Introduction
The advanced glycosylation end product-specific
receptor (RAGE) is a single-transmembrane, multi-ligand receptor
that belongs to the immunoglobulin superfamily. It was initially
cloned in 1992 and termed receptor for advanced glycosylation end
products (AGEs) of proteins (1).
The RAGE gene generates extensive splice variants in
humans and other mammals (2,3). In
addition to the full-length RAGE, there are two major splice
variants: Variant 1 (C-truncated RAGE) and variant 2 (N-truncated
RAGE). N-truncation deletes the V-type domain, the ligand binding
domain, from the full-length RAGE. C-truncation removes the
transmembrane and C-terminal domains of the full-length RAGE and
contains only the extracellular domain of RAGE (2), rendering RAGE free (secreted) and
soluble. One variant of soluble RAGE (sRAGE), endogenous secretory
RAGE (esRAGE), also termed RAGE variant 1, is generated through
alternative RNA splicing (4,5). In
humans, full-length RAGE is the most prevalent form of RAGE,
followed by esRAGE, the most predominant soluble form of the sRAGE
isoforms (2,5). In addition to alternative RNA
splicing, sRAGE may be generated via proteolytic cleavage of the
full-length RAGE (6,7). This type of RAGE is termed cleaved
RAGE (cRAGE). However, esRAGE and cRAGE are able to bind the same
RAGE ligands, and are therefore functionally equivalent (5).
RAGE binds a variety of ligands, including AGEs,
high mobility group protein B1 (HMGB1), serum amyloid A (SAA), and
S100 family proteins (8–11). RAGE is highly expressed in normal
lungs, particularly in alveolar epithelial type I cells, compared
with other tissues (12,13). A recent study demonstrated that the
absence of RAGE abolished the majority of the typical pathological
indicators of asthma, including airway hypersensitivity,
eosinophilic inflammation and airway remodeling, in a house dust
mite-RAGE knockout mouse model of asthma/allergic airway disease,
suggesting that RAGE may be a principal mediator of asthma
pathogenesis (14). In addition,
increased expression of RAGE ligands in asthma has been reported,
including HMGB1, SAA and S100 family proteins (8–11).
Conceivably, suppression of RAGE activation and/or expression may
be a promising approach to decrease the airway inflammation
commonly observed in asthma.
sRAGE may act as a decoy receptor to compete with
RAGE ligands for binding with the full-length RAGE, thereby
suppressing the activation of RAGE at the cell surface (15). Accumulating evidence has
demonstrated that sRAGE may be associated with various chronic
diseases, including metabolic, cardiovascular, neurodegenerative
and inflammatory disorders (16–19).
Previous studies have demonstrated that sRAGE was decreased in
patients with chronic obstructive pulmonary disease (COPD) and was
correlated with the severity of emphysema and neutrophilic
inflammatory conditions (20–25).
RAGE genetic variability additionally results from
single-nucleotide polymorphisms (SNPs). A total of >30 SNPs have
been identified within the RAGE gene and are associated with
diabetes mellitus (26). Among
these SNPs, RAGE genetic variation rs2070600 (Gly82Ser, G82S),
which causes a glycine to serine substitution at amino acid number
82 of the ligand-binding V-domain (Gly82Ser) in the extracellular
domain, is of particular interest, as it has been associated with
circulating RAGE levels (27,28)
and COPD (21). In addition,
genome-wide association studies have revealed an association
between the Gly82Ser polymorphism and pulmonary function (29,30).
However, to date, there have been few studies, which
have examined the association between sRAGE and asthma. These
studies had small sample sizes and their results were contradictory
(11,20,31).
For example, Sukkar et al (20) reported that sRAGE was deficient in
neutrophilic asthma. Other studies, however, reported elevated
levels of sRAGE in asthmatics (11,31).
The potential reason for the discrepancy may be attributable to the
fact that different asthma inflammatory phenotypes, which are
classified as neutrophilic and non-neutrophilic based on induced
sputum inflammatory cells (20),
are associated with RAGE genetic variants.
Therefore, the primary purpose of the present study
was to determine plasma sRAGE levels in asthmatics with different
inflammatory phenotypes, correlate plasma sRAGE levels with related
clinical characteristics, and assess the association between plasma
sRAGE and the RAGE G82S genetic variant.
Materials and methods
Study population
A total of 96 patients were recruited from the
outpatient clinic of Nanfang Hospital, Southern Medical University
(Guangzhou, China), between September 2011 and September 2012, and
97 patients from The People's Hospital of Zhongshan City
(Zhongshan, China) between September 2014 and December 2015. The
characteristics of the subjects are displayed in Table I. All patients were >18 years
old and newly-diagnosed with asthma via positive bronchodilator
reversibility test or airway hyper-responsiveness to methacholine.
A positive bronchodilator reversibility test is defined as an
increase in forced expiratory volume in 1 sec (FEV1) of >12% and
>200 ml from baseline, 10–15 min subsequent to the inhalation of
200–400 μg albuterol or equivalent. Airway hyper-responsiveness to
methacholine is defined as a decrease in FEV1 from baseline of ≥20%
with standard doses of methacholine (32). As a control group, 118 healthy
individuals without a history of chronic respiratory disease,
allergies, cardiovascular disease, diabetes and neurological
diseases, and without evidence of airflow obstruction measured by
spirometry, were recruited between September 2014 and December
2015. The present study was approved by the Medical Ethics
Committees of Nanfang Hospital and The People's Hospital of
Zhongshan City. All subjects provided written informed consent for
participation in the study.
 | Table I.Subject characteristics. |
Table I.
