Introduction
Pancreatic cancer continues to be the fourth leading
cause of cancer-related death worldwide. It is characterized by
late clinical presentation, malignant behavior and absence of
effective therapeutic approaches, the 5-year overall survival rate
is still less than 7% (1–3). Despite significant advance in
diagnosis and treatment, the treatment efficacy is still
unsatisfactory (4,5). Hence, the development of reliable
methods for early detection and new therapeutic strategies to
improve the outcome for patients with pancreatic cancer is urgently
needed.
Circular RNAs (circRNAs) are a novel class of
endogenous non-coding RNA, which are first discovered in RNA
viruses as early as the 1970s (6).
Unlike linear RNAs, circRNAs are terminated with 5′ caps and 3′
tails, circRNAs form covalently closed loop structures with neither
5′-3′ polarities nor polyadenylated tails. Accumulating studies
have demonstrated that circRNAs are an abundant, stable, diverse
and conserved class of RNA molecules (7–10).
Originally, circRNAs gained little attention from researchers and
the related studies were still in infancy. However, emerging
evidence showed that circRNAs were implicated in multiple
biological processes, including proliferation, differentiation,
apoptosis and angiogenesis. Recent reports have showed the
dysregulated circRNAs expression in diverse cancers, indicating
crucial roles of circRNAs in carcinogenesis and progression of
human cancer (11–13). With the development of
biotechnology (e.g., high throughput RNA sequencing technology and
bioinformatics), the characteristics of circRNAs are gradually
revealed. Recent studies showed that circRNAs could regulate
alternative splicing and targeted gene expression via microRNA
(miRNA) sponge (14,15). Meanwhile, circRNAs dysregulation
was engaged in carcinogenesis and cancer progression, including
breast cancer, colorectal cancer, liver cancer and gastric cancer
(16–19). The above findings suggest that
circRNAs as a novel type of endogenous non-coding RNA become a new
hotspot in non-coding RNA field and may play very important role in
the progression of malignant tumors.
In this study, we investigated circRNAs expression
profile in 20 pancreatic cancer tissues and corresponding
paracancerous tissues. We found that circRNAs expression profile
was significantly different between pancreatic cancer tissue and
paracancerous tissue, and circRNAs could act as a miRNA sponge to
modulate gene expression in pancreatic cancer. Our data indicated
that circRNAs dysregulation may be associated with initiation and
progression of pancreatic cancer and provide more potential
biomarkers and new insights for pancreatic cancer.
Materials and methods
Samples
The Ethics Review Board of Southwest Hospital, the
Third Military Medical University approved this study (no. 2016
Scientific Research no. 13), and the methods were carried out in
accordance with the approved guidelines. All patients provided
written informed consent. Twenty fresh-frozen pancreatic cancer
tissues and corresponding paracancerous tissues were collected from
Institute of Hepatopancreatobiliary Surgery, Southwest Hospital,
the Third Military Medical University from April 2014 to November
2014 (clinical information of pancreatic cancer patients in
Table I). All patients didn't
undergo preoperative chemotherapy or radiotherapy. The pathologists
from Department of Pathology, Southwest Hospital performed
pathological diagnosis. According to tumor-node-metastasis (TNM)
staging criteria from Union for International Cancer Control
(UICC), tumor clinical stages were performed. All samples were
stored in liquid nitrogen.
 | Table I.Clinical characteristics of pancreatic
cancer patients. |
Table I.
Clinical characteristics of pancreatic
cancer patients.
| No. | Sex | Age (years) | Diabetes | Jaundice | Histological
grade | TNM stage |
|---|
| 1 | Male | 67 | Yes | Yes | G2 | T3N1M0 |
| 2 | Female | 50 | No | No | G1 | T2N0M0 |
| 3 | Male | 50 | No | No | G3 | T4N1M0 |
| 4 | Male | 62 | Yes | No | G2 | T4N1M0 |
| 5 | Male | 46 | No | No | G2 | T3N1M0 |
| 6 | Female | 71 | Yes | No | G2 | T3N1M0 |
| 7 | Male | 49 | No | No | G2 | T4N1M0 |
| 8 | Female | 47 | No | No | G2 | T3N1M0 |
| 9 | Female | 71 | No | No | G2 | T4N1M0 |
| 10 | Female | 72 | No | No | G2 | T3N0M0 |
| 11 | Male | 64 | Yes | No | G2 | T3N0M0 |
| 12 | Female | 61 | No | Yes | G1 | T3N0M0 |
| 13 | Male | 60 | No | No | G3 | T3N1M0 |
| 14 | Male | 73 | No | No | G1 | T3N1M0 |
| 15 | Female | 52 | No | No | G2 | T3N1M0 |
| 16 | Male | 61 | No | No | G2 | T3N1M0 |
| 17 | Male | 36 | No | No | G2 | T3N0M0 |
| 18 | Male | 54 | Yes | No | G2 | T3N0M0 |
| 19 | Female | 60 | No | Yes | G2 | T3N0M0 |
| 20 | Male | 60 | No | No | G2 | T3N0M0 |
RNA isolation
Total RNA from pancreatic cancer tissues and
paracancerous tissues was extracted using TRIzol reagent
(Invitrogen, Carlsbad, CA, USA). The RNA extractions were stored at
−80°C.
RNA sample quality control (QC)
The purity and concentration of total RNA from each
sample were quantified using a NanoDrop ND-1000. The integrity of
RNA was assessed by electrophoresis on a denaturing agarose gel.
Sample preparation and microarray hybridization were performed
based on Arraystar's standard protocols.
RNA labeling and hybridization
Briefly, total RNA was digested with RNase R
(Epicentre; Illumina, Inc., San Diego, CA, USA) to remove linear
RNAs and enrich for circRNAs. The enriched circRNAs were amplified
and transcribed into fluorescent cRNA utilizing a random priming
method (Arraystar Super RNA Labeling kit; Arraystar, Inc.,
Rockville, MD, USA). The labeled cRNAs were purified by RNeasy Mini
kit (Qiagen GmbH, Hilden, Germany). The concentration and specific
activity of the labeled cRNAs (pmol Cy3/µg cRNA) were measured by
NanoDrop ND-1000. Each labeled cRNA (1 µg) was fragmented by adding
5 µl 10X Blocking Agent and 1 µl of 25X fragmentation buffer, and
then heated the mixture at 60°C for 30 min. Finally, 25 µl 2X
Hybridization buffer was added to dilute the labeled cRNA. 50 µl of
hybridization solution was dispensed into the gasket slide and
assembled to the circRNA expression microarray slide. The labeled
cRNAs were hybridized into an Arraystar Human circRNA array (8×15K;
Arraystar, Inc.) and incubated for 17 h at 65°C in an Agilent
Hybridization Oven (Agilent Technologies, Inc., Santa Clara, CA,
USA). After the slides were washed, the arrays were scanned using
an Agilent Scanner G2505C.
Microarray analysis and quality
control
Agilent Feature Extraction software (version
11.0.1.1) was used to analyze acquired raw array images. Quantile
normalization and subsequent data processing were performed using
the R software package (R version 3.1.2). After quantile
normalization of the raw data, low intensity filtering was
performed, and circRNAs that at least 15 out of 40 samples have
flags in ‘P’ or ‘M’ (‘All Targets Value’) were retained for further
analyses. When comparing two groups of profile differences (such as
disease vs. control), the ‘fold change’ (i.e., the ratio of the
group averages) between the groups for each circRNA is computed.
The statistical significance of the difference may be conveniently
estimated by t-test. CircRNAs having fold changes ≥2 and P-values
≤0.05 are selected as significantly different expression. According
to fold change, P-value, one can filter the analysis outputs and
rank the differentially expressed circRNAs using Microsoft Excel's
Data/Sort and Filter functionalities. The Statistical differences
in circRNA expression between the two groups were determined with
volcano plot filtering. Differentially expressed circRNAs between
the two sample sets were identified by fold change filtering.
