Introduction
Alcoholic liver disease (ALD) is a worldwide health
concern. The World Health Organization states that there are 3.3
million alcohol-associated mortalities each year (1). Excessive alcohol consumption can lead
to acute and chronic fatty liver diseases, including steatosis,
alcoholic hepatitis, liver fibrosis, liver cirrhosis and
hepatocellular carcinoma (2).
Steatosis occurs during the early stage of ALD and is characterized
by excessive fat accumulation in hepatocytes (3). There are numerous mechanisms
underlying fat accumulation, including de novo lipogenesis,
impaired β-oxidation of fatty acids and uptake of triglyceride-rich
lipoproteins (4–6). Furthermore, previous studies have
demonstrated that ethanol-induced oxidative stress and associated
production of cytokines have a role in the development of ALD
(7,8).
Benign steatosis can be reversed by abstinence from
alcohol; however, there are currently no effective clinical
approaches for the treatment of ALD (2,3).
Thus, it is necessary to identify compounds that may provide
protection against ALD. Myricitrin (3,4′,5′,5,7-five
hydroxyflavone-3-O-α-L-rhamnoside), a naturally occurring
polyphenol hydroxy flavonoid present in a number of berries,
vegetables and various edible and/or medicinal herbs, has been
reported to exhibit a variety of beneficial properties, including
anti-inflammatory, antinociceptive, anticarcinogenic and
antimicrobial activities (9–13).
Myricitrin exhibits oxidative resistance and free radical
scavenging activities due to its polyhydroxy structure (14). Myricitrin has been reported to
suppress acrylamide-mediated cytotoxicity in human Caco-2 cells by
inhibiting the production of reactive oxygen species (ROS)
(15). Shimosaki et al
(13) confirmed that pretreatment
with high-performance liquid chromatography-purified myricitrin
from Myrica extract can inhibit production of tumor necrosis
factor-α (TNF-α) in lipopolysaccharide-stimulated RAW264.7
macrophages. Myricitrin inhibits oxidative stress-induced
endothelial damage and atherosclerosis (16,17).
It has been demonstrated that myricitrin exhibits anti-oxidative,
anti-inflammatory and antifibrotic effects in carbon
tetrachloride-intoxicated mice (18). Myricitrin decreased hepatic lipid
peroxidation, increased levels of glutathione, reduced
cyclooxygenase-2 and TNF-α overexpression, and inflammation in the
liver, and inhibited hepatic expression of transforming growth
factor-β1 (TGF-β1) and liver fibrosis (18). The above studies demonstrated
anti-oxidative and anti-inflammatory activities of myricitrin under
various disease conditions. However, little is known about the
protective effect of myricitrin against alcoholic liver disease.
The aim of the present study was to evaluate the effect of
myricitrin on ethanol-induced steatosis in mouse AML12 liver cells
and to identify possible underlying molecular mechanisms.
Materials and methods
Cell culture
AML12 mouse liver cells were obtained from American
Type Culture Collection (Manassas, VA, USA) and cultured in
Dulbecco's modified Eagle medium/nutrient mixture F12 (Hyclone; GE
Healthcare Life Sciences, Logan, UT, USA) containing 100 units/ml
penicillin, 100 µg/ml streptomycin and 10% fetal bovine serum
(Hyclone; GE Healthcare Life Sciences) at 37°C in an incubator with
5% CO2.
Cell viability
Cell viability was assessed by an MTT assay.
Briefly, AML12 cells were seeded in 96-well plates at the density
of 1×104 cells/well. The following day, cells were
treated with 800 mM ethanol and co-cultured with 5, 10, 20 or 40 µM
myricitrin for 16 h. Subsequently MTT solution (5 mg/ml) was added
to each well and the cells were cultured for another 4 h. MTT
formazan precipitate was dissolved in dimethyl sulfoxide (DMSO) and
the absorbance was measured at a wavelength of 570 nm using a
microplate reader (Varioskan Flash; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). The vehicle control cells were treated with
ultrapure water rather than ethanol, and DMSO rather than
myricitrin. Cell viability values are expressed as percentage of
the vehicle control (100%). All experiments were performed in
triplicate.
Oil red O staining
AML12 cells were seeded into 24-well plates at a
density of 1×105/well. The following day, cells were
stimulated with 100 mM ethanol and co-treated with 20 µM
myricitrin. Following 36 h of treatment, cells were washed with
ice-cold PBS buffer, fixed with 10% formalin at room temperature
for 10 min, and stained with oil red O at room temperature for 30
min to detect lipid droplets in cells. Following staining, the
cultures were washed and the dye was extracted by isopropanol.
Quantification was performed by measuring optical density at a
wavelength of 510 nm.
Detection of ROS in AML12 cells
AML12 cells were seeded in 24-well plates at a
density of 1×105 cells/well. After 24 h, cells were
stimulated with 100 mM ethanol and co-cultured with 20 µM
myricitrin. A total of 48 h after ethanol and myricitrin treatment,
cells were washed and resuspended in PBS and incubated with 10 µM
2′,7′-dichlorofluorescin diacetate (DCF-DA) for 1 h at 37°C.
Subsequently, 1×106 cells/200 µl/ well were seeded in a
96-well microplate and DCF fluorescence was measured using a
fluorescence microplate reader (Varioskan Flash; Thermo Fisher
Scientific, Inc.) at an excitation wavelength of 485 nm and an
emission wavelength of 530 nm.
Cell lipid peroxidation assay
AML12 cells were plated and treated with ethanol and
myricitrin as described above. At 48 h after ethanol and myricitrin
treatment, total lipoperoxides were measured as thiobarbituric acid
reactive substances (TBARS) in culture medium using a Lipid
Peroxidation MDA Assay kit (Beyotime Institute of Biotechnology,
Haimen, China), according to the manufacturer's protocol. TBARS
were quantified using malondialdehyde (MDA) present in each
sample.
