Introduction
Preeclampsia (PE) is a pregnancy-specific
hypertensive syndrome affecting ~2–7% of pregnant women globally,
developing subsequent to 20 weeks of gestational age (1). The clinical manifestations of PE
include hypertension, placental hypoxia, proteinuria, endothelial
dysfunction, end-organ ischemia and increased vascular permeability
(2). These conditions are the
leading causes of maternal and fetal morbidity and mortality
(3).
Although there have been previous studies into the
mechanism of PE, its exact pathogenic mechanism remains unknown
(4). It has been widely accepted
that dysfunction of the placenta, which is the organ facilitating
the exchange of nutrients and waste between the mother and fetus,
may result in PE (5). The placenta
begins to develop upon invasion of the blastocyst into the
endometrium. The outer layer of the blastocyst becomes the
trophoblast, which serves an essential role in the formation of the
placenta. During normal pregnancy, appropriate trophoblast invasion
produces spiral arteries to facilitate the exchange of gases
between mother and fetus (6).
However, PE begins with incomplete trophoblast invasion at the
early stages of pregnancy, which disrupts correct placental
formation. A reduction of trophoblastic invasion at the decidual
and myometrial levels, and a failure of trophoblast cells to
replace the spiral artery, have been observed in the PE placenta
(7). High blood pressure with low
blood flow are generated in PE, resulting in reduced uteroplacental
blood flow. Incomplete placentation additionally produces increased
placental oxidative stress, contributing to the development of
systemic endothelial dysfunction in later phases of the disease
(8).
During pregnancy, the placenta is the primary
endocrine organ for maintaining pregnancy and fetal growth.
Hormones released by the placenta regulate the growth and
differentiation of the placental trophoblast, growth and maturation
of the placental vascular tree, and uterine endovascular invasion
by the extravillous cytotrophoblast (9). To successfully establish pregnancy, a
number of steroid hormones are synthesized and secreted through
steroidogenesis in the placenta. Progesterone (P4) and estrogen
(E2) are the principal steroid hormones produced by the placenta in
primate pregnancy (10). The serum
levels of P4 and E2 increase throughout pregnancy (11).
Steroidogenesis is the biological process through
which cholesterol is converted into multiple steroid hormones
(12). The pathway of
steroidogenesis is mediated by steroidogenic enzymes (13). The conversion of cholesterol into
pregnenolone (PG) by cholesterol side-chain cleavage enzyme
(CYP11A1) represents the initiation of steroidogenesis (14). Following its production, PG is
converted into P4 or dehydroepiandrosterone (DHEA) by 3
β-hydroxysteroid dehydrogenase/δ5 4-isomerase type 1 (HSD3B1) or
steroid 17-α-hydroxylase/17,20 lyase (CYP17A1), respectively.
Androgens, including testosterone (T), may be synthesized from DHEA
and P4, mediated by testosterone 17-β-dehydrogenase 3 (HSD17B3) and
HSD3B1 (15). Aromatase (CYP19A1)
and HSD17B3 catalyze the final steps of E2 biosynthesis from
androgens. These steps require a number of factors and complex
processes comprising networks of intracellular signaling pathways
(16).
In previous studies, the levels of steroid hormones
in patients with PE have been compared with those in normal
pregnant women to reveal the mechanism of PE (17,18).
However, only the levels in serum have been focused on, while the
placental steroid concentrations and comparisons with those from
serum have not been addressed. Therefore, the present study
investigated the expression of steroidogenic enzymes and analyzed
the concentration of steroid hormones in the placenta and serum.
The association between PE and steroid hormones from the placenta
and serum may provide insights into the pathophysiological
characteristics. In addition, the results of the present study may
suggest potential biomarkers to predict PE and possible therapeutic
methods to treat women with PE.
Materials and methods
Tissue and blood collection
Human placental tissues and blood samples were
collected and immediately stored at −80°C, which were divided into
normal women (n=21) and patients with PE (n=20). The samples were
obtained between 29 and 40 weeks of gestation from Jan to Dec of
2015 and provided by the Biobank of Pusan National University
Hospital (Busan, Korea), a member of the Korea Biobank Network. The
present study was approved by the Institutional Review Board of the
Pusan National University Hospital Clinical Trials Center (no.
H-1302-005-015), and all participants gave written informed
consent. Patients with PE were defined as having systolic and
diastolic blood pressures >140 and 90 mm Hg, respectively,
measured at least 6 h apart, in addition to proteinuria >300
mg/24 h or >1+ as determined by the dipstick method. Blood was
collected in plastic tubes under aseptic conditions with EDTA as an
anti-coagulant and centrifuged at 18,472 × g for 10 min at 4°C in
order to separate the serum. Clinical details of the patients are
presented in Table I.
 | Table I.Clinical characteristics of pregnancy
women. |
Table I.
Clinical characteristics of pregnancy
women.
| Characteristic | Normal (n=21) | Preeclampsia
(n=20) | P-value |
|---|
| Age, years |
31.6±3.58 |
33.9±3.05 | N.S |
| Gestational age,
weeks |
35.1±1.8 |
35.6±2.6 | N.S |
| Weight, kg |
66.1±8.4 |
74.9±14.0 | <0.05 |
| BMI,
kg/m2 |
25.8±3.0 |
28.6±5.8 | N.S |
| Systolic BP,
mmHg |
107.1±7.8 |
147.9±12.7 | <0.05 |
| Diastolic BP,
mmHg |
67.1±7.2 |
96.8±10.0 | <0.05 |
| Parity |
0.8±0.7 |
0.6±0.7 | N.S |
| Gravidity |
2.6±1.4 |
2.3±1.3 | N.S |
| Maternal BMI,
kg/m2 |
15.8±3.0 |
28.6±5.8 | N.S |
| Infant birth weight,
g |
2485.7±439.3 |
2130.0±686.2 | <0.05 |
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA from placenta tissue was extracted using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), according to the manufacturer's protocol. The
concentration of total RNA was measured using a spectrophotometer.
