Introduction
Bladder cancer is the most frequent malignancy of
the urinary tract and the seventh most common cancer worldwide
(1,2). There are ~429,800 new cases and
165,100 mortalities associated with bladder cancer every year
worldwide (3). The morbidity of
bladder cancer is higher in developed countries than in developing
countries, due to the aging population, and the increase in
occupational chemical exposure and the use of cigarettes (4,5).
Despite advancements in current therapeutic treatments, the
clinical outcome of patients with bladder cancer remains poor. The
5-year overall survival rate for patients with non-muscle invasive
bladder cancer is ~90%, however, it is ~60% for patients with
muscle-invasive bladder cancer (6,7). A
major issue associated with bladder cancer therapy is that
following conventional treatments including surgical resection,
chemotherapy and radiotherapy, a large proportion of patients
suffer recurrence and metastasis, however, there have been a few
advances in clinical practice (8).
Therefore, it is important to elucidate the mechanisms underlying
bladder cancer initiation and progression, as they may provide
novel therapeutic targets for the treatments of bladder cancer.
MicroRNAs (miRNAs or miRs) are a large class of
small, endogenous, non-coding and single-stranded RNA molecules of
~22 nucleotides (9). Based on
miRBase version 21, released in June 2014 (http://www.mirbase.org/), 1,881 miRNA precursors and
2,588 mature miRNAs have been identified in the human genome.
Mature miRNAs negatively regulate gene expression through imperfect
complementary sequence pairing to the 3′-untranslated regions
(3′UTRs) of their target genes, subsequently promoting
translational inhibition or mRNA degradation, which results in
moderate protein expression levels (9,10).
Functionally, miRNAs are implicated in a range of biological
processes, including cell proliferation and cycling, apoptosis,
differentiation, generation and metastasis (11). In addition, abnormal expression of
miRNAs has been frequently observed in a number of different types
of human cancer, including bladder (12), prostate (13), gastric (14) and colorectal cancers (15). Previous studies have demonstrated
that miRNAs can act as tumor suppressors or oncogenes in human
cancers, and are therefore potential therapeutic targets for cancer
diagnosis, treatment and prognosis (16–18).
miR-539 has been studied in multiple different types
of human cancers (19–21). However, there is currently no
information available concerning miR-539 in bladder cancer. In the
present study, the expression levels of miR-539 were determined in
bladder cancer tissues and cell lines. In vitro functional
assays were performed to investigate the effects of miR-539 on
bladder cancer cell proliferation and invasion. In addition, the
molecular mechanism underlying the effects of miR-539 on cell
proliferation and invasion was also evaluated.
Materials and methods
Clinical specimens
The present study was approved by the Medical Ethics
Committee of the Affiliated Hospital of Guizhou Medical University
(Guizhou, China). Informed written consent was also obtained from
all subjects. All experimental protocols were carried out in
accordance with the approved guidelines (22). Bladder cancer tissues (n=49) and
matched adjacent normal bladder tissues (n=49) were obtained from
patients (n=49; age range, 46–78 years; Table I) who underwent surgery in the
Affiliated Hospital of Guizhou Medical University from May 2012 to
March 2014. All tissues were freshly frozen in liquid nitrogen and
stored at −80°C until required.
 | Table I.Associations between miR-539
expression and clinicopathological features in patients with
bladder cancer. |
Table I.
Associations between miR-539
expression and clinicopathological features in patients with
bladder cancer.
|
|
| miR-539
expression |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
feature | Number of cases
(n) | Low (n) | High (n) | P-value |
|---|
| Sex |
|
|
| 0.079 |
|
Male | 33 | 16 | 17 |
|
|
Female | 16 | 12 | 4 |
|
| Age (years) |
|
|
| 0.620 |
|
<60 | 23 | 14 | 9 |
|
|
≥60 | 26 | 14 | 12 |
|
| Tumor number |
|
|
| 0.665 |
|
Single | 32 | 19 | 13 |
|
|
Multiple | 17 | 9 | 8 |
|
| Tumor grade |
|
|
| 0.804 |
|
I–II | 22 | 13 | 9 |
|
|
III | 27 | 15 | 12 |
|
| Tumor stage |
|
|
| 0.013a |
|
T1-T2 | 25 | 10 | 15 |
|
|
T3-T4 | 24 | 18 | 6 |
|
| Lymph node
metastasis |
|
|
| 0.017a |
|
Positive | 26 | 19 | 7 |
|
|
Negative | 23 | 9 | 14 |
|
Cell lines and cell culture
Bladder cancer cell lines (T24, 5637 and TCCSUP) and
the normal bladder epithelial cell line (SV-HUC-1) were purchased
from the Shanghai Institute of Biochemistry and Cell Biology,
Chinese Academy of Sciences (Shanghai, China). All cells were grown
in Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) containing 10% heat-inactivated
fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.),
100 U/ml penicillin G and 100 mg/ml streptomycin in a 5%
CO2 incubator at 37°C. Cell passage was performed once
the cell density had reached 90%.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) assay
Total RNA was isolated from all tissues and cells
(1×106) using TRIzol® reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
instructions. The One Step SYBR® PrimeScript™ miRNA
RT-PCR kit (Takara Biotechnology Co., Ltd., Dalian, China) was used
to analyze the levels of miR-539 expression, according to the
manufacturer's instructions. The thermocycling conditions were as
follows: 42°C for 5 min, 95°C for 10 sec, followed by 40 cycles of
95°C for 5 sec, 55°C for 30 sec and 72°C for 30 sec. To quantify
insulin like growth factor 1 receptor (IGF-1R) mRNA expression,
reverse transcription was performed using the Moloney Murine
Leukemia Virus Reverse Transcription system (Promega Corporation,
Madison, WI, USA), followed by qPCR using the SYBR Green I mix
(Takara Biotechnology Co., Ltd.), according to the manufacturer's
instructions. The thermocycling conditions were as follows: 95°C
for 10 min, followed by 40 cycles of 95°C for 15 sec and 60°C for 1
min. U6 and GADPH were used as reference genes for miR-539 and
IGF-1R, respectively. The following primers were used: miR-539,
forward, 5′-GAAGAGGCTAACGTGAGGTTG-3′ and reverse,
5′-CACCATGACCAAGCCACGTAG-3′; U6, forward,
5′-CTCGCTTCGGCAGCACATATACT-3′ and reverse,
5′-ACGCTTCACGAATTTGCGTGTC-3′; IGF-1R, forward,
5′-GGCATACCTCAACGCCAATA-3′ and reverse, 5′-CAGCCCTTTCCCTCCTTT-3′;
GAPDH, forward, 5′-ATAGCACAGCCTGGATAGCAACGTAC-3′ and reverse,
5′-CACCTTCTACAATGAGCTGCGTGTG-3′. Each sample was analyzed in
triplicate and experiments were repeated three times. The relative
expression was analyzed using the 2−ΔΔCq method
(23).