Determination of cytokine secretion in
ethanol-intoxicated cell culture
AML12 cells were plated and treated with ethanol and
myricitrin as described above. A total of 48 h after ethanol and
myricitrin treatment, the cells were centrifuged at 400 × g at 4°C
for 5 min, the supernatant was collected and samples were stored at
−20°C until cytokine levels were measured. The concentrations of
TNF-α, interleukin (IL)-6 and TGF-β1 in cell supernatants were
tested using the following ELISA kits: TNF-α (cat. no. ml002095),
IL-6 (cat. no. ml002293) and TGF-β1 (cat. no. ml002115). All kits
were purchased from Shanghai Enzyme-linked Biotechnology Co., Ltd.
(Shanghai, China), and performed according to the manufacturer's
protocol.
Quantitative analyses of mRNA
expression by reverse transcription-quantitative polymerase chain
reaction (RT-qPCR)
Total RNA was isolated from AML12 cells using the
TRIpure reagent (Roche Applied Science, Mannheim, Germany)
according to the manufacturer's protocol. cDNA was synthesized from
hepatic mRNA using RevertAid First Strand cDNA Synthesis kit
(Thermo Fisher Scientific, Inc.). The following temperature
protocol was used for cDNA synthesis: Incubation for 5 min at 25°C,
followed by 60 min at 42°C and then termination of the reaction via
incubation for 5 min at 70°C. Hepatic sterol regulatory element
binding protein-1c (SREBP-1c), fatty acid synthase (FAS), TNF-α,
IL-6, and TGF-β1 were analyzed using specific primers listed in
Table I. qPCR was performed using
FastStart Universal SYBR Green Master (Rox; Roche Applied Science).
The following thermocycling conditions were used for qPCR: Initial
denaturation at 95°C for 10 min; 35 cycles of 95°C for 15 sec,
53–60°C for 30 sec and 72°C for 30 sec; and a final extension at
72°C for 10 min. Relative expression of each gene was normalized to
β-actin and was calculated using the comparative 2−∆∆Cq
method (19).
 | Table I.Primer sequences used for
amplification of mRNA by quantitative polymerase chain
reaction. |
Table I.
Primer sequences used for
amplification of mRNA by quantitative polymerase chain
reaction.
|
| Primer sequence
(5′-3′) |
|---|
|
|
|
|---|
| Gene | Forward | Reverse |
|---|
| TNF-α |
CGTGCTCCTCACCCACAC |
GGGTTCATACCAGGGTTTGA |
| IL-6 |
ACAACCACGGCCTTCCCTACTT |
GTGTAATTAAGCCTCCGACT |
| TGF-β1 |
CAACTTCTGTCTGGGACCCT |
TAGTAGACGATGGGCAGTGG |
| SREBP-1c |
ATCGGCGCGGAAGCTGTCGGGGTAGCGTC |
ACTGTCTTGGTTGTTGATGAGCTGGAGCAT |
| FAS |
TGCTCCCAGCTGCAGGC |
GCCCGGTAGCTCTGGGTGTA |
| β-actin |
CTATTGGCAACGAGCGGTTCC |
GCACTGTGTTGGCATAGAGGTC |
Western blotting
Cells were homogenized in ice-cold
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology). Total protein concentration in extracts was
determined using a bicinchoninic acid protein assay kit (Beyotime
Institute of Biotechnology), according to the manufacturer's
protocol. Each protein sample (40 µg/lane) was loaded and separated
using 10% SDS-PAGE and transferred to polyvinylidene difluoride
membranes. Membranes were blocked with 5% non-fat milk in TBS
containing 0.1% Tween-20 for 1 h at room temperature and
subsequently incubated with anti-phosphorylated (p)-adenosine
5′-phosphate-activated protein kinase (AMPKα; cat. no. 2535;
1:1,000 dilution), anti-AMPKα (cat. no. 2532; 1:1,000 dilution;
both Cell Signaling Technology, Inc., Danvers, MA, USA) or GAPDH
(cat. no. G9545; 1:2,000 dilution; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) antibodies at 4°C overnight. Subsequently,
horseradish peroxidase-conjugated anti-rabbit immunoglobulin G
secondary antibody (cat. no. A6154; 1:2,000 dilution;
Sigma-Aldrich; Merck KGaA) was used for an incubation at room
temperature for 2 h. Detection was performed using a Supersignal
Chemiluminescent Substrate kit (Beyotime Institute of
Biotechnology).
Statistical analysis
All data were processed and analyzed using GraphPad
software (version 5.0; GraphPad Software, Inc., La Jolla, CA, USA)
and are presented as the mean ± standard error of the mean. One-way
analysis of variance with Bonferroni's multiple comparison test
were used for analyses. P<0.05 was considered to indicate a
statistically significant difference.
