Introduction
Cholangiocarcinoma (CC) is a malignant tumor
originating from biliary epithelia within intrahepatic and
extrahepatic tracts (1,2). Although the incidence of CC is rare
globally, its prevalence and mortality rate is growing annually
(2). The challenges put forward by
this cancer are hardly diagnosed early for its ease of migration
and perineural invasion (3). The
only present treatment for long-term survival for patients with CC
is surgical resection (4,5). However, the recurrence rate of this
cancer following surgery is high and current chemotherapy practices
have not been able to increase the survival rate of this cancer
(4,6). Alterations in apoptotic thresholds
induced by a chronic inflammatory state are an important indicator
during the development of CC (7).
Primary sclerosing cholangitis and primary biliary
cirrhosis are the important risk factors for CC (8–10).
The inflammatory environment in these conditions leads to further
cell damage, inducing dysregulation of apoptosis (10).
Recently, regarding the multivariate and multi-stage
processes of cancer, interfering with or inhibiting one or several
factors leading to these processes is an effective method of
preventing, or providing therapy for cancer (11). However, in order to inhibit these
processes, effective molecular targets are required. According to
epidemiological investigations, nuclear factor kappa B (NF-κB) is a
widely distributed and important transcription factor participating
in various biological processes, including immune responses,
inflammatory reactions, apoptosis, tumorigenesis and tumor
proliferation (12,13). Activation of NF-κB-mediated gene
transcription, including viral proteins, cell mitogens and tumor
necrosis factor-α, is stimulated by a variety of factors (14). The majority of the cytoplasmic form
of NF-κB was associated with the members of the inhibitor of NF-κB
family, including NF-κB inhibitor α (IκBα). NF-κB is released when
IκBα is phosphorylated by cellular stimulation, and is translocated
to the nucleus where it stimulates the transcription of genes that
have an NF-κB binding site (14,15).
It is well known that IκB kinases (IKK), RAC-α
serine/threonine-protein kinase (AKT), mitogen-activated protein
kinase and casein kinase II are regulated by
serine/threonine-protein phosphatase 2A (PP2A), a serine-threonine
phosphatase family member involved in the cell cycle, metabolism,
cell growth, transcription, translation and apoptosis (16,17).
PP2A holoenzymes consist of a structural subunit A, a regulatory
subunit B and a catalytic subunit C (17). Previous investigations demonstrated
that cantharidin (CAN), derived from cantharis, which has been
widely used in traditional Chinese medicine, is a strong inhibitor
of PP2A (18,19). CAN and its derivatives have a
marked effect on the inhibition of various types of cancer,
including gallbladder carcinoma, bladder cancer, leukemia and
hepatoma (17,18). Clinical applications have indicated
that CAN exhibits unique efficacy in cancer treatment, particularly
for advanced liver cancer, by increasing leukocyte numbers
(20).
The majority of cases of CC are diagnosed at an
advanced stage (21). Therefore,
CAN may be used for the treatment of CC due to its specific
efficacy in advanced cancer, and it is necessary to investigate its
molecular mechanisms. In addition, a previous study revealed that
the inhibitory effects of CAN in cancer may involve the inhibition
of cellular migration and invasion (22). The aim of the present study was to
examine the precise molecular mechanisms inducing the inhibitory
effect of CAN on the migration and invasion of a CC cell line. The
present study suggested that CAN may inhibit cellular migration and
invasion in QBC939 cells by stimulating the IKKα/IκBα/NF-κB
signaling pathway, and by regulating the expression of proteins
associated with cellular migration and invasion, including
metalloproteinase inhibitors (TIMPs) and matrix metalloproteinases
(MMPs).
Materials and methods
Cell culture
Human cholangiocarcinoma cell lines QBC939 and
Hucc-t1, as well as human intrahepatic biliary epithelial cells
(HiBECs) were obtained from the Cell Bank of the Type Culture
Collection of the Chinese Academy of Sciences (Shanghai, China) and
ScienCell Research Laboratories, Inc. (San Diego, CA, USA),
respectively. QBC939, Hucc-t1 and HiBECs were cultured in RPMI-1640
medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
containing 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.) and antibiotics, at 37°C under a humidified
atmosphere with 5% CO2 and 95% air.
