h group. Bcl2, B
cell lymphoma 2; CIA, collagen II-induced arthritis; E003,
daphnetin; FLS, fibroblast-like synoviocytes; si-Bcl2, short
interfering RNA targeting Bcl2; STAT3, signal transducer and
activator of transcription 3. |
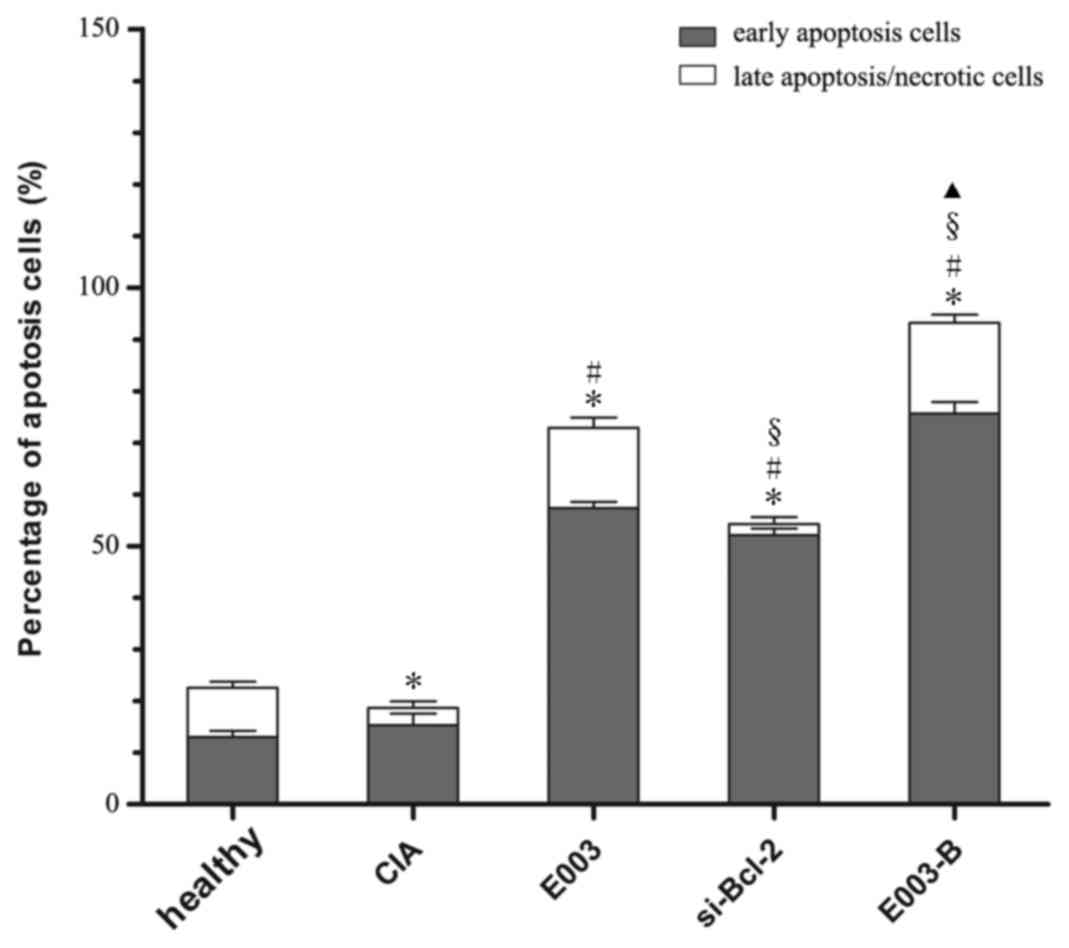
Effects of daphnetin combined with
si-Bcl2 on apoptosis of RAFLS derived from CIA rats
The apoptotic rate of RAFLS derived from CIA rats
was also determined by flow cytometry. The results demonstrated
that the apoptotic rate of FLS in the E003-B group was
significantly higher than that of other groups (P<0.05; Fig. 4).
Discussion
RA is a common systemic autoimmune disease that is
characterised by chronic joint inflammation, synovial hyperplasia
and progressive destruction of cartilage and bone. This process is
sustained and recurs so that the affected joints swell and lose
functionality. If RA is not treated in a timely manner, it may
result in severe joint deformity, leading to multiple joint
dysfunction and other organ diseases. RA and complications caused
by RA, such as cancer (20,21),
are a major cause of disability or lost labour in China. However,
the pathogenesis of RA remains to be elucidated. Recently, studies
have revealed that RA may be associated with an abnormal autoimmune
process mediated by T cells and/or macrophages and the abnormal
proliferation of synovial cells (22,23).
