Introduction
Diabetes mellitus (DM) is one of the most common
chronic diseases around the world. The prevalence of diabetes has
increased in recent decades (1).
Diabetic cystopathy (DCP), which is a common disorder associated
with diabetes in the urologic system, accounts for ~80% of the
diabetic population (2). The
pathogenesis of DCP is a complex, multifactorial and time-dependent
process. Myogenic, neurogenic and urothelial changes are the main
possible causes for DCP (3,4).
Much evidence has suggested that the structure and function of
bladder detrusor muscle is changed by DM (5–7).
Much research has attempted to elucidate the mechanism of DCP, but
it is still unclear, and so, the treatments for DCP are
limited.
In DCP, the contraction of the bladder is damaged
depending on the diabetes duration (7). However, the exact mechanisms are far
from clear. Calcium is a very important factor in the regulation of
bladder smooth muscle cell (BSMC) contraction. One contraction
mechanism of the SMC is driven by the release of Ca2+
from the sarcoplasmic reticulum or the endoplasmic reticulum (ER).
Store-operated Ca2+ entry (SOCE) is so termed because
calcium influxes through the plasma membrane when the ER
Ca2+ store is exhausted. These channels are called
store-operated calcium channels (SOCCs) (8). Following the description of SOCE for
two decades, stromal interaction molecule 1 (STIM1) and Orai1 have
been confirmed as two critical molecular components of SOCCs,
according to use of RNAi technology (9,10).
Presently, it is clear that SOCE participates in a
wide range of pathophysiologic processes in muscles such as
proliferation, growth, differentiation and migration (11,12).
However, in bladder detrusor muscle cells, limited research has
been conducted. Recently, some studies demonstrated that SOCCs were
involved in detrusor overactivity, and the amount of urothelial ATP
release could be suppressed by SOCE (13,14).
According to clinical studies, detrusor overactivity and detrusor
hyperreflexia accounts for half of the DCP population (2,15).
Therefore, the authors hypothesized that SOCCs might be involved in
the progression of DCP. In the current study, the authors
investigated the role of SOCCs in the regulation of bladder
contraction and intracellular Ca2+ of BSMCs in
STZ-induced diabetic rats at different time points.
Materials and methods
Experimental animals
A total of 60 female Sprague-Dawley rats (weight,
230–270 g; age, 4–6 weeks) were used and matched by date of birth.
Animals were caged in a 12 h light/dark cycle, constant temperature
and humidity room with free access to water and food. The rats were
randomly divided into six groups: DM group for 4 weeks (DM4W), for
8 weeks (DM8W) and for 12 weeks (DM12W); the normal control group
for 4 weeks (N4W), for 8 weeks (N8W) and for 12 weeks (N12W).
Diabetes was induced after a 24 h fast by a single intraperitoneal
injection of 50 mg/kg STZ (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) dissolved in 0.1 M citrate buffer solution, pH 4.5. The
normal controls were treated with vehicle only. At 3 days after the
treatment, blood samples were collected by cutting the tail to
evaluate the blood glucose level with an automatic glucometer
(Accu-Check; Roche Diagnostics, Basel, Switzerland). Provided the
blood glucose lever was >300 mg/dl, the rats were considered to
have reached the standard of the diabetic rat model and were
suitable for the following study. The body weights and blood
glucose levels of all the rats were measured before the STZ
injection and after the rats were sacrificed by a single
intraperitoneal injection of pentobarbital (200 mg/kg), when the
wet bladder weight was recorded. The bladders harvested from each
rat were sliced into three parts for further studies. The ventral
part of the bladder was prepared for bladder smooth muscle strips.
The rest of the bladder was divided into two parts equally. One of
these was prepared for bladder smooth muscle cells. Meanwhile, the
other tissue was rapidly frozen in liquid nitrogen and stored at
−4°C for western blotting and reverse transcription-quantitative
polymerase chain reaction (RT-qPCR). The experimental protocol was
approved by the Laboratory Animal Research Committee of Shanxi
Medical University (Taiyuan, China).
Preparation for bladder smooth muscle
strips in vitro
The different experimental rats were sacrificed at
4, 8 and 12 weeks respectively. Bladders were harvested and dipped
into 4°C Krebs (containing 119 mM NaCl, 4.7 mM KCl, 1.2 mM
KH2PO4, 1.2 mM
MgSO4.7H2O, 25 mM NaHCO3, 2.5 mM
CaCl2 and 11 mM glucose, pH adjusted to 7.35 with NaOH)
solution immediately. The urothelium and submucosa layers were
removed by using microinstruments under a microscope (Motic,
SMZ-168 Series; China Group Co., Ltd., Xiamen, China; http://www.motic.com/Indu_Stereo/product_432.html) and
were longitudinally cut into 3×3×8 mm strips from the ventral
bladder. Bladder strips were fixed between electrodes and an
automatic organ bath (Panlab; Harvard Apparatus, Holliston, MA,
USA) containing 10 ml Krebs solution inflated with 95%
O2 and 5% CO2 at 37°C, as described
previously (16). Following 30
min, the SOCCs agonist cyclopiazonic acid (CPA, 10 µM)
(Sigma-Aldrich; Merck KGaA) was added into the Krebs solution and
the inhibitor SKF-96365 (10 µM; Sigma-Aldrich; Merck KGaA) was
added into the Krebs solution after 5 min. The spontaneous
contractive frequency and amplitude of BSM strips were recorded ~5
min for each intervention after the spontaneous contractions of
bladder strips were detected by PowerLab system and the data were
analyzed by LabChart version 7.3.7 (AD Instruments, Bella Vista,
Australia).