Cell viability assay
QBC939 cells and HiBECs were incubated in CAN
(Biomol; Enzo Life Sciences, Inc., Farmingdale, NY, USA) at 3, 6,
10, 20 and 40 µM, as well as the p65 inhibitor caffeic acid
phenethyl ester (CAPE; 1 µM), the PP2A inhibitor okadaic acid (OA;
1 nM) or the PP2A activator D-erythro-sphingosine (DES; 10 nM) for
12, 24, 48 and 72 h. Thereafter the cytotoxic or anti-proliferative
effect of CAN was investigated using a MTT assay (Roche Applied
Science, Penzberg, Germany), according to the manufacturer's
protocol. The precipitated formazan was dissolved in 150 µl
dimethyl sulfoxide and the wavelength used to measure the formazan
was 570 nm.
PP2A activity assay
QBC939 cells (1×104) and HiBECs
(1×104) were incubated with CAN at 6 and 10 µM for 6,
12, 24, 48 h, followed by treatment with the PP2A inhibitor okadaic
acid (OA; 1 nM) or the PP2A activator D-erythro-sphingosine (DES;
10 nM) for 24 h at 37°C. The activity of PP2A was subsequently
measured with a nonradioactive serine/threonine-phosphatase assay
kit (Promega Corporation, Madison, WI, USA), according to the
manufacturer's protocol. Cell lysate was removed by a Sephadex G-25
column twice and was seeded on a 96-well plate, the reaction
substrate of PP2A, RRA(pT)VA, was added (23), and was incubated with molybdate dye
at 25°C for 30 min. Plates were read using a SpectraMax®
M5/M5e (Molecular Devices LLC, Sunnyvale, CA, USA) at 630 nm. The
relative activity of PP2A was expressed as the percentage of
QBC939- and HiBEC-positive cells.
Cell experiments
QBC939 cells grown to 90% confluency were incubated
with CAN at 0 (control), 2, 6 and 10 µM for 24 h, and subsequently
harvested for the following assays.
Reactive oxygen species (ROS)
assay
Cells (1×105) incubated with CAN were
collected and washed with PBS. Subsequently, cells were incubated
in a solution containing 10 µM dichloro-dihydro-fluorescein
diacetate (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at 37°C
for 1 h. Cells were washed three times. ROS production was
determined by flow cytometry by measuring the alterations in
fluorescence (emission, 510 nm; excitation, 488 nm).
Transwell cell migration assay
In a sterile environment, cells were detached from
the plate using 0.25% trypsin-EDTA solution, harvested following
centrifugation at 500 × g for 5 min at 4°C, and washed twice to
remove the serum. Cells resuspended with Dulbecco's modified
Eagle's medium (DMEM, Gibco; Thermo Fisher Scientific, Inc.) were
adjusted to 1×106 cells/ml, and 300 µl cell solution was
added to the top chamber of the Transwell (BD Biosciences, Franklin
Lakes, NJ, USA) and placed on a 24-well plate for 2 h at 37°C.
Finally, 500 µl DMEM containing 2.5% FBS was added to the bottom
chamber of the Transwell and incubated for 24 h at 37°C. Following
incubation, the migratory cells were fixed and stained with 0.1%
crystal violet dye for 20 min at room temperature. Following
dissolving in 200 µl 33% acetic acid, the absorbance value at 570
nm was read with the Spectra Max® M5/M5e. The migration
rate was calculated as a percentage of the control.
Transwell cell invasion assay
Cells were harvested as previously described,
resuspended with DMEM and adjusted to 1×106 cells/ml.
Cell migration was determined using the ECM554 invasion kit
(Chemicon; EMD Millipore, Billerica, MA, USA), according to the
manufacturer's protocol. The top chamber of the Transwell was
placed on a 24 well plate and 300 µl serum free medium was added,
and DMEM medium containing 2.5% FBS was added into the bottom
chamber. Following incubation for 10 min at 37°C, 250 µl DMEM in
the top chamber was replaced with 250 µl cell solution and
incubated for 24 h at 37°C. The invaded cells were stained using
0.1% crystal violet dye for 20 min at room temperature. The number
of invaded cells was counted under an inverted microscope at a
magnification of ×200. The invasion rate was expressed as a
percentage of the control.
