Introduction
Corneal disease is the second leading cause of
blindness worldwide, as the cornea serves an important role in the
refraction of light through the eye (1). However, local factors or systemic
diseases can affect corneal transparency, which leads to the
formation of corneal neovascularization (CNV), causing decreased
visual acuity, corneal scar, lipid deposition and induced corneal
transplant rejection (2). CNV can
be induced by alkali burns. Fats and proteins are dissolved within
alkali burns, which cause cellular decomposition and necrosis, and
consequent corneal degeneration, necrosis, ulceration, perforation
and NV. It is very important to control the inflammatory reaction
and the invasion of CNV in order to reduce corneal injury and
complications. The clinical use of anti-inflammatory
glucocorticoids can inhibit CNV but several side effects have been
reported, including intraocular pressure, infection and resistance
(3). Such treatment cannot be
administered onto the ocular surface safely for long durations. The
present study aimed to investigate the use of catalpol to treat CNV
in a safe and effective manner.
Catalpol, which is a member of the iridoid
glycosides family, is a chemical component isolated from the
Scrophulariaceae rehmannia root (4). A recent study suggested that catalpol
exerts protective effects against cerebral ischemia, dementia,
inflammation, capillary permeability, tumor, laxation and blood
glucose levels, and other pharmacological properties associated
with high safety and low toxicity (5). It has been reported that catalpol can
protect neurons from cytotoxic damage, reduce neuronal cell
apoptosis following cerebral ischemia (6) and alleviate neuropathic pain
(7). Our previous studies have
demonstrated that netrin-1 suppressed corneal and retinal NV
(8–10).
Numerous neuroprotective and anti-inflammatory
effects exhibited by catalpol have been reported; however, the
underlying mechanism has yet to be determined. In the present
study, corneal alkali-burn rat models of CNV were employed to
investigate the effects of catalpol on angiogenesis and
inflammation; the potential underlying anti-angiogenic mechanism
was investigated in vitro.
Materials and methods
Reagents and cell culture
Catalpol was isolated from the traditional Chinese
medicinal root, Scrophulariaceae rehmannia. Catalpol was purchased
from Shanghai Yuanye Biotechnology Co., Ltd. (Shanghai, China).
Catalpol was dissolved in PBS to generate various concentrations,
0.1, 0.5, 1, 2, 5, 10 and 20 mM; preservatives were not used in the
present study. Human umbilical vein endothelial cells (HUVECs) were
obtained from the Cell Line Bank of the Chinese Academy of Sciences
(Shanghai, China) and cultured as previously described (11). HUVECs were used after 2–6
passages.
Cell viability assay
The Cell Counting kit-8 (CCK-8; Dojindo Molecular
Technologies, Inc., Kumamoto, Japan) assay was used to quantify
cell according to the manufacturer's protocol. Briefly,
5×103 HUVECs/well were seeded into 96-well plates in
triplicate and incubated at 37°C for 24 h. Catalpol was applied to
the EGM-2 medium (cat. no: CC-3156; Lonza Group, Ltd., Basel,
Switzerland), at a dosage of 0.1, 0.5, 1, 2, 5, 10 and 20 mM for 72
h. Cell viability was determined using 10 µl CCK-8 solution
(Dojindo Molecular Technologies, Inc.), according to the
manufacturer's protocol. Optical density was determined with a
universal microplate reader at 450 and 570 nm (BioTek Instruments,
Winooski, VT, USA),
Wound closure assay
HUVEC migration was investigated as previously
described (10). HUVECs
(1×105/well) were seeded onto 1% gelatin-coated 24-well
plates (Corning Life Sciences, Amsterdam, Netherlands). A scratch
wound was made in confluent cell culture in two perpendicular
directions with a sterile pipet tip (200 µl). Floating cells were
washed with PBS and the remaining cells were cultured with
experimental medium (with or without 5 mM catalpol) for an
additional 24 h.
In vitro tube formation assay
HUVECs were serum-starved for 12 h and seeded onto
growth factor-depleted Matrigel (BD Biosciences, Franklin Lakes,
NJ, USA) coated 24-well plates at a density of 10,000 cells/well.
Cells were incubated at 37°C with 5 mM catalpol or PBS (control)
for 6 h and fixed with 4% paraformaldehyde (PFA). Tube structures
within ≥5 microscopic fields were imaged and quantified; tube
length was analyzed via ImageJ software version 2X (National
Institutes of Health, Bethesda, MD, USA) (7).
Alkali-burned rat cornea model
Alkali burns were applied to Sprague Dawley rats
(180–220 g; 2 months old; male; n=60; Shanghai Shilaike Laboratory
Animal Co., Ltd., Shanghai, China) as previously reported (8). The rats were individually housed in
hanging wire cages (changed weekly) with in a room maintained at
22±2°C, relative humidity of 30–70%, 10 changes of air per hour,
and a 12-h light/dark cycle. Tap water and standard diet were
supplied ad libitum. Briefly, 60 anesthetized rats (10% chloral
hydrate, 3 ml/kg) received topical administration of a drop of 0.5%
tetracaine. Alkali burns were induced by placing 3.5 mm diameter
round filter paper soaked with 1 M NaOH onto the center of the
corneal surface for 30 sec, followed by a rinse of 10 ml PBS.
Alkali-burned animals were randomly divided into the
PBS and catalpol groups (n=30 rats/group). Topical administrations
of 10 µl PBS or 5 mM catalpol were applied four times per day for
14 days; treatments were applied every 6 h (6:00 a.m., 12:00 p.m.,
6:00 p.m. and 12:00 a.m.). Eyes were examined on days 1, 4, 7, 10
and 14 by slit lamp microscopy to evaluate CNV, inflammation and
damage. Rats were sacrificed on postoperative day 7 or 14, and
corneal samples were collected for histological examination,
protein extraction or stored at −80°C until use.
Animal experiments were carefully performed in
accordance with the guidelines of the Association for Research in
Vision and Ophthalmology (Rockville, MD, USA) Statement for the Use
of Animals in Ophthalmic and Vision Research (9), and the present study was approved by
the Experimental Animal Committee of Xiamen University (Xiamen,
China; approval ID: XMUMC2015-02-1).
Slit lamp microscopy examination
Corneal epithelial alterations were determined by
0.1% fluorescein sodium staining under cobalt blue light. Images
were processed with Image Pro Plus version 6.0 (Media Cybernetics,
Silver Spring, MD, USA). CNV area (S) was quantified using the
following formula: S=C/12π × [r2-(r-I)2];
where C is time, I is the vessel radius and r is the cornea radius
(8). The inflammatory index was
evaluated based on various parameters as previously described,
including ciliary hyperemia, peripheral and central corneal edema
(12).
