Introduction
Ocular neovascularization (NV) is the primary cause
of blindness in a wide range of ocular diseases, including diabetic
retinopathy and age-related macular degeneration among others
(1). Endothelial progenitor cells
(EPCs) have been experimentally and clinically verified as a
contributor to both re-endothelialization and NV processes
(2–4). EPCs are implicated in an early stage
of embryogenesis and express a broad range of endothelial markers,
including fetal liver kinase-1 (FLK-1) and low-density lipoprotein
(LDL) receptor, which are integrated in the newly formed blood
vessels during vasculogenesis and angiogenesis.
In addition, endostatin is a cytokine that was first
discovered as a secretory product in the media of non-metastasizing
mouse cells. Endostatin is a 20 kDa C-terminal fragment of collagen
XVIII, and is an endogenous inhibitor of angiogenesis (5,6).
Endostatin may constitute a potent anti-angiogenic molecule for
treating ocular NV. Endostatin inhibits endothelial cell
proliferation, particularly in vitro, and inhibits
angiogenesis and tumor growth in animal models (5). However, large-scale protein
production is required in order to achieve a therapeutic effect
with regards to angiogenesis inhibition. Exogenous endostatin
protein has a short half-life in circulation, low peptide stability
in vivo and is costly, thus the clinical applications of
exogenous endostatin have been limited (7). Gene therapy may be the best way to
explore practical application of endostatin in anti-angiogenesis. A
previous study revealed that intravitreous injection of adenoviral
vector samples containing sig-mEndo transgenes, in order to
increase endostatin amounts, reduced laser-induced choroidal NV and
retinal NV in a mouse model (8).
Both EPCs and endostatin are implicated in NV. EPCs
genetically modified with endostatin may represent an effective
therapeutic intervention strategy for the treatment of patients
with ocular NV. However, in order for a gene-delivery system to be
successful, highly efficient gene transfection and stable,
long-term expression of the proteins are required. Therefore, the
present study aimed to investigate the possibility of the
generating a stable effective transfection of endostatin in EPCs,
and to detect the vascular endothelial growth factor (VEGF)
secretion for the observation of the anti-angiogenic effect of
endostatin-transfected EPCs.
Materials and methods
Ethics statement
All of the animal experiments in the present study
were approved by the Institutional Animal Care and Ethics Committee
of Zhejiang University (Hangzhou, China). All surgeries were
performed under anesthesia and all possible efforts were made to
minimize animal discomfort and stress. The methods in the present
study were performed in accordance with approved guidelines and
regulations.
EPC culture
EPCs were cultured according to our previous study
(9). Five Sprague-Dawley rats
(age, 2–3 months; 3 male and 2 female; 250–300 g; Laboratory Animal
Centre of Zhejiang University, Hangzhou, China) were kept in a 12 h
light/dark cycle at 22±1°C, 50–60% humidity with free access to
food and water. Animals were fasted 12 h before the experiment and
were given no water on the day of the experiment. Rats were
anesthetized for 10 min prior to each experiment via
intraperitoneal injection of sodium pentobarbital (Merck KGaA,
Darmstadt, Germany) at a dose of 30 mg/kg. Following this, 5 ml of
peripheral blood was collected from the right ventricle of each
anesthetized rat. Post-blood collection, the rats were then
sacrificed and their carcasses were handled in accordance with
approved guidelines. The blood samples were heparinized, diluted
with phosphate buffer saline and then separated using Ficoll
solution (GE Healthcare Bio-Sciences, Pittsburgh, PA, USA). The
layer of peripheral blood mononuclear cells was isolated via
density centrifugation, and was then re-suspended in EGM-2MV medium
(Lonza Group, Ltd., Basel, Switzerland) within a fibronectin-coated
vessel. Unattached cells were subsequently removed following 4 days
of culture at 37°C and then periodically removed every 2 days
thereafter. At 80–90% confluence, the cells were trypsinized using
0.25% trypsin (Solarbio Life Sciences, Beijing, China) and then
sub-cultured for 7 days onto glass cover slips at ~1×105
cells/cm2.
EPC characterization
Following sub-culture, the cells were then incubated
with 1,1-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine-labeled
acetylated LDL (Dil-Ac-LDL; 12 µg/ml; cat. no. L3484; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) for 10 h at room temperature in
dark. Subsequently, cells were fixed by 4% polyoxymethylene at room
temperature for 20 min and counterstained with fluorescein
isothiocyanate-conjugated Ulex europaeus lectin (FITC-UEA-1;
10 µg/ml; cat. no. L9006; Sigma-Aldrich; Merck KGaA) for 1 h at
room temperature. Nuclei were counterstained with DAPI (cat. no.
C1002; 0.1 µg/ml; Beyotime Institute of Biotechnology, Shanghai,
China) at room temperature for 10 min. Micrographs were acquired by
fluorescence microscopy (magnification, ×100) (Motic Incorporation,
Ltd., Causeway Bay, Hong Kong). Cells with double-positive
fluorescence were considered to be EPCs. Following staining, the
total number of double-positive Dil-Ac-LDL/FITC-UEA-1 cells was
calculated by counting the cells in each visual field, which were
then expressed as the percentage of EPCs marked positive for merged
Dil-Ac-LDL/FITC-UEA-1 dual staining. In addition, flow cytometry
was used to analyze the expression of CD34 and CD133 progenitors,
as well as FLK-1 and CD31 endothelial lineage markers (10–14).