Results
Myricitrin exerts a protective effect
against ethanol-induced cytotoxicity
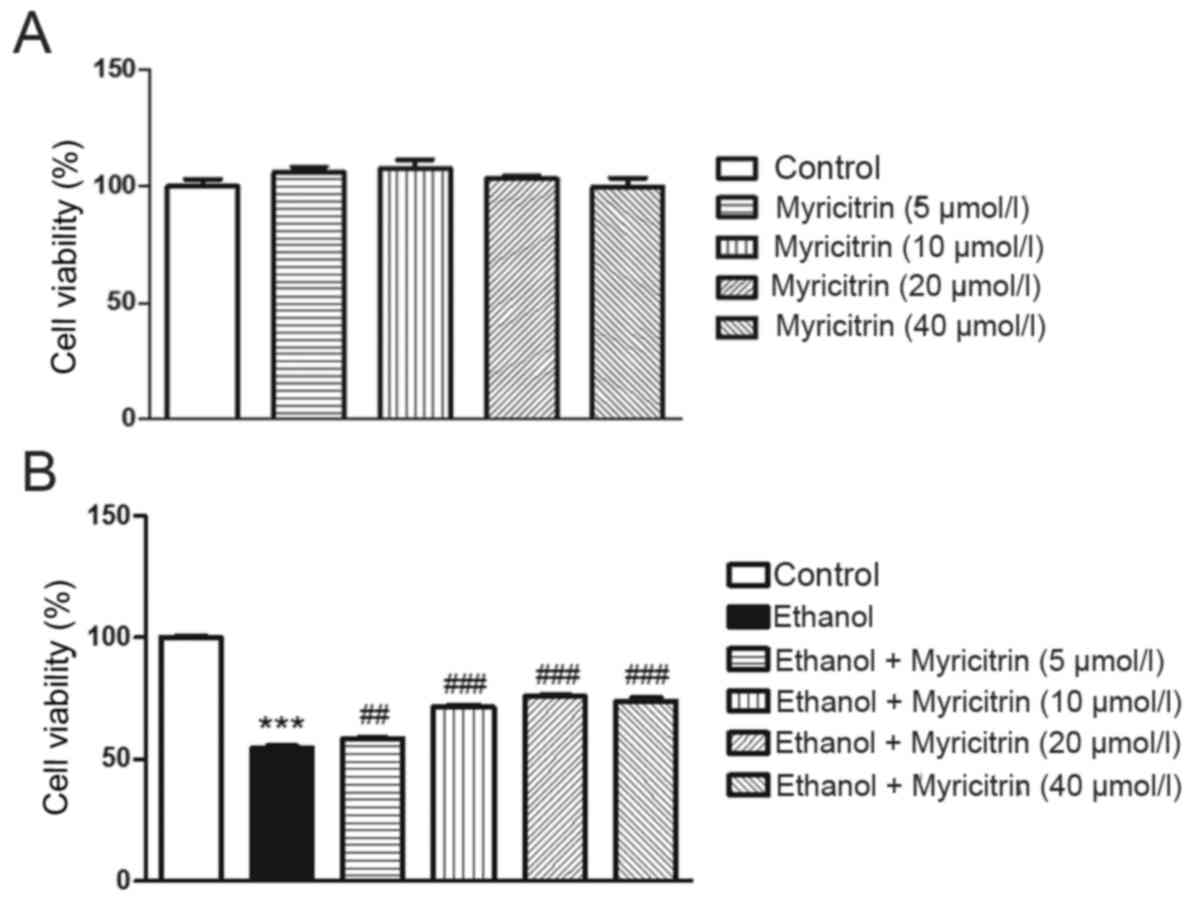
The present study aimed to determine the effect of
various concentrations of myricitrin (5, 10, 20 and 40 µM) on
ethanol-induced hepatotoxicity in AML12 cells. Myricitrin alone at
5, 10, 20, or 40 µM did not exert a cytotoxic effect on AML12 cells
(Fig. 1A). Incubation of AML12
cells with 800 mM ethanol for 16 h induced ~45.5% growth inhibition
compared with the untreated control (P<0.001; Fig. 1B). Administration of 5, 10, 20, and
40 µM myricitrin to ethanol-stimulated cells exerted a protective
effect and reduced cell viability inhibition to ~41.6 (P<0.01),
28.5 (P<0.001), 24.2 (P<0.001) and 25.9% (P<0.001),
respectively. The above data suggest that myricitrin exerts a
significant protective effect against ethanol-induced cytotoxicity.
Myricitrin at a dose of 20 µM exerted the most beneficial effect on
ethanol-induced cytotoxicity and therefore, this dose was used in
the subsequent experiments.
Myricitrin attenuates ethanol-induced
steatosis in AML12 cells
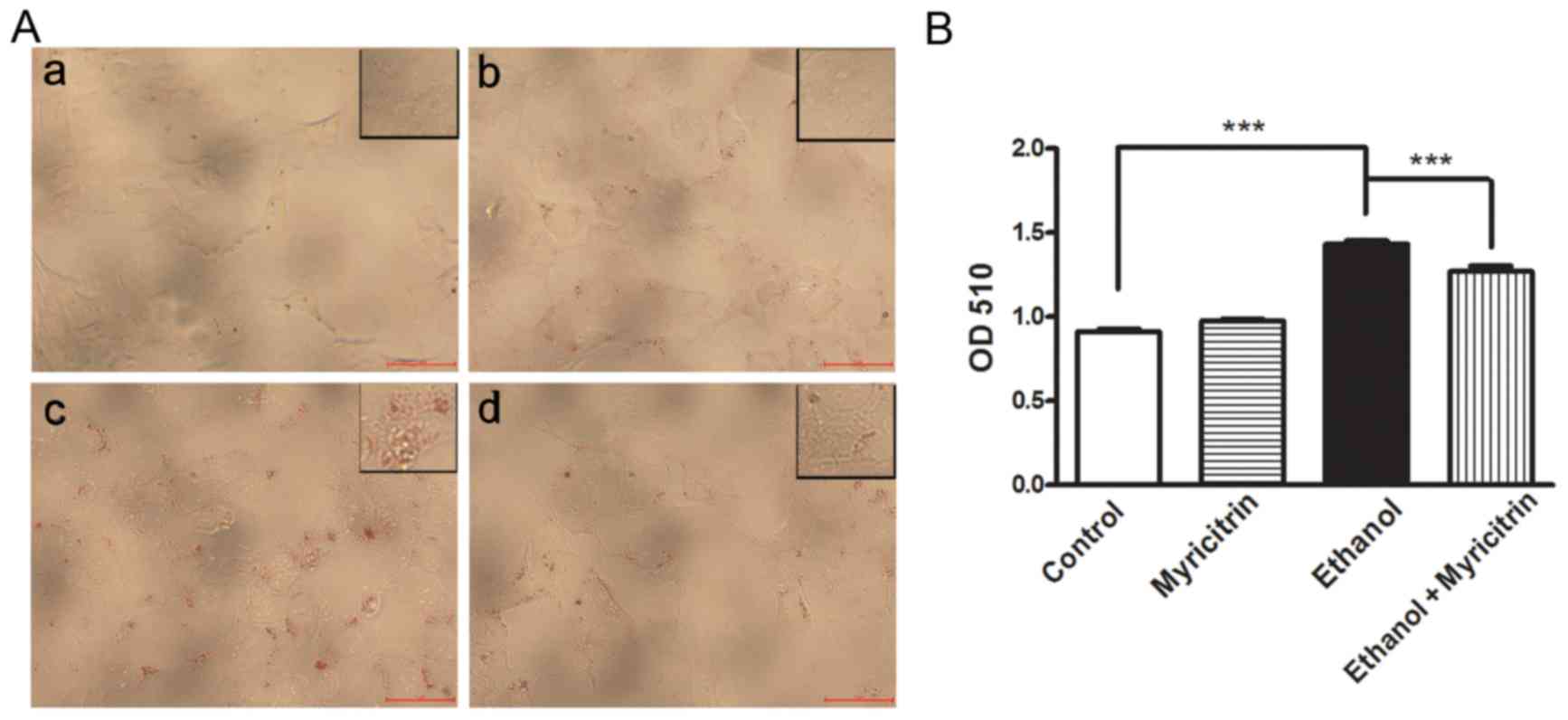
Previous studies demonstrated that ethanol may