Isolation and culture of bladder
smooth muscle cells
Bladder smooth muscle cells (BSMCs) were isolated by
enzymatic dissociation method. Briefly, BSM tissues were cut into
pieces and digested in D-Hanks solution containing 0.4 mg/ml
type-II collagenase and 0.4 mg/ml bovine serum albumin at 37°C, 5%
CO2 and 95% O2 condition for 40–60 min in
HERAcell 150i (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
BMSCs were cultured in glass-bottom cell culture dishes at 37°C, 5%
CO2 and 95% O2 condition for confocal
microscopy. BMSCs from each animal bladder were cultured in one
dish for 24 h.
Measurement of cytosolic
Ca2+ in BMSCs
Cells cultured for 24 h were loaded with 4 µM
Fluo-4AM. Then, cells were washed with Hanks solution three times,
10 min for each wash. Finally, dishes were perfused with Tyrode
solution (118 mM NaCl, 6.1 mM glucose, 24 mM NaHCO3, 4.0
mM KCl, 1.0 mM MgCl2, 0.4 mM
NaH2PO4, 1.8 mM CaCl2 and 5.0 mM
sodium pyruvate at pH 7.35) and cells were scanned (1 image for 5
sec) under confocal microscopy (FlouView-FV1000; Olympus
Corporation, Tokyo, Japan). The method followed was as reported
previously (14). The SOCCs
agonist CPA (10 µM) and the inhibitor SKF-96365 (10 µM) were added
into the Tyrode solution after 5 min. The fluorescence values of
BSMCs were collected ~5 min after each intervention. The ratios of
fluorescence values of BSMCs (F1) and background fluorescence
values (F0) were calculated for comparison in different groups. The
data was collected and analyzed with FluoView 1.7a software
(Olympus Corporation).
RT-qPCR
The bladder tissue from each animal was homogenized
and total RNA was extracted from the bladder tissues using TRIzol
reagent (Sangon Biotech Co., Ltd., Shanghai, China). The extracted
RNA was dissolved in diethylpyrocarbonate-treated water. The cDNA
was synthesized using a reverse transcription kit (cat. no. K1622;
Thermo Fisher Scientific, Inc.). The sequences of the primers used
are listed in Table I. RT-qPCR was
performed with FastStart Universal SYBR-Green Master (Roche Applied
Science, Penzberg, Germany) using the following cycle settings:
Heating to 94°C for 10 min and amplification for 45 cycles at 94°C
for 15 sec and 60°C for 60 sec. The relative mRNA expression of the
Orai1 and STIM1 gene was normalized to abundance of mRNA for
β-actin. The data was analyzed using the 2−∆∆Cq method
(17).
 | Table I.Primer sequences and product
length. |
Table I.
Primer sequences and product
length.
| Gene | Primer
sequences | Product length
(bp) |
|---|
| Orai1 | Forward:
5′-ccataagacggaccgacagt-3′ | 132 |
|
| Reverse:
5′-gggaaggtgaggacttaggc-3′ |
|
| STIM1 | Forward:
5′-tggagctgccacagtatgag-3′ | 196 |
|
| Reverse:
5′-tgattgtggcgagtcaagag-3′ |
|
| β-actin | Forward:
5′-gtcaggtcatcactatcggcaat-3′ | 147 |
|
| Reverse:
5′-agaggtctttacggatgtcaacgt-3′ |
|
Western blot analysis
The bladder tissue lysis solution was prepared using
an extraction reagents kit (cat. no. PROTTOT-1KT; Sigma-Aldrich;
Merck KGaA), according to the manufacturer's protocol. The protein
concentrations were determined by using a bicinchoninic acid
protein assay kit (cat. no. P1551; Applygen Technologies Inc.,
Beijing, China). Equal amounts of protein extract (40 µg/lane) from
all specimens were separated by 15% SDS-PAGE for STIM1 and 10%
SDS-PAGE for Orai1 and β-actin, then electroblotted onto a 0.45 µm
polyvinylidene fluoride membrane in a transfer buffer. The
membranes were blocked with 5% fat-free milk for 1 h and incubated
overnight at 4°C with primary antibodies: Oria1 (cat. no. sc-68895;
diluted 1:200; Santa Cruz Biotechnology, Inc., Dallas, TX, USA);
STIM1 (cat. no. ab108994; diluted 1:1,000; Abcam, Cambridge, UK);
β-actin (cat. no. sc-47778; diluted 1:1,000; Santa Cruz
Biotechnology, Inc.). After being washed with TBST (TBS, 0.1%
Tween-20), the incubation with secondary antibody (goat anti-mouse,
cat. no. sc-2005 and goat anti-rabbit, cat. no. sc-2004; diluted
1:10,000; Santa Cruz Biotechnology, Inc.) conjugated to horseradish
peroxidase was performed for 1 h at room temperature. Then, the
immunoreactive bands were shown with a chemiluminescence detection
solution (KL140454; Pierce; Thermo Fisher Scientific, Inc.). The
film was scanned and analysed with Scion imaging software version
4.0.3.2 (Scion Corporation, Frederick, MD, USA). β-actin was used
as a loading control.
Statistical analysis
All data were presented as mean ± standard
deviation. Data were analyzed by Student's t-test and one-way
analysis of variance tests. P<0.05 was considered to indicate a
statistically significant difference. The data were analyzed with
SPSS software (version, 13.0; SPSS, Inc., Chicago, IL, USA).
Results
General characteristics
All of the 30 STZ-induced DM rats were established
successfully according to the standard of a fasting blood glucose
level of >300 mg/dl. A total of 4 rats died in the first 3 days
after the injection with STZ, possibly due to the intolerance to
the toxicity of STZ. Another 3 rats in the DM groups died from the
intestinal obstruction. A total of 3 rats in the control groups
died in the first week before treatment, and the etiology of these
unexpected deaths may be environmental changes. Table II presents the mean blood glucose
levels, initial and terminal body weights, and the bladder weights.
