Introduction
Nicotinamide phosphoribosyltransferase (Nampt),
which is also termed pre-B-cell colony-enhancing factor 1 or
visfatin, is a protein with a molecular weight of 52-kDa that
exhibits various functions (1–3). Two
isoforms of Nampt exist, which include intracellular Nampt (iNampt)
and extracellular, or secreted Nampt (eNampt). iNampt catalyses the
rate-limiting step in the nicotinamide adenine dinucleotide (NAD)
salvage pathway. At present, four pathways for NAD biosynthesis
have been identified. NAD may be synthesized from tryptophan, which
is a primary mechanism of biosynthesis, or via NAD salvage pathways
from various niacins, including nicotinamide (NAM), nicotinic acid
and nicotinamide riboside. In mammals, 90% of the substrates for
Nampt activity originate from NAM (4,5).
Although it is a central metabolic enzyme, eNampt is
considered to be an important secreted cytokine. eNampt is
primarily secreted by adipose tissues; however, it may also be
secreted by other cell types, including macrophages, hepatocytes,
chondrocytes, leucocytes, inflamed endothelial cells, human
peripheral blood lymphocytes, chronic lymphocytic leukaemia
lymphocytes, gastric cancer cells and antigen-presenting cells
(6). Despite this, the mechanism
of eNampt secretion has not yet been fully elucidated. Notably, the
3T3-L1 murine adipose cell line secretes high levels of eNampt
(7). iNampt is a cytosolic protein
that localises outside of intracellular vesicles. The secretion of
eNampt is not inhibited by brefeldin A or monensin, which indicates
that eNampt is not secreted by a classical endoplasmic
reticulum-Golgi apparatus-mediated pathway and instead occurs via
an alternative pathway (8).
Furthermore, it has been reported that sirtuin (SIRT)1 is required
for eNampt secretion; in the white and brown adipose tissue of
wild-type FVB mice, deacetylation of iNampt by the mammalian
NAD+-dependent deacetylase SIRT1 mediates the secretion
of the protein by adipocytes (7).
It has been previously reported that iNampt
functions in numerous oncogenic cellular activities, including DNA
repair, autophagy, apoptosis, tumour metastasis, inflammation and
angiogenesis (2,5–8). In
addition, iNampt has a central role in maintaining the equilibrium
of NAD degradation and NAD synthesis at stable levels. Through this
function, iNampt stimulates numerous NAD-dependent enzymes and
transcriptional factors, including SIRT1, CD38, BRCA1 and poly(ADP
ribose) polymerase 1, and influences the downstream pathways of
those proteins (6). Furthermore,
eNampt has been proven to circulate in the blood and exhibits
robust NAD biosynthetic activity (9). Importantly, the Nampt receptor has
not yet been identified.
Despite numerous reports concerning the action of
eNampt on various aspects of organs and the function of regulatory
systems, limited data is currently available concerning its
involvement in the regulation of the hypothalamic-pituitary-adrenal
(HPA) axis. eNampt is present in human serum and cerebrospinal
fluid (CSF); however, the levels of eNampt in the CSF are much
lower (~10%) compared with levels in the serum. The origin of the
CSF eNampt is not known (10).
Regarding this, 24 h after administration of Nampt protein into the
arcuate nucleus of the rat hypothalamus, increased food intake was
observed alongside reduced corticotropin-releasing hormone (CRH)
and cocaine and amphetamine-regulated transcript (CART) gene
expression, while proopiomelanocortin (POMC) gene expression
remained unaltered (11).
A limited amount of experimental data has indicated
the presence of an association between adrenocorticotropic hormone
(ACTH) and Nampt. ACTH stimulation reduces the Nampt gene
expression level in white adipose tissue cell culture by 60% after
4 h of treatment (12). In
addition, in adrenocortical cells, ACTH-stimulated accumulation of
reduced NAD (NADH) induced the transcription of cytochrome P450
family 17 subfamily A member 1 by promoting the dissociation of
corepressor carboxyl-terminal-binding proteins from the promoter
(13). Recently, Reverchon et
al (14) demonstrated that
eNampt induces and improves in vitro insulin-like growth
factor (IGF)1-induced steroidogenesis in bovine ovary primary cell
culture. They reported that human recombinant eNampt (10 ng/ml)
enhanced cellular progesterone and oestradiol output, an effect
that was associated with an increase in the protein expression of
steroidogenic acute regulatory protein (STAR), increased
hydroxy-Δ-5-steroid dehydrogenase, 3 β- and steroid Δ-isomerase 1
(HSD3B) activity and enhanced phosphorylation of the IGF1 receptor
(IGF1R) and the mitogen-activated protein kinase (MAPK)
extracellular signal-regulated kinase (ERK)1/2 in the presence or
absence of IGF1 (10 nM) (14).
These reports indicate that eNampt may directly affect
steroidogenic cells (bovine ovary primary cell culture). As iNampt
is a rate-limiting enzyme for NAD+ production, which is
used by the cytochrome P450 family in steroidogenesis, a similar
effect may be observed in the adrenal cortex. Concerning this, to
the best of our knowledge, no data are currently available, and the
present study therefore aimed to investigate the role of Nampt in
the regulation of rat HPA axis activity.
Materials and methods
Animals and reagents
Wistar rats were obtained from the Laboratory Animal
Breeding Centre of the Department of Toxicology, Poznan University
of Medical Sciences (Poznan, Poland). The animals were maintained
under standard light conditions (14:10 h light/dark cycle,
illumination onset at 6.00 a.m.) at 23°C, 50–60% air humidity, 8–10
air changes per hour (mechanical, via HEPA filters) with free
access to standard pellets and tap water. The studies were
performed on total of 90 sexually mature wistar rats. 15 rats were
used for in vivo experiments while 75 were used for all
in vitro experiments. The number of rats, their sex, age and
weight used in the current study are given in the descriptions of
the individual experiments or descriptions of the figures. The
study protocol was approved by the independent Local Ethics
Committee for Animal Studies in Poznań (protocol no. 75/2016). If
not otherwise stated, all reagents were obtained from Sigma-Aldrich
(Merck KGaA, Darmstadt, Germany) or Avantor Performance Materials
Poland S.A. (Gliwice, Poland). Recombinant human Nampt protein was
purchased from BioVendor R&D Products (Brno, Czech Republic)
and the specific Nampt inhibitor FK866 was purchased from ApexBio
Technology (Houston, TX, USA).
eNampt and ACTH administration in
vivo
Experiments were performed on 15 adult (3–4 months
old, 250–300 g body weight) male rats. The eNampt protein was
administered by intraperitoneal (i.p.) injection at a dose of 4
µg/100 g, while ACTH (Cortrosyn®; Organon
Pharmaceuticals; Merck KGaA, Darmstadt, Germany) was given at a
dose of 2.5 µg/100 g. Rats in the control group were administered
with 0.2 ml physiological saline (in each group-control, eNampt and
ACTH-n=5). Rats were decapitated 1 h after injection. Trunk blood
was collected on EDTA (150 mM, pH 8, 300 µl/5 ml) and centrifuged
at 1,000 × g, for 10 min at 4°C. The serum was collected in fresh
tubes and stored at −20°C until analysis. Simultaneously, the
adrenal glands, pituitary glands, hypothalami and periadrenal
adipose tissue were collected, preserved in RNAlater™ (ThermoFisher
Scientific, Inc., Waltham, MA, USA) and stored at −20°C until RNA
isolation. Doses of administered proteins were selected according
to previous studies (9,15).
Adrenal compartment isolation and
freshly isolated rat adrenocortical cells for in vitro
experiments
The methods described by Trejter et al
(16) were followed. Immediately
after decapitation, adrenal glands of intact 15 adult males, ~3–4
months old, ~250–300 g body weight, rats were removed and freed of
adherent fat. Subsequently, under a stereomicroscope, glands were
decapsulated to separate the zona glomerulosa (ZG) from the zona
fasciculata/reticularis (ZF/R). Pieces of connective tissue capsule
with adjacent ZG cells and pieces of the ZF/R compartment were
mechanically chopped using surgical scissors. The mechanically
isolated compartments were then digested with collagenase
(collagenase type I; Sigma-Aldrich; Merck KGaA) in Krebs-Ringer
solution (1 mg/ml). Digestion was performed at 37°C for 60 min in a
water bath with continuous shaking (300 oscillations per min). The
obtained suspensions were subjected to mechanical grinding by
pipetting (10–15 times) and filtered through a nylon membrane.
Cells were centrifuged (200 × g for 10 min at room temperature) and
washed three times in Krebs-Ringer solution supplemented with 0.3%
glucose and 0.2% bovine serum albumin (Sigma-Aldrich; Merck KGaA).
Finally, cells were suspended in Dulbecco's modified Eagle's medium
(DMEM)/F12 medium without phenol red (Sigma-Aldrich; Merck KGaA)
with 10% fetal bovine serum (FBS, F7524, Sigma-Aldrich; Merck KGaA)
and Antibiotic Antimycotic Solution (A5955, Sigma-Aldrich; Merck
KGaA). Incubation of cells (5,000 cells/ml) with eNampt (0.1 and 10
nM) and/or ACTH (1 µM) was performed in a water bath at 37°C for 2
h. This is a modified version of the technique described in greater
detail by Hinson et al (17), Malendowicz et al (18,19)
and Rucinski et al (20).
The concentrations of eNampt were selected based on a previous
study by Benito-Martin et al (21). The incubation medium was
centrifuged (200 × g for 10 min at room temperature) and the
collected cells were frozen at −36°C. Cells were treated with TRI
Reagent (Sigma-Aldrich; Merck KGaA) for RNA isolation. The obtained
material was used for further experiments.
Primary culture of rat adrenocortical
cells
The method used for culturing rat adrenocortical
cells has been described previously (16,22).