A previous study demonstrated that synovial cells produce a variety
of pro-inflammatory cytokines following activation and a number of
matrix-destructing enzymes through inflammatory signals, causing
persistent inflammation and progressive synovial tissue destruction
in RA patients (24). It is now
considered that FLS cells do not only fill space, but they are also
directly responsible for cartilage destruction and drive both
inflammation and autoimmunity. Active RAFLS cells stimulate the
expression of proinflammatory cytokines, such as interleukin (IL)-8
and IL-6, and also form a complex that constantly changes the
expression levels of various molecules without external stimulation
by altering the expression of regulatory factors, signalling
molecules and apoptosis-associated molecules (25,26).
RAFLS cells, which are characterised by incomplete conversion,
exhibit tumour-like proliferation with sustained activation,
although they may escape from the inflammatory environment and have
strong invasive properties (27),
and this is closely associated with impaired apoptosis of RAFLS.
Impaired apoptosis prolongs the life of RAFLS, causing joint
cartilage and bone destruction (28).
In the clinic, many diseases are associated with
impaired apoptosis of lesion cells. FLS cells in RA are tumour-like
proliferation (27). The induction
of cell apoptosis is an efficient strategy with which to inhibit
proliferation. Inducing the apoptotic pathway with active
ingredients in Chinese medicine has become a popular target for
inhibition of tumour or tumour-like growth. The present study used
daphnetin extracted from natural D. odora, which has many
pharmacological applications, such as anti-inflammatory, analgesic,
antitumoural and immune regulatory properties (29–32).
A previous study by the authors of the present study demonstrated
that daphnetin not only inhibited immunised foot swelling and
inflammation but also relieved the degeneration of articular
chondrocytes, increased the number of forkhead box P3+
regulatory T cells and regulated T helper 17 cells to achieve
immune balance in CIA (33). In
2012, it was also demonstrated that daphnetin treatment
demethylated the apoptosis genes death receptor 3, programmed cell
death 5, Fas ligand and the promoter region of p53 in CIA (13).
RNAi-associated RNA silencing pathways constitute a
group of small-RNA-based silencing mechanisms that is conserved in
eukaryotic cells and affect their whole existence (34). RNA silencing requires a number of
enzymes, and different species require different enzymes, generally
including argonaute (which binds to siRNA), and P-element induced
wimpy testis protein (35). RNAi
is an efficient sequence-specific gene knockout technology that has
been rapidly developed for applications in infectious diseases and
gene therapy areas of cancer. It may be a novel idea that an
efficient siRNA may be used to treat RA alone or in combination
with biological agents or other medicines. However, studies have
focused on using RNAi for anti-inflammation-based treatments of RA
(36,37). The use of RNAi to remove or turn
off specific genes highly expressed in RAFLS, such as the
antiapoptosis protein Bcl2, has not been previously reported. The
present study investigated the ability of combined treatment with a
Bcl2-specific RNAi with daphnetin, an active ingredient of
traditional Chinese medicine, to downregulate antiapoptosis gene
expression, aiming to promote RAFLS apoptosis and reduce the
over-proliferation of these cells.
In the present study, daphnetin was combined with
si-Bcl2 to explore the effect of this combined treatment on
antiapoptotic gene expression and the underlying molecular
mechanism of RAFLS of CIA rats. Bcl2 exerts an antiapoptotic effect
by blocking common signalling pathways of apoptosis. Previous
studies have demonstrated that Bcl2 gene expression is
significantly higher in the synovial tissue of RA patients and that
it inhibits apoptosis by preventing cytochrome C (Cyto C) release
from mitochondria and also by regulating caspase activity following
Cyto C release (38). A report by
Ferdek et al (39) revealed
that Bcl2 serves important roles in Ca2+ signalling by
influencing inositol triphosphate receptors and regulating
Ca2+−induced Ca2+ release. In the present
study, it was demonstrated that Bcl2 was highly expressed in
synovial cells in CIA rats, and treatment with daphnetin or si-Bcl2
alone resulted in mRNA and protein expression levels that were
significantly lower than those in the CIA group. There was clear
synergy between daphnetin and si-Bcl2 in downregulating Bcl2 mRNA
and protein expression levels.