Hierarchical clustering is one of the simplest and widely used
clustering techniques for analysis of gene expression data. Cluster
analysis arranges samples into groups based on their expression
levels, which allows us to hypothesize about the relationships
among samples. The dendrogram shows the relationships among the
expression levels of samples. Here, hierarchical clustering was
performed based on ‘All Targets Value-CircRNAs’. Our experiment
consists of 40 different samples, and hierarchical clustering was
performed to show a distinguishable circRNA genes expression
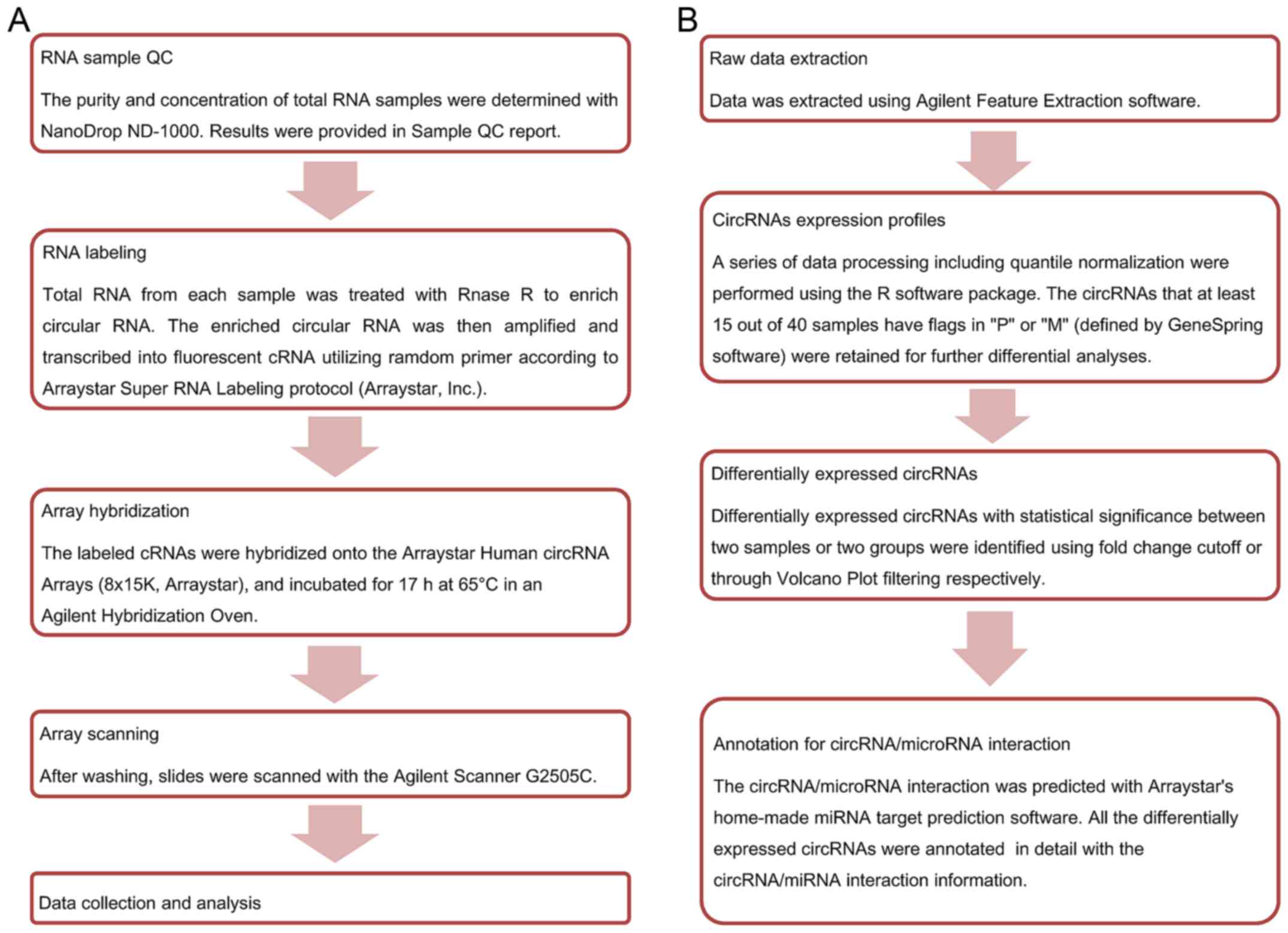
profile among samples. The experiment workflow and data analysis
flowchart are shown in Fig. 1.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) validation assay for
circRNAs
Total RNA from 10 pairs human pancreatic cancer
tissue and corresponding paracancerous tissue was isolated using
TRIzol reagent (Invitrogen). The cDNA synthesis of RNA was
performed using SuperScript™ III Reverse Transcriptase
(Invitrogen). 10 differentially expressed circRNAs were
respectively measured by qRT-PCR using 2X PCR Master Mix
(Arraystar, Inc.) in pancreatic cancer tissue and corresponding
paracancerous tissue. The reaction condition was as follows: 95°C
for 10 min, 40 cycles of 95°C for 10 sec, 60°C for 60 sec, 95°C for
15 sec. The RNA levels were normalized to β-actin. All of the
quantitative PCR reactions were conducted in triplicate. The
related information of primers is shown in Table II. The specificity of PCR primers
is validated using BLAST.
 | Table II.Primers used for reverse
transcription-quantitative polymerase chain reaction analysis of
circRNAs, miR-15a and miR-506. |
Table II.
Primers used for reverse
transcription-quantitative polymerase chain reaction analysis of
circRNAs, miR-15a and miR-506.
| ID | Primer sequence
(5′-3′) | AT (°C) |
|---|
| β-actin
(Human) | F:
GTGGCCGAGGACTTTGATTG | 60 |
|
| R:
CCTGTAACAACGCATCTCATATT |
|
|
hsa_circRNA_102619 | F:
GGGCATCTATTACATTCCATTCT | 60 |
|
| R:
ATTATTCTCCGCAGCATCAGT |
|
|
hsa_circRNA_102049 | F:
GAAGCATTTCATCAATAACCCTC | 60 |
|
| R:
CAAAGCCACAGTCCATCACAG |
|
|
hsa_circRNA_000167 | F:
TGAGCTTCGGGGAGGTGAGT | 60 |
|
| R:
CAAGGGACATGGGAGTGGAGT |
|
|
hsa_circRNA_100433 | F:
CTTACCCATTCAGCCCATTCC | 60 |
|
| R:
CGTGGCAAGGCTCTTTCTTCT |
|
|
hsa_circRNA_103390 | F:
CTGGGCTGACTACCTCAAACA | 60 |
|
| R:
ACTCCACTGCACAGGGAAGAT |
|
|
hsa_circRNA_101717 | F:
GTCCTGTTTCTCAGATCGCTCAC | 60 |
|
| R:
GAAGTCGGGGTTGCTGGTATT |
|
|
hsa_circRNA_103076 | F:
TCCTCCGTACAGCACATTCATTA | 60 |
|
| R:
CACTCGATCGGCTTCACAAA |
|
|
hsa_circRNA_104084 | F:
GAGTGAGGATCAATGGGAAGAA | 60 |
|
| R:
GGATGACTTGGTGACGGAAA |
|
|
hsa_circRNA_102051 | F:
CCTGAAACAAGCAGAGGAAGC | 60 |
|
| R:
CACTCCTCCTTGGTCTTGGTG |
|
|
hsa_circRNA_104270 | F:
CGACAGCGGGTCTACTCACTCT | 60 |
|
| R:
GGTACTTCCCACAAATCCTTGC |
|
| miR-506 | F:
TAAGGCACCCTTCTGAGTAGA | 60 |
|
| R:
GCGAGCACAGAATTAATACGAC |
|
| miR-15a | F:
GGGGTAGCTTATCAGACTG | 60 |
|
| R:
AGTGCGTGTCGTGGAGTC |
|
Quantitative real-time PCR for
miRNAs
Total RNA from pancreatic cancer tissues was
isolated using TRIzol reagent (Invitrogen) according to the
manufacturer's instructions. miRNA-specific primers were purchased
from Ambion; Thermo Fisher Scientific, Inc. (Waltham, MA, USA). For
miR-15a and miR-506 detection, cDNA synthesis was performed using
the High Capacity cDNA Synthesis kit (Applied Biosystems, Foster
City, CA, USA). Real-time qRT-PCR was carried out with
miRNA-specific primers using the TaqMan Gene Expression Assay
(Applied Biosystems). The reaction condition was as follows: 95°C
for 10 min, 40 cycles of 95°C for 15 sec, 60°C for 60 sec, 4°C
forever. β-actin was used as an endogenous control. The
2−ΔΔCt method was used to calculate expression relative
to the endogenous control. The primer sequences of miR-15a and
miR-506 were listed in Table
II.
