Introduction
Prostate cancer is the most frequently occurring
cancer and the second leading cause of cancer-associated
mortalities in men in the United States. In 2014, estimated newly
diagnosed cases and fatalities from the disease were 233,000 and
29,480, respectively (1). The rate
of tumor growth varies from very slow to moderately rapid, and
various patients may have prolonged survival even following cancer
metastasis to distant sites, including bone (2,3). The
5-year relative survival rate for men diagnosed in the United
States from 2001–2007 with local or regional disease (where tumors
have not metastasized from their point of origin) was 100%, and the
rate for distant disease (where tumors that have metastasized to
further sites around the body) was 28.7%. The great survival rate
gap between local or regional tumors and advanced stages of the
disease where the tumors have metastasized, is of primary concern,
and has resulted in various research efforts to investigate the
metastatic process of the disease and the mechanisms underlying it.
Considerable progress has been made in the last decade to
understand the disease at the molecular and genetic level (4,5),
however understanding of the process of metastasis remains to be
fully elucidated.
miRNAs are non-protein-coding RNAs that regulate
genes and genomes. This regulation may occur at various important
levels of genome functioning, including chromatin structure,
chromosome segregation, transcription, RNA processing, RNA
stability and translation (6).
miRNAs may regulate the expression of up to 90% of human genes
(7). They bind by complimentary
base pairing to the 3′-untranslated region (3′-UTR) of their target
mRNA to post-transcriptionally suppress gene expression (8).
The downregulation of miR-138 may be associated with
cancer progression, and the tumor suppressive role has been
reported in lung, kidney, tongue, head and neck malignant diseases
(9–12). miR-138 modulates tumor growth,
migration and invasion. The molecular mechanism underlying the
functions of miR-138 remain to be elucidated, despite current
research efforts. Furthermore, whether miR-138 exhibits the ability
to act as a tumor suppressor in prostate cancer is as of yet
unknown.
The present study aimed to investigate the function
of miR-138 in prostate cancer and to identify the associated
molecular mechanism by which it may exhibit tumor suppressive
effects. It was demonstrated that miR-138 was downregulated in PC3
and C4-2B aggressive prostate cancerous cell lines, compared with
cancerous cell lines that are less metastatic. Furthermore,
following miR-138 overexpression, PC3 and C4-2B cell lines
exhibited impaired invasion and migration. Additionally, E-cadherin
was upregulated and vimentin was downregulated. It was revealed
that miR-138 overexpression suppressed β-catenin activation, and
inhibition of the activation of the β-catenin pathway reversed the
anti-tumor effect of miR-138. These results suggest that miR-138 is
a tumor suppressor in prostate cancer and its associated anti-tumor
effect is dependent on β-catenin pathway inhibition.
Materials and methods
Cell lines and transfection
PC3, DU145 and LNCaP cells were obtained from Type
Culture Collection of Chinese Academy of Sciences (Shanghai,
China); PC3 is an aggressive prostate cancerous cell line whereas
DU145 and LNCaP cells are non-aggressive prostate cancerous cell
lines. The cells were maintained in F-12, modified Eagle's medium
and RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 10% FBS (Gibco; Thermo Fisher Scientific,
Inc., USA) and penicillin/streptomycin (Gibco; Thermo Fisher
Scientific, Inc.). The cells were maintained in an incubator
containing 5% CO2 at 37°C and 100% humidity. C4-2B cell
lines were purchased from UroCor, Inc. (Shanghai, China) and
maintained in RPMI-1640 supplemented with 10% FBS. miRNA-138 mimics
(GCC GGA CUA AGU GUU GUG GUC GA; 50 nM) were purchased from
Sigma-Aldrich; Merck KGaA (Darmstadt, Germany) and β-catenin
overexpression plasmid (100 nM) was obtained from Addgene Inc.
(Cambridge, MA, USA), (Plasmid 17,198). Transient transfection was
performed using the Lipofectamine 2000 reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's
protocol.
Invasion and migration assays
Assays were performed in BioCoat transwell chambers
(BD Pharmingen; BD Biosciences, Franklin Lakes, NJ, USA) with
uncoated porous filters (pore size 8 µm) to evaluate cell
migration, or Matrigel coated porous filters to exam cell invasion.
C4-2B and PC3 cells (5×105/ml) in serum-free medium were
seeded into the upper chamber of each insert, and complete medium
was added to the lower chamber. Following 12 h of incubation at
37°C, cells in the upper chambers were removed using cotton tips.