Similar to other investigations, the mean blood glucose levels and
bladder weights in the diabetic groups increased significantly,
while the mean body weights were decreased significantly, compared
with the control groups. Because a number of 10 rats were lost, 47
rats were used for the study (7 rats in the D12W group, 8 rats each
in the other five groups).
 | Table II.Body weight, bladder weight, and
blood glucose in diabetic and control groups. |
Table II.
Body weight, bladder weight, and
blood glucose in diabetic and control groups.
|
| Body weight |
|
|
|
|---|
|
|
|
|
|
|
|---|
| Group | Before STZ
injection (g) | At the time point
of sacrifice (g)a | Bladder weight
(mg)a | Bladder to body
weight (mg/g)a | Blood glucose
(mg/dl)a |
|---|
| N4W (n=10) |
213.5±7.4 |
298.5±13.8 |
111.5±4.7 |
0.374±0.012 |
106.9±9.1 |
| N8W (n=8) |
244.9±6.8 |
387.0±13.8 |
125.2±3.2 |
0.324±0.011 |
110.2±11.3 |
| N12W (n=9) |
251.6±6.0 |
449.4±14.6 |
132.2±5.1 |
0.294±0.012 |
96.86±10.5 |
| DM4W (n=8) |
220.9±5.7 |
201.4±13.1 |
201.7±10.8 |
1.000±0.048 |
435.9±17.1 |
| DM8W (n=8) |
245.4±6.2 |
227.9±9.0 |
218.8±14.1 |
0.960±0.053 |
452.6±11.8 |
| DM12W (n=7) |
257.3±6.8 |
271.9±11.0 |
245.8±9.6 |
0.904±0.032 |
432.5±15.1 |
The frequencies of contractions in DM
rats changed significantly after activation or inhibition of
SOCCs
The changes of contractions of bladder detrusor
strips are presented in Fig. 1.
The frequency variations of spontaneous contraction were increased
significantly in the DM4W group (t=3.776, P=0.002, n=8) and the
DM8W (t=2.282, P=0.039, n=8) group following activation of SOCC
with CPA (10 µM), compared with the control groups (Fig. 1C). Among these three DM groups, the
DM12W group was significantly lower than the other two groups
(DM12W vs. DM4W, P=0.039; DM12W vs. DM8W, P=0.012, n: 8 for DM4W, 8
for DM8W, and 7 for DM12W). Despite there being no significant
differences between DM groups and control groups, the amplitudes of
contractions decreased in all experiment groups after activation of
SOCCs.
After the addition of SKF-96365 (10 µM) to the bath,
the frequencies of spontaneous contraction decreased and the
amplitudes of spontaneous contraction increased in all experimental
groups, compared to the time of adding CPA only. The frequency
variations were significantly changed in the DM4W group (t=−3.750,
P=0.02, n=8) and the DM8W group (t=−2.593, P=0.021, n=8), compared
with the control groups (Fig. 1D).
Among these three DM groups, the DM12W group was significantly
higher than the other two groups (DM12W vs. DM4W, P=0.004; DM12W
vs. DM8W, P=0.021, n=8 for DM4W, n=8 for DM8W and n=7 for DM12W).
The amplitudes increased to some extent compared to CPA only, and
there were no significant differences between DM groups and control
groups.
The intracellular Ca2+ was
changed significantly in BSMCs of DM rats by activation or
inhibition of SOCCs
The changes of fluorescence values in BSMCs are
indicated in Fig. 2. Following
activation of SOCCs with CPA, the F1/F0 increased in DM4W and DM8W
groups and reached its peak in the 8th week, then went down to the
control level at the time of 12th week. Compared with control
groups, the F1/F0 elevated significantly in DM4W (n=8, t=4.867,
P=0.0002) and DM8W groups (n=8, t=4.203, P=0.001) (Fig. 2C). The F1/F0 decreased, when SOCCs
inhibited by SKF-9365. However, the fluorescence intensity of BSMCs
was still significantly higher in the DM4W (n=8, t=3.141, P=0.007)
and DM8W groups (n=8, t=3.871, P=0.002) than that of control groups
(Fig. 2D). There was no
significant difference between the DM12W group and the N4 W group
after addition of CPA (t=−0.536, P=0.601, n=7 for DM12W, n=8 for
N4W) or SKF-96365 (t=−0.186, P=0.857, n=7 for DM12W, n=8 for N4W).
The fluorescence intensity in the DM12W group was significantly
lower than the other DM groups after activation (DM12W vs. DM4W,
P=0.001; DM12W vs. DM8W, P=0.0004, DM8W vs. DM4W, P=0.680; n=8 for
DM4W, n=8 for DM8W and n=7 for DM12W) or inhibition of SOCCs (DM12W
vs. DM4W, P=0.003; DM12W vs. DM8W, P=0.002, DM8W vs. DM4W, P=0.914;
n=8 for DM4W, n=8 for DM8W and n=7 for DM12W).