Briefly, adrenal glands were obtained from 35 ~60–80 g, 21-day-old,
male rats. Glands were immediately transferred into a vessel with
culture medium (DMEM/F12 without phenol red), mechanically chopped
and digested with collagenase type I (1 mg/ml in DMEM/F12 medium;
Sigma Aldrich; Merck KGaA) in a water bath at 37°C for 30 min. The
suspension was further mechanically disintegrated using a glass
pipette and poured through a nylon filter into a test tube,
followed by centrifugation (200 × g for 10 min at room
temperature). The collected cells were subsequently suspended in
DMEM/F12 with 10% FBS (FBS, F7524, Sigma-Aldrich; Merck KGaA) and
Antibiotic Antimycotic Solution (A5955, Sigma-Aldrich; Merck KGaA)
and plated on culture plates (Nalge Nunc International, Penfield,
NY, USA) at 1×104/well. The culture was incubated in
37°C and 5% CO2. The culture medium was changed every 24
h. At day 4 of culture, the test substances, including eNampt (1
and 100 nM), ACTH (1 µM) and FK866 (10 nM), were added and cells
were harvested after 24 h incubation. For the group treated with a
combination of eNampt + FK866, 1 µM eNampt and 10 nM FK866 were
employed. The incubation medium was centrifuged (200 × g for 10 min
at room temperature) and frozen at −36°C. Obtained cells were
treated with TRI Reagent for RNA isolation.
Pituitary gland explants
Rat pituitary glands from 20 ~3–4 months old,
250–300 g, male rats were collected immediately following
decapitation. Following neural lobe removal, the glands were
halved, preincubated for 30 min (without test substances) and
incubated at 37°C, with continuous shaking (300 oscillations per
min), in DMEM/F12 supplemented with 10% FBS and the addition of
test substances, including eNampt (10 nM), CRH (1 µM; CRH
Ferring®; Ferring Pharmaceuticals, Saint-Prex,
Switzerland) and FK866 (10 nM, Selleck Chemicals, Houston, TX,
USA). Pituitary glands were harvested after 2 h of incubation.
Pituitary gland halves were treated with TRI Reagent
(Sigma-Aldrich; Merck KGaA) for RNA isolation. Incubation medium
was frozen at −36°C.
Hypothalamic explants
Details of this method have been described
previously by Rucinski et al (23). Briefly, rat hypothalami were
collected from 20 ~3–4 months old, 250–300 g, male rats immediately
following decapitation, preincubated for 30 min (without test
substances) and incubated at 37°C, with continuous shaking (300
oscillations per min), in DMEM/F12 supplemented with 10% FBS and
the addition of eNampt (10 nM), KCl (60 mM) or both. Hypothalami
were harvested after 2 h of incubation. Explants were treated with
TRI Reagent for RNA isolation. Incubation medium was frozen at
−36°C.
Hormone level detection
The sera and incubation media were analysed by ELISA
to determine the concentration of aldosterone (cat. no. DE5298
Demeditec Diagnostics GmbH, Kiel, Germany), corticosterone (cat.
DEV9922 Demeditec Diagnostics GmbH), ACTH (cat. no. EK-001-21
Phoenix Europe GmbH, Karlsruhe, Germany) and CRH (cat. no.
OKEH00625 Aviva Systems Biology, Corp., San Diego, CA, USA). All
determinations were performed according to the manufacturers'
protocols.
RNA isolation
The methods used have been described previously
(24–28). Total RNA was extracted from
collected cells, samples of adrenal zones, entire adrenal glands,
periadrenal adipose tissue, pituitaries and hypothalami using TRI
Reagent and subsequently purified on columns (NucleoSpin RNA XS;
Macherey-Nagel, Düren, Germany). The amount of total mRNA was
determined by optical density at 260 nm and its purity was
estimated by the 260/280 nm absorption ratio (>1.8; NanoDrop
ND-1000 spectrophotometer; Thermo Fisher Scientific, Inc.).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RT was performed using the Transcriptor First Strand
cDNA Synthesis Kit (cat. no 04379012001 Roche Diagnostics, Basel,
Switzerland). RT was performed according to the manufacturer's
protocol. The primers used for qPCR (Table I) were designed by Primer 3
software (version 0.4.0, Whitehead Institute for Biomedical
Research, Cambridge, MA, USA) and purchased from the Laboratory of
DNA Sequencing and Oligonucleotide Synthesis, Institute of
Biochemistry and Biophysics, Polish Academy of Sciences (Warsaw,
Poland). qPCR was performed using a LightCycler 2.0 instrument 4.05
software version (Roche Diagnostics). Using the aforementioned
primers, a SYBR Green detection system was applied, as described
previously (24–29). Every 20 µl reaction mixture
contained 2 µl template cDNA (standard or control), 0.5 µM specific
primers and a previously determined optimum MgCl2
concentration (3.5 µM for one reaction). The LightCycler FastStart
DNA Master SYBR-Green I mix (Roche Applied Science, Penzberg,
Germany) was used. The qPCR program included a 10 min denaturation
step at 95°C to activate the Taq DNA polymerase, followed by a 45
cycles of three-step amplification program: Denaturation at 95°C
for 10 sec, annealing at 56°C for 5 sec and extension at 72°C for
10 sec. The specificity of the reaction products was checked by
determination of the melting points (0.1°C/sec transition rate).
The gene expression was normalized to HPRT with Pfaffl Ratio method
(30) by 4.05 LightCycler 2.0
software.
 | Table I.Primer sequences used for
quantitative polymerase chain reaction. |
Table I.
Primer sequences used for
quantitative polymerase chain reaction.
| Gene name | Forward primer
(5′-3′) | Reverse primer
(5′-3′) | GenBank accession
number | Product length,
bp |
|---|
| Nampt |
TGATCCCAACAAAAGGTCGAA |
CCCACTCACACAAAAGCCTA | NM_177928 | 238 |
| POMC |
CATGACGTACTTCCGGGGAT |
TCACCACGGAAAGCAACCTG | XM_017594033 | 192 |
| Fos |
TTTCAACGCGGACTACGAG |
AGTTGGCACTAGAGACGGAC | NM_022197 | 164 |
| HPRT |
ATAGAAATAGTGATAGGTCCA |
TCTGCATTGTTTTACCAGT | XM_008773659 | 177 |
Statistical analysis
Data are presented as the mean ± standard error of
the mean. For multiple comparisons, statistical analysis of the
data was performed by using one-way analysis of variance (ANOVA)
followed by Tukey's post-hoc test. Calculations were performed
using R ×64 3.4.1 software with the multcomp library. Following
one-way ANOVA, if P<0.05 was obtained, Tukey's post-hoc test was
performed and differences were considered to be statistically
significant when P<0.05. On the figures, the results of the
Tukey's post-hoc test are marked by letters. Groups sharing the
same letter are not significantly different to each other,
according to the Tukey's post-hoc test. When only two groups were
compared, a statistical evaluation of the differences was performed
using Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
In vivo experiments
At 1 h after i.p. eNampt administration, and in the
saline-treated control group, CRH was not detectable in the serum
of rats (data not shown). At this time-point, serum ACTH levels
remained unchanged in the eNampt group compared with the control
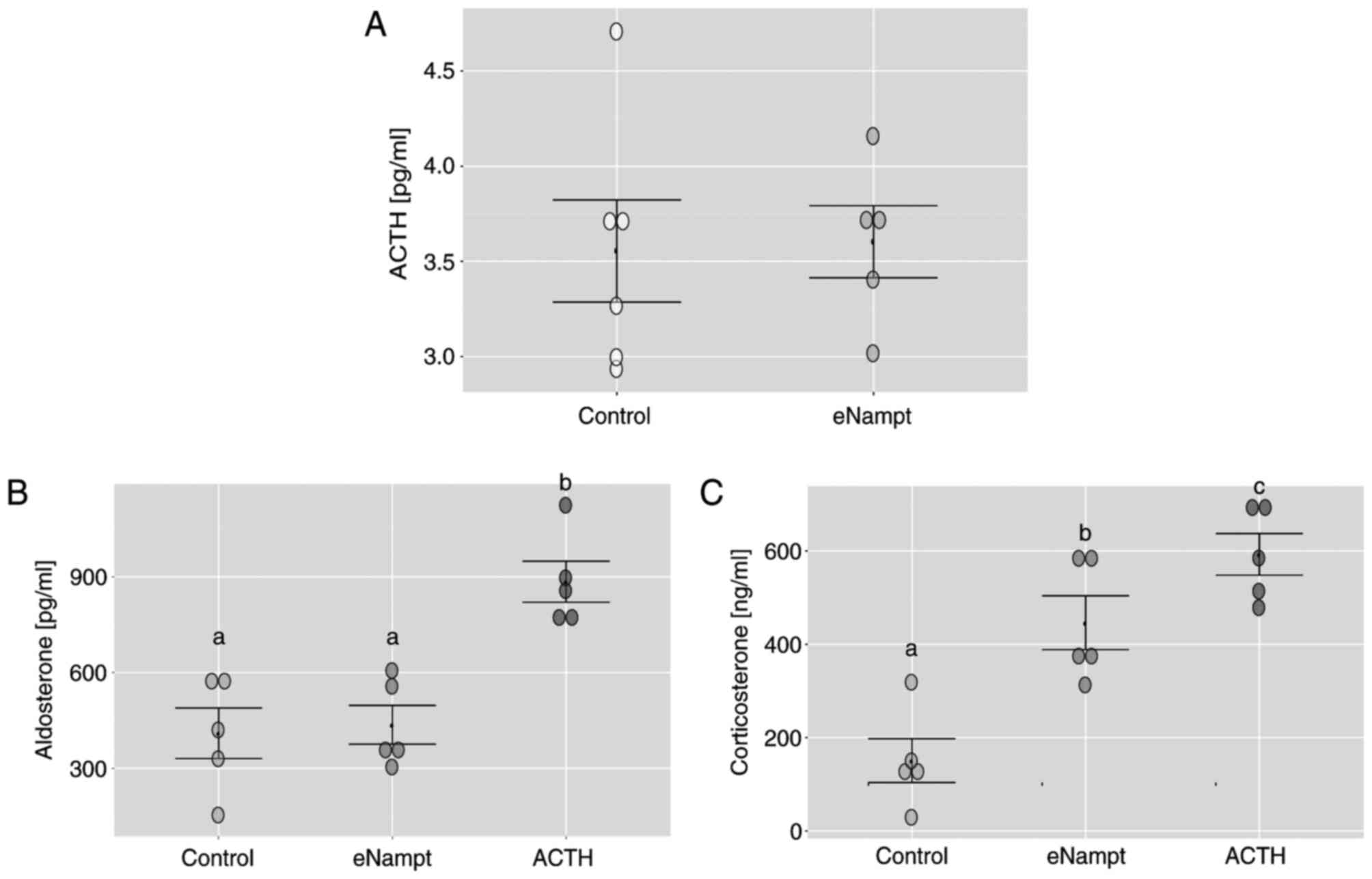
group (Fig. 1A). Notably, the i.p.
eNampt injection did not affect serum aldosterone concentration
compared with the control group; however, corticosterone levels
were notably elevated in the eNampt group compared with the control
group, reaching levels comparable to those induced by ACTH
administration (Fig. 1B and
C).
