Introduction
Osteoarthritis is a common and frequently occurring
disease in orthopedics (1). The
main pathological manifestations include articular cartilage
degeneration, joint edge and subchondral bone hyperosteogeny
(2). OA is one of the most serious
arthrosis diseases that affect the health and life of human beings
(3). It is more common in the
middle and old aged people over 50 years old, seriously endanger
the physical health of middle-aged and elderly people and affect
their life quality. Also, it causes heavy burdens for individuals,
families and the society (1).
miRNA is a single stranded non-coding RNA containing
19–25 molecules, commonly existing in plants, animals, and
microorganisms, and involving in regulating a variety of
physiological activities in the body (4). In 1993, the first miRNA was found in
Caenorhabditis elegans (5). miRNA
has a wide range of biological functions in the body, and can
participate in the regulation of many physiological activities,
including cell division, differentiation, apoptosis and organ
formation. The research on the function of miRNA has become a hot
topic in the field of life science. The expression of miRNA is
strictly regulated by the body, and has strict time specificity and
tissue specificity (5). Meantime,
the biological function of miRNA is also strictly regulated.
Studies have shown that many miRNAs play an important role in the
regulation of OA (6). Through gene
chip and in situ hybridization, we found that the expression
of nine miRNAs such as miRNA-483, miRNA-22, and miRNA-377 were
significantly up-regulated in OA of human knee joint, and the
expression of seven miRNAs was obviously downregulated (7).
Matrix metalloproteinases (MMPs) are synthesized by
synovial and cartilage cells (8).
They play a decisive role in the imbalance between extracellular
matrix synthesis and degradation of osteoarthritis (OA) articular
cartilage (8). Therefore, MMPs
play a key role in OA. Its pathological function is mainly tissue
degradation in OA (8). MMP-17 is
one of the MMPs gene family members (9). It has strong matrix degradation
activity and extensive subtract specificity. These substrates
include type IV collagen, laminin, fibronectin, proteoglycans, type
I gelatin and soluble elastin (9).
Insulin-like growth factor-I (IGF-I) is a
pro-synthetic cytokine (10). It
can promote cell proliferation, differentiation and migration, and
inhibit apoptosis (10). Thus, it
can regulate body metabolism, growth and development, reproduction
and immunity, and delay aging (10). Cartilage cell can secrete IGF-I in
the manners of paracrine and autocrine. In addition, it can
activate the intracellular pathway after binding with its cell
surface IGF-I receptor (11).
Moreover, it can stimulate division and proliferation, maintain
stable phenotype and suppress apoptosis of cartilage cell. Thus, it
can promote synthesis of cartilage matrix proteoglycans and
collagen (12). Meanwhile, it can
actively participate in cartilage matrix repair (11). Consequently, it can delay or
prevent articular cartilage degeneration. However, IGF-I is
regulated by insulin-like growth factor binding protein (IGFBP)
(12). Of them, insulin-like
growth factorbinding protein-5 (IGFBP-3) can bind with IGF-I
(12). In this way, it can inhibit
the affinity of IGF-I to IGF-I receptor (IGF-IR) (12,13).
Moreover, it will affect the physiological function of IGF-I and
inhibit the production of ECM proteoglycans and collagen (13).
Peroxisome proliferator-activated receptor γ (PPARγ)
can regulate various biological processes, including lipid
metabolism, fat formation, cell division and apoptosis (14). In recent years, it has been found
that ligand activated PPARγ has beneficial effects on resisting
obesity, hypertension, atherosclerosis, diabetes, cancer and other
diseases, which makes the research on the receptor function and
ligand screening of PPARγ become a hot spot in the field of
biomedicine and pharmacology (14,15).
Here, we investigated the role of miR-27a on arthritis production
and its mechanism.
Materials and methods
Experimental animals and
collagen-induced arthritis
Female Wistar rats (150–200 g, 6 weeks) was received
water and food ad libitum, and were kept at room temperature
(22–24°C) and 55–60% relative humidity in a standard
microbiological regime in a dark-light cycle. All rats were
randomly distributed into two groups: Control and arthritis model
groups. In arthritis model group, rats were subcutaneously injected
with 200 µg bovine collagen type II from the tail base with 1:1
(v/v) and Incomplete Adjuvant (IFA) (200 µl/one week;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 3 week.
After collagen-induced arthritis, rat was sacrificed
under anesthesia and arthritis were acquired and fixed with 4%
paraformaldehyde for 72 h. Then samples were quickly dissected and
cut into frozen sections (5 µm). Samples was stained using H&E
sassy for 15 min and observed by microtome (Leica CM1900 UV; Leica,
Solms, Germany). Clinical signs was scored as follows: 0 score: no
change; 0–5 scores: redness and swelling of the ankle; 6–10 scores:
redness and swelling in the paw; 11–20 scores: inflammation of the
hind paw multiple joints involved, and/or deformation of joints
with function impairment.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from serum of rat with
arthritis using a total RNA extraction kit (Omega, Norcross, GA,
USA). cDNA was composed using total RNA using a High Capacity cDNA
Reverse Transcription kit (Thermo Fisher Scientific, Waltham, MA,
USA). miR-27a expression was amplified using SensiFAST SYBR-Green
Master mix (Bioline, London, UK) by an ABI7900HT machine, under the
following cycling conditions: polymerase activation for 10 min at
95°C followed by 40 cycles at 95°C for 30 sec and 60°C for 30 sec.
miR-27a: primers, 5′-GGCTTAGCTGCTTGTGAGCA-3′; reverse,
5′-GCGGAACTTAGCCACTGTGA-3′. The relative expression of the target
gene was calculated as 2−ΔΔCq.
Cell culture and cell
transfection
A bone marrow-derived human MSC (hMSC) line
(BM025SS13) were cultured in Dulbecco's modifed Eagle's medium
supplemented with 10% FBS (both from Gibco-BRL; Thermo Fisher
Scientific) and antibiotics (100 U/ml of penicillin G and 100 µg/ml
of streptomycin) at 37°C in a humidified atmosphere of 5%
CO2. miR-27a, anti-miR-27a and negative were synthesized
by Shanghai GenePharma Co., Ltd. (Shanghai, China). The cells were
then transfected with miR-27a mimics, anti-miR-27a mimics and
negative mimics using Lipofectamine™ 2000 (Invitrogen; Thermo
Fisher Scientific). After transfection for 24 h, cell was cultured
with 2 ml of fresh DMEM supplemented with TNF-α (20 ng/ml;
Invitrogen; Thermo Fisher Scientific).
Enzyme-linked immunosorbent assay
(ELISA)
Cell was collected at 500 g for 10 min, proteins
were extracted using RIPA buffer, and protein concentrations were
determined using BCA Protein assay kit (P0009; Beyotime Institute
of Biotechnology, Haimen, China). Proteins of 5 µg were used to
analyze IL-1β (PI303) and IL-6 (PI328) level using ELISA kit
(Beyotime Institute of Biotechnology).
MTT assay
After transfection for 24, 48 and 72 h, the MTT
solution (20 µl, 5 mg/ml) was added to each well for 4 h at 37°C.
In the last day, old medium was removed and 150 µl DMSO was added
to dissolve purple crystals for 20 min at 37°C. Absorbance was
analyzed by Bio-Plex Suspension array (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) at 492 nm.
Flow cytometry
After induction of arthritis for 48 h, cell were
washed twice with PBS and stained with 5 µl Annexin V-fluorescein
isothiocyanate and 5 µl propidium iodide (BD Biosciences, Franklin
Lakes, NJ, USA) for 15 min at darkness. Apoptosis rate was analyzed
using flow cytometry (BD C6 flow cytometer; BD Biosciences).
Western blot analysis
Cell was collected at 500 × g for 10 min, proteins
were extracted using RIPA buffer, and protein concentrations were
determined using BCA Protein assay kit (Beyotime, Beijing, China).