First-strand cDNA was prepared from total RNA (3 µg) via reverse
transcription at 37°C using Moloney murine leukemia virus reverse
transcriptase (Invitrogen; Thermo Fisher Scientific, Inc.) and
random primers (9-mers; Takara Bio Inc., Otsu, Japan), according to
the manufacturer's instructions and stored at −20°C until use. qPCR
was performed with the cDNA template (2 µl) and 2X Power SYBR Green
(6 µl; Toyobo Life Science, Osaka, Japan) containing specific
primers. Primer sequences for cytochrome c1 heme protein
mitochondrial (CYC1), β-actin, CYP11A1, CYP17A1, HSD17B3, HSD3B1,
and CYP19A1 are presented in Table
II. qPCR was performed for 40 cycles using the following
parameters: Denaturation at 95°C for 15 sec, followed by annealing
and extension at 70°C for 60 sec. Fluorescence intensity was
measured at the end of the extension phase of each cycle. The
threshold value for the fluorescence intensities of all samples was
set manually. The reaction cycle at which the PCR products exceeded
this fluorescence intensity threshold during the exponential phase
of PCR amplification was considered to be the threshold cycle (Cq).
Expression of the target gene was quantified relative to that of
CYC1 and β-actin, which are housekeeping genes, based on comparison
of the Cqs at a constant fluorescence intensity as previously
described (19).
 | Table II.Primer sequences for the reverse
transcription-quantitative polymerase chain reaction. |
Table II.
Primer sequences for the reverse
transcription-quantitative polymerase chain reaction.
| Gene name | Orientation | Sequence, 5′-
3′ | Fragment, bp |
|---|
| β-actin | Forward |
GGACTTCGAGCAAGAGATGG | 234 |
|
| Reverse |
AGCACTGTGTTGGCGTACAG |
|
| CYC1 | Forward |
CCAGCTACCATGTCCCAGAT | 185 |
|
| Reverse |
TATGCCAGCTTCCGACTCTT |
|
| CYP11A1 | Forward |
GCAACGTGGAGTCGGTTTAT | 229 |
|
| Reverse |
ATTGCAGCATCTTGCTTGTG |
|
| CYP17A1 | Forward |
CTGATGCAAGCCAAGATGAA | 222 |
|
| Reverse |
GCTGAAACCCACATTCTGGT |
|
| HSD17B3 | Forward |
ATCCAGAGCCTCATCCATTG | 164 |
|
| Reverse |
AACGCCTTGGAAGCTGAGTA |
|
| HSD3B1 | Forward |
AGAGGCCTGTGTCCAAGCTA | 152 |
|
| Reverse |
TTTTGCTGTGTGGGTATGGA |
|
| CYP19A1 | Forward |
CCAGTGAAAAAGGGGACAAA | 175 |
|
| Reverse |
CCATGGCGATGTACTTTCCT |
|
Western blot analysis
Protein samples of the placenta tissues were
extracted with Pro-prep solution (Intron Biotechnology, Inc.,
Seongnam, Korea), according to the manufacturer's protocol. The
concentration of the protein was determined via a bicinchoninic
acid assay; a total of 20 µg protein, was loaded and separated by
SDS-PAGE on an 8–10% gel and transferred onto nitrocellulose
membranes (Daeillab Lab Service Co., Ltd., Seoul, Korea). Membranes
were blocked for 2 h with 5% skimmed milk (Difco™; BD
Biosciences, Franklin Lakes, NJ, USA) in PBS with 0.05% Tween-20
(PBS-T) at room temperature. Following blocking, membranes were
incubated with anti-CYP11A1 (1:100; cat. no. sc-18040, Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), CYP17A1 (1:500; cat. no.
sc-46084, Santa Cruz Biotechnology, Inc.), HSD17B3 (1:2,000; cat.
no. ab55268, Abcam, Cambridge, UK), HSD3B1 (1:2,000; sc-30820,
Santa Cruz Biotechnology, Inc.), and CYP19A1 (1:500; cat no.
sc-14244, Santa Cruz Biotechnology, Inc.) antibodies overnight at
4°C, followed by horseradish peroxidase-conjugated secondary
antibodies (all 1:2,000; cat. nos. sc-2313, sc-2005, sc-2020, Santa
Cruz Biotechnology, Inc.) in 5% skimmed milk with PBS-T for 1 h at
room temperature. Luminol reagent (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) was used to visualize antibody binding.
Membranes were subsequently probed with an antibody against β-actin
(Cell Signaling Technology Inc., Danvers, MA, USA; diluted 1:3,000;
cat. no. 967) as an internal control. Blots were scanned using Gel
Doc 1000, version 1.5 (Bio-Rad Laboratories, Inc.), and band
intensities were normalized to β-actin levels.
ELISA analysis
Concentrations of PG (cat. no. KA1912, Abnova,
Taoyuan, Taiwan)), P4 (cat. no. 582601; Cayman Chemical Company,
Ann Arbor, MI, USA;), DHEA (cat. no. 20-DHEHU-E01, Enzo Life
Sciences, Inc., Farmingdale, NY, USA;), T (cat. no. 582701; Cayman
Chemical Company), and E2 (cat. no. 582251; Cayman Chemical
Company) were measured using competitive enzyme immunoassay kits,
according to the manufacturers' protocols. Serum and tissues were
added to a 96-well plate following tissue homogenization with
Pro-prep solution (Intron biotechnology, Seoul, South Korea),
according to the manufacturer's protocols. The plate was incubated
for 1 h at room temperature on an orbital shaker. Following
incubation at room temperature for 90 min with Ellman's reagent,
optical density values were measured spectrophotometrically at 420
nm. The final concentrations were calculated using standard curve
analysis.