induce steatosis (20,21). To determine the effect of
myricitrin on ethanol-induced steatosis, cells were treated with
100 mM ethanol or 20 µM myricitrin, or both. Oil red O staining
demonstrated lipid accumulation in ethanol-stimulated cells while
the control cells did not exhibit steatosis (P<0.001; Fig. 2). Following co-culture with
myricitrin, lipid accumulation in ethanol-stimulated cells was
significantly attenuated (P<0.001). The results indicate that
myricitrin can suppress the development of ethanol-induced
steatosis in hepatocytes.
Myricitrin reduces ethanol-induced
oxidative stress and decreases mRNA expression of certain cytokines
in AML12 cells
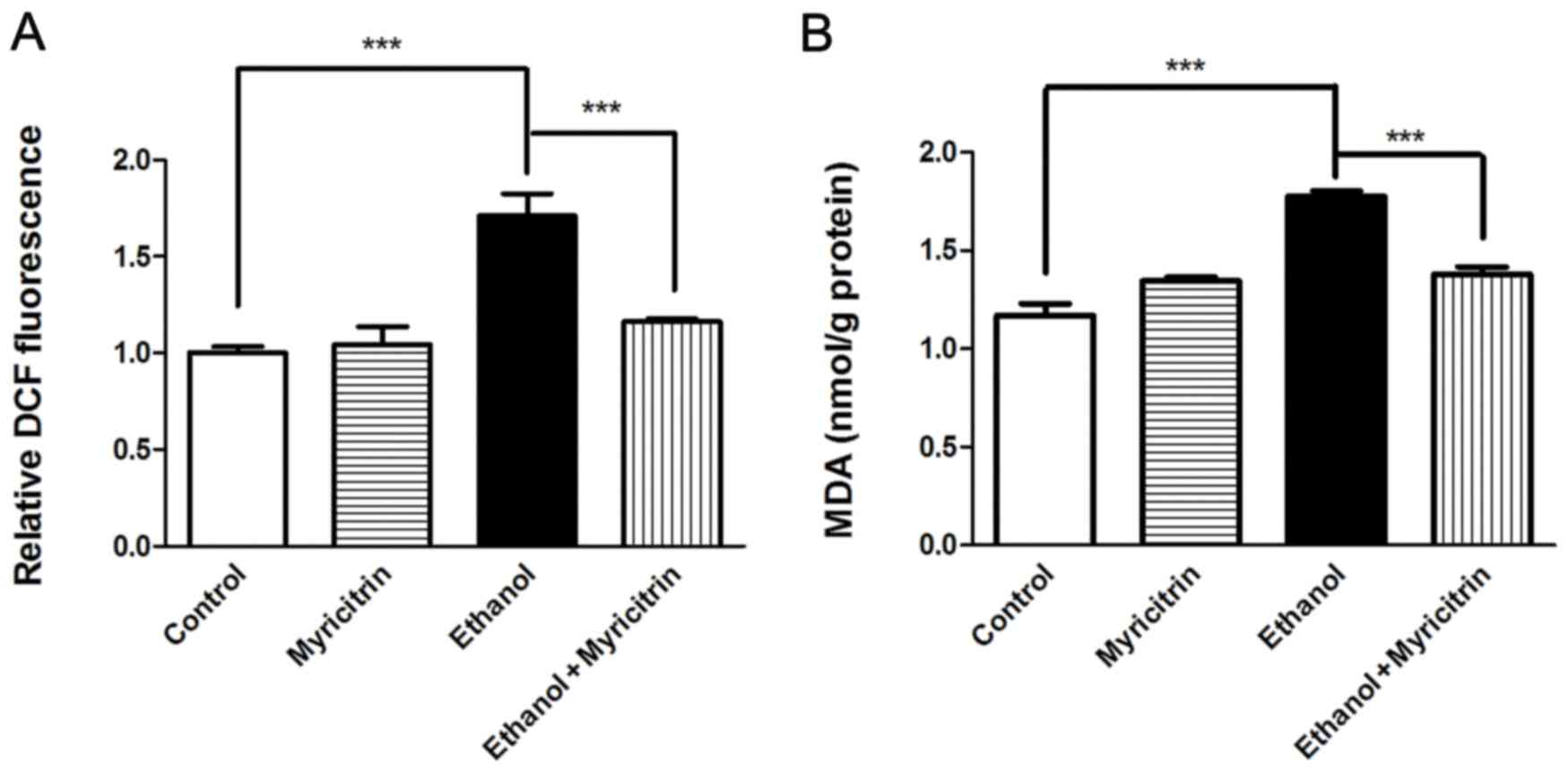
Oxidative stress is reported to be involved in the
development of ethanol-induced steatosis (2). The present study investigated the
effect of myricitrin treatment on MDA and ROS production in AML12
cells. Treatment with myricitrin resulted in a 32.1% decrease in
ROS levels (P<0.001) and 22.4% decrease in MDA levels
(P<0.001) in ethanol-stimulated AML12 cells (Fig. 3). The above data indicate that
myricitrin alleviates ethanol-induced oxidative stress in liver
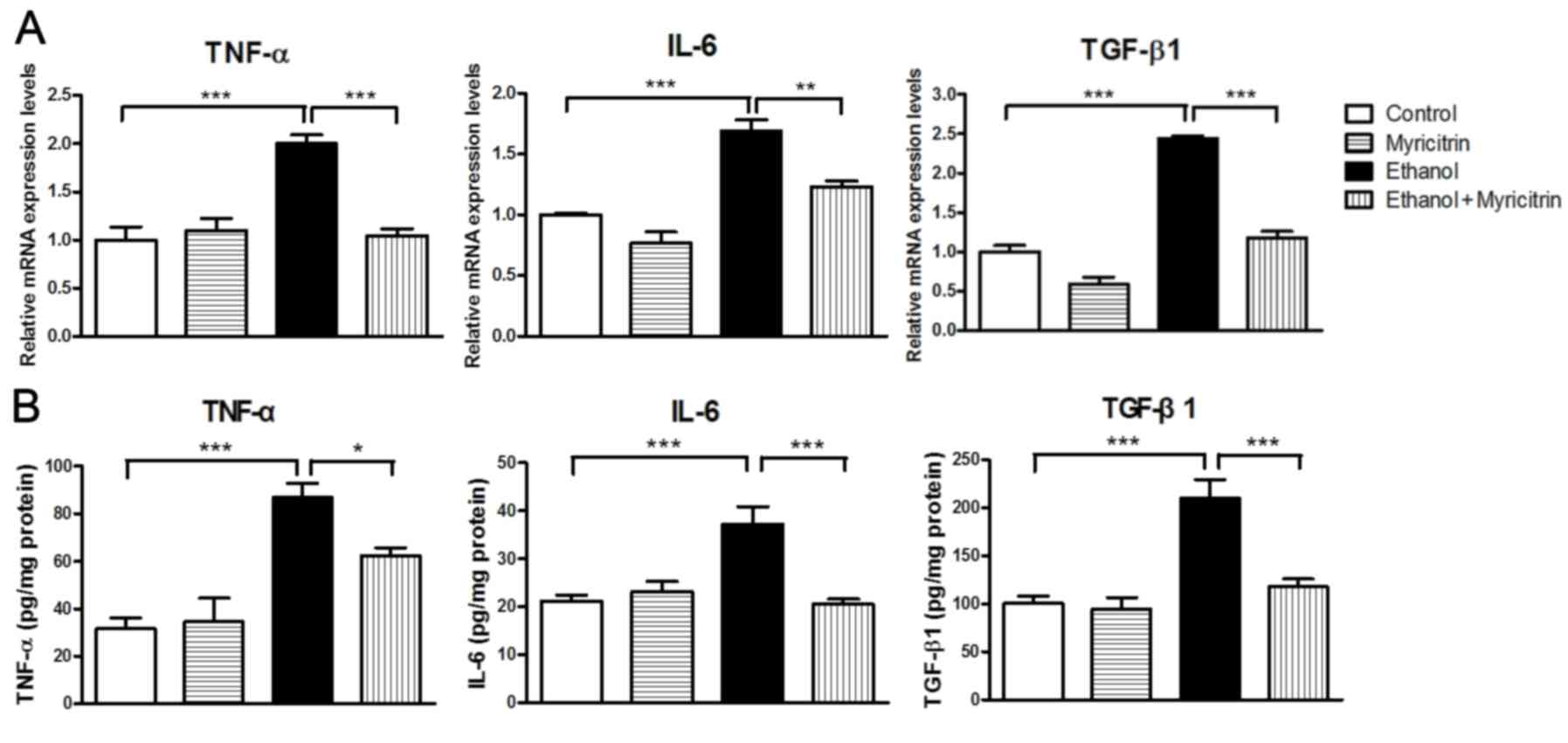