Filters were fixed in 4% paraformaldehyde and stained at room
temperature for 24 h with 0.1% crystal violet (Sigma-Aldrich; Merck
KGaA) for counting. Independent experiments were repeated at least
three times. Values for cell migration or invasion are expressed as
the average number of cells per microscopic field (CX23 microscope;
Olympus Corporation, Tokyo, Japan) over five fields for each
filter.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from t C4-2B and PC3 cells
using TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.) according
to the manufacturer's protocol. The relative expression levels of
miR-138 were determined by RT-qPCR using mirVana™ qPCR microRNA
Detection kit, according to the manufacturer's protocol. An Applied
Biosystems® 7500 thermal cycler, (Thermo Fisher
Scientific, Inc.) was used and the reaction conditions were as
follows: 95°C for 10 min, 40 cycles at 95°C for 15 sec and 60°C for
30 sec. For the PCR reaction, 1 µl cDNA solution, 10 µl PCR master
mix, 2 µl of primers and 5 µl H2O were mixed to obtain a
final reaction volume of 20 µl. Specific primer sets for miR-138
and U6 were obtained from Genecopoeia (Applied Biosystems; Thermo
Fisher Scientific, Inc.). The mRNA expression levels of LIMK1 were
detected by RT-qPCR using the standard SYBR-Green RT-PCR kit
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. Primers were synthesized by Invitrogen
(Thermo Fisher Scientific, Inc.) were as follows: miR-138; sense
5′-GCGAACTGTTTGCAGAGG-3′, antisense 5′-CAGTGCGTGTCGTGGAGT-3′; U6
forward, 5′-TGCGGGTGCTCGCTTCGGCAGC-3′ and reverse,
5′-CCAGTGCAGGGTCCGAGGT-3′ and GAPDH forward,
5′-TGTGGGCATCAATGGATTTGG-3′ and reverse,
5′-ACACCATGTATTCCGGGTCAAT-3′. The relative expression levels
miR-138 were quantified using GraphPad Prism 5.0 software (GraphPad
Software, Inc., San Diego, CA, USA), and the 2-ΔΔCq method
(13).
Immunoblotting assay
Whole-cell extracts were obtained by lysis of cells
in an appropriate volume of ice-cold radioimmunoprecipitation assay
buffer (Thermo Fisher Scientific, Inc.). The BCA Protein Assay kit
(Thermo Fisher Scientific, Inc.) was used to determine protein
concentration. Protein samples (20 µg) were separated by 10% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis and were
transferred to a polvinylidene difluoride (PVDF) membrane (Thermo
Fisher Scientific, Inc.) and blocked in phosphate-buffered
saline/Tween-20 containing 5% non-fat milk for 1 h at room
temperature. Subsequently, the PVDF membrane was incubated with
mouse anti-E-cadherin antibody (ab1416; 1:200; Abcam, Cambridge,
UK), mouse anti-vimentin antibody (ab8978; 1:200; Abcam), mouse
anti-β-catenin antibody (ab22656; 1:200; Abcam), mouse
anti-active-β-catenin antibody (05–665; 1:200; Sigma-Aldrich; Merck
KGaA), anti-lamin B1 (ab8982; 1:200; Abcam) and mouse
anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) monoclonal
antibody (ab8245; 1:50; Abcam) at 4°C overnight. The membranes were
washed with TBS containing 0.1% Tween-20, and incubated with a
corresponding horseradish peroxidase conjugated secondary antibody
(ab6789; 1:3,000; Abcam) at 37°C for 1 h. Following extensive
washing, proteins were visualized by enhanced chemiluminescence
(ab5801; Abcam) and exposure to film (Fujifilm, Tokyo, Japan).
TOP/FOP Luciferase reporter assay
Cells were transiently transfected with 1 µg of a
constitutively active vector encoding Renilla luciferase (Promega
Corporation, Madison, WI, USA) and 10 µg of β-catenin-responsive
firefly luciferase reporter plasmid TopFlash (EMD Millipore,
Billerica, MA, USA) or the negative control FopFlash (EMD
Millipore) using the Lipofectamine 2000 reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol.
Cells were harvested following 24 h in culture and firefly and
Renilla luciferase activity was measured using a Dual-Luciferase
Reporter Assay System (Promega Corporation) according to the
manufacturer's protocol. The firefly luciferase activity was
normalized against the Renilla luciferase activity and fold
increase in TOPFlash activity compared to FOPFlash is reported.
Statistical analysis
Data are presented as the mean ± standard deviation.
