Introduction
Colon cancer is one of the most common malignant
carcinomas, the morbidity of which is the second highest among
gastroenteric tumors worldwide (1). The majority of patients with colon
cancer suffer from hemafecia, diarrhea and pain. Colon cancer
affects human health. Due to the late stage of diagnosis and poor
efficacy of treatment, the incidence and mortality rates are 8–9%
(2). The 5-year survival rate of
early-stage colon cancer exceeds 70–90%, although the survival rate
of advanced colon cancer is unsatisfactory. National Comprehensive
Cancer Network guidelines recommend surgical treatment for patients
with primary-stage colon cancer. However, in patients with
advanced-stage disease, it is challenging to completely remove the
tumor via surgical procedures due to the ubiquitous invasion and
migration. Therefore, palliative surgery combined with chemotherapy
is more frequently applied (3).
Chemotherapy is given priority among comprehensive treatments
(4). However, anti-drug
sensitivity or severe adverse reactions occasionally limit the
clinical effectiveness. It is therefore necessary to develop novel
drugs exhibiting fewer anti-drug sensitivity effects and adverse
reactions. Genistein is a potential drug candidate for the
treatment of colon cancer.
The initiation and progression of carcinogenesis
occurs when genetic and epigenetic modifications accumulate and
interact. DNA methylation of CpG islands in the promoter region of
tumor suppressor genes is considered to be an epigenetic mechanism
underlying cancer development (4,5). As
a highly conserved metabolism-associated pathway, the Wnt signaling
pathway is involved in metabolic processes, including embryonic
development, cell proliferation, metastasis and differentiation.
The Wnt signaling pathway is inactive in normal cells, and the
abnormal activation may induce tumor development. Wnt inhibitory
factor 1 (WIF1) is a tumor suppressor, which interacts with Wnt
protein to inhibit the canonical and non-canonical Wnt pathways. It
has been reported that silencing of WIF1 via methylation is
associated with cancers, such as colon cancer (6). A previous study additionally reported
that WIF1 inhibited the Wnt signaling pathway by inhibiting
β-catenin accumulation, suppressed esophageal cancer and decreased
the expression of E2F transcription factor 1, cyclin D1 and c-Myc
proto-oncogene protein (c-Myc) to promote the apoptosis of hepatoma
cells (7).
Recent studies demonstrated that
methylation-mediated epigenetic silencing of genes may be reversed
by drugs (8,9). Genistein, a soy-derived isoflavone,
is reported to upregulate the mRNA expression of a number of tumor
suppressor genes, to antagonize function of growth stimulating
factors and to inhibit cellular malignancy. Genistein has a
broad-spectrum anti-cancer effect on breast, prostate, esophageal,
pancreatic, gastric and colon cancer, and metrocarcinoma, lymphoma
and neuroblastoma; and therefore, it is becoming a novel therapy
for the treatment of tumors (10–12).
However, whether genistein is able to upregulate the mRNA
expression of WIF1 through demethylation in colon cancer remains to
be elucidated.
The present study investigated the effect of
treatment with genistein on WIF1 in colon cancer cells,
particularly on cell invasion and migration. The present study
suggested a mechanism underlying the genistein-mediated
demethylation of the WIF1 promoter region, and its influence on
downstream molecular factors in the associated pathways. The
results of the present study may contribute to the treatment of
colon cancer.
Materials and methods
Patients and tissue samples
Written informed consent was obtained from all
participants prior to the present study. A total of 52 patients
(36–79 years old; median age, 55 years), 32 males and 20 females,
with colon cancer from Changzhou No. 2 People's Hospital
(Changzhou, China) were included in the present study, from
November 2015 to November 2016. The present study included two
patients with well-differentiated tumors, 31 patients with
moderately-differentiated tumors and 19 patients with
poorly-differentiated tumors. Of those patients, a total of nine
were in the T1+T2 stage, 22 patients were in the T3 stage and 21
patients were in the T4 stage. Preoperative clinical and
pathological follow-up data were completed by all patients. All
tissue samples of patients were collected according to procedures
approved by the institutional review board of the independent
ethics committee of Changzhou No. 2 People's Hospital. Adjacent
normal colon tissues were also collected as negative controls.
Reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) and western blot analyses were performed to detect the
mRNA and protein expression levels of WIF1 in colon cancer tissues
and adjacent normal colon tissues. Detailed procedures are
described in the designated sections below.
Cell culture
Human colon mucosal epithelial cells NCM460 and
colon cancer cell lines (HT29, SW620, LOVO, HCT116) were purchased
from the American Type Culture Collection (Manassas, VA, USA) and
cultured in RPMI 1640 medium (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) containing 10% fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Inc.) with 1%
penicillin/streptomycin (Invitrogen; Thermo Fisher Scientific,
Inc.) at 37°C with 5% CO2. Cells in the logarithmic
phase were used for subsequent experimentation.
RT-qPCR and western blot analyses were performed to
detect the mRNA and protein expression of WIF1 in the above colon
cancer cell lines and the normal colon cell line NCM1460.
Cell viability assay
The effect of genistein on HT29 cell viability was
detected using the Cell Counting Kit-8 (CCK8; Beyotime Institute of
Biotechnology, Haimen, China). HT29 cells were seeded in 96-well
plates at the density of 5×103 cells/well and incubated
for 24 h at 37°C. Genistein (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) was subsequently added to each well at different final
concentrations, including 0, 5, 10, 20, 40 and 60 µmol/l and
incubated for 12, 24, 48 and 72 h at 37°C. Subsequently, 10 µl CCK8
solution was added to each well and incubated for a further 4 h at
37°C with 5% CO2. The optical density (OD) values were
measured at a wavelength of 450 nm using a microplate reader
(Thermo Fisher Scientific, Inc.). Data are presented as the ratio
of viable cells to control cells.
RT-qPCR and western blot analyses were performed to
detect the mRNA and protein expression of WIF1 in HT29 colon cancer
cells and genistein-treated cells (10, 20 and 60 µmol/l).
Methylation specific PCR (MSP)
Following a 72 h-treatment with 10, 20 and 60 µmol/l
genistein independently, HT29 cells (1.5×104
cells/sample) were subjected to bisulfite conversion using the EZ
DNA Methylation-Startup kit (Zymo Research Corp., Irvine, CA, USA),
according to the manufacturer's protocol. Subsequently, 30 ng
converted DNA of each sample was used for the MSP amplification.
