Introduction
Chondrosarcoma is a malignant cartilage-forming
cancer, which is the most common primary malignant bone tumor in
adults (1,2). Currently, wide local excision is the
most effective treatment since the treatment of patients with
chondrosarcomas displays barely response to both chemotherapy and
radiation therapy (3,4). Moreover, most chemotherapy drugs for
chondrosarcoma are associated with strong toxicities for normal
tissues (5). Thus, effective
therapeutic approaches to improve chondrosarcoma clinical outcome
are still under investigation.
B-cell lymphoma-2 associated athanogene 3 (BAG3) is
a member of BAG family of co-chaperones (6). It has a modular structure that
contains a BAG domain, a WW domain, a proline-rich (PxxP) domain
interacting with proteins (7). In
addition, the WW domains connect BAG3 to SH3 domains of its binding
proteins (7). The functions of
BAG3 in cancers have been described. BAG3 protein is known to
interact with the ATPase domain of the heat shock protein (Hsp)70
through BAG domain (8,9). A study reported that the Hsp70-BAG3
interactions regulate multiple cancer-related signaling networks
(9). It has illustrated that BAG3
interacts with the SH3 domain of Src, thereby mediating the effects
of Hsp70 on Src signaling (9).
BAG3 has been reported to associate with GRP78, resulting in
sensitization of cancer cells to DNA damaging agents (10). Moreover, it has been demonstrated
that inhibition of endogenous BAG3 by siRNA could enhance the
effectiveness of chemotherapy (11), suggesting that BAG3 has the
potential to be a therapeutic target of human malignancies.
However, the roles of BAG3 in chondrosarcoma remain unclear.
The potential roles and mechanisms of the BAG3 in
modulation of chondrosarcoma malignancies have been largely unknown
previously. In this study, we investigated the functions of BAG3 in
human chondrosarcoma by comparison of the expressions of BAG3 in
normal cartilage tissue, benign chondroma and chondrosarcoma.
Meanwhile, we assessed the roles of BAG3 in the proliferation and
migration of chondrosarcoma cells. Our study identified the
potential roles of BAG3 in chondrosarcoma, which will contribute to
the development of novel therapeutics for the treatments of
chondrosarcoma patients.
Materials and methods
Patient specimens and information
Fifty-nine cases of chondrosarcoma, thirty cases of
naturally express cartilage tumors and eight cases of normal
cartilage tissues were randomly obtained from the Department of
Pathology at the First Affiliated Hospital of Sun Yat-Sen
University (Guangzhou, China) between January 2006 and November
2015. The chondrosarcoma tissues were from 33 males and 26 females.
There are thirty-two cases under the age of forty-three years and
twenty-seven cases over the age of forty-three years. Moreover,
according to the Enneking surgical staging for musculoskeletal
neoplasms classification, there are 30 cases belong to stage I, 25
cases belong to stage II and four cases belong to stage III.
According to WHO histology grading: There were 31 cases in grade I,
22 cases in grade II~III and 6 cases of undifferentiated. Patients
with preoperative radiotherapy or chemotherapy were excluded. In
addition, normal cartilage tissues were obtained at 2–5 cm away
from the tumors. The material had been fixed in 4% buffered
formalin and embedded in paraffin. Prior to the research, patient's
written informed consent and approval from the Institute Research
Ethics Committee of The First Affiliated Hospital of Sun Yat-Sen
University was obtained, and the Ethics Committee approval number
is 201301.
Cell culture
The human chondrosarcoma cell line SW1353 was
purchased from the Type Culture Collection of the Chinese Academy
of Sciences (Shanghai, China). The normal chondrocyte cell line
HC-a was purchased from Shanghai Yu Bo Biological Technology Co.,
Ltd. (Shanghai, China). SW1353 cells were cultivated in Dulbecco's
modified Eagle's medium (DMEM) supplemented with 10%
heat-inactivated fetal bovine serum (FBS). HC-a cells were cultured
in RPMI-1640 supplemented with 10% FBS. All cultures contained 1%
penicillin/streptomycin and were incubated in an incubator with 5%
CO2 at 37°C.
Antibodies and reagents
The antibodies were purchased from: Rabbit
polyclonal Anti-BAG3 antibody (cat. no. ab47124; Abcam, Cambridge,
UK); rabbit polyclonal anti-RUNX2 antibody (cat. no. ab28931;
Abcam); Rabbit monoclonal Anti-GAPDH antibody (cat. no. 2118; Cell
Signaling Technology, Inc., Danvers, MA, USA) and β-catenin were
purchased form the epithelial-mesenchymal transition (EMT) Antibody
Sampler kit (cat. no. 9782; Cell Signaling Technology, Inc.).
Plasmid DNA and siRNA
transfections
Expression vector containing wild type BAG3 were
constructed from pcDNA3.1 according to the previous report
(10). The vector was double
digested at XhoI and BamHI sites. SiRNA
oligonucleotides for BAG3 was purchased from Invitrogen with a
scrambled siRNA as a control. The siBAG3 sequences were described
as following: sense: 5′-AAGGUUCAGACCAUCUUGGAA-3′; antisense:
5′-TTCCGTGGTCTGCCTT-3′. Cells were transfected using the
Lipofectamine® 2000 Transfection reagent (Invitrogen)
according to the manufacturer's protocol. siRNA transfection was
performed with 100 nmol/l and plasmid DNA was transfected with 2
µg. At 48 h after transfection, whole-cell lysates were prepared
for further analysis.
Polymerase chain reaction (PCR) and
reverse transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted after homogenization of
cells and tissues using RNeasy mini kit (Qiagen Sciences, Inc.,
Germantown, MD, USA). Total RNA (1 µg) was reversely transcribed
with the High Capacity cDNA Reverse Transcription kit (Applied
Biosystems; Thermo Fisher Scientific, Inc., Waltham, MA, USA). The
cDNA reaction was diluted to 1:10 for use as template for RT-qPCR.
TaqMan Gene Expression Assays primers and probes specific to BAG3
or β-catenin were used for expression analysis and 18S ribosomal
primers and probes (Applied Biosystems; Thermo Fisher Scientific,
Inc.) were used as internal controls. PCR amplifications were
performed in a final reaction volume of 10 µl containing, 5.5 µl of
TaqMan Universal PCR Master Mix (Applied Biosystems; Thermo Fisher
Scientific, Inc.), 0.5 µl of the primers and probes mix and 4.5 µg
of the cDNA diluted solution. The primers for qPCR are:
BAG3-forward: 5′-TGGGAGATCAAGATCGACCC-3′; BAG3-reverse:
5′-GGGCCATTGGCAGAGGATG-3′. All qPCR reactions were carried out in
triplicate and repeated at least twice. The ΔCq for mRNA expression
was calculated relative to the Cq (quantitation cycle) of 18S
ribosomal RNA. Relative mRNA expression was calculated using the
formula 2−ΔΔCq.
Cell growth assay
For the measurements of cell growth, a total of
2.5×103 cells/well were seeded in 24-well plates. Cells
were transfected with either control siRNA, siBAG3 or
overexpression vector of BAG3 for 48 h. Cell growth was determined
by the 3-(4,5 dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
(MTT) assay according to the previous report (10). Absorbance was measured
spectrophotometrically at 490 nm by using the Universal Microplate
Reader EL800 (BioTek Instruments, Inc., Winooski, VT, USA). All
experiments were performed in triplicate.