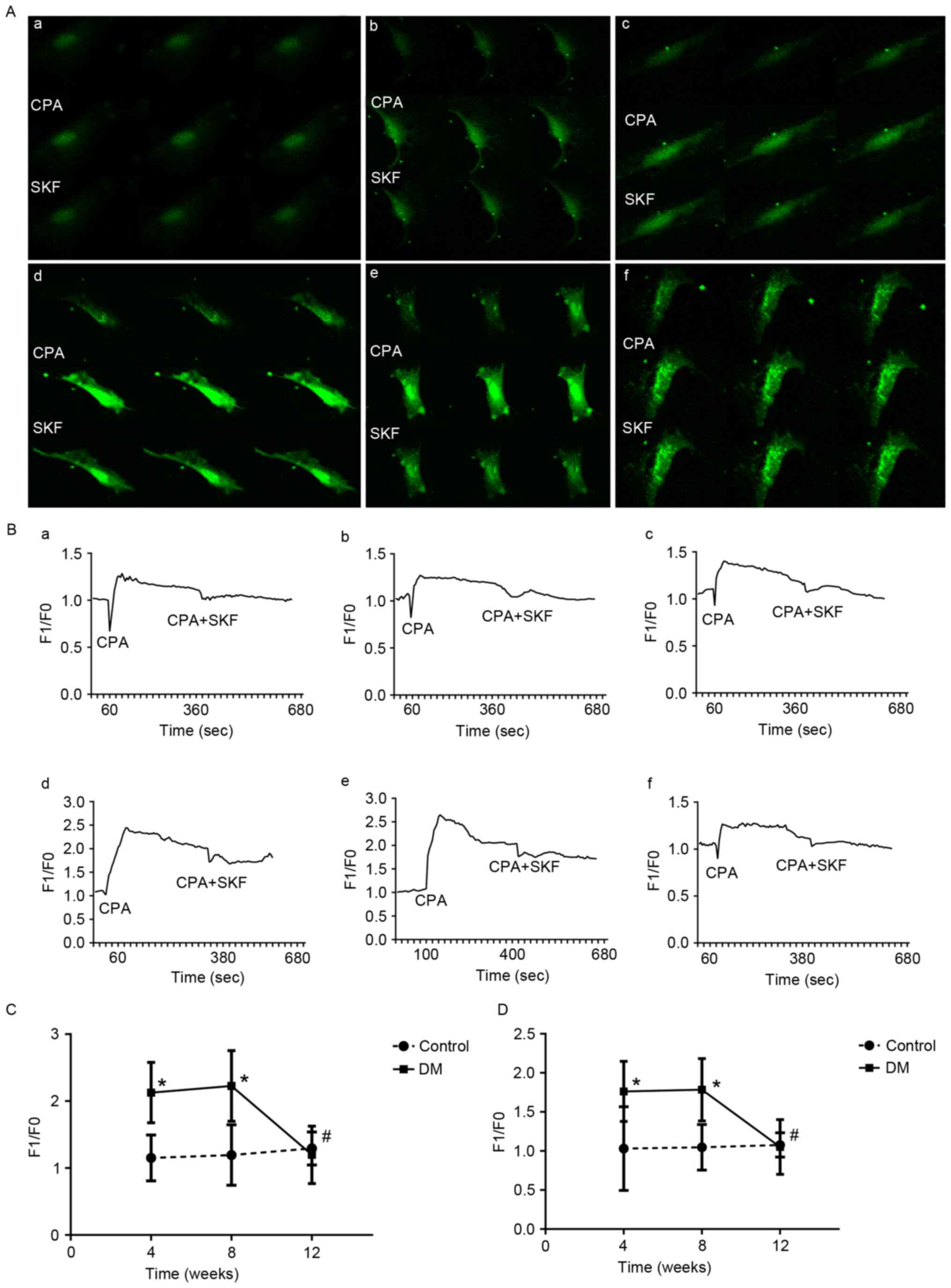 | Figure 2.Changes of intracellular
Ca2+ in BSMCs after activation (CPA, 10 µM) and
inhibition (SKF-96365, 10 µM) SOCCs. (A) shown as green
fluorescence intensity of Fluo-4 AM at 516 nm. CPA, after using
CPA; SKF, after using SKF-96365. (A-d, A-e and A-f) represent DM4W,
DM8W and DM12W group, respectively. (A-a, A-b and A-c) represent
the control groups. Magnification, ×200. (B) The fluorescent line
graphs of real-time changes of intracellular Ca2+ in
BSMCs. (B-d, B-e and B-f) represent DM4W, DM8W and DM12W group
respectively. (B-a, B-b and B-c) represent the control groups. (C)
*P<0.05, vs. control groups after using CPA. (D) *P<0.05 vs.
control groups after using SKF-96365. #P<0.05, DM12W
group compared with other two DM groups. BSMCs, bladder smooth
muscle cells; CPA, cyclopiazonic acid; F1, the measured values of
fluorescence intensity in BSMCs; F0, the background values of
fluorescence intensity; DM, diabetes mellitus; SKF, SKF-96365. |
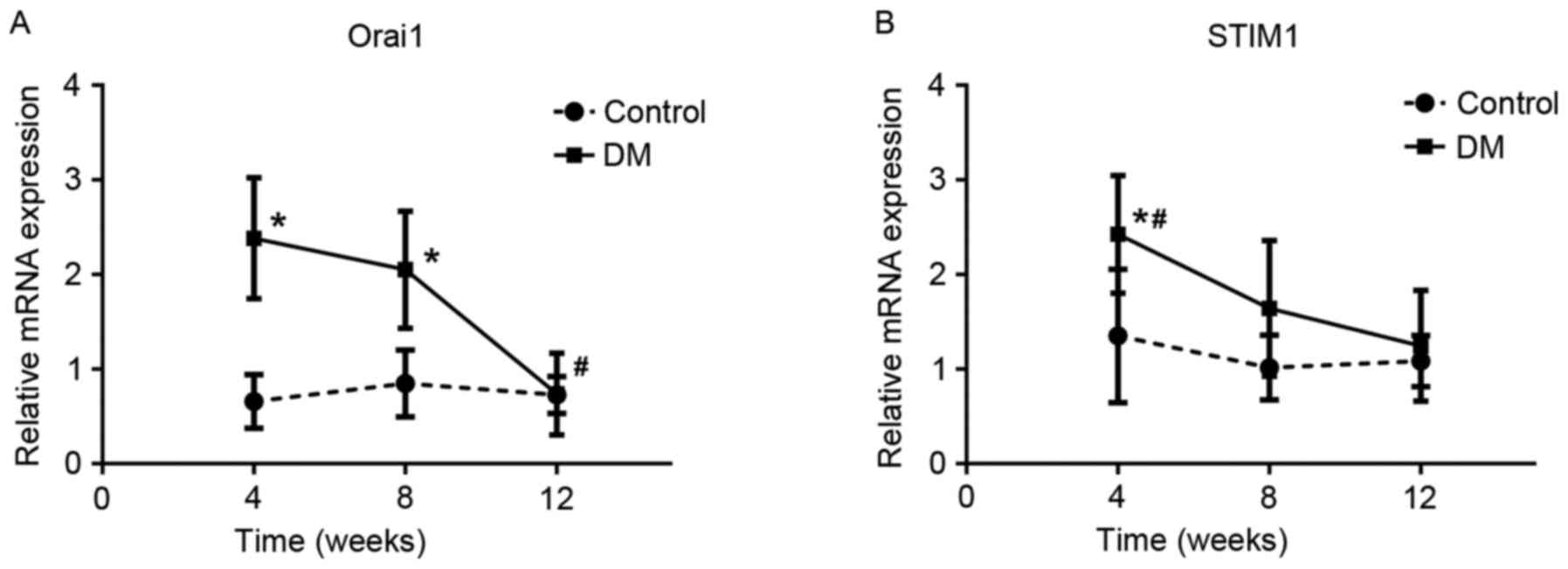
RT-qPCR analysis of Orai1 and
STIM1
The expressions of Orai1 and STIM1 mRNA showed a