Statistical analysis
Results are presented as the mean ± standard
deviation. Data were analyzed using Sigma Plot 10.0 (Systat
Software, Inc., San Jose, CA, USA). Repeats were performed ≥3
times; differences between groups were calculated using a Student's
t-test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Concentration of steroid hormones in
PE serum and placenta
The characteristics of the patients with PE are
summarized in Table I. Patients
with PE exhibited high blood pressure (>140/90 mmHg), and the
average blood pressure was 147.9±12.7/96.8±10.0 mmHg. Patients with
PE exhibited an increased body weight, while infant weight was
markedly decreased. Parity, gravidity and maternal body mass index
were not notably different between normal patients and patients
with PE, which was consistent with other studies (20,21).
To investigate the association between steroid hormones and PE, the
concentrations of steroid hormones were evaluated to verify whether
or not the PE placenta is under different endocrinal conditions
compared with the normal placenta. The concentrations of PG, P4,
DHEA, T and E2 in the serum and placenta were analyzed using ELISA
kit (Figs. 1 and 2). Serum levels of PG, P4, DHEA and E2
tended to decline in women with PE (with significant results for P4
and E2), whereas the concentrations of T did not exhibit any
significant difference in PE compared with normal serum. The
concentrations of PG, DHEA and E2 in the serum were increased
compared with the placenta, whereas the levels of P4 and T were
similar. Hormonal levels in placental tissues exhibited similar
results compared with the serum. Levels of PG, P4, DHEA and E2 were
reduced in the PE placenta, although the results were not
significant.
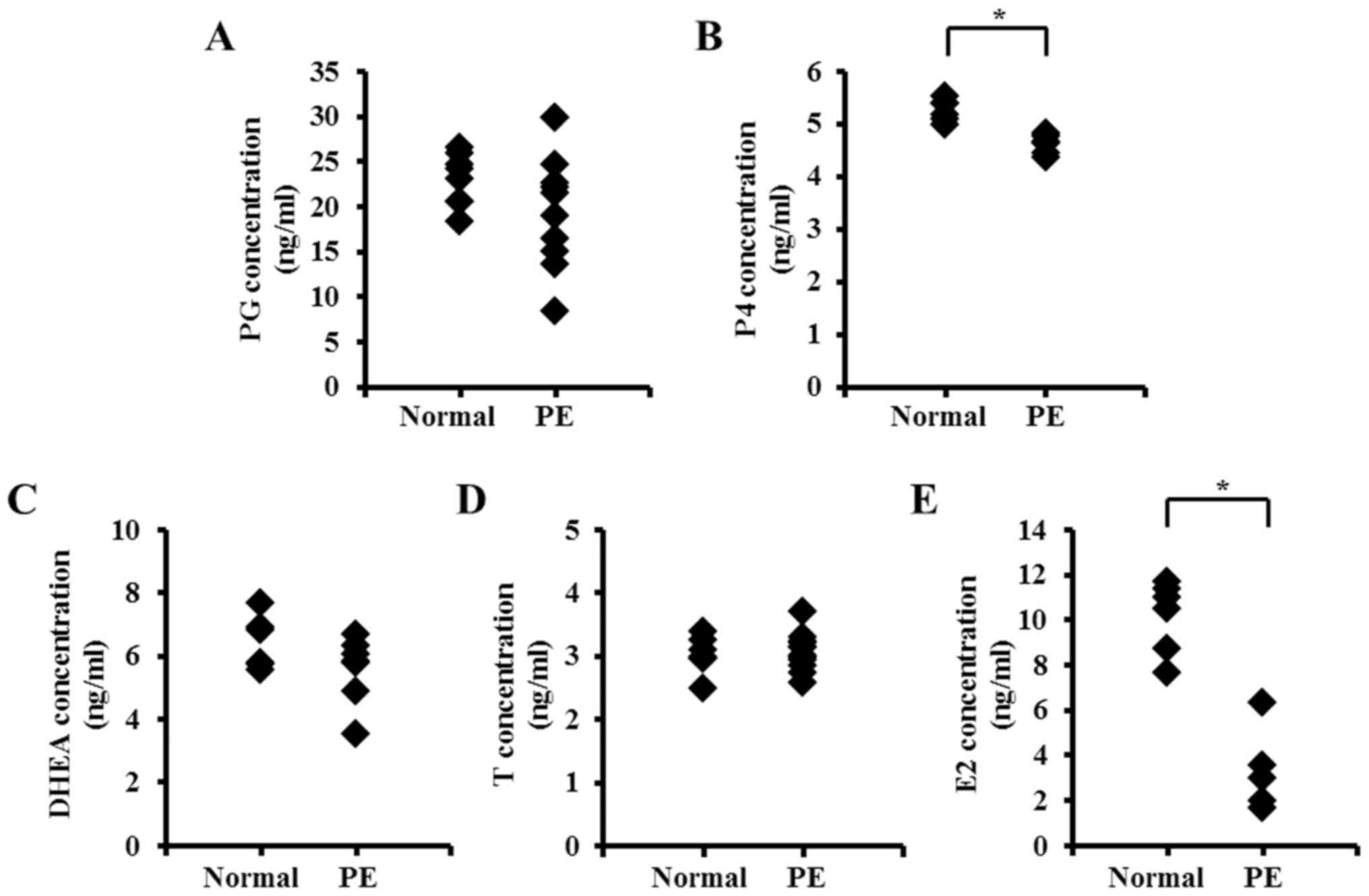 | Figure 1.Serum concentration of steroid