Cells were harvested and washed with ice cold PBS. Cells were
subsequently blocked with 10% goat serum (cat. no. SL038; Beijing
Solarbio Science & Technology Co., Ltd., Beijing, China) at
room temperature for 30 min. Anti-CD34 (cat. no. sc-7324; dilution
1:200; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), anti-CD133
(cat. no. 18470-1-AP; 1:500; ProteinTech Group, Inc., Chicago, IL,
USA) and anti-FLK-1 (cat. no. ab2349; dilution 1:1,000; Abcam,
Cambridge, UK) primary antibodies were incubated with the cells at
4°C for 2 h. Subsequently, cells were washed with PBS and incubated
with the phycoerythin-conjugated goat anti-rabbit IgG (cat. no.
sc-3739; 1:200; Santa Cruz Biotechnology, Inc.) or fluorescein
isothiocyanate (FITC)-conjugated goat anti-mouse IgG secondary
antibodies (cat. no. sc-2010; 1:200; Santa Cruz Biotechnology,
Inc.), or incubated directly with FITC-conjugated anti-CD31 primary
antibody (cat. no. ab33858; 1:100; Abcam) at 4°C for 30 min in the
dark. Finally, the cells were washed with PBS and resuspended in
PBS; 1×106 cells (20 µl) were used each time. Passage 3
cultures were used for all experiments. Quantitative fluorescence
analysis was performed with a FACSCalibur flow cytometer (BD
Biosciences, Franklin Lakes, NJ, USA), and the data was analyzed
with CellQuest Pro software (version 5.1; BD Biosciences).
Endostatin expression plasmid
construction
The endostatin fragment was amplified between the
NotI and BamHI restriction sites via polymerase chain
reaction (PCR) using the following primer sequences: B2085CEF,
5′-AGGGTTCCAAGCTTAAGCGGCCGCGCCACCATGCATACTCATCAGGAT-3′ and
B2085CER, 5′-ATCAGTAGAGAGTGTCGGATCCTTATTTGGAGAAAGAGGTCATGAAG-3′.
Pfu DNA polymerase (cat. no. D7216; 0.3 µl; Beyotime Institute of
Biotechnology) was used and the thermocycling conditions were as
follows: 95°C for 3 min; followed by 30 cycles of 94°C for 30 sec,
55°C for 30 sec and 72°C for 30 sec and final extension at 72°C for
5 min. The product was then verified via DNA sequencing. The
endostatin fragment was subsequently cloned into a lentiviral (LV)
vector [LV5-EF1a-green fluorescent protein (GFP)+PURO] (Shanghai
GenePharma Co., Ltd., Shanghai, China) using
ClonExpress® Entry One Step Cloning kit (cat. no. C114;
Vazyme Biotech Co., Ltd., Nanjing, China) for 30 min at 37°C. The
recombinant LV vector (LV5-EF1a-GFP+PURO-Endostatin) was then
produced via co-transfection of 293T cells with three plasmids
[pLV/helper-SL3 (4 µg), pLV/helper-SL4 (4 µg), pLV/helper-SL5 (4
µg)] and endostatin plasmid (4 µg) using Lipofectamine®
2000 reagent (Thermo Fisher Scientific, Inc.) for 24 h at 37°C. The
medium was then changed and following 48 h of further incubation,
cell culture supernatants were then collected and centrifuged at
2,500 × g for 15 min at 37°C. The recombinant LV vector solution
was then concentrated and stored at −80°C. Following flow
cytometric assessment for GFP levels, vector titers were expressed
as transduction units per ml.
Stable transduction of EPCs
At 80–90% confluence, the primary EPCs were
transferred into 6-well plates at 1×106 cells/well for
lentiviral transduction. A medium containing the lentiviral vector
(LV-Endostatin-GFP) and polybrene (5 µg/ml; Merck KGaA, Germany)
was added at a multiplicity of infection of 100 in order to improve
infection efficiency, and then mixed with the cells. Following
incubation for 24 h, the cell culture medium was then removed and
replaced with Dulbecco's modified Eagle's medium (Hyclone; GE
Healthcare Life Sciences, Logan, UT, USA) supplemented with 10%
fetal bovine serum. At 80–90% confluence, the transfected EPCs and
control cells were subjected to puromycin (1 µg/ml) selection.
Cells transduced with LV-Endostatin-GFP were subsequently used as
the endostatin overexpression (OE) groups. Cells that did not
undergo transduction were used as the blank control groups. Cells
transduced with GFP alone were used as the negative control (NC)
groups.
RNA extraction
Total RNA was obtained from EPCs by
phenol-chloroform extraction and ethanol precipitation using the
TRIzol reagent (Thermo Fisher Scientific, Inc.) following the
manufacturer's protocol. RNA quantities and purities were
determined spectrophotometrically using a Nano Drop®
instrument (Thermo Fisher Scientific, Inc.), and RNA purity was
further assessed by determining the optical density (OD) ratio at
260:280 nm. RNA integrity was determined by 1% agarose gel
electrophoresis and visualized with ethidium bromide (0.5
µg/ml).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RT-qPCR was used for the determination of endostatin
and VEGF gene expression levels. Reverse transcription to cDNA was
performed using the Takara PrimeScript® RT Reagent kit
(Takara Biotechnology Co., Ltd., Dalian, China). The reaction
conditions were as follows: 42°C for 30 min and 85°C for 10 min.