Apoptosis is initiated by environmental stimuli and
induces a variety of other gene regulation mechanisms. It serves an
important role in eliminating cells that have aged in the body or
have the potential for uncontrolled growth, and also maintains
homeostasis of organs. The antiapoptotic gene Bcl2 is primarily
involved in the mitochondrial pathway and endoplasmic reticulum
pathway. STAT3, an important member of the STAT family, is commonly
expressed in human tumours and is widely involved in many
processes, such as tumour invasion and metastasis, angiogenesis,
apoptosis resistance and immune evasion. STAT3, located upstream of
Bcl2 gene regulation in many apoptotic signalling pathways, may
regulate the expression of Bcl2 to block apoptosis. Li et al
(40) reported that downregulation
of STAT3 expression using RNAi significantly reduced Bcl2 and
cyclin D expression and thereby induced glioblastoma stem cell
apoptosis and inhibited cell growth. Lee et al (41) demonstrated that STAT3-mediated
IL-17 expression induces the upregulation of Bcl2 expression, and
IL-17 promotes proliferation of RAFLS through the STAT3 apoptosis
pathway. In the present study, it was revealed that the apoptosis
of FLS cells increased with daphnetin treatment alone or with
si-Bcl2 treatment alone. In the mitochondrial apoptotic signalling
pathway, there was a corresponding decline in STAT3 expression, and
there was also an enhanced association between daphnetin and
si-Bcl2 in downregulating STAT3 expression.
The results of the present study suggested that
there may be an interaction between daphnetin and si-Bcl2 in
reducing the expression levels of Bcl2 and STAT3, which promoted
FLS apoptosis in CIA rats. The effects of daphnetin combined with
sibcl-2 in the present study may provide a new direction for the
treatment of RA. However, the mechanism of these effects on FLS in
RA remains to be elucidated.
Acknowledgements
The authors would like to thank Chuan Liu and Qiong
Wu at the Second Affiliated Hospital of Nanchang University
(Nanchang, China) for their assistance. The present study was
supported by The National Natural Science Foundation of China
(grant no. 31460239) and by The Natural Science Foundation of
Jiangxi Province (grant no. 20122BAB205058).
References
|
1
|
Isozaki T, Rabquer BJ, Ruth JH, Haines GK
Ш and Koch AE: ADAM-10 Is Overexpressed in rheumatoid arthritis
synovial tissue and mediates angiogenesis. Arthritis Rheum.
65:98–108. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Szabó-Taylora KÉ, Eggleton P, Turner CA,
Faro ML, Tarr JM, Tóth S, Whiteman M, Haigh RC, Littlechild JA and
Winyard PG: Lymphocytes from rheumatoid arthritis patients have
elevated levels of intracellular peroxiredoxin 2 and a greater
frequency of cells with exofacial peroxiredoxin 2, compared with
healthy human lymphocytes. Int J Biochem Cell Biol. 44:1223–1231.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stanford SM, Maestre MF, Campbell AM,
Bartok B, Kiosses WB, Boyle DL, Arnett HA, Mustelin T, Firestein GS
and Bottini N: Protein tyrosine phosphatase expression profile of
rheumatoid arthritis fibroblast-like synoviocytes: A novel role of
SH2 domain-containing phosphatase 2 as a modulator of invasion and
survival. Arthritis Rheum. 65:1171–1180. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Garcia S, Liz M, Gómez-Reino JJ and Conde
C: Akt activity protects rheumatoid synovial fibroblasts from
Fas-induced apoptosis by inhibition of Bid cleavage. Arthritis Res
Ther. 12:R332010. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xu K, Xu P, Yao JF, Zhang YG, Hou WK and
Lu SM: Reduced apoptosis correlates with enhanced autophagy in
synovial tissues of rheumatoid arthritis. Inflamm Res. 62:229–237.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Korb A, Pavenstädt H and Pap T: Cell death
in rheumatoid arthritis. Apoptosis. 14:447–454. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lawlor KE, Nieuwenhuijze AV, Parker KL,
Drake SF, Campbell IK, Smith SD, Vince JE, Strasser A and Wicks IP:
Bcl-2 overexpression ameliorates immune complex-mediated arthritis
by altering FcγRIIb expression and monocyte homeostasis. J Leukoc
Biol. 93:585–597. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bartok B and Firestein GS: Fibroblast-like
synoviocytes: Key effector cells in rheumatoid arthritis. Immunol
Rev. 233:233–255. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim SK, Park KY, Yoon WC, Park SH, Park
KK, Yoo DH and Choe JY: Melittin enhances apoptosis through
suppression of IL-6/sIL-6R complex-induced NF-κB and STAT3
activation and Bcl-2 expression for human fibroblast-like
synoviocytes in rheumatoid arthritis. Joint Bone Spine. 78:471–477.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shu Q, Fu YY, Lu T and Yu YY: Study on
anti-tumor effect of the Daphne odora var.