Proteins of 50 µg were loaded onto 8–12% sodium dodecyl
sulfate-polyacrylamide gels (SDS-PAGE) and transferred to
polyvinylidene difluoride (PVDF) membranes. Membranes were
incubated with the primary antibodies overnight at 4°C: IGFBP-5,
PPARγ, MMP-17 and GAPDH (both from Santa Cruz Biotechnology,
Dallas, TX, USA) followed by incubation with horseradish
peroxidase-conjugated rabbit anti-rabbit IgG (Santa Cruz
Biotechnology) at 37°C for 1 h. Protein blank was examined using a
chemiluminescence detection kit (Amersham Pharmacia Biotech,
Piscataway, NJ, USA).
Statistical analysis
All quantified results are presented as the mean ±
SD of at least three experiments. The differences among multiple
groups with normal distribution were evaluated using one-way ANOVA
with a Tukey's post-hoc test. Differences were regarded as
statistically significant when P<0.05.
Results
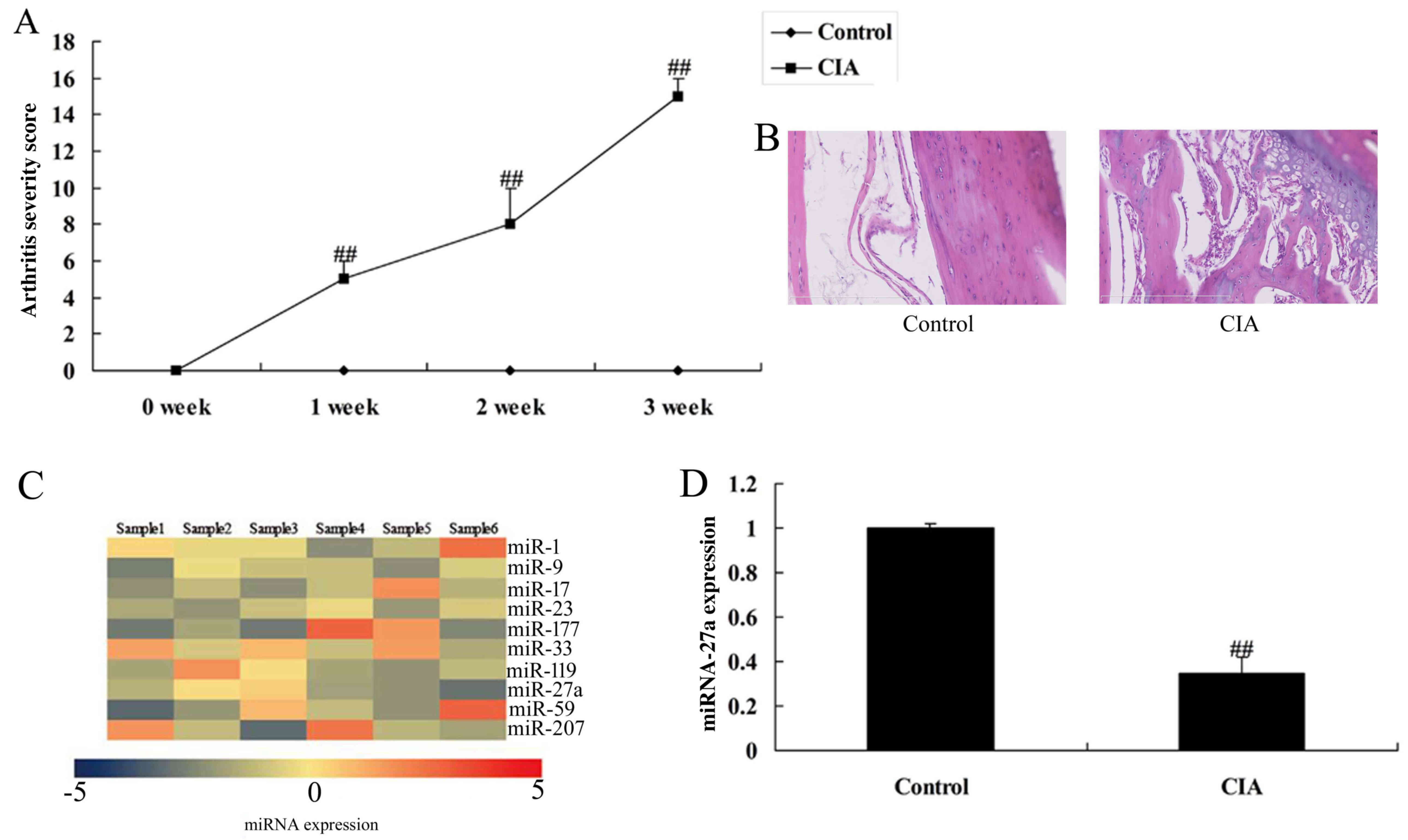
miR-27a expression of rate with
arthritis
RT-qPCR was used to analyze miR-27a expression in
rate with arthritis and to verify the microarray analysis. There
was a significant increases of clinical signs in collagen-induced
arthritis, compared with control group (Fig. 1A). Fig. 1B showed that there was some cavity
in collagen-induced arthritis, compared with control group. As
showed in Fig. 1C and D, miR-27a
expression of arthritis in rat with arthritis was reduced, compared
with control normal group, which showed that miR-27a maybe
correlated with arthritis happen.
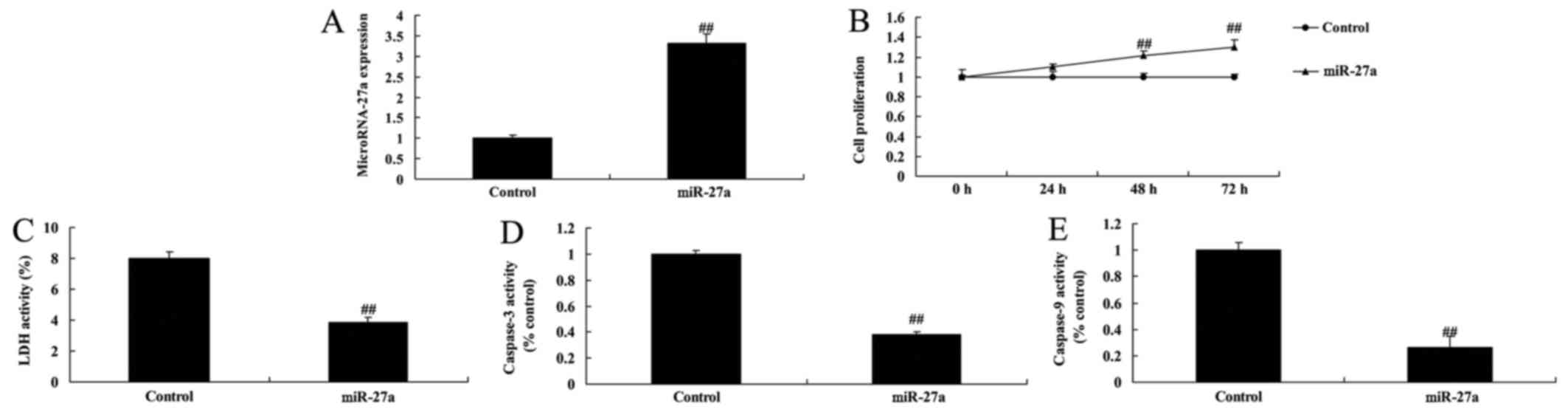
miR-27a overexpression increased cell
proliferation of osteoblast-like cell in vitro model of
arthritis
We transfected human osteoblast-like cell MG-63 cell
with miR-27a mimics to detect the interaction between miR-27a and
bone cell proliferation of in vitro model of arthritis.
miR-27a mimics increased significantly increased miR-27a
expression, increased cell proliferation, and reduced LDH activity
and caspase-3/9 activity of osteoblast-like cell in vitro
model of arthritis (Fig. 2). So,
this study showed that miR-27a promoted bone cell growth and
inhibited cell apoptosis in arthritis, and this may be a way for
treatment of arthritis.