PCR was performed using SYBR Premix Ex Taq™ II (TliRNaseH Plus;
cat. no. RR820A; Takara Biotechnology Co., Ltd.) according to the
manufacturer's instructions. Taq DNA polymerase (cat. no. MB3408;
2.5 U/µl; Melone Biological Technology Co., Ltd., Dalian, China)
was used with the following thermocycling conditions: 95°C for 3
min, followed by 40 cycles of 95°C for 12 sec and 62°C for 40 sec.
The bands were normalized using GAPDH as a housekeeping gene
control (internal control). The relative expression levels of
endostatin genes were quantified with the 2−ΔΔCq method
(15). The following primers were
used: endostatin forward, 5′-TCTCCCAAGTCGAAGACCCT-3′ and reverse,
5′-GAACAGCAGCGAAAAGTCCC-3′; VEGF forward, 5′-GTGAGCCTTGTTCAGAGCG-3′
and reverse, 5′-GACGGTGACGATGGTGG-3′; GAPDH forward,
5′-TCTCTGCTCCTCCCTGTTCT-3′ and reverse, 5′-ATCCGTTCACACCGACCTTC-3′.
PCR was performed in triplicate in each cDNA sample.
Western blot assay
The expression levels of endostatin and VEGF
proteins in the supernatants of cell cultures were determined using
a western blot assay. EPCs were lysed with radioimmunoprecipitation
assay lysis buffer (cat. no. P0013; Beyotime Institute of
Biotechnology). Total protein was obtained from the supernatants of
cell lysates and the concentration was determined with a
bicinchoninic acid protein assay kit. Samples were subjected to
SDS-PAGE loading buffer, heated at 100°C for 5 min, cooled and then
centrifuged at 10,000 × g for 10 min. Equal amounts of protein (20
µg) were resolved by 10% SDS-PAGE, electro-transferred onto
polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA,
USA), and blocked with Tris-buffered saline containing 0.05%
Tween-20 and 5% non-fat dry milk at room temperature for 2 h.
Membranes were then incubated with primary mouse anti-endostatin
antibody (dilution 1:1,000; cat. no. ab64569; Abcam) and rabbit
anti-rat VEGF antibody (dilution 1:1,000; cat. no. 19003-1-AP;
ProteinTech Group, Inc.) at 4°C overnight. Following rinsing with
Tris-buffered saline containing Tween-20 (TBST; China National