hormones in women with PE. Serum steroid hormone concentrations of
(A) PG, (B) P4, (C) DHEA, (D) T and (E) E2 were analyzed by ELISA,
comparing normal serum with PE serum. *P<0.05 vs. Normal. PE,
preeclampsia; PG, pregnenolone; P4, progesterone; DHEA,
dehydroepiandrosterone; T, testosterone; E2, estrogen. |
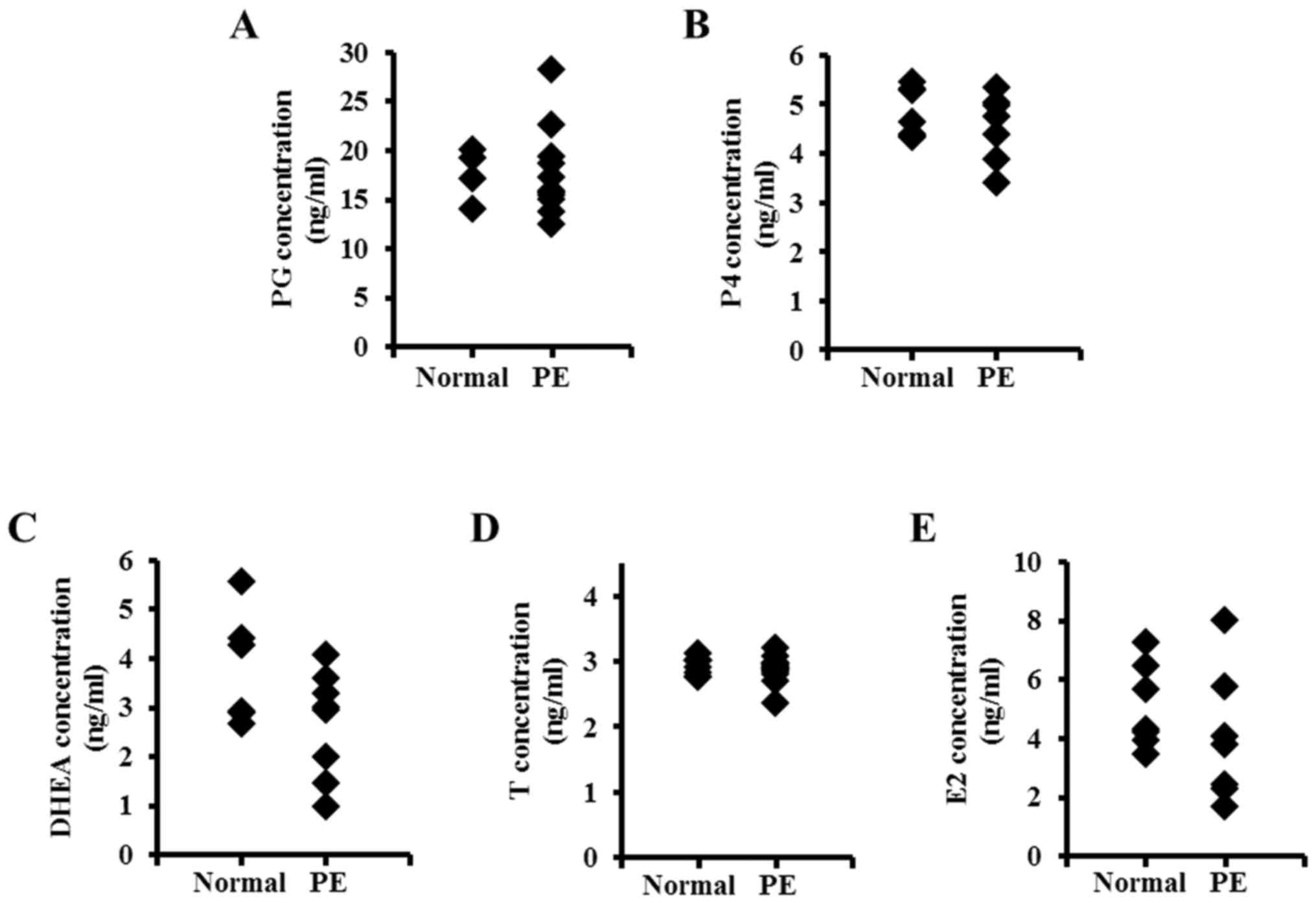 | Figure 2.Placental concentration of steroid
hormones in women with PE. Steroid hormone concentrations of (A)
PG, (B) P4, (C) DHEA, (D) T and (E) E2 were analyzed by ELISA,
comparing the normal placenta with the PE placenta. PE,
preeclampsia; PG, pregnenolone; P4, progesterone; DHEA,
dehydroepiandrosterone; T, testosterone; E2, estrogen. |
Expression of steroidogenic enzymes in
the PE placenta
Since the concentrations of steroid hormones were
altered in PE serum and placentas, the expression of steroidogenic
enzymes in the PE placenta was subsequently investigated. The mRNA
expression of CYP11A1, CYP17A1, HSD17B3, HSD3B1 and CYP19A1, which
are salient steroidogenic enzymes, were examined by RT-qPCR
analysis (Fig. 3). The mRNA level
of HSD3B1 was decreased in PE compared with the normal placenta,
whereas that of CYP19A1 was elevated by ~2-fold in the PE placenta
(Fig. 3D and E). Transcription
levels of other steroidogenic enzymes were not significantly
altered. The translation levels of steroidogenic enzymes were
analyzed by western blotting (Fig.
4). The protein levels of HSD3B1 exhibited similar patterns
compared with the mRNA by significantly decreasing (Fig. 4A). The CYP19A1 protein level
increased, although the result was not statistically significant
(Fig. 4D). CYP11A1 and CYP17A1
mRNA levels were unaltered in the PE group (Fig. 4A and B). Notably, the protein level
of HSD17B3 was different compared with the mRNA level, which was
downregulated by 2.7-fold in the PE placenta compared with the
control (Fig. 4C).