Analysis was performed using SPSS software version 20.0 (IBM Corp,
Armonk, NY, USA). All data were assessed using the Student's t-test
or one-way analysis of variance followed by Newman Keuls post hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
miR-138 is downregulated in aggressive
prostate cancer cell lines
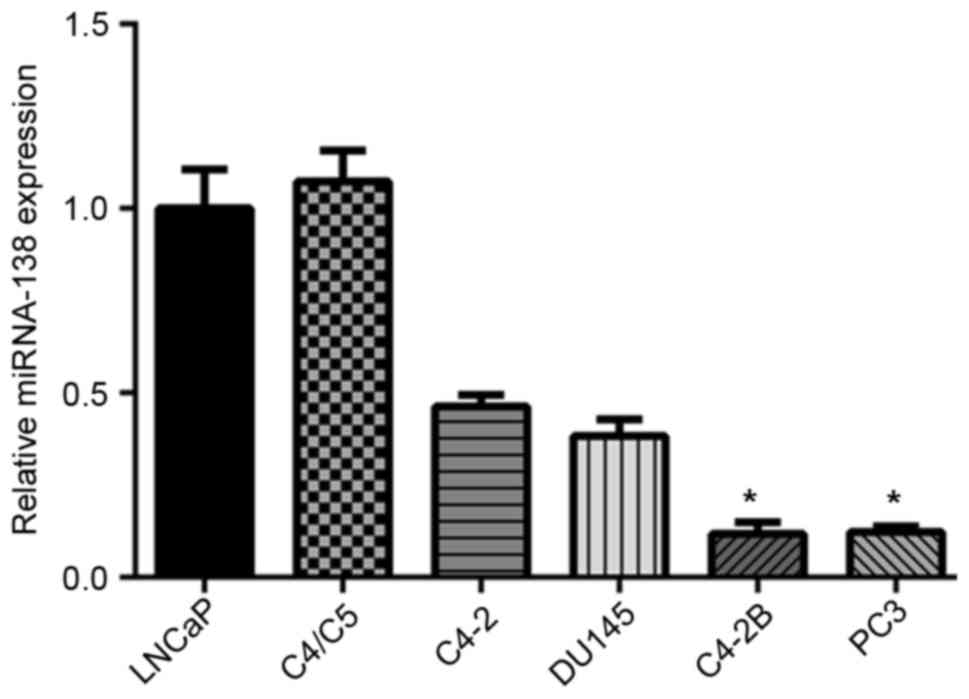
To determine the role of miR-138 in prostate cancer,
the present study firstly investigated miR-138 expression in six
prostate cancer cell lines (LNCaP, C4/C5, C4-2, DU145, C4-2B and
PC3) using RT-qPCR. It was demonstrated that miR-138 expression was
associated with the aggressiveness, or, metastatic capabilities of
tumor cell lines. In the prostate cancer cell lines C4-2B and PC3
with metastatic abilities, miR-138 expression levels were
significantly decreased, compared with LNCaP, C4/C5, C4-2 and DU145
cell lines (Fig. 1). These results
suggested that miR-138 expression may be associated with prostate
cancer progression, and miR-138 may function as a tumor
suppressor.
miR-138 negatively regulates invasion