Oligonucleotide transfection
miR-539 mimics, the corresponding negative control
mimics (miR-NC), small interfering RNA of IGF-1R (si-IGF-1R) and
the corresponding negative controls (si-NC) were obtained from
Shanghai GenePharma Co., Ltd., Shanghai, China. The sequences were
as follows: miR-539 mimics, 5′-GGAGAAAUUAUCCUUGGUGUGU-3′; miR-NC,
5′-UUCUCCGAACGUGUCACGUTT-3′; si-IGF-1R, 5′-CAACGGCCTATTGTCAGGT-3′;
and si-NC, 5′-UUCUCCGAACGUGUCACGUTT-3′. For functional assays, T24
cells were seeded into 6-well plates at a density of 60–70%
confluency. Cells were then transfected with miR-539 mimics (100
pmol), miR-NC (100 pmol), si-IGF-1R (100 pmol) or si-NC (100 pmol)
using Lipofectamine 2000 reagent (Thermo Fisher Scientific, Inc.)
following to the manufacturer's instructions. A MTT assay was
performed at 24 h post-transfection, and RT-qPCR and cell invasion
assays were performed at 48 h post-transfection. Following 72 h
after transfection, western blot analysis was used to detect
protein expression.
MTT assay
Cell proliferation was determined by performing an
MTT assay (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Briefly,
transfected cells were re-seeded in 96-well culture plates at a
density of 1×103 cells/well. Cells were then incubated
at 37°C for 24, 48, 72 or 96 h. At each time point, 10 µl MTT (5
mg/ml) was added into each well and incubated for an additional 4
h. The solution containing the MTT regent was then carefully
removed and replaced with 150 µl DMSO (Sigma-Aldrich; Merck KGaA).
The optical density was determined at a wavelength of 490 nm using
an enzyme-linked immunosorbent assay reader (Elx800; Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Cell invasion assay
Matrigel (BD Biosciences, San Jose, CA, USA) coated
Transwell chambers (8 µm; Costar; Thermo Fisher Scientific, Inc.)
were used to perform cell invasion assay. Briefly, 5×104
transfected cells were cultured in DMEM with 2% FBS (Gibco; Thermo
Fisher Scientific, Inc.) and re-seeded into the top chambers, while
the lower chambers were filled with DMEM containing 20% FBS (Gibco;
Thermo Fisher Scientific, Inc.) as a chemoattractant. Following 48
h of incubation at 37°C, cells that had not crossed over the
Matrigel were removed carefully using cotton swabs. The invaded
cells were fixed with 100% methanol (Beyotime Institute of
Biotechnology, Haimen, China) for 10 min at room temperature,
stained with 0.5% crystal violet (Beyotime Institute of
Biotechnology) for 10 min at room temperature, washed in PBS
(Gibco; Thermo Fisher Scientific, Inc.), photographed and then
counted using an Olympus fluorescence microscope (Olympus
Corporation, Tokyo, Japan). Five visual fields of each membrane
were counted for every Transwell chamber (original magnification,
×100).
Bioinformatics analysis
TargetScan (version 7.1; www.targetscan.org/) and miRanda (www.microrna.org/microrna/) were used to analyze
the potential targets of miR-539.
Luciferase reporter assay
Luciferase reporter vectors, psiCHECK-IGF-1R-3′UTR
wile type (WT) and psiCHECK-IGF-1R-3′UTR mutant (MUT), were
synthesized and confirmed by Shanghai GenePharma Co., Ltd. For the
luciferase reporter assay, psiCHECK-IGF-1R-3′UTR WT or
psiCHECK-IGF-1R-3′UTR MUT were co-transfected with miR-539 mimics
or miR-NC into HEK293T cells (1×105 cells/well)
(Shanghai Institute of Biochemistry and Cell Biology) using
Lipofectamine 2000 reagent (Thermo Fisher Scientific, Inc.).
Following 48 h incubation at 37°C, cells were collected and
luciferase activities were determined using the
Dual-Luciferase® Reporter Assay system (Promega
Corporation) in accordance with the manufacturer's instructions.
Firefly luciferase activity was normalized to Renilla
luciferase activity. Each assay was performed in triplicate.