Histology
Eye samples were fixed in 4% PFA in PBS overnight at
4°C, dehydrated in a series of alcohol and embedded in paraffin.
Tissue samples were cut into 5 µm sections and were subsequently
stained with hematoxylin and eosin examined using an Eclipse 50i
clinical microscope (Nikon Corporation, Tokyo, Japan).
Immunofluorescent staining
Cryosections of 4 µm were air-dried at room
temperature for 30 min and fixed in acetone for 10 min at −20°C.
Then, sections were rehydrated in PBS, and incubated in 0.2% Triton
X-100 for 10 min
Following three rinses with PBS for 5 min each and
preincubation with 2% BSA to block nonspecific staining, samples
were incubated with anti-rabbit VEGF (cat. no: ab46154; 1:200;
Abcam, Cambridge, MA, USA) and anti-rabbit PEDF (cat. no: sc-25594;
1:200; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) antibodies
for 16 h at 4°C. Following three washes with PBS for 15 min,
samples were incubated with a FITC-conjugated secondary antibody
(goat anti-rabbit IgG; cat. no. F-6005; Lot: 065k6224; 1:100,
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 1 h. Following
three additional PBS washes, the sections were counterstained with
propidium iodide (1:1,000) and then counterstained with DAPI
(Vector Laboratories, Inc., Burlingame, CA, USA), mounted, and
photographed using the Leica upright microscope (DM2500; Leica
Microsystems GmbH, Wetzlar, Germany).
For analysis of integrated optical density
expression of positive immunostaining, images from immunostained
(VEGF and PEDF proteins) sections were processed using
image-processing software (Image Pro Plus version 6.0; Media
Cybernetics, Bethesda, MD).
Western blot analysis
Corneal tissues were dissected and ground in cold
radioimmunoprecipitation assay buffer with proteinase inhibitor
cocktail (Merck KGaA, Darmstadt, Germany). Total protein was
quantified using a bicinchoninic acid assay and 20 µg were loaded
onto 10% Bis-Tris SDS-PAGE gels under reducing conditions at (80 V,
3 h, room temperature) and then transferred onto nitrocellulose
membranes. The membranes were blocked with ChemiBlocker for 1 h and
the blots are subsequently incubated overnight at 4°C with primary
antibodies against VEGF (cat. no. ab46154; 1:200; Abcam, Cambridge,
MA, USA), PEDF (cat. no. sc-25594; 1:200; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA), TNF-α (cat. no. ab66579; 1:200; Abcam) and
phosphorylated p-NF-κB p65 (cat. no. sc-3033S; 1:200; Santa Cruz
Biotechnology, Inc.) overnight. β-actin (cat. no. A5316; 1:10,000;
Sigma-Aldrich; Merck KGaA) was used as the loading control
overnight at 4°C. Subsequently, membranes were incubated with
horseradish peroxidase-conjugated secondary antibodies (Goat Anti
Rabbit IgG HRP Affinity, cat. no. HAF008; 1:5,000; R&D Systems,
Inc., Minneapolis, MN, USA) for 1 h at 37°C and were visualized by
enhanced chemiluminescence (ECL, lot no:161203-85, Advansta Inc.,
Menlo Park, CA, USA). The results were visualized and recorded on
film by a Chemi DOC™ XRS Imaging System (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Apoptosis detection assay