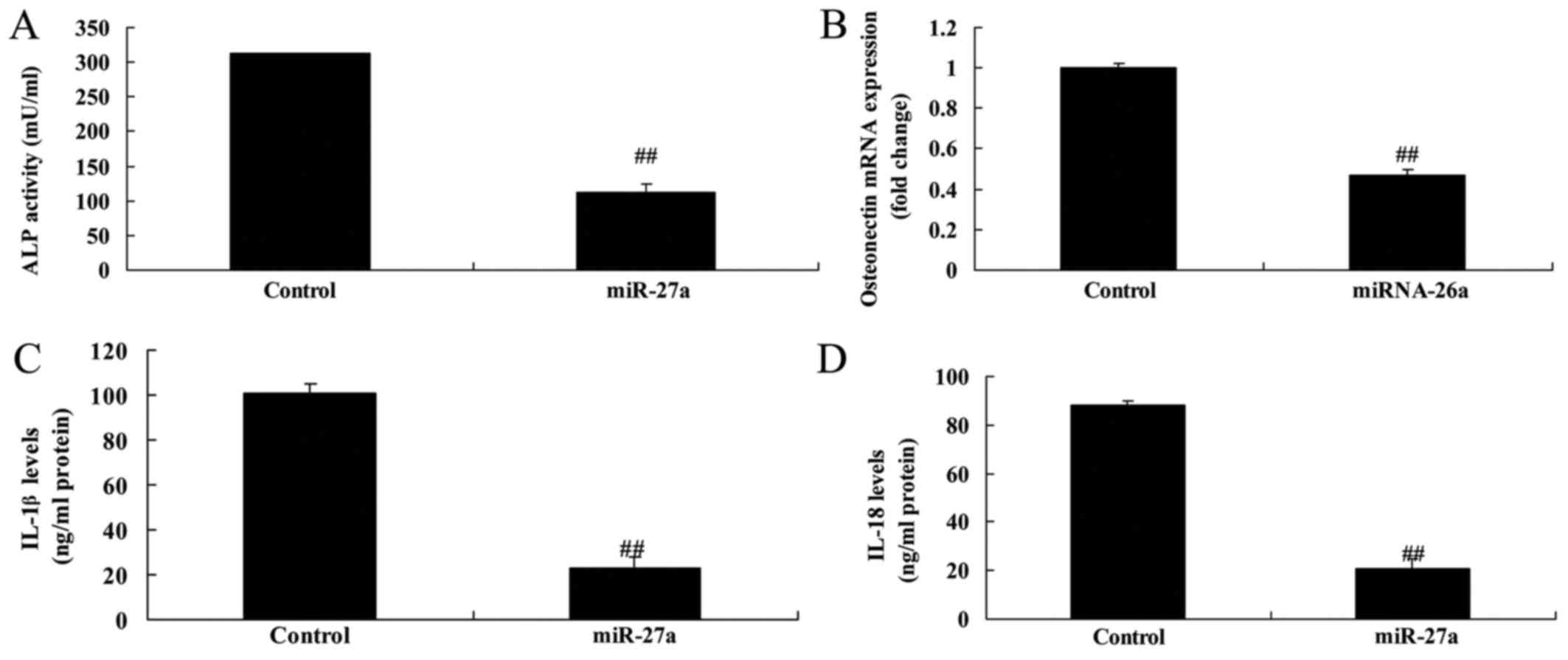
miR-27a overexpression promoted
osteogenic differentiation and inhibited inflammation
Next, we detected osteogenic differentiation and
inflammation by miR-27a overexpression. In addition, significantly
promotion of osteogenic differentiation and alkaline phosphatase
(ALP) and osteoporosis (OST) content, and inflammation in
vitro model of arthritis by miR-27a overexpression (Fig. 3). These results showed that miR-27a
overexpression promoted osteogenic differentiation, increased cell
proliferation and inhibited inflammation.
miR-27a overexpression affects
IGFBP-5, PPARγ and MMP-17 protein expression
We also examined the mechanism of miR-27a on
arthritis, IGFBP-5, PPARγ and MMP-17 protein expression were
analyze using western blot analysis. Fig. 4A showed that putative miR-27a
binding sites predicted by TargetScan in 3′-UTR of PPARγ gene. The
result of western blotting showed that IGFBP-5 protein expression
was induced, and PPARγ and MMP-17 protein expression was suppressed
in vitro model of arthritis by miR-27a overexpression
(Fig. 4B-F). miR-27a regulates
arthritis through PPARγ/MMP-17 signaling pathway by IGFBP-5.
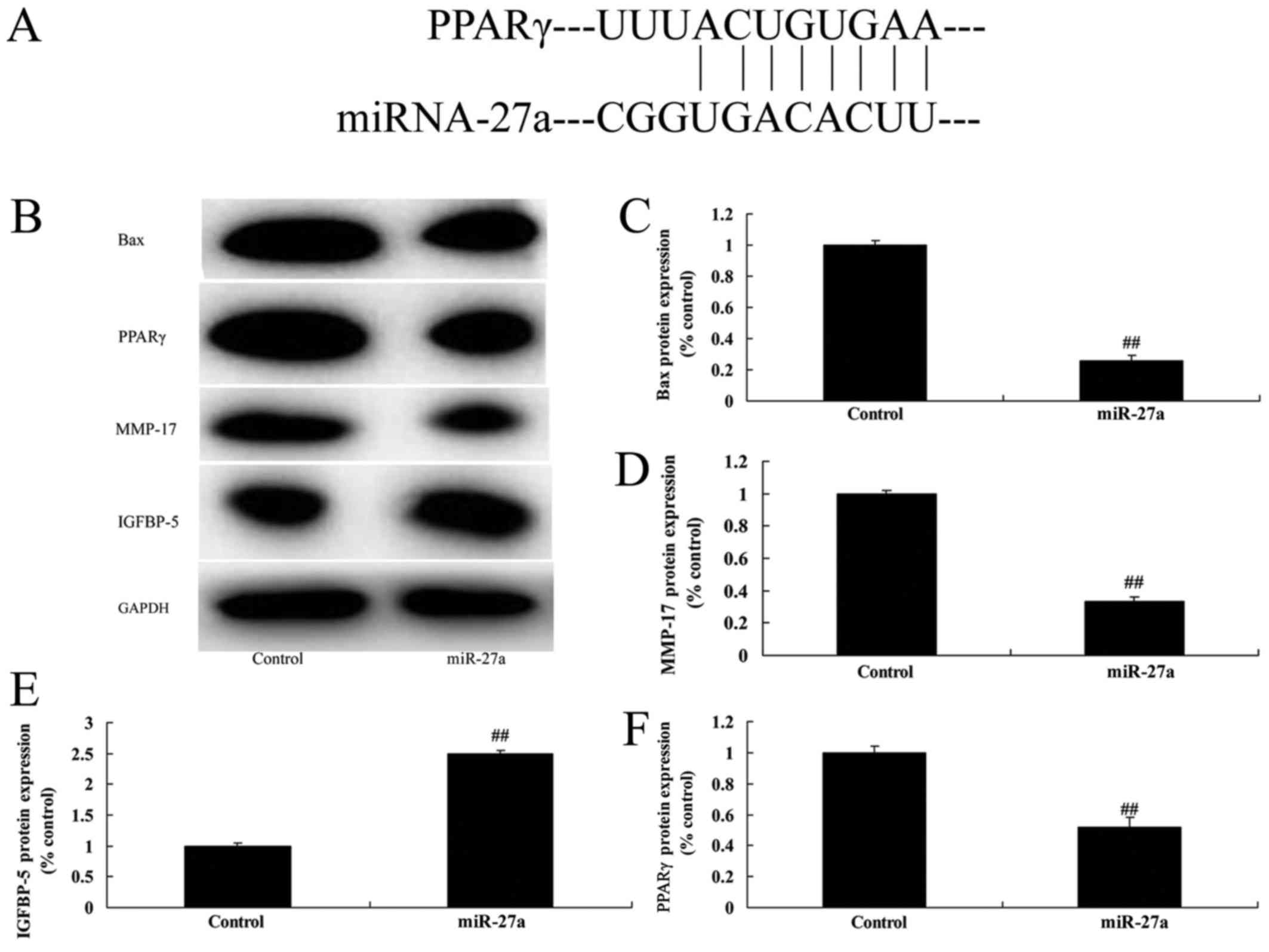 | Figure 4.miR-27a overexpression affects
IGFBP-5, PPARγ and MMP-17 protein expression. (A) Putative miR-27a
binding sites predicted by TargetScan in 3′-UTR of PPARγ gene, (B)
Bax, PPARγ, IGFBP-5 and MMP-17 protein expression using western
blot analysis. The results of the western blotting were quantified
for (C) Bax, (D) MMP-17, (E) IGFBP-5 and (F) PPARγ. All quantified