Western blot analysis
Total protein was extracted from transfected cells
(6-well plates; 1×106 cells/well) using ice-cold
radioimmunoprecipitation lysis buffer (Beyotime Institute of
Biotechnology). The concentration of total proteins was quantified
using a Bicinchoninic Acid Protein Assay kit (Pierce; Thermo Fisher
Scientific, Inc.). Equal proteins (20 µg) were separated by 10%
SDS-PAGE and transferred onto polyvinylidene difluoride (PVDF)
membranes (EMD Millipore, Billerica, MA, USA). The PVDF membranes
were blocked in 5% skim milk containing Tris-buffered saline with
0.05% Tween-20 (TBST) for 1 h at room temperature. Membranes were
then incubated overnight at 4°C with the following antibodies:
Mouse anti-human monoclonal IGF-1R (cat. no. sc-81464; 1:1,000
dilution; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), mouse
anti-human monoclonal extracellular signal-regulated kinases (ERK;
cat. no. sc-514302; 1:1,000 dilution; Santa Cruz Biotechnology,
Inc.), mouse anti-human monoclonal phosphorylated (p)-ERK (cat. no.
sc-81492; 1:1,000 dilution; Santa Cruz Biotechnology, Inc.), rabbit
anti-human polyclonal protein kinase B (AKT; cat. no. sc-8312;
1:1,000 dilution; Santa Cruz Biotechnology, Inc.), mouse anti-human
monoclonal p-AKT (cat. no. sc-514032; 1:1,000 dilution; Santa Cruz
Biotechnology, Inc.) and mouse anti-human monoclonal GADPH (cat.
no. sc-166574; 1:1,000 dilution; Santa Cruz Biotechnology, Inc.).
Following 3 washes with TBST every 10 min, the membranes were
probed with the corresponding horseradish peroxidase-conjugated
secondary antibody (cat. nos. sc-2004 for AKT reactions and sc-2005
for the remaining primary antibodies; 1:5,000 dilution; Santa Cruz
Biotechnology, Inc.) for 1 h at room temperature and washed again
three times with TBST every 10 min. Visualization was performed
using an enhanced chemiluminescence solution (Pierce; Thermo Fisher
Scientific, Inc.). ImageJ v1.49 (National Institutes of Health,
Bethesda, MD, USA) was used to perform densitometry. GADPH was used
as an internal control and three experimental repeats were
performed.
Statistical analysis
All data are expressed as the mean ± standard
deviation. Analysis was performed with SPSS software (version 13.0;
SPSS Inc., Chicago, IL, USA). A paired Student's t-test and
Pearson's Χ2 test were applied. P<0.05 was considered
to indicate a statistically significant difference.
Results
miR-539 is poorly expressed in
clinical bladder cancer tissues and cell lines
To explore the biological roles of miR-539 in
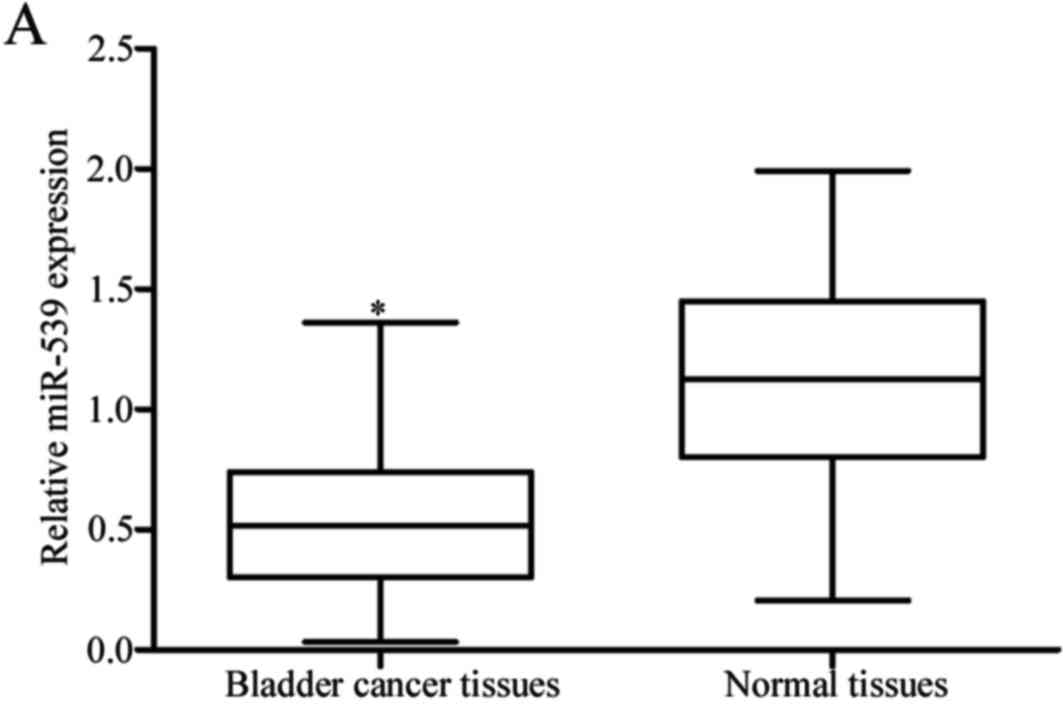
bladder cancer, miR-539 expression was measured in bladder cancer
tissues using RT-qPCR. The results revealed that the expression
levels of miR-539 were reduced in bladder cancer tissues when
compared with the matched adjacent normal bladder tissues
(P<0.05; Fig. 1A). The
expression of miR-539 was further determined in the bladder cancer
cell lines T24, 5637 and TCCSUP), and the normal bladder epithelial
cell line SV-HUC-1. As observed in the collected tissues, miR-539
was significantly downregulated in all 3 bladder cancer cell lines
when compared with the SV-HUC-1 cell line (P<0.05; Fig. 1B).
Association between miR-539 expression
and clinicopathological features of bladder cancer
The associations between miR-539 expression and the
clinicopathological features of bladder cancer were then evaluated.