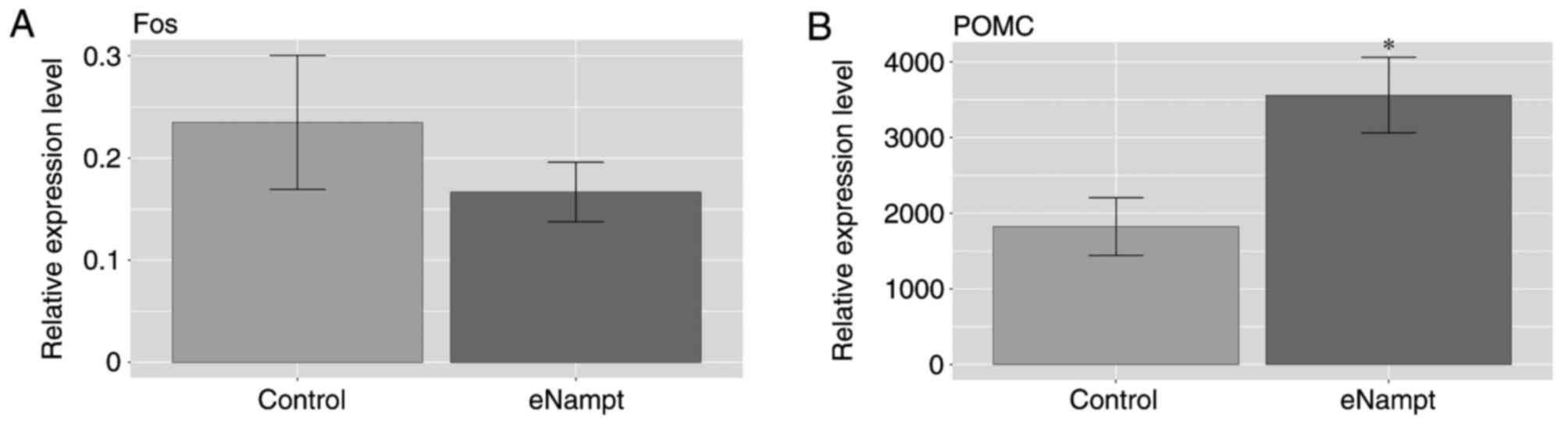
In the hypothalami of eNampt-treated rats, the mRNA
expression levels of Fos proto-oncogene (Fos), also termed c-Fos,
were not significantly different compared with levels in the
saline-treated group (Fig. 2A). By
contrast, in the pituitary glands of eNampt-treated rats, the mRNA
expression level of POMC was significantly higher compared with
levels in the saline-treated control group (Fig. 2B).
In addition, the present study also investigated
whether i.p. administration of eNampt affected Nampt gene
expression in the hypothalamus, pituitary glands, adrenal glands
and adipose tissue. In eNampt-treated rats, Nampt gene expression
was unchanged in the hypothalamus (Fig. 3A), markedly elevated in the
pituitary and adrenal glands (Fig. 3B
and C), and lowered in the periadrenal adipose tissue (Fig. 3D), compared with the control group.
In addition, following administration of ACTH, the expression of
Nampt mRNA was increased in the adipose tissue compared with the
control group.
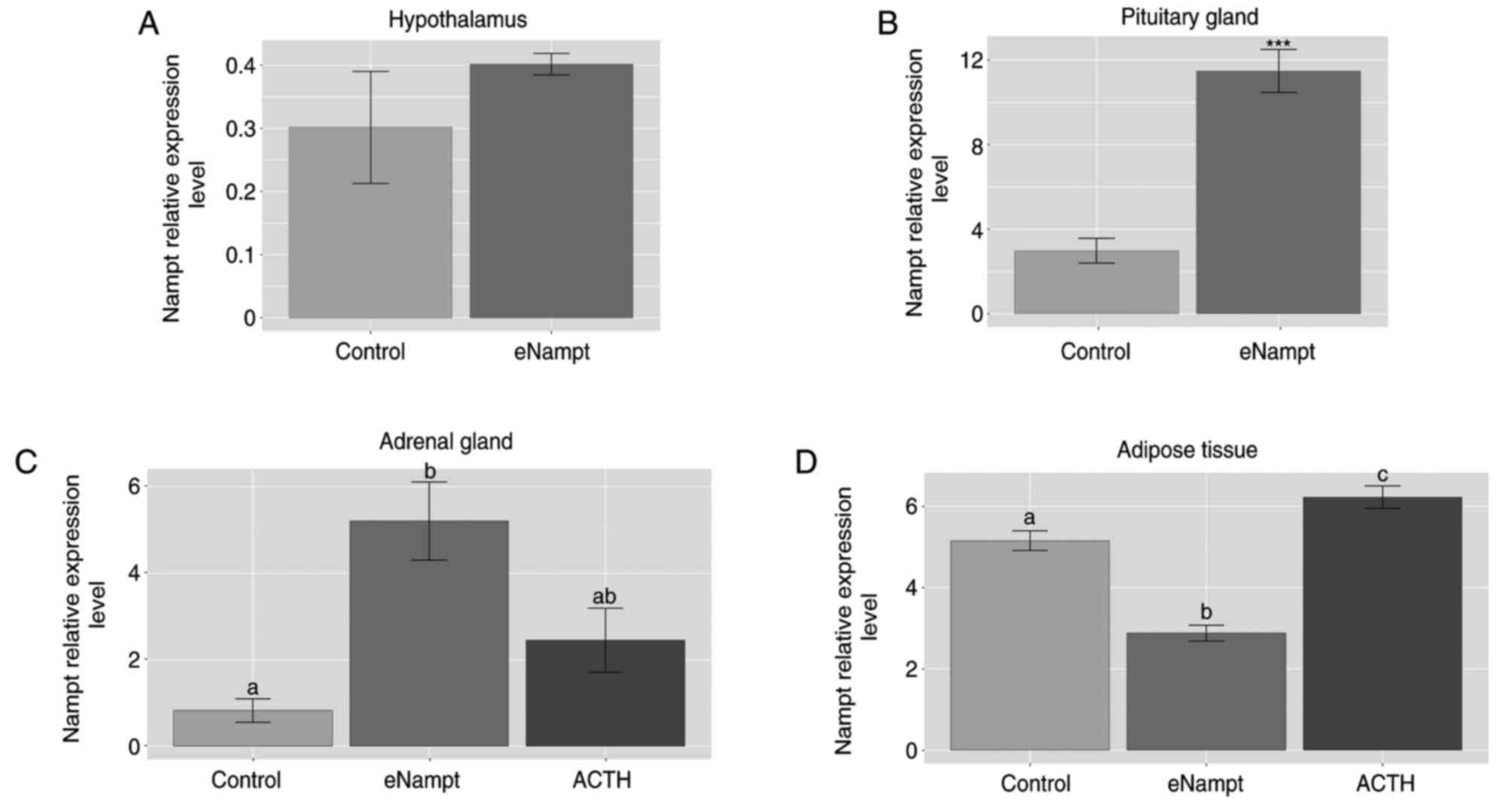 | Figure 3.Relative mRNA expression of the Nampt
gene in the hypothalamus, pituitary gland, adrenal glands and
periadrenal adipose tissue following i.p. eNampt administration in
rats. At 1 h after i.p. eNampt (4 µg/100 g), ACTH (2.5 µg/100 g) or
0.2 ml physiological saline administration in rats, reverse
transcription-quantitative polymerase chain reaction was performed
to determine the mRNA expression levels of Nampt in the (A)
hypothalamus, (B) pituitary gland, (C) adrenal gland and (D)
periadrenal adipose tissue. n=5 per group. Data are presented as
the mean ± standard error of the mean. In part B, ***P<0.001 vs.
control group; in parts C and D, groups sharing the same letter are
not significantly different, while different letters indicate
groups that are significantly different to each other, with
P<0.05. Nampt, nicotinamide phosphoribosyltransferase; i.p.
intraperitoneal; eNampt, extracellular Nampt; ACTH,
adrenocorticotropic hormone. |
In vitro hypothalamic explants
Treatment of hypothalamic explants with eNampt
protein exhibited no effect on CRH release into the incubation
medium, compared with the control group (Fig. 4A). As expected, treatment with KCl
notably increased CRH release compared with the control group, an
effect that was prevented by the addition of eNampt. Furthermore,
in hypothalamic explants exposed to eNampt, there was a significant
increase in Fos and Nampt gene expression compared with the control
group (Fig. 4B and C).