Marginata's Extracts on SMMC-7721 and MCF-7 cells. Lishizhen
Med Materia Med Res. 9:2149–2150. 2009.
|
|
11
|
Li H, Li WP, Liu X, Wang JP and Yang SJ:
Experimental study of daphnetin and its derivatives influence on
microcirculation of type 2 diabetic rats. Jilin Med. 30:2981–2983.
2010.
|
|
12
|
Yao R, Fu Y, Li S, Tu L, Zeng X and Kuang
N: Regulatory effect of daphnetin, a coumarin extracted from
Daphne odora, on the balance of Treg and Th17 in
collagen-induced arthritis. Eur J Pharmacol. 670:286–294. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang ZQ: The demethylation molecular
mechanism and the effect of daphnetin on the proapoptotic genes in
the collagen-induced arthritis rats synovial cells (unpublished PhD
thesis). Nanchang University. 2012.
|
|
14
|
Ye C, Bhan AK, Deshpande V, Shankar P and
Manjunath N: Silencing TNF-α in macrophages and dendritic cells for
arthritis treatment. Scand J Rheumatol. 42:266–269. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Davis ME, Zuckerman JE, Choi CH, Seligan
D, Tolcher A, Alabi CA, Yen Y, Heidel JD and Ribas A: Evidence of
RNAi in human from systemically administered siRNA Via targeted
nanoparticles. Nature. 464:1067–1070. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lan ZY, Bai XZ and Han XR: Tak1 siRNA
impact on mmp99 and tinp1 expression in synovial cells of patients
with rheumatoid arthritis. J China Med Univ. 41:540–543. 2012.
|
|
17
|
Kang EH, Kim DJ, Lee EY, Lee YJ, Lee EB
and Song YW: Downregulation of heat shock protein 70 protects
rheumatoid arthritis fibroblast-like synoviocytes from nitric
oxide-induced apoptosis. Arthritis Res Ther. 11:R1302009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Toh ML, Gonzales G, Koenders MI, Tournadre
A, Boyle D, Lubberts E, Zhou Y, Firestein GS, van den Berg WB and
Miossec P: Role of Interleukin 17 in arthritis chronicity through
survival of synoviocytes via regulation of synoviolin expression.
PLoS One. 5:e134162010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen YJ, Chang YT, Wang CB and Wu CY: The
risk of cancer in patients with rheumatoid arthritis: A nationwide
cohort study in Taiwan. Arthritis Rheum. 63:352–358. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Perkins S, Cohen M, Rahme E and Bernasky
S: Melanoma and rheumatoid arthritis (brief report). Clin
Rheumatol. 31:1001–1003. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang L, Wang CH, Jia JF, Ma XK, Li Y, Zhu
HB, Tang H, Chen ZN and Zhu P: Contribution of Cyclophilin A to the
regulation of inflammatory processes in rhuematiod arthritis. J
Clin Immunol. 30:24–33. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yu P, Long L, Wang SY, Li R and Zhang XP:
Expression and function of MiR-155 in fibroblasts of rheumatoid
arthritis. Chin J Rheumatol. 14:460–463. 2010.
|
|
24
|
Guma M, Hammaker D, Topolewski K, Corr M,
Boyle DL, Karin M and Firestein GS: Antiinflammatory functions of
p38 in mouse models of rheumatoid arthritis: Advantage of targeting
upstream kinases MKK-3 or MKK-6. Arthritis Rheum. 64:2887–2895.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mor A, Abramson SB and Pillinger MH: The
fibroblast-like synovial cell in rheumatoid arthritis: A key player
in inflammation and joint destruction. Clin Immunol. 115:118–128.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huber LC, Distler O, Tarner I, Gay RE, Gay
S and Pap T: Synovial fibroblasts: Key players in rheumatoid
arthritis. Rheumatology (Oxford). 45:669–675. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fox DA, Gizinski A, Morgan R and Lundy SK:
Cell-cell Interactions in rheumatoid arthritis synovium. Rheum Dis
Clin North Am. 36:311–323. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Baier A, Meinekel I, Gay S and Pap T:
Apoptosis in rheumatoid arthritis. Curt Opin Rheumatol. 15:274–279.