similar trend at the three time points in SZT-induced diabetic
rats, peaking at the 4th week and reaching its lowest level at the
12th week (Fig. 3). Compared with
the control groups, the expressions of Orai1 mRNA were
significantly higher in the DM4W (n=6, t=6.049, P=0.0001) and DM8W
groups (n=6, t=4.122, P=0.002) and the expressions of STIM1 mRNA
were significantly higher in the DM4W group (n=6, t=2.799,
P=0.019). Among DM groups, the expressions of Orai1 and STIM1 mRNA
showed a declining trend and reached their lowest levels in the
12th week. The expression of Orai1 mRNA in the 12th week was
significantly lower than the other two DM groups (DM12W vs. DM4W,
P=0.0001; DM12W vs. DM8W, P=0.001, DM8W vs. DM4W, P=0.329; n=6 for
every group). The expression of STIM1 mRNA in the 4th week was
significantly higher than the other two DM groups (DM4W vs. DM8W,
P=0.013; DM4W vs. DM12W, P=0.002, DM8W vs. DM12W, P=0.409; n=6 for
every group).
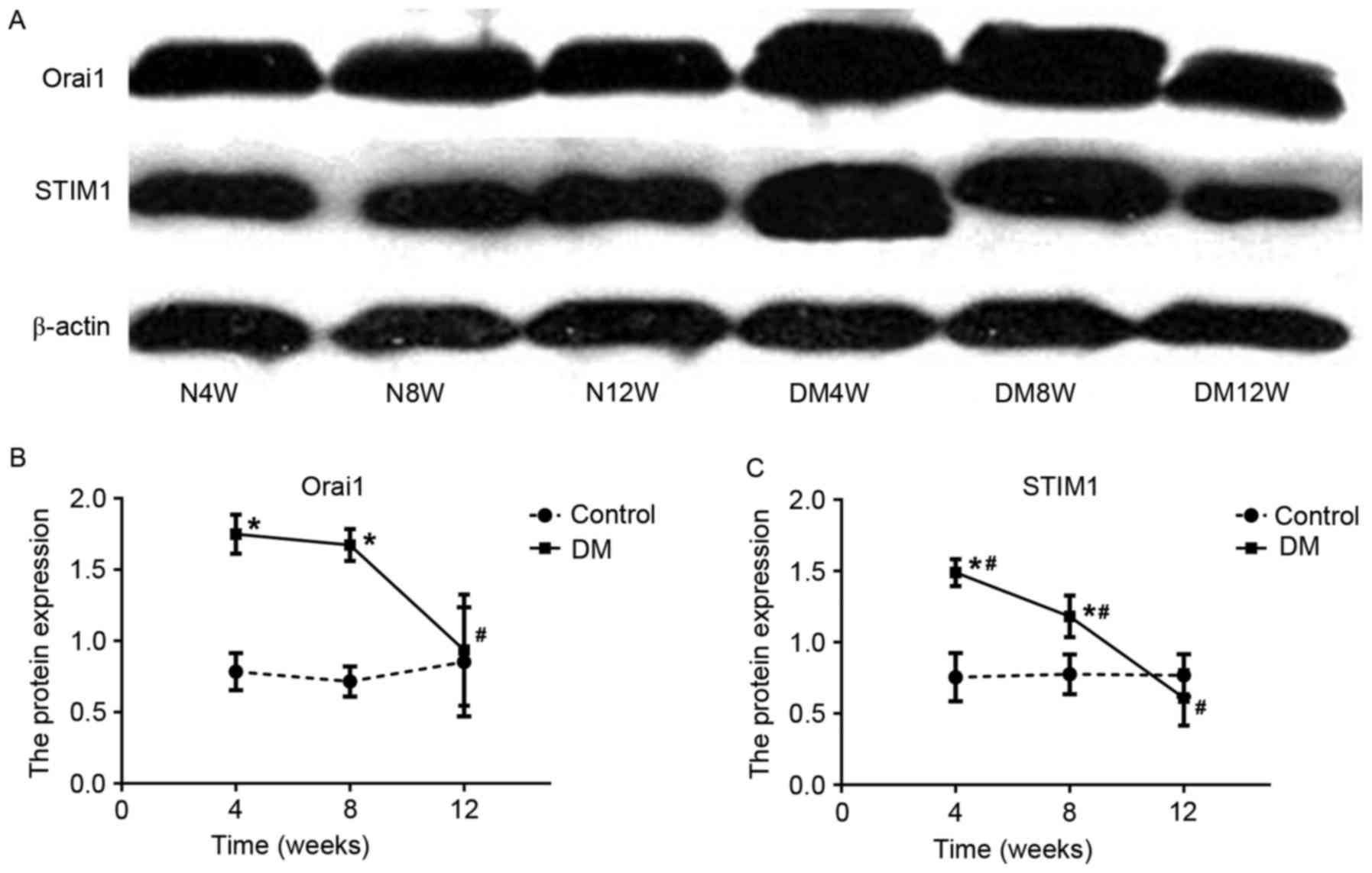
Western blotting of Orai1 and
STIM1
The results of western blotting indicated that Orai1
and STIM1 proteins in bladder tissues as single bands migrating at
38 and 77 kDa, respectively (Fig.