Statistical analysis
Data was extracted using Agilent Feature Extraction
software. A series of data processing including quantile
normalization were performed using the R software package. The
circRNAs that at least 15 out of 40 samples have flags in ‘P’ or
‘M’ (defined by GeneSpring software) and were retained for further
differential analyses. Differentially expressed circRNAs with
statistical significance between two samples or two groups were
identified using fold change. The statistical significance of the
difference was conveniently estimated by paired t-test. CircRNAs
having fold changes ≥2 and P-values ≤0.05 were selected as the
significantly differentially expressed. The expression level of
each circRNA was represented as fold change by 2−ΔΔCq
method. The circRNAs/microRNA interaction was predicted with
Arraystar's home-made miRNA target prediction software. All
differentially expressed circRNAs were annotated in detail with the
circRNAs/miRNA interaction information.
Results
Summary of circRNAs expression
profile
The expression profile of circRNAs was performed in
20 pancreatic cancer tissues and corresponding paracancerous
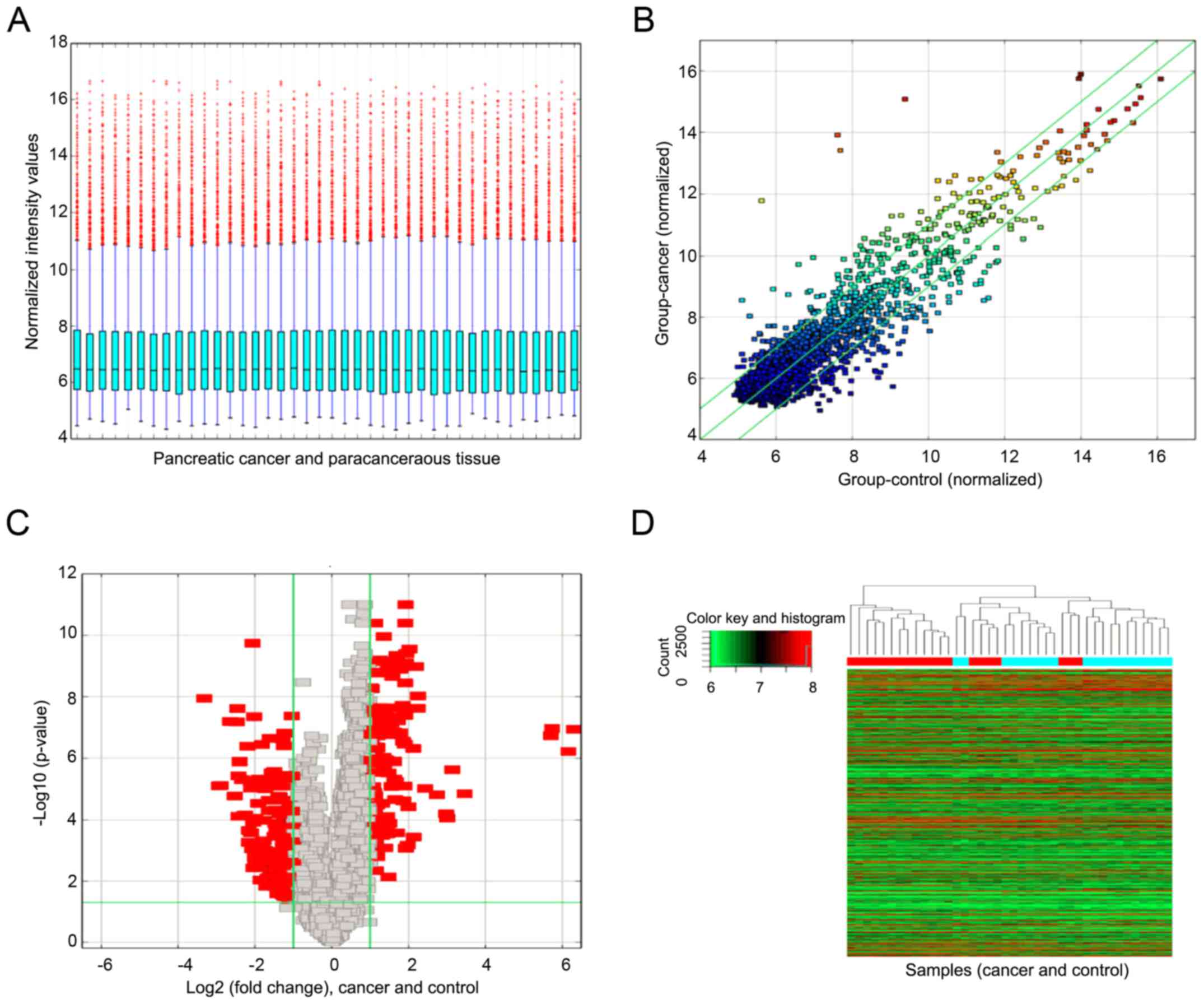
tissues using Arraystar Human CircRNA Array Analysis. The box plot
is a convenient way to quickly visualize the distribution of a
dataset. Here, it is used to compare the distributions of
expression values for the samples in an experiment after
normalization (Fig. 2A). The
scatter-plot is a visualization method used for assessing circRNA
expression variation (or reproducibility) between two compared
samples or two compared groups of samples. The green lines are fold
change lines. The data indicated that the expressions of circRNAs
above the top green line and below the bottom green line are more
than 2.0 fold changes between two compared samples (Fig. 2B). The volcano plot is useful tool
for visualizing differential expression between two different
conditions. The vertical lines respectively correspond to 2.0-fold
up and down, and the horizontal line represents a P-value of 0.05.
The red point in the plot represents the differentially expressed
circRNAs with statistical significance (Fig. 2C). Hierarchical clustering is one
of the simplest and widely used clustering techniques for analysis
of gene expression data. Hierarchical clustering analysis shows a
distinguishable circRNA expression profile among samples (Fig. 2D). Our results showed that there
was a distinctly distinguishable circRNAs expression profile
between pancreatic cancer tissue and paracancerous tissue.
Meanwhile, we also indentified the characteristics of top 10
differently expressed circRNAs and 12 circRNAs associated with
miR-15a and miR-506 (Table III).
Among our results, 128 circRNAs were upregulated and 161 circRNAs
were downregulated compared to paracancerous tissue (fold change
≥2.0 and P<0.05). Additionally, we validated the category of
dysregulated circRNAs. Among these, upregulated circRNAs included
111 exonic, 9 intronic, 5 intragenic and 3 antisense, and
downregulated circRNAs contained 122 exonic, 25 intronic, 13
intragenic and 1 antisense. We had submitted the microarray data to
National Center for Biotechnology Information (NCBI) Gene
Expression Omnibus (GEO). The GEO accession no. is GSE79634
(www.ncbi.nlm.nih.gov/geo/query/acc.cgi?token=cvyzseqoflahdmv&acc=GSE79634).
 | Table III.The characteristics of top 10
differently expressed circRNAs and 12 circRNAs associated with
miR-15a and miR-506. |
Table III.