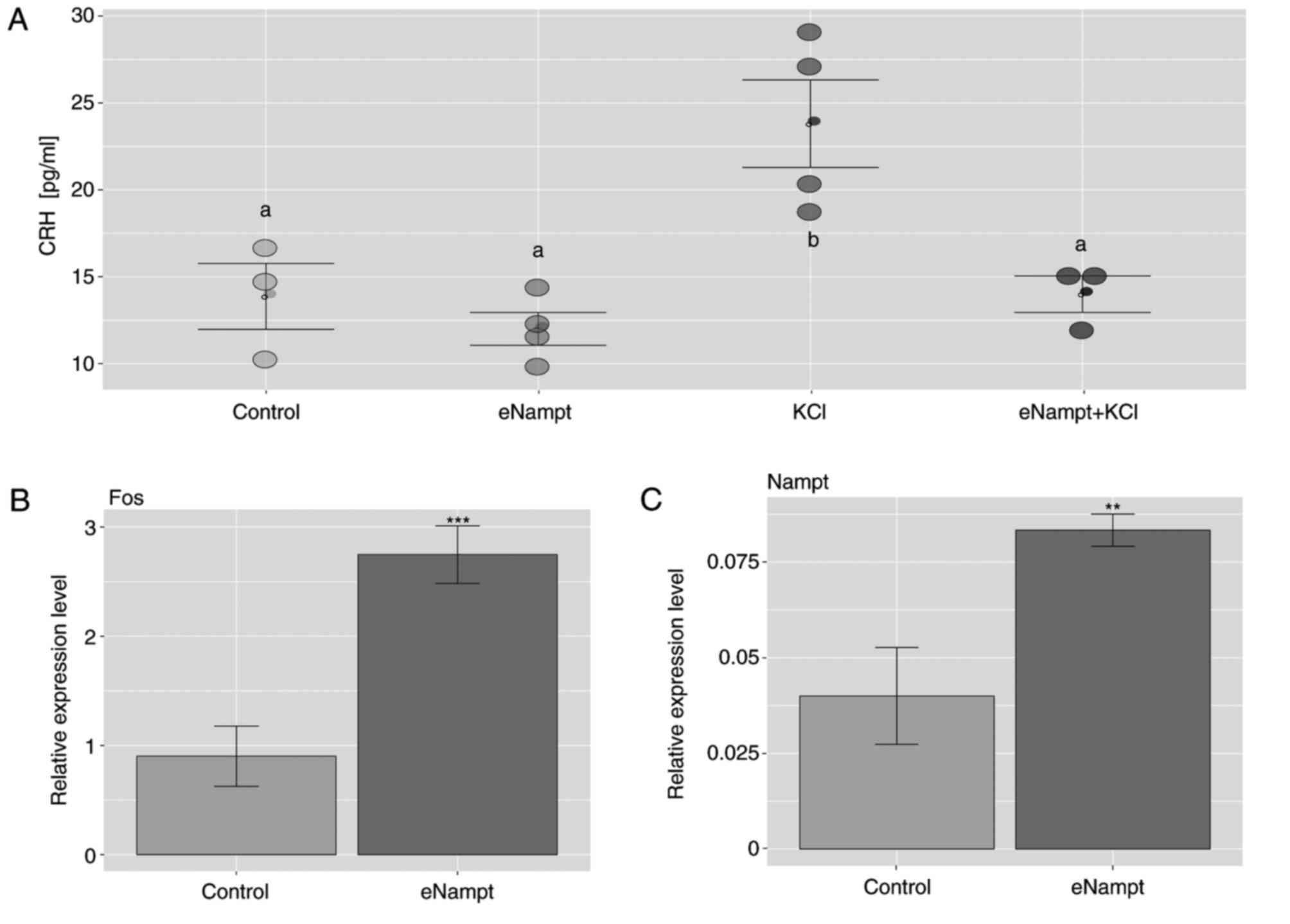 | Figure 4.Effects of eNampt on CRH release by
hypothalamic explants and the expression of Fos and Nampt genes.
(A) ELISA was performed on incubation media following 2 h exposure
of hypothalamic explants to eNampt (10 nM), KCl (60 mM) or a
combination of both to determine the level of CRH release. Each dot
represents an individual result. After 2 h exposure of hypothalamic
explants to eNampt (10 nM), KCl (60 mM) or a combination of both,
reverse transcription-quantitative polymerase chain reaction was
performed to determine the mRNA expression of (B) Fos and (C) Nampt
in the hypothalamic explants. Data are presented as the mean ±
standard error of the mean. n=4 per group. In part A, groups
sharing the same letter are not significantly different, while
different letters indicate groups that are significantly different
to each other, with P<0.05; in parts B and C, **P<0.01,
***P<0.001 vs. control group. Nampt, nicotinamide
phosphoribosyltransferase; eNampt, extracellular Nampt; CRH,
corticotropin-releasing hormone; Fos, Fos proto-oncogene. |
In vitro pituitary gland explants
The secretion of ACTH by the pituitary explants was
increased in the presence of eNampt, compared with the control
group; however, this increase was not statistically significant. By
contrast, CRH significantly increased the release of ACTH into the
incubation medium, compared with the control group, and this effect
was not modified by the presence of eNampt (Fig. 5A). The stimulatory effect of eNampt
on the expression of POMC in the pituitary glands was inhibited by
administration of FK866, a specific Nampt inhibitor (Fig. 5B). Furthermore, in the pituitary
glands, eNampt or CRH administration alone did not alter the level
of Nampt gene expression, while joint administration of the two
substances significantly increased the level of Nampt gene
expression compared with the control group (Fig. 5C).
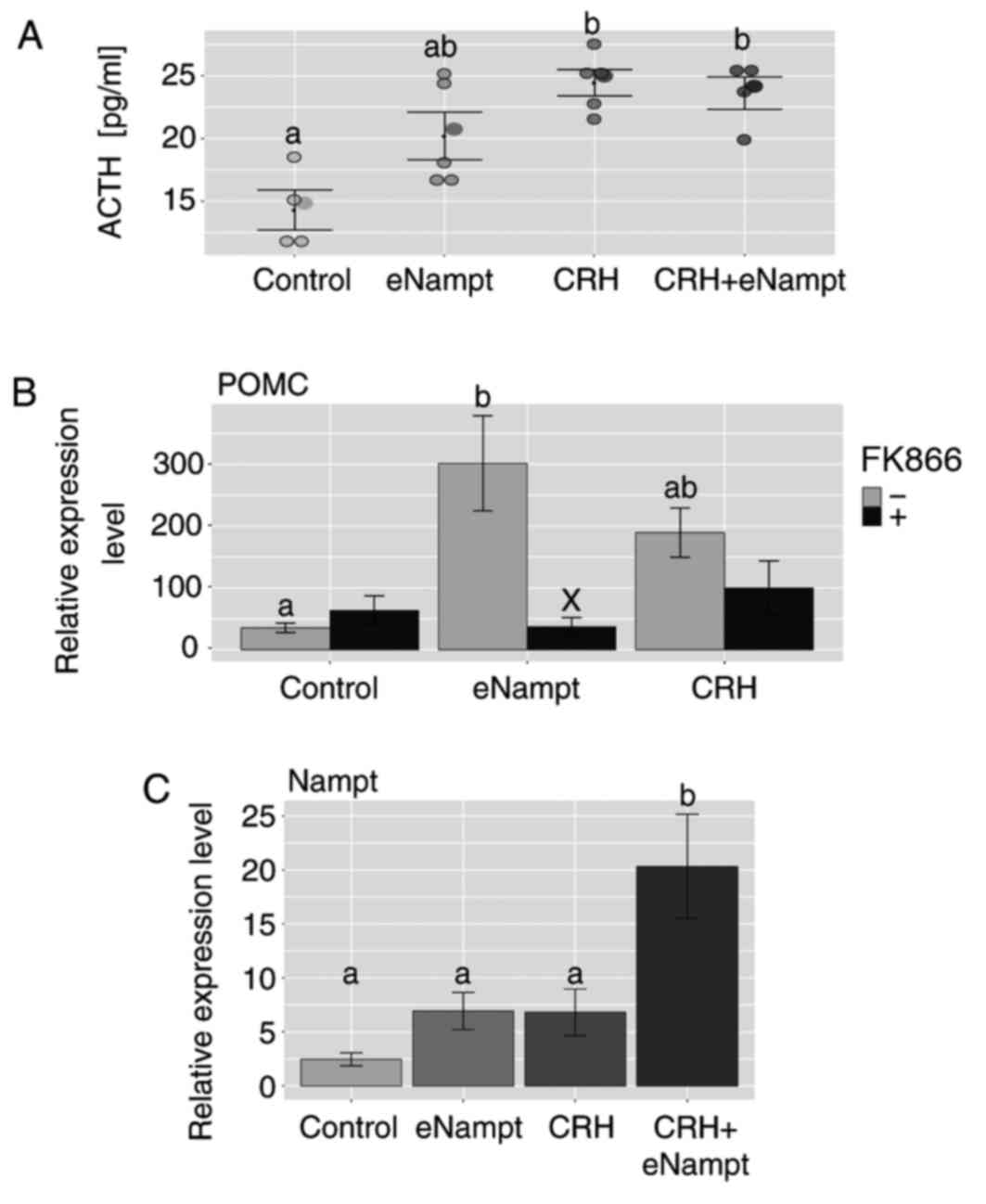 | Figure 5.Effects of eNampt, CRH and FK866 on
ACTH release by rat pituitary gland explants and the expression of
POMC and Nampt genes. (A) ELISA was performed on incubation media
following 2 h exposure of pituitary gland explants to eNampt (10
nM), CRH (1 µM) or a combination of both to determine the level of
ACTH release. Each dot represents an individual result. (B) After 2
h exposure of pituitary gland explants to eNampt (10 nM) or CRH (1
µM), with or without FK866 (10 nM), RT-qPCR was performed to
determine the mRNA expression of POMC in the pituitary gland
explants. (C) After 2 h exposure of pituitary gland explants to
eNampt (10 nM), CRH (1 µM) or a combination of both, RT-qPCR was
performed to determine the mRNA expression of Nampt in the
pituitary gland explants. Data are presented as the mean ± standard
error of the mean. n=4 per group. Groups sharing the same letter
are not significantly different, while different letters indicate
groups that are significantly different to each other, with