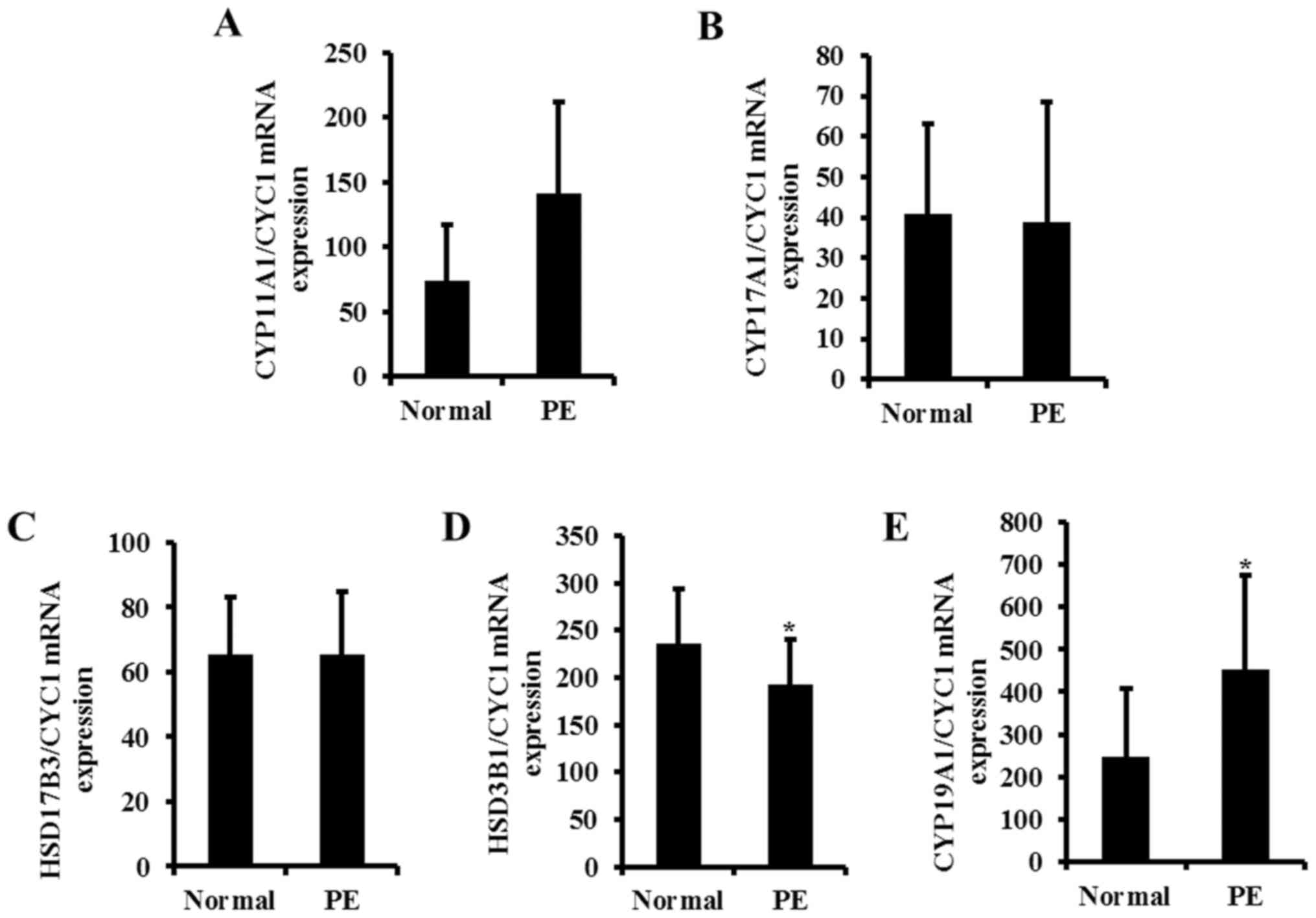 | Figure 3.Transcriptional level of
steroidogenesis in the human placenta. The mRNA levels of (A)
CYP11A1, (B) CYP17A1, (C) HSD17B3, (D) HSD3B1 and (E) CYP19A1, in
normal and PE placentas, were analyzed by the reverse
transcription-quantitative polymerase chain reaction. Total mRNA
was harvested from human normal and PE placentas subsequent to the
onset of labor. The total mRNA expression level was normalized to
that of CYC1. Data are expressed as the mean ± standard deviation.
*P<0.05 vs. Normal. CYP11A1, cholesterol side-chain cleavage
enzyme; CYP17A1, steroid 17-α-hydroxylase/17,20 lyase; HSD17B3,
testosterone 17-β-dehydrogenase 3; HSD3B1, 3 β-hydroxysteroid
dehydrogenase/δ5 4-isomerase type 1; CYP19A1, aromatase; CYC1,
cytochrome c1 heme protein mitochondrial; PE, preeclampsia. |
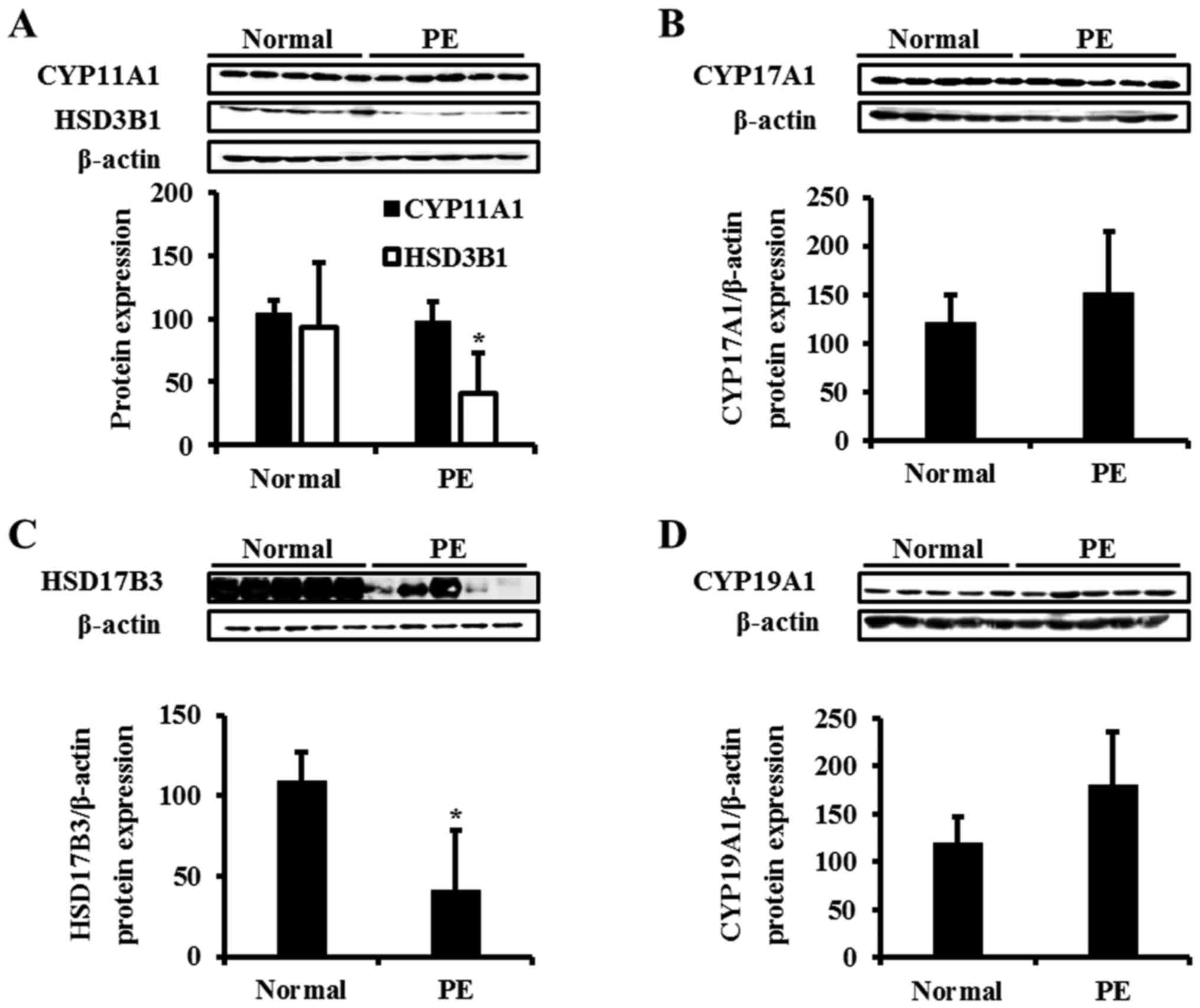 | Figure 4.Translational level of steroidogenesis
in the human placenta. The protein levels of (A) CYP11A1 and HSD3B1
(B) CYP17A1, (C) HSD17B3 and (D) CYP19A1 in normal and PE placentas
were examined. Total proteins were harvested from human normal and
PE placentas subsequent to the onset of labor. Proteins were
processed for western blot analysis. The total protein expression
level was normalized to that of β-actin. The images of CYP11A1,
HSD3B1 and β-actin were derived from same blot. Data are expressed
as the mean ± standard deviation. *P<0.05 vs. Normal. CYP11A1,
cholesterol side-chain cleavage enzyme; CYP17A1, steroid
17-α-hydroxylase/17,20 lyase; HSD17B3, testosterone
17-β-dehydrogenase 3; HSD3B1, 3 β-hydroxysteroid dehydrogenase/δ5
4-isomerase type 1; CYP19A1, aromatase; CYC1, cytochrome c1 heme
protein mitochondrial; PE, preeclampsia. |
Discussion
PE is a systemic syndrome of pregnancy characterized
by hypertension and proteinuria (22). Although efforts have been made, the
etiology of PE is remains to be completely understood. Due to the
life-threatening risks and lack of effective treatment, early
diagnostic biomarkers of PE are required. Recently, it was reported
that the serum concentrations of steroid hormone, including E2, may
be associated with PE (23).
However, the mechanism of alteration has not been studied. Steroid