The characteristics of top 10
differently expressed circRNAs and 12 circRNAs associated with
miR-15a and miR-506.
| circRNA | P-value | FDR | FC | Regulation | circRNA type | Chrom | Strand | Gene symbol | miRNA binding
sites |
|---|
|
hsa_circRNA_102051 | 0.00000011389 | 0.00000181 | 78.901931 | Up | Exonic | chr17 | + | TADA2A |
miR-302c-3p/miR-520d-3p |
|
|
|
|
|
|
|
|
|
|
miR-302b-3p/miR-302d-3p/miR-197-5p |
|
has_circRNA_102619 | 0.00000060084 | 0.000006232 | 71.3388052 | Up | Exonic | chr2 | − | NOL10 |
miR-452-5p/miR-874-3p |
|
|
|
|
|
|
|
|
|
|
miR-218-1-3p/miR-767-5p/miR-218-2-3p |
|
hsa_circRNA_104270 | 0.00000010672 | 0.000001743 | 53.0782362 | Up | Exonic | chr6 | + | FAM120B |
miR-450a-1-3p/miR-661 |
|
|
|
|
|
|
|
|
|
|
miR-493-5p/miR-208a-5p/miR-612 |
|
hsa_circRNA_102049 | 0.00000018328 | 0.000002511 | 52.0619239 | Up | Exonic | chr17 | + | TADA2A |
miR-103a-2-5p/miR-455-3p |
|
|
|
|
|
|
|
|
|
|
miR-302c-3p/miR-520d-3p/miR-302-3p |
|
hsa_circRNA_104227 | 0.00001350199 | 0.00006792 | 10.96655 | Up | Exonic | chr6 | + | MTHFD1L |
miR-329-3p/miR-593-5p |
|
|
|
|
|
|
|
|
|
|
miR-641/miR-103a-2-5p/miR-19b-1-5p |
|
hsa_circRNA_000167 | 0.00000001124 | 0.000000389 | 10.010543 | Down | Intragenic | chr14 | − | RPPH1 |
miR-330-5p/miR-328-3p |
|
|
|
|
|
|
|
|
|
|
miR-127-5p/miR-326/miR-1296-5p |
|
hsa_circRNA_103809 | 0.00000741477 | 0.000042676 | 7.6595391 | Down | Exonic | chr5 | − | ZFR |
miR-511-5p/miR-130b-5p |
|
|
|
|
|
|
|
|
|
|
miR-642a-5p/miR-532-3p/miR-329-5p |
|
hsa_circRNA_104700 | 0.00000727569 | 0.000042007 | 7.4515712 | Down | Exonic | chr8 | − | PTK2 |
miR-141-5p/miR-500a-5p |
|
|
|
|
|
|
|
|
|
|
miR509-3p/miR-619-3p/miR578 |
|
hsa_circRNA_001846 | 0.00000006353 | 0.000001136 | 6.2821101 | Down | Intragenic | chr14 | − | RPPH1 |
miR-328-3p/miR-1296-5p |
|
|
|
|
|
|
|
|
|
|
miR-146b-3p/miR-330-5p/miR-608 |
|
hsa_circRNA_102728 | 0.00001617324 | 0.000076928 | 5.7878202 | Down | Exonic | chr2 | − | NBAS |
miR-105-5p/miR-18a-5p |
|
|
|
|
|
|
|
|
|
|
miR-455-3p/miR-624-5p/miR-7-2-3p |
|
hsa_circRNA_103076 | 0.00000004087 | 0.000000904 | 2.6239863 | Up | Exonic | chr20 | − | CSE1L |
miR-330-3p/miR-155-5p |
|
|
|
|
|
|
|
|
|
|
miR-330-5p/miR-146-3p/miR-15a-3p |
|
hsa_circRNA_100435 | 0.00000048773 | 0.000005267 | 2.1392264 | Up | Exonic | chr1 | − | DSTYK |