Pharmaceutical Group Corporation, Beijing, China), then with
horseradish peroxidise-conjugated goat anti-rabbit or goat
anti-mouse IgG secondary antibodies [dilution 1:20,000; cat. nos.
GAR0072 and GAM0072, respectively; MultiSciences (Lianke) Biotech,
Co., Ltd., Hangzhou, China] for 2 h at 37°C. Protein expression was
normalized to GAPDH and expressed as relative densitometry units.
Detection of immunoreactive bands was carried out using the
Enhanced Chemiluminescence System (GE Healthcare Bio-Sciences).
Gel-Pro Analyzer software (version 4; Media Cybernetics, Inc.,
Rockville, MD, USA) was used for data analysis.
Statistical analysis
SPSS version 19.0 software (IBM Corp., Armonk, NY,
USA) was used to analyze all data via one-way analysis of variance
followed by a Least Significant Difference post-hoc test. All data
were expressed as the mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant difference. Each
in vitro experiment was repeated at least three times.
Results
Characterization of EPCs
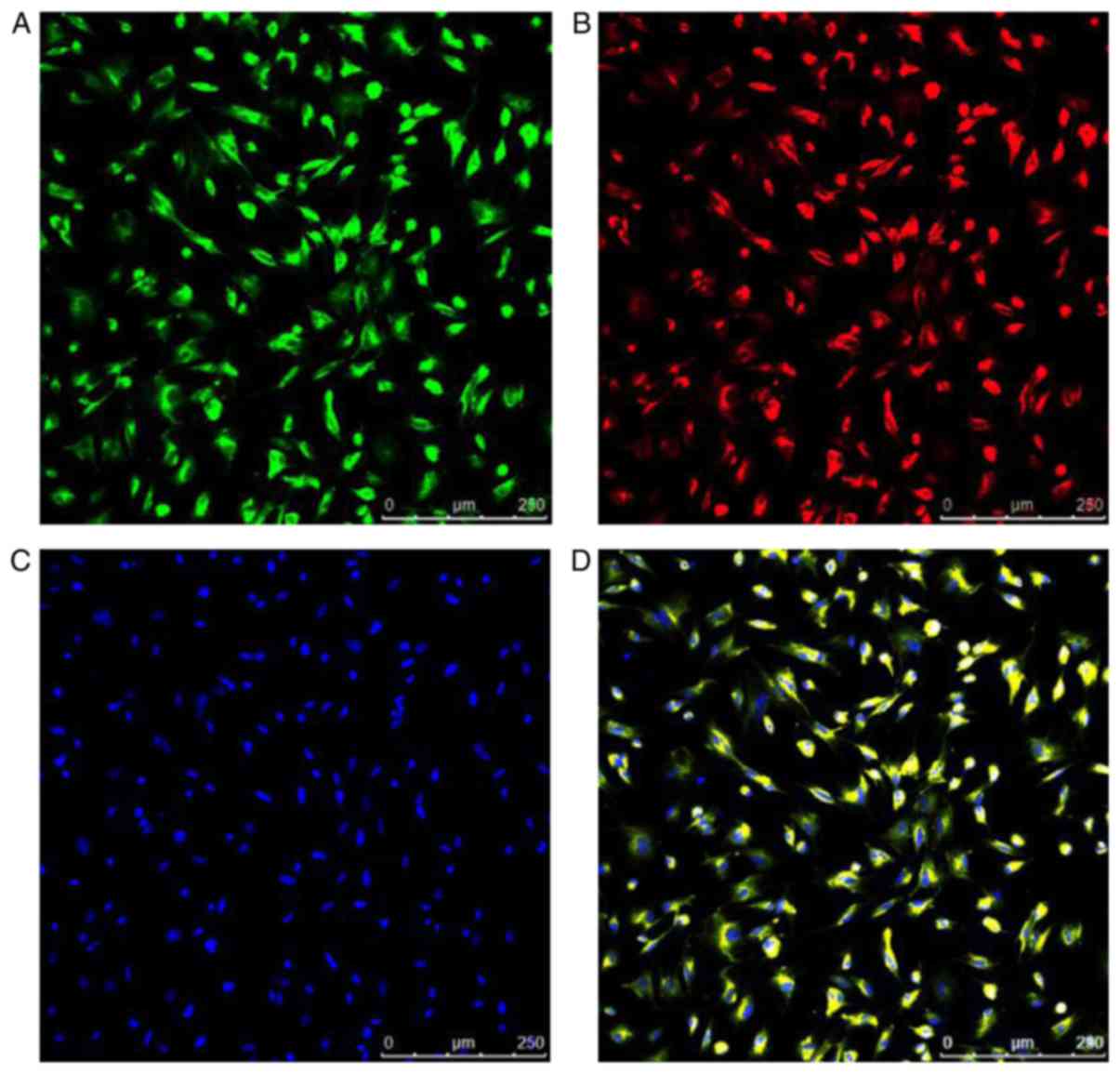
EPCs are able to take up Dil-Ac-LDL and FITC-UEA-1,
the levels of which were demonstrated by fluorescent staining.
Nuclei counterstained with DAPI produced blue fluorescence.
Positive staining with Dil-Ac-LDL and FITC-UEA-1 produced red
fluorescence and green fluorescence, respectively; double-positive
Dil-Ac-LDL and FITC-UEA-1 appeared as yellow fluorescence staining.
The percentage of double positive cells in the total number of
cells was 98.87±0.29% (Fig.
1).
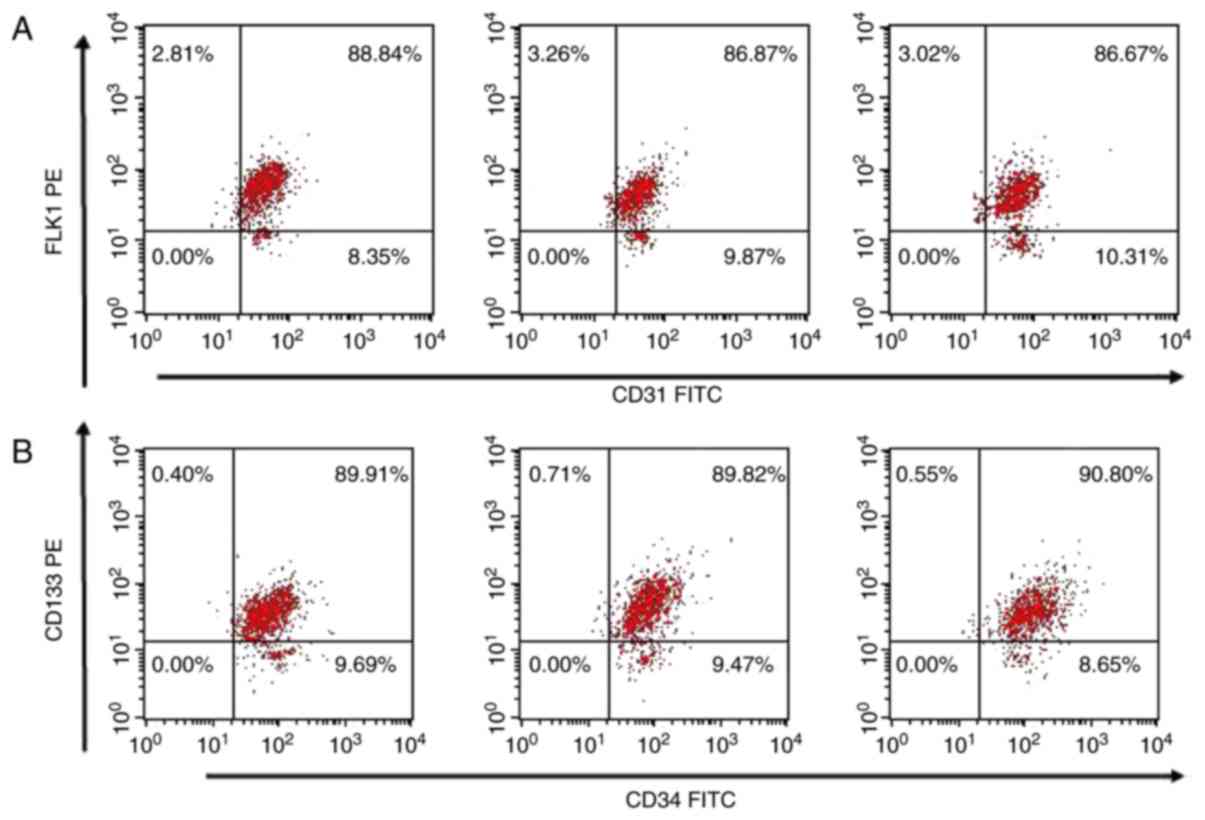
The expression levels of CD34, CD133, CD31 and FLK-1
EPC surface markers were investigated using flow cytometry. Flow
cytometry revealed that the expression levels of
CD31+/FLK1−,
CD31−/FLK1+,
CD34+/CD133−, and
CD34−/CD133+ were 9.91±1.20, 2.94±0.75,
9.03±1.35, and 0.65±0.19%, respectively. The
CD34+/CD133+ double-positive cells rate
amounted to 90.32±1.18%, and the CD31+/FLK-1+
double-positive cells rate amounted to 87.16±0.96% (Table I; Fig.