Corneal cell apoptosis was analyzed using frozen
corneal sections and terminal deoxynucleotidyl transferase-mediated
nick end labeling (TUNEL; DeadEndä Fluorometric TUNEL system;
Promega Corporation, Madison, WI, USA) according to the
manufacturer's protocol following 4% PFA fixation overnight at 4°C.
Nuclei were counterstained with DAPI and 3 fields of view per
sample were mounted in H-1200 (Vector Laboratories, Inc.,
Burlingame, CA, USA) and observed under a confocal microscope
(Fluoview FV1000; Olympus Corporation, Tokyo, Japan).
Statistical analysis
Data are presented as the mean ± standard deviation.
The inflammatory index and CNV area were analyzed with one-way
analysis of variance followed by a Bonferroni post hoc comparison.
One-way analysis of variance followed by a post hoc Tukey test were
used to analyze HUVEC viability, migration and tube formation
between groups. Statistical analyses were conducted using GraphPad
software version 5 and analyzed using t-test (GraphPad Software,
Inc., La Jolla, CA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
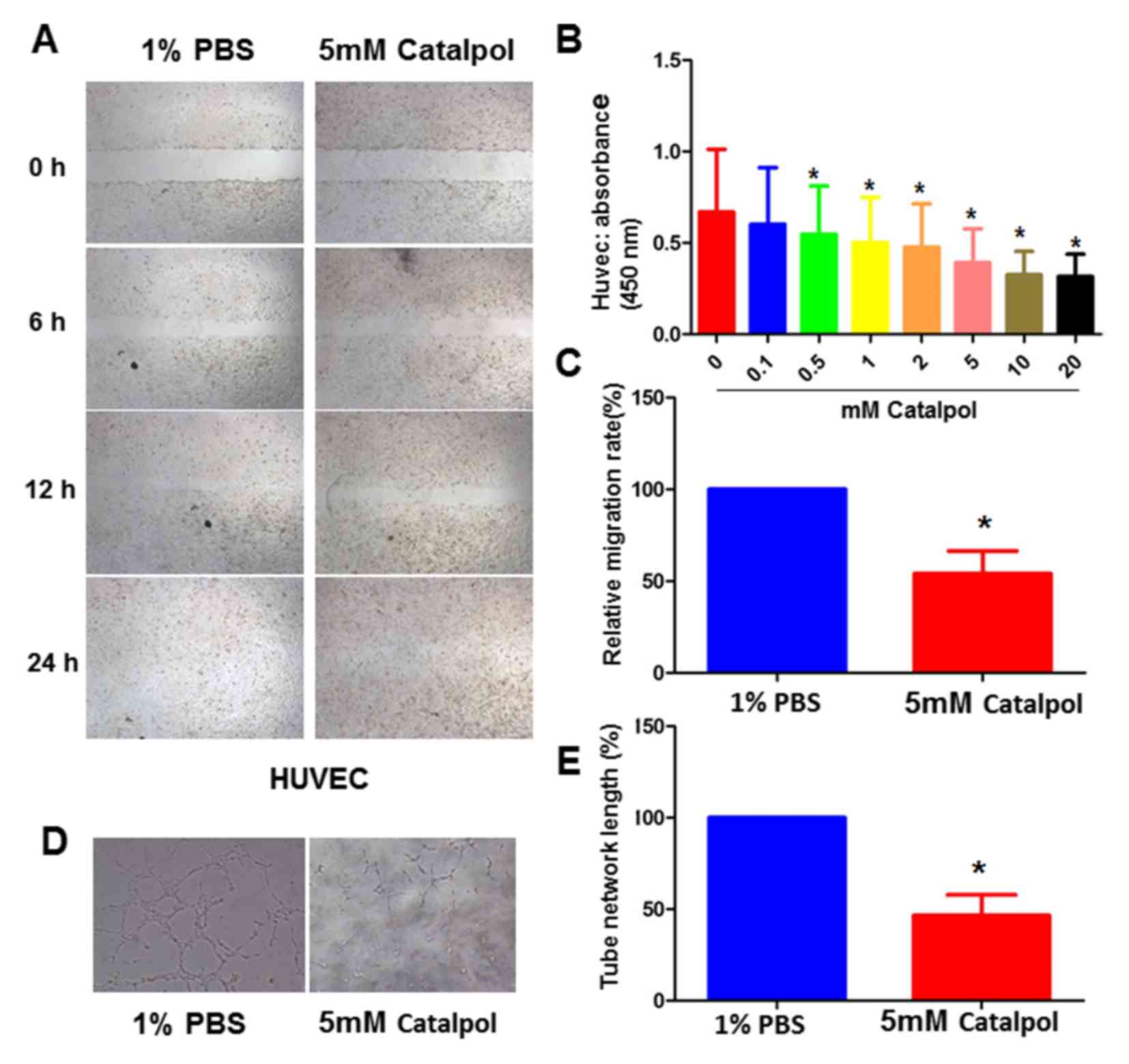
Catalpol decreases HUVEC viability in
a dosage-dependent manner
The present study performed CCK-8 assays to
determine cell viability in the presence of various concentrations
of catalpol (0, 0.1, 0.5, 1, 2, 5, 10 and 20 mM). Treatment with
≥0.5 mM catalpol inhibited HUVEC survival in vitro (Fig. 1A). In addition, in the
pre-experiment, 5 mM catalpol was reported to exert an effect on
the rat model in vivo. In the pre-experiment, 5 mM catalpol reduced
CNV and inflammation in alkali-burned rats. The CNV area at day 4,
7,10 and 14 was ~37.8, 53, 68 and 65mm2 in the control
group, and was ~25.2, 32, 35 and 30 mm2 in the
catalpol-treated group. The inflammation index at day 4, 7,10 and
14 was ~0.58, 0.63, 0.59 and 0.55 in the control group, and was
~0.43, 0.35, 0.31 and 0.24 in the catalpol-treated group (data not
shown). Therefore, 5 mM catalpol was chosen for subsequent
experiments.
Catalpol inhibits HUVEC migration and
tube formation
In order to confirm the role of catalpol in cell
proliferation, the present study assessed its effects on HUVEC
migration using a scratch-wound model. Wound closure was detected
in the presence of absence of catalpol. In the PBS control group,
wound closure was detected within ~12 h. However, wounds in the
catalpol group did not heal within 12 h; 50% of the wound remained
unhealed in the catalpol-treated group at 12 h. The migration rates
at 12 h were 100 and 50% in the control and 5 mM catalpol-treated
groups, respectively (Fig. 1B and
C).
Subsequently, the effects of catalpol on HUVEC tube
formation were assessed using a previously established in
vitro tubulogenesis assay (13). The results demonstrated that 5 mM
catalpol significantly inhibited HUVEC tube formation (Fig. 1D and E).
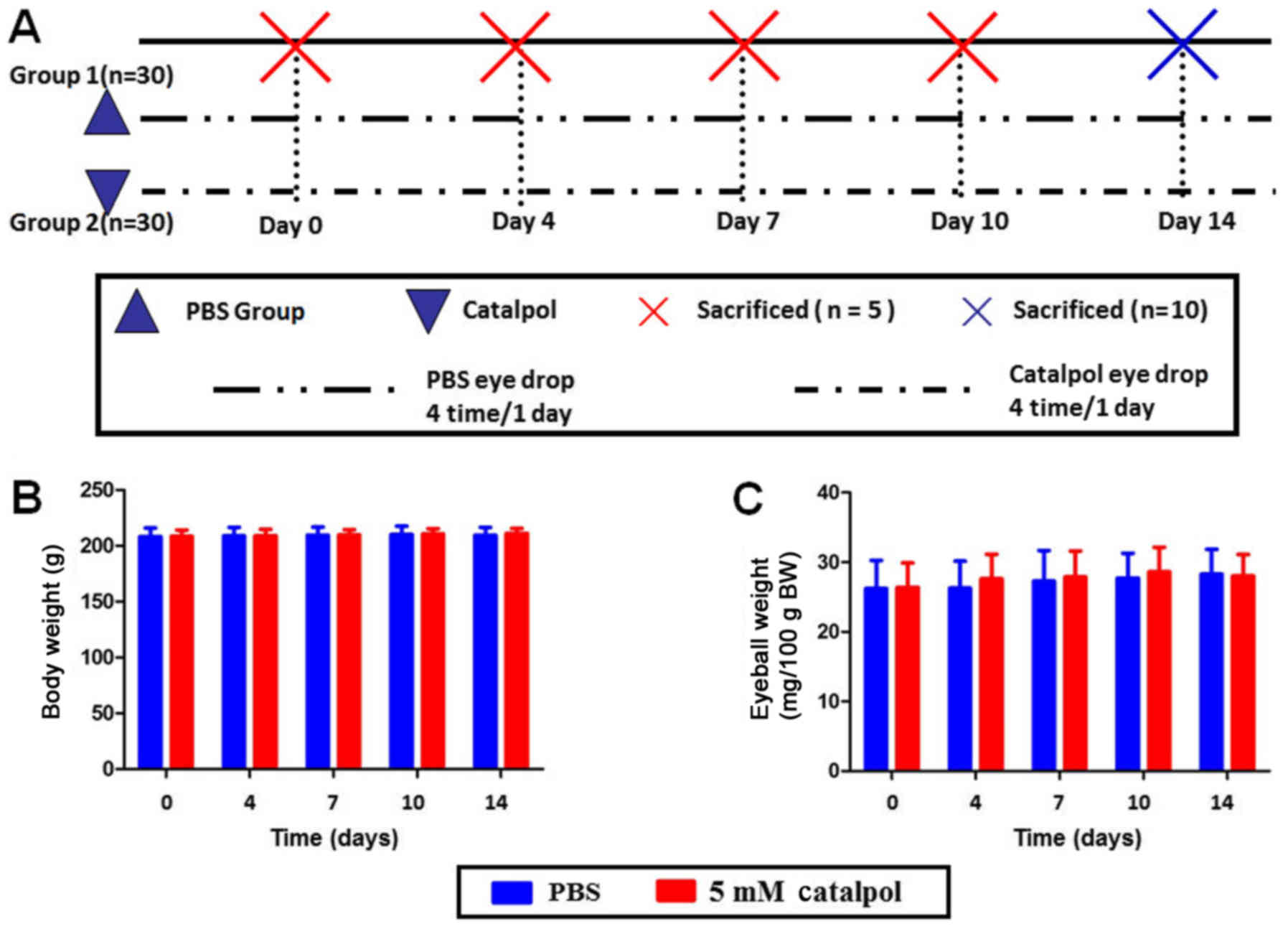
Metabolic conditions in Sprague Dawley
rats
A total of 60 weight-matched Sprague Dawley rats
(weight, 200–220g; age, 8–10 weeks; male) were randomly divided
into two groups, which were treated with PBS (control) or catalpol.