and migration in prostate cancer cells
To further investigate the miR-138 function in
prostate cancer, the present study transfected miR-138 mimics into
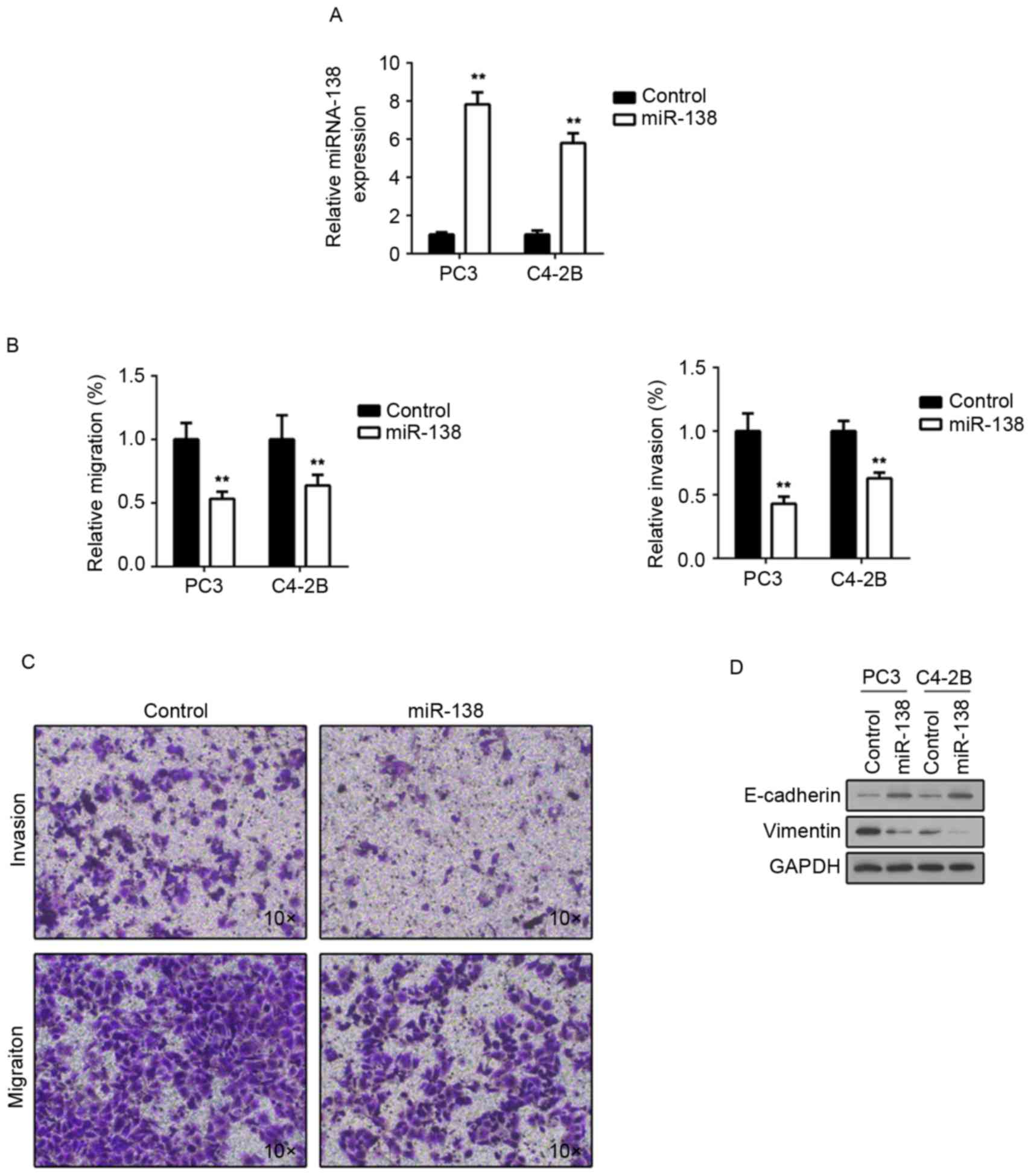
C4-2B and PC3 cells. As presented in Fig. 2A, following transfection, miR-138
expression was significantly upregulated in the two cell lines. A
Transwell assay was then performed to examine whether there was an
invasive or migratory alteration following miR-138 overexpression.
It was demonstrated that C4-2B and PC3 cells transfected with
miR-138 mimics exhibited impaired invasion and migration (Fig. 2B and C), compared with transfected
control miRNA. Epithelial marker E-cadherin expression was then
investigated, and it was revealed that miR-138 overexpression
induced E-cadherin expression in the two cell lines (Fig. 2D). Furthermore, expression of the
mesenchymal marker vimentin was investigated, following miR-138
overexpression. Consistent with E-cadherin alteration, vimentin
expression was downregulated (Fig.
2D). These results suggested that miR-138 may participate in
tumor metastasis regulation.