2).
 | Table I.Flow cytometry analyses of
endothelial progenitor cell surface markers. |
Table I.
Flow cytometry analyses of
endothelial progenitor cell surface markers.
| A,
FITC-CD31/PE-FLK1 antibodies |
|---|
| Cell type | Percentage (mean ±
standard deviation) |
|---|
|
CD31−FLK1− | 0.00±0.00 |
|
CD31+FLK1− | 9.51±1.03 |
|
CD31−FLK1+ | 3.03±0.25 |
|
CD31+FLK1+ | 87.46±1.20 |
|
| B,
FITC-CD34/PE-CD133 antibodies |
|
| Cell
type | Percentage (mean
± standard deviation) |
|
|
CD34−CD133− | 0.00±0.00 |
|
CD34+CD133− | 9.27±0.55 |
|
CD34−CD133+ | 0.55±0.16 |
|
CD34+CD133+ | 90.18±0.54 |
RNA integrity, purity and
concentration
RNA integrity was confirmed with the clear bands at
28s ribosomal (r)RNA and 18s rRNA markers on agarose gels. RNA
purity was reflected by an OD 260:280 nm value of 1.9–2.2 (Fig. 3).
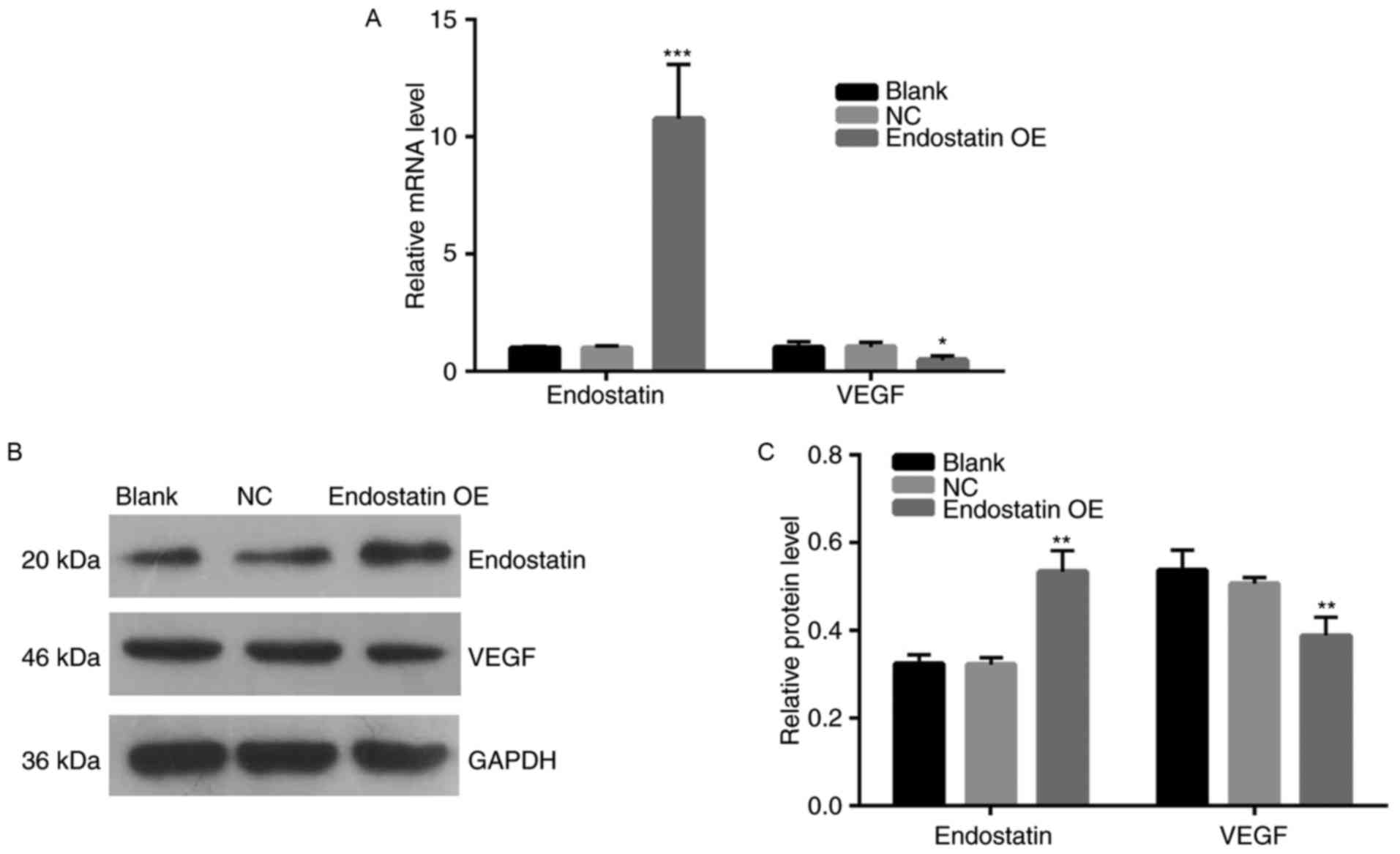
Endostatin expression
Compared with the NC group, the endostatin mRNA
expression of the OE group was significantly increased
(P<0.001). However, there was no significant difference in the
mRNA expression between the blank control group and NC group
(Fig. 4A). Fig. 4B and C present the results of the
western blot assay. Compared with the NC group, the endostatin
protein expression of the OE group increased significantly
(P<0.01). However, there was no difference in endostatin protein
expression between the blank control and NC groups.