The WIF1 promoter region was identified using the online software
MethPrimer 2.0 (Chinese Academy of Medical Sciences, Beijing,
China), with methylated [(M)] allele-specific primers, forward (F)
5′-CGTTTTATTGGGCGTATCGT-3′ and reverse (R)
5′-ACTAACGCGAACGAAATACGA-3′; and unmethylated [(U)] allele-specific
primers, F5′-GGGTGTTTTATTGGGTGTATTGT-3′ and R
5′-AAAAAAACTAACACAAACAAAATACAAAC-3′. MSP amplification was
performed using the following thermocycling conditions: Initial
denaturation for 1 min at 94°C; 35 (M) or 40 (U) cycles of
denaturation at 94°C for 1 min, and annealing at 65°C (M) or 56°C
(U) for 30 sec; and final extension at 72°C for 1 min.
Cell invasion and migration assay
Cell invasion and migration assays were performed
using 24-well Transwell chambers with polycarbonate filters
(Corning Incorporated, Corning, NY, USA). Matrigel chambers (BD
Biosciences, Franklin Lakes, NJ, USA) were applied to evaluate the
effect of genistein (10, 20 and 60 µmol/l) on invasiveness,
according to the manufacturer's protocol. Cells treated with
genistein for 48 h were collected and resuspended in serum-free
medium and transferred to diluted Matrigel chambers
(5×104 cells/well). The chambers were incubated for 24 h
prior to examination, with the bottom chambers containing culture
medium with 10% FBS. The invaded cells on the lower surface that
passed through the filter were stained using 0.1% crystal violet
for 30 min at room temperature. Finally, invading cells were
counted in five randomly-selected high power fields under a light
microscope (Olympus Corporation, Tokyo, Japan, magnification, ×100)
and the cell number was calculated. A cell migration assay was
performed using the same procedure as described above for the cell
invasion assay, although cell medium without Matrigel was used.
RT-qPCR and western blot analyses were performed to
detect the mRNA and protein expression of the
invasion/migration-associated factors E-cadherin, matrix
metalloproteinase (MMP) 2, MMP9, tissue inhibitor of
metalloproteinase inhibitor 1 (TIMP1), β-catenin, c-Myc
proto-oncogene protein (c-Myc) and cyclin D1, serving a role in the
Wnt/β-catenin signaling pathway.
Small interfering (si)RNA
transfection
siRNA transfection was performed to validate the
effect of WIF1 on tumor invasion and the effect of treatment with
genistein on tumor suppression. siRNA-WIF1 was designed and
synthesized by Shanghai GenePharma Co., Ltd. (Shanghai, China). For
the siRNA transfection, cells were seeded onto 12-well cell culture
plates at an initial density of 6×104 cells/well. When
cells were 70% confluent, they were transfected with 1 µg WIF1
siRNA (siWIF1 group) or nonspecific siRNA (mock group) with
FlexiTube siRNA Premix transfection reagent (Qiagen, Inc.,
Valencia, CA, USA) according to the manufacturer's protocols. HT29
cells without any treatment served as the control group. The
following siRNA sequences were used: siWIF1,
5′-CUCAUAGGAUUUGAAGAAG-3′; and nonspecific siRNA,
5′-UCACUGCGCUCGAUGCAGUTT-3′.
RT-qPCR and western blot analyses were performed to
measure the interference efficiency 48 h following transfection.
Cell invasion/migration assays were performed as a forentioned to
evaluate the effect of siWIF1 transfection among the control, mock,
siWIF1 and siWIF1+genistein (siWIF1 group treated with 60 µmol/l
genstein) groups. The above experiments were performed
independently.
RT-qPCR analysis
Total RNA was extracted from cells by ZR RNA
MiniPrep kit (Zymo Research Corp., Irvine, CA USA), and
reverse-transcribed into cDNA using a first strand cDNA kit
(Sigma-Aldrich; Merck KGaA), according to the manufacturer's
protocol. The following thermocycling conditions were used for the
PCR: Initial denaturation for 30 sec at 95°C; 40 cycles of
denaturation at 95°C for 5 sec, annealing/extension at 60°C for 30
sec and final extension at 72°C for 10 min. An ABI 7300
thermocycler (Applied Biosystems; Thermo Fisher Scientific, Inc.)
and the SYBR Premix Ex Taq kit (Takara Biotechnology Co., Ltd.,
Dalian, China) were used. The quantification was identified by
2−ΔΔCq (13). Primer
sequences used for the PCR are listed in Table I.
 | Table I.Primers used for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primers used for reverse
transcription-quantitative polymerase chain reaction.
|
| Primer sequence |
|---|
|
|
|
|---|
| Gene | Forward (5′→3′) | Reverse (5′→3′) |
|---|
| WIF1 |
ATCATCTTCTTAACTGGCATTGTG |
AAAAATAAAAAAAACACGCT |
| E-cadherin |
CTGAAGTGACTCGTAACGAC |
CATGTCAGCCAGCTTCTTGAAG |
| TIMP1 |
TCGTCATCAGGGCCAAGTTC |
TCCACAAGCAATGAGTGCCA |
| MMP2 |
ATGCAGTGGGGGCTTAAGAA |
TCTGGGGCAGTCCAAAGAAC |
| MMP9 |
CATCCGGCACCTCTATGGTC |
CATCGTCCACCGGACTCAAA |
| β-catenin |
ATAAGAGCTCCTTGTGCGGC |
GGCCATGTCCAACTCCATCA |
| c-Myc |
GCCACGTCTCCACACATCAG |
TGGTGCATTTTCGGTTGTTG |
| CyclinD1 |
GCTGGAGGTCTGCGAGGA |
ACAGGAAGCGGTCCAGGTAGT |
| GAPDH |
TGACTTCAACAGCGACACCCA |
CACCCTGTTGCTGTAGCCAAA |
Western blot analysis
Tissues or cells were lysed by lysis buffer (50 mM
Tris-Cl, 150 mM NaCl, 0.02% NaN2, 100 µg/ml phenylmethanesulfonyl
fluoride, 1 µg/ml aprotinin, and 1% Triton X-100) and the
supernatant was collected. Protein concentrations were determined
using a bicinchoninic acid assay (Beyotime Institute of
Biotechnology, China). A total of 20 µg proteins were subjected to
10% SDS-PAGE and electroblotted onto polyvinylidene fluoride
membranes (PVDF). Following blocking with 5% skimmed dried milk in
PBS for 1 h at room temperature, blotting membranes were probed
overnight at 4°C with the following primary antibodies: Rabbit
anti-WIF1 (1:2,000; ab186845; Abcam, Cambridge, UK),
anti-E-cadherin (1:500; ab15148; Abcam), anti-MMP2 (1:1,000;
ab92536; Abcam), anti-MMP9 (1:1,000; ab38898; Abcam), anti-TIMP1
(1:1,000; ab61224; Abcam), anti-β-catenin (1:5,000; ab16051;
Abcam), anti-C-Myc (1:1,000; ab39688; Abcam), and anti-Cyclin D1
(1:2,000; ab226977; Abcam), GAPDH (1:2,000; ab9485; Abcam). GAPDH
was used for the loading control. Subsequently, the membranes were
probed with the appropriate horseradish peroxidase (HRP)-conjugated
secondary antibodies: Goat anti-rabbit immunoglobulin G H&L HRP
(1:5,000; ab205718 and ab6721; Abcam) for 1 h at room temperature.