A total of 4, 7, 10 and 14 days following administration of PBS or
catalpol eye drops, body weight and eyeball weight were measured
(Fig. 2A). No significant
difference was observed between the experimental and control groups
with regards to alterations in body weight and eyeball weight
(Fig. 2B and C).
Catalpol reduces CNV in alkali-burned
rat corneas
Alkali burn is a well-established model used to
study CNV. The present study evaluated the effects of catalpol on
CNV using an alkali burn model. Few peripheral neovascularization
were detected on day 1 post-alkali burn (Fig. 3A and B). The inflammatory index was
slightly decreased from day 4 to day 14 in the PBS group, whereas
it was markedly reduced in the catalpol-treated group. There were
significant differences between the two groups on days 4, 7, 10 and
14 (Fig. 3C).
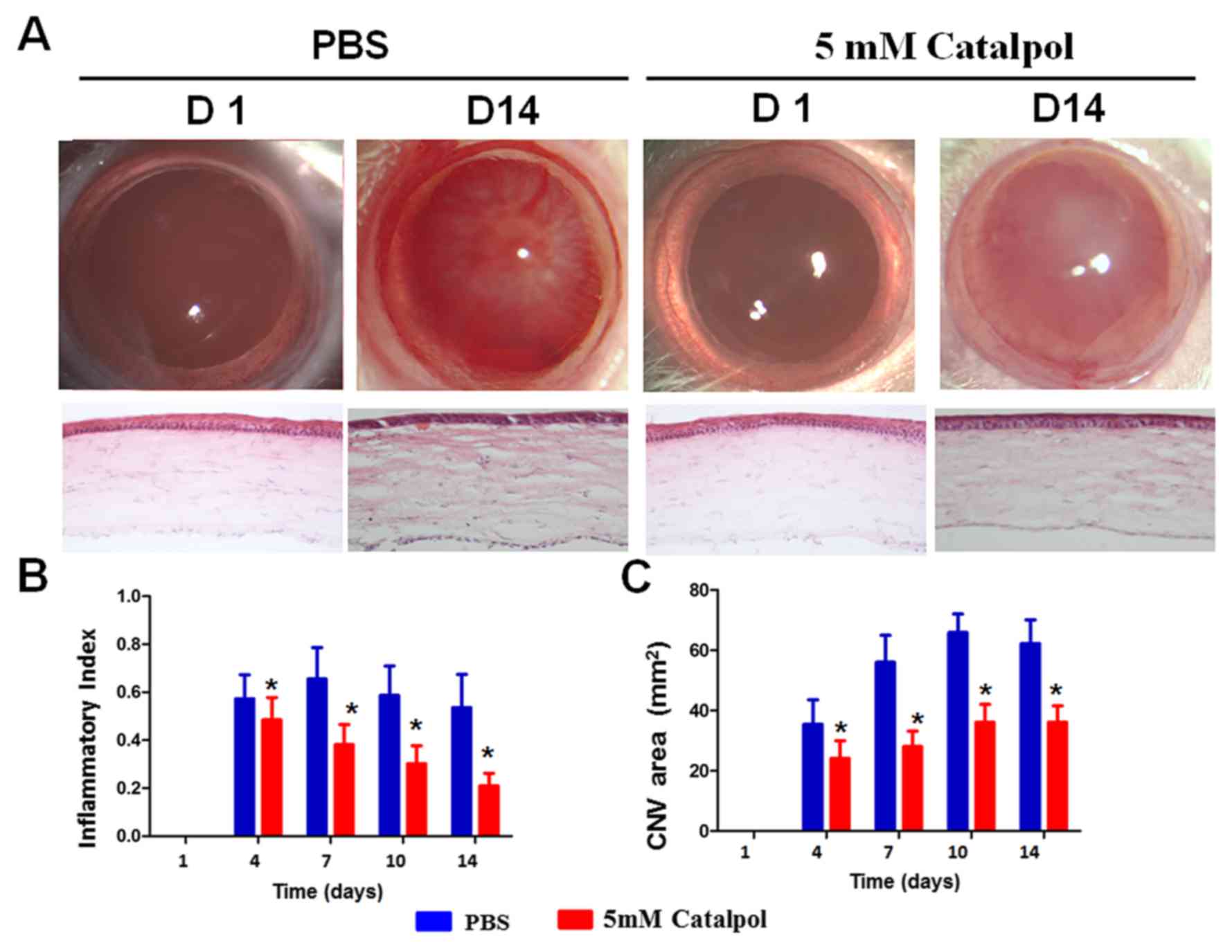 | Figure 3.Catalpol reduces CNV and inflammation
post-alkali burn. (A) On day 14, newly formed blood vessels
approached the central part of the cornea in the control group, and
corneal transparency was markedly decreased. However, only a few
newly formed blood vessels in the limbus were detected in the
catalpol-treated group, and the corneas remained transparent on day
14. Hematoxylin and eosin staining demonstrated that new blood
vessels (white arrows) were present in the limbus and the central
corneas in the control group on day 14, whereas in the
catalpol-treated group, few blood vessels (white arrows) were
detected in the limbus, and none were present in the peripheral and
central corneas, magnification 20x. (B) CNV area in the control
group was increased from day 0 to 14; however, there was a mild
decrease on day 14 post-alkali burn. Conversely, the
catalpol-treated group exhibited a mild but significant decrease in
CNV area on days 4, 7, 10 and day 14. *P<0.05 vs. PBS group. (C)
Inflammatory index was reduced from day 0 to 14 in both groups.
However, it was significantly lower in the catalpol-treated group
on days 4, 7, 10 and 14. *P<0.05. CNV, corneal
neovascularization. |
In the PBS group, angiogenesis was detected in the
peripheral corneas at around day 4, and approached the central
corneas on day 7; the newly formed blood vessels were detectable on
day 14 (Fig. 3A and B).