Statistical analysis demonstrated that the expression levels of
miR-539 were significantly associated with tumor stage (P=0.013)
and lymph node metastasis (P=0.017; Table I). However, there were no
significant associations with sex, age, tumor number or tumor grade
(all P>0.05; Table I).
miR-539 represses cell proliferation
and invasion in bladder cancer
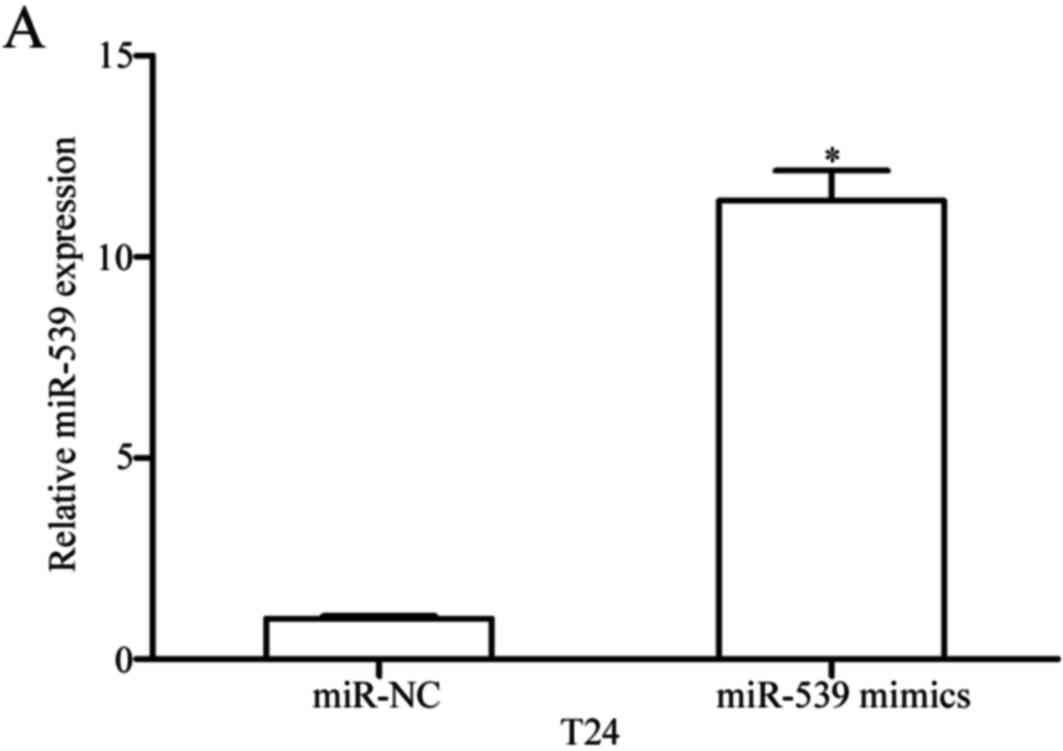
To examine the roles of miR-539 in bladder cancer,
miR-539 mimics were transfected into T24 cells to increase miR-539
expression (P<0.05; Fig. 2A).
The effect of miR-539 overexpression on cell proliferation was
investigated using an MTT assay. As demonstrated in Fig. 2B, transfection of miR-539 mimics
significantly inhibited T24 cell proliferation following 96 h
(P<0.05). The cell invasive capacity was evaluated by performing
a cell invasion assay. The results revealed that upregulation of
miR-539 decreased the invasive ability of T24 cells (P<0.05;
Fig. 2C). These results suggested
that miR-539 inhibits cell proliferation and invasion in bladder
cancer.
IGF-1R is a direct target of miR-539
in bladder cancer
Bioinformatics analysis was used to predict the
potential targets of miR-539. Among these candidate target genes,
the 3′UTR of the IGF-1R gene contains a putative region that
matches the seed sequence of miR-539 (Fig. 3A). IGF-1R was subsequently chosen
for further analysis as it has previously been reported to be
upregulated in bladder cancer (24) and be involved in tumorigenesis and
the progression of bladder cancer (25–28).
To confirm our hypothesis, a luciferase reporter assay was
performed in HEK293T cells co-transfected with miR-539 mimics or
miR-NC, and psiCHECK-IGF-1R-3′UTR WT or psiCHECK-IGF-1R-3′UTR MUT.
As shown in Fig. 3B, miR-539
expression significantly decreased luciferase activities in the
vector with the wild-type construct (P<0.05), however, not in
the mutant IGF-1R 3′UTR construct. IGF-1R mRNA and protein
expression were then assessed in T24 cells transfected with miR-539
mimics or miR-NC. As presented in Fig.
3C and D, miR-539 mimic transfection significantly suppressed
IGF-1R mRNA and protein expression (P<0.05). These findings
indicated that IGF-1R may be a direct target gene of miR-539.