The PVDF membranes were exposed to X-ray film and immunoreactive
bands were detected by enhanced chemiluminescence detection system
reagents (Amersham; GE Healthcare, Chicago, IL, USA). Lab Works
Image Acquisition and Analysis software (Visionworks®
LS, UVP, LLC, Phoenix, AZ, USA) was used to quantify band
intensities.
Statistical analysis
Data are presented as the mean ± standard deviation
of three independent experiments. Statistical analysis was
performed using SPSS software (version 13.0; SPSS, Inc., Chicago,
IL, USA) and data were analyzed using one-way analysis of variance,
followed by Dunnett's test. P<0.05 was considered to indicate a
statistically significant difference.
Results
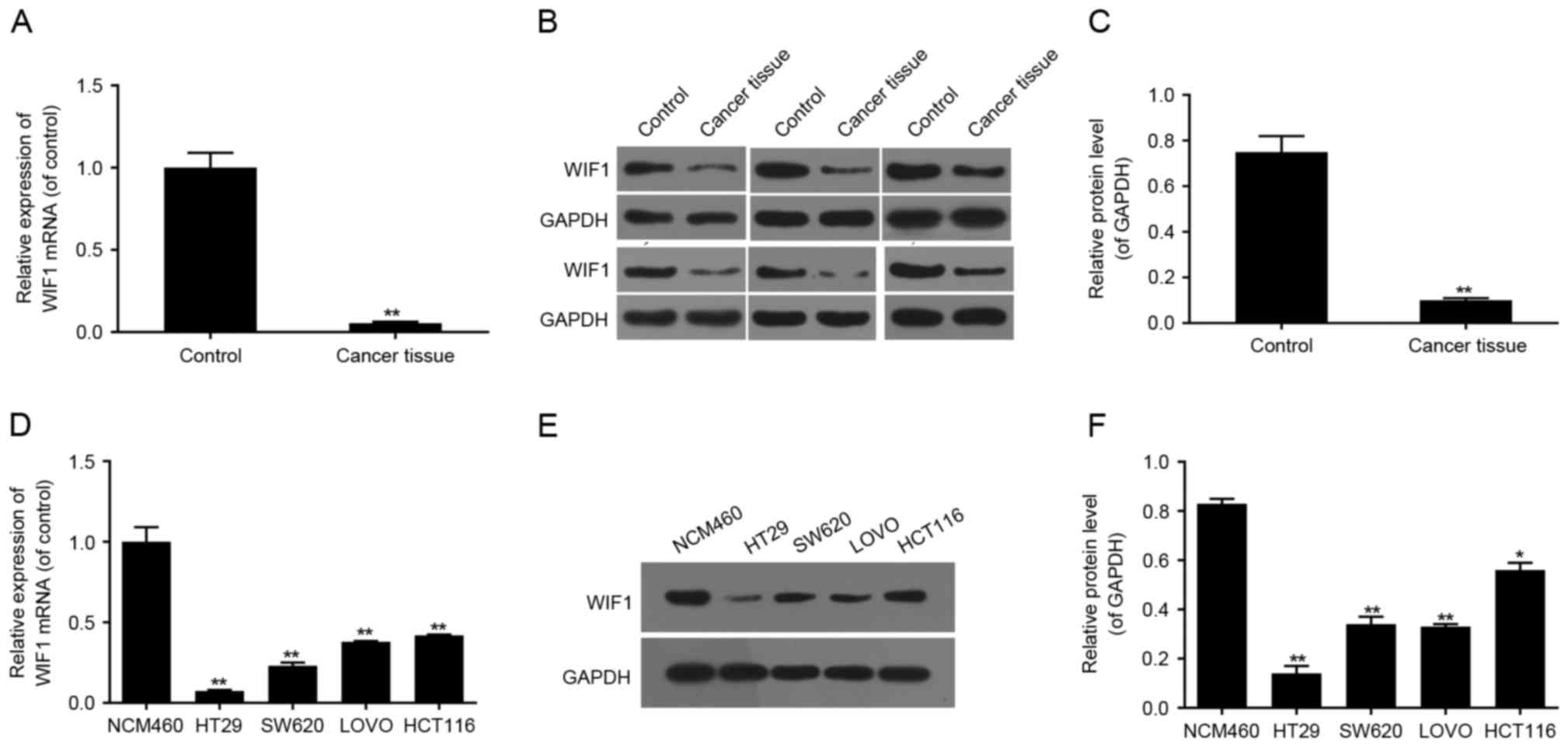
Low expression of WIF1 in colon cancer
tissues and cell lines
To verify the biological role of WIF1 in colon
cancer, the mRNA and protein expression levels of WIF1 in colon
cancer tissues and colon cancer cell lines (HT29, SW620, LOVO,
HCT116) were detected by RT-qPCR and western blot analyses. The
expression of WIF1 in the adjacent normal colon tissues and in the
colon epithelial cell line NCM460 was detected as the control. The
results demonstrated that the mRNA and protein expression of WIF1
was decreased in colon cancer tissues compared with the adjacent
normal tissues (both P<0.01; Fig.
1A-C). The mRNA and protein expression of WIF1 was additionally
downregulated in colon cancer cells (HT29, SW620, LOVO and HCT116),
compared with normal colon NCM460 cells (Fig. 1D-F). The above results indicated
that downregulated expression of WIF1 may be associated with the
initiation and/or progression of colon cancer. The HT29 cell line
was selected for subsequent experiments as it exhibited the lowest
expression of WIF1, compared with the other cell lines included in
the present study.