miR-138 negatively regulates β-catenin
activation in prostate cancer cells
The Wnt/β-catenin pathway is involved in tumor
metastasis (14). To further
investigate whether miR-138 affects the Wnt/β-catenin pathway in
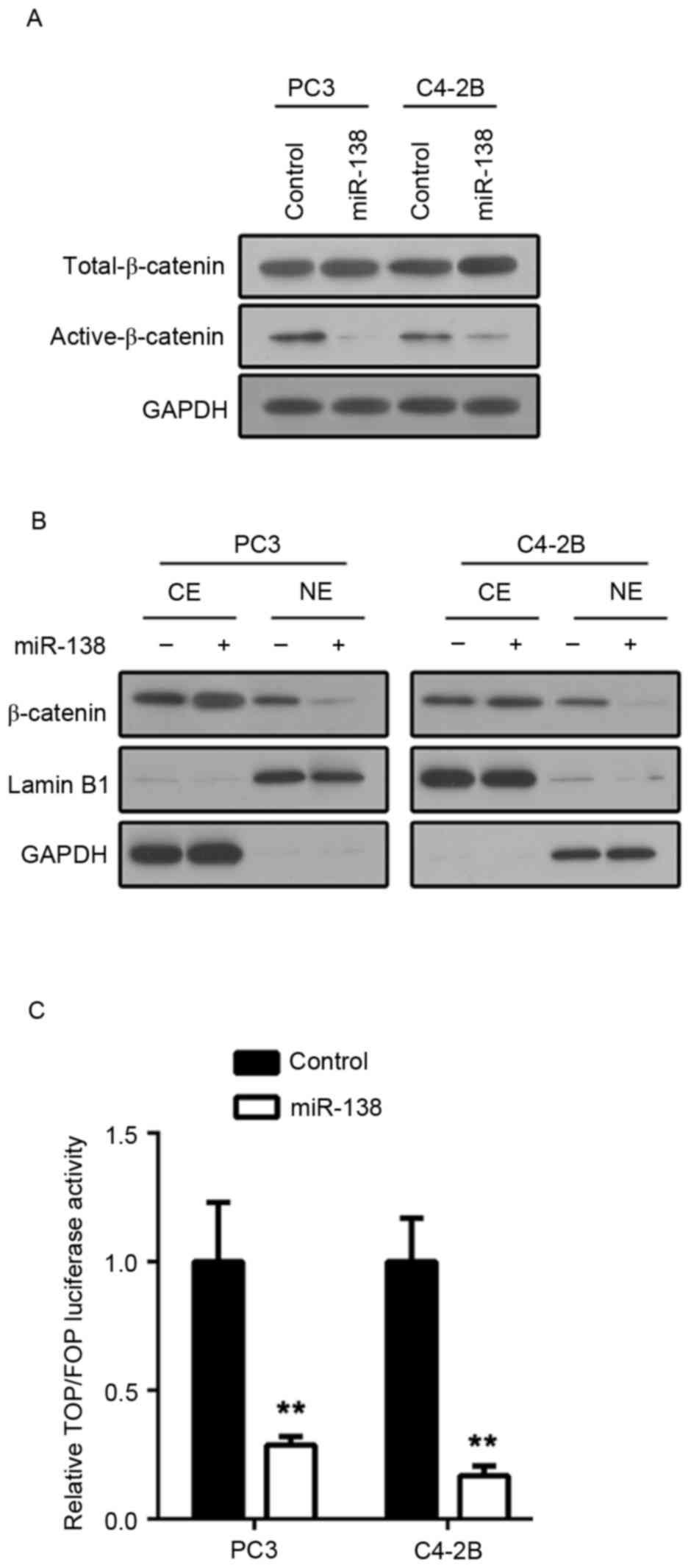
prostate cancer, the present study examined the Wnt/β-catenin
pathway activation status. β-catenin dephosphorylation and
translocation to the nucleus are the core events in Wnt/β-catenin
pathway activation. β-catenin phosphorylation status was
investigated. It was demonstrated that the active form of β-catenin
(dephosphorylated) was downregulated in C4-2B and PC3 cells
transfected with miR-138 mimics (Fig.
3A). To determine β-catenin localization, nuclear protein and
cytoplasmic proteins from C4-2B and PC3 cell lines transfected with
either miR-138 mimics or control mimics were extracted. Then,
β-catenin expression was examined by immunoblot assay. In
accordance with previous results, nuclear localization of β-catenin
in C4-2B and PC3 cells transfected with miR-138 mimics was
downregulated compared with controls (Fig. 3B). Furthermore, lymphoid enhancer
factor/T-cell factor (LEF/TCF) transcription activity was examined
via luciferase activity assay. Following miR-138 overexpression,
C4-2B and PC3 cells indicated weakened LEF/TCF transcription
activity (Fig. 3C). These results
suggested that miR-138 suppresses the Wnt/β-catenin pathway in
prostate cancer.