Apoptosis assay
Cell apoptosis assay was performed using Apoptosis
kit with Annexin V FITC and PI from Invitrogen (Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
Briefly, cells were transfected with either control siRNA, siBAG3
or overexpression vector of BAG3 for 48 h, cells were trypsinized
and 1–5×106 cells of each group were collected for the
apoptosis assay. After staining with Annexin V-FITC and PI, samples
were analyzed by using fluorescence-activated cell scanner
(FACScan) flow cytometer (BD Biosciences, Franklin Lakes, NJ,
USA).
Clonogenic assay
SW1353 cells were transfected with either control
siRNA, siBAG3 or overexpression vector of BAG3 for 48 h,
1×103 cells were seeded on 10 cm dish with regular cell
culture medium for two weeks and the colonies were stained with
gentian violet after methanol fixation, and visible colonies
(>50 cells) were counted. Colonies from randomly-selected image
areas of three replicate wells were enumerated.
Cell migration assay
The cell migration assay was performed using
Transwell cell culture inserts (Invitrogen; Thermo Fisher
Scientific, Inc.). The cells were transfected with control siRNA,
siBAG3 or overexpression vector of BAG3 for 48 h then cells were
trypsinized and 5×103 cells of each group were plated to
transwell insert to migrate for 24 h. The passaged cells were
stained with crystal violet solution, and absorbance was measured
at 595 nm. In the wound-healing assays, cell motility was assessed
by measuring the movement of cells toward the scratch. The speed of
wound closure was monitored after 24 h by measuring the ratio of
the distance of the wound at 0 h. Each experiment was performed in
triplicate.
Immunohistochemistry
For IHC, the tissue samples were cut in 4-micro
meter sections. After antigen retrieval, the sections were
incubated with anti-BAG3 or anti-β-catenin antibodies at 4°C
overnight, followed by a HRP-labeled second antibody. The staining
was photographed under an inverted light microscope (Olympus,
Tokyo, Japan). The scoring criteria for staining were as follows:
staining was scored by integrating the staining intensity of
positive cells (trichotomy) and the percentage of positive cells in
the tumor area (quartering). The scoring criteria for staining
intensity were regarded as: 0, no staining; 1, light yellow
staining; 2, yellow staining; and 3, brown staining. Areas: 0, no
staining; 1, ≤10%; 2, 10–25%; 3, 25–50%; and 4, >50%. Two scores
were multiplied to obtain a score of 0 to 12, setting 6 as the
threshold by the log-rank test. Low expression, score <6; high
expression score ≥6. β-catenin was judged by the Maruyama standard
(12) and mainly expressed in the
cell membrane. The expression of membrane was normal when ≥70% and
decreased when <70%, respectively. The expression of membrane
was decreased and abnormal when ≥10% plasma or nuclear expression.
Immunohistochemical results were independently judged by three
pathologists, and disagreeable immunohistochemical results were
finally decided after discussion.
Western blotting
Cells were lysed with RIPA buffer (Thermo Fisher
Scientific, Inc.). The concentration of samples was measured using
the BCA kit (Pierce; Thermo Fisher Scientific, Inc.) as instructed.
The lysates were separated by 10% SDS-PAGE gels, and
polyvinylidenefluoride (PVDF) membranes (EMD Millipore, Billerica,
MA, USA) were used for protein transfer. The PVDF membranes were
then blocked by 5% skim milk containing 0.1% Tween-20 at room
temperature for 1 h, and then incubated over night at 4°C with
primary antibody (1:1,000). After washing with PBST the following
day, the membranes were incubated with secondary antibodies
(1:5,000; Invitrogen; Thermo Fisher Scientific, Inc.) at room
temperature for 1 h. Then, bands were captured using BeyoECL Plus
(Beyotime Institute of Biotechnology, Haimen, China) and detected
using a BioImaging System. The protein expression criteria referred
to previous studies (12).
Statistical analysis
Data were analyzed using the Statistical Package for
the Social Sciences (SPSS), version 18 (SPSS, Inc., Chicago, IL,
USA). The Pearson's Chi-square test was used to analyze the
relationship between BAG3 expression and clinical pathological
characteristics. One-way analysis of variance (ANOVA), Student's
t-test, or Chi-square tests were used to compare differences
between groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
BAG3 was significantly upregulated in
human chondrosarcoma
Multiple studies have identified functions of BAG3
which is correlated with malignant phenotypes of cancers. To
uncover the roles of BAG3 in human chondrosarcoma, we sought to
compare the expression levels of BAG3 in human chondrosarcoma cell
lines, SW1353 and normal chondrocyte cell line, HC-a. As we
expected, BAG3 was significantly upregulated in chondrosarcoma
cells at protein and mRNA levels (Fig.
1A and B). To evaluate whether BAG3 is a potential oncogene in
human chondrosarcoma tissues, we measured the mRNA levels of BAG3
in normal human cartilage tissue, benign cartilaginous tumor
chondroma and chondrosarcoma tissues. Consistently, the expression
of BAG3 in chondrosarcoma patient tumor samples was significantly
upregulated compared with normal human cartilage and chondroma
tissues (Fig. 1C). In addition, we
observed that the expressions of BAG3 in human chondrosarcoma were
elevated according to the tumor malignant stages (Fig. 1D, Table I), indicating that the upregulated
BAG3 expression might be the target for anti-chondrosarcoma
therapy.