miR-539 represses the AKT and ERK
signaling pathways
Previous studies have demonstrated that IGF-1R
serves important roles in biological processes associated with the
downstream phosphoinositide 3-kinase (PI3K)/AKT and
mitogen-activated protein kinase (MAPK)/ERK signaling pathways
(29,30). The present study further determined
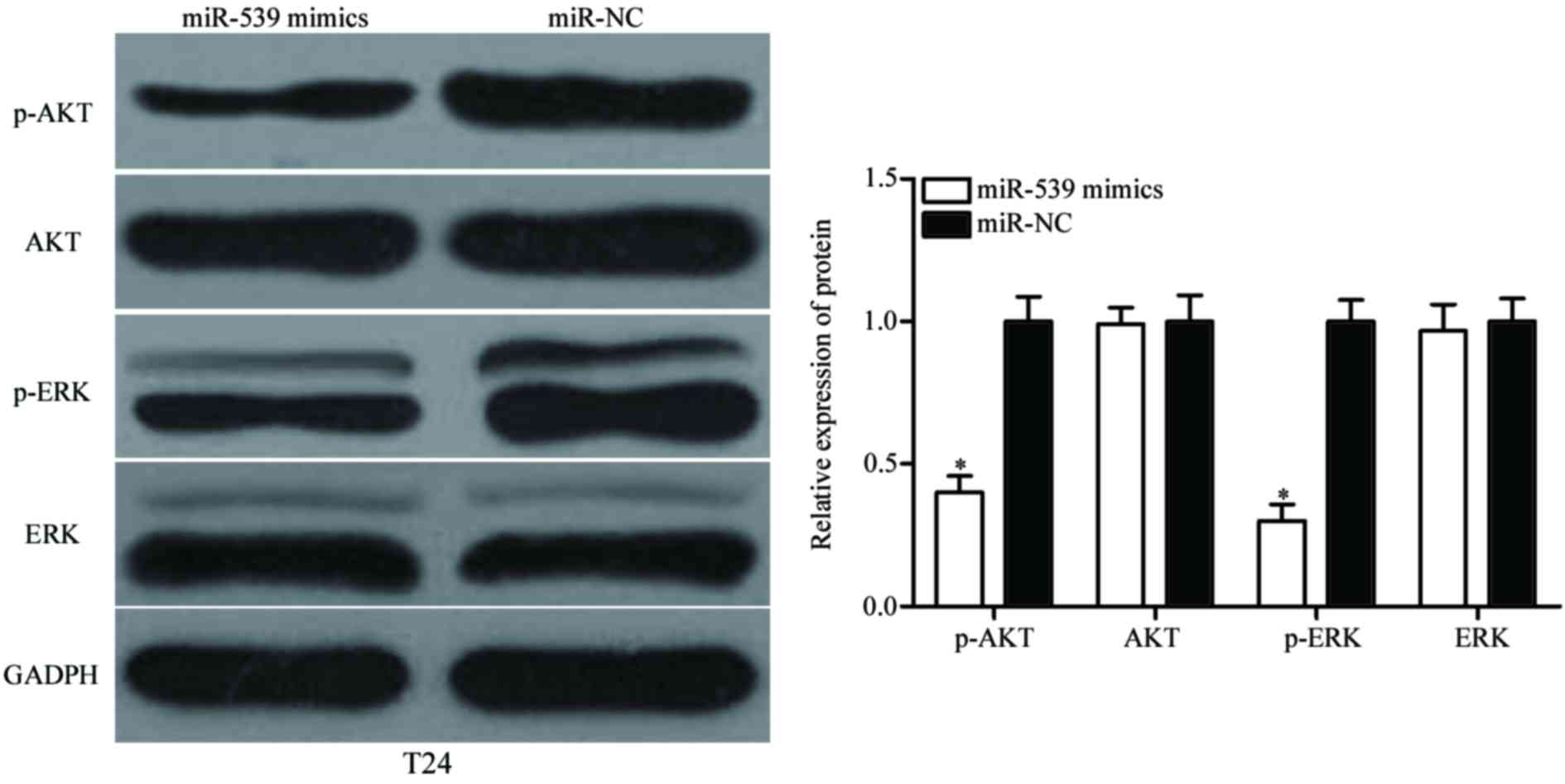
whether miR-539 is involved in the AKT and EKR signaling pathways.
Western blotting was performed to detect ERK, p-ERK, AKT and p-AKT
expression in T24 cells following transfection with miR-539 mimics
or miR-NC. As shown in Fig. 4,
p-ERK and p-AKT were downregulated in miR-539 mimics-transfected
T24 cells (P<0.05), however, miR-539 expression did not affect
ERK and AKT expression. These results suggested that miR-539 may
inhibit bladder cancer cell proliferation and invasion by
activating the AKT and ERK signaling pathways.
Inverse correlation between miR-539
and IGF-1R in clinical bladder cancer tissues
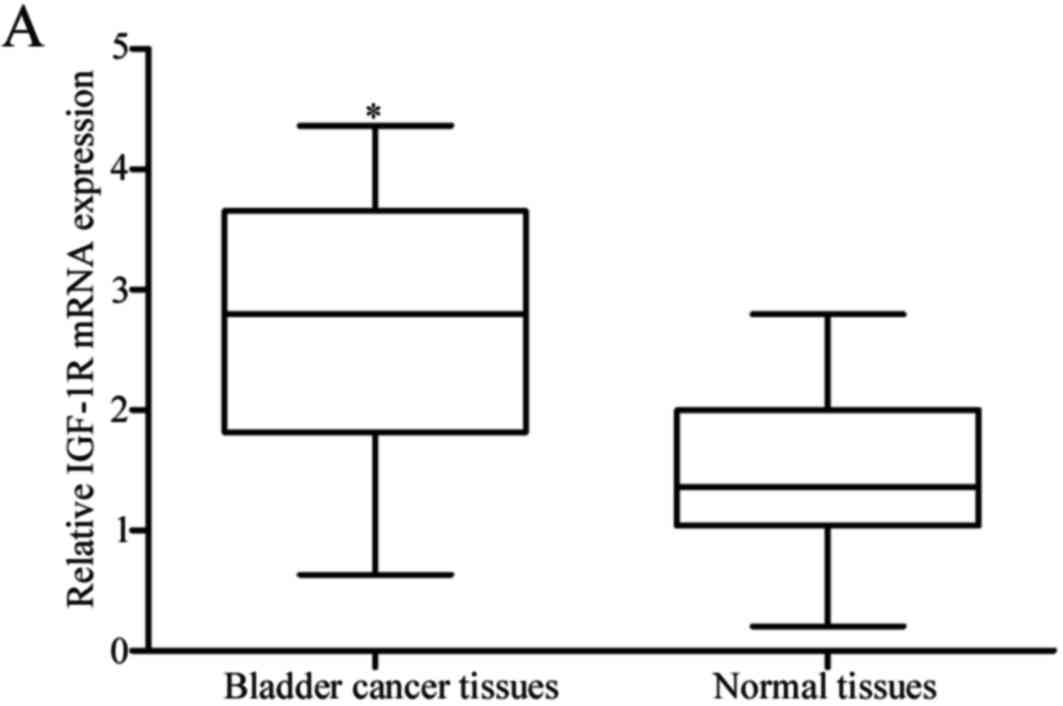
To further elucidate the correlation between miR-539
and IGF-1R, the expression levels of IGF-1R mRNA and protein were
detected in bladder cancer tissues and matched adjacent normal
bladder tissues. The results revealed that IGF-1R mRNA and protein
were significantly elevated in bladder cancer tissues in comparison
to those observed in adjacent normal bladder tissues (P<0.05;
Fig. 5A and B). In addition,
IGF-1R mRNA expression was negatively correlated with the level of
miR-539 expression in bladder cancer tissues (r=−0.5838,
P<0.001; Fig. 5C).