4A). The densitometric analysis of the bands for these proteins
indicated that the expressions of Orai1 and STIM1 protein were
similar with that of their mRNA levels in DM groups, which peaked
in the 4th week and reached its lowest point in the 12th week.
Compared with control groups (Fig. 4B
and C), the expressions of Orai1 and STIM1 protein were
significantly higher in DM4W and DM8W groups (Orai1: DM4W vs. N4W
n=6, t=8.862, P=0.001; DM8W vs. N8W n=6, t=10.803, P=0.0004; STIM1:
DM4W vs. N4W n=6, t=6.540, P=0.003; DM8W vs. N8W n=6, t=3.495,
P=0.025). Among DM groups, the expression of Orai1 protein at the
12th week was significant lower than that at 4 and 8th weeks (DM12W
vs. DM4W, P=0.007; DM12W vs. DM8W, P=0.011, DM8W vs. DM4W, P=0.717;
n=6 for every group). However, the expressions of STIM1 proteins
were significantly different at all three time points (DM12W vs.
DM4W, P=0.0003; DM12W vs. DM8W, P=0.003, DM8W vs. DM4W, P=0.046;
n=6 for every group).
Discussion
SOCCs were discovered in muscle cells and were found
to be involved in the contraction of SMCs (18,19).
In the coronary artery, smooth muscle contractions via SOCCs
induced by CPA were identified and that these contractions can be
suppressed by pre-treated with hydrogen peroxide (18). In pulmonary arteries, CPA-induced
contraction was increased in chronic hypoxia and
monocrotaline-treated rats and this effect was inhibited by
ginsenoside Rb1 (19). In
addition, SOCCs have been proved to serve a crucial role in the
progression of diabetic complications, such as diabetic nephropathy
(20), diabetic vascular disease
(21,22) and diabetic cardiomyopathy (23). CPA is a sarcoplasmic endoplasmic
Ca2+-ATPase pump inhibitor, and is widely used to
deplete intracellular stores in various tissues. SKF-96365 may
cause a significant reduction of the transient Ca2+
release initiated by CPA and directly inhibit Ca2+ entry
through SOCC (24). CPA and
SKF-96365 are commonly used SOCC agents and have been used as
pharmacological tools to study SOCE (25,26).
In the present study, the authors found that the
contractive frequencies of BSM strips elevated significantly in the
4th and 8th week and declined to the control level in the 12th week
in DM groups by the activation of SOCCs, which implied that the
SOCCs played a critical role in regulation of the contractive
frequency of the bladder in diabetic rats. However, the effect of
diabetes on bladder smooth muscle contraction was inconsistent.
Waring and Wendt (27) reported
significantly more force in CPA-treated diabetic muscles at 8th
week. Compared with the control group, a reduced CPA induced
bladder contraction was discovered in diabetic rats at 12th week
(28). According to the
International Consultation on Incontinence-Research Society panel
recommendation (29), the
time-dependent design should be developed in future research to
investigate the diabetic uropathy in DM. Daneshgari et al
(7) suggested that the contraction
changes of DSM strips in diabetic rats were time-dependent.
Diabetic bladders may undergo a transition from a compensated to a
decompensated state and transition in the streptozotocin rat model
may begin 9 to 12 weeks following induction. The discrepancies of
these reports may be due to the different experimental conditions
and the duration of diabetes (30).
Calcium homeostasis is a very important factor for
the contraction function of SMCs. The authors measured the changes
of intracellular Ca2+ concentration in BMSCs in order to
explore the potential mechanisms of the contractive frequency
changes. Waring and Wendt (27)
suggested that there were no major impairments in either
intracellular calcium regulation or contractile function in bladder
smooth muscle after 8 weeks of STZ-induced diabetes. Mustafa
(28) found that the CPA induced
contraction of bladder strips decreased in diabetic rats at 12th
week and suggested that the Ca2+-ATPase pumps were
impaired after 12 weeks of STZ-induced diabetes. In the current
study, the changes of F1/F0 increased in the 4th and 8th week and
declined to the control level in the 12th week in DM groups by the
activation of SOCCs, which decreased when SKF-96365 was added. The
results were consistent with the contraction frequency of bladder
detrusor muscle strips. These results may suggest that the elevated
contraction frequency was triggered by the changes of intracellular
Ca2+ concentration via SOCCs.
In previous studies (30,31),
the voltage-gated calcium channels (VGCCs) were found to play a
major role in contractile responses of bladder strips to
depolarizing stimuli in STZ-induced diabetic mice (type 1 diabetes)
and db/db mice (type 2 diabetes). However, the interaction effect
among Ca2+ related channels is poorly understood. Some
researchers suggested that the Cav1.2 channel, a subtype
of L-VGCC, could be inhibited by STIM1induced store depletion
(32,33). Nguyen et al (34) found an inhibitory interaction
between STIM1 and Cav3.1, a T-type Ca2+
channel. Moreover, mitochondria were suggested to be involved in
the modulation of SOCE in Alzheimer's disease (35). Mitochondria serve an important role
in calcium buffering and can modulate SOCE through multiple
interaction pathways, to prolong SOCE for example, but the
interaction pathways are not well understood (8). One limitation of these
Ca2+ concentration study results is that we are unable
to rule out the contribution of non-SOCE influx in the fluorescent
values. However, the increased fluorescent values induced by CPA
were partly blocked by SKF-96365, which might suggest that the SOCE
was involved in the process.