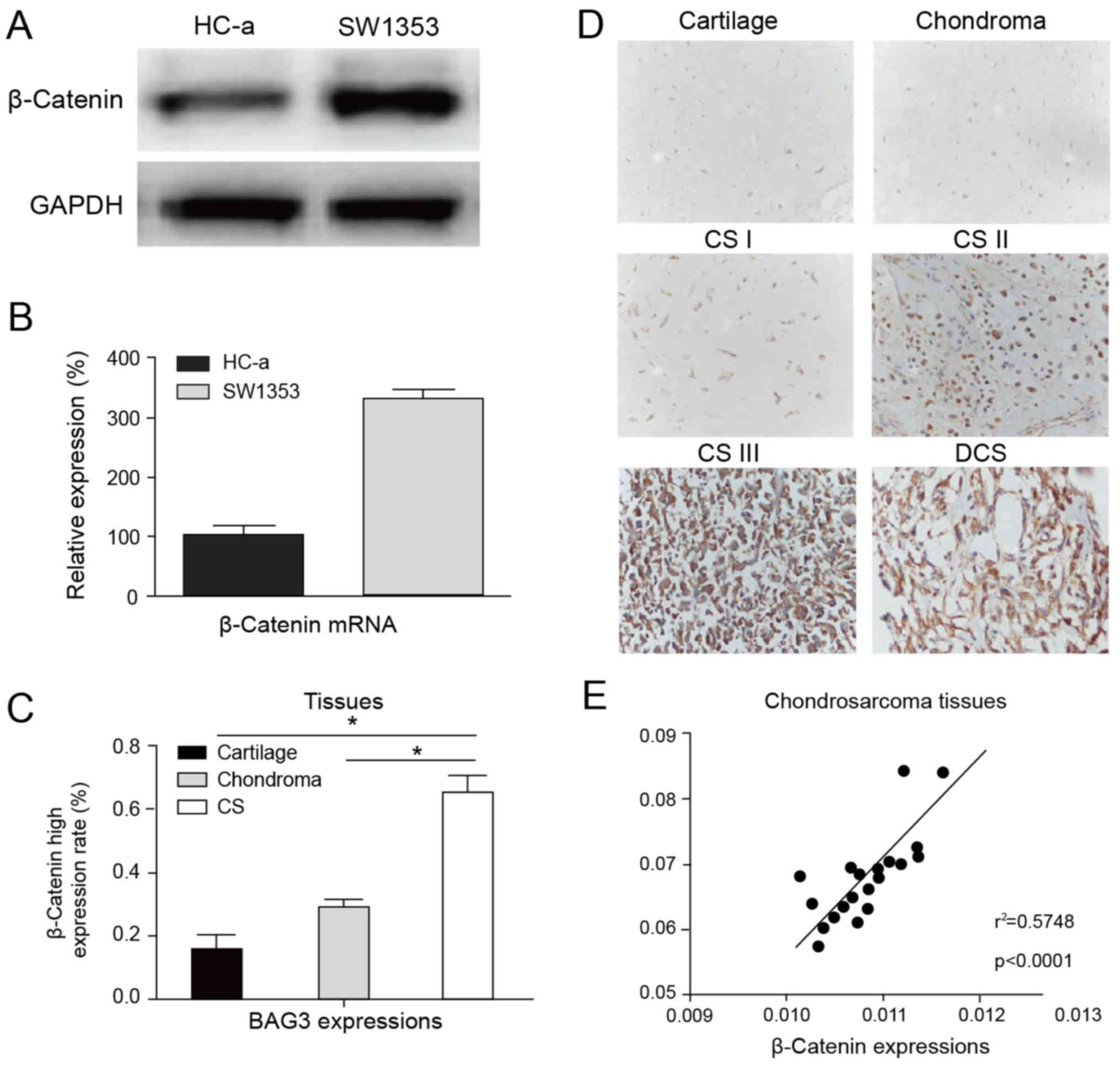
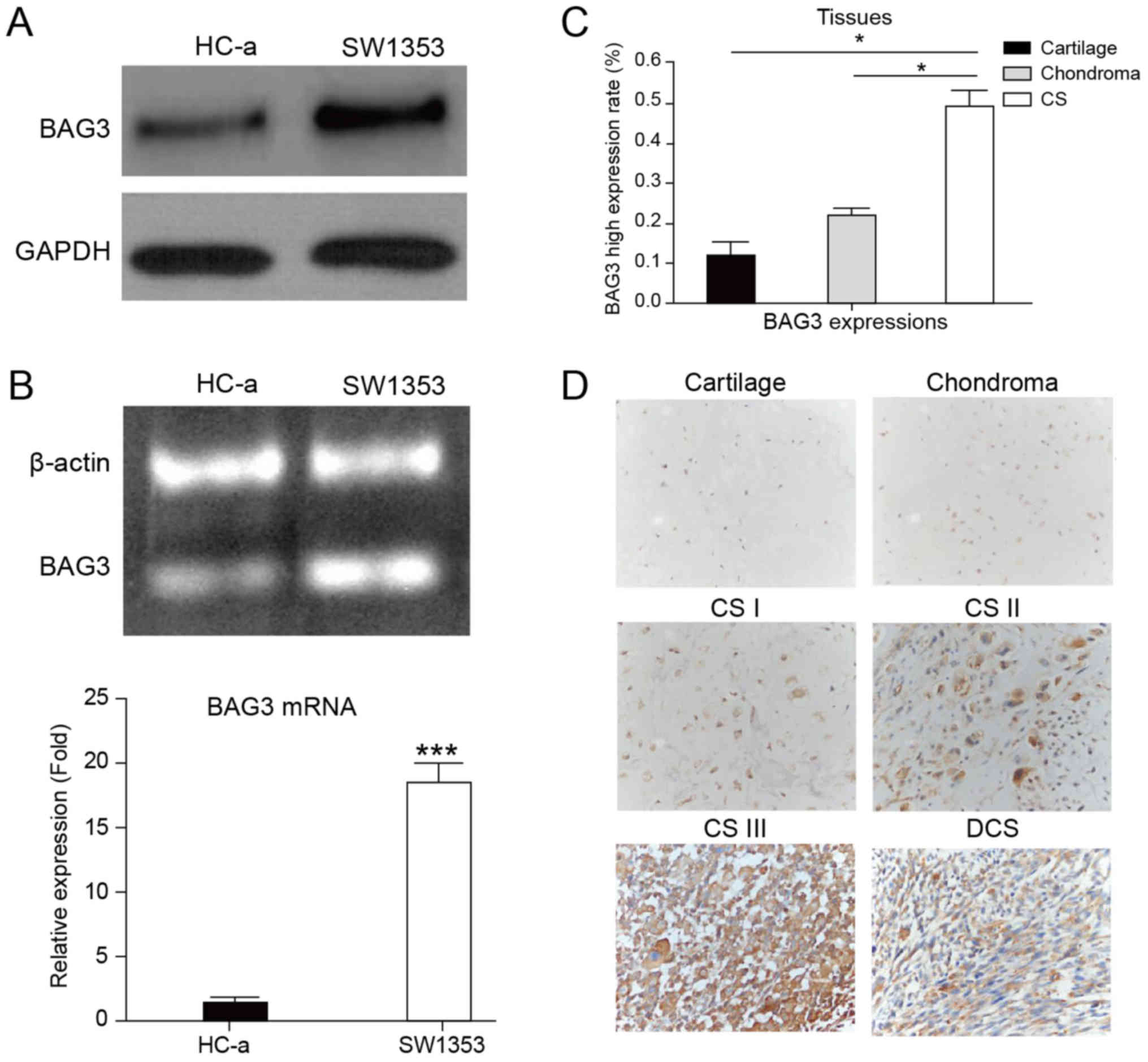 | Figure 1.Upregulation of BAG3 in human
chondrosarcoma. (A) The protein expression of BAG3 in the SW1353
chondrosarcoma cell line and the normal chondrocyte cell line, HC-a
by western blotting; GAPDH was used as a loading control. (B) The
mRNA expression of BAG3 in the SW1353 chondrosarcoma cell line and
normal chondrocyte cell line, HC-a by RT-qPCR; β-actin was used as
a loading control. (C) The mRNA expression of BAG3 in human
cartilage tissues, chondroma tissues and chondrosarcoma tumors by
RT-qPCR. (D) Immunohistochemical staining for BAG3 expression in
human cartilage tissues, chondroma tissues, chondrosarcoma tumors
from grade I to III and DCS. The micrographs shown represent the
range of staining observed in tissues (magnification, ×200). Data
are presented as the mean ± standard error of three independent
experiments. *P<0.05, as indicated; ***P<0.001 vs. HC-a.
BAG3, B-cell lymphoma-2 associated athanogene 3; DCS,
dedifferentiated chondrosarcoma; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; CS,
chondrosarcoma. |
 | Table I.Demographic characteristics of the 59
patients with chondrosarcoma. |
Table I.