results are presented as the mean ± SD. PPARγ, peroxisome
proliferators-activated receptors-γ; IGFBP-5, insulin like growth
factor binding protein-5; MMP-17, matrix metalloproteinase-17;
control, control negative group; miR-27a, miR-27a overexpression
group. ##P<0.01 compared with control group. |
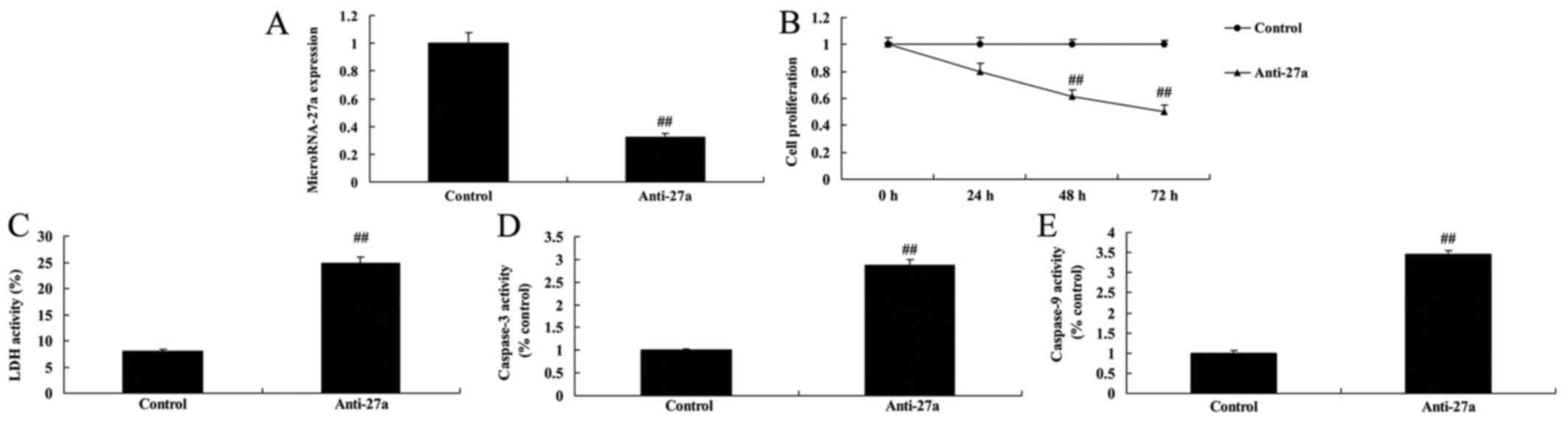
miR-27a downregulation decreased cell
proliferation of osteoblast-like cell in vitro model of
arthritis
Moreover, to further verify the function of miR-27a
on arthritis, cell proliferation of osteoblast-like cell by
anti-miR-27a was measured by MTT sassy. As showed in Fig. 5, anti-miR-27a mimics inhibited
miR-27a expression in vitro model of arthritis, decreased
cell proliferation, and increased LDH activity and caspase-3/9
activity of osteoblast-like cell in vitro model of
arthritis.
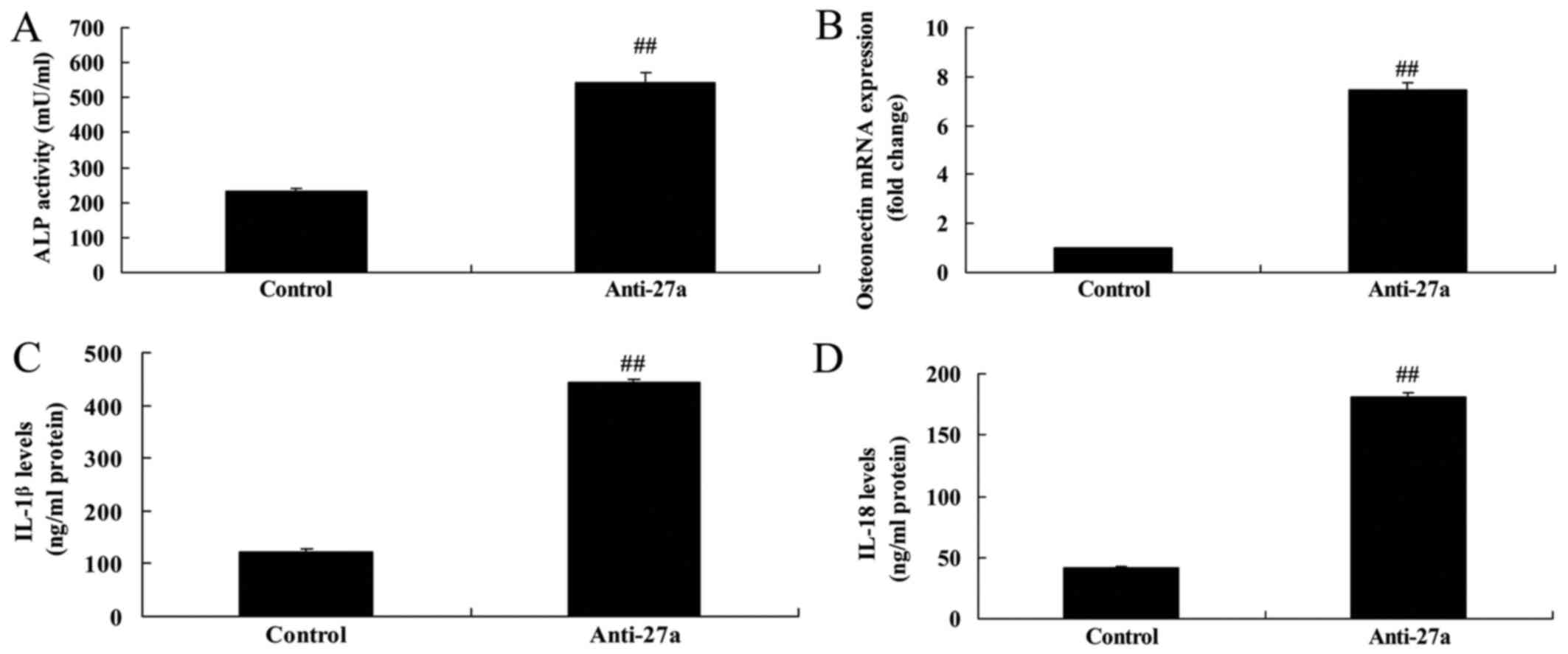
miR-27a downregulation suppressed
osteogenic differentiation and increased inflammation
Next, in vitro model of arthritis by
anti-miR-27a mimics, osteogenic differentiation and ALP and OST
content were suppressed, and inflammation also increased (Fig. 6).
miR-27a downregulation affects on
IGFBP-5, PPARγ and MMP-17 protein expression
Lastly, anti-miR-27a mimics suppressed IGFBP-5, and
induced PPARγ and MMP-17 protein expression in osteoblast-like
cell. These results suggested that miR-27a affects on PPARγ
expression in arthritis through anti-inflammation and osteogenic
differentiation (Fig. 7). These
results also showed that downregulation of miR-27a regulate PPARγ
and MMP-17 protein expression by IGFBP-5 to promote arthritis.