Conversely, corneas treated with catalpol exhibited a mild increase
in newly formed blood vessels, which were maintained at low levels
throughout the study period (Fig.
3A). The area of the newly formed blood vessels was much lower
in the catalpol-treated group compared with in the control group on
day 14 (Fig. 3A). Persistent
angiogenesis was detected in the anterior region of the corneal
stroma in the control group on day 14, as determined by hematoxylin
and eosin staining, and clearly indicated by the red blood cells in
the blood vessels. However, in the catalpol-treated group only a
few blood vessels developed in the limbus and none were detected in
the peripheral or central corneas (Fig. 3A).
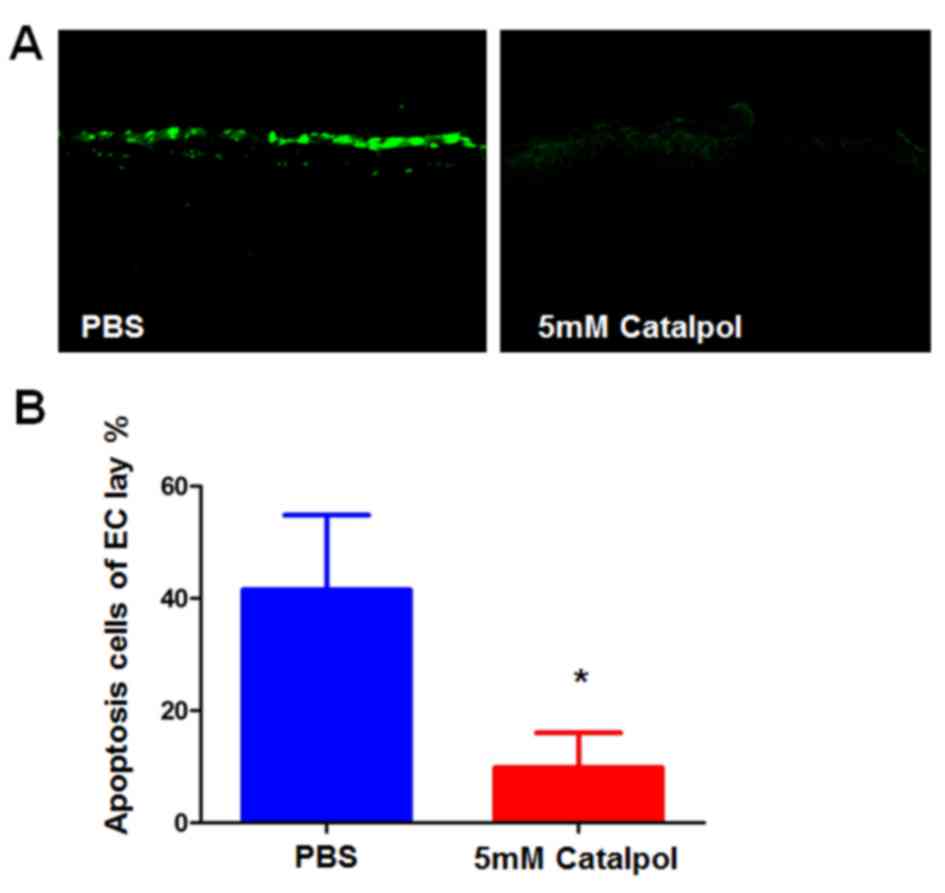
Catalpol reduces the alkali burn-induced apoptosis
of corneal cells. Alkali burns may directly damage corneal
epithelia by inducing apoptosis of underlying stromal cells, which
promotes the infiltration of inflammatory cells that can cause
further damage. To determine the effects of catalpol on alkali
burn-induced apoptosis, a TUNEL assay was conducted. The majority
of cells in the central corneas were TUNEL-positive on day 7
post-alkali burn in the PBS group. Conversely, the number of
apoptotic cells was significantly reduced in the catalpol-treated
corneas at day 7 compared with in the PBS group (Fig. 4A and B).
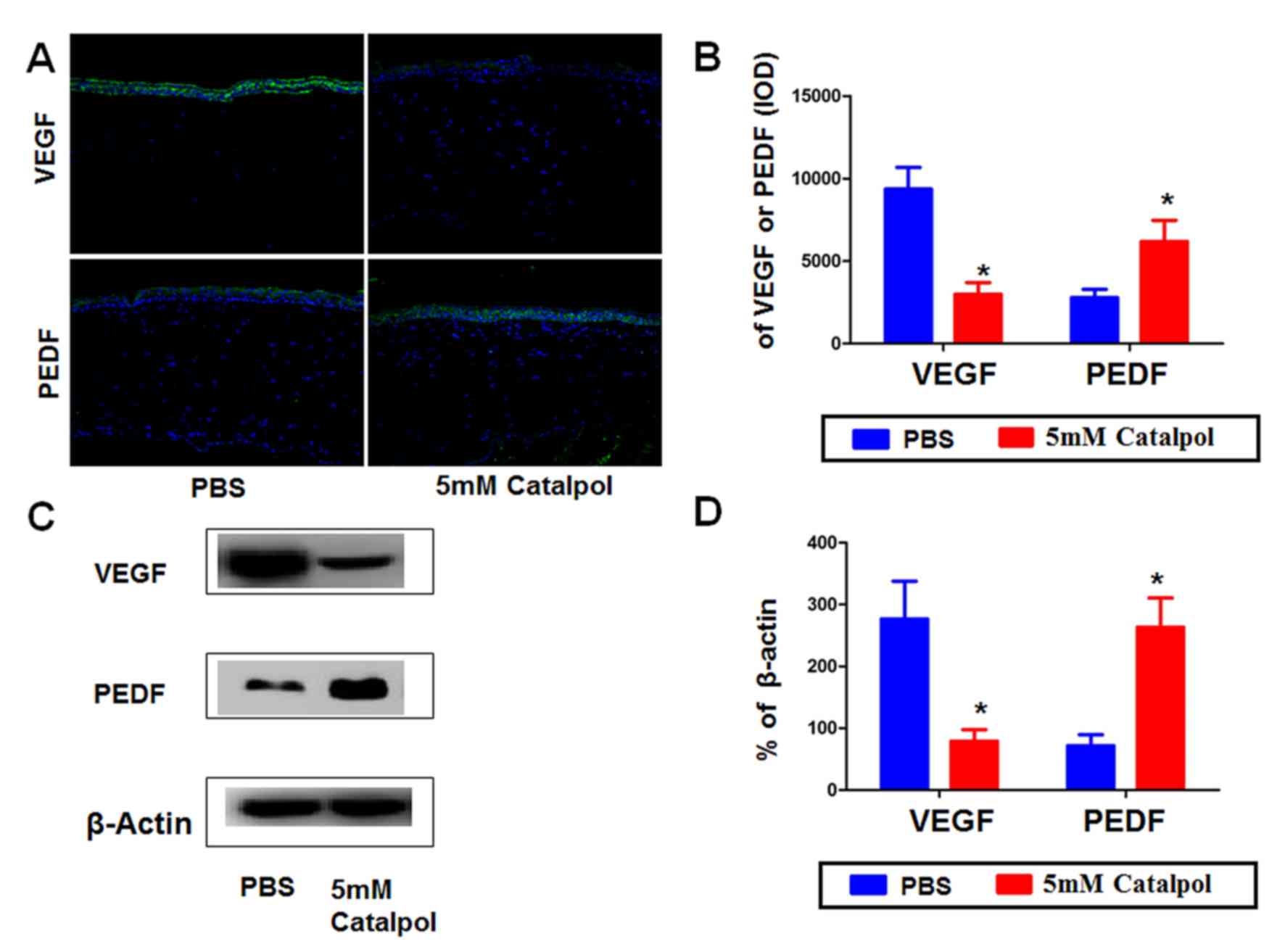
Catalpol alters the expression of VEGF
and PEDF in alkali-burned rat corneas
CNV is controlled by the balance between pro- and
antiangiogenic factors, including VEGF and PEDF. To investigate how
catalpol prevents CNV in the alkali burn model, the present study
analyzed the expression levels of VEGF and PEDF by western blotting
and immunofluorescence (Fig. 5A).