P<0.05. In part B, X is used to indicate a statistically
significant difference compared with the respective FK866 negative
group, with P<0.001. Nampt, nicotinamide
phosphoribosyltransferase; eNampt, extracellular Nampt; CRH,
corticotropin-releasing hormone; ACTH, adrenocorticotropic hormone;
POMC, proopiomelanocortin; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction. |
Freshly isolated adrenocortical cells
and primary rat adrenocortical cell cultures
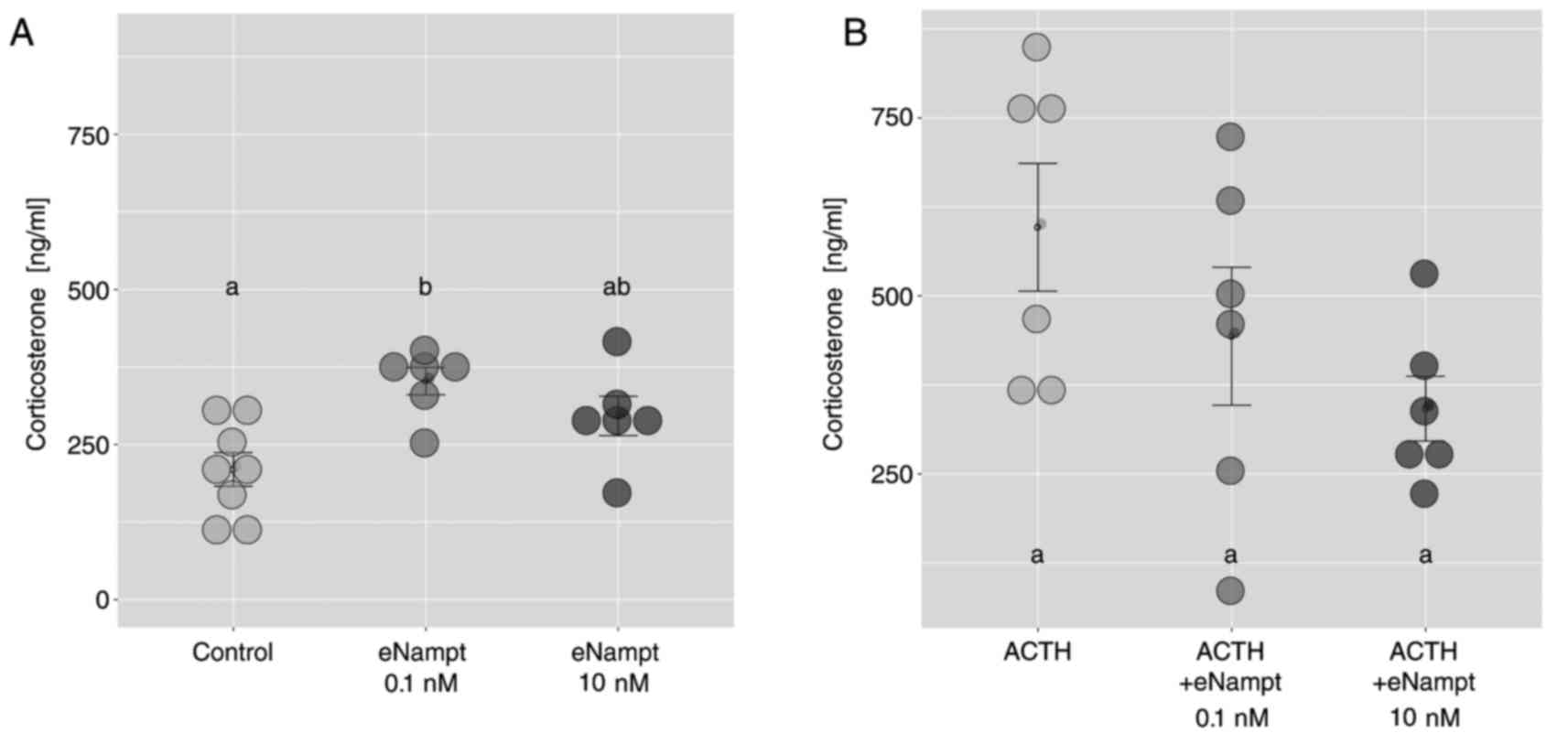
In freshly isolated ZF/R cells, eNampt at a
concentration of 0.1 nM led to an increase in the corticosterone
output compared with the control group; an effect that was not
observed at the higher eNampt concentration 10 nM (Fig. 6A). Notably, in freshly isolated
adrenocortical cells, eNampt did not affect ACTH-stimulated
corticosterone secretion (Fig.
6B).
Subsequent experiments were performed on primary rat
adrenocortical cell cultures, and the cells were exposed to test
substances at day 4 for 24 h. The results demonstrated that eNampt
at concentrations of 1 and 100 nM did not alter basal aldosterone
and corticosterone secretion from primary adrenocortical cell
cultures, while the cell response to ACTH was retained (Fig. 7A and B). In cells exposed to
eNampt, an increase in Nampt gene expression levels was observed
compared with the control group; however, ACTH administration did
not affect the expression level of this gene (Fig. 7C).
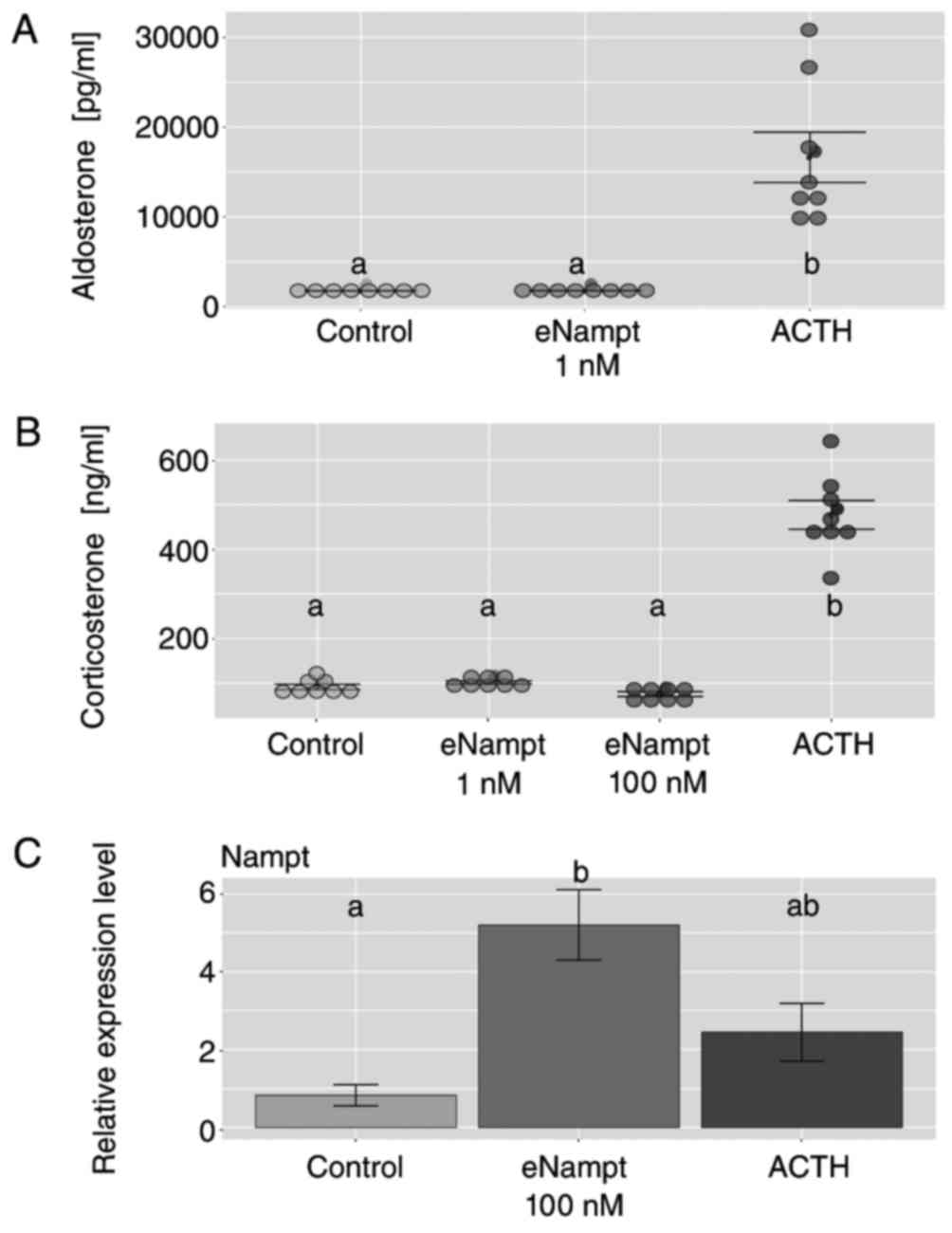 | Figure 7.Effects of eNampt on aldosterone and
corticosterone output in primary cultures of rat adrenocortical
cells. At day 4 of culture, cells were exposed to eNampt (1 and 100
nM) or ACTH (1 µM), and medium was collected after 24 h to perform
ELISA to measure the levels of (A) aldosterone and (B)
corticosterone in the media. Each dot represents an individual
result. (C) At day 4 of culture, cells were exposed to eNampt (100
nM) or ACTH (1 µM), and cells were collected after 24 h for reverse
transcription-quantitative polymerase chain reaction analysis of
Nampt mRNA expression. Data are presented as the mean ± standard
error, n=8. Groups sharing the same letter are not significantly
different, while different letters indicate groups that are
significantly different to each other, with P<0.05. Nampt,
nicotinamide phosphoribosyltransferase; eNampt, extracellular
Nampt; ACTH, adrenocorticotropic hormone. |
To assess the potential role of iNampt in the
regulation of adrenal steroidogenesis, the present study also
performed experiments using FK866, a specific iNampt inhibitor.
Exposure of cultured rat adrenocortical cells to FK866 notably
lowered basal aldosterone output compared with the control group,
and these effects were not altered by the addition of eNampt
(Fig. 8A). Furthermore, the
stimulatory effect of ACTH on aldosterone output in cultured rat
adrenocortical cells was eliminated by FK866 administration
(Fig. 8B). Similar effects were
observed on corticosterone output following FK866 treatment
(Fig. 8C and D).