hormones serve vital roles, including the maintenance of pregnancy
in females in addition to regulation of the estrus cycle and
puberty (24). Numerous studies
have reported that the concentrations of these steroid hormones in
PE serum contribute to pathogenesis of PE (17,18,25,26).
Since sera are collected from women with different characteristics,
including age, body weight and clinical histories, hormone levels
vary and the reliability of data is decreased. Therefore, the
regulation of steroid hormones in PE women is controversial. For
instance, case-control and cross-sectional studies have reported an
association between vitamin D status and PE, although evidence has
been inconsistent (27,28). In addition, antenatal
corticosteroid therapy in women with PE may be associated with
fetal maturation (17). However,
the present study focused on sex steroid hormone, since the levels
of sex steroid hormones are dynamically regulated during pregnancy.
Additionally, there are previous studies demonstrating that sex
steroid hormones may be associated with PE. A previous study
reported that serum levels of DHEA and E2 in patients with PE were
decreased compared with those in normal pregnant woman, whereas P4
production was markedly increased in PE (17). However, Iou et al (26) indicated that serum levels of P4
were decreased in women with PE during the third trimester.
In the present study, serum and placental levels of
steroid hormones, including PG, P4, DHEA, T and E2, were examined
in patients with PE and normal pregnant women. The assessed steroid
hormones were selected as they are known to be involved in the
regulation of pregnancy. It has been reported that PG is a
precursor of other steroid hormones such as P4 and DHEA (29) P4 is known to reduce vascular
resistance by decreasing the sensitivity of angiotensin and
increasing the production of endothelial vasodilator, which
directly affects muscles (30).
Additionally, P4 stimulates the production and secretion of
substances required for the growth and development of the conceptus
(31). E2 stimulates the
expression of VEGF and angiogenesis in the uterus during normal
gestation, serving important roles in implantation, pregnancy
maintenance and embryonic development (11,32).