VEGF expression
Fig. 4A presents
the results of VEGF mRNA expression analysis. Compared with the NC
group, VEGF mRNA expression of the OE group decreased significantly
(P<0.05). However, there was no significant difference in the
level of VEGF mRNA expression between the blank control and NC
groups. Fig. 4B and C present the
results of VEGF protein expression levels. Compared with the NC
group, VEGF protein expression of the OE group decreased
significantly (P<0.01). However, there was no significant
difference in the level of VEGF protein expression between the
blank control and NC groups.
Discussion
EPCs are considered to be circulating cells with
significant pathological and therapeutic properties. EPCs have the
ability to migrate to areas where NV occurs, and participate in
either NV or endothelial repair. Such cells contribute to
neovasculature by differentiating into endothelial cells (ECs) via
the process of vasculogenesis (16), which contributes to postnatal
vascular remodeling and NV (17–20).
Despite EPCs contributing to NV, it is yet to be investigated
whether or not a transplant of normal healthy EPCs has the
potential to aggregate NV of diabetic retinopathy (21). In addition, ischemic vascular
damage may be repaired by healthy and nondiabetic EPCs (22). Furthermore, intravitreal injections
of EPCs have been confirmed to rescue degenerated retinas; healthy
EPCs may repair unhealthy NV tissue and indirectly inhibit ocular
NV (23). EPCs can be purified,
expanded in vitro and administered to patients as autologous
cells to revascularize ischemic tissues. Thus, EPCs may serve as a
potential therapeutic agent for use in future clinical therapy.
The present study successfully isolated and cultured
EPCs in vitro, and determined the typical expression levels
of EPC surface markers (CD34, CD133, CD31 and FLK-1). CD133 is the
most promising candidate for use as a specific EPC marker, as it is
expressed solely in early EPCs and lost once the EPCs have
differentiated into mature ECs (10–14).
Furthermore, differentiating EPCs have the ability to take up
Dil-Ac-LDL and FITC-UEA-1 simultaneously (12,24),
and the present study demonstrated that the Dil-Ac-LDL and
FITC-UEA-1 double-positive staining percentage of EPCs was
98.87±0.29%, therefore verifying that the cultured cells were in
fact differentiating EPCs.
Furthermore, the present study also successfully
developed an endostatin overexpressing EPC line for increasing
long-term expression of endostatin, which has previously been
revealed to be an endogenous inhibitor possessing anti-angiogenic
activity, and to be responsible for suppressing retinal vascular
leakage (25,26). Endostatin administration may offer
an innovative, preventative pharmaceutical strategy for ocular NV,
whilst demonstrating anti-tumor effects when delivered continuously
(27,28). However, the use of endostatin in
clinical trials for NV therapy has previously been hindered by
difficulties regarding the production of large quantities of the
protein, the loss of endostatin's biological activity during
long-term storage and cumbersome daily administration requirements
(6). The present investigation
into endostatin production may provide new insight with regards to
potential therapeutic endostatin use, and may resolve these
difficulties.
In addition, EPCs may also promote regeneration of
the vasculature and damaged tissue via increased expression of
VEGF, hepatocyte growth factor (HGF) and other growth factors
(29). Furthermore, multiple
growth factors and cytokines have been demonstrated as being able
to recruit EPCs from the EPC rich bone marrow into neovascular
sites. Such factors include VEGF, HGF, insulin-like growth factor-1
and others (15). VEGF induces
angiogenesis via high-affinity tyrosine kinase receptors, such as
VEGF receptor 1 and VEGF receptor 2 (VEGFR-2). VEGFR-2 is a
predominant EPC surface marker (30) and mediates the effects of VEGF
(31). The interaction between
VEGF and VEGFR-2 induces microvascular EC proliferation and
migration, thus promoting angiogenesis (31–33).
It has been shown that in proliferative diabetic retinopathy, when
NV occurs, VEGF expression is increased in the vitreous and
sub-retinal fluid (34–36). Therefore, if angiogenic factors,
including VEGF, could be successfully inhibited, ocular NV
therapies may significantly progress. The present study revealed
that the expression of VEGF decreased significantly in the stable,
endostatin-transfected EPC line.
As previously aforementioned, it has been
hypothesized that EPC may serve as a vehicle for continuous
delivery of endostatin to tissues undergoing NV, and the present
study successfully developed an endostatin-overexpressing EPC line.
The results of this study suggest that the anti-angiogenic and
angiogenic agents may achieve autocrine and paracrine effects on
angiogenesis by increasing expression of endostatin via a gene
transfer system directly targeted to EPC, or by inhibition of VEGF
expression via the paracrine effects of endostatin.