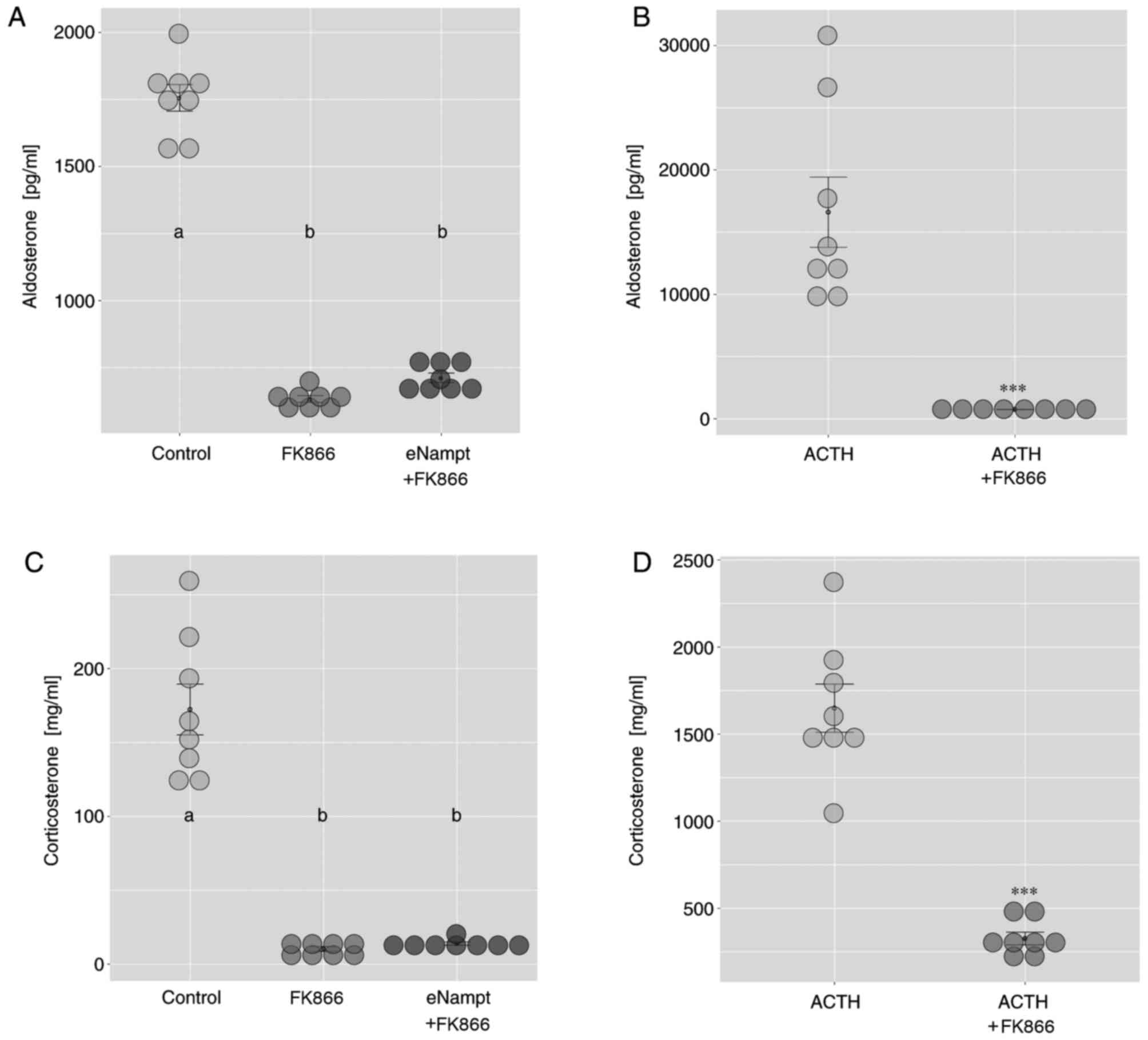 | Figure 8.Effects of FK866 on basal and
ACTH-stimulated aldosterone and corticosterone output in primary
cultures of rat adrenocortical cells. At day 4 of culture, cells
were exposed to FK866 (10 nM) with or without eNampt (1 µM), or
ACTH (1 µM) with or without FK866 (10 nM), and medium was collected
after 24 h. The effect of FK866 on (A) basal and (B)
ACTH-stimulated aldosterone release was determined by ELISA. The
effect of FK866 on (C) basal and (D) ACTH-stimulated corticosterone
release was determined by ELISA. Each dot represents an individual
result. Data are presented as the mean ± standard error, n=8. In
parts A and C, groups sharing the same letter are not significantly
different, while different letters indicate groups that are
significantly different to each other, with P<0.05; in parts B
and D, ***P<0.001 vs. ACTH alone group. ACTH,
adrenocorticotropic hormone; eNampt, extracellular Nampt. |
Discussion
iNampt has an important role in the regulation of
intracellular NAD levels and, due to this function, is essential
for various metabolic processes. Secreted Nampt (eNampt) is a newly
discovered hormone whose function is not yet fully understood. As a
result, the role of eNampt in the regulation of the HPA axis
remains to be established. Therefore, the current study aimed to
investigate the role of Nampt (both iNampt and eNampt) in the
regulation of the HPA axis in the rat. The results demonstrated
that i.p. injection of eNampt increased the serum levels of
corticosterone, potentially by acting at the pituitary level.
Furthermore, the results of in vitro experiments in the
present study indicated that iNampt may exert a tonic stimulatory
effect on the basal secretion of aldosterone and corticosterone
from cultured rat adrenocortical cells. Furthermore, normal iNampt
levels were required to retain the normal response of cultured rat
adrenocortical cells to ACTH.
As previously mentioned, administration of eNampt by
i.p. injection in rats resulted in a large increase in the serum
corticosterone concentration, while the aldosterone levels remained
unchanged. This observation indicates that eNampt may stimulate
hypothalamic CRH and/or pituitary ACTH secretion. Although eNampt
is present in the CSF, its origin has not been explained (10). In the brain, Nampt is primarily
expressed in neurons with a cytoplasmic localization (31,32)
and it is unlikely that this large protein is able to pass through
the blood-brain barrier, which may explain the lack of effect of
eNampt on CRH and ACTH secretion at 1 h after injection of this
protein. In the present study, at 1 h after i.p. eNampt
administration and in control rats, serum CRH was undetectable, and
serum ACTH levels remained unchanged in the eNampt group compared
with the control group. As the half-life of CRH and ACTH is short
(33,34), the current study also investigated
the expression of Fos in the hypothalamus and POMC in the pituitary
gland of experimental rats. Fos, an immediate-early response gene,
is expressed in neurons, and the expression of this gene is
employed as a marker of neuroendocrine cell activation (35,36).
In the current study, following i.p. eNampt administration, the
expression level of Fos in the hypothalamus was unaltered, while
POMC gene expression in the pituitary gland was significantly
increased, compared with the control group. These results indicate
that injected eNampt may not be able to activate hypothalamic CRH
neurons, at least acutely. This lack of activation may be due to
the fact that large eNampt molecules do not cross the blood-brain
barrier. This hypothesis is further supported by the observation
that, in hypothalamic explants, eNampt significantly stimulated the
expression of the Fos gene following 2 h treatment.
The literature concerning the effect of eNampt on
the hypothalamus is scarce. One group reported that administration
of eNampt directly into the arcuate nucleus of the rat hypothalamus
increased food intake within 24 h, and this effect was accompanied
by decreased CRH and CART gene expression, while POMC gene
expression remained unaltered (11). Importantly, in their study, the
mRNA was isolated from the entire hypothalamus. Another group
recently reported that in mouse hypothalamic explants, eNampt
enhanced NAD+/SIRT1 and the neural activity of cells, as
estimated by Fos gene expression (7). Thus, regarding the interaction
between eNampt and the Fos gene in hypothalamic explants, the
observations in rats in the present study confirm previous
observations in mice.
Based on the results of the current study and the
available literature, it may be hypothesised that the primary site
of action of exogenous eNampt within the HPA axis is the pituitary
gland. To test this hypothesis, the present study performed
experiments in vitro with rat pituitary glands. These
experiments demonstrated that the exposure of pituitary explants to
eNampt did not significantly stimulate ACTH secretion, but the
expression of the POMC gene in the pituitary gland was
significantly increased, compared with the control group. The
effect of eNampt on POMC gene expression was inhibited by the
specific inhibitor of Nampt enzymatic activity, FK866 (37). Notably, based on these results, it
is unclear whether eNampt enzymatic activity is responsible for the
biological effect exerted by eNampt. In addition, whether the
enzymatic activity of eNampt is inhibited by FK866 is also
debatable, as it is not clear yet whether eNAMPT actually carries
out its enzymatic activity in the extracellular environment. The
plasma concentrations of the Nampt substrates (Nam, ATP and PRPP)
are insufficient to support the Nampt reaction (38) as well as the NMN presence in plasma
remains controversial (9,38–40).
Therefore, the mechanism of eNampt action remains unclear. Despite
this, the results of in vivo and in vitro experiments
in the current study indicate that eNampt may exhibit a direct
stimulatory effect on the secretion of ACTH by the pituitary and on
POMC gene expression in this gland.
In the existing literature, several publications
have reported the presence of iNampt in the pituitary gland;
however, they do not provide any information regarding the
association of iNampt and eNampt with ACTH. For example, in the
pars tuberalis of the sheep pituitary gland, Nampt gene expression
was reported to be regulated by melatonin (41,42).
Importantly, the pars tuberalis is a structurally distinct part of
the adenohypophysis with few or no ACTH cells (43).
The role of eNampt in the regulation of
steroidogenesis has been previously investigated in relation to the
ovaries and testes and, to a lesser extent, the adrenal cortex.
Data have indicated that eNampt is involved in the regulation of
reproductive function. Nampt gene expression was identified in
primary human granulosa cells and in a human ovarian granulosa-like
tumour cell line (44). In these
cells, eNampt increased IGF-1-induced steroidogenesis (secretion of
progesterone and oestradiol) and cell proliferation. The Nampt gene
was also reported to be expressed in bovine granulosa, theca and
luteal cells (42). In cultured
bovine granulosa cells, eNampt also exhibited a stimulatory effect
on the secretion of progesterone and oestradiol, an effect that was
associated with increased protein levels of STAR and HSD3B activity
(42). The same group also
conducted research on the role of eNampt in the gonads of chickens
and turkeys (45,46). The expression of the Nampt gene in
chicken and turkey steroidogenic cells was similar to that
described in humans and cattle. However, in cultured chicken
granulosa cells, eNampt notably inhibited progesterone secretion,
an effect that was associated with decreased STAR and HSD3B protein
levels. In another type of steroidogenic cell, Leydig cells of the
testis, the expression of the Nampt gene has also been reported in
prepubertal and adult chicken testes (47). However, in the existing literature,
no reliable data concerning the effect of eNampt on the secretion
of steroid hormones by the Leydig cells was available.
To the best of our knowledge, there is only one
report concerning the association between Nampt and steroidogenesis
in the adrenal glands (48). The
authors investigated the role of eNampt in the H295R human adrenal
cell line. In these cells, eNampt markedly induced steroid release
into the cell culture media within 4 h, an effect that was
associated with the upregulation of STAR mRNA and protein
expression. The authors also provided data indicating that the
effects of eNampt on steroidogenesis may be mediated through the
MAPK and phosphatidylinositol 3-kinase/Akt signalling pathways.