Demographic characteristics of the 59
patients with chondrosarcoma.
|
| BAG3 |
|
|
|---|
|
|
|
|
|
|---|
| Characteristics | Low expression | High expression | Chi-square | P-value |
|---|
| Age (years) |
|
| 0.817 | 0.366 |
| ≤42 | 18 | 14 |
|
|
|
>42 | 12 | 15 |
|
|
| Sex |
|
| 0.410 | 0.522 |
| Male | 18 | 15 |
|
|
|
Female | 12 | 14 |
|
|
| WHO histology |
|
| 2.958 | 0.228 |
| I | 19 | 12 |
|
|
|
II–II | 9 | 13 |
|
|
| D- | 2 | 4 |
|
|
| Enneking stage |
|
| 13.926 | 0.001 |
| 1 | 23 | 7 |
|
|
| 2 | 7 | 18 |
|
|
| 3 | 0 | 4 |
|
|
| T
classification |
|
| 4.094 | 0.043 |
| 1 | 8 | 2 |
|
|
| 2 | 22 | 27 |
|
|
| Distant |
|
|
|
|
| metastasis |
|
| 3.270 | 0.071 |
| 0 | 30 | 26 |
|
|
| 1 | 0 | 3 |
|
|
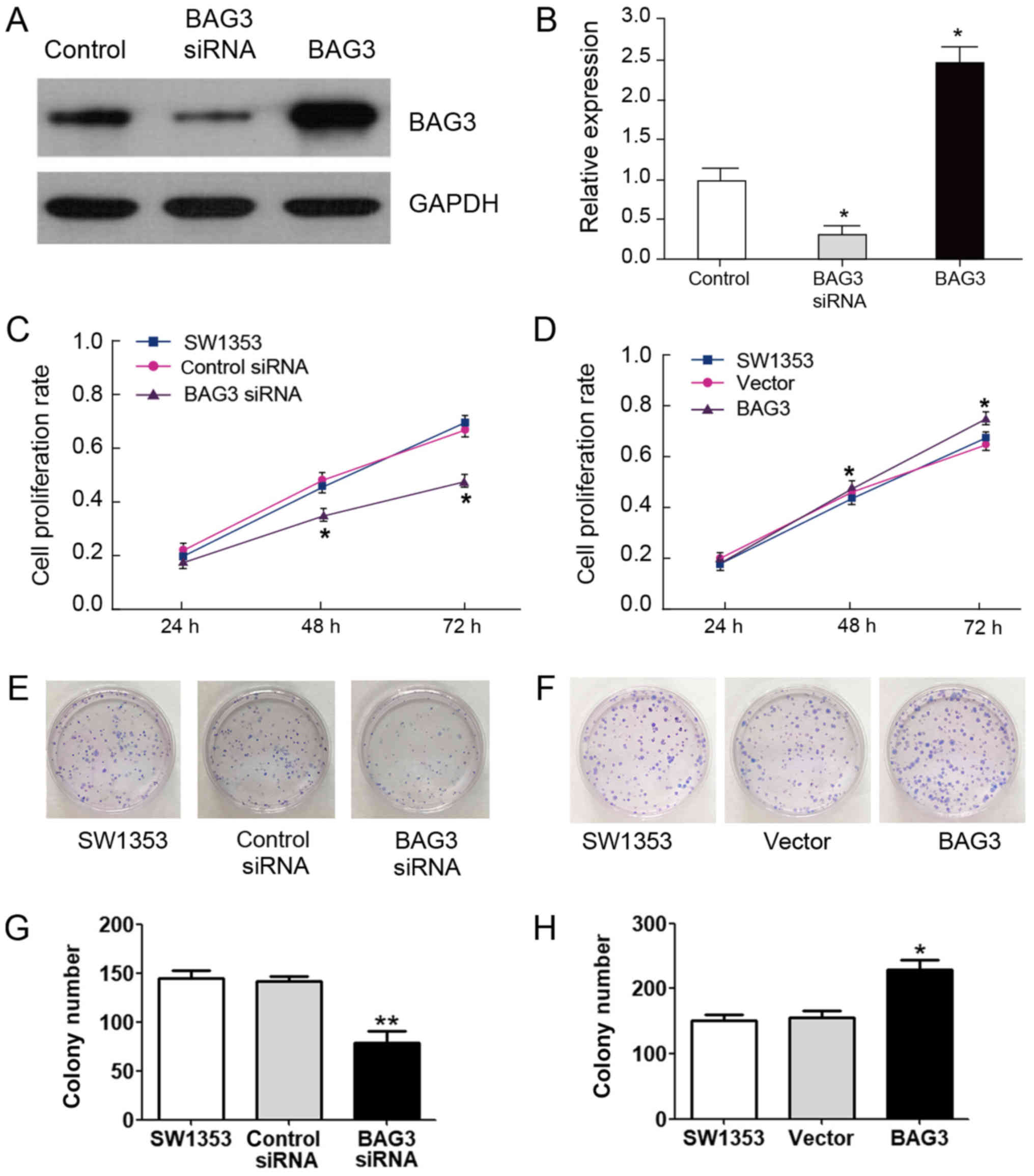
BAG3 promoted chondrosarcoma cells growth, migration
and inhibited cells apoptosis. Our above results demonstrated that
BAG3 might possess onco-protein functions. We next investigated the
role of BAG3 in chondrosarcoma cell proliferation and tumor growth.
SW1353 cells were transfected with siRNA to knock down BAG3 or
overexpression vector of BAG3 (Fig. 2A
and B). MTT assays results indicated that knockdown BAG3
resulted in the inhibition of cell proliferation compared with the
control siRNA-transfected cells (Fig.
2C). In contrast, overexpression of BAG3 promoted the cell
proliferation rates (Fig. 2D). To
further confirm the role of BAG3 in chondrosarcoma cell growth
in vitro, we performed clonogenic assays. Knock down of BAG3
suppressed colony formation (Fig.
2E) and overexpression of BAG3 notably increased colony
formation in fourteen days (Fig.