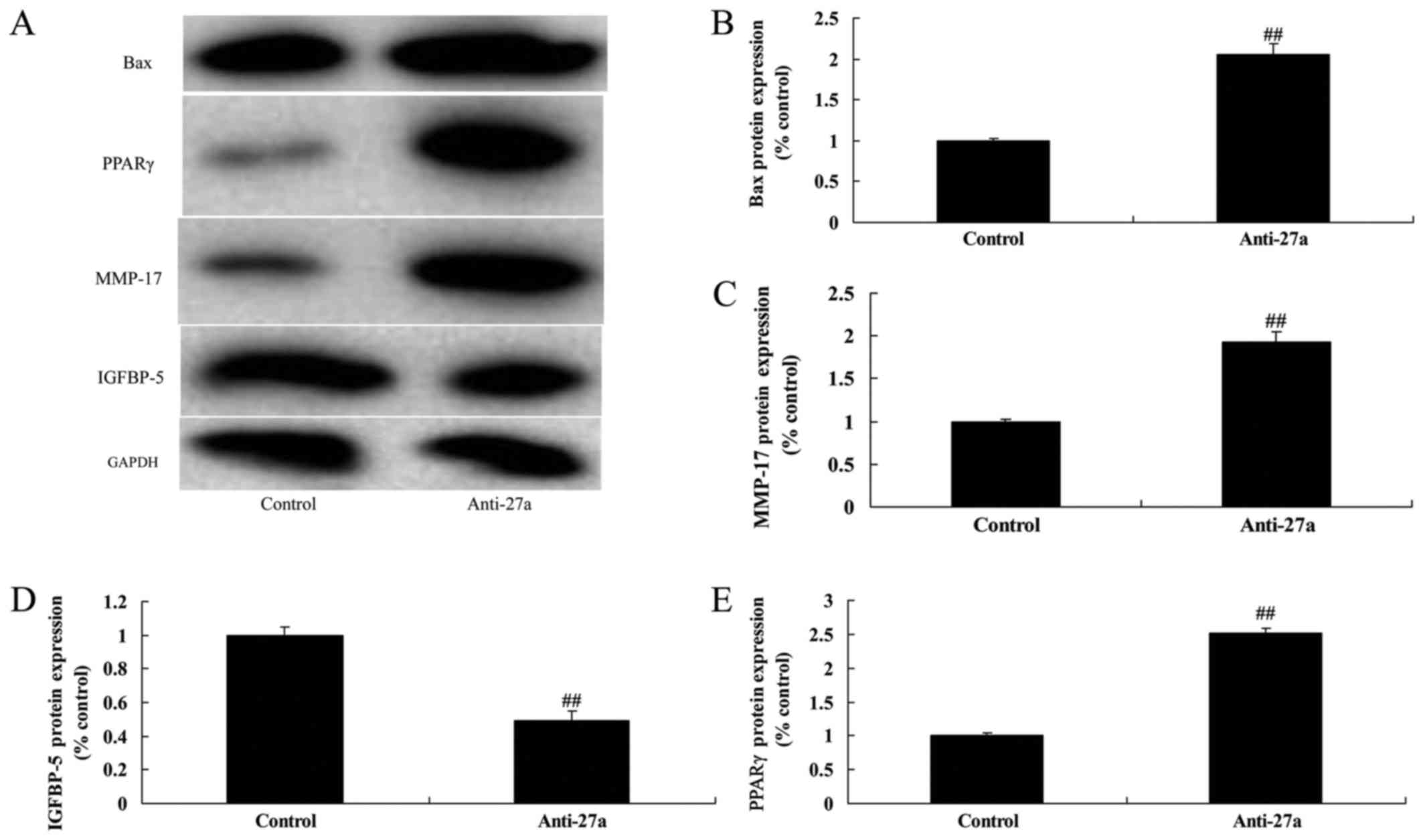 | Figure 7.miR-27a downregulation affects on
IGFBP-5, PPARγ and MMP-17 protein expression. (A) Bax, PPARγ,
IGFBP-5 and MMP-17 protein expression using western blot analysis.
The results of the western blotting were quantified for (B) Bax,
(C) MMP-17, (D) IGFBP-5 and (E) PPARγ. All quantified results are
presented as the mean ± SD. PPARγ, peroxisome
proliferators-activated receptors-γ; IGFBP-5, insulin like growth
factor binding protein-5; MMP-17, matrix metalloproteinase-17;
control, control negative group; anti-27a, miR-27a downregulation
group. ##P<0.01 compared with control group. |
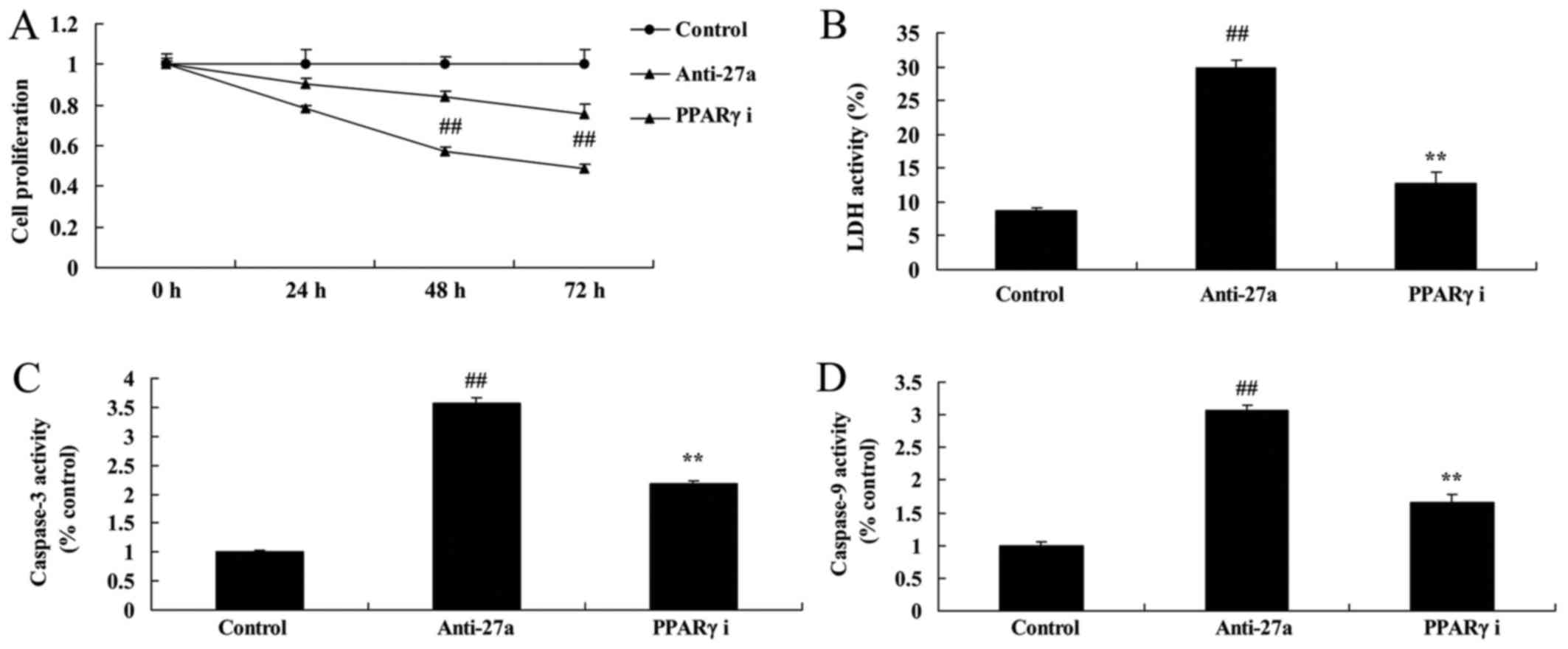
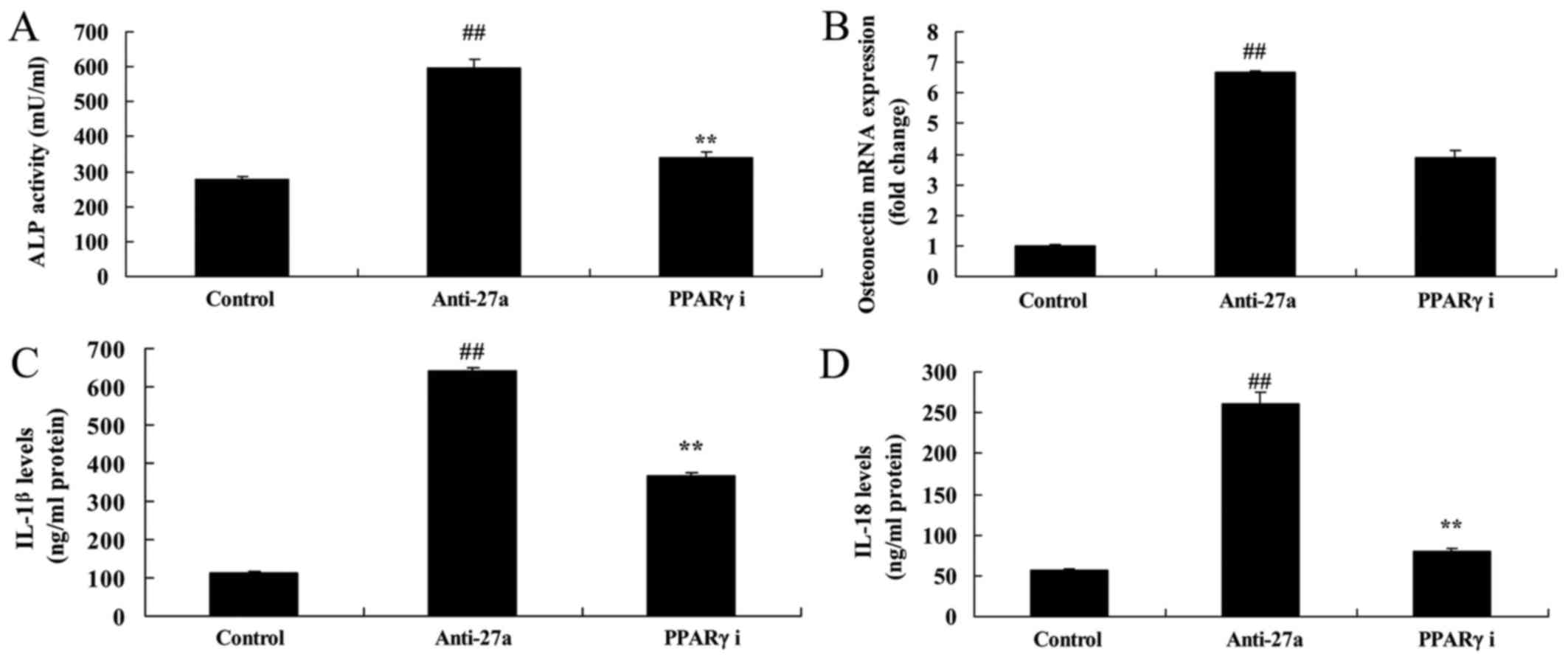
PPARγ inhibitor reduced the function
of miR-27a downregulation on arthritis
We next examined whether PPARγ participated in the
unction of miR-27a downregulation on arthritis. As showed in
Fig. 8, PPARγ inhibitor suppressed
PPARγ and MMP-17 protein expression, and induced IGFBP-5 protein
expression in vitro model of arthritis. The inhibition of
PPARγ inhibited the effects of miR-27a downregulation on cell
proliferation, LDH activity and caspase-3/9 activity in
vitro model of arthritis following miR-27a downregulation,
compared with miR-27a downregulation group (Fig. 9). The PPARγ inhibition the effects
of miR-27a downregulation on osteogenic differentiation and ALP and
OST content in vitro model of arthritis following miR-27a
downregulation, compared with miR-27a downregulation group
(Fig. 10).
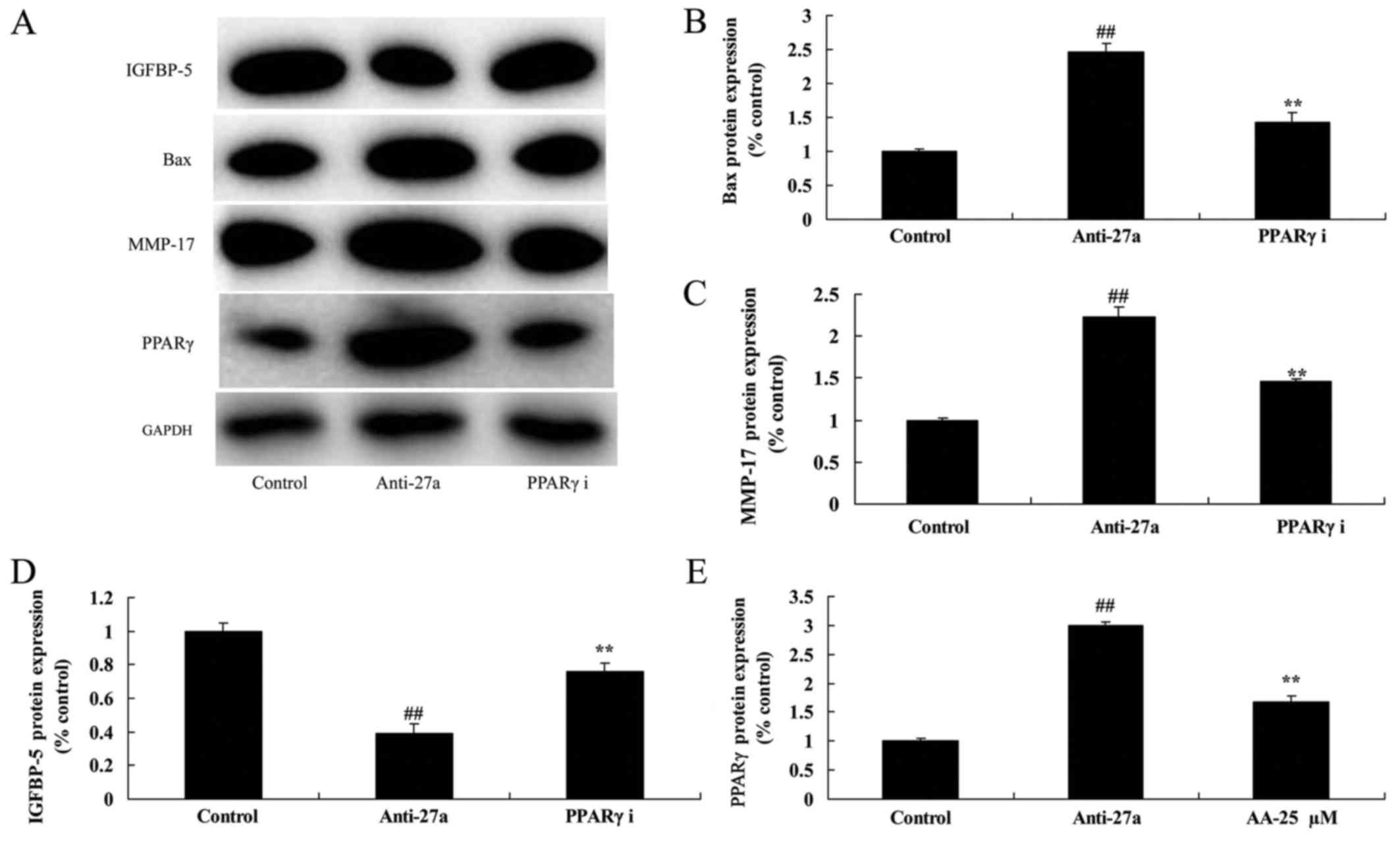 | Figure 8.PPARγ inhibitor reduced the function