The results demonstrated that VEGF was expressed at low levels in
normal rat corneas, and was markedly increased on day 14
post-alkali burn in the control group (data not shown). In the
catalpol group, the expression of VEGF was much lower than in the
control group on day 14. Conversely, PEDF was markedly decreased on
day 14 post-alkali burn; however, PEDF was restored to some degree
in the catalpol treatment group, although it was still somewhat
lower than the expression in normal corneas (data not shown).
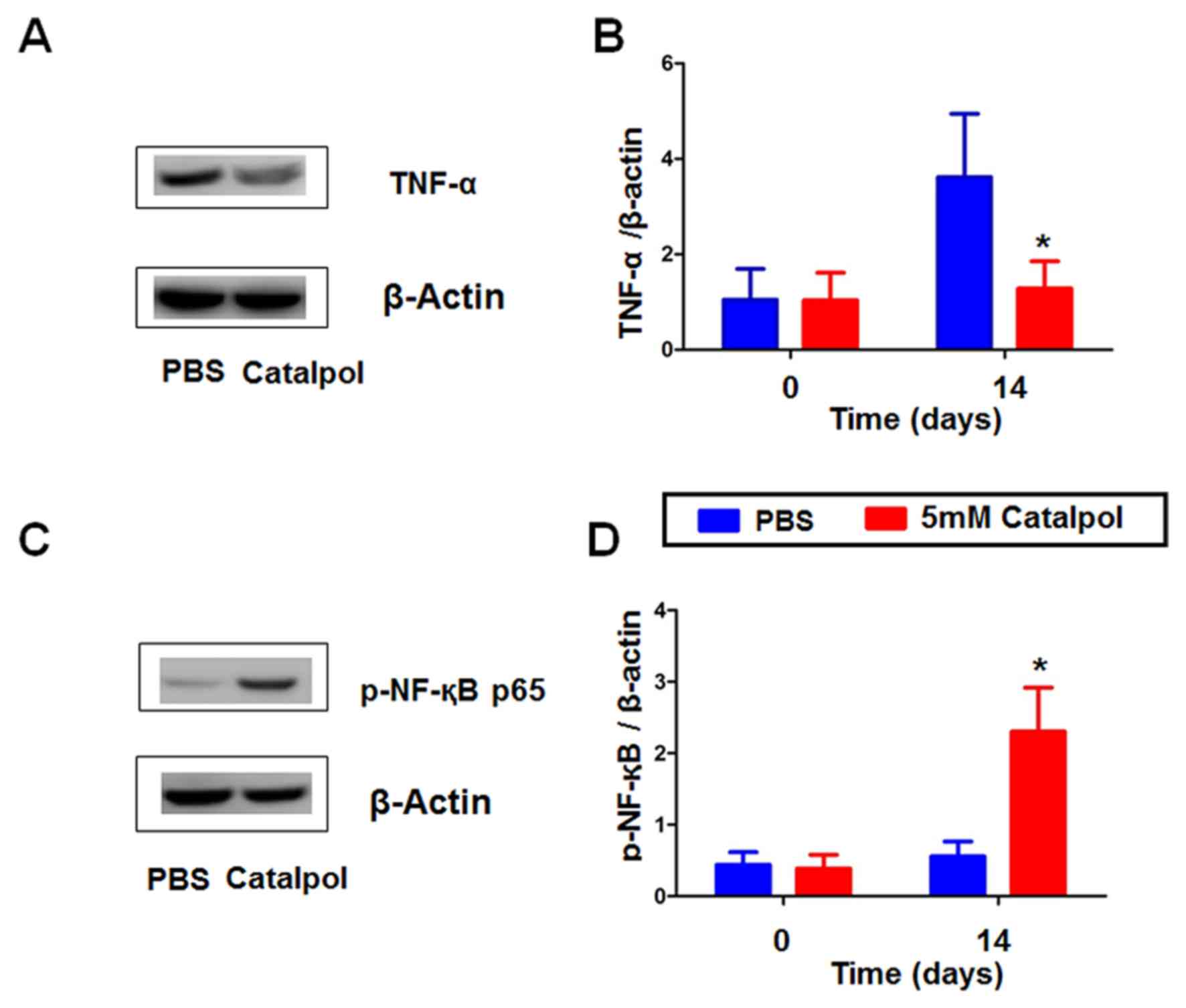
Catalpol alters the expression of
TNF-α and p-NF-κB p65 in alkali-burned rat corneas
The present study investigated the anti-inflammatory
effects of catalpol post-alkali burn by measuring the expression
levels of the inflammatory factors TNF-α and p-NF-κB p65 by western
blotting (Fig. 6). On day 14 TNF-α
expression was lower in the catalpol group vs. the PBS group
(Fig. 6A and B). Furthermore,
TNF-α was downregulated in catalpol group. Conversely, p-NF-κB p65
expression was increased in the catalpol-treated group (Fig. 6C and D). Catapol can inhibit
inflammation induced by alkali-burn rat cornea.
Discussion
Corneal alkali burns result in the generation of
progressive ocular disease, which aggravates inflammation and
tissue injury, causing CNV-associated complications and ulceration.