The molecular components of SOCCs have not been
completely clarified. Some of the canonical transient receptor
potential proteins have been suggested to contribute to SOCE under
certain conditions, but conflicting results have been reported
(36). Moreover, mammals express
two STIM homologs, STIM1-2, and three Orai homologs, Orai1-3.
However, less is known concerning the functions of STIM2, Orai2,
and Orai3 (37). As the
combination of STIM1-Orai1 is undisputed, the expressions of Orai1
and STIM1 were therefore studied. Chaudhari et al (20) provided a molecular basis for
increased store-operated Ca2+ entry under high glucose
and diabetes conditions in mesangial cells. They identified that
the STIM1 and Orai1 protein levels increased following
administration of high glucose (25 mM) for 7 days. The upregulation
of these two proteins were further verified in rats with 4 weeks of
STZ treated type 1 diabetic rats and high-fat diet induced type 2
diabetic rats. Another study (38)
reported that increased abundance of Orai1-3 and STIM1-2 was
detected with enhanced SOCE in vascular endothelial cells in high
glucose (25 mM) treated for 3 days. Furthermore, the expression
levels of Orai1-3 and STIM1-2 mRNAs were significantly upregulated
in the aortae of Akita diabetic and STZ-induced diabetic mice.
However, Estrada et al (21) reported that the expression of STIM1
protein was decreased in coronary endothelial cells in diabetic
mice at 6 weeks after STZ injection. Moreover, overexpression of
STIM1 alleviated the impaired ER Ca2+ refilling in
diabetic coronary endothelial cells. The present study demonstrated
that the expressions of Orai1 and STIM1 peaked in the 4th week and
returned to the level of the control group in the 12th week, and
this also paralleled the changes of intracellular Ca2+
concentration.
Taking these results into consideration together,
the authors concluded that the SOCCs were involved in the
pathological mechanism of DCP. However, the detailed signal pathway
of SOCCs in the progression of DCP should be explored in future
studies.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81370861).
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cheng YJ, Imperatore G, Geiss LS, Wang J,
Saydah SH, Cowie CC and Gregg EW: Secular changes in the
age-specific prevalence of diabetes among U.S. adults: 1988–2010.
Diabetes Care. 36:2690–2696. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kaplan SA, Te AE and Blaivas JG:
Urodynamic findings in patients with diabetic cystopathy. J Urol.
153:342–344. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sasaki K, Chancellor MB, Phelan MW,
Yokoyama T, Fraser MO, Seki S, Kubo K, Kumon H, Groat WC and
Yoshimura N: Diabetic cystopathy correlates with a long-term
decrease in nerve growth factor levels in the bladder and
lumbosacral dorsal root Ganglia. J Urol. 168:1259–1264. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hanna-Mitchell AT, Ruiz GW, Daneshgari F,
Liu G, Apodaca G and Birder LA: Impact of diabetes mellitus on
bladder uroepithelial cells. Am J Physiol Regul Integr Comp
Physiol. 304:R84–R93. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang D, Yuan X, Hu C, Zhang B, Gao H, Wang
D, Chi J, Jing Q, Wu S and Wu CL: Endoplasmic reticulum stress is
involved in apoptosis of detrusor muscle in streptozocin-induced
diabetic rats. Neurourol Urodyn. 36:65–72. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Changolkar AK, Hypolite JA, Disanto M,
Oates PJ, Wein AJ and Chacko S: Diabetes induced decrease in
detrusor smooth muscle force is associated with oxidative stress
and overactivity of aldose reductase. J Urol. 173:309–313. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Daneshgari F, Liu G and Imrey PB: Time
dependent changes in diabetic cystopathy in rats include
compensated and decompensated bladder function. J Urol.
176:380–386. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Prakriya M and Lewis RS: Store-operated
calcium channels. Physiol Rev. 95:1383–1436. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang SL, Yu Y, Roos J, Kozak JA, Deerinck
TJ, Ellisman MH, Stauderman KA and Cahalan MD: STIM1 is a
Ca2+ sensor that activates CRAC channels and migrates
from the Ca2+ store to the plasma membrane. Nature.