miR-539 suppresses cell proliferation
and invasion of bladder cancer by regulating IGF-1R
As IGF-1R is a direct target of miR-539, it was
hypothesized that miR-539 may have tumor suppressive roles in
bladder cancer via the regulation of its target, IGF-1R. To confirm
this, si-IGF-1R was used to knockdown IGF-1R expression in T24
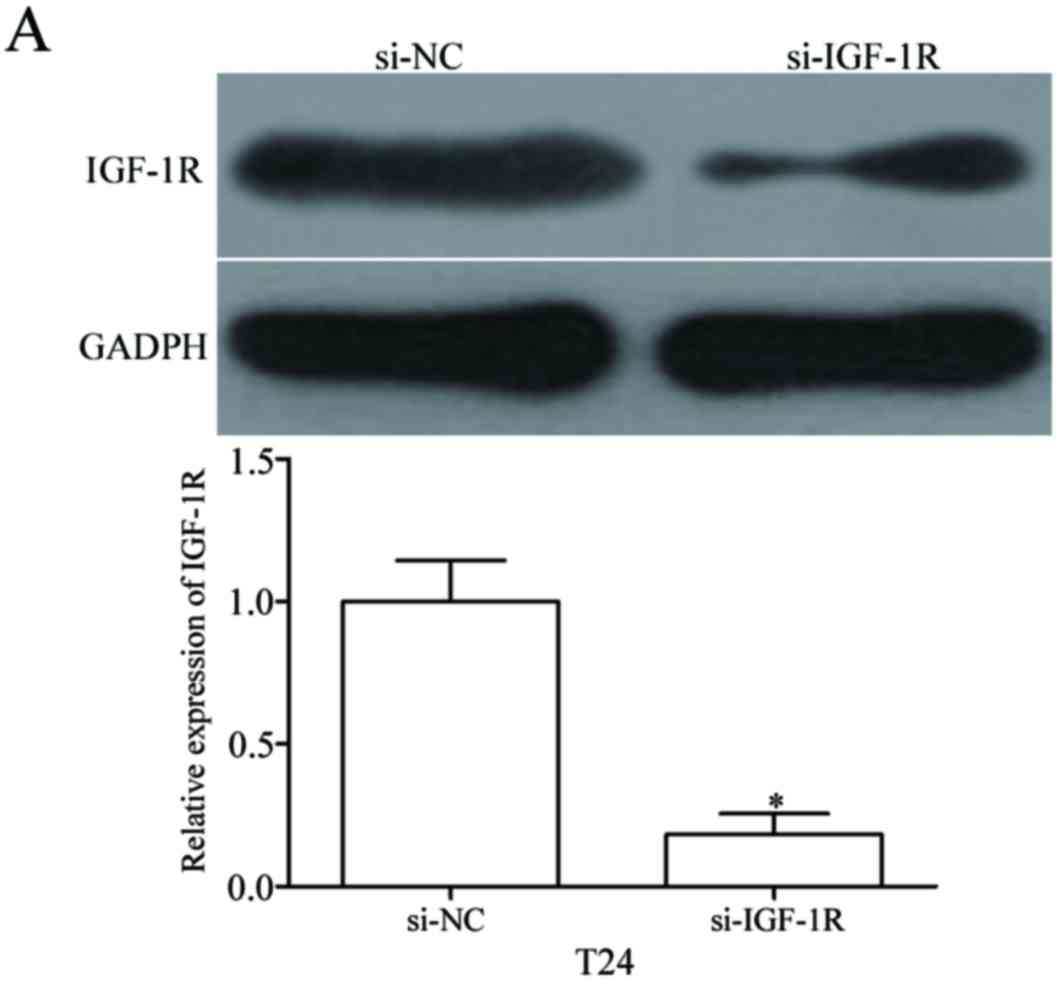
cells (P<0.05; Fig. 6A). The
effects of IGF-1R knockdown on cell proliferation and invasion were
evaluated using MTT and cell invasion assays, respectively. As
shown in Fig. 6B and C, IGF-1R
knockdown markedly suppressed the proliferation and invasion of T24
cells (P<0.05). These results indicated that the underexpression
of IGF-1R induced by miR-539 may contribute, at least in part, to
the suppression of bladder cancer cell proliferation and
invasion.
Discussion
Aberrant expression of miR-539 has been detected in
a number of different types of human cancers. For example, in
osteosarcoma, there is a low level of miR-539 expression in MG-63
cells when compared with osteoblast cells (31). Jin and Wang (32) demonstrated that miR-539 was
downregulated in osteosarcoma tissues and cell lines. In addition,
a previous study by Mirghasemi et al (19) revealed that low miR-539 expression
was correlated with advanced Tumor, Node and Metastasis staging,
and metastasis or recurrence in patients with osteosarcoma.