2F), suggesting BAG3 promoted chondrosarcoma cell
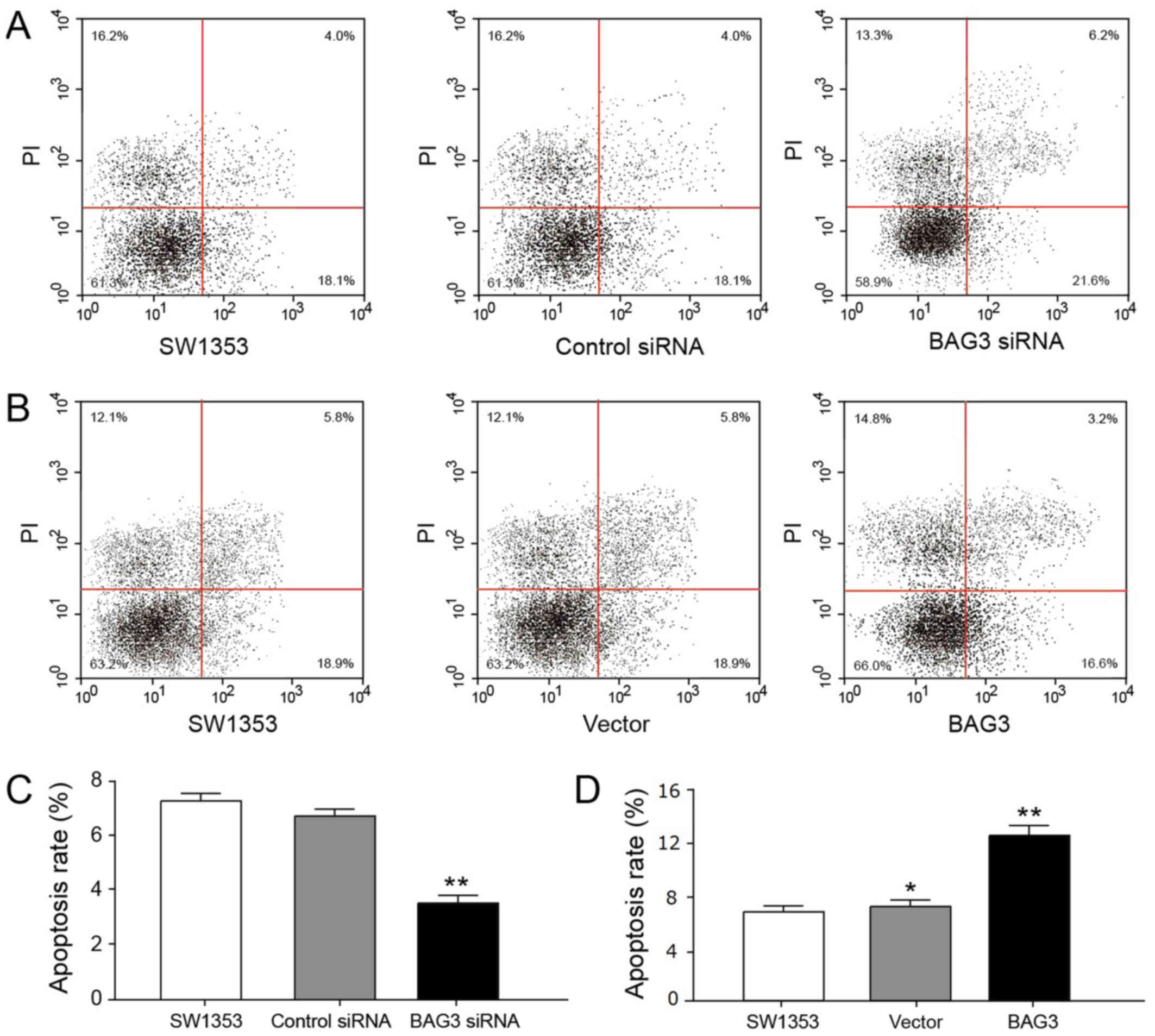
proliferation. We evaluated the roles of BAG3 in cell apoptosis.
Consistently, results in Fig. 3A and
B revealed that BAG3 is required to prevent chondrosarcoma
cells apoptosis. To investigate the effect of BAG3 on the viability
of chondrosarcoma cells, we counted the cell number in colony
formation experiment (Fig. 2G and
H) and evaluated the apoptosis rate of chondrosarcoma cells in
apoptosis experiment (Fig. 3C and
D).
BAG3 promotes chondrosarcoma cells migration
(Fig. 4A and B). The number of
migration cells in the control siRNA and BAG3 siRNA were 12 and 73,
respectively. On the contrary, the number of migration cells in the
control vector and BAG3 overexpression vector were 112 and 255,
respectively (Fig. 4C). The
maximum migration distance of BAG3 siRNA was 2,189 µm, the distance
in the control siRNA and the control vector group as well as the
control group were 568, 578 and 523 µm, respectively, and the
minimum distance of BAG3 overexpression vector was 300 µm (Fig. 4D).
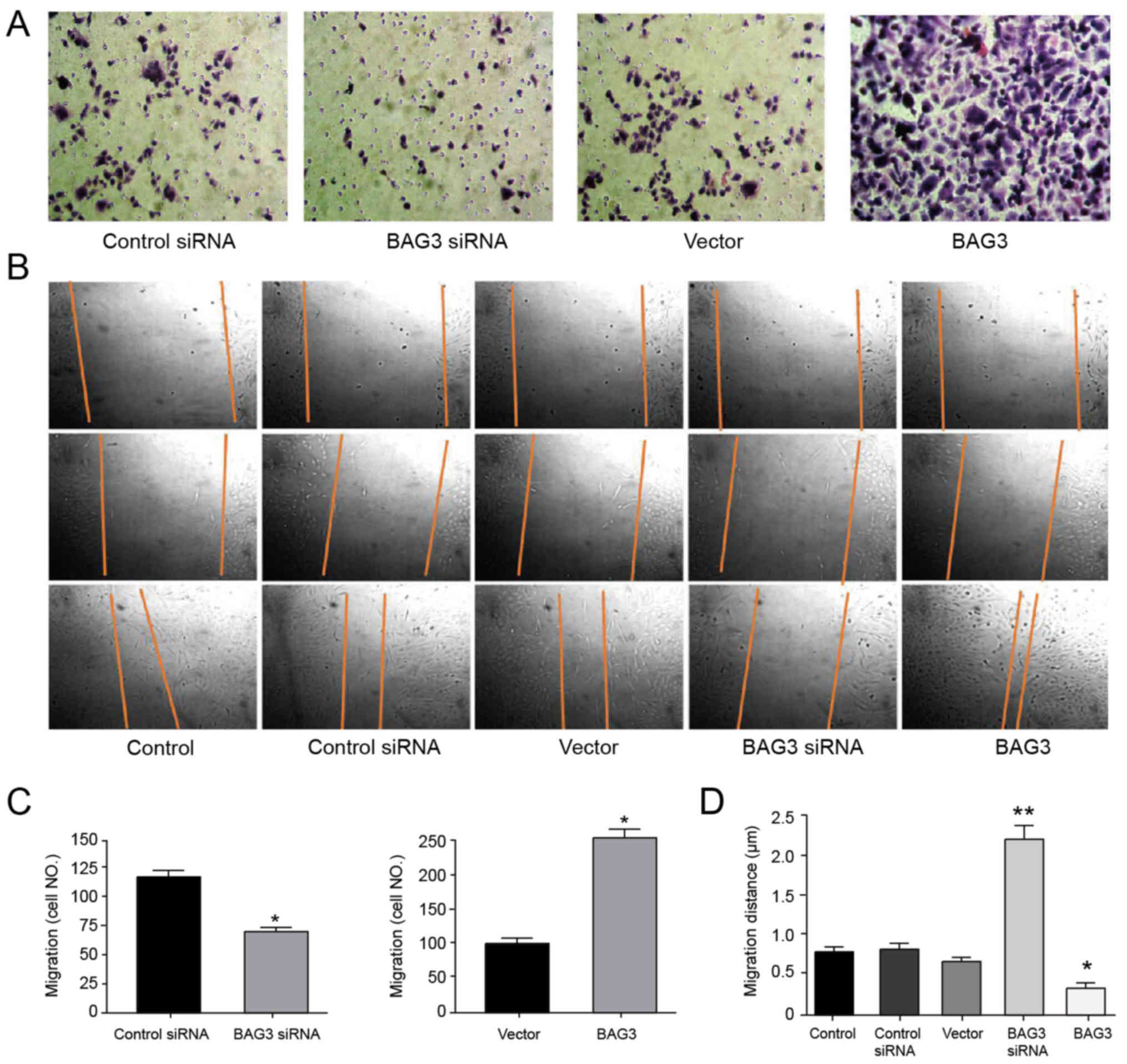 | Figure 4.BAG3 promoted chondrosarcoma cell
migration and invasion. (A) SW1353 cells were transfected with
control siRNA, BAG3 siRNA, control vector or BAG3 overexpression
vector for 48 h, which was followed by measurements of cell
invasion. (B) Migration in SW1353 cells with or without
transfection of control, control siRNA, vector, BAG3 siRNA or BAG3
overexpression vector for 48 h (magnification, ×100). (C) The
number of migratory SW1353 cells. (D) The migration distance of the
SW1353, control siRNA, BAG3 siRNA, vector or BAG3 groups. Data are
presented as the mean ± standard error of three independent
experiments. *P<0.05 and **P<0.005 vs. control. BAG3, B-cell
lymphoma-2 associated athanogene 3; siRNA, small interfering
RNA. |
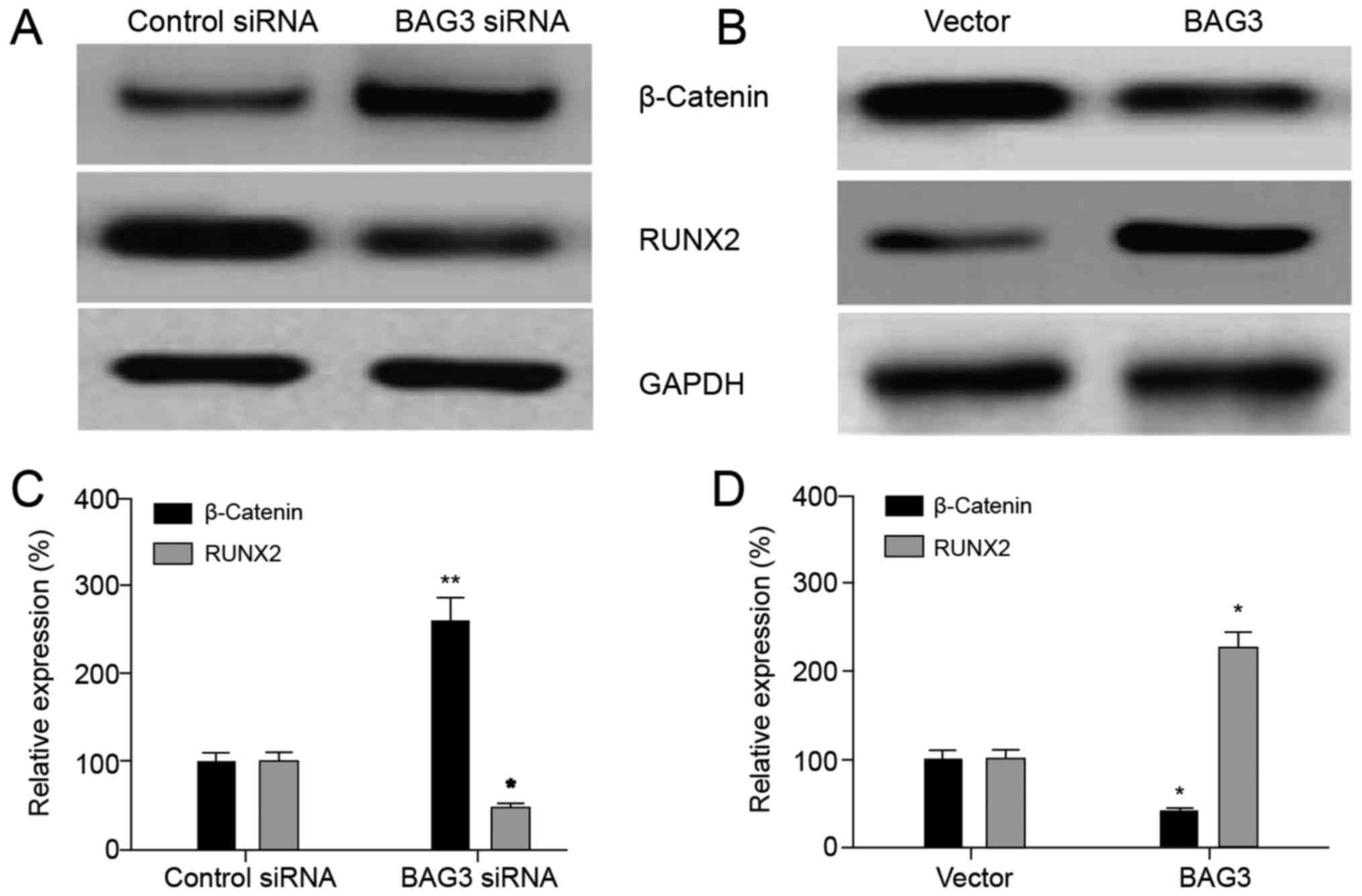
The RUNX2 in chondrosarcoma cells were
upregulated by BAG3
RUNX2 was a member of the Run-related transcription
factor family and is a key factor of osteoblast differentiation
(13,14). Studies found that mutations in the
RUNX 2 gene were closely related to bone formation in mouse model
and human body. In this study, we examined the effect of BAG3 on
the expression of RUNX2 gene. It was found that the expression
level of RUNX2 was significantly decreased after silencing BAG3
compared with the control group and increased after overexpressing
BAG3 compared with the blank load group (Fig. 5A and B), indicating that BAG3 could
upregulate the expression of RUNX2, as well as β-Catenin. The
relative expression levels of β-Catenin and RUNX2 proteins also
showed consistent results (Fig. 5C and
D).
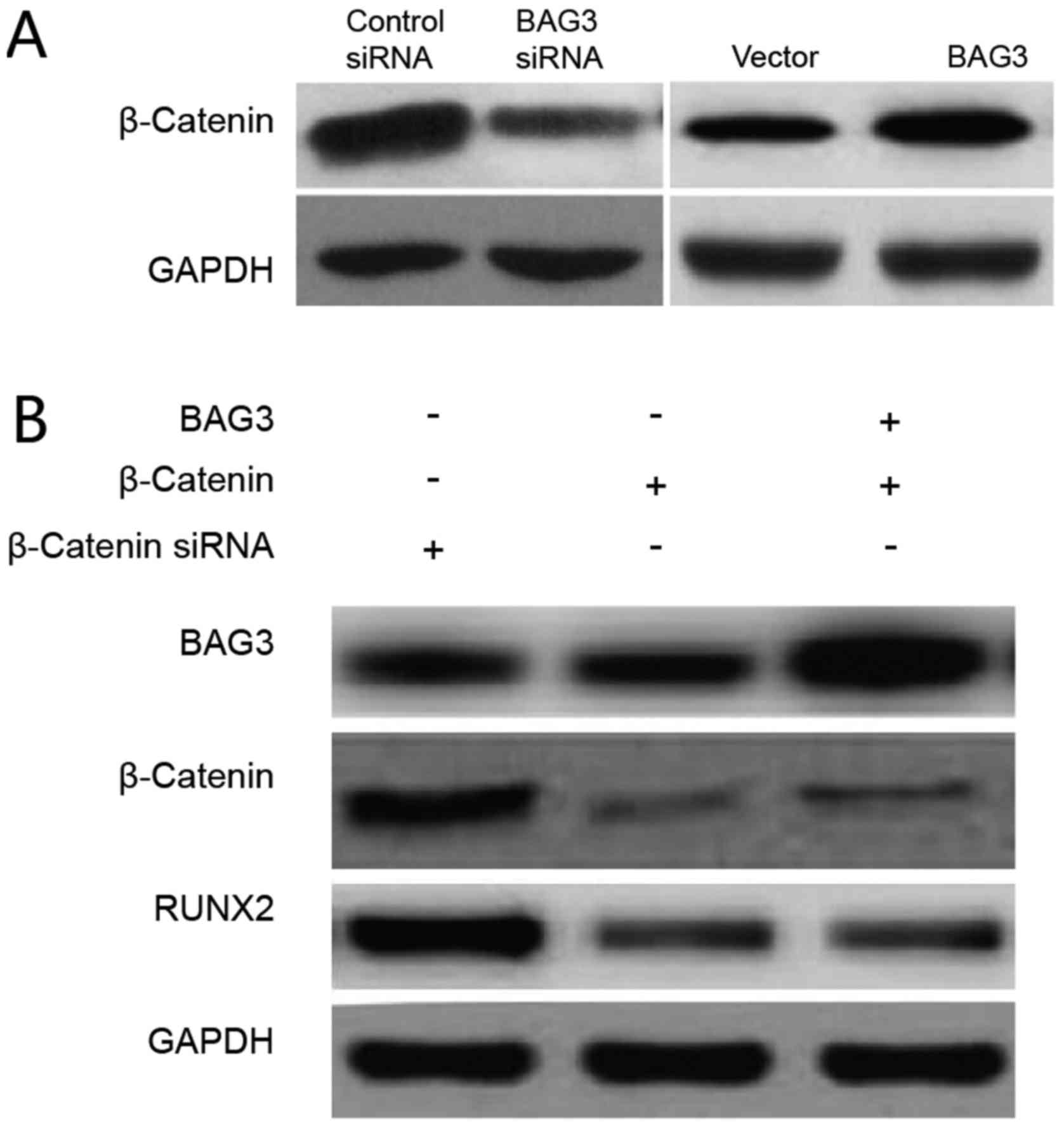
BAG3 promoted the expression of RUNX2
through upregulation of β-catenin
To further investigate signaling pathways that