of miR-27a downregulation on arthritis. (A) Bax, PPARγ, IGFBP-5 and
MMP-17 protein expression using western blot analysis. The results
of the western blotting were quantified for (B) Bax, (C) MMP-17,
(D) IGFBP-5 and (E) PPARγ. All quantified results are presented as
the mean ± SD. PPARγ, peroxisome proliferators-activated
receptors-γ; IGFBP-5, insulin like growth factor binding protein-5;
MMP-17, matrix metalloproteinase-17; control, control negative
group; anti-27a, miR-27a downregulation group; PPARγ i, miR-27a
downregulation and PPARγ inhibitor group. ##P<0.01
compared with control group; **P<0.01 compared with the miR-27a
downregulation group. |
Discussion
OA is a chronic degenerative osteoarticular disease
which seriously endangers human health (16). The degenerative changes in the
pathology is mainly expressed as the destruction of articular
cartilage degeneration, subchondral bone sclerosis, cystic
degeneration joint marginal hyperostosis, synovial hyperplasia,
contracture of joint capsule, ligament laxity, muscle weakness and
atrophy(2). The incidence of OA is high, commonly occurring in the
elderly patients, and female patients are more than male patients
(16). The prevalence rate of
people under the age of 40 is ~5%, and the prevalence rate of
people aged 60–75 years old is up to 50, and 80% among people over
75 years old. There is an increasing trend with age (17). Therefore, we found that miR-27a
expression of arthritis in rat with arthritis was reduced, compared
with control normal group.