miR-138 tumor suppressor role is
dependent on Wnt/β-catenin pathway inhibition
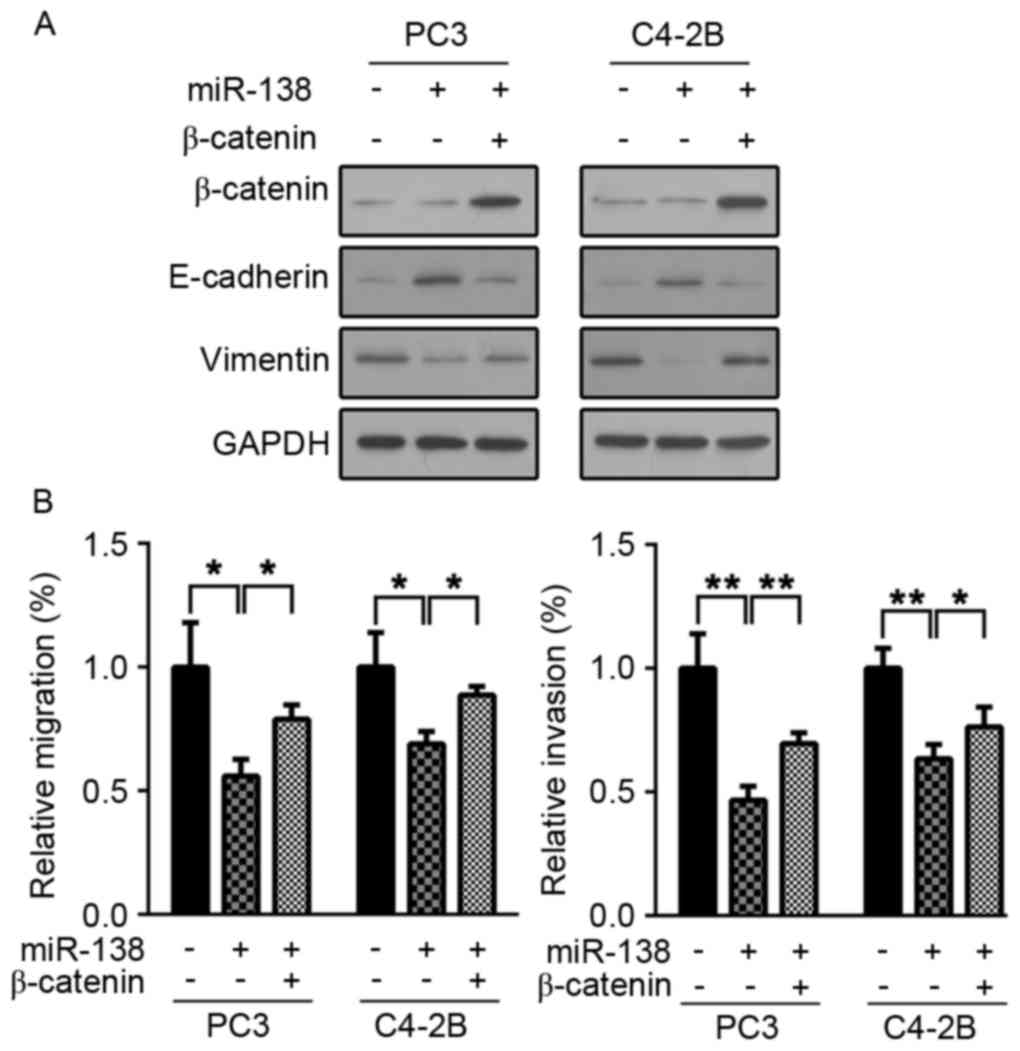
To further investigate whether the miR-138
anti-tumor effects were Wnt/β-catenin pathway dependent, β-catenin
was overexpressed in C4-2B and PC3 cells, to activate the
Wnt/β-catenin signaling pathway. It was then examined whether
miR-138 overexpression induced an anti-tumor effect. As presented
in Fig. 4A, β-catenin was
overexpressed efficiently in C4-2B and PC3 cells. β-catenin
overexpression restored C4-2B and PC3 cell invasion and migration
capacity (Fig. 4B). Furthermore,
E-cadherin and vimentin expression were examined following
β-catenin overexpression. In accordance with previous results, the
expression levels of the two proteins were restored (Fig. 4B). These results suggested that the
miR-138 tumor suppressor role is dependent on Wnt/β-catenin pathway
inhibition.
Discussion
The acquired capability of tumor cells to exhibit
tissue invasion and metastasis has been defined as a ‘hallmark of
cancer’ (15,16). Localized prostate tumors may be
treated and do not frequently result in patient mortality. However,
patients with prostate cancer may develop metastatic tumors in
different organs, including bone, lung, brain and liver which may
be fatal (3,17). Therefore, understanding the process
of prostate cancer metastasis is of primary concern, and a
prerequisite for improving prostate cancer therapeutics.
The present study revealed a novel invasion and
migration regulator in prostate cancer. miR-138 was downregulated
in aggressive tumor cell lines compared with those which were less
aggressive in their metastatic capabilities. miR-138 overexpression
induced impaired invasion and migration in C4-2B and PC3 cells.