The results of the present study suggest that a
cell-based therapeutic approach may prove useful in clinical
settings for the treatment of patients with NV, as endostatin is a
key anti-angiogenic factor and EPCs may be important for the future
of NV treatment. EPCs can be modified to produce endostatin via
gene transfer in vitro, thus avoiding frequent protein
administration. Furthermore, the results of the present study
indicate that EPCs constitute an optimal vehicle for the delivery
of anti-angiogenic protein molecules, as well as providing a strong
basis for the development of anti-angiogenic EPCs for NV treatment.
This strategy (autologous EPCs with overexpressed anti-angiogenic
agents) could be used for each stage of clinical ocular NV as well
as several other varieties of ocular vasculopathy. However, the
results of the in vitro experiment in the present study
cannot be extrapolated directly to human treatment of NV, and
therefore animal studies and clinical trials should be performed in
order to verify the results of the present study.
Acknowledgements
Not applicable.
Funding
This study was supported by Zhejiang Provincial
Natural Science Foundation of China (grant no. LQ14H120001),
Zhejiang Provincial Natural Science Foundation of China (grant no.
LZ18H180001), Zhejiang Key Laboratory Fund of China (grant no.
2011E10006), Project of National Clinical Key Discipline of Chinese
Ministry of Health and Zhejiang Province Key Research and
Development Program (grant no. 2015C03042), Zhejiang Provincial
Natural Science Foundation of China (grant no. LY14H120004), and
the National Natural Science Foundation of China (grant nos.
81371001, 81570822, 81500760 and 81500694).
Availability of data and materials
The analyzed datasets generated during the study are
available from the corresponding author on reasonable request.
Authors' contributions
KY and JA conceived, designed and supervised the
research. JA, JHS and TW performed the experiments. JM and LF
performed data analyses. JA and KY wrote the manuscript. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
All of the animal experiments in the present study
were approved by the Institutional Animal Care and Ethics Committee
of Zhejiang University (Hangzhou, China). The methods in the
present study were performed in accordance with approved guidelines
and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhang SX and Ma JX: Ocular
neovascularization: Implication of endogenous angiogenic inhibitors
and potentialtherapy. Prog Retin Eye Res. 26:1–37. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kong D, Melo LG, Gnecchi M, Zhang L,
Mostoslavsky G, Pratt RE and Dzau VJ: Cytokine-induced mobilization
of circulating endothelial progenitor cells enhances repair of
injured arteries. Circulation. 110:2039–2046. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hu Y, Davison F, Zhang Z and Xu Q:
Endothelial replacement and angiogenesis in arteriosclerotic
lesions of allografts are contributed by circulating progenitor
cells. Circulation. 108:3122–3127. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Abe Y, Ozaki Y, Kasuya J, Yamamoto K, Ando
J, Sudo R, Ikeda M and Tanishita K: Endothelial progenitor cells
promote directional three-dimensional endothelial network formation
by secreting vascular endothelial growth factor. PLoS One.
8:e820852013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
O'Reilly MS, Boehm T, Shing Y, Fukai N,
Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR and Folkman J:
Endostatin: An endogenous inhibitor of angiogenesis and tumor
growth. Cell. 88:277–285. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dudek AZ, Bodempudi V, Welsh BW, Jasinski
P, Griffin RJ, Milbauer L and Hebbel RP: Systemic inhibition of
tumour angiogenesis by endothelial cell-based gene therapy. Br J
Cancer. 97:513–522. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dhanabal M, Ramchandran R, Volk R,
Stillman IE, Lombardo M, Iruela-Arispe ML, Simons M and Sukhatme
VP: Endostatin: Yeast production, mutants, and antitumor effect in
renal cell carcinoma. Cancer Res. 59:189–197. 1999.PubMed/NCBI
|
|
8
|
Mori K, Duh E, Gehlbach P, Ando A,
Takahashi K, Pearlman J, Mori K, Yang HS, Zack DJ, Ettyreddy D, et
al: Pigment epithelium-derived factor inhibits retinal and
choroidal neovascularization. J Cell Physiol. 188:253–263. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sun JH, Zhang YL, Nie CH, Qian SP, Yu XB,
Xie HY, Zhou L and Zheng SS: In vitro labeling of endothelial
progenitor cells isolated from peripheral blood with
superparamagnetic ironoxide nanoparticles. Mol Med Rep. 6:282–286.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hristov M, Erl W and Weber PC: Endothelial
progenitor cells: Isolation and characterization. Trends Cardiovasc
Med. 13:201–206. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun W, Zheng L, Han P and Kang YJ:
Isolation and characterization of endothelial progenitor cells from
Rhesus monkeys. Regen Med Res. 2:52014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dome B, Dobos J, Tovari J, Paku S, Kovacs
G, Ostoros G and Timar J: Circulating bone marrow-derived
endothelial progenitor cells: characterization, mobilization and
therapeutic considerations in malignant disease. Cytometry A.