DHEA additionally increases vascular endothelial proliferation,
migration and vascular tube formation by activating the
extracellular signal-regulated kinase 1/2 pathway (33). Therefore, a lack of these steroid
hormones may lead to abnormalities in the development of the
placenta, leading to the pathogenesis of PE. According to the
results of the present study, the serum levels of P4 and E2 were
significantly decreased in women with PE. In addition, PG and DHEA
levels were slightly decreased in PE serum samples.
To completely elucidate the mechanism of
steroidogenesis, the present study quantified steroid hormones in
the human placenta. In normal women, the levels of PG, DHEA and E2
were increased in the serum compared with the placenta, while the
levels of P4 and T were similar, suggesting that other reproductive
organs from the mother or fetus may contribute to the
concentrations of hormones. However, the altered patterns of
hormones in women with PE in the placenta were similar to the
results from the serum. In PE placentas, steroid hormones, apart
from T, tended to decrease compared with the normal placenta, and
this regulation was even more apparent in the serum. These results
indicated that the serum concentration of steroid hormones is
primarily regulated by the placenta and that the reduced hormones
may contribute the physiological features of women with PE.
For the following experiment, the expression of
enzymes associated with synthesis of steroid hormones in the
placenta was analyzed. The expression levels of CYP11A1 and CYP17A1
were not altered in PE compared with normal placentas. The serum PG
concentration was not altered in women with PE compared with the
normal group, and this may be explained by the results of the
analysis of CYP11A1 and CYP17A1 gene expression. CYP11A1 and
CYP17A1 are involved in the process of PG metabolism and their
expression was not significantly altered in the PE placenta. Since
the expression of HSD3B1, the enzyme converting DHEA to
androstenedione, was decreased, it was hypothesized that the
concentration of DHEA in the maternal serum may have been enhanced.
However, the results of the present study demonstrated that DHEA
was decreased in the serum and placenta. During pregnancy,
steroidogenesis is a complex process occurring in a number of
organs, including the maternal uterus, placenta, fetal membrane,
and the maternal and fetal hypothalamic-pituitary-adrenal (HPA)
axis, although the placenta is the most important of these. During
normal pregnancy, increased production of corticotropin releasing
hormone from decidual, trophoblastic and fetal membranes leads to
an increase in circulating cortisol in midgestation (34). The fetal HPA axis, activated by
cortisol, results in enhanced fetal pituitary adrenocorticotropin
hormone secretion, which leads to release of the abundant C19
estrogen precursor DHEA sulfate from the fetal adrenal zone. The
results of the present study demonstrated that the serum
concentration of DHEA in normal women and patients with PE was
increased compared with the placenta, suggesting that the fetal
production of DHEA is important for the regulation of maternal DHEA
concentration. It has also been reported that the synthesis of DHEA
by the fetal adrenal gland is important for placental steroid
production (35).
The synthesis of T from P4 and DHEA, catalyzed by
HSD17B3, was markedly reduced in the PE placenta, suggesting that
the products of these enzymes, including E2, P4 and T, may be
reduced. Indeed, the hormone data in the present study demonstrated
that the concentration of E2 was decreased in the serum and
placenta. Notably, the expression of CYP19A1, the final enzyme
involved in the synthesis of E2, was enhanced in the PE placenta,
in contrast to the reduced concentrations of hormones. This may be
a compensatory response of the placental tissue to elevate the
serum levels of E2 in patients with PE.
In conclusion, the results of the present study
provided novel insights into the association between steroid
hormones and PE. In the PE placenta, the synthesis of steroid
hormones, including P4 and E2, was decreased via the regulation of
steroidogenic enzymes.
Acknowledgements
The present study was supported by a grant from the
Korea Health Technology R&D Project through the Korea Health
Industry Development Institute (KHIDI), funded by the Ministry of
Health and Welfare (grant no. HI16C0313).
References
|
1
|
Ushida T, Kotani T, Tsuda H, Imai K,
Nakano T, Hirako S, Ito Y, Li H, Mano Y, Wang J, et al: Molecular
hydrogen ameliorates several characteristics of preeclampsia in the
Reduced Uterine Perfusion Pressure (RUPP) rat model. Free Radic
Biol Med. 101:524–533. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Venkatesha S, Toporsian M, Lam C, Hanai J,
Hanai JI, Mammoto T, Kim YM, Bdolah Y, Lim KH, Yuan HT, et al:
Soluble endoglin contributes to the pathogenesis of preeclampsia.
Nat Med. 12:642–649. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Duska D and Anja HF: Low
catechol-O-methyltransferase and 2-methoxyestradiol in
preeclampsia: More than a unifying hypothesis. Nephrol Dial
Transplant. 24:31–33. 2009.PubMed/NCBI
|
|
4
|
Tian M, Zhang Y, Liu Z, Sun G, Mor G and
Liao A: The PD-1/PD-L1 inhibitory pathway is altered in
pre-eclampsia and regulates T cell responses in pre-eclamptic rats.
Sci Rep. 6:276832016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brownfoot FC, Tong S, Hannan NJ, Binder
NK, Walker SP, Cannon P, Hastie R, Onda K and Kaitu'u-Lino TJ:
Effects of pravastatin on human placenta, endothelium, and women
with severe preeclampsia. Hypertension. 66:687–697, Discussion 445.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Whitley GS and Cartwright JE: Cellular and
molecular regulation of spiral artery remodelling: Lessons from the
cardiovascular field. Placenta. 31:465–474. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gunel T, Hosseini MK, Gumusoglu E,
Dolekcap I and Aydinli K: Future perspective of preeclampsia by
miRNA. Global J Hum Genet Gene Ther. 2:53–67. 2014.
|
|
8
|
Zuniga FA, Ormazabal V, Gutierrez N,
Aguilera V, Radojkovic C, Veas C, Escudero C, Lamperti L and Aguayo
C: Role of lectin-like oxidized low density lipoprotein-1 in
fetoplacental vascular dysfunction in preeclampsia. Biomed Res Int.