2003. View Article : Google Scholar
|
|
29
|
Liang SC, Ge GB, Liu HX, Zhang YY, Wang
LM, Zhang JW, Yin L, Li W, Fang ZZ, Wu JJ, et al: Identification
and Characterization of Human UDP-Glucuronosyltransferases
responsible for the in vitro glucuronidation of daphnetin. Drug
Metab Dispos. 38:973–980. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shu Q, Fu YY and Liu T: The study of
active ingredients of Daphne odero as Daphne flava-I and Daphnetin
application on SMMC-7721 and MCF-7 cells. Materia Med.
20:2149–2150. 2009.
|
|
31
|
Xu J, Fu YY, Huang BH, Zeng XP and Kuang
NZ: The effect of Daphne odero extract anti-tumor in vitro and
vivo. Chin J New Drugs. 17:2019–2026. 2008.
|
|
32
|
Huang BH, Fu YY and Xu J: Daphne
Odora impact on immune function of mice with S180 tumor.
Materia Med. 19:2376–2377. 2008.
|
|
33
|
Tu LN, Li S, Fu Y, Yao R, Zhang Z, Yang S,
Zeng X and Kuang N: The therapeutic effects of daphnetin in
collagen-induced arthritis involve its regulation of Th17 cells.
Int Immunopharmacol. 13:417–423. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dumesic PA, Natarajan P, Chen C,
Drinnenberg IA, Schiller BJ, Thompson J, Moresco JJ, Yates JR III,
Bartel DP and Madhani HD: Stalled spliceosomes are a signal for
RNAi-mediated genome defense. Cell. 152:957–968. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tabach Y, Billi AC, Hayes GD, Newman MA,
Zuk O, Gabel H, Kamath R, Yacoby K, Chapman B, Garcia SM, et al:
Identification of small RNA pathway genes using patterns of
phylogenetic conservation and divergence. Nature. 493:694–698.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chi PL, Chen YW, Hsiao LD, Chen YL and
Yang CM: Heme oxygenase 1 attenuates interleukin-1β-induced
cytosolic phospholipase A2 expression via a decrease in NADPH
oxidase/reactive oxygen species/activator protein 1 activation in
rheumatoid arthritis synovial fibroblasts. Arthritis Rheum.
64:2114–2125. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Park JS, Yang HN, Jeon SY, Woo DG, Kim MS
and Park KH: The use of anti-COX2 siRNA coated onto PLGA
nanoparticles loading dexamethasone in the treatment of rheumatoid
arthritis. Biomaterials. 33:8600–8612. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gao Wei, Feng Xin, Ren Li-Bing, et al:
Expressions and significance of apoptosis-related factors Bcl-xl,
Bcl-2 and Bax in synovial tissues of rats with rheumatoid athritis.
Chin Gen Med. 15:2776–2779. 2012.
|
|
39
|
Ferdek PE, Gerasimenko JV, Peng S, Tepikin
AV, Petersen OH and Gerasimenko OV: A novel role for Bcl-2 in
regulation of cellular calcium extrusion. Curr Biol. 22:1241–1246.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li GH, Wei H, LV SQ, JI H and Wang DL:
Knockdown of STAT3 expression by RNAi suppresses growth and induces
apoptosis and differentiation in glioblastoma stem cells. Int J
Oncol. 37:103–110. 2010.PubMed/NCBI
|
|
41
|
Lee SY, Kwok SK, Son HJ, Ryu JG, Kim EK,
Oh HJ, Cho ML, Ju JH, Park SH and Kim HY: IL-17-mediated Bcl-2
expression regulates survival of fibroblast like synoviocytes in
rheumatoid arthritis through STAT3 activation. Arthritis Res Ther.
15:R312013. View
Article : Google Scholar : PubMed/NCBI
|