Subject characteristics.
|
Characteristics | Neutrophilic
asthmatics (n=85) | Non-neutrophilic
asthmatics (n=109) | Healthy controls
(n=118) | P-value |
|---|
| Male/female, n | 61/47 | 56/29 | 76/42 | 0.328 |
| Age, y | 46.26±14.86 |
38.41±13.23a | 48.75±18.31 | <0.001 |
| BMI,
kg/m2 | 22.35±3.26 | 22.15±3.00 | 21.78±2.56 | 0.600 |
| Smoker
(yes/no) | 45/40 | 44/65 | 51/67 | 0.196 |
| FEV1% Pre | 62.77±25.00 | 67.11±24.06 |
97.26±9.73a | <0.001 |
| Sputum eosinophils,
% | 2.5 (9.5) | 15.5
(28.8)a | 0 (4.5) | <0.001 |
| Sputum neutrophils,
% | 87.0
(18.12)a | 41.5 (20.5) | 46.0 (54.0) | <0.001 |
Study design
All participants completed a questionnaire to
collect data about age, sex, weight, height and smoking history.
Nonsmokers were defined as those who had a <2-pack-year
(pack-year, cigarette packs per day multiplied by the number of
years smoking) smoking history during their lifetime, and had not
smoked for at least 1 year prior to participation. Smokers were
defined as having a smoking history of 2 pack-years or more. All
participants received pulmonary function testing, sputum induction
and collection of a 5-ml EDTA-preserved blood specimen following
recruitment. All asthmatics were divided into a neutrophilic asthma
group (neutrophils ≥65%) and non-neutrophilic asthma group
(neutrophils <65%) based on induced sputum inflammatory cells.
This 65% cut-off value was selected according to a previous report
(20).
Sample preparation and measurement of
sRAGE
Blood collection and sputum induction and their
processing were performed as per a previous report (8). Sputum smears in which the ratio of
squamous epithelial cells/nuclear cells was <20% were considered
to be adequate specimens. Following processing of the blood
samples, plasma was stored at −80°C until use. Plasma sRAGE levels
were detected by ELISA analysis using Quantikine sRAGE ELISA, (cat.
no. DRG00; R&D Systems, Inc., Minneapolis, MN, USA), according
to the manufacturer's protocol. This ELISA kit measures the total
pool of sRAGE, including esRAGE and cRAGE (2).
DNA preparation and genotype analyses
of RAGE G82S
Genomic DNA was prepared from peripheral blood
samples using a pure gene DNA purification kit (Tiangen Biotech
Co., Ltd., Beijing, China), according to the manufacturer's
protocol. The RAGE SNP (rs2070600; G82S) was determined using the
Sanger sequencing method by BGI (Shenzhen, China).
Statistical analysis
SPSS 16.0 software (SPSS, Inc., Chicago, IL, USA)
was used for statistical analysis. Continuous variables were
evaluated for a normal distribution using the Kolmogorov-Smirnov
test. Parametric data are presented as mean ± standard deviation.
Categorical variables are presented as either medians
(interquartile ranges) or numbers (percentages), as appropriate.
Comparisons among groups were performed using one-way analysis of
variance. For the post hoc multiple comparisons, least significant
difference test was used when equal variances were assumed and
Tamhane's T2 test was used when equal variances were not assumed.
Categorical variables were examined using the χ2 test.
Correlations were assessed using Spearman or Pearson rank
correlation coefficients. The Hardy-Weinberg equilibrium of the
G82S polymorphism was examined using the Executive SNP Analyzer 1.0
(BGI, Shenzhen, China). All statistical analyses were two-sided,
and P<0.05 was considered to indicate a statistically
significant difference.
Results
Subject characteristics
A total of 194 newly-diagnosed asthmatics and 118
healthy controls were included in the analysis. Of the asthma
patients, 85 were neutrophilic and 109 were non-neutrophilic. The
characteristics of the subjects are displayed in Table I. There was no significant
difference in sex, body mass index (BMI) and smoking history
between asthmatic and control subjects. The mean age of the
non-neutrophilic asthma group was lower compared with that of the
neutrophilic and control groups. Asthmatics had a decreased FEV1%
predicted (FEV1% Pre) compared with healthy controls, although
there was no difference between the two asthma groups.