2014:3536162014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pepe G and Albrecht E: Steroid
endocrinology of pregnancy. Glob Libr Women's Med. 5:382008.
|
|
10
|
Young SM, Gryder LK, Zava D, Kimball D and
Benyshek DC: Presence and concentration of 17 hormones in human
placenta processed for encapsulation and consumption. Placenta.
43:86–89. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim SC, Park MN, Lee YJ, Joo JK and An BS:
Interaction of steroid receptor coactivators and estrogen receptors
in the human placenta. J Mol Endocrinol. 56:239–247. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Miller WL: Steroid hormone synthesis in
mitochondria. Mol Cell Endocrinol. 379:62–73. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sato K, Iemitsu M, Matsutani K, Kurihara
T, Hamaoka T and Fujita S: Resistance training restores muscle sex
steroid hormone steroidogenesis in older men. FASEB J.
28:1891–1897. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Arukwe A: Steroidogenic acute regulatory
(StAR) protein and cholesterol side-chain cleavage
(P450scc)-regulated steroidogenesis as an organ-specific molecular
and cellular target for endocrine disrupting chemicals in fish.
Cell Biol Toxicol. 24:527–540. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Miller WL and Auchus RJ: The molecular
biology, biochemistry, and physiology of human steroidogenesis and
its disorders. Endocr Rev. 32:81–151. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tremblay JJ: Molecular regulation of
steroidogenesis in endocrine Leydig cells. Steroids. 103:3–10.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hertig A, Liere P, Chabbert-Buffet N, Fort
J, Pianose A, Eychennee B, Cambourge A, Schumacher M, Berkane N,
Lefevre G, et al: Steroid profiling in preeclamptic women: Evidence
for aromatase deficiency. Am J Obstet Gynecol. 205:477.e1–e9. 2010.
View Article : Google Scholar
|
|
18
|
Zeisler H, Jirecek S, Hohlagschwandtner M,
Knöfler M, Tempfer C and Livingston JC: Concentrations of estrogens
in patients with preeclampsia. Wien Klin Wochenschr. 114:458–461.
2002.PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Clausen T, Slott M, Solvoll K, Drevon CA,
Vollset SE and Henriksen T: High intake of energy, sucrose, and
polyunsaturated fatty acids is associated with increased risk of
preeclampsia. Am J Obstet Gynecol. 185:451–458. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Canakci V, Canakci CF, Canakci H, Canakci
E, Cicek Y, Ingec M, Ozgoz M, Demir T, Dilsiz A and Yagiz H:
Periodontal disease as a risk factor for pre-eclampsia: A case
control study. Aust N Z J Obstet Gynaecol. 44:568–573. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang A, Rana S and Karumanchi SA:
Preeclampsia: The role of angiogenic factors in its pathogenesis.
Physiology (Bethesda). 24:147–158. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zheng JJ, Wang HO, Huang M and Zheng FY:
Assessment of ADMA, Estradiol, and progesterone in severe
preeclmpsia. Clin Exp Hypertens. 38:347–351. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hong SH, Lee JE, Kim HS, Jung YJ, Hwang D,
Lee JH, Yang SY, Kim SC, Cho SK and An BS: Effect of vitamin D3 on
production of progesterone in porcine granulosa cells by regulation
of steroidogenic enzymes. J Biomed Res. 30:203–208. 2016.PubMed/NCBI
|
|
25
|
Chen D, Dong M, Fang Q, He J, Wang Z and
Yang X: Alterations of serum resistin in normal pregnancy and
pre-eclampsia. Clin Sci (Lond). 108:81–84. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Iou SG, Eskandari M and Dabiri A:
Evaluation of androgen and progesterone levels of women with
preeclampsia in third trimester. Med J Islamic World Acad Sci.
15:19–22. 2005.
|
|
27
|
Welberg L: Addiciton: Pregnenolone limits
effects of cannabis. Nat Rev Neurosci. 15:66–67. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Purswani JM, Gala P, Dwarkanath P, Larkin
HM, Kurpad A and Mehta S: The role of vitamin D in pre-eclampsia: A
systematic review. BMC Pregnancy Childbirth. 17:2312017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vestgaard M, Secher AL, Ringholm L, Jensen
J, Damm P and Mathiesen ER: Vitamin D insufficiency, preterm
delivery and preeclampsia in women with type 1 diabetes-an
observational study. Acta Obstet Gynecol Scand. 96:1197–1204. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Albrecht ED and Pepe GJ: Estrogen
regulation of placental angiogenesis and fetal ovarian development
during primate pregnancy. Int J Dev Biol. 54:397–408. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Steinhausera CB, Bazerb FW, Burghardta RC
and Johnsona GA: Expression of progesterone receptor in the porcine
uterus and placenta throughout gestation: Correlation with
expression of uteroferrin and osteopontin. Domest Anim Endocrinol.
58:19–29. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Feng Y, Zhang P, Zhang Z, Shi J, Jiao Z
and Shao B: Endocrine disrupting effects of triclosan on the
placenta in pregnant rats. PloS One. 11:e01547582016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu D, Iruthayanathan ML, Homan L, Wang Y,
Yang L, Wang Y and Dillon JS: Dehydroepiandrosterone stimulates
endothelial proliferation and angiogenesis through extracellular
signal-regulated kinase 1/2-mediated mechanisms. Endocrinology.
149:889–898. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lockwood CJ, Radunovic N, Nastic D,
Petkovic S, Aigner S and Berkowitz GS: Corticotropin-releasing
hormone and related pituitary-adrenal axis hormones in fetal and
maternal blood during the second half of pregnancy. J Perinat Med.
24:243–51. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hoffman Sage Y, Lee L, Thomas AM, Benson
CB and Shipp TD: Fetal adrenal gland volume and preterm birth: A
prospective third-trimester screening evaluation. J Matern Fetal
Neonatal Med. 29:1552–1555. 2016. View Article : Google Scholar : PubMed/NCBI
|


