miR-424-5p/miR-15a-5p |
|
|
|
|
|
|
|
|
|
|
miR-10b-3p/miR-650/miR-15b-5p |
|
hsa_circRNA_103309 | 0.00122306572 | 0.002970302 | 2.127291 | Up | Exonic | chr3 | + | RBMS3 |
miR-330-5p/miR-15a-5p |
|
|
|
|
|
|
|
|
|
|
miR-429/miR-424-5p/miR-200b-3p |
|
hsa_circRNA_000780 | 0.00841633735 | 0.015881187 | 2.1957639 | Down | Intronic | chr10 | − | FAM107B |
miR-651-3p/miR-15a-3p |
|
|
|
|
|
|
|
|
|
|
miR-381-3p/miR-522-3p/miR-300 |
|
hsa_circRNA_101252 | 0.00007537652 | 0.000272424 | 2.3359115 | Down | Exonic | chr13 | − | FOXO1 |
miR-654-3p/miR-15a-3p |
|
|
|
|
|
|
|
|
|
|
miR-762/miR-1301-3p/miR-145-5p |
|
hsa_circRNA_102374 | 0.00258652534 | 0.005756195 | 2.8567112 | Down | Exonic | chr18 | + | WDR7 |
miR-503-5p/miR-15a-5p |
|
|
|
|
|
|
|
|
|
|
miR-16-5p/miR-424-5p/miR-497-5p |
|
hsa_circRNA_104433 | 0.00051721594 | 0.001427983 | 2.0563844 | Down | Exonic | chr7 | + | ARPC1B |
miR-545-3p/miR-15a-5p |
|
|
|
|
|
|
|
|
|
|
miR-636/miR-335-3p/miR-497-5p |
|
hsa_circRNA_104882 | 0.00005024961 | 0.000192181 | 2.3947053 | Down | Exonic | chr9 | + | HSDL2 |
miR-16-5p/miR-15a-5p |
|
|
|
|
|
|
|
|
|
|
miR-135b-5p/miR-424-5p/miR-135a-5p |
|
hsa_circRNA_101717 | 0.0000322857 | 0.00013679 | 2.2591213 | Up | Exonic | chr16 | + | ABCC1 |
let-7e-5p/miR-124-3p |
|
|
|
|
|
|
|
|
|
|
miR-877-5p/miR-506-3p/let-7c-5p |
|
hsa_circRNA_104084 | 0.00028685355 | 0.000860561 | 2.7046096 | Up | Exonic | chr6 | + | LINC00340 |
miR-644a/miR-605-3p |
|
|
|
|
|
|
|
|
|
|
miR-124-3p/miR-506-3p/miR-1224-3p |
|
hsa_circRNA_100646 | 0.00000451139 | 0.000029215 | 3.0830469 | Up | Exonic | chr10 | − | CPEB3 |
miR-34a-5p/miR-499a-5p |
|
|
|
|
|
|
|
|
|
|
miR-146a-5p/miR-506-5p/miR-146b-5p |
|
hsa_circRNA_102213 | 0.0000001515 | 0.000002296 | 2.214269 | Up | Exonic | chr17 | − | USP36 |
miR-873-5p/miR-544a |
|
|
|
|
|
|
|
|
|
|
miR-25-5p/miR-506-5p/miR-152-5p |
Verification for circRNAs
expression
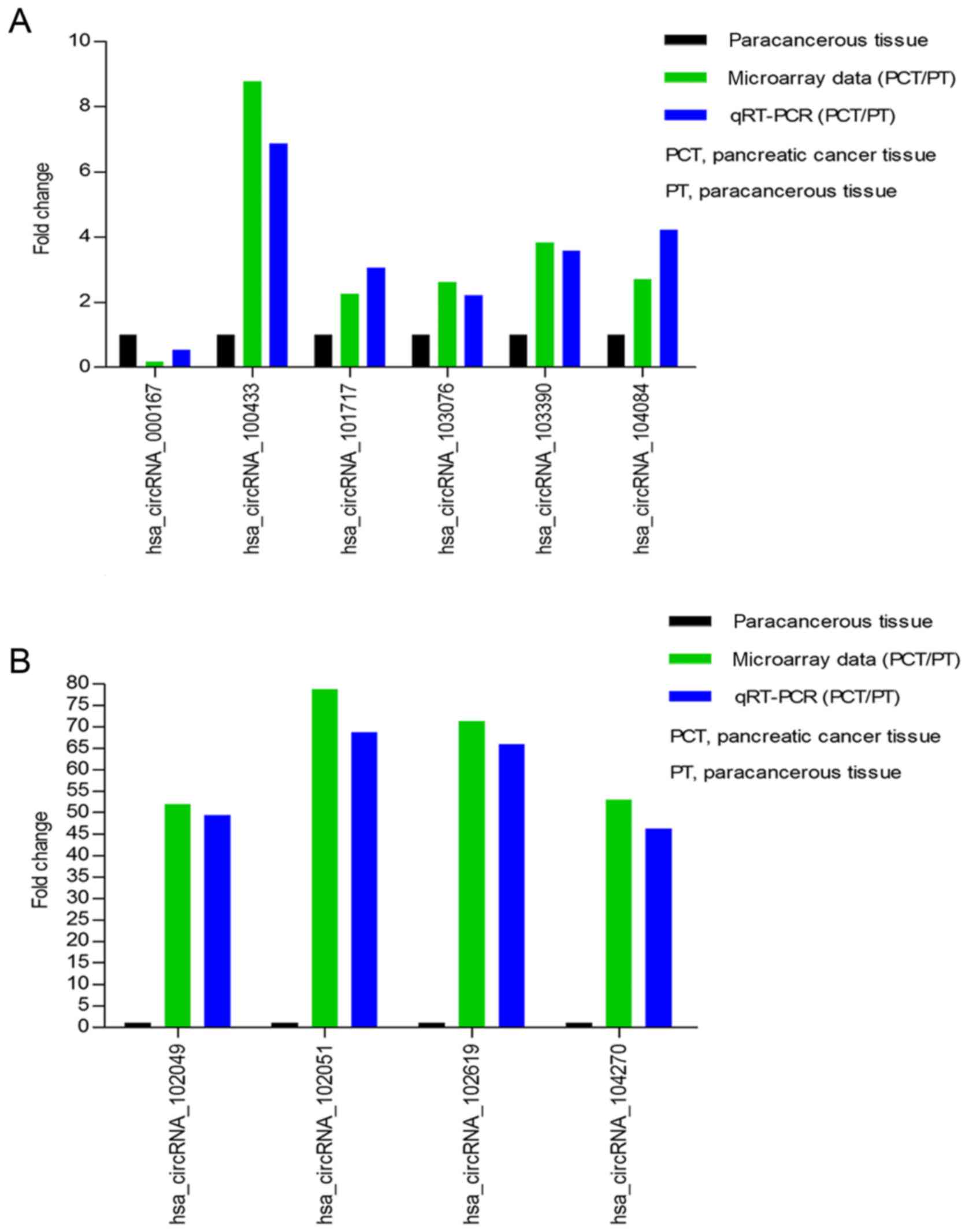
Our circRNAs expression profile revealed that the
expression of circRNAs was significantly different in pancreatic
cancer tissue compared to paracancerous tissue. To validate our
microarray data, we randomly selected 10 differently expressed
circRNAs, including one downregulated circRNA (circRNA_000167) and
nine upregulated circRNAs (circRNA_100433, circRNA_101717,