Although CNV is conducive to the elimination of pathogens and
tissue repair, it is also associated with reduced corneal
transparency, thus resulting in damage to eye structure and visual
function. In addition, CNV has been reported to be the main
clinical cause of blindness (14).
At present, the aim of future research is to develop novel, safe
and effective anti-inflammatory and anti-angiogenic therapies for
the treatment of CNV.
Catalpol has been reported to possess antioxidative,
anti-inflammatory, anticancer, neuroprotective, diuretic,
hypoglycemic, anti-hepatitis, hemostatic and antispasmodic
properties (15,16). Ocular inflammatory factors and
proapoptotic factors within ocular alkali-induced burns activate
the apoptotic pathway, and simultaneously introduce a large number
of polymorphonuclear and inflammatory cells to aggravate
inflammation and injury. Choi et al (17) reported that catalpol alleviates the
inflammatory response of THP-1 cells by inhibiting the activity of
NF-κB. Basal expression of NF-κB has been reported in all cell
types, and is known to serve a physiological role in the
development of the cornea and normal physiological activities
(16). Upon stimulation of the
cornea by external factors, the NF-κB/inhibitory κB complex
dissociates, and NF-κB is translocated into the nucleus where it
binds to corresponding sites on target genes, thus activating gene
transcription.
TNF-α is mainly produced by monocytes and
macrophages, and can inhibit the activity of nitric oxide synthase,
promote the expression of endothelial cell adhesion molecules,
activate vascular endothelial cells, release platelet-derived
growth factor and induce apoptosis of endothelial cells.
Consequently, TNF-α can cause vascular proliferation and necrosis
(17). Recently, it has been
demonstrated that catalpol can reduce TNF-α and p-NF-κB expression
(18). The results of the present
study indicated that catalpol treatment reduced inflammation
associated with alkali burns of the cornea and downregulated the
expression of TNF-α and p-NF-κB.
VEGF is a specific marker protein of vascular
endothelial cells, which is involved in the regulation of capillary
vessel angiogenesis (19). VEGF
can induce proliferation, response to chemokines and permeability
of vascular endothelial cells, and participates in the formation of
new blood vessels. In addition, VEGF can promote adhesion,
migration and differentiation of mononuclear macrophages, and
maintains these processes (20).
Previously, it has been reported that that VEGF is an effective
factor in the initiation and regulation of angiogenesis (21). PEDF is a specific anti-angiogenic
factor and neurotrophic factor, which is expressed within the
retinal pigment epithelium, iris and cornea (22). It has been reported that PEDF can
promote endothelial cell apoptosis; however, it also inhibits
migration of vascular endothelial cells and lumen formation
(23). PEDF has a strong
inhibitory effect on CNV and may be an important factor in
controlling the progression of the disease (24). Evidence has demonstrated that the
proangiogenic effects of catalpol may be associated with the
upregulation of VEGF expression (25). Low doses of catalpol exert an
inhibitory effect on the integrity of vascular endothelial cells.
Studies have also reported that the protective effects of catalpol
on vascular endothelial cells are dose-dependent. Low doses of
catalpol exert an inhibitory effect on the integrity of vascular
endothelial cells; however high doses of catalpol provide
protective effects (26,27). In the present study, low
concentrations of catalpol were administered as low doses of
catalpol exert an inhibitory effect on the integrity of vascular
endothelial cells; however high doses of catalpol provide
protective effects. Western blotting revealed that catalpol can
downregulate the expression levels of VEGF and upregulate the
expression levels of PEDF to inhibit the formation of new blood
vessels. In addition, catalpol was confirmed to inhibit the
proliferation, invasion, migration and tube formation of HUVECs,
which is associated with the inhibition of CNV (Fig. 1B).
As one of the main causes of blindness, CNV is also
a risk factor for graft rejection following corneal allograft
transplantation. Corneal alkali burns provide an integrated model
of severe ocular surface disease, which results in corneal
epithelial defects, keratitis, CNV and decreased corneal
transparency (28,29). Such models are used to investigate
the underlying mechanism and treatment of inflammation and
angiogenesis due to ease of use and observation (30,31).
The present study generated a corneal alkali-burned rat model, and
the effects of the traditional Chinese medicine catalpol were
determined. The results demonstrated that catalpol can inhibit the
formation of CNV and the inflammatory response within alkali-burned
rats, and confirmed that catalpol can inhibit HUVEC cell migration,
tube formation, proliferation and apoptosis in vitro.
Catalpol exhibits effects on corneal alkali burns via VEGF
inhibition and PEDF upregulation. In addition, catalpol may reduce
the expression of TNF and p-NF-kB-p65 to relieve the inflammatory
response. Studies on the anti-inflammatory activity of catalpol
further suggests that catalpol exerts therapeutic activity through
attenuation of NF-κB activity (6,32).
The present study provided a novel experimental basis for the
treatment of CNV, as catalpol was demonstrated to inhibit the
progression of CNV; however, the effects are dose dependent and
further investigation into optimal dosage is required.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81300729,
81160118, 81460092 and 81660152), the Natural Science Foundation of
Fujian Province (grant no. 2015J05170) and the ShanHai Foundation
of China (grant no. 2013SH008).
References
|
1
|
Whitcher JP, Srinivasan M and Upadhyay MP:
Corneal blindness: A global perspective. Bull World Health Organ.
79:214–221. 2001.PubMed/NCBI
|
|
2
|
Hayashi K, Hooper LC, Detrick B and Hooks
JJ: HSV immune complex (HSV-IgG: IC) and HSV-DNA elicit the
production of angiogenic factor VEGF and MMP-9. Arch Virol.
154:219–226. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang MC and Bian F: Emphasizing the
prevention and anti-inflammation research of dry eye disease.
Zhonghua Yan Ke Za Zhi. 49:6–7. 2013.(In Chinese). PubMed/NCBI
|
|
4
|
Liu YR, Lei RY, Wang CE, Zhang BA, Lu H,
Zhu HC and Zhang GB: Effects of catalpol on ATPase and amino acids
in gerbils with cerebral ischemia/reperfusion injury. Neurol Sci.
35:1229–1233. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang JH, Zou L, Wan D, Zhu HF, Wang Y and
Qin L: Review of Catalpol's pleiotropic signaling pathways. Zhong
Guo Yao Li Xue Tong Bao Bian Ji Bu. 9:1189–1194. 2015.(In
Chinese).
|
|
6
|
Bi J, Jiang B, Zorn A, Zhao RG, Liu P and
An LJ: Catalpol inhibits LPS plus IFN-γ-induced inflammatory
response in astrocytes primary cultures. Toxicol In Vitro.