Studies have shown that miRNA is associated with
disease progression and pathogenesis (5). For example, miRNA plays an important
role in the pathogenesis of cancer and cardiovascular disease
(18). Research on tissue
specificity miRNA in mice also showed that miRNA could have both
pathogenic and tissue protective functions (19). Along with the gradually deepened
research on miRNA in terms of the regulation of cell function in
science, and the discovery of new miRNA targets, increasing
research has shown that miRNA can regulate the expression of
OA-related genes in multiple aspects (18). Our study showed that miRNA-27a
overexpression increased cell proliferation and inhibited bone cell
apoptosis in vitro model of arthritis.
In the process of OA development, matrix degradation
and severe damage is often accompanied by excessive apoptosis of
cartilage cells (20). Cell
apoptosis, also known as programmed death, refers to the
physiological process that nucleated cells trigger programmed cell
death under the regulation of gene, which causes the natural death
of cells and automatical removal of the non-functional, damaged and
senescent cells (21). There is a
small amount of apoptosis in the normal cartilage tissue, which is
usually confined to the surface of cartilage tissue (22). It is an essential physiological
process to ensure the normal growth and development of cartilage,
regulate function and maintain homeostasis (22). The excessive apoptosis in cartilage
cells indicates pathological changes of cartilage tissue, which is
considered to be the key factor leading to the onset of OA
(20). In this study, we found
that miR-27a overexpression promoted osteogenic differentiation
in vitro model of arthritis.
Cartilage abnormity is a key link in the
pathogenesis of OA, and closely related the mechanical properties,
cartilage cell proliferation and apoptosis, inflammatory factor and
the secretion of MMPs (23). It
has been reported that IL-1β can stimulate the release of NO from
the cartilage cells so as to promote apoptosis. Inflammatory factor
and its harm are important mechanisms of OA (23). In the process of OA, the
pathological changes of cartilage tissue can lead to the increase
of inflammatory factor expression, while the increased inflammatory
factors in turn intensify the cartilage tissue lesions by mediating
signal activities, forming a vicious circle (24). Inflammatory factors can affect the
expression of ECM catabolic proteins, such as MMPs and integrins,
so that the contents will be significantly increased, which leads
to ECM metabolism disorder and promotes the development of OA
(25). We found that miR-27a
overexpression inhibited inflammation in vitro model of
arthritis. Wang et al suggested that microRNA-27a mediate
physcion 8-O-β-glucopyranoside-induced apoptosis through MMPs
expression in osteosarcoma cells (26). These results showed that
microRNA-27a negatively modulates inflammatory response in
lipopolysaccharide-stimulated microglia.
Insulin-like growth factor-1 (IGF-1) is one of the
major mediators in the synthesis and metabolism of articular
cartilage (27). OA model showed
that IGF-1 can reduce the destruction of articular cartilage
(28). Insulin-like growth factor
binding proteins (IGFBPs) are an important factor in regulating and
maintaining the activity of anabolic factors IGF-1 (29). The increase of IGFBP5 concentration
in joints will enhance IGF-1 (28). Also, it has been found that the
expression level of IGFBP5 in OA cartilage was significantly lower
than that in normal subjects (29). This study showed that miR-27a
overexpression induced IGFBP-5 protein expression, and suppressed
PPARγ and MMP-17 protein expression in vitro model of
arthritis. Tardif el al reported that miRNA-27 regulated the
IGFBP-5 and MMP-13 genes in human osteoarthritic chondrocytes
(30).
The inhibitory effect of PPARγ on OA is mainly
expressed as inhibition of inflammatory reaction and regulation of
cell proliferation and migration (31). PPARγ ligand or agonist can reduce
the expression of inflammatory factor IL-1β, IL-6, tumor necrosis
factor-α (TNF-α), inducible nitric oxide synthase (iNOS) and matrix
metalloproteinase-9 (MMP-9) (32).
Also, it can inhibit the activity of monocyte/macrophage
transcription factor AP-1, NF-κB and transcription activator (Stat)
(33). The activated PPARγ may
regulate the inflammatory response in OA (32). In this study, we found that PPARγ
inhibitor reduced the function of miR-27a downregulation on
arthritis. Xie et al demonstrated that miR-27a mediates
endothelin-1-induced PPARγ reduction and proliferation of pulmonary
artery smooth muscle cells (34).
In conclusion, we found that miRNA-27 play essential
roles in regulating osteoblast differentiation and bone apoptosis
by arthritis through PPARγ expression in vivo and in
vitro model. miRNA-27 has been proposed as a candidate
therapeutic modality to treat for arthritis, and provides novel
insights into the pathogenesis of AI and potential preventative or
therapeutic interventions.
References
|
1
|
Cakir S, Hepguler S, Ozturk C, Korkmaz M,
Isleten B and Atamaz FC: Efficacy of therapeutic ultrasound for the
management of knee osteoarthritis: A randomized, controlled, and
double-blind study. Am J Phys Med Rehabil. 93:405–412. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McAlindon TE, LaValley MP, Harvey WF,
Price LL, Driban JB, Zhang M and Ward RJ: Effect of Intra-articular
triamcinolone vs saline on knee cartilage volume and pain in
patients with knee osteoarthritis: A randomized clinical trial.
JAMA. 317:1967–1975. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schumacher HR, Pullman-Mooar S, Gupta SR,
Dinnella JE, Kim R and McHugh MP: Randomized double-blind crossover
study of the efficacy of a tart cherry juice blend in treatment of
osteoarthritis (OA) of the knee. Osteoarthritis Cartilage.