involve in the BAG3-promoted the expression of RUNX2 through
upregulation of β-catenin in chondrosarcoma cells, we measured the
β-catenin expression in response to BAG3 knock down or
overexpression in SW1353 cells. Our results in Fig. 6A demonstrated that BAG3 upregulated
both protein and mRNA levels of β-catenin, intriguing us to
investigate whether BAG3 promoted the expression of RUNX2 through
the upregulation of β-catenin. SW1353 cells were transfected with
control vector, β-catenin siRNA or β-catenin siRNA plus BAG3
overexpressing vector. As we expected, overexpression of BAG3 could
not rescue the β-catenin expression in β-catenin knocking down
cells (Fig. 6B), indicating
β-catenin was a downstream signal molecule of BAG3. Moreover, knock
down of β-catenin significantly impeded the expression of RUNX2,
which could not be rescued by overexpression of BAG3 (Fig. 6B), suggesting that BAG3promoted the
expression of RUNX2 through the BAG3 downstream effector,
β-catenin.
An obviously positive correlation
between BAG3 expression and β-catenin in chondrosarcoma
tissues
To evaluate the clinical significance of the
BAG3-regulated β-catenin expression in chondrosarcoma, we first
compared the expressions of β-catenin in normal chondrocyte, HC-a
and chondrosarcoma cell line, SW1353. We observed a significantly
upregulation of β-catenin in tumor cells (Fig. 7A and B). The expression of
β-catenin were upregulated in chondrosarcoma tissues compared with
normal cartilage and chondroma tissues (Fig. 7C). Moreover, the expression of
β-catenin was positively correlated with the malignant stages (CS
I–III and DCS) of chondrosarcoma tissues in immunohistochemistry
stating (Fig. 7D), which was
consistent with previous reports that β-catenin acted as an
oncoprotein in human cancers (15). We next evaluated whether there is
positive correlation between BAG3 and β-catenin in chondrosarcoma
patient specimens. We analyzed twenty chondrosarcoma tissues, and
it was observed that BAG3 was correlatively expressed with
β-catenin (P<0.0001, r2=0.5748) (Fig. 7E). Meanwhile, it was found that in
BAG3 low-expressed chondrosarcoma tissues, β-catenin was at lower
potential to be dysregulated (40%) (Table II). Meanwhile, in BAG3
high-expressed chondrosarcoma tissues, the probability of the
abnormal expression of β-catenin was much higher (75.86%) (Table II). Our statistical analysis
showed that there was an obvious correlation between BAG3 and
β-catenin expression in chondrosarcoma patient specimens.
 | Table II.Associations between B-cell
lymphoma-2 associated athanogene 3 and β-catenin expression in
chondrosarcoma. |
Table II.
Associations between B-cell
lymphoma-2 associated athanogene 3 and β-catenin expression in
chondrosarcoma.
|
| BAG3
expression |
|
|
|---|
|
|
|
|
|
|---|
| β-catenin
expression | Low | High | Chi-square | P-value |
|---|
| Normal | 18 | 7 | 7.766 | 0.005 |