cells. Certain pro-inflammatory cytokines, including TNF-α, IL-6
and TGF-β1 have been reported to be associated with the development
of ethanol-induced hepatic steatosis (8,22).
Expression levels of TNF-α, IL-6 and TGF-β1 were also been
determined in the present study. Cells stimulated with ethanol
exhibited markedly elevated mRNA expression levels of TNF-α,
compared with control cells (Fig.
4A). However, ethanol-induced increase in TNF-α mRNA levels was
significantly attenuated in cells treated with myricitrin (47.9%;
P<0.001). Similarly, cells stimulated with ethanol exhibited
markedly increased expression of IL-6 and TGF-β1 mRNA compared with
control cells. This ethanol-induced elevation of mRNA levels of
IL-6 and TGF-β1 was significantly attenuated, by 27.1% (P<0.01)
and 51.8% (P<0.001) respectively, in cells treated with
myricitrin. Furthermore, ethanol-induced secretion of TNF-α, IL-6
and TGF-β1 proteins was also suppressed by 28.3% (P<0.01), 44.6%
(P<0.05) and 43.8% (P<0.01), respectively, in cells treated
with myricitrin (Fig. 4B). The
results suggest that myricitrin may reduce oxidative stress and
affect the production of pro-inflammatory cytokines to prevent
ethanol-induced steatosis in AML12 liver cells.