circRNA_102049, circRNA_102051, circRNA_102619, circRNA_103076,
circRNA_103390, circRNA_104084, circRNA_104270). We respectively
verified their expression levels using qRT-PCR in 10 pancreatic
cancer tissues and corresponding paracancerous tissues. The qRT-PCR
results showed that the expression levels of circRNAs were
significantly different in pancreatic cancer tissue compared with
corresponding paracancerous tissue. The log2 fold-changes were
calculated for microarray data and qRT-PCR results. Our results
illustrated that microarray data was consistent with the qRT-PCR
results upon the expression levels of the ten circRNAs (Fig. 3A and B).
CircRNAs could act as a miRNA sponge to modulate
gene expression in pancreatic cancer. Recent reports demonstrated
that circRNAs played very vital role in regulating gene expression
via miRNAs sponge, and could bind some miRNAs associated with
disease to affect disease initiation and progression (20). For instance, circRNA, ciRS-7,
contains multiple, tandem miRNA-7 binding sites, and regulates
miRNA-7 functions via an endogenous miRNA sponge (21). CiRS-7 as a critical factor probably
is engaged in modulating the function of neuron as well as a
responsible candidate in neurological disorder and tumor
development (12,22). To explore the association between
circRNAs and miRNAs in pancreatic cancer, we predicted
circRNA/miRNA interaction using Arraystar's home-made miRNA target
prediction software based on TargetScan and miRanda (23,24).
The differentially expressed circRNAs within all the comparisons
were annotated in detail with the circRNA/miRNA interaction
information. What's more, combined with our previous studies
(25,26), we specially focused on the
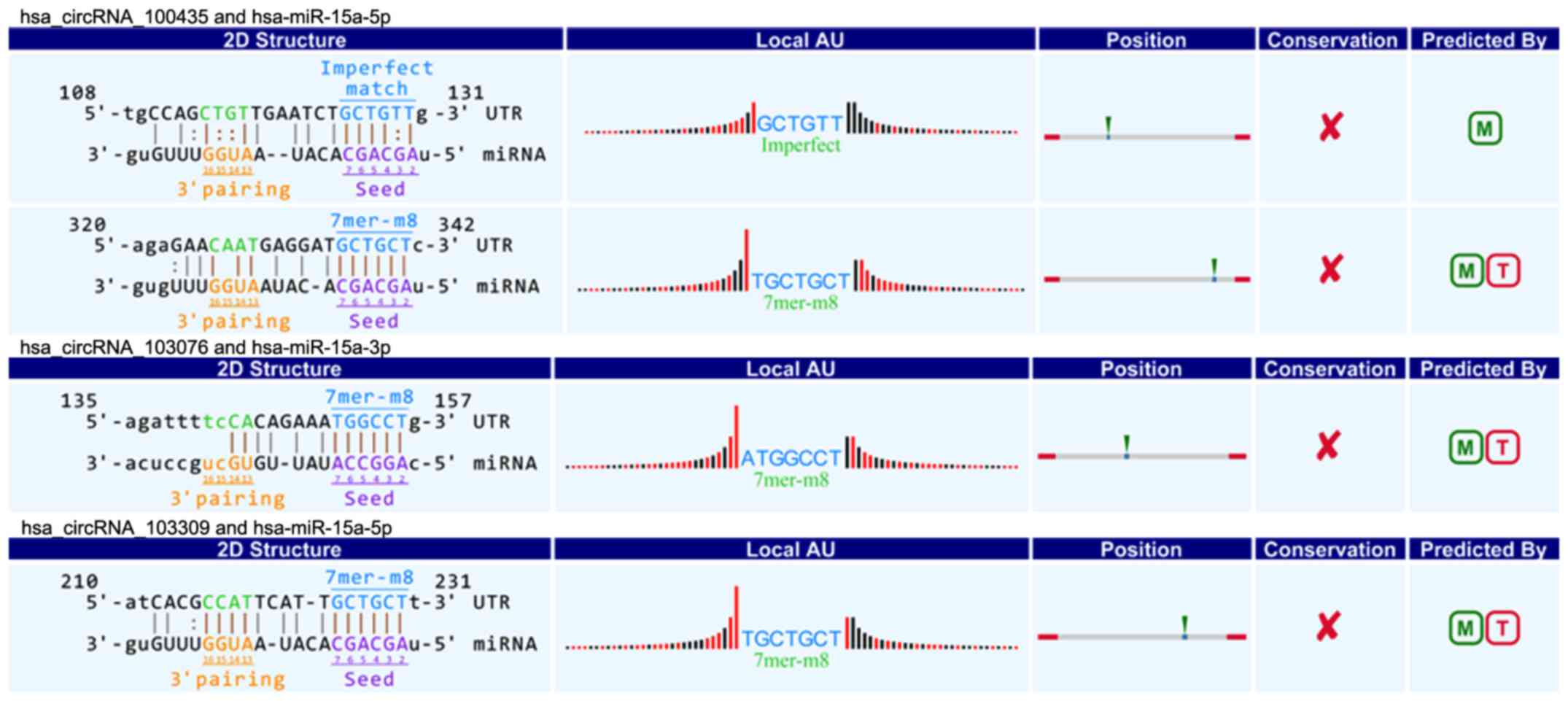
interaction between circRNAs and miR-15a/miR-506. Among our data,
we found that eight circRNAs including three upregulated circRNAs
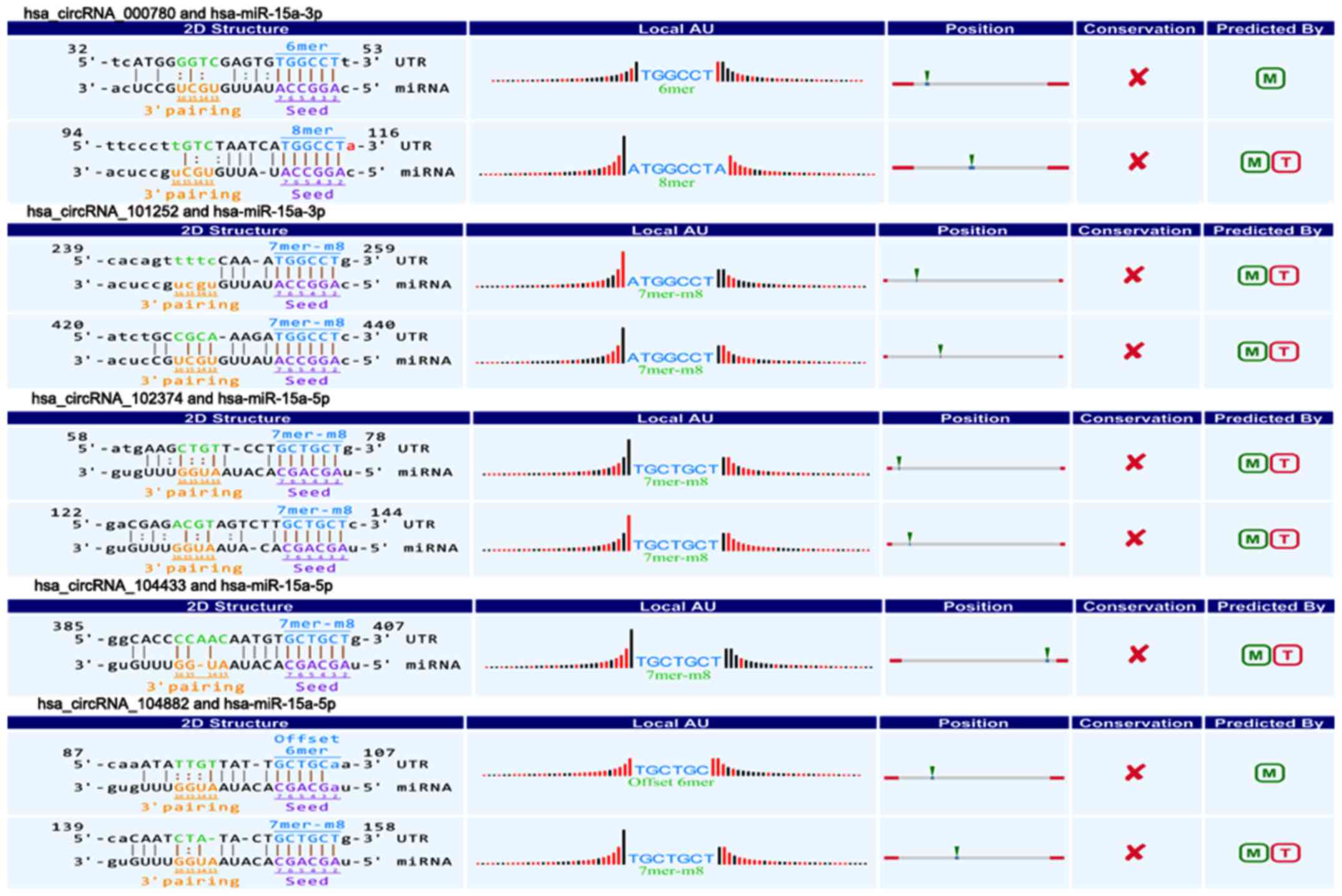
(circRNA_100435, circRNA_103076, circRNA_103309; Fig. 4) and five downregulated circRNAs
(circRNA_000780, circRNA_101252, circRNA_102374, circRNA_104433,
circRNA_104882; Fig. 5) were
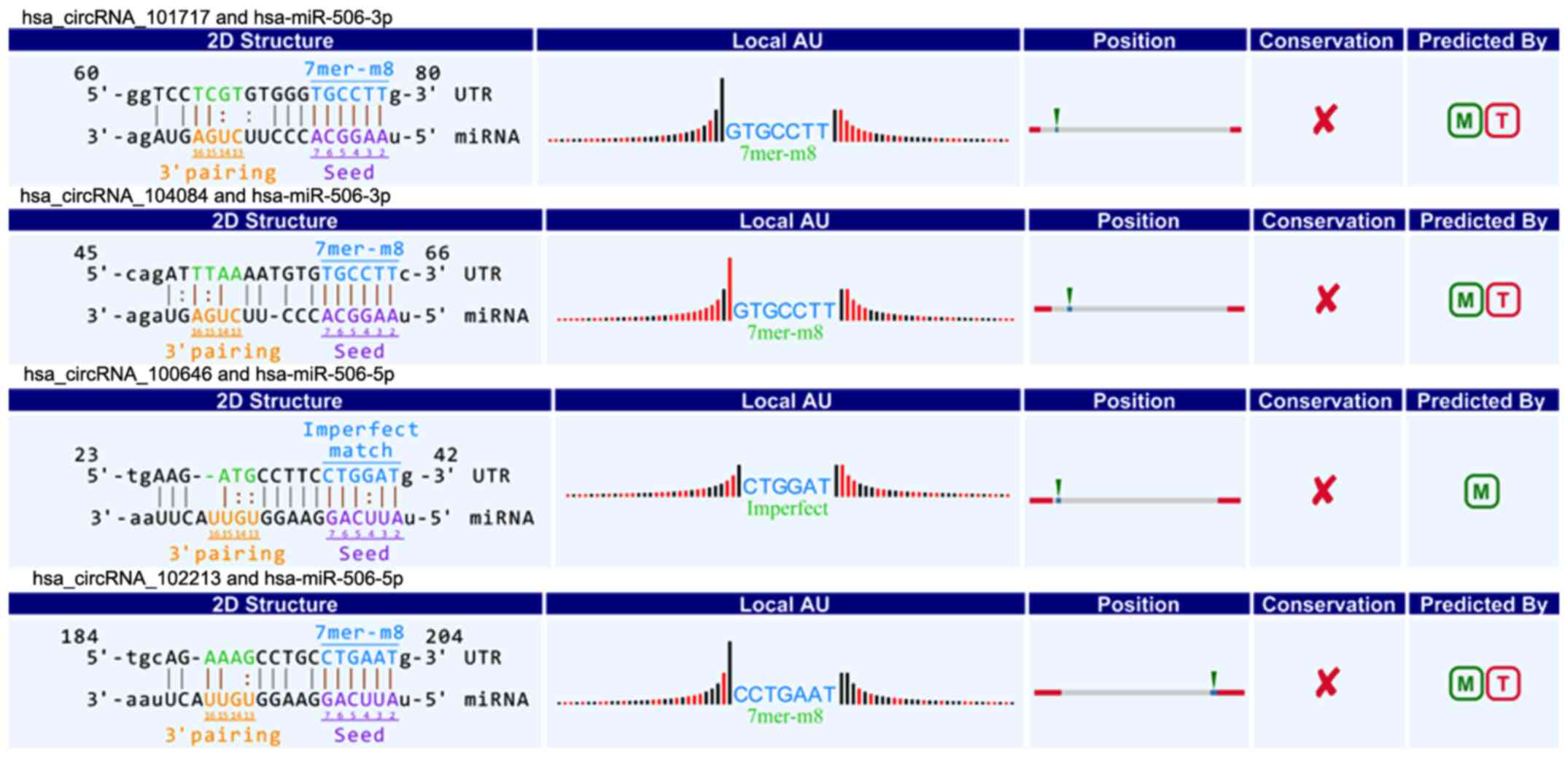
respectively related with miR-15a. Four upregulated circRNAs
(circRNA_101717, circRNA_104084, circRNA_100646, circRNA_102213)
were associated with miR-506 (Fig.