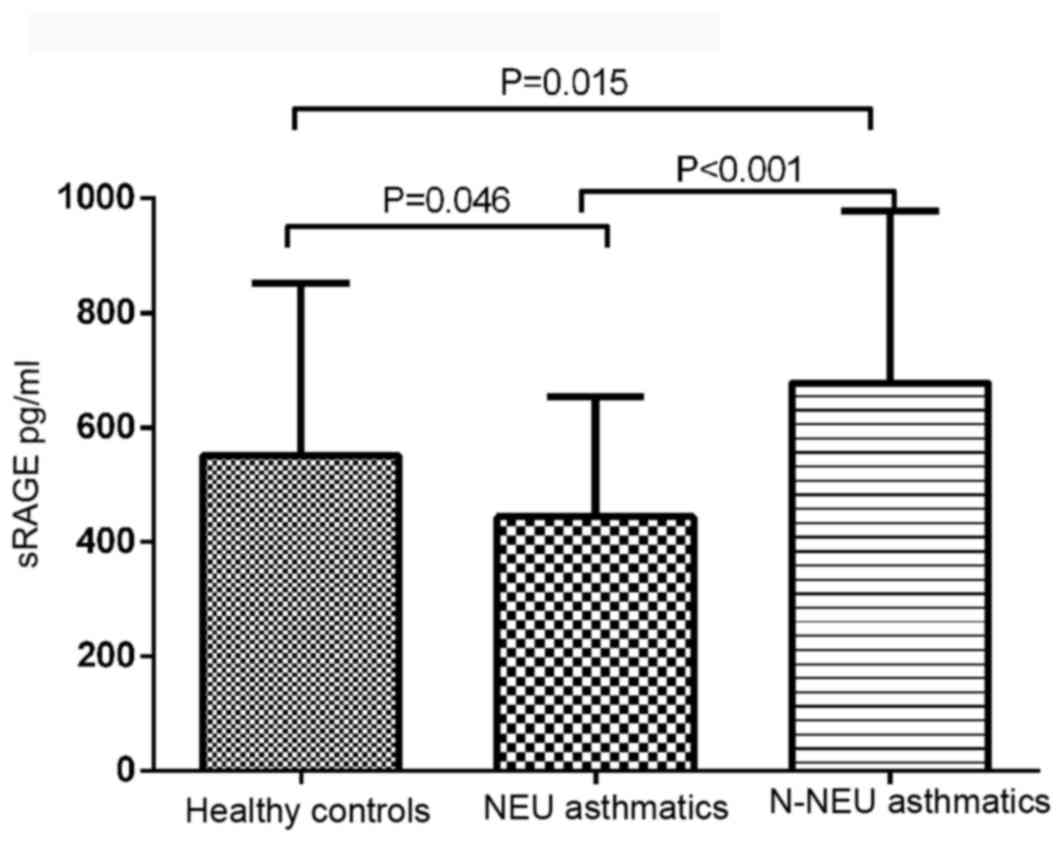
Plasma sRAGE levels
There was a significant difference in plasma sRAGE
levels between asthmatic patients and normal controls (F=15.925;
P<0.001). The mean plasma sRAGE level was significantly
decreased in neutrophilic asthmatics (443.67±208.9 pg/ml) and
increased in non-neutrophilic asthmatics (677.63±300.75 pg/ml),
compared with the control group (550.02±300.83 pg/ml; Fig. 1).
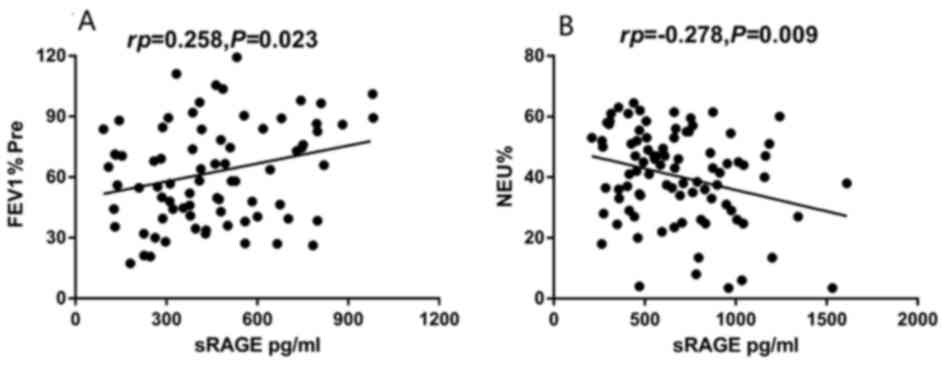
Correlation of plasma sRAGE with
clinical characteristics of asthmatic patients
The plasma sRAGE level of neutrophilic asthmatics
was positively correlated with FEV1% Pre (rp=0.258;
P=0.023; Fig. 2A), while no
significant correlation was observed in non-neutrophilic asthmatics
(P=0.406). The plasma sRAGE level of non-neutrophilic asthmatics
was inversely correlated with the percentage of sputum neutrophils
(rp=−0.278; P=0.009; Fig.
2B), although no significant correlation was observed in
neutrophilic asthmatics (P=0.183). There was no significant
correlation between plasma sRAGE level and sex, BMI, age, smoking
history or and FVC% Pre, or the percentage of sputum eosinophils
(P>0.05), in the two asthma groups.