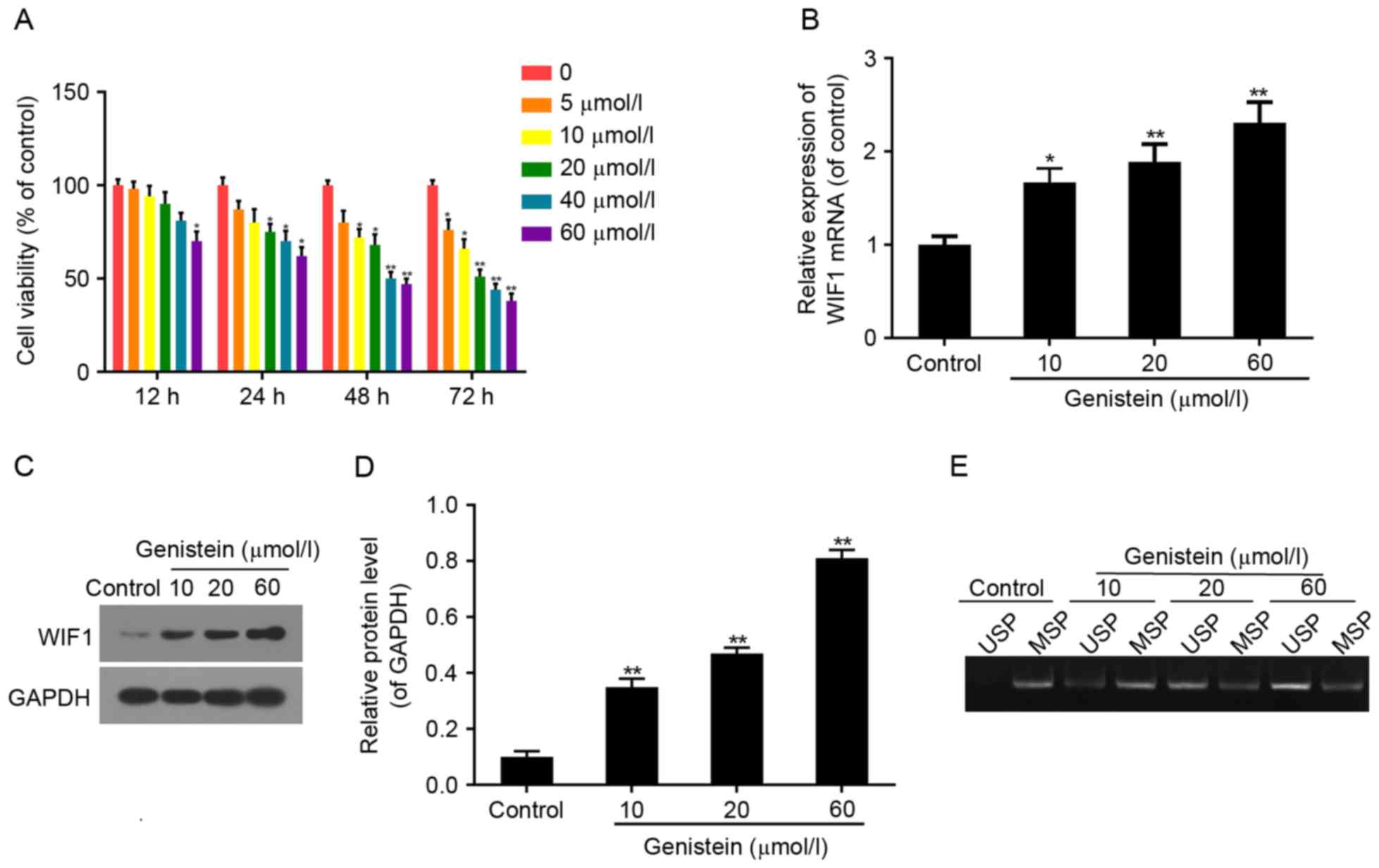
Effect of genistein on HT29 cell
viability, and WIF1 expression and promoter methylation
The effect of genistein on HT29 cell viability was
measured by CCK8 assay. Cell viability was suppressed in the
genistein-treated groups in a dose-dependent manner compared with
the control group (Fig. 2A). Cell
viability was respectively 66, 51, 44 and 38% of the control when
treated with 10, 20, 40 and 60 µmol/l genistein for 72 h (all
P<0.01). HT29 cells treated with 10, 20 and 60 µmol/l genistein
respectively for 72 h were subjected to the subsequent
experimentation.
RT-qPCR and western blot analyses demonstrated that
the mRNA and protein expression of WIF1 notably increased in
genistein-treated cells in a dose-dependent manner (Fig. 2B-D). The above results confirmed
that genistein could recover the expression and function of WIF1 to
inhibit cell viability.
As detected by MSP, following 72 h of treatment with
10, 20 and 60 µmol/l genistein, levels of demethylated WIF1
increased compared with the levels of methylated WIF1. These
effects occurred in a dose-dependent manner, compared withthe
respective control groups (Fig.
2E). The above results indicated that the role served by
genistein in colon cancer may be associated with demethylation of
WIF1, to recover its tumor suppressor function.
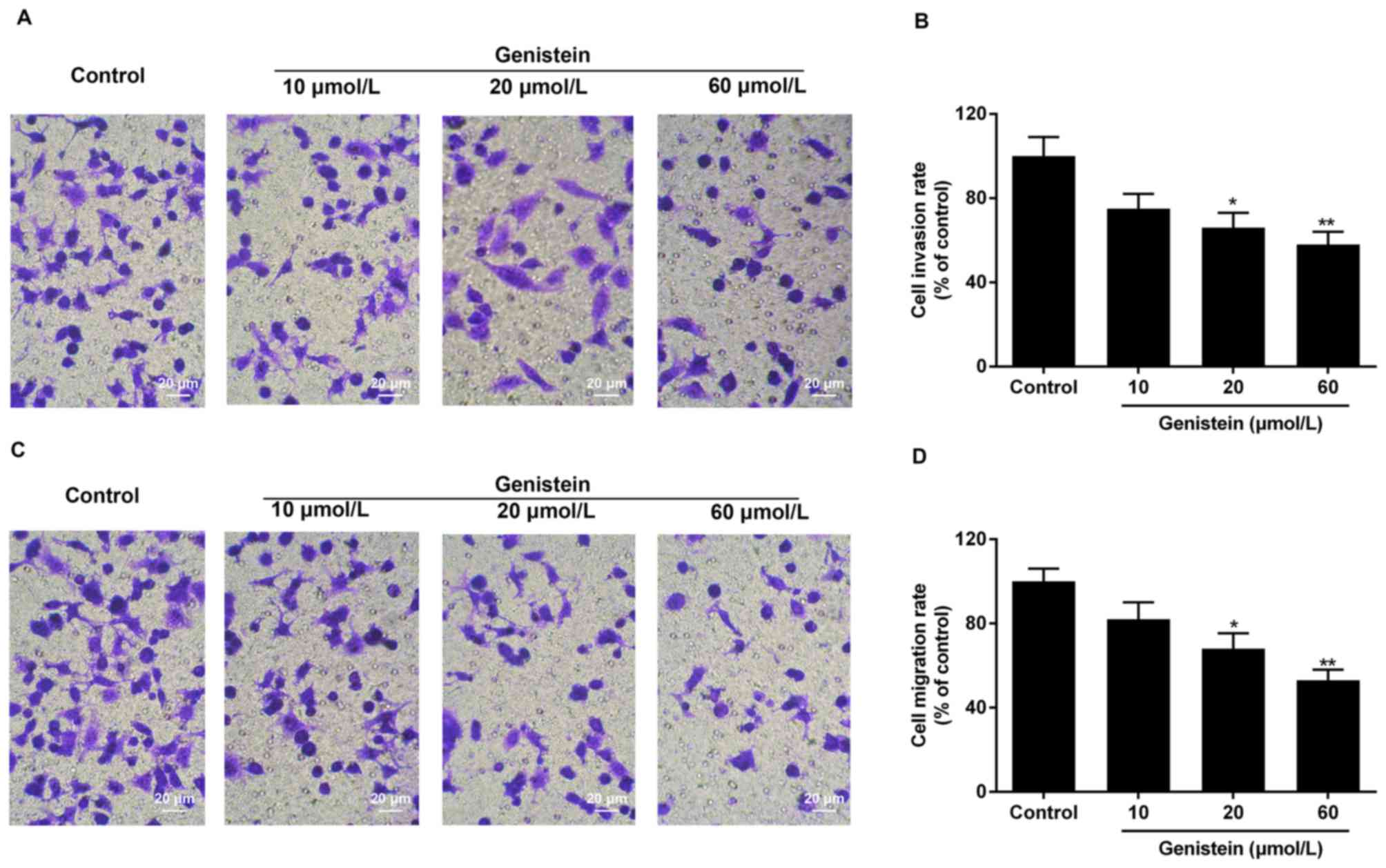
Effect of genistein on HT29 cell
invasion and migration
The invasive and migratory ability of HT29 cells
treated with genistein was identified by Transwell assay. The
results of the assay demonstrated that the invasive/migratory
abilities of cells treated with 10, 20 and 60 µmol/l genistein for
72 h decreased notably in a dose-dependent manner, compared with
the control group. Invasion rates in groups treated with 10, 20 and
60 µmol/l genistein were ~75, 66 and 58%, respectively, compared
with the control group (Fig. 3A and
B). Migration rates in groups treated with 10, 20 and 60 µmol/l
genistein were ~82, 69 and 53%, respectively, compared with the
control group (Fig. 3C and D).