6). The above circRNAs respectively had complementary sequence
with miR-15a/miR-506 and diverse binding sites at the seed region,
while the characteristics and differences were shown in Table III. It indicated that these
circRNAs could modulate miR-15a/miR-506 expression via miRNA sponge
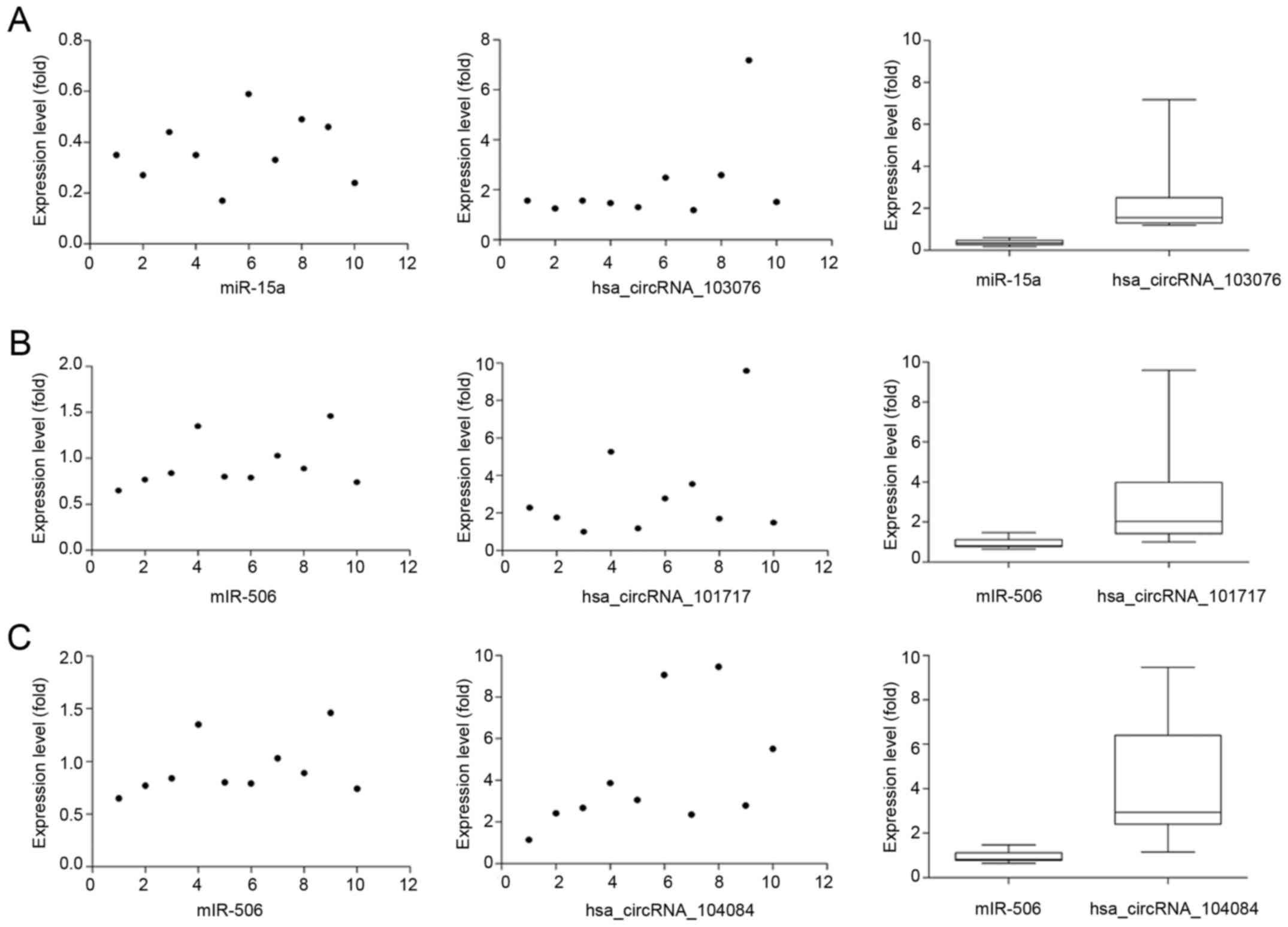
in pancreatic cancer. To verify our predicted result, we selected
three differently expressed circRNAs interacted with miR-15a and
miR-506 from our prediction result, and then respectively performed
qRT-PCR to quantify miR-15a and miR-506 expression levels in
pancreatic cancer tissues showing altered expression of circRNAs.
Our result showed that the above three circRNAs respesctively were
negatively correlated with miR-15a and miR-506 (Fig. 7). Taken together, we speculated
that circRNAs could regulate gene expression via miRNA sponge and
play very significant role in the progression of pancreatic
cancer.
Discussion
In recent years, the relationship has been
extensively explored between non-coding RNA and cancer, such as
miRNAs, lncRNAs and circRNAs. Emerging studies have clarified that
non-coding RNA plays very significant role in the carcinogenesis
and cancer progression (27–29).
However, the role of circRNAs and molecular mechanism remain to be
investigated in pancreatic cancer. With the development of
biotechnology (e.g., high throughput RNA sequencing and
bioinformatics), accumulating evidence showed that circRNAs as a
novel type of endogenous non-coding RNA were an abundant, stable
and conserved class of RNA molecules (7–10).
What is more, recent studies revealed that circRNAs may regulate
diverse biological processes and be engaged in multiple diseases,
especially malignant tumors including hepatocellular carcinoma,
colorectal cancer, gastric cancer, non-small cell lung cancer
(14–19). Therefore, these findings indicate
that circRNAs may play very important role in carcinogenesis and
cancer progression.
In this study, we systematically explored the
expression profile of circRNAs in 20 pancreatic cancer tissues and
corresponding paracancerous tissues. Our results indicated that
circRNAs expression profile was significantly different between
pancreatic cancer tissue and paracancerous tissue. The expression
profile showed that 289 circRNAs expression were aberrantly
expressed in pancreatic cancer compared to paracancerous tissue.
circRNAs (161) were downregulated and 128 circRNAs were
upregulated. We also indentified the circRNAs type, chromosome
location, gene symbol, and sequence. In addition, we confirmed that
our microarray data was consistent with qRT-PCR result,
illustrating that our microarray data was available.
Recently, some studies have demonstrated that
circRNAs can play very crucial role in biological process and
progression of disease via miRNA sponge (20,21).