As mentioned earlier, in the present study, i.p.
administration of eNampt significantly increased the serum
corticosterone levels within 1 h, but not aldosterone levels. In
these rats, the serum corticosterone levels were comparable to
those induced by ACTH administration. In addition, in the adrenal
gland, Nampt gene expression levels were notably elevated following
eNampt administration, compared with the control group. Based on
these results, it was further investigated, using in vitro
experiments, whether eNampt exerts a direct effect on adrenal
steroidogenesis. In freshly isolated ZF/R cells of the rat adrenal
cortex, eNampt at a concentration of 0.1 nM, but not 10 nM, led to
an increase in corticosterone output. In addition, none of the
eNampt concentrations influenced the ACTH-stimulated corticosterone
output of freshly isolated adrenocortical cells. Based on these
results, in freshly isolated adrenocortical cells, the effects of
eNampt on adrenal steroidogenesis may depend on the concentration
of this adipokine; lower doses of eNampt appear to exert a
stimulatory effect on steroidogenesis. However, the role of iNampt
in the regulation of steroidogenesis in the adrenal glands of rats
remains an open question. To investigate this, the present study
performed experiments involving FK866, a specific Nampt enzymatic
action inhibitor. Exposure of cultured rat adrenocortical cells to
FK866 markedly lowered basal aldosterone and corticosterone output
within 24 h, and these effects were also observed in the presence
of 1 µM eNampt. Furthermore, in the presence of FK866, the
stimulatory effects of ACTH on aldosterone and corticosterone
secretion were eliminated. To exclude a potential toxic effect of
FK866 on cultured rat adrenocortical cells, the proliferation rate
of cells was also investigated (data not shown); the results
demonstrated that FK866 did not interfere with the proliferation or
survival rates of cells.
Therefore, regarding the effect of eNampt on adrenal
steroidogenesis in the rat, the results of the current study
indicate that low concentrations of this adipokine may stimulate
the secretion of corticosteroids by cultured adrenocortical cells.
Furthermore, for the first time, the present study obtained
indicating a physiological role of iNampt in the regulation of
steroidogenesis. iNampt appeared to be necessary for the normal
responses of adrenocortical cells to ACTH. Finally, the results of
the current study also suggest that iNampt may exert a tonic
stimulatory effect on the secretion of aldosterone and
corticosterone by cultured rat adrenocortical cells, evidence of
which is provided by the reduction in the basal secretion of these
hormones in the presence of FK866, a specific Nampt enzymatic
activity inhibitor (40).
In the steroidogenic pathway, cytochrome P450
enzymes are responsible for the hydroxylation and cleavage of the
steroid intermediates and hormones, and their function is dependent
on reduced NAD phosphate (49).
The mitochondria of cells that secrete steroid hormones are able to
generate NADP from NAD in a reaction that is catalysed by
nicotinamide nucleotide transhydrogenase (NNT) (50). Thus, the availability of NAD in
adrenocortical cells may determine normal steroidogenesis.
Importantly, in this regard, FK866 is a highly specific
non-competitive inhibitor of iNampt enzymatic action (40). Therefore, in the present study, the
decline in the basal secretion of aldosterone and corticosterone by
rat adrenocortical cells cultured in the presence of FK866
indicates that iNampt may exert a tonic stimulatory effect on the
secretion of corticosteroids. This hypothesis is consistent with
the latest clinical reports that indicate that NNT mutations maybe
responsible for primary adrenal insufficiency, which refers to
combined mineralocorticoid and glucocorticoid deficiency (14,42–53).
Patients with these genetic mutations also suffer from oxidative
stress.
Another important observation described in the
current study is the stimulation of Nampt gene expression by
eNampt. This effect was observed in hypothalamic and pituitary
gland explants, and in cultured rat adrenocortical cells. A similar
effect was also observed in primary bovine ovary cells exposed to
eNampt, as reported in a previous study (14). It is possible that this effect may
be associated with the regulation of redox imbalance and oxidative
stress in these cells (54–56).
However, in the present study, Nampt gene expression following i.p.
administration of eNampt differed notably compared with in
vitro results. In the experimental model, Nampt gene expression
was unchanged in the hypothalamus, notably elevated in pituitary
and adrenal glands, and lowered in adipose tissue. These results
support our suggestion that eNampt administered by i.p. injection
does not cross the blood-brain barrier. However, the results of
in vivo experiments may not be comparable to the results
obtained in in vitro experiments. It is established that
alterations in vivo reflect the eNampt-induced alterations
in the whole body and may not necessarily represent a direct impact
of eNampt on a specific organ.
In conclusion, the present study has demonstrated
that, in the rat, i.p. injection of eNampt increased the serum
levels of corticosterone, which may occur via action at the
pituitary gland level. The results of in vitro experiments
also demonstrated that eNampt treatment increased the level of POMC
gene expression in isolated pituitary glands and induced ACTH
secretion. It was also indicated that iNampt may exert a tonic
stimulatory effect on the basal secretion of aldosterone and
corticosterone from cultured rat adrenocortical cells. In addition,
the results indicate that physiological iNampt enzymatic activity
is required to maintain the normal response of adrenocortical cells
to ACTH. Finally, proper understanding of endocrine crosstalk
between hypothalamo-pituitary-adrenal axis and other tissues could
help better understand mechanisms of metabolic and mineral
disorders.
Acknowledgements
The present study was supported by PRELUDIUM (grant
no. 2016/21/N/NZ4/00122) from the National Science Centre (Poland,
Kraków).
References
|
1
|
Samal B, Sun Y, Stearns G, Xie C, Suggs S
and McNiece I: Cloning and characterization of the cDNA encoding a
novel human pre-B-cell colony-enhancing factor. Mol Cell Biol.
14:1431–1437. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Araki T, Sasaki Y and Milbrandt J:
Increased nuclear NAD biosynthesis and SIRT1 activation prevent
axonal degeneration. Science. 305:1010–1013. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Revollo JR, Grimm AA and Imai S: The NAD
biosynthesis pathway mediated by nicotinamide
phosphoribosyltransferase regulates Sir2 activity in mammalian
cells. J Biol Chem. 279:50754–50763. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bogan KL and Brenner C: Nicotinic acid,
nicotinamide, and nicotinamide riboside: A molecular evaluation of
NAD+ precursor vitamins in human nutrition. Annu Rev Nutr.
28:115–130. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Houtkooper RH, Cantó C, Wanders RJ and
Auwerx J: The secret life of NAD+: An old metabolite controlling
new metabolic signaling pathways. Endocr Rev. 31:194–223. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen H, Wang S, Zhang H, Nice EC and Huang
C: Nicotinamide phosphoribosyltransferase (Nampt) in
carcinogenesis: New clinical opportunities. Expert Rev Anticancer
Ther. 16:827–838. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yoon MJ, Yoshida M, Johnson S, Takikawa A,
Usui I, Tobe K, Nakagawa T, Yoshino J and Imai S: SIRT1-mediated
eNAMPT secretion from adipose tissue regulates hypothalamic NAD+
and function in mice. Cell Metab. 21:706–717. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tanaka T and Nabeshima Y:
Nampt/PBEF/Visfatin: A new player in beta cell physiology and in
metabolic diseases? Cell Metab. 6:341–343. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Revollo JR, Korner A, Mills KF, Satoh A,
Wang T, Garten A, Dasgupt B, Sasaki Y, Wolberger C, Townsend RR, et
al: Nampt/PBEF/Visfatin regulates insulin secretion in beta cells
as a systemic NAD biosynthetic enzyme. Cell Metab. 6:363–375. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hallschmid M, Randeva H, Tan BK, Kern W
and Lehnert H: Relationship between cerebrospinal fluid visfatin
(PBEF/Nampt) levels and adiposity in humans. Diabetes. 58:637–640.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brunetti L, Recinella L, Di Nisio C,
Chiavaroli A, Leone S, Ferrante C, Orlando G and Vacca M: Effects
of visfatin/PBEF/NAMPT on feeding behaviour and hypothalamic
neuromodulators in the rat. J Biol Regul Homeost Agents.
26:295–302. 2012.PubMed/NCBI
|
|
12
|
Iwen KA, Senyaman O, Schwartz A, Drenckhan
M, Meier B, Hadaschik D and Klein J: Melanocortin crosstalk with
adipose functions: ACTH directly induces insulin resistance,
promotes a pro-inflammatory adipokine profile and stimulates UCP-1
in adipocytes. J Endocrinol. 196:465–472. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dammer EB and Sewer MB: Phosphorylation of
CtBP1 by cAMP-dependent protein kinase modulates induction of CYP17
by stimulating partnering of CtBP1 and 2. J Biol Chem.
283:6925–6934. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Reverchon M, Rame C, Bunel A, Chen W,
Froment P and Dupont J: VISFATIN (NAMPT) improves In Vitro
IGF1-induced steroidogenesis and IGF1 receptor signaling through
SIRT1 in bovine granulosa cells. Biol Reprod. 94:542016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Paschke L, Zemleduch T, Rucinski M,
Ziolkowska A, Szyszka M and Malendowicz LK: Adiponectin and
adiponectin receptor system in the rat adrenal gland: Ontogenetic
and physiologic regulation, and its involvement in regulating
adrenocortical growth and steroidogenesis. Peptides. 31:1715–1724.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Trejter M, Hochol A, Tyczewska M,
Ziolkowska A, Jopek K, Szyszka M, Malendowicz LK and Rucinski M:
Visinin-like peptide 1 in adrenal gland of the rat. Gene expression
and its hormonal control. Peptides. 63:22–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hinson JP, Kapas S, Orford CD and Vinson
GP: Vasoactive intestinal peptide stimulation of aldosterone
secretion by the rat adrenal cortex may be mediated by the local
release of catecholamines. J Endocrinol. 133:253–258. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Malendowicz LK, Nussdorfer GG, Warchol JB,
Markowska A, MacChi C, Nowak KW and Butowska W: Effects of
neuromedin-K on the rat hypothalamo-pituitary-adrenal axis.
Neuropeptides. 29:337–341. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Malendowicz LK, Rebuffat P, Nussdorfer GG
and Nowak KW: Corticotropin-inhibiting peptide enhances aldosterone
secretion by dispersed rat zona glomerulosa cells. J Steroid
Biochem Mol Biol. 67:149–152. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rucinski M, Ziolkowska A, Szyszka M and
Malendowicz LK: Cerebellin and des-cerebellin exert ACTH-like
effects on corticosterone secretion and the intracellular signaling
pathway gene expression in cultured rat adrenocortical cells-DNA
microarray and QPCR studies. Int J Mol Med. 23:539–546.