21:1035–1041. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li B, Bai L, Shen P, Sun Y, Chen Z and Wen
Y: Identification of differentially expressed microRNAs in knee
anterior cruciate ligament tissues surgically removed from patients
with osteoarthritis. Int J Mol Med. 40:1105–1113. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Soyocak A, Kurt H, Ozgen M, Turgut Cosan
D, Colak E and Gunes HV: miRNA-146a, miRNA-155 and JNK expression
levels in peripheral blood mononuclear cells according to grade of
knee osteoarthritis. Gene. 627:207–211. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xu B, Li YY, Ma J and Pei FX: Roles of
microRNA and signaling pathway in osteoarthritis pathogenesis. J
Zhejiang Univ Sci B. 17:200–208. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Min Z, Zhang R, Yao J, Jiang C, Guo Y,
Cong F, Wang W, Tian J, Zhong N, Sun J, et al: MicroRNAs associated
with osteoarthritis differently expressed in bone matrix gelatin
(BMG) rat model. Int J Clin Exp Med. 8:1009–1017. 2015.PubMed/NCBI
|
|
8
|
Pajak A, Kostrzewa M, Malek N, Korostynski
M and Starowicz K: Expression of matrix metalloproteinases and
components of the endocannabinoid system in the knee joint are
associated with biphasic pain progression in a rat model of
osteoarthritis. J Pain Res. 10:1973–1989. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rikimaru A, Komori K, Sakamoto T, Ichise
H, Yoshida N, Yana I and Seiki M: Establishment of an
MT4-MMP-deficient mouse strain representing an efficient tracking
system for MT4-MMP/MMP-17 expression in vivo using
beta-galactosidase. Genes Cells. 12:1091–1100. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ni Q, Tan Y, Zhang X, Luo H, Deng Y,
Magdalou J, Chen L and Wang H: Prenatal ethanol exposure increases
osteoarthritis susceptibility in female rat offspring by
programming a low-functioning IGF-1 signaling pathway. Sci Rep.
5:147112015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shi S, Mercer S, Eckert GJ and Trippel SB:
Growth factor transgenes interactively regulate articular
chondrocytes. J Cell Biochem. 114:908–919. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yates MP, Settle SL, Yocum SA, Aggarwal P,
Vickery LE, Aguiar DJ, Skepner AP, Kellner D, Weinrich SL and
Sverdrup FM: IGFBP-5 metabolism is disrupted in the rat medial
meniscal tear model of osteoarthritis. Cartilage. 1:43–54. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Loffredo FS, Pancoast JR, Cai L, Vannelli
T, Dong JZ, Lee RT and Patwari P: Targeted delivery to cartilage is
critical for in vivo efficacy of insulin-like growth factor
1 in a rat model of osteoarthritis. Arthritis Rheumatol.
66:1247–1255. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qu Y, Zhou L and Wang C: Mangiferin
inhibits IL-1β-induced inflammatory response by activating PPAR-γ
in human osteoarthritis chondrocytes. Inflammation. 40:52–57. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vasheghani F, Zhang Y, Li YH, Blati M,
Fahmi H, Lussier B, Roughley P, Lagares D, Endisha H, Saffar B, et
al: PPARγ deficiency results in severe, accelerated osteoarthritis
associated with aberrant mTOR signalling in the articular
cartilage. Ann Rheum Dis. 74:569–578. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bennell KL, Dobson F, Roos EM, Skou ST,
Hodges P, Wrigley TV, Kyriakides M, Metcalf B, Hunt MA and Hinman
RS: Influence of biomechanical characteristics on pain and function
outcomes from exercise in medial knee osteoarthritis and varus
malalignment: Exploratory analyses from a randomized controlled
trial. Arthritis Care Res (Hoboken). 67:1281–1288. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
King MR, Haussler KK, Kawcak CE,
McIlwraith CW, Reiser RF II, Frisbie DD and Werpy NM: Biomechanical
and histologic evaluation of the effects of underwater treadmill
exercise on horses with experimentally induced osteoarthritis of
the middle carpal joint. Am J Vet Res. 78:558–569. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li YH, Tavallaee G, Tokar T, Nakamura A,
Sundararajan K, Weston A, Sharma A, Mahomed NN, Gandhi R, Jurisica
I and Kapoor M: Identification of synovial fluid microRNA signature
in knee osteoarthritis: Differentiating early- and late-stage knee
osteoarthritis. Osteoarthritis Cartilage. 24:1577–1586. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kolhe R, Hunter M, Liu S, Jadeja RN,
Pundkar C, Mondal AK, Mendhe B, Drewry M, Rojiani MV, Liu Y, et al:
Gender-specific differential expression of exosomal miRNA in
synovial fluid of patients with osteoarthritis. Sci Rep.
7:20292017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu J, Meng Q, Jing H and Zhou S:
Astragaloside IV protects against apoptosis in human degenerative
chondrocytes through autophagy activation. Mol Med Rep.
16:3269–3275. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang P: Ginsenoside-Rg5 treatment
inhibits apoptosis of chondrocytes and degradation of cartilage
matrix in a rat model of osteoarthritis. Oncol Rep. 37:1497–1502.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gao H, Gui J, Wang L, Xu Y, Jiang Y, Xiong
M and Cui Y: Aquaporin 1 contributes to chondrocyte apoptosis in a
rat model of osteoarthritis. Int J Mol Med. 38:1752–1758. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu CC, Zhang Y, Dai BL, Ma YJ, Zhang Q,
Wang Y and Yang H: Chlorogenic acid prevents inflammatory responses
in IL-1β-stimulated human SW-1353 chondrocytes, a model for
osteoarthritis. Mol Med Rep. 16:1369–1375. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Feng Z, Zheng W, Li X, Lin J, Xie C, Li H,
Cheng L, Wu A and Ni W: Cryptotanshinone protects against
IL-1β-induced inflammation in human osteoarthritis chondrocytes and
ameliorates the progression of osteoarthritis in mice. Int
Immunopharmacol. 50:161–167. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Santoro A, Conde J, Scotece M, Abella V,
Lois A, Lopez V, Pino J, Gomez R, Gomez-Reino JJ and Gualillo O:
SERPINE2 Inhibits IL-1α-Induced MMP-13 expression in human
chondrocytes: Involvement of ERK/NF-κB/AP-1 pathways. PLoS One.
10:e01359792015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang Z and Yang H: EMMPRIN, SP1 and
microRNA-27a mediate physcion 8-O-β-glucopyranoside-induced
apoptosis in osteosarcoma cells. Am J Cancer Res. 6:1331–1344.
2016.PubMed/NCBI
|
|
27
|
Tie K, Zhang X, Tan Y, Deng Y, Li J, Ni Q,
Wang H and Chen L: Intrauterine low-functional programming of IGF1
by prenatal nicotine exposure mediates the susceptibility to
osteoarthritis in female adult rat offspring. FASEB J. 30:785–797.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ekenstedt KJ, Sonntag WE, Loeser RF,
Lindgren BR and Carlson CS: Effects of chronic growth hormone and
insulin-like growth factor 1 deficiency on osteoarthritis severity
in rat knee joints. Arthritis Rheum. 54:3850–3858. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Morales TI: The quantitative and
functional relation between insulin-like growth factor-I (IGF) and
IGF-binding proteins during human osteoarthritis. J Orthop Res.
26:465–474. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tardif G, Hum D, Pelletier JP, Duval N and
Martel-Pelletier J: Regulation of the IGFBP-5 and MMP-13 genes by
the microRNAs miR-140 and miR-27a in human osteoarthritic
chondrocytes. BMC Musculoskelet Disord. 10:1482009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fahmi H, Pelletier JP, Di Battista JA,
Cheung HS, Fernandes JC and Martel-Pelletier J: Peroxisome
proliferator-activated receptor gamma activators inhibit MMP-1
production in human synovial fibroblasts likely by reducing the
binding of the activator protein 1. Osteoarthritis Cartilage.
10:100–108. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zheru D, Peiliang F, Yuli W, Haishan W,
Qirong Q, Xiaohua L, Hui Z, Bo W and Qiwei F: Association of PPARγ
gene polymorphisms with osteoarthritis in a southeast Chinese
population. J Genet. 93:719–723. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen YJ, Chan DC, Lan KC, Wang CC, Chen
CM, Chao SC, Tsai KS, Yang RS and Liu SH: PPARγ is involved in the
hyperglycemia-induced inflammatory responses and collagen
degradation in human chondrocytes and diabetic mouse cartilages. J
Orthop Res. 33:373–381. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xie X, Li S, Zhu Y, Liu L, Pan Y, Wang J,
Shi W, Song Y, Yang L, Gao L, et al: MicroRNA-27a/b mediates
endothelin-1-induced PPARγ reduction and proliferation of pulmonary
artery smooth muscle cells. Cell Tissue Res. May 8–2017.(Epub ahead
of print). View Article : Google Scholar
|