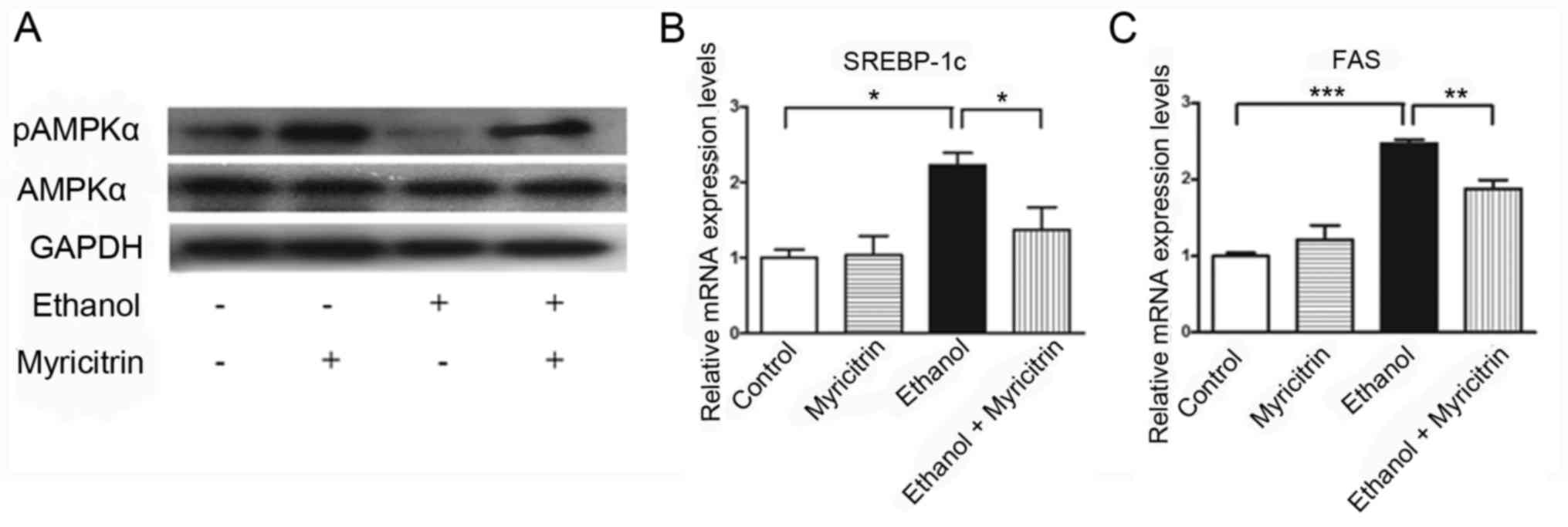
Myricitrin activates AMPK and reduces
the ethanol-induced elevation of SREBP-1c and FAS mRNA expression
in AML12 cells
Previous studies indicated that AMPK inactivation
may be involved in the development of ethanol-induced hepatic
steatosis (23,24). To determine the mechanism
underlying myricitrin-induced amelioration of ethanol-induced
steatosis, the present study investigated the effect of treatment
with myricitrin on AMPK activity. pAMPK protein expression levels
in ethanol-stimulated AML12 cells treated with myricitrin increased
compared with myricitrin untreated ethanol-stimulated cells,
indicating that myricitrin treatment activated AMPK in
ethanol-stimulated AML12 cells (Fig.
5A). Furthermore, the effect of treatment with myricitrin on
mRNA expression of SREBP-1c and FAS was determined. SREBP-1c and
FAS genes are regulated by AMPK and control the synthesis of fatty
acids in ethanol-stimulated liver cells (21,25,26).
Treatment with 100 mM ethanol resulted in a significant increase in
SREBP-1c mRNA expression levels compared with the control group and
this increase was significantly attenuated following treatment of
ethanol-stimulated cells with myricitrin (P<0.05; Fig. 5B). mRNA expression of FAS
significantly decreased in myricitrin and ethanol-stimulated cells
compared with the ethanol treatment group (P<0.01; Fig. 5C). The above results indicate that
treatment with myricitrin may ameliorate ethanol-induced steatosis
in AML12 cells through activation of AMPK and subsequent inhibition
of SREBP-1c-regulated lipogenesis.
Discussion
Previous studies have demonstrated that myricitrin
may protect against liver injury by attenuating oxidative stress in
CCl4-intoxicated mice (18). In the present study, myricitrin
suppressed ethanol-induced production of MDA and ROS in mouse AML12
liver cells. The results of the present study further indicate that
myricitrin may attenuate oxidative stress in chemically intoxicated
cells. Oxidative stress has been hypothesized to serve a role in
pathogenesis of ALD (2). The
results of the present study suggest that myricitrin may protect
against ALD by attenuating oxidative stress.
The incidence and development of alcoholic liver
disease are associated with overexpression of a number of
cytokines, including TNF-α, IL-6 and TGF-β1 (8,27,28).
Previous studies have demonstrated that expression of the
inflammatory cytokine TNF-α is elevated in patients with alcoholic
liver disease (8,29). TNF-α has been reported to induce
hepatic steatosis in mice by affecting hepatic lipogenic metabolism
(30). Other studies have
indicated that expression of IL-6 is elevated in patients with
alcoholic liver diseases (27,28).
Persistent activation of IL-6 signaling may be detrimental to the
liver and may cause liver tumors (31). The present study demonstrated that
myricitrin may reduce mRNA expression of TNF-α, IL-6 and TGF-β1,
indicating that alleviation of ethanol-induced steatosis in liver
cells caused by myricitrin may be partially due to inhibition of
ethanol-induced overexpression of TNF-α, IL-6 and TGF-β1 mRNA.