Effect of genistein on the expression
of factors associated with cell invasion and the Wnt/β-catenin
signaling pathway in HT29 cells
RT-qPCR and western blot analyses were performed to
detect the mRNA and protein expression of
invasion/migration-associated factors, including E-cadherin, MMP2,
MMP9 and TIMP1, and factors associated with the Wnt/β-catenin
signaling pathway: β-catenin, c-Myc and cyclin D1.
The results of the present study demonstrated that
the mRNA and protein expression levels of E-cadherin and TIMP1
increased significantly, while the expression levels of MMP2 and
MMP9 decreased significantly, in a dose-dependent manner in
genistein-treated cells (72 h), compared with the control group
(Fig. 4A-C). A significant
dose-dependent decrease in mRNA and protein expression levels of
β-catenin, c-Myc and cyclin D1 was observed in cells treated with
genistein, compared with the control group (Fig. 4D-F). The results of the present
study indicated that the inhibitory properties of genistein on HT29
cell invasion and migration may be associated with the expression
of invasion/migration-associated factors, including E-cadherin,
MMP2, MMP9 and TIMP1, and factors associated with the Wnt/β-catenin
signaling pathway (β-catenin, c-Myc and cyclin D1).
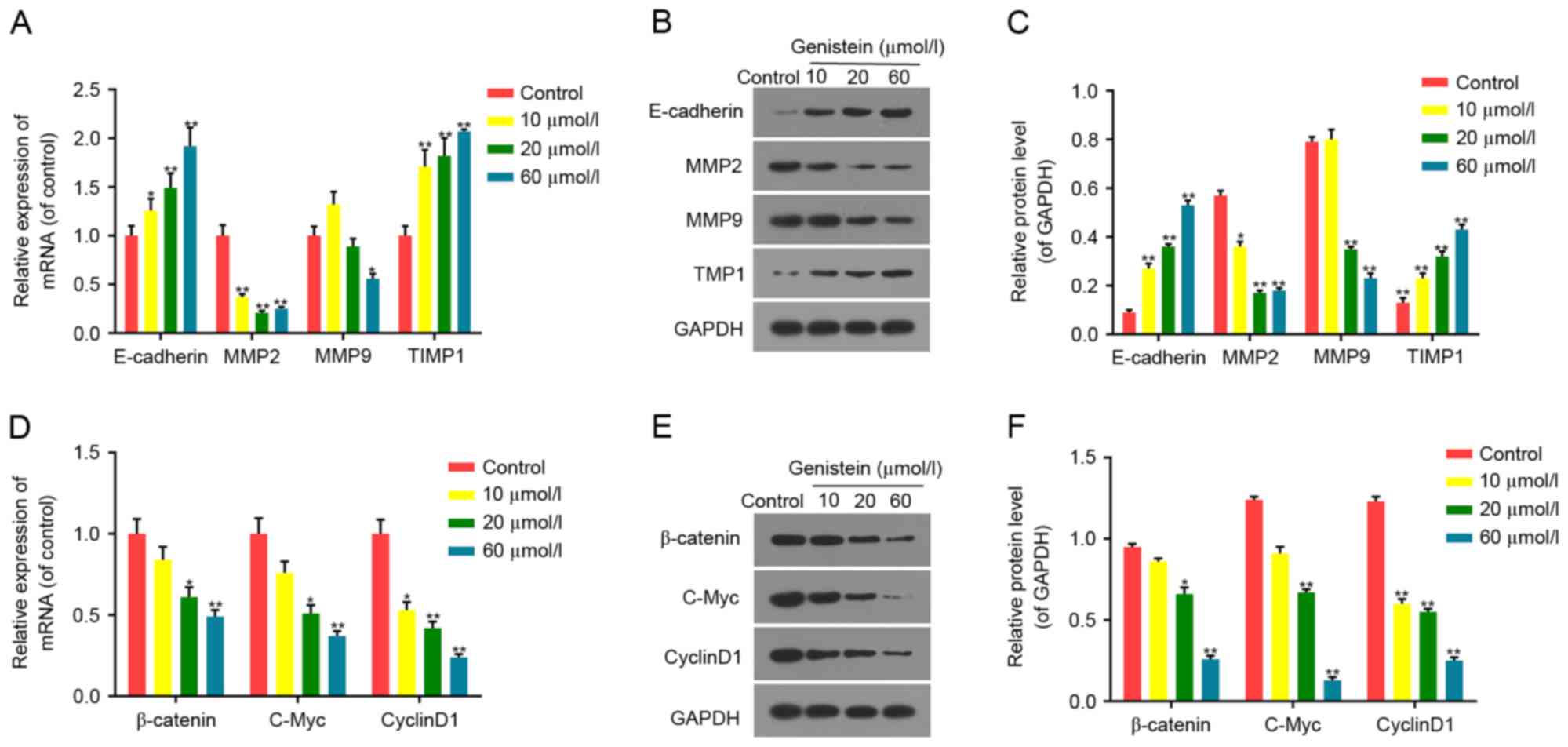 | Figure 4.Effect of genistein on the expression
of tumor-associated factors. Following treatment with 10, 20 and 60
µmol/l genistein for 72 h, the (A) mRNA and (B) protein expression
of invasion/migration-associated factors (E-cadherin, MMP2, MMP9
and TIMP1) was detected in HT29 cells, and (C) quantified. The (D)
mRNA and (E) protein expression of Wnt/β-catenin-associated
factors, including β-catenin, c-Myc and cyclinD1 in HT29 cells. (F)
The protein levels of β-catenin, c-Myc and cyclinD1 in HT29 cells
were quantified. GAPDH was detected as the control of sample
loading. Data were presented as the mean ± standard deviation. n=6.