Kaplan-Meier survival analysis and a log-rank test revealed that a
low expression of miR-539 was significantly associated with a
reduction in the overall survival rate of patients with
osteosarcoma. The Multivariate Cox proportional hazards model
demonstrated that decreased miR-539 expression was an independent
prognostic marker of overall survival in patients with osteosarcoma
(19). Gu and Sun (20) reported that miR-539 expression
levels were reduced in thyroid cancer tissues and cell lines when
compared with the respective control. In addition, in
nasopharyngeal carcinoma, miR-539 levels decreased in tumor tissues
when compared with normal tissues (21). These findings suggested that
miR-539 may be an effective diagnostic and prognostic marker in
these types of cancer.
Dysregulation of miR-539 is thought to contribute to
the malignant phenotype of several types of tumor. In thyroid
cancer, enforced expression of miR-539 suppressed cell migration
and invasion by negatively regulating caspase recruitment
domain-membrane-associated guanylate kinase protein 1 (20). In osteosarcoma, upregulation of
miR-539 decreased cell growth and metastasis by directly targeting
matrix metalloproteinase 8 (32).
Lv et al (21) demonstrated
that ectopic expression of miR-539 repressed cell growth in
vitro and in vivo via a blockade of cyclin-dependent
kinase 4. Zhang et al (33)
reported that, in prostate cancer, miR-539 targeted sperm
associated antigen 5 to inhibit cell proliferation, migration and
invasion in vitro, and suppress tumor growth and metastasis
in vivo. These findings indicated that miR-539 may act as a
novel therapeutic target for the treatment of these types of
cancer.
The present study revealed that miR-539
re-expression inhibited cell proliferation and invasion in bladder
cancer. Subsequently, the potential molecular mechanism underlying
the miR-539-induced inhibition of bladder cancer cell proliferation
and invasion was determined. An important molecular association
between miR-539 and IGF-1R was observed in bladder cancer.
Initially, bioinformatics analysis predicted that IGF-1R contained
a miR-539 seed match at the 3′UTR of IGF-1R. The luciferase
reporter assay further demonstrated that miR-539 directly targeted
the IGF-1R 3′UTR. RT-qPCR and western blot analysis was then
performed and revealed that miR-539 negatively regulated IGF-1R
expression in bladder cancer cells at the mRNA and protein level.
IGF-1R mRNA was significantly upregulated in bladder cancer tissues
and was negatively correlated with miR-539 level. In addition,
IGF-1R knockdown suppressed cell proliferation and invasion,
similar to the effect of miR-539 overexpression in bladder cancer
cells. Identification of miR-539 targets is essential for
understanding its role in bladder cancer formation and progression.
In addition, it is important for developing novel therapeutic
targets for the treatment of patients with bladder cancer.
IGF-1R, a transmembrane tyrosine kinase receptor of
the insulin receptor family, contains two extracellular α subunits
with a ligand-binding site and two transmembrane β subunits with
intracellular tyrosine kinase activity (34). The IGF-1R itself has been
frequently observed to be upregulated in various types of human
cancers, including bladder cancer (24,35).
In addition, IGF-1R expression in bladder cancer was significantly
correlated with tumor grade, stage and recurrence (24). Furthermore, there is a growing body
of evidence that supports the biological roles of IGF-1R in
promoting tumorigenesis and progression of bladder cancer. Sun
et al (25) reported that
downregulation of IGF-1R suppressed the growth of T24 cells,
induced apoptosis and improved cell chemosensitivity to mitomycin.
Metalli et al (26)
demonstrated that IGF-1R enhanced cell motility and invasion in
bladder cancer. These results suggested that targeting IGF-1R could
serve as a novel therapeutic method in bladder cancer.
To the best of our knowledge, this is the first
study to provide experimental evidence that miR-539 was
downregulated in bladder cancer, and its expression was associated
with tumor stage and lymph node metastasis in patients with bladder
cancer. Restoration of miR-539 expression inhibits bladder cancer
cell proliferation and invasion. In addition, IGF-1R was identified
as a direct target of miR-539 and miR-539 was observed to regulate
the AKT and ERK signaling pathways. This newly identified
miR-539/IGF-1R pathway may provide novel insights into the
initiation and progression of bladder cancer, and may serve as a
potential therapeutic target for the treatment of patients with
bladder cancer.
References
|
1
|
Burger M, Catto JW, Dalbagni G, Grossman
HB, Herr H, Karakiewicz P, Kassouf W, Kiemeney LA, La Vecchia C,
Shariat S and Lotan Y: Epidemiology and risk factors of urothelial
bladder cancer. Eur Urol. 63:234–241. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu D, Niu X, Pan H, Zhou Y, Qu P and Zhou
J: MicroRNA-335 is downregulated in bladder cancer and inhibits
cell growth, migration and invasion via targeting ROCK1. Mol Med
Rep. 13:4379–4385. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ploeg M, Aben KK and Kiemeney LA: The
present and future burden of urinary bladder cancer in the world.
World J Urol. 27:289–293. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen WQ, Zeng HM, Zheng RS, Zhang SW and
He J: Cancer incidence and mortality in china, 2007. Chin J Cancer
Res. 24:1–8. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cornu JN, Neuzillet Y, Herve JM, Yonneau
L, Botto H and Lebret T: Patterns of local recurrence after radical
cystectomy in a contemporary series of patients with
muscle-invasive bladder cancer. World J Urol. 30:821–826. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang J, Wang S, Han F, Li J, Yu L, Zhou
P, Chen Z, Xue S, Dai C and Li Q: MicroRNA-542-3p suppresses
cellular proliferation of bladder cancer cells through
post-transcriptionally regulating survivin. Gene. 579:146–152.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kaufman DS, Shipley WU and Feldman AS:
Bladder cancer. Lancet. 374:239–249. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Borel C, Deutsch S, Letourneau A,
Migliavacca E, Montgomery SB, Dimas AS, Vejnar CE, Attar H,
Gagnebin M, Gehrig C, et al: Identification of cis- and
trans-regulatory variation modulating microRNA expression levels in
human fibroblasts. Genome Res. 21:68–73. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Phuah NH and Nagoor NH: Regulation of
microRNAs by natural agents: New strategies in cancer therapies.