437:902–905. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Prakriya M, Feske S, Gwack Y, Srikanth S,
Rao A and Hogan PG: Orai1 is an essential pore subunit of the CRAC
channel. Nature. 443:230–233. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Spinelli AM, González-Cobos JC, Zhang X,
Motiani RK, Rowan S, Zhang W, Garrett J, Vincent PA, Matrougui K,
Singer HA and Trebak M: Airway smooth muscle STIM1 and Orai1 are
upregulated in asthmatic mice and mediate PDGF-activated SOCE, CRAC
currents, proliferation, and migration. Pflugers Arch. 464:481–492.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Seth M, Li T, Graham V, Burch J, Finch E,
Stiber JA and Rosenberg PB: Dynamic regulation of sarcoplasmic
reticulum Ca(2+) stores by stromal interaction molecule 1 and
sarcolipin during muscle differentiation. Dev Dyn. 241:639–647.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Matsumoto-Miyai K, Kagase A, Yamada E,
Yoshizumi M, Murakami M, Ohba T and Kawatani M: Store-operated
Ca2+ entry suppresses distention-induced ATP release
from the urothelium. Am J Physiol Renal Physiol. 300:F716–F720.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao B, Zhong X, Bai X, Wang Q, Song B and
Li L: Changes in store-operated calcium channels in rat bladders
with detrusor overactivity. Urology. 84:491.e1–e6. 2014. View Article : Google Scholar
|
|
15
|
Bansal R, Agarwal MM, Modi M, Mandal AK
and Singh SK: Urodynamic profile of diabetic patients with lower
urinary tract symptoms: Association of diabetic cystopathy with
autonomic and peripheral neuropathy. Urology. 77:699–705. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
He P, Deng J, Zhong X, Zhou Z, Song B and
Li L: Identification of a hyperpolarization-activated cyclic
nucleotide-gated channel and its subtypes in the urinary bladder of
the rat. Urology. 79:1411.e7–e13. 2012. View Article : Google Scholar
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Weirich J, Dumont L and Fleckenstein-Grün
G: Contribution of capacitative and non-capacitative
Ca2+-entry to M3-receptor-mediated contraction of
porcine coronary smooth muscle. Cell Calcium. 38:457–467. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang RX, He RL, Jiao HX, Dai M, Mu YP, Hu
Y, Wu ZJ, Sham JS and Lin MJ: Ginsenoside Rb1 attenuates
agonist-induced contractile response via inhibition of
store-operated calcium entry in pulmonary arteries of normal and
pulmonary hypertensive rats. Cell Physiol Biochem. 35:1467–1481.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chaudhari S, Wu P, Wang Y, Ding Y, Yuan J,
Begg M and Ma R: High glucose and diabetes enhanced store-operated
Ca(2+) entry and increased expression of its signaling proteins in
mesangial cells. Am J Physiol Renal Physiol. 306:F1069–F1080. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Estrada IA, Donthamsetty R, Debski P, Zhou
MH, Zhang SL, Yuan JX, Han W and Makino A: STIM1 restores coronary
endothelial function in type 1 diabetic mice. Circ Res.
111:1166–1175. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Curtis TM, Major EH, Trimble ER and
Scholfield CN: Diabetes-induced activation of protein kinase C
inhibits store-operated Ca2+ uptake in rat retinal
microvascular smooth muscle. Diabetologia. 46:1252–1259. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pang Y, Hunton DL, Bounelis P and Marchase
RB: Hyperglycemia inhibits capacitative calcium entry and
hypertrophy in neonatal cardiomyocytes. Diabetes. 51:3461–3467.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Peng G, Lu W, Li X, Chen Y, Zhong N, Ran P
and Wang J: Expression of store-operated Ca2+ entry and transient
receptor potential canonical and vanilloid-related proteins in rat
distal pulmonary venous smooth muscle. Am J Physiol Lung Cell Mol
Physiol. 299:L621–L630. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Peng G, Ran P, Lu W, Zhong N and Wang J:
Acute hypoxia activates store-operated Ca(2+) entry and increases
intracellular Ca(2+) concentration in rat distal pulmonary venous
smooth muscle cells. J Thorac Dis. 5:605–612. 2013.PubMed/NCBI
|
|
26
|
Liu H, Hughes JD, Rollins S, Chen B and
Perkins E: Calcium entry via ORAI1 regulates glioblastoma cell
proliferation and apoptosis. Exp Mol Pathol. 91:753–760. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Waring JV and Wendt IR: Effects of
streptozotocin-induced diabetes mellitus on intracellular calcium
and contraction of longitudinal smooth muscle from rat urinary
bladder. J Urol. 163:323–330. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mustafa S: Effect of diabetes on the ion
pumps of the bladder. Urology. 81:211.e17–e21. 2013. View Article : Google Scholar
|
|
29
|
Kirschner-Hermanns R, Daneshgari F, Vahabi
B, Birder L, Oelke M and Chacko S: Does diabetes mellitus-induced
bladder remodeling affect lower urinary tract function? ICI-RS
2011. Neurourol Urodyn. 31:359–364. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Leiria LO, Mónica FZ, Carvalho FD,
Claudino MA, Franco-Penteado CF, Schenka A, Grant AD, De Nucci G
and Antunes E: Functional, morphological and molecular
characterization of bladder dysfunction in streptozotocin-induced
diabetic mice: Evidence of a role for L-type voltage-operated
Ca2+ channels. Br J Pharmacol. 163:1276–1288. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jiang X, Luttrell I, Chitaley K and Yang
CC: T- and L-type voltage-gated calcium channels: Their role in
diabetic bladder dysfunction. Neurourol Urodyn. 33:147–152. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang Y, Deng X, Mancarella S, Hendron E,
Eguchi S, Soboloff J, Tang XD and Gill DL: The calcium store
sensor, STIM1, reciprocally controls Orai and CaV1.2 channels.
Science. 330:105–109. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Park CY, Shcheglovitov A and Dolmetsch R:
The CRAC channel activator STIM1 binds and inhibits L-type
voltage-gated calcium channels. Science. 330:101–105. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nguyen N, Biet M, Simard E, Béliveau E,
Francoeur N, Guillemette G, Dumaine R, Grandbois M and Boulay G:
STIM1 participates in the contractile rhythmicity of HL-1 cells by
moderating T-type Ca(2+) channel activity. Biochim Biophys Acta.
1833:1294–1303. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ma T, Gong K, Yan Y, Song B, Zhang X and
Gong Y: Mitochondrial modulation of store-operated Ca(2+) entry in
model cells of Alzheimer's disease. Biochem Biophys Res Commun.
426:196–202. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Parekh AB and Putney JW Jr: Store-operated
calcium channels. Physiol Rev. 85:757–810. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hoth M and Niemeyer BA: The neglected CRAC
proteins: Orai2, Orai3, and STIM2. Curr Top Membr. 71:237–271.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Daskoulidou N, Zeng B, Berglund LM, Jiang
H, Chen GL, Kotova O, Bhandari S, Ayoola J, Griffin S, Atkin SL, et
al: High glucose enhances store-operated calcium entry by
upregulating ORAI/STIM via calcineurin-NFAT signalling. J Mol Med
(Berl). 93:511–521. 2015. View Article : Google Scholar : PubMed/NCBI
|