AMPK has a role in cellular energy homeostasis
(32). Inhibition of AMPK
suppresses fatty acid oxidation and stimulates lipogenesis
(33). Ethanol has been reported
to inhibit AMPK activity in vitro (24) and in vivo (34). Inhibition of AMPK by ethanol has a
role in the development of hepatosteatosis induced by alcohol
consumption (23,24). SREBP-1c is a regulator of hepatic
lipid metabolism and is involved transcription of a number of genes
involved in liver triglyceride and fatty acid synthesis, such as
FAS (35–37). Increased expression of SREBP-1c
induced by ethanol may cause hepatic lipid accumulation in
alcoholic fatty liver (24). AMPK
activators have been demonstrated to reduce the expression of
SREBP-1c (24). In the present
study, myricitrin activated AMPK in ethanol-stimulated AML12 liver
cells. The results also demonstrated that myricitrin suppressed
mRNA expression of SREBP-1c and FAS. Suppression of SREBP-1c
expression and activation of AMPK were observed in ethanol-induced
cells following treatment with myricitrin. The results of the
present study indicate that myricitrin regulates the AMPK/SREBP-1c
signaling pathway and reduces lipid formation in liver cells.
However, specific inhibitors or gene specific-short hairpin RNAs
may be used in the future to alter the activity of AMPK following
treatment with myricitrin to further validate the specificity of
myricitrin to the AMPK/SREBP signaling pathway.
Aldose reductase (AKR1B1) is an enzyme that
catalyzes the reduction of glucose to sorbitol with the aid of
co-factor NADPH in the polyol pathway (38). We previously reported that AKR1B1
inhibitor ameliorates ethanol-induced steatosis in vitro and
in vivo by activating AMPK and suppressing expression of
SREBP-1c and FAS (21,25). Myricitrin is an inhibitor of AKR1B1
(39,40). In the present study, myricitrin
ameliorated ethanol-induced steatosis via activation of AMPK and
suppression of expression of SREBP-1c and FAS, thus indicating that
the beneficial effect of myricitrin on ethanol-induced steatosis
may be at least partially attributable to inhibition of AKR1B1.
In conclusion, myricitrin ameliorated
ethanol-induced lipid abnormalities in AML12 liver cells, which was
partially due to suppression of ethanol-induced oxidative stress
and associated with suppression of ethanol-induced overexpression
of certain cytokines. The present study also demonstrated that
myricitrin activated AMPK and suppressed SREBP-1c and FAS mRNA
overexpression to alleviate the development of hepatic steatosis.
The present study may provide theoretical basis for therapeutic use
of AKR1B1 inhibitor in the treatment of fatty liver disease.
Acknowledgements
The authors would like to thank Mrs. Ailing Dai and
Mrs. Chunhua Wei of Longyan University for their technical
support.
Funding
The present study was supported in part by Natural
Science Foundation of Fujian Province, China (grant no. 2015J01619)
and by Fujian Provincial Undergraduate Training Programs for
Innovation and Entrepreneurship, China (grant. no.
201611312026).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LQ conceived and designed the experiments, and wrote
the manuscript; JG, SC, ZQ, LF and LZ performed the experiments and
analyzed the data; LQ, CG and TC contributed
reagents/materials/analysis tools and analyzed the data.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chacko KR and Reinus J: Spectrum of
alcoholic liver disease. Clin Liver Dis. 20:419–427. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Louvet A and Mathurin P: Alcoholic liver
disease: Mechanisms of injury and targeted treatment. Nat Rev
Gastroenterol Hepatol. 12:231–242. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Osna NA, Donohue TM Jr and Kharbanda KK:
Alcoholic liver disease: Pathogenesis and current management.
Alcohol Res. 38:147–161. 2017.PubMed/NCBI
|
|
4
|
Sun X, Tang Y, Tan X, Li Q, Zhong W, Sun
X, Jia W, McClain CJ and Zhou Z: Activation of peroxisome
proliferator-activated receptor-gamma by rosiglitazone improves
lipid homeostasis at the adipose tissue-liver axis in ethanol-fed
mice. Am J Physiol Gastrointest Liver Physiol. 302:G548–G557. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ji C, Chan C and Kaplowitz N: Predominant
role of sterol response element binding proteins (SREBP) lipogenic
pathways in hepatic steatosis in the murine intragastric ethanol
feeding model. J Hepatol. 45:717–724. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang Z, Dou X, Li S, Zhang X, Sun X, Zhou
Z and Song Z: Nuclear factor (erythroid-derived 2)-like 2
activation-induced hepatic very-low-density lipoprotein receptor
overexpression in response to oxidative stress contributes to
alcoholic liver disease in mice. Hepatology. 59:1381–1392. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ishii H, Kurose I and Kato S: Pathogenesis
of alcoholic liver disease with particular emphasis on oxidative
stress. J Gastroenterol Hepatol. 12:S272–S282. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kawaratani H, Tsujimoto T, Douhara A,
Takaya H, Moriya K, Namisaki T, Noguchi R, Yoshiji H, Fujimoto M
and Fukui H: The effect of inflammatory cytokines in alcoholic
liver disease. Mediators Inflamm: 495156. 2013. View Article : Google Scholar
|
|
9
|
Cushnie TP and Lamb AJ: Antimicrobial
activity of flavonoids. Int J Antimicrob Agents. 26:343–356. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kale A, Gawande S and Kotwal S: Cancer
phytotherapeutics: Role for flavonoids at the cellular level.
Phytother Res. 22:567–577. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Meotti FC, Fachinetto R, Maffi LC, Missau
FC, Pizzolatti MG, Rocha JB and Santos AR: Antinociceptive action
of myricitrin: Involvement of the K+ and Ca2+ channels. Eur J
Pharmacol. 567:198–205. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qi S, Feng Z, Li Q, Qi Z and Zhang Y:
Myricitrin modulates NADPH oxidase-dependent ROS production to
inhibit endotoxin-mediated inflammation by blocking the JAK/STAT1
and NOX2/p47phox pathways. Oxid Med Cell Longev.