| Abnormal | 12 | 22 |
|
|
Discussion
This study was to explore the role of BAG3 in
chondrosarcoma and the possible mechanisms for the development of
tumorigenesis. BAG3 protein has been found to be associated with
anti-apoptotic functions recently. BAG3 gene contained 588 amino
acids, located in 10 q25, coded one kind of Bcl-2 related proteins,
and could combine with HSP70. Recent studies have shown that BAG3
is upregulated in a variety of malignant tumor, especially
adenocarcinoma such as ovarian cancer (16), colon cancer (17), prostate cancer (18), pancreatic cancer (19), breast cancer (20) and thyroid carcinoma (21). Our study revealed the oncogenic
roles of BAG3 in chondrosarcoma, suggesting anti-BAG3 might
contribute to development of anti-chondrosarcoma drugs. It was
confirmed that the expression of BAG3 was correlated with the
expression of β-catenin, and previous studies have proved that the
accumulation of β-catenin promoted the physiological process of
Chondrosarcoma (22). Therefore,
we can guess that the targeted drugs of BAG3 was studied to reduce
the distribution of BAG3 in chondrosarcoma and its expression,
which may decrease the accumulation of β-catenin in order to
achieve low expression level of β-catenin in chondrocytes, so as to
control the occurrence of chondrosarcoma. We found that the BAG3
mRNA and protein expressions were significantly upregulated in
chondrosarcoma cells compared with normal cartilage cell line HC-a.
Importantly, BAG3 protein expression was upregulated in 59 cases of
chondrosarcoma compared with in 30 cases of patients with
endogenous chondroma and 8 cases of normal cartilage. We also
analyzed BAG3 protein expression levels in chondrosarcoma regarding
the patient's clinical pathological parameters, clinical stage and
the survival time. Therefore, it is necessary to investigate the
functions and mechanisms of BAG3 during the malignant development
in chondrosarcoma. We measured the cell proliferation, the colony
formation, apoptosis and migration by knocking down or
overexpression of BAG3 in chondrosarcoma cells. All these results
consistently demonstrated that BAG3 possesses an oncogenic function
in chondrosarcoma.
It has been found that β-catenin as an adhesion
factor played an important role in the development of some tumors,
such as breast cancer and prostate cancer (23,24).
It demonstrated that BAG3 promoted the expression of RUNX2 in
chondrosarcoma and that RUNX22 was involve in the migration of
multiple bone tumors. Importantly, it was observed that β-catenin
was a key inducing factor of RUNX2 and was upregulated by BAG3 at
both mRNA and protein levels. Knockout of β-catenin in
chondrosarcoma cells could not result in high expression of RUNX2
through overexpression of BAG3, indicating that BAG3 upregulated
RUNX2 expression through mediating the expression of β-catenin.