2009.PubMed/NCBI
|
|
21
|
Benito-Martin A, Ucero AC, Izquierdo MC,
Santamaria B, Picaroste B, Carrasco S, Lorenzo O, Ruiz-Ortega M,
Egido J and Ortiz A: Endogenous NAMPT dampens chemokine expression
and apoptotic responses in stressed tubular cells. Biochim Biophys
Acta. 1842:293–303. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ziolkowska A, Rucinski M, Tyczewska M and
Malendowicz LK: Orexin B inhibits proliferation and stimulates
specialized function of cultured rat calvarial osteoblast-like
cells. Int J Mol Med. 22:749–755. 2008.PubMed/NCBI
|
|
23
|
Rucinski M, Ziolkowska A, Szyszka M,
Hochol A and Malendowicz LK: Evidence suggesting that ghrelin
O-acyl transferase inhibitor acts at the hypothalamus to inhibit
hypothalamo-pituitary-adrenocortical axis function in the rat.
Peptides. 35:149–159. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rucinski M, Albertin G, Spinazzi R,
Ziolkowska A, Nussdorfer GG and Malendowicz LK: Cerebellin in the
rat adrenal gland: Gene expression and effects of CER and
[des-Ser1]CER on the secretion and growth of cultured
adrenocortical cells. Int J Mol Med. 15:411–415. 2005.PubMed/NCBI
|
|
25
|
Rucinski M, Spinazzi R, Ziolkowska A,
Nussdorfer GG and Malendowicz LK: Effects of beacon on the rat
pituitary-adrenocortical axis response to stress. Int J Mol Med.
16:297–299. 2005.PubMed/NCBI
|
|
26
|
Rucinski M, Tortorella C, Ziolkowska A,
Nowak M, Nussdorfer GG and Malendowicz LK: Steroidogenic acute
regulatory protein gene expression, steroid-hormone secretion and
proliferative activity of adrenocortical cells in the presence of
proteasome inhibitors: In vivo studies on the regenerating rat
adrenal cortex. Int J Mol Med. 21:593–597. 2008.PubMed/NCBI
|
|
27
|
Tyczewska M, Rucinski M, Ziolkowska A,
Szyszka M, Trejter M, Hochol-Molenda A, Nowak KW and Malendowicz
LK: Enucleation-induced rat adrenal gland regeneration: Expression
profile of selected genes involved in control of adrenocortical
cell proliferation. Int J Endocrinol. 2014:1303592014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tyczewska M, Rucinski M, Ziolkowska A,
Trejter M, Szyszka M and Malendowicz LK: Expression of selected
genes involved in steroidogenesis in the course of
enucleation-induced rat adrenal regeneration. Int J Mol Med.
33:613–623. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jopek K, Celichowski P, Szyszka M,
Tyczewska M, Milecka P, Malendowicz LK and Rucinski M:
Transcriptome Profile of rat adrenal evoked by gonadectomy and
testosterone or estradiol replacement. Front Endocrinol (Lausanne).
8:262017.PubMed/NCBI
|
|
30
|
Pfaffl MW: A new mathematical model for
relative quantification in real-time RT–PCR. Nucleic Acids Res.
29:e452001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Owens K, Park JH, Schuh R and Kristian T:
Mitochondrial dysfunction and NAD(+) metabolism alterations in the
pathophysiology of acute brain injury. Transl Stroke Res.
4:618–634. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang XQ, Lu JT, Jiang WX, Lu YB, Wu M,
Wei EQ, Zhang WP and Tang C: NAMPT inhibitor and metabolite protect
mouse brain from cryoinjury through distinct mechanisms.
Neuroscience. 291:230–240. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
López FJ and Negro-Vilar A: Estimation of
endogenous adrenocorticotropin half-life using pulsatility
patterns: A physiological approach to the evaluation of secretory
episodes. Endocrinology. 123:740–746. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kleine Bernhard RWG: Hormones and the
Endocrine System. Springer; New York, NY: 2016, View Article : Google Scholar
|
|
35
|
Dampney RA and Horiuchi J: Functional
organisation of central cardiovascular pathways: Studies using
c-fos gene expression. Prog Neurobiol. 71:359–384. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hoffman GE, Smith MS and Verbalis JG:
c-Fos and related immediate early gene products as markers of
activity in neuroendocrine systems. Front Neuroendocrinol.
14:173–213. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hasmann M and Schemainda I: FK866, a
highly specific noncompetitive inhibitor of nicotinamide
phosphoribosyltransferase, represents a novel mechanism for
induction of tumor cell apoptosis. Cancer Res. 63:7436–7442.
2003.PubMed/NCBI
|
|
38
|
Hara N, Yamada K, Shibata T, Osago H and
Tsuchiya M: Nicotinamide phosphoribosyltransferase/visfatin does
not catalyze nicotinamide mononucleotide formation in blood plasma.
PLoS One. 6:e227812011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ratajczak J, Joffraud M, Trammell SA, Ras
R, Canela N, Boutant M, Kulkarni SS, Rodrigues M, Redpath P, Migaud
ME, et al: NRK1 controls nicotinamide mononucleotide and
nicotinamide riboside metabolism in mammalian cells. Nat Commun.
7:131032017. View Article : Google Scholar
|
|
40
|
Carbone F, Liberale L, Bonaventura A,
Vecchie A, Casula M, Cea M, Monacelli F, Caffa I, Bruzzone S,
Montecucco F and Nencioni A: Regulation and function of
extracellular nicotinamide phosphoribosyltransferase/visfatin.
Compr Physiol. 7:603–621. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Dupré SM, Burt DW, Talbot R, Downing A,
Mouzaki D, Waddington D, Malpaux B, Davis JR, Lincoln GA and Loudon
AS: Identification of melatonin-regulated genes in the ovine
pituitary pars tuberalis, a target site for seasonal hormone
control. Endocrinology. 149:5527–5539. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
West A, Dupre SM, Yu L, Paton IR,
Miedzinska K, McNeilly AS, Davis JR, Burt DW and Loundon AS: Npas4
is activated by melatonin, and drives the clock gene Cry1 in the
ovine pars tuberalis. Mol Endocrinol. 27:979–989. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Morgan PJ and Williams LM: The pars
tuberalis of the pituitary: A gateway for neuroendocrine output.
Rev Reprod. 1:153–161. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Reverchon M, Cornuau M, Cloix L, Rame C,
Guerif F, Royere D and Dupont J: Visfatin is expressed in human
granulosa cells: Regulation by metformin through AMPK/SIRT1
pathways and its role in steroidogenesis. Mol Hum Reprod.
19:313–326. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Diot M, Reverchon M, Rame C, Baumard Y and
Dupont J: Expression and effect of NAMPT (visfatin) on progesterone
secretion in hen granulosa cells. Reproduction. 150:53–63. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Diot M, Reverchon M, Rame C, Froment P,
Brillard JP, Brière S, Levêque G, Guillaume D and Dupont J:
Expression of adiponectin, chemerin and visfatin in plasma and
different tissues during a laying season in turkeys. Reprod Biol
Endocrinol. 13:812015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ocón-Grove OM, Krzysik-Walker SM,
Maddineni SR, Hendricks GL III and Ramachandran R: NAMPT (visfatin)
in the chicken testis: Influence of sexual maturation on cellular
localization, plasma levels and gene and protein expression.
Reproduction. 139:217–226. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kaur J, Ramanjaneya M, Chen J and Randeva
H: The adipokine, Pre-B cell colony enhancing factor
(PBEF)/visfatin, activates steroidogenic acute regulatory protein
(StAR) protein expression and steroid production in human
adrenocortical-H295R-cells via MAPK and PI3/AkT signalling
pathways. Endocrine Abstracts. 19:3142009.
|
|
49
|
Payne AH and Hales DB: Overview of
steroidogenic enzymes in the pathway from cholesterol to active
steroid hormones. Endocr Rev. 25:947–970. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hanukoglu I and Rapoport R: Routes and
regulation of NADPH production in steroidogenic mitochondria.
Endocr Res. 21:231–241. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Roucher-Boulez F, Mallet-Motak D,
Samara-Boustani D, Jilani H, Ladjouze A, Souchon PF, Simon D, Nivot
S, Heinrichs C, Ronze M, et al: NNT mutations: A cause of primary
adrenal insufficiency, oxidative stress and extra-adrenal defects.
Eur J Endocrinol. 175:73–84. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Weinberg-Shukron A, Abu-Libdeh A, Zhadeh
F, Carmel L, Kogot-Levin A, Kamal L, Kanaan M, Zeligson S, Renbaum
P, Levy-Lahad E, et al: Combined mineralocorticoid and
glucocorticoid deficiency is caused by a novel founder nicotinamide
nucleotide transhydrogenase mutation that alters mitochondrial
morphology and increases oxidative stress. J Med Genet. 52:636–641.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Meimaridou E, Goldsworthy MG, Chortis V,
Fragoulis T, Foster PA, Arlt W, Cox RD and Metherell LA: OR24-2:
Role of Nicotinamide Nucleotide Transhydrogenase in the Control of
Steroidogenesis in Mouse Adrenals. Proceedings of Endocrine
Society's 98th Annual Meeting and Expo. Boston. 2016;
|
|
54
|
Buldak RJ, Gowarzewski M, Buldak L,
Skonieczna M, Kukla M, Polaniak R and Zwirska-Korczala K: Viability
and oxidative response of human colorectal HCT-116 cancer cells
treated with visfatin/eNampt in vitro. J Physiol Pharmacol.
66:557–566. 2015.PubMed/NCBI
|
|
55
|
Oita RC, Ferdinando D, Wilson S, Bunce C
and Mazzatti DJ: Visfatin induces oxidative stress in
differentiated C2C12 myotubes in an Akt- and MAPK-independent,
NFkB-dependent manner. Pflugers Arch. 459:619–630. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Song SY, Jung EC, Bae CH, Choi YS and Kim
YD: Visfatin induces MUC8 and MUC5B expression via p38
MAPK/ROS/NF-κB in human airway epithelial cells. J Biomed Sci.
21:492014. View Article : Google Scholar : PubMed/NCBI
|