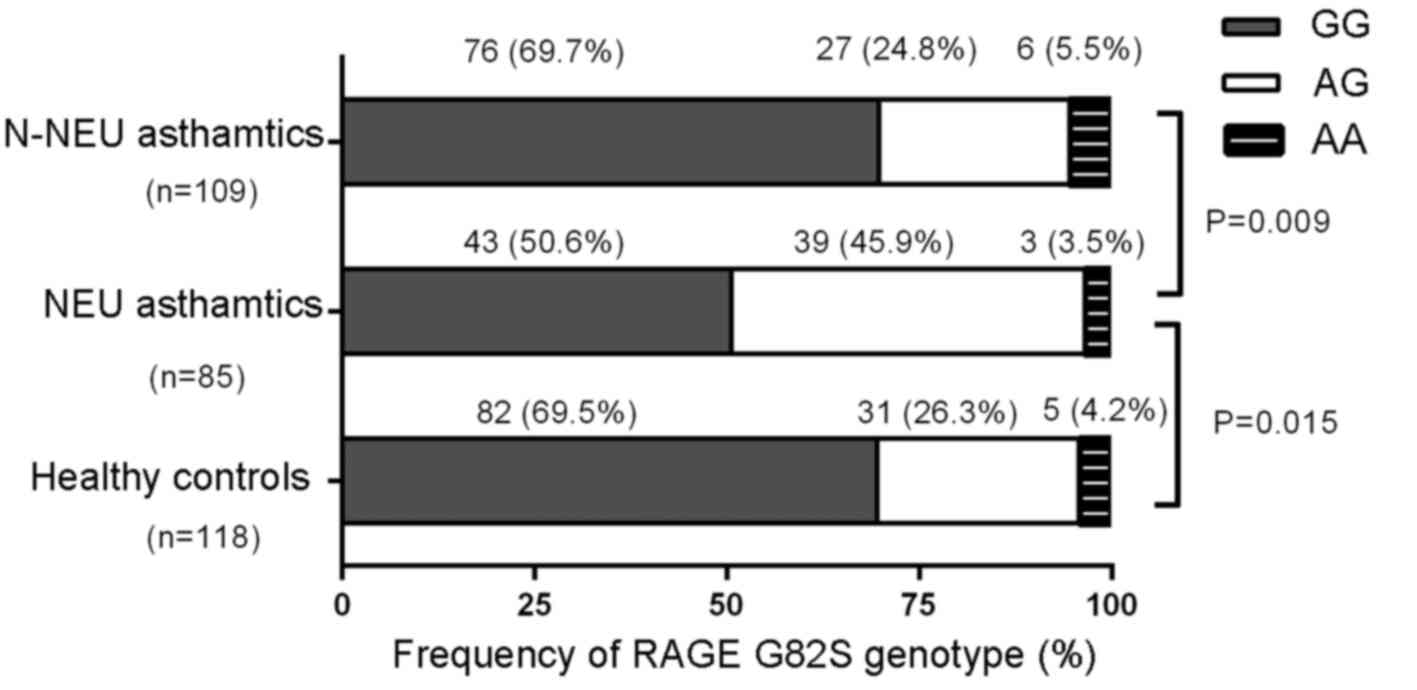
Frequencies of RAGE G82S
polymorphism
The RAGE G82S genotype distribution among
neutrophilic asthmatics (G/G, 50.6%; A/G, 45.9%; A/A, 3.5%),
non-neutrophilic asthmatics (G/G, 69.7%; A/G, 24.8%; A/A, 5.5%),
and controls (G/G, 69.5%; A/G, 26.3%; A/A, 4.2%) did not deviate
significantly from Hardy-Weinberg equilibrium (χ2=2.71,
P=0.100; χ2=2.682, P=0.102; and χ2=0.851,
P=0.356, respectively) (Fig. 3).
The frequency of the G82S genotype was significantly different
between neutrophilic and non-neutrophilic asthmatics
(χ2=9.510, P=0.009), and between neutrophilic asthmatics
and healthy controls (χ2=8.441, P=0.015), while no
significant difference between non-neutrophilic asthmatics and
healthy controls was noted (χ2=0.238, P=0.888).
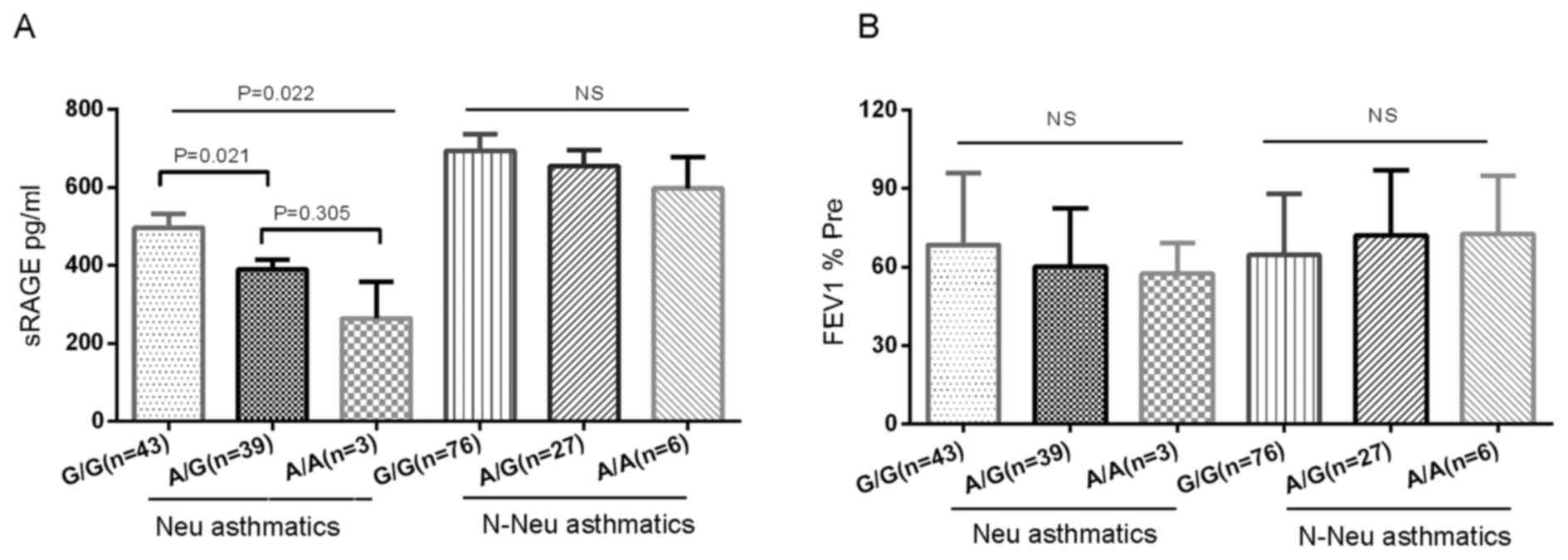
Association of RAGE G82S genotypes
with plasma sRAGE and FEV1% Pre
The present study analyzed the association between
RAGE G82S genotypes and plasma sRAGE distribution (Fig. 4A), and demonstrated a significant
association between G82S genotypes and plasma sRAGE levels in
neutrophilic asthmatics. Following adjustment for age, subjects
with the G/G genotype exhibited a significantly increased sRAGE
level (498.64±235.37 pg/ml) compared with those with the A/G
genotype (389.83±150.37 pg/ml) and A/A genotype (264.59±161.74
pg/ml) (P=0.022). Only 3 neutrophil subjects had an A/A genotype,
and they had very low plasma sRAGE levels. Neutrophilic asthmatics
with the RAGE A/G or A/A genotype displayed a trend toward lower
FEV1% Pre compared with those with the G/G genotype (60.14±22.36%
vs. 64.51±27.37%; Fig. 4B).
However, significant differences in sRAGE between the different
genotypes, and associations with FEV1% Pre, were not observed in
non-neutrophilic asthmatics and healthy controls.
Discussion
The present study demonstrated that plasma sRAGE
levels were altered in different asthma inflammatory phenotypes. At
initial diagnosis, plasma sRAGE levels were decreased in patients
with neutrophilic asthma and increased in patients with
non-neutrophilic asthma compared with healthy subjects. In
addition, the plasma sRAGE level was positively correlated with
FEV1% Pre in neutrophilic asthmatics. In terms of G82S genotype,
the frequencies of G82S genotypes were different between
neutrophilic asthmatics and healthy controls. G82S genotype
exhibited a trend that correlated with the severity of pulmonary
dysfunction. To the best of our knowledge, the present study was
the first to report a likely association between RAGE G82S gene
variants and plasma sRAGE levels in patients with asthma.