27:543–550. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang Y, Zhang R, Xie J, Lu J and Yue Z:
Analgesic activity of catalpol in rodent models of neuropathic
pain, and its spinal mechanism. Cell Biochem Biophys. 70:1565–1571.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Han Y, Shao Y, Lin Z, Qu YL, Wang H, Zhou
Y, Chen W, Chen Y, Chen WL, Hu FR, et al: Netrin-1 simultaneously
suppresses corneal inflammation and neovascularization. Invest
Ophthalmol Vis Sci. 53:1285–1295. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Policy statements adopted by the Governing
Council of the American Public Health Association, November 15,
2000. Am J Public Health. 91:476–521. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Han Y, Shao Y, Liu T, Qu YL, Li W and Liu
Z: Therapeutic effects of topical netrin-4 inhibits corneal
neovascularization in alkali-burn rats. PLoS One. 10:e01229512015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu Y, Zou J, Han Y, Quyang L, He H, Hu P,
Shao Y and Tu P: Effects of intravitreal injection of netrin-1 in
retinal neovascularization of streptozotocin-induced diabetic rats.
Drug Des Devel Ther. 9:6363–6377. 2015.PubMed/NCBI
|
|
12
|
Huang X, Han Y, Shao Y and Yi JL: Efficacy
of the nucleotide-binding oligomerzation domain 1 inhibitor
Nodinhibit-1 on corneal alkali burns in rats. Int J Ophthalmol.
8:860–865. 2015.PubMed/NCBI
|
|
13
|
Arnaoutova I and Kleinman HK: In vitro
angiogenesis: Endothelial cell tube formation on gelled basement
membrane extract. Nat Protoc. 5:628–635. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Voiculescu OB, Voinea LM and Alexandrescu
C: Corneal neovascularization and biological therapy. J Med Life.
8:444–448. 2015.PubMed/NCBI
|
|
15
|
Ismailoglu UB, Saracoglu I, Harput US and
Sahin-Erdemli I: Effects of phenylpropanoid and iridoid glycosides
on free radical-induced impairment of endothelium-dependent
relaxation in rat aortic rings. J Ethnopharmacol. 79:193–197. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li DQ, Zhou N, Zhang L, Ma P and
Pflugfelder SC: Suppressive effects of azithromycin on
zymosan-induced production of proinflammatory mediators by human
corneal epithelial cells. Invest Ophthalmol Vis Sci. 51:5623–5629.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Choi HJ, Jang HJ, Chung TW, Jeong SI, Cha
J, Choi JY, Han CW, Jang YS, Joo M, Jeong HS and Ha KT: Catalpol
suppresses advanced glycation end-products-induced inflammatory
responses through inhibition of reactive oxygen species in human
monocytic THP-1 cells. Fitoterapia. 86:19–28. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kleemann R, Zadelaar S and Kooistra T:
Cytokines and atherosclerosis: A comprehensive review of studies in
mice. Cardiovasc Res. 79:360–376. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shao Y, Zhang Y, Yu Y, Xu TT, Wei R and
Zhou Q: Impact of catalpol on retinal ganglion cells in diabetic
retinopathy. Int J Clin Exp Med. 9:17274–17280. 2016.
|
|
20
|
Leung DW, Cachianes G, Kuang WJ, Goeddel
DV and Ferrara N: Vascular endothelial growth factor is a secreted
angiogenic mitogen. Science. 246:1306–1309. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ferrara N and Davis-Smyth T: The biology
of vascular endothelial growth factor. Endocr Rev. 18:4–25. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Phillips GD, Stone AM, Jones BD, Schultz
JC, Whitehead RA and Knighton DR: Vascular endothelial growth
factor (rhVEGF165) stimulates direct angiogenesis in the rabbit
cornea. In Vivo. 8:961–965. 1994.PubMed/NCBI
|
|
23
|
Liu JT, Chen YL, Chen WC, Chen HY, Lin YW,
Wang SH, Man KM, Wan HM, Yin WH, Liu PL and Chen YH: Role of
pigment epithelium-derived factor in stem/progenitor
cell-associated neovascularization. J Biomed Biotechnol.
2012:8712722012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ortego J, Escribano J, Becerra SP and
Coca-Prados M: Gene expression of the neurotrophic pigment
epithelium-derived factor in the human ciliary epithelium.
Synthesis and secretion into the aqueous humor. Invest Ophthalmol
Vis Sci. 37:2759–2767. 1996.PubMed/NCBI
|
|
25
|
Becerra SP: Focus on Molecules: Pigment
epithelium-derived factor (PEDF). Exp Eye Res. 82:739–740. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhu HF, Wan D, Luo Y, Zhou JL, Chen L and
Xu XY: Catalpol increases brain angio-genesis and up-regulates VEGF
and EPO in the rat after permanent middle cerebral artery
occlusion. Int J Biol Sci. 6:443–453. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu JY: Catalpol protect diabetic vascular
endothelial function by inhibiting NADPH oxidase. Zhongguo Zhong
Yao Za Zhi. 39:2936–2941. 2014.(In Chinese). PubMed/NCBI
|
|
28
|
Liu X, Lin Z, Zhou T, Zong R, He H, Liu Z,
Ma JX, Liu Z and Zhou Y: Anti-angiogenic and anti-inflammatory
effects of SERPINA3K on corneal injury. PLoS One. 6:e167122011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Saika S, Miyamoto T, Yamanaka O, Kato T,
Ohnishi Y, Flanders KC, Ikeda K, Nakajima Y, Kao WW, Sato M, et al:
Therapeutic effect of topical administration of SN50, an inhibitor
of nuclear factor-kappaB, in treatment of corneal alkali burns in
mice. Am J Pathol. 166:1393–1403. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen M, Matsuda H, Wang L, Watanabe T,
Kimura MT, Igarashi J, Wang X, Sakimoto T, Fukuda N, Sawa M and
Nagase H: Pretranscriptional regulation of Tgf-beta1 by PI
polyamide prevents scarring and accelerates wound healing of the
cornea after exposure to alkali. Mol Ther. 18:519–527. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mochimaru H, Usui T, Yaguchi T, Nagahama
Y, Hasegawa G, Usui Y, Shimmura S, Tsubota K, Amano S, Kawakami Y
and Ishida S: Suppression of alkali burn-induced corneal
neovascularization by dendritic cell vaccination targeting VEGF
receptor 2. Invest Ophthalmol Vis Sci. 49:2172–2177. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang A, Hao S, Bi J, Bao Y, Zhang X, An L
and Jiang B: Effects of catalpol on mitochondrial function and
working memory in mice after lipopolysaccharide-induced acute
systemic inflammation. Exp Toxicol Pathol. 61:461–469. 2009.
View Article : Google Scholar : PubMed/NCBI
|