Notably, E-cadherin and vimentin, the epithelial mesenchymal
transition (EMT) markers, were additionally regulated by miR-138.
EMT is a process where epithelial cells acquire mesenchymal
characteristics with defined morphology, protein expression and
gene signatures. This process has been reported in a wide range of
cancers and enables malignant cells to acquire the ability of
invasion and migration (18–21).
EMT has additionally been reported in prostate cancer (22–25).
The results of the present study suggested that miR-138 may be an
EMT regulator and therefore regulate invasion and migration in
prostate cancer.
Wnt/β-catenin pathway activation has been widely
reported in prostate cancer and its aberrant activation may
participate in numerous processes associated with tumor progression
(26–28), including invasion and migration.
The results demonstrated that miR-138 overexpression significantly
inhibited the activation of the Wnt/β-catenin pathway in C4-2B and
PC3 cells. The active form of β-catenin was downregulated in C4-2B
and PC3 cells transfected with miR-138 mimics. β-catenin
localization in the nucleus was additionally reduced. Furthermore,
miR-138 overexpression weakened LEF/TCF transcription activity.
Therefore, the present study reported a novel Wnt/β-catenin pathway
inhibitor. Downregulation of miR-138 in prostate cancer may
contribute to the aberrant activation of the Wnt/β-catenin pathway
in the disease. However, further investigation is necessary in
order to verify if miR-138 is downregulated and elucidate the
underlying molecular mechanisms regarding the association between
the downregulation of miR-138 and the Wnt/β-catenin pathway
activation in prostate cancer.
The results revealed the importance of Wnt/β-catenin
signaling in prostate cancer. Identification of these therapeutic
targets may be of clinical relevance in the future. These results
indicate that drugs and inhibitors targeting this pathway may have
clinical potential in the treatment of prostate cancer. Various
inhibitors are currently at developmental stages (29–32),
however require further evaluation regarding their role in prostate
cancer.
In conclusion, the present study demonstrated a
novel mechanism that regulates invasion and migration in prostate
cancer. miR-138 acts as a Wnt/β-catenin pathway inhibitor to
maintain prostate cancer cell epithelial status. However, the exact
targets of miR-138 and its application in tumor therapy require
further investigation.
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hess KR, Varadhachary GR, Taylor SH, Wei
W, Raber MN, Lenzi R and Abbruzzese JL: Metastatic patterns in
adenocarcinoma. Cancer. 106:1624–1633. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sturge J, Caley MP and Waxman J: Bone
metastasis in prostate cancer: Emerging therapeutic strategies. Nat
Rev Clin Oncol. 8:357–368. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Corn PG, Wang F, McKeehan WL and Navone N:
Targeting fibroblast growth factor pathways in prostate cancer.
Clin Cancer Res. 19:5856–5866. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schoenborn JR, Nelson P and Fang M:
Genomic profiling defines subtypes of prostate cancer with the
potential for therapeutic stratification. Clin Cancer Res.
19:4058–4066. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Carthew RW and Sontheimer EJ: Origins and
mechanisms of miRNAs and siRNAs. Cell. 136:642–655. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Miranda KC, Huynh T, Tay Y, Ang YS, Tam
WL, Thomson AM, Lim B and Rigoutsos I: A pattern-based method for
the identification of MicroRNA binding sites and their
corresponding heteroduplexes. Cell. 126:1203–1217. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
He L and Hannon GJ: MicroRNAs: Small RNAs
with a big role in gene regulation. Nat Rev Genet. 5:522–531. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu X, Jiang L, Wang A, Yu J, Shi F and
Zhou X: MicroRNA-138 suppresses invasion and promotes apoptosis in
head and neck squamous cell carcinoma cell lines. Cancer Lett.
286:217–222. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jiang L, Dai Y, Liu X, Wang C, Wang A,
Chen Z, Heidbreder CE, Kolokythas A and Zhou X: Identification and
experimental validation of G protein alpha inhibiting activity
polypeptide 2 (GNAI2) as a microRNA-138 target in tongue squamous
cell carcinoma. Hum Genet. 129:189–197. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yamasaki T, Seki N, Yamada Y, Yoshino H,
Hidaka H, Chiyomaru T, Nohata N, Kinoshita T, Nakagawa M and
Enokida H: Tumor suppressive microRNA-138 contributes to cell
migration and invasion through its targeting of vimentin in renal
cell carcinoma. Int J Oncol. 41:805–817. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang H, Zhang H, Zhao M, Lv Z, Zhang X,
Qin X, Wang H, Wang S, Su J, Lv X, et al: MiR-138 inhibits tumor
growth through repression of EZH2 in non-small cell lung cancer.