*P<0.05 and **P<0.01 vs. the control group. MMP, matrix
metalloproteinase; WIF1, Wnt inhibitory factor 1; TIMP1, tissue of
metalloproteinase inhibitor 1; c-Myc, c-Myc proto-oncogene
protein. |
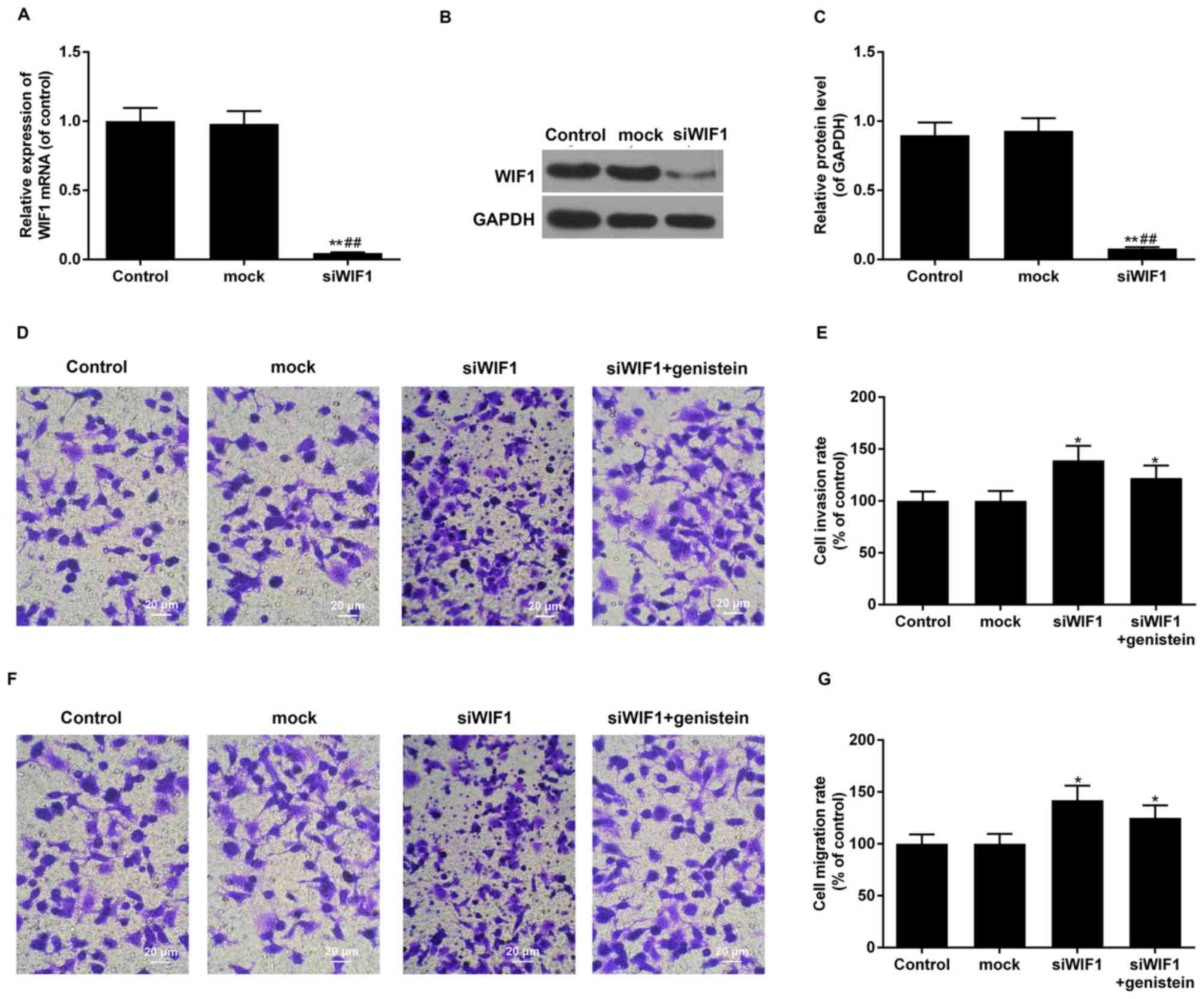
Effect of WIF1-siRNA transfection on
HT29 cell invasion and migration
WIF1 knockdown and the interference efficiency were
identified using RT-qPCR and western blot analyses. Transfection of
siRNA-WIF1 resulted in a significant decline in WIF1 mRNA and
protein expression levels in the siRNA-WIF1 group compared with the
control and mock groups, which confirmed that siRNA-WIF1 was
effective in silencing the expression of WIF1 (all P<0.01;
Fig. 5A-C).
The invasive and migratory abilities of the siWIF1,
siWIF1+genistein, mock and HT29 control groups were identified by
Transwell assay. The results of the above experiment demonstrated
that the invasive/migratory abilities of the siWIF1and
siWIF1+genistein groups increased significantly, compared with the
control group and mock group (all P<0.05). The invasion rate of
the siWIF1 and siWIF1+genistein groups was 139 and 122%,
respectively, compared with the control group (Fig. 5D and E). The migration rates of the
siWIF1 and siWIF1+genistein groups were 142 and 125%, respectively,
compared with the control group (Fig.
5F and G). It was confirmed that genistein was able to partly
reverse the function of siWIF1 in promoting cell
invasion/migration.
Discussion
Colon cancer is one of the most common malignant
carcinomas, demonstrating elevated invasion and migration rates
(10). DNA methylation of CpG
islands inpromoter regions of tumor suppressor genes is considered
to be an epigenetic mechanism underlying cancer development
(11). Abnormal activation of the
Wnt signaling pathway could induce tumor development (12). Previous studies reported that
silencing of WIF1 via methylationis associated with colon cancer
(5,14,15).