73:186–193. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rafii S, Lyden D, Benezra R, Hattori K and
Heissig B: Vascular and haematopoietic stem cells: Novel targets
for anti-angiogenesis therapy? Nat Rev Cancer. 2:826–835. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Asahara T, Murohara T, Sullivan A, Silver
M, van der Zee R, Li T, Witzenbichler B, Schatteman G and Isner JM:
Isolation of putative progenitor endothelial cells for
angiogenesis. Science. 275:964–967. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Friedrich EB, Walenta K, Scharlau J,
Nickenig G and Werner N: CD34/CD133+/VEGFR-2+
endothelial progenitor cell subpopulation with potent
vasoregenerative capacities. Circ Res. 98:e20–e25. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Paczkowska E, Gołąb-Janowska M,
Bajer-Czajkowska A, Machalińska A, Ustianowski P, Rybicka M, Kłos
P, Dziedziejko V, Safranow K, Nowacki P, et al: Increased
circulating endothelial progenitor cells in patients with
haemorrhagic and ischaemic stroke: The role of endothelin-1. J
Neurol Sci. 325:90–99. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Grant MB, May WS, Caballero S, Brown GA,
Guthrie SM, Mames RN, Byrne BJ, Vaught T, Spoerri PE, Peck AB, et
al: Adult hematopoietic stem cells provide functional hemangioblast
activity during retinal neovascularization. Nat Med. 8:607–612.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tomita M, Yamada H, Adachi Y, Cui Y,
Yamada E, Higuchi A, Minamino K, Suzuki Y, Matsumura M and Ikehara
S: Choroidal neovascularization is provided by bone marrow cells.
Stem Cells. 22:21–26. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tsai SH, Huang PH, Chang WC, Tsai HY, Lin
CP, Leu HB, Wu TC, Chen JW and Lin SJ: Zoledronate inhibits
ischemia-induced neovascularization by impairing the mobilization
and function of endothelial progenitor cells. PLoS One.
7:e410652012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Butler JM, Guthrie SM, Koc M, Afzal A,
Caballero S, Brooks HL, Mames RN, Segal MS, Grant MB and Scott EW:
SDF-1 is both necessary and sufficient to promote proliferative
retinopathy. J Clin Invest. 115:86–93. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Losordo DW and Dimmeler S: Therapeutic
angiogenesis and vasculogenesis for ischemic disease: Part II:
Cell-based therapies. Circulation. 109:2692–2697. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Caballero S, Sengupta N, Afzal A, Chang
KH, Li Calzi S, Guberski DL, Kern TS and Grant MB: Ischemic
vascular damage can be repaired by healthy, but not diabetic,
endothelial progenitor cells. Diabetes. 56:960–967. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liew A, Barry F and O'Brien T: Endothelial
progenitor cells: Diagnostic and therapeutic considerations.
Bioessays. 28:261–270. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bai YJ, Huang LZ, Zhou AY, Zhao M, Yu WZ
and Li XX: Antiangiogenesis effects of endostatin in retinal
neovascularization. J Ocul Pharmacol Ther. 29:619–626. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen R, Yu H, An YL, Chen HJ, Jia Z and
Teng GJ: Endothelial progenitor cells combined with cytosine
deaminase-endostatin for suppression of liver carcinoma. J Biomed
Nanotechnol. 12:1174–1182. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Baharivand N, Zarghami N, Panahi F, Dokht
Ghafari MY, Mahdavi Fard A and Mohajeri A: Relationship between
vitreous and serum vascular endothelial growth factor levels,
control of diabetes and microalbuminuria in proliferative diabetic
retinopathy. Clin Ophthalmol. 6:185–191. 2012.PubMed/NCBI
|
|
28
|
Szary J and Szala S: Intra-tumoral
administration of naked plasmid DNA encoding mouse endostatin
inhibits renal carcinoma growth. Int J Cancer. 91:835–839. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Otani A, Kinder K, Ewalt K, Otero FJ,
Schimmel P and Friedlander M: Bone marrow-derived stem cells target
retinal astrocytes and can promote or inhibit retinal angiogenesis.
Nat Med. 8:1004–1010. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hansen TM, Moss AJ and Brindle NP:
Vascular endothelial growth factor and angiopoietins in
neurovascular regeneration and protection following stroke. Curr
Neurovasc Res. 5:236–245. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hirashima M, Kataoka H and Nishikawa S,
Matsuyoshi N and Nishikawa S: Maturation of embryonic stem cells
into endothelial cells in an in vitro model of vasculogenesis.
Blood. 93:1253–1263. 1999.PubMed/NCBI
|
|
32
|
Eichmann A, Corbel C, Nataf V, Vaigot P,
Bréant C and Le Douarin NM: Ligand-dependent development of the
endothelial and hemopoietic lineages from embryonic mesodermal
cells expressing vascular endothelial growth factor receptor 2.
Proc Natl Acad Sci USA. 94:pp. 5141–5146. 1997; View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dimmeler S, Dernbach E and Zeiher AM:
Phosphorylation of the endothelial nitric oxide synthase at
ser-1177 is required for VEGF-induced endothelial cell migration.
FEBS Lett. 477:258–262. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Connolly DT, Heuvelman DM, Nelson R,
Olander JV, Eppley BL, Delfino JJ, Siegel NR, Leimgruber RM and
Feder J: Tumor vascular permeability factor stimulates endothelial
cell growth and angiogenesis. J Clin Invest. 84:1470–1478. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dieudonné SC, La Heij EC, Diederen RM,
Kessels AG, Liem AT, Kijlstra A and Hendrikse F: Balance of
vascular endothelial growth factor and pigment epithelial growth
factor prior to development of proliferative vitreoretinopathy.
Ophthalmic Res. 39:148–154. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Funatsu H, Noma H, Mimura T, Eguchi S and
Hori S: Association of vitreous inflammatory factors with diabetic
macular edema. Ophthalmology. 116:73–79. 2009. View Article : Google Scholar : PubMed/NCBI
|