Western blot assay
QBC939 cells were incubated in CAN at 3, 6, 10, 20
and 40 µM, as well as CAPE (1 µM), OA (1 nM) for 12, 24, 48 and 72
h. Following this, cells were harvested and washed with ice-cold
PBS, and solubilized in lysis buffer containing protease inhibitors
(Sigma-Aldrich; Merck KGaA). Total protein was extracted and
measured using a bicinchoninic acid protein assay kit (Pierce;
Thermo Fisher Scientific, Inc.), following the manufacturer's
protocol. Nucleoprotein was extracted using NE-PER Nuclear and
Cytoplasmic Extraction reagent (Pierce; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. Protein (50
µg/lane) was separated by 10% SDS-PAGE and subsequently transferred
to polyvinylidene fluoride membranes (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Following blocking in 5% non-fat dry milk for 1
h at room temperature, the membranes were incubated with primary
antibodies at 4°C overnight. The protein level was measured using a
horseradish peroxidase-conjugated secondary antibody (cat no.
P0162; 1:100; Dako, Agilent Technologies, Inc., Santa Clara, CA,
USA) for 1 h at room temperature. Blots were developed using an
enhanced chemiluminescence detection kit from Amersham (GE
Healthcare, Chicago, IL, USA). The primary antibodies were as
follows: NF-κB/p65 (cat no. D14E12; 1:1,000), phosphorylated
(p-)IKKα (cat no. C84E11; 1:1,000), IKKα (cat no. 3G12; 1:1,000),
p-IκBα (cat no. 5A5; 1:1,000), IκBα (cat no. L35A5; 1:1,000),
β-actin (cat no. D6A8; 1:1,000), PP2A (cat no. 52F8; 1:1,000),
p-p65 (cat no. 93H1; 1:1,000), MMP-9 (cat no. D6O3H; 1:1,000),
MMP-2 (cat no. D4M2N; 1:1,000), TIMP-1 (cat no. D10E6; 1:1,000),
TIMP-2 (cat no. D18B7; 1:1,000) and Lamin B (cat no. D9V6H;
1:1,000; all CST Biological Reagents Co., Ltd., Shanghai, China).
β-actin was used as the internal control.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) assay
Total RNA was isolated from cells using a
commercially available RNeasy mini-kit (Qiagen, Inc., Valencia, CA,
USA), according to the manufacturer's protocol. Total RNA (1 µg)
was reverse transcribed using an NCode VILO miRNA cDNA Synthesis
kit (Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. The primer sequences for RT-qPCR were
purchased from Integrated DNA Technologies, Inc. (Coralville, IA,
USA). Quantification of relative mRNA was performed using iQ
SYBR-Green Supermix on the iCycler iQ thermal cycler (Bio-Rad
Laboratories, Inc.). The thermocycling conditions for qPCR were as
follows: 45°C for 10 min, 95°C for 10 min, followed by 50 cycles of
95°C for 15 sec and 60°C for 45 sec. The amplification products of
the PCR were verified by melting curve analysis. Results were
calculated using the ΔΔCq method (24). Relative expression of mRNA was
normalized to β-actin expression levels. Primers were as follows:
MMP-2 forward, 5′-GGCCGTGTTTGCCATCTGTT-3′ and reverse,
5′-TGCAGGGAGCAGAGATTCGG-3′; MMP-9 forward,
5′-CCAGTCCACCCTTGTGCTCT-3′ and reverse, 5′-CTCTCCACGCATCTCTGCCA-3′;
TIMP-2 forward, 5′-CGACTGGTCCAGCTCTGACA-3′ and reverse,
5′-TGGCAGAGGGAGGATGGGAT-3′; β-actin forward,
5′-GGCACTCTTCCAGCCTTCCT-3′ and reverse,
5′-GCACTGTGTTGGCGTACAGG-3′.
Dual-luciferase reporter gene
assay
The cells were seeded into 96-well plates at 70%
confluence. After 16 h, cells were transfected with NF-κB
luciferase reporter plasmid (Promega Corporation) and
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). Following incubation for 48 h at 37°C, cells
were collected to measure the luciferase activity with the
Dual-Luciferase Reporter Assay System kit (Promega Corporation).
The transcriptional activities of genes were expressed as the ratio
between firefly luciferase and Renilla luciferase.
Statistical analysis
Data are expressed as the mean ± standard deviation.
Results were analyzed by two-tailed and unpaired Student's t-test.
Multiple comparisons between three or more groups were performed
using one-way analysis of variance, followed by Tukey's post-hoc
test. SPSS 22.0 software (IBM Corp., Armonk, NY, USA) was used to
perform the statistical analysis. P<0.05 was considered to
indicate a statistically significant difference.