The primary aim of the present study was to
elucidate the molecular mechanism underlying genistein-mediated
inhibition of invasion and migration of colon cancer associated
with WIF1 demethylation. Preliminary observations demonstrated that
the expression of WIF1 decreased markedly in colon cancer tissues
and cells, including HT29, SW620, LOVO and HCT116 at different
rates. The expression levels of WIF1 decreased in HCT116, SW620 and
LOVO cells compared with the control. The HT29 cell line
demonstrated the lowest expression levels of WIF1 compared with all
other cells and, therefore, was selected for further analysis.
Future studies could investigate the mechanism underlying the
association between genistein and cancer in order to verify the
results obtained in the present study.
Low expression of WIF1 may induce abnormal
activation of the Wnt signaling pathway, promoting cell
proliferation and resulting in tumor development (16,17).
In the present study, the proliferation of HT29 colon cancer cells
treated with genistein was inhibited in a dose-dependent manner.
The mRNA and protein expression levels of WIF1 increased in HT29
cells treated with genistein in a dose-dependent manner, suggesting
that the function of genistein on cell proliferation was associated
with the regulation of WIF1 expression. In order to further
elucidate the mechanism underlying the modulation of expression of
WIF1 in colon cancer cells, an MSP assay was performed to evaluate
methylation levels in the promoter region of the WIF1 gene.
Decreased methylation levels in the group treated with genistein,
compared with the control group indicated that genistein-mediated
upregulation of WIF1 expression maybe associated with the
demethylation of CpG islands.
A number of factors are involved in the process of
tumor development. The Transwell assay performed in the present
study demonstrated that genistein inhibited the migration and
invasion of HT29 cells in a dose-dependent manner.
It was additionally demonstrated that the mechanism
underlying the above observations was associated with the
regulation of invasion/migration-associated factors. MMP is a
family of enzymes regulating the proteolysis of extracellular
matrix-associated structural proteins, dysregulation of MMPs in
cells can result in the degradation of the histological barrier
that normally prevents cell invasion and migration (18–20).
MMPs serve a number of roles in tumor cell invasion and migration,
and have been investigated in a recent study (21). Type IV collagenases are a
sub-family of MMPs that includes two members, glycosylated MMP9 and
non-glycosylated MMP2. Previous studies demonstrated that Type IV
collagenases are implicated in tumor cell invasion and migration
processes (22,23). MMPs are controlled by endogenous
tissue specific inhibitors, also known as tissue inhibitors of
metalloproteinases (TIMPs) (24).
TIMP1 can inhibit tumor progression by suppressing MMP9-mediated
release of vascular endothelial growth factor from the matrix.
E-cadherin, a family member of Ca2+-dependent
transmembrane glycoproteins, is an important tumor suppressor
participating in intercellular signal transduction (25). E-cadherin is able to conjugate to
β-catenin in the cytoplasm and inhibit its function in the Wnt
signaling pathway. The results of the present study demonstrated
that treatment with genistein resulted in decreased expression of
MMP9 and MMP2, and increased expression of TIMP1 and E-cadherin in
HT29 cells, compared with the control group. The above results
indicated that genistein regulates tumor invasion and migration by
influencing the expression levels of MMP2, MMP9, TIMP1 and
E-cadherin.
The Wnt/β-catenin signaling pathway is involved in
growth-associated processes and tumor development. WIF1 is an
inhibitor of the Wnt/β-catenin signaling pathway and, therefore,
silencing of WIF1 may activate the pathway and induce tumor
development (26). β-catenin is a
scaffold protein linking the cytoplasmic tail of classical
cadherins of the endothelium to the actin cytoskeleton.
Upregulation of β-catenin may cause tumor metastasis (27). Cyclin D1 is a cell cycle-related
factor associated with G1/S stage transition. C-Myc encodes
phosphorylated proteins, which facilitate cell proliferation and
differentiation (28). Cyclin D1
and c-Myc are downstream target genes of the Wnt/β-catenin
signaling pathway (29). The mRNA
and protein expression of β-catenin, c-Myc and cyclin D1 decreased
in HT29 cells treated with genistein, compared with the control
group. It may be hypothesized that demethylation of WIF1 inhibited
tumor progression by downregulating the expression of factors
associated with the Wnt/β-catenin signaling pathway (β-catenin,
c-Myc and cyclin D1).
Following transfection with WIF1-siRNA, the mRNA and
protein levels of WIF1 in siWIF1 cells decreased markedly. The
invasive and migratory abilities of the siWIF1 and siWIF1+genistein
groups increased markedly compared with the control and mock
groups, and genistein was able to partly reverse the function of
siWIF1 in promoting cell invasion/migration.
In conclusion, the present study demonstrated that
the expression of WIF1 was suppressed by methylation of CpG islands
in the promoter region, in human colon cancer tissues and cells.
Additionally, genistein recovered the expression of WIF1 in the
HT29 colon cancer cell line via demethylation of WIF1, and
suppressed tumor cell invasion and migration. The above
observations may be associated with the regulation of cell invasion
and migration-associated genes, including MMP9, MMP2, TIMP1,
E-cadherin, β-catenin, c-Myc and cyclin D1. Further in vivo
studies are required to confirm the results of the present study.
Novel evidence may, in the future, confirm the administration of
genistein to be an effective treatment for patients with colon
cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analysed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
JZ contributed to the conception of the study. JR
contributed significantly to conducting of the experiments,
analysis and manuscript preparation. LT performed the data analyses
and wrote the manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from all
participants prior to the present study. All tissue samples of
patients were collected according to procedures approved by the
institutional review board of the independent ethics committee of
Changzhou No. 2 People's Hospital (Changzhou, China).
Consent for publication
All participants provided written informed consent
prior to the present study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Xu S, Yao H, Pei L, Hu M, Li D, Qiu Y,
Wang G, Wu L, Yao H, Zhu Z and Xu J: Design, synthesis, and
biological evaluation of NAD(P)H: Quinone oxidoreductase
(NQO1)-targeted oridonin prodrugs possessing indolequinone moiety
for hypoxia-selective activation. Eur J Med Chem. 132:310–321.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Choi Y, Sateia HF, Peairs KS and Stewart
RW: Screening for colorectal cancer. Semin Oncol. 44:34–44. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hu J, Li J, Yue X, Wang JC, Wang JF, Liu
JZ and Kong DL: Targeting BCRP/ABCG2 by RNA interference enhances
the chemotherapy sensitivity of human colon cancer side population
cells. J Huazhong Univ Sci Technolog Med Sci. 37:231–236. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ahmed I, Roy BC, Subramaniam D, Ganie SA,
Kwatra D, Dixon D, Anant S, Zargar MA and Umar S: An ornamental
plant targets epigenetic signaling to block cancer stem cell-driven
colon carcinogenesis. Carcinogenesis. 37:385–396. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Patai ÁV, Valcz G, Hollósi P, Kalmár A,
Péterfia B, Patai Á, Wichmann B, Spisák S, Barták BK, Leiszter K,
et al: Comprehensive DNA methylation analysis reveals a common
ten-gene methylation signature in colorectal adenomas and
carcinomas. PLoS One. 10:e01338362015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sánchez-Vega F, Gotea V, Chen YC and
Elnitski L: CpG island methylator phenotype in adenocarcinomas from
the digestive tract: Methods, conclusions and controversies. World
J Gastrointest Oncol. 9:105–120. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chan SL, Cui Y, van Hasselt A, Li H,
Srivastava G, Jin H, Ng KM, Wang Y, Lee KY, Tsao GS, et al: The
tumor suppressor Wnt inhibitory factor 1 is frequently methylated
in nasopharyngeal and esophageal carcinomas. Lab Invest.