Few studies have examined sRAGE levels and asthma
inflammatory phenotypes. In accordance with the results of the
present study, Watanabe et al (11) reported elevated levels of sRAGE in
the sputum of asthmatic patients. In that study, the subjects
exhibited low levels of sputum neutrophils (median, 24%; upper
limit, 47%), and high eosinophil counts (median, 9.4%; lower limit,
4.5%). However, Sukkar et al (20) reported that sRAGE was deficient in
neutrophilic asthma, which is consistent with the results of the
present study. Notably, the patients in the study of Sukkar et
al (20) were clinically
stable older asthmatics, and 68% of them used inhaled
corticosteroids; in the present study, all the patients were
newly-diagnosed asthmatics, suggesting that plasma sRAGE levels,
although altered in different asthma inflammatory phenotypes, may
remain relatively stable regardless of medical therapy. Similar to
the findings adults (7,11,16,20),
Eli-Seify et al (33)
reported that children with >2% eosinophils and ≥40% neutrophils
exhibited lower sRAGE levels compared with children with >2%
eosinophils and <40% neutrophils; this suggests that the sRAGE
level may remain relatively stable in different asthma inflammatory
phenotypes regardless of age.
Previous studies have demonstrated that decreased
plasma levels in sRAGE were associated with increased progression
of airflow limitations in patients with COPD patients and asthmatic
children (24,33). However, Watanabe et al
(11) and Zhou et al
(31) observed that sRAGE levels
were not associated with asthma severity in adult patients with
asthma, possibly as their patients were all non-neutrophilic
asthmatics. In the present study, no significant association
between plasma sRAGE levels and asthma severity was observed in
non-neutrophilic asthmatics. However, it was demonstrated that
decreased plasma sRAGE levels were significantly associated with
FEV1% Pre in neutrophilic asthmatics, suggesting that plasma sRAGE
may be a potential systemic biomarker for neutrophilic asthma
severity, although not for patients with non-neutrophilic
asthma.
In the present study, the sRAGE levels measured
comprised all types of sRAGE, including esRAGE and cRAGE. esRAGE
has been identified to be the primary soluble variant of RAGE, as
demonstrated by Sukkar et al (20) in neutrophilic and non-neutrophilic
asthmatics. esRAGE is generated as a splice variant of the RAGE
gene. The association of the G82S variant with blood sRAGE levels
has been previously reported in a number of diseases, including
diabetes mellitus and COPD (21,27,34).