We analyzed 59 cases of chondrosarcoma, 30 cases of
naturally express cartilage tumors and 8 cases of normal cartilage
tissues using immunohistochemical method and semi-quantitative
analysis. Among them, statistical analysis showed that there was an
obvious positive correlation between BAG3 and β-catenin expression,
suggesting BAG3 might be involved in the development of
chondrosarcoma by regulating β-catenin clinically. In summary, our
study revealed oncogenic roles of BAG3 in chondrosarcoma and
provided mechanisms that the BAG3-modulated the expression of RUNX2
through upregulation of β-catenin. This study provides a new
foundation for molecule-targeted therapy of human
chondrosarcoma.
Acknowledgements
The authors would like to thank the Department of
Pathology (The First Affiliated Hospital of Sun Yat-Sen University,
Guangdong, China) for supplying the patients' data.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81502327
and 81502021).
Availability of data and materials
All data generated and analyzed during this study
are included in this published article.
Authors' contributions
WC, YD, XL and WZ collected the data. HS conducted
the experiments, and drafted the manuscript and revised it
critically for important intellectual content. LW analyzed and
interpreted the data.
Ethics approval and consent to
participate
Patient's written informed consent and approval from
the Institute Research Ethics Committee of The First Affiliated
Hospital of Sun Yat-Sen University (Guangzhou, China) were obtained
(Ethics Committee approval no. 201301).
Consent for publication
All patients provided written informed consent.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gelderblom H, Hogendoorn PC, Dijkstra SD,
van Rijswijk CS, Krol AD, Taminiau AH and Bovée JV: The clinical
approach towards chondrosarcoma. Oncologist. 13:320–329. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xu J, Li D, Xie L, Tang S and Guo W:
Mesenchymal chondrosarcoma of bone and soft tissue: A systematic
review of 107 patients in the past 20 years. PLoS One.
10:e01222162015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wagner MJ, Livingston JA, Patel SR and
Benjamin RS: Chemotherapy for bone sarcoma in adults. J Oncol
Pract. 12:208–216. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Onishi AC, Hincker AM and Lee FY:
Surmounting chemotherapy and radioresistance in chondrosarcoma:
Molecular mechanisms and therapeutic targets. Sarcoma.
2011:3815642011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Italiano A, Mir O, Cioffi A, Palmerini E,
Piperno-Neumann S, Perrin C, Chaigneau L, Penel N, Duffaud F, Kurtz
JE, et al: Advanced chondrosarcomas: Role of chemotherapy and
survival. Ann Oncol. 24:2916–2922. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rosati A, Graziano V, De Laurenzi V,
Pascale M and Turco MC: BAG3: A multifaceted protein that regulates
major cell pathways. Cell Death Dis. 2:e1412011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fuchs M, Poirier DJ, Seguin SJ, Lambert H,
Carra S, Charette SJ and Landry J: Identification of the key
structural motifs involved in HspB8/HspB6-Bag3 interaction. Biochem
J. 425:245–255. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li X, Colvin T, Rauch JN, Acosta-Alvear D,
Kampmann M, Dunyak B, Hann B, Aftab BT, Murnane M, Cho M, et al:
Validation of the Hsp70-Bag3 protein-protein interaction as a
potential therapeutic target in cancer. Mol Cancer Ther.
14:642–648. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Colvin TA, Gabai VL, Gong J, Calderwood
SK, Li H, Gummuluru S, Matchuk ON, Smirnova SG, Orlova NV,
Zamulaeva IA, et al: Hsp70-Bag3 interactions regulate
cancer-related signaling networks. Cancer Res. 74:4731–4740. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kong DH, Zhang Q, Meng X, Zong ZH, Li C,
Liu BQ, Guan Y and Wang HQ: BAG3 sensitizes cancer cells exposed to
DNA damaging agents via direct interaction with GRP78. Biochim
Biophys Acta. 1833:3245–3253. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Habata S, Iwasaki M, Sugio A, Suzuki M,
Tamate M, Satohisa S, Tanaka R and Saito T: BAG3-mediated Mcl-1
stabilization contributes to drug resistance via interaction with
USP9X in ovarian cancer. Int J Oncol. 49:402–410. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Maruyama K, Ochiai A, Akimoto S, Nakamura
S, Baba S, Moriya Y and Hirohashi S: Cytoplasmic beta-catenin
accumulation as a predictor of hematogenous metastasis in human
colorectal cancer. Oncology. 59:302–309. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Komori T: Regulation of skeletal
development by the Runx family of transcription factors. J Cell
Biochem. 95:445–453. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Banerjee C, McCabe LR, Choi JY, Hiebert
SW, Stein JL, Stein GS and Lian JB: Runt homology domain proteins
in osteoblast differentiation: AML3/CBFA1 is a major component of a
bone-specific complex. J Cell Biochem. 66:1–8. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lemieux E, Cagnol S, Beaudry K, Carrier J
and Rivard N: Oncogenic KRAS signalling promotes the Wnt/β-catenin
pathway through LRP6 in colorectal cancer. Oncogene. 34:4914–4927.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Suzuki M, Iwasaki M, Sugio A, Hishiya A,
Tanaka R, Endo T, Takayama S and Saito T: BAG3 (BCL2-associated
athanogene 3) interacts with MMP-2 to positively regulate invasion
by ovarian carcinoma cells. Cancer Lett. 303:65–71. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shi H, Xu H, Li Z, Zhen Y, Wang B, Huo S,
Xiao R and Xu Z: BAG3 regulates cell proliferation, migration and
invasion in human colorectal cancer. Tumour Biol. 37:5591–5597.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Staibano S, Mascolo M, Di Benedetto M,
Vecchione ML, Ilardi G, Di Lorenzo G, Autorino R, Salerno V, Morena
A, Rocco A, et al: BAG3 protein delocalisation in prostate
carcinoma. Tumour Biol. 31:461–469. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Falco A, Rosati A, Festa M, Basile A, De
Marco M, d'Avenia M, Pascale M, Dal Piaz F, Tavano F, Di Mola FF,
et al: BAG3 is a novel serum biomarker for pancreatic
adenocarcinomas. Am J Gastroenterol. 108:1178–1180. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Felzen V, Hiebel C, Koziollek-Drechsler I,
Reißig S, Wolfrum U, Kögel D, Brandts C, Behl C and Morawe T:
Estrogen receptor α regulates non-canonical autophagy that provides
stress resistance to neuroblastoma and breast cancer cells and
involves BAG3 function. Cell Death Dis. 6:e18122015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chiappetta G, Ammirante M, Basile A,
Rosati A, Festa M, Monaco M, Vuttariello E, Pasquinelli R, Arra C,
Zerilli M, et al: The antiapoptotic protein BAG3 is expressed in
thyroid carcinomas and modulates apoptosis mediated by tumor
necrosis factor-related apoptosis-inducing ligand. J Clin
Endocrinol Metab. 92:1159–1163. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen C, Zhou H, Zhang X, Ma X, Liu Z and
Liu X: Elevated levels of Dickkopf-1 are associated with
beta-catenin accumulation and poor prognosis in patients with
chondrosarcoma. PLoS One. 9:e1054142014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nowicki A, Sporny S and Duda-Szymanska J:
β-catenin as a prognostic factor for prostate cancer (PCa). Central
European J Urol. 65:119–123. 2012. View Article : Google Scholar
|
|
24
|
Calaf GM, Alvarado ME and Hei TK: Beta
catenin is associated with breast cancer progression in vitro. Int
J Oncol. 26:913–921. 2005.PubMed/NCBI
|