87:644–650. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bilir B, Sharma NV, Lee J, Hammarstrom B,
Svindland A, Kucuk O and Moreno CS: Effects of genistein
supplementation on genome-wide DNA methylation and gene expression
in patients with localized prostate cancer. Int J Oncol.
51:223–234. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sundaram MK, Ansari MZ, Mutery AA, Ashraf
M, Nasab R, Rai S, Rais N and Hussain A: Genistein induces
alterations of epigenetic modulatory signatures in human cervical
cancer cells. Anticancer Agents Med Chem. Sep 18–2017.(Epub ahead
of print). PubMed/NCBI
|
|
10
|
Chen G, Zhou T, Li Y, Yu Z and Sun L: p53
target miR-29c-3p suppresses colon cancer cell invasion and
migration through inhibition of PHLDB2. Biochem Biophys Res Commun.
487:90–95. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang L, Wu H, Xiao X, Li K, Zhang Y,
Zhang L and Wen T: Analysis on regulatory network linked to Hpa
gene in invasion and metastasis of colon cancer. Saudi J Biol Sci.
24:504–507. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Luo X, Xiong X, Shao Q, Xiang T, Li L, Yin
X, Li X, Tao Q and Ren G: The tumor suppressor interferon
regulatory factor 8 inhibits β-catenin signaling in breast cancers,
but is frequently silenced by promoter methylation. Oncotarget.
8:48875–48888. 2017.PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang LS, Kuo CT, Huang TH, Yearsley M,
Oshima K, Stoner GD, Yu J, Lechner JF and Huang YW: Black
raspberries protectively regulate methylation of Wnt pathway genes
in precancerous colon tissue. Cancer Prev Res (Phila). 6:1317–1327.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Afgar A, Fard-Esfahani P, Mehrtash A,
Azadmanesh K, Khodarahmi F, Ghadir M and Teimoori-Toolabi L:
MiR-339 and especially miR-766 reactivate the expression of tumor
suppressor genes in colorectal cancer cell lines through DNA
methyltransferase 3B gene inhibition. Cancer Biol Ther.
17:1126–1138. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang SH, Li SL, Dong ZM and Kan QC:
Epigenetic inactivation of Wnt inhibitory factor-1 in human
esophageal squamous cell carcinoma. Oncol Res. 20:123–130. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stewart DJ: Wnt signaling pathway in
non-small cell lung cancer. J Natl Cancer Inst. 106:djt3562014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stanciu AE, Zamfir-Chiru-Anton A, Stanciu
MM, Popescu CR and Gheorghe DC: Imbalance between matrix
metalloproteinases and tissue inhibitors of metalloproteinases
promotes invasion and metastasis of head and neck squamous cell
carcinoma. Clin Lab. 63:1613–1620. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Das A, Monteiro M, Barai A, Kumar S and
Sen S: MMP proteolytic activity regulates cancer invasiveness by
modulating integrins. Sci Rep. 7:142192017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shi Y, An D, Liu Y, Feng Q, Fang X, Pan G
and Wang Q: Propoxur enhances MMP-2 expression and the
corresponding invasion of human breast cancer cells via the
ERK/Nrf2 signaling pathway. Oncotarget. 8:87107–87123.
2017.PubMed/NCBI
|
|
21
|
Cheng Z, Limbu MH, Wang Z, Liu J, Liu L,
Zhang X, Chen P and Liu B: MMP-2 and 9 in chronic kidney disease.
Int J Mol Sci. 18:2762017. View Article : Google Scholar :
|
|
22
|
Shen EY, Wang WG, Li Y, Zhang SH and Zhen
YS: Inhibitory effect of endoplasmic reticulum-retained anti-type
IV collagenase intrabody on invasion and proliferation of cancer
cell. Ai Zheng. 23:1005–1010. 2004.(In Chinese). PubMed/NCBI
|
|
23
|
Lu L, Li X, Xu P, Zheng Y and Wang X:
Tenuigenin down-regulates the release of nitric oxide, matrix
metalloproteinase-9 and cytokines from
lipopolysaccharide-stimulated microglia. Neurosci Lett. 650:82–88.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Thiele ND, Wirth JW, Steins D, Koop AC,
Ittrich H, Lohse AW and Kluwe J: TIMP-1 is upregulated, but not
essential in hepatic fibrogenesis and carcinogenesis in mice. Sci
Rep. 7:7142017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gulati N, Rathore AS, Juneja S and Rastogi
P: Expression of E-cadherin and B-cell lymphoma 2 in oral cancer: A
ratio-based planning for targeted therapy. Indian J Dent Res.
28:3–9. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang M, Chen C, Geng J, Han D, Wang T,
Xie T, Wang L, Wang Y, Wang CH, Lei Z and Chu X: Targeting KDM1A
attenuates Wnt/β-catenin signaling pathway to eliminate
sorafenib-resistant stem-like cells in hepatocellular carcinoma.
Cancer Lett. 398:12–21. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vega OA, Lucero CMJ, Araya HF, Jerez S,
Tapia JC, Antonelli M, Salazar-Onfray F, Las Heras F, Thaler R,
Riester SM, et al: Wnt/β-catenin signaling activates expression of
the bone-related transcription factor RUNX2 in select human
osteosarcoma cell types. J Cell Biochem. 118:3662–3674. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Strindlund K, Troiano G, Sgaramella N,
Coates PJ, Gu X, Boldrup L, Califano L, Fahraeus R, Muzio LL,
Ardito F, et al: Patients with high c-MYC expressing squamous cell
carcinomas of the tongue show better survival than those with
low-and medium-expressing tumours. J Oral Pathol Med. 46:967–971.
2017.PubMed/NCBI
|
|
29
|
Hong F, Ze Y, Zhou Y, Hong J, Yu X, Sheng
L and Wang L: Nanoparticulate TiO2 -mediated inhibition of the Wnt
signaling pathway causes dendritic development disorder in cultured
rat hippocampal neurons. J Biomed Mater Res A. 105:2139–2149. 2017.
View Article : Google Scholar : PubMed/NCBI
|