In the present study, it was demonstrated that the G82S variant was
significantly correlated with plasma sRAGE levels, patients with
the A allele had lower plasma sRAGE levels, and G82S variant was
correlated with the severity of pulmonary dysfunction in
neutrophilic asthmatics. By contrast, these findings were not
observed in non-neutrophilic asthmatics and healthy controls.
Furthermore, in a genome wide association study performed in a
Japanese population, the RAGE G82S variant was reported to not be
significantly associated with asthma susceptibility, although
another single nucleotide polymorphism (rs404860) located in close
proximity was associated with asthma susceptibility (35). These results raised the possibility
that sRAGE levels may be determined by genetic variation in the
RAGE locus, and the RAGE locus may be differentially regulated
between neutrophilic and non-neutrophilic asthma patients.
sRAGE may be consumed by competitively binding to
the ligands of RAGE, including HMGB1 and SAA; therefore, the levels
of RAGE ligands may influence plasma sRAGE levels. Previous studies
have reported that plasma and sputum HMGB1 and SAA were increased
in patients with asthma and COPD, and correlated with higher
degrees of airway neutrophilia and systemic inflammation (20,25,36–38).
Neutrophilic asthmatics exhibited more severe airway neutrophil
inflammation compared with non-neutrophilic asthmatics, and it was
hypothesized that a higher percentage of sputum neutrophils and
increased RAGE ligands may be additional reasons for the decreased
plasma sRAGE levels in neutrophilic asthmatics. However, no
significant correlation was observed between plasma sRAGE levels
and the percentage of sputum neutrophils in neutrophilic
asthmatics, and it was previously reported that there were no
significant differences in plasma HMGB1 levels between different
asthma inflammatory phenotypes (8). These results further suggested that
the G82S variant may be the primary reason for decreased plasma
sRAGE levels.
It is unknown why the plasma sRAGE level was
increased in non-neutrophilic asthmatics. In the present study, no
significant association was observed between plasma sRAGE levels
and G82S variants. It was demonstrated that the plasma sRAGE level
was inversely correlated with the percentage of sputum neutrophils
in non-neutrophilic asthmatics; however, a higher percentage of
sputum neutrophils has been previously reported to be associated
with lower plasma sRAGE levels (20,39),
thus it may be hypothesized that there may exist other factors
which influence the sRAGE level in non-neutrophilic asthmatics. In
addition to esRAGE, sRAGE has another isoform, cRAGE, which is
produced via proteolytic cleavage of membrane-bound RAGE by
metalloproteinases (6,7). Increased expression levels of RAGE
and metalloproteinases in airway and inflammatory cells may induce
an increase in plasma sRAGE in asthmatic patients. However, no
research into this has yet been reported.
There are a number of limitations to the present
study. The sample size was relatively small. The levels of RAGE
ligands were not examined, which may influence the plasma sRAGE
level. In addition, the reasons for the increased plasma sRAGE
level in non-neutrophilic asthmatics were not determined.
In conclusion, plasma sRAGE is decreased in
neutrophilic asthmatics and is correlated with the severity of
neutrophilic asthma and G82S genotype variant. The importance of
and the mechanism underlying the increased plasma sRAGE level in
non-neutrophilic asthmatics requires further study.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81470228
and 81300029), the National Program on Key Basic Research Project
(973 Program; grant no. 2012CB518203), the Natural Science
Foundation of Guangdong Province (grant no. 2015A030313236), the
Scientific and Technological Projects of Guangdong Province (grant
no. 2016A020215117), the Medicine Foundation of Guangdong Province
(grant no. A2015007), and the Science and Technology Program of
Zhongshan City (grant no. 2015B1103).
Glossary
Abbreviations
Abbreviations:
|
RAGE
|
advanced glycosylation end
product-specific receptor
|
|
sRAGE
|
soluble advanced glycosylation end
product-specific receptor
|
|
esRAGE
|
endogenous secretory advanced
glycosylation end product-specific receptor
|
|
cRAGE
|
cleaved advanced glycosylation end
product-specific receptor
|
|
AGE
|
advanced glycosylation end product
|
|
HMGB1
|
high mobility group protein B1
|
|
FEV1
|
forced expiratory volume in 1 sec
|
|
COPD
|
chronic obstructive pulmonary
disease
|
|
BMI
|
body mass index
|
|
SAA
|
serum amyloid A
|
|
SNP
|
single nucleotide polymorphism
|
References
|
1
|
Neeper M, Schmidt AM, Brett J, Yan SD,
Wang F, Pan YC, Elliston K, Stern D and Shaw A: Cloning and
expression of a cell surface receptor for advanced glycosylation
end products of proteins. J Biol Chem. 267:14998–15004.