Cell Physiol Biochem. 31:56–65. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nguyen DX, Chiang AC, Zhang XH, Kim JY,
Kris MG, Ladanyi M, Gerald WL and Massagué J: WNT/TCF signaling
through LEF1 and HOXB9 mediates lung adenocarcinoma metastasis.
Cell. 138:51–62. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Doolan G, Benke G and Giles G: An update
on occupation and prostate cancer. Asian Pac J Cancer Prev.
15:501–516. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
De Craene B and Berx G: Regulatory
networks defining EMT during cancer initiation and progression. Nat
Rev Cancer. 13:97–110. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Han RF, Ji X, Dong XG, Xiao RJ, Liu YP,
Xiong J and Zhang QP: An epigenetic mechanism underlying
doxorubicin induced EMT in the human BGC-823 gastric cancer cell.
Asian Pac J Cancer Prev. 15:4271–4274. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu MM, Mao GX, Liu J, Li JC, Huang H, Liu
YF and Liu JH: Low expression of the FoxO4 gene may contribute to
the phenomenon of EMT in non-small cell lung cancer. Asian Pac J
Cancer Prev. 15:4013–4018. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Byles V, Zhu L, Lovaas JD, Chmilewski LK,
Wang J, Faller DV and Dai Y: SIRT1 induces EMT by cooperating with
EMT transcription factors and enhances prostate cancer cell
migration and metastasis. Oncogene. 31:4619–4629. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hance MW, Dole K, Gopal U, Bohonowych JE,
Jezierska-Drutel A, Neumann CA, Liu H, Garraway IP and Isaacs JS:
Secreted Hsp90 is a novel regulator of the epithelial to
mesenchymal transition (EMT) in prostate cancer. J Biol Chem.
287:37732–37744. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen CL, Mahalingam D, Osmulski P, Jadhav
RR, Wang CM, Leach RJ, Chang TC, Weitman SD, Kumar AP, Sun L, et
al: Single-cell analysis of circulating tumor cells identifies
cumulative expression patterns of EMT-related genes in metastatic
prostate cancer. Prostate. 73:813–826. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zurita AJ, Bischoff FZ, Gorlov I, Mayer
JN, Pircher TJ, Mikolajezyk S, Logothetis C and Lin SH: Cadherin-11
(Cad11) for detection of epithelial-mesenchymal transition (EMT) in
circulating prostate cancer (PCa) cells. J Clin Oncol.
31:e220472013.
|
|
26
|
Yu CY, Liang GB, Du P and Liu YH: Lgr4
promotes glioma cell proliferation through activation of Wnt
signaling. Asian Pac J Cancer Prev. 14:4907–4911. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yuan JB, Yang LY, Tang ZY, Zu XB and Qi L:
Down-regulation of EZH2 by RNA interference inhibits proliferation
and invasion of ACHN cells via the Wnt/β- catenin pathway. Asian
Pac J Cancer Prev. 13:6197–6201. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Clevers H and Nusse R: Wnt/β-catenin
signaling and disease. Cell. 149:1192–1205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Arcaroli JJ, Quackenbush KS, Purkey A,
Powell RW, Pitts TM, Bagby S, Tan AC, Cross B, McPhillips K, Song
EK, et al: Tumours with elevated levels of the Notch and Wnt
pathways exhibit efficacy to PF-03084014, a γ-secretase inhibitor,
in a preclinical colorectal explant model. Br J Cancer.
109:667–675. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yan Z, Zhu Z, Wang J, Sun J, Chen Y, Yang
G, Chen W and Deng Y: Synthesis, characterization, and evaluation
of a novel inhibitor of WNT/β-catenin signaling pathway. Mol
Cancer. 12:1162013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Basu D, Lettan R, Damodaran K, Strellec S,
Reyes-Mugica M and Rebbaa A: Identification, mechanism of action,
and antitumor activity of a small molecule inhibitor of hippo,
TGF-β, and Wnt signaling pathways. Mol Cancer Ther. 13:1457–1467.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jiang Y, Dai J, Zhang H, Sottnik JL,
Keller JM, Escott KJ, Sanganee HJ, Yao Z, McCauley LK and Keller
ET: Activation of the Wnt pathway through AR79, a GSK3β inhibitor,
promotes prostate cancer growth in soft tissue and bone. Mol Cancer
Res. 11:1597–1610. 2013. View Article : Google Scholar : PubMed/NCBI
|