20147:97387452017.
|
|
13
|
Shimosaki S, Tsurunaga Y, Itamura H and
Nakamura M: Anti-allergic effect of the flavonoid myricitrin from
Myrica rubra leaf extracts in vitro and in vivo. Nat Prod
Res. 25:374–380. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu JH, Huang CY, Tung YT and Chang ST:
Online RP-HPLC-DPPH screening method for detection of
radical-scavenging phytochemicals from flowers of Acacia
confusa. J Agric Food Chem. 56:328–332. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen W, Feng L, Shen Y, Su H, Li Y, Zhuang
J, Zhang L and Zheng X: Myricitrin inhibits acrylamide-mediated
cytotoxicity in human Caco-2 cells by preventing oxidative stress.
Biomed Res Int: 724183. 2013. View Article : Google Scholar
|
|
16
|
Sun GB, Qin M, Ye JX, Pan RL, Meng XB,
Wang M, Luo Y, Li ZY, Wang HW and Sun XB: Inhibitory effects of
myricitrin on oxidative stress-induced endothelial damage and early
atherosclerosis in ApoE-/- mice. Toxicol Appl Pharmacol.
271:114–126. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qin M, Luo Y, Meng XB, Wang M, Wang HW,
Song SY, Ye JX, Pan RL, Yao F, Wu P, et al: Myricitrin attenuates
endothelial cell apoptosis to prevent atherosclerosis: An insight
into PI3K/Akt activation and STAT3 signaling pathways. Vascul
Pharmacol. 70:23–34. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Domitrović R, Rashed K, Cvijanović O,
Vladimir-Knežević S, Škoda M and Višnić A: Myricitrin exhibits
antioxidant, anti-inflammatory and antifibrotic activity in carbon
tetrachloride-intoxicated mice. Chem Biol Interact. 230:21–29.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Altamirano J and Bataller R: Alcoholic
liver disease: Pathogenesis and new targets for therapy. Nat Rev
Gastroenterol Hepatol. 8:491–501. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qiu L, Cai C, Zhao X, Fang Y, Tang W and
Guo C: Inhibition of aldose reductase ameliorates ethanol induced
steatosis in HepG2 cells. Mol Med Rep. 15:2732–2736. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Thiele DL: Tumor necrosis factor, the
acute phase response and the pathogenesis of alcoholic liver
disease. Hepatology. 9:497–499. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sid B, Verrax J and Calderon PB: Role of
AMPK activation in oxidative cell damage: Implications for
alcohol-induced liver disease. Biochem Pharmacol. 86:200–209. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
You M, Matsumoto M, Pacold CM, Cho WK and
Crabb DW: The role of AMP-activated protein kinase in the action of
ethanol in the liver. Gastroenterology. 127:1798–1808. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shi C, Wang Y, Gao J, Chen S, Zhao X, Cai
C, Guo C and Qiu L: Inhibition of aldose reductase ameliorates
alcoholic liver disease by activating AMPK and modulating oxidative
stress and inflammatory cytokines. Mol Med Rep. 16:2767–2772. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hong SH, Lee H, Lee HJ, Kim B, Nam MH,
Shim BS and Kim SH: Ethanol extract of pinus koraiensis leaf
ameliorates alcoholic fatty liver via the activation of LKB1-AMPK
signaling in vitro and in vivo. Phytother Res. 31:783–791. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Neuman MG, Maor Y, Nanau RM, Melzer E,
Mell H, Opris M, Cohen L and Malnick S: Alcoholic liver disease:
Role of cytokines. Biomolecules. 5:2023–2034. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Prystupa A, Kiciński P, Sak J,
Boguszewska-Czubara A, Torun-Jurkowska A and Zaluska W:
Proinflammatory cytokines (IL-1alpha, IL-6) and hepatocyte growth
factor in patients with alcoholic liver cirrhosis. Gastroenterol
Res Pract. 2015:5326152015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
McClain CJ and Cohen DA: Increased tumor
necrosis factor production by monocytes in alcoholic hepatitis.
Hepatology. 9:349–351. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Endo M, Masaki T, Seike M and Yoshimatsu
H: TNF-alpha induces hepatic steatosis in mice by enhancing gene
expression of sterol regulatory element binding protein-1c
(SREBP-1c). Exp Biol Med (Maywood). 232:614–621. 2007.PubMed/NCBI
|
|
31
|
Schmidt-Arras D and Rose-John S: IL-6
pathway in the liver: From physiopathology to therapy. J Hepatol.
64:1403–1415. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Garcia D and Shaw RJ: AMPK: Mechanisms of
cellular energy sensing and restoration of metabolic balance. Mol
Cell. 66:789–800. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Viollet B, Guigas B, Leclerc J, Hébrard S,
Lantier L, Mounier R, Andreelli F and Foretz M: AMP-activated
protein kinase in the regulation of hepatic energy metabolism: From
physiology to therapeutic perspectives. Acta Physiol (Oxf).
196:81–98. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
García-Villafranca J, Guillén A and Castro
J: Ethanol consumption impairs regulation of fatty acid metabolism
by decreasing the activity of AMP-activated protein kinase in rat
liver. Biochimie. 90:460–466. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ferré P and Foufelle F: SREBP-1c
transcription factor and lipid homeostasis: Clinical perspective.
Horm Res. 68:72–82. 2007.PubMed/NCBI
|
|
36
|
Ferré P and Foufelle F: Hepatic steatosis:
A role for de novo lipogenesis and the transcription factor
SREBP-1c. Diabetes Obes Metab. 12 Suppl 2:83–92. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Horton JD, Goldstein JL and Brown MS:
SREBPs: Activators of the complete program of cholesterol and fatty
acid synthesis in the liver. J Clin Invest. 109:1125–1131. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hers HG: [Aldose reductase]. Biochim
Biophys Acta. 37:120–126. 1960. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Varma SD, Mikuni I and Kinoshita JH:
Flavonoids as inhibitors of lens aldose reductase. Science.
188:1215–1216. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Veeresham C, Rama Rao A and Asres K:
Aldose reductase inhibitors of plant origin. Phytother Res.
28:317–333. 2014. View Article : Google Scholar : PubMed/NCBI
|