1992.PubMed/NCBI
|
|
2
|
Hudson BI, Carter AM, Harja E, Kalea AZ,
Arriero M, Yang H, Grant PJ and Schmidt AM: Identification,
classification, and expression of RAGE gene splice variants. FASEB
J. 22:1572–1580. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
López-Díez R, Rastrojo A, Villate O and
Aguado B: Complex tissue-specific patterns and distribution of
multiple RAGE splice variants in different mammals. Genome Biol
Evol. 5:2420–2435. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yonekura H, Yamamoto Y, Sakurai S, Petrova
RG, Abedin MJ, Li H, Yasui K, Takeuchi M, Makita Z, Takasawa S, et
al: Novel splice variants of the receptor for advanced glycation
end-products expressed in human vascular endothelial cells and
pericytes, and their putative roles in diabetes-induced vascular
injury. Biochem J. 370:1097–1109. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Maillard-Lefebvre H, Boulanger E, Daroux
M, Gaxatte C, Hudson BI and Lambert M: Soluble receptor for
advanced glycation end products: A new biomarker in diagnosis and
prognosis of chronic inflammatory diseases. Rheumatology (Oxford).
48:1190–1196. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Raucci A, Cugusi S, Antonelli A, Barabino
SM, Monti L, Bierhaus A, Reiss K, Saftig P and Bianchi ME: A
soluble form of the receptor for advanced glycation endproducts
(RAGE) is produced by proteolytic cleavage of the membrane-bound
form by the sheddase a disintegrin and metalloprotease 10 (ADAM10).
FASEB J. 22:3716–3727. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang L, Bukulin M, Kojro E, Roth A, Metz
VV, Fahrenholz F, Nawroth PP, Bierhaus A and Postina R: Receptor
for advanced glycation end products is subjected to protein
ectodomain shedding by metalloproteinases. J Biol Chem.
283:35507–35516. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hou C, Zhao H, Liu L, Li W, Zhou X, Lv Y,
Shen X, Liang Z, Cai S and Zou F: High mobility group protein B1
(HMGB1) in asthma: Comparison of patients with chronic obstructive
pulmonary disease and healthy controls. Mol Med. 17:807–815. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ozseker F, Buyukozturk S, Depboylu B,
Yilmazbayhan D, Karayigit E, Gelincik A, Genc S, Colakoglu B, Dal M
and Issever H: Serum amyloid A (SAA) in induced sputum of
asthmatics: A new look to an old marker. Int Immunopharmacol.
6:1569–1576. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang Z, Yan WX, Cai H, Tedla N, Armishaw
C, Di Girolamo N, Wang HW, Hampartzoumian T, Simpson JL, Gibson PG,
et al: S100A12 provokes mast cell activation: A potential
amplification pathway in asthma and innate immunity. J Allergy Clin
Immunol. 119:106–114. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Watanabe T, Asai K, Fujimoto H, Tanaka H,
Kanazawa H and Hirata K: Increased levels of HMGB-1 and endogenous
secretory RAGE in induced sputum from asthmatic patients. Respir
Med. 105:519–525. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Buckley ST and Ehrhardt C: The receptor
for advanced glycation end products (RAGE) and the lung. J Biomed
Biotechnol. 2010:9171082010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Demling N, Ehrhardt C, Kasper M, Laue M,
Knels L and Rieber EP: Promotion of cell adherence and spreading: A
novel function of RAGE, the highly selective differentiation marker
of human alveolar epithelial type I cells. Cell Tissue Res.
323:475–488. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Milutinovic PS, Alcorn JF, Englert JM,
Crum LT and Oury TD: The receptor for advanced glycation end
products is a central mediator of asthma pathogenesis. Am J Pathol.
181:1215–1225. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sukkar MB, Ullah MA, Gan WJ, Wark PA,
Chung KF, Hughes JM, Armour CL and Phipps S: RAGE: A new frontier
in chronic airways disease. Br J Pharmacol. 167:1161–1176. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Park L, Raman KG, Lee KJ, Lu Y, Ferran LJ,
Chow WS, Stern D and Schmidt AM: Suppression of accelerated
diabetic atherosclerosis by the soluble receptor for advanced
glycation endproducts. Nat Med. 4:1025–1031. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vazzana N, Santilli F, Cuccurullo C and
Davì G: Soluble forms of RAGE in internal medicine. Intern Emerg
Med. 4:389–401. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nin JW, Jorsal A, Ferreira I, Schalkwijk
CG, Prins MH, Parving H, Tarnow L, Rossing P and Stehouwer CD:
Higher plasma soluble receptor for advanced glycation end products
(sRAGE) levels are associated with incident cardiovascular disease
and all-cause mortality in type 1 diabetes: A 12-year follow-up
study. Diabetes. 59:2027–2032. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Thomas MC, Söderlund J, Lehto M, Mäkinen
VP, Moran JL, Cooper ME, Forsblo C and Groop PH; FinnDiane Study
Group, : Soluble receptor for AGE (RAGE) is a novel independent
predictor of all-cause and cardiovascular mortality in type 1
diabetes. Diabetologia. 54:2669–2677. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sukkar MB, Wood LG, Tooze M, Simpson JL,
McDonald VM, Gibson PG and Wark PA: Soluble RAGE is deficient in
neutrophilic asthma and COPD. Eur Respir J. 39:721–729. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cheng DT, Kim DK, Cockayne DA, Belousov A,
Bitter H, Cho MH, Duvoix A, Edwards LD, Lomas DA, Miller BE, et al:
Systemic soluble receptor for advanced glycation endproducts is a
biomarker of emphysema and associated with AGER genetic variants in
patients with chronic obstructive pulmonary disease. Am J Respir
Crit Care Med. 188:948–957. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Miniati M, Monti S, Basta G, Cocci F,
Fornai E and Bottai M: Soluble receptor for advanced glycation end
products in COPD: Relationship with emphysema and chronic cor
pulmonale: A case-control study. Respir Res. 12:372011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hoonhorst SJ, Lo Tam Loi AT, Pouwels SD,
Faiz A, Telenga E, Van den Berge M, Koenderman L, Lammers JW,
Boezen HM, van Oosterhout M, et al: Advanced glycation endproducts
and their receptor in different body compartments in COPD. Respir
Res. 17:462016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Iwamoto H, Gao J, Pulkkinen V, Toljamo T,
Nieminen P and Mazur W: Soluble receptor for advanced glycation
end-products and progression of airway disease. BMC Pulm Med.