Furthermore, our previous studies have confirmed that miR-15a can
inhibit pancreatic cancer cell proliferation and EMT by
downregulated BMI-1, and miR-506 can suppress the progression and
chemoresistance (25,26). To identify whether there was the
interaction between circRNAs and miR-15a/miR-506, we performed
Arraystar's home-made miRNA target prediction based on TargetScan
and miRanda, and differentially expressed circRNAs were annotated
in detail with circRNA/miRNA interaction information. We found that
multiple circRNAs had complementary sequence with miR-15a/miR-506
and diverse miRNA binding sites, and our qRT-PCR result also
validated this point. Altogether, we speculated that circRNAs could
interact with miR-15a/miR-506 via miRNA sponge to modulate the
progression of pancreatic cancer. However, we should perform
further in-depth functional studies to indicate whether circRNAs
acting as miRNA sponge play very important role in progression of
pancreatic cancer in future.
In conclusion, we demonstrated that circRNAs
expression profile was significantly different in pancreatic cancer
tissue compared to paracancerous tissue. We also validated that
dysregulated circRNAs expression in pancreatic cancer tissue using
qRT-PCR, which was consistent with our microarray data.
Additionally, combined with our previous studies, we successfully
predicted that multiple circRNAs had complementary sequence with
miR-15a/miR-506 and diverse miRNA binding sites at the seed region.
We speculated that circRNAs may play very crucial role in
modulating the progression of pancreatic cancer via miRNA sponge,
and then we validated this point using qRT-PCR. Therefore, these
findings may provide more potential biomarkers and new treatment
strategies for pancreatic cancer. However, in future, we need to
perform further studies to explore circRNAs' function and molecular
mechanism in pancreatic cancer.
Acknowledgements
The present study was supported by grants from
National Natural Science Foundation of China (grant no. 81502550),
National Natural Science Foundation of China (grant no. 81672382)
and Southwest Hospital Research Project (grant no.
SWH2016JCYB-45).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vincent A, Herman J, Schulick R, Hruban RH
and Goggins M: Pancreatic cancer. Lancet. 378:607–620. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen W, Zheng R, Zeng H and Zhang S: The
incidence and mortality of major cancers in China, 2012. Chin J
Cancer. 35:732016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hartwig W, Werner J, Jäger D, Debus J and
Büchler MW: Improvement of surgical results for pancreatic cancer.
Lancet Oncol. 14:e476–e485. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Garrido-Laguna I and Hidalgo M: Pancreatic
cancer: From state-of-the-art treatments to promising novel
therapies. Nat Rev Clin Oncol. 12:319–334. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sanger HL, Klotz G, Riesner D, Gross HJ
and Kleinschmidt AK: Viroids are single-stranded covalently closed
circular RNA molecules existing as highly base-paired rod-like
structures. Proc Natl Acad Sci USA. 73:3852–3856. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Salzman J, Gawad C, Wang PL, Lacayo N and
Brown PO: Circular RNAs are the predominant transcript isoform from
hundreds of human genes in diverse cell types. PLoS One.
7:e307332012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Salzman J, Chen RE, Olsen MN, Wang PL and
Brown PO: Cell-type specific features of circular RNA expression.
PLoS Genet. 9:e10037772013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jeck WR, Sorrentino JA, Wang K, Slevin MK,
Burd CE, Liu J, Marzluff WF and Sharpless NE: CircularRNAs are
abundant, conserved and associated with ALU repeats. RNA.
19:141–157. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Memczak S, Jens M, Elefsinioti A, Torti F,
Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer
M, et al: Circular RNAs are a large class of animal RNAs with
regulatory potency. Nature. 495:333–338. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
You X, Vlatkovic I, Babic A, Will T,
Epstein I, Tushev G, Akbalik G, Wang M, Glock C, Quedenau C, et al:
Neural circular RNAs are derived from synaptic genes and regulated
by development and plasticity. Nat Neurosci. 18:603–610. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hansen TB, Kjems J and Damgaard CK:
Circular RNA and miR-7 in cancer. Cancer Res. 73:5609–5612. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Boeckel JN, Jaé N, Heumüller AW, Chen W,
Boon RA, Stellos K, Zeiher AM, John D, Uchida S and Dimmeler S:
Identification and characterization of hypoxia-regulated
endothelial circular RNA. Circ Res. 117:884–890. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang Y, Zhang XO, Chen T, Xiang JF, Yin
QF, Xing YH, Zhu S, Yang L and Chen LL: Circular intronic long
noncoding RNAs. Mol Cell. 51:792–806. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li Z, Huang C, Bao C, Chen L, Lin M, Wang
X, Zhong G, Yu B, Hu W and Dai L: Exon-intron circular RNAs
regulate transcription in the nucleus. Nat Struct Mol Biol.
22:256–264. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bachmayr-Heyda A, Reiner AT, Auer K,
Sukhbaatar N, Aust S, Bcahleitner-Hofmann T, Mesteri I, Grunt TW,
Zeillinger R and Pils D: Correlation of circular RNA abundance with
proliferation-exemplified with colorectal and ovarian cancer,
idiopathic lung fibrosis and normal human tissues. Sci Rep.
5:80572015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Westholm JO, Miura P, Olson S, Shenker S,
Joseph B, Sanfilippo P, Celniker SE, Graveley BR and Lai EC:
Genome-wide analysis of Drosophila circular RNAs reveals
their structural and sequence properties and age-dependent neural
accumulation. Cell Rep. 9:1966–1980. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bahn JH, Zhang Q, Li F, Chan TM, Lin X,
Kim Y, Wong DT and Xiao X: The landscape of microRNA,
Piwi-interacting RNA and circular RNA in human saliva. Clin Chem.
61:221–230. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li P, Chen S, Chen H, Mo X, Li T, Shao Y,
Xiao B and Guo J: Using circular RNA as a novel type of biomarker
in the screening of gastric cancer. Clin Chim Acta. 444:132–136.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ghosal S, Das S, Sen R, Basak P and
Chakrabarti J: Circ2Traits: A comprehensive database for circular
RNA potentially associated with disease and traits. Front Genet.
4:2832013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lukiw WJ: Circular RNA (circRNA) in
Alzheimer's disease (AD). Front Genet. 4:3072013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hansen TB, Jensen TI, Clausen BH, Bramsen
JB, Finsen B, Damgaard CK and Kjems J: Natural RNA circles function
as efficient microRNA sponges. Nature. 495:384–388. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Enright AJ, John B, Gaul U, TuschI T,
Sander C and Marks DS: MicroRNA targets in Drosophila.
Genome Biol. 5:R12003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pasquinelli AE: MicroRNAs and their
targets: Recognition, regulation and an emerging reciprocal
relationship. Nat Rev Genet. 13:271–282. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Guo S, Xu X, Tang Y, Zhang C, Li J, Ouyang
Y, Ju J, Bie P and Wang H: miR-15a inhibits cell proliferation and
epithelial to mesenchymal transition in pancreatic cancer by
down-regulating Bmi-1 expression. Cancer Lett. 344:40–46. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li J, Wu H, Li W, Yin L, Guo S, Xu X,
Ouyang Y, Zhao Z, Liu S, Tian Y, et al: Downregulated miR-506
expression facilitates pancreatic cancer progression and
chemoresistance via SPHK1/Akt/NF-κB signaling. Oncogene.
35:5501–5514. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xiong G, Feng M, Yang G, Zheng S, Song X,
Cao Z, You L, Zheng L, Hu Y, Zhang T and Zhao Y: The underlying
mechanisms of non-coding RNAs in the chemoresistance of pancreatic
cancer. Cancer Lett. 397:94–102. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu YF, Hannafon BN and Ding WQ: microRNA
regulation of human pancreatic cancer stem cells. Stem Cell
Investig. 4:52017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gupta Chandra S and Tripathi Nandan Y:
Potential of long non-coding RNAs in cancer patients: From
biomarkers to therapeutic targets. Int J Cancer. 140:1955–1967.
2017. View Article : Google Scholar : PubMed/NCBI
|