Biomed Res Int. 2014:8045102014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xiao J, Lin HY, Zhu YY, Zhu YP and Chen
LW: MiR-126 regulates proliferation and invasion in the bladder
cancer BLS cell line by targeting the PIK3R2-mediated PI3K/Akt
signaling pathway. Onco Targets Ther. 9:5181–5193. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yoo HI, Kim BK and Yoon SK:
MicroRNA-330-5p negatively regulates ITGA5 expression in human
colorectal cancer. Oncol Rep. 36:3023–3029. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu K, Ma L and Zhu J: miR-483-5p promotes
growth, invasion and self-renewal of gastric cancer stem cells by
Wnt/β-catenin signaling. Mol Med Rep. 14:3421–3428. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chandrasekaran KS, Sathyanarayanan A and
Karunagaran D: MicroRNA-214 suppresses growth, migration and
invasion through a novel target, high mobility group AT-hook 1, in
human cervical and colorectal cancer cells. Br J Cancer.
115:741–751. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ventura A and Jacks T: MicroRNAs and
cancer: Short RNAs go a long way. Cell. 136:586–591. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kong YW, Ferland-McCollough D, Jackson TJ
and Bushell M: microRNAs in cancer management. Lancet Oncol.
13:e249–e258. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cortés-Sempere M and Ibáñez de Cáceres I:
microRNAs as novel epigenetic biomarkers for human cancer. Clin
Transl Oncol. 13:357–362. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mirghasemi A, Taheriazam A, Karbasy SH,
Torkaman A, Shakeri M, Yahaghi E and Mokarizadeh A: Down-regulation
of miR-133a and miR-539 are associated with unfavorable prognosis
in patients suffering from osteosarcoma. Cancer Cell Int.
15:862015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gu L and Sun W: MiR-539 inhibits thyroid
cancer cell migration and invasion by directly targeting CARMA1.
Biochem Biophys Res Commun. 464:1128–1133. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lv LY, Wang YZ, Zhang Q, Zang HR and Wang
XJ: miR-539 induces cell cycle arrest in nasopharyngeal carcinoma
by targeting cyclin-dependent kinase 4. Cell Biochem Funct.
33:534–540. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Montie JE, Bahnson RR, Cohen SM, Drucker
B, Eisenberger MA, El-Galley R, Herr HW, Hudes GR, Kuzel TM, Lange
PH, et al: Bladder cancer. Clinical practice guidelines in
oncology. J Natl Compr Canc Netw. 3:4–5, 19–34. 2005.PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xie QX, Lin XC, Zhang MF, Han CX and Guo
YH: Expression of IGF-I and IGF-IR in bladder cancer. Ai Zheng.
23:707–709. 2004.(In Chinese). PubMed/NCBI
|
|
25
|
Sun HZ, Wu SF and Tu ZH: Blockage of
IGF-1R signaling sensitizes urinary bladder cancer cells to
mitomycin-mediated cytotoxicity. Cell Res. 11:107–115. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Metalli D, Lovat F, Tripodi F, Genua M, Xu
SQ, Spinelli M, Alberghina L, Vanoni M, Baffa R, Gomella LG, et al:
The insulin-like growth factor receptor I promotes motility and
invasion of bladder cancer cells through Akt- and mitogen-activated
protein kinase-dependent activation of paxillin. Am J Pathol.
176:2997–3006. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen Z, Li S, Huang K, Zhang Q, Wang J, Li
X, Hu T, Wang S, Yang R, Jia Y, et al: The nuclear protein
expression levels of SNAI1 and ZEB1 are involved in the progression
and lymph node metastasis of cervical cancer via the
epithelial-mesenchymal transition pathway. Hum Pathol.
44:2097–2105. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ran J, Lin DL, Wu RF, Chen QH, Huang HP,
Qiu NX and Quan S: ZEB1 promotes epithelial-mesenchymal transition
in cervical cancer metastasis. Fertil Steril. 103:1606–1614.e1-e2.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Osaki LH and Gama P: MAPKs and signal
transduction in the control of gastrointestinal epithelial cell
proliferation and differentiation. Int J Mol Sci. 14:10143–10161.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cao Z, Liu LZ, Dixon DA, Zheng JZ,
Chandran B and Jiang BH: Insulin-like growth factor-I induces
cyclooxygenase-2 expression via PI3K, MAPK and PKC signaling
pathways in human ovarian cancer cells. Cell Signal. 19:1542–1553.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hu H, Zhang Y, Cai XH, Huang JF and Cai L:
Changes in microRNA expression in the MG-63 osteosarcoma cell line
compared with osteoblasts. Oncol Lett. 4:1037–1042. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jin H and Wang W: MicroRNA-539 suppresses
osteosarcoma cell invasion and migration in vitro and targeting
Matrix metallopeptidase-8. Int J Clin Exp Pathol. 8:8075–8082.
2015.PubMed/NCBI
|
|
33
|
Zhang H, Li S, Yang X, Qiao B, Zhang Z and
Xu Y: miR-539 inhibits prostate cancer progression by directly
targeting SPAG5. J Exp Clin Cancer Res. 35:602016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hu Q, Gong JP, Li J, Zhong SL, Chen WX,
Zhang JY, Ma TF, Ji H, Lv MM, Zhao JH and Tang JH: Down-regulation
of miRNA-452 is associated with adriamycin-resistance in breast
cancer cells. Asian Pac J Cancer Prev. 15:5137–5142. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rochester MA, Patel N, Turney BW, Davies
DR, Roberts IS, Crew J, Protheroe A and Macaulay VM: The type 1
insulin-like growth factor receptor is over-expressed in bladder
cancer. BJU Int. 100:1396–1401. 2007. View Article : Google Scholar : PubMed/NCBI
|