14:682014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Smith DJ, Yerkovich ST, Towers MA, Carroll
ML, Thomas R and Upham JW: Reduced soluble receptor for advanced
glycation end-products in COPD. Eur Respir J. 37:516–522. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hudson BI, Stickland MH and Grant PJ:
Identification of polymorphisms in the receptor for advanced
glycation end products (RAGE) gene: Prevalence in type 2 diabetes
and ethnic groups. Diabetes. 47:1155–1157. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Miller S, Henry AP, Hodge E, Kheirallah
AK, Billington CK, Rimington TL, Bhaker SK, Obeidat M, Melén E,
Merid SK, et al: The Ser82 RAGE variant affects lung function and
serum RAGE in smokers and sRAGE production in vitro. PLoS One.
11:e1640412016. View Article : Google Scholar
|
|
28
|
Gaens KH, Ferreira I, van der Kallen CJ,
van Greevenbroek MM, Blaak EE, Feskens EJ, Dekker JM, Nijpels G,
Heine RJ, 't Hart LM, et al: Association of polymorphism in the
receptor for advanced glycation end products (RAGE) gene with
circulating RAGE levels. J Clin Endocrinol Metab. 94:5174–5180.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hancock DB, Eijgelsheim M, Wilk JB, Gharib
SA, Loehr LR, Marciante KD, Franceschini N, van Durme YM, Chen TH,
Barr RG, et al: Meta-analyses of genome-wide association studies
identify multiple loci associated with pulmonary function. Nat
Genet. 42:45–52. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Repapi E, Sayers I, Wain LV, Burton PR,
Johnson T, Obeidat M, Zhao JH, Ramasamy A, Zhai G, Vitart V, et al:
Genome-wide association study identifies five loci associated with
lung function. Nat Genet. 42:36–44. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhou Y, Jiang YQ, Wang WX, Zhou ZX, Wang
YG, Yang L and Ji YL: HMGB1 and RAGE levels in induced sputum
correlate with asthma severity and neutrophil percentage. Hum
Immunol. 73:1171–1174. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bateman ED, Hurd SS, Barnes PJ, Bousquet
J, Drazen JM, FitzGerald M, Gibson P, Ohta K, O'Byrne P, Pedersen
SE, et al: Global strategy for asthma management and prevention:
GINA executive summary. Eur Respir J. 31:143–178. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
El-Seify MY, Fouda EM and Nabih ES: Serum
level of soluble receptor for advanced glycation end products in
asthmatic children and its correlation to severity and pulmonary
functions. Clin Lab. 60:957–962. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu TL, Tsai CC, Wang YY, Ho KY, Wu YM,
Hung HC and Lin YC: The association between the RAGE G82S
polymorphism, sRAGE and chronic periodontitis in Taiwanese
individuals with and without diabetes. J Periodontal Res.
50:881–889. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hirota T, Takahashi A, Kubo M, Tsunoda T,
Tomita K, Doi S, Fujita K, Miyatake A, Enomoto T, Miyagawa T, et
al: Genome-wide association study identifies three new
susceptibility loci for adult asthma in the Japanese population.
Nat Genet. 43:893–896. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Iwamoto H, Gao J, Koskela J, Kinnula V,
Kobayashi H, Laitinen T and Mazur W: Differences in plasma and
sputum biomarkers between COPD and COPD-asthma overlap. Eur Respir
J. 43:421–429. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bozinovski S, Hutchinson A, Thompson M,
Macgregor L, Black J, Giannakis E, Karlsson AS, Silvestrini R,
Smallwood D, Vlahos R, et al: Serum amyloid a is a biomarker of
acute exacerbations of chronic obstructive pulmonary disease. Am J
Respir Crit Care Med. 177:269–278. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ferhani N, Letuve S, Kozhich A, Thibaudeau
O, Grandsaigne M, Maret M, Dombret MC, Sims GP, Kolbeck R, Coyle
AJ, et al: Expression of high-mobility group box 1 and of receptor
for advanced glycation end products in chronic obstructive
pulmonary disease. Am J Respir Crit Care Med. 181:917–927. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yerkovich ST, Chang AB, Carroll ML, Petsky
HL, Scrivener G and Upham JW: Soluble receptor for advanced
glycation end products (sRAGE) is present at high concentrations in
the lungs of children and varies with age and the pattern of lung
inflammation. Respirology. 17:841–846. 2012. View Article : Google Scholar : PubMed/NCBI
|