Results
CAN inhibits the viability of human
cholangiocarcinoma cell lines
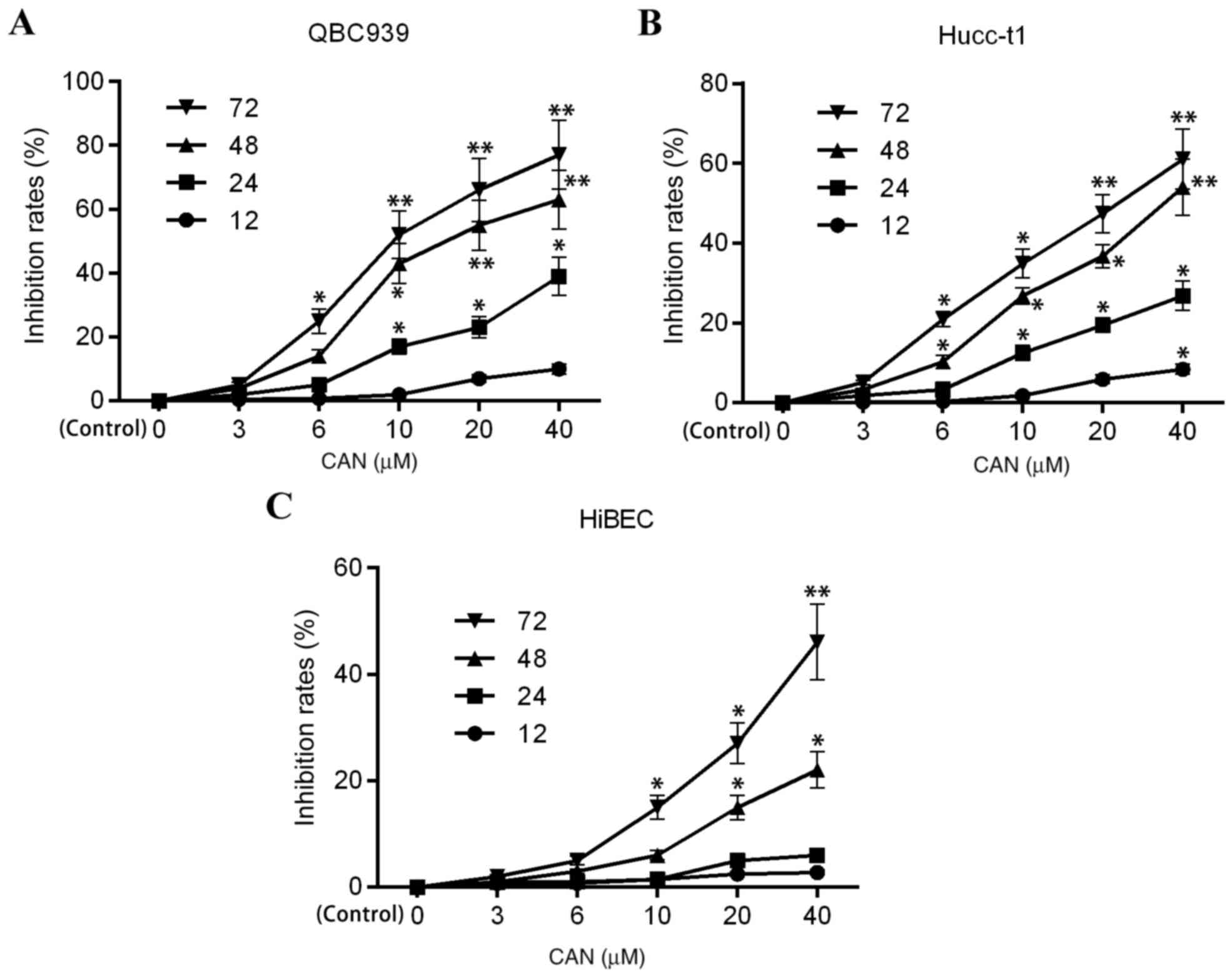
Fig. 1 illustrates
the cell viability of CAN-treated human cholangiocarcinoma QBC939,
Hucc-t1 and HiBECs, as demonstrated by MTT assay. The profiles of
the inhibition rates of QBC939, Hucc-t1 and HiBECs following
treatment with CAN were dose- and time-dependent. However, the
inhibition rates in QBC939 and Hucc-t1 cells following treatment
with CAN were significant increased compared with HiBECs, which
indicated increased cytotoxicity of CAN inhuman cholangiocarcinoma
cell lines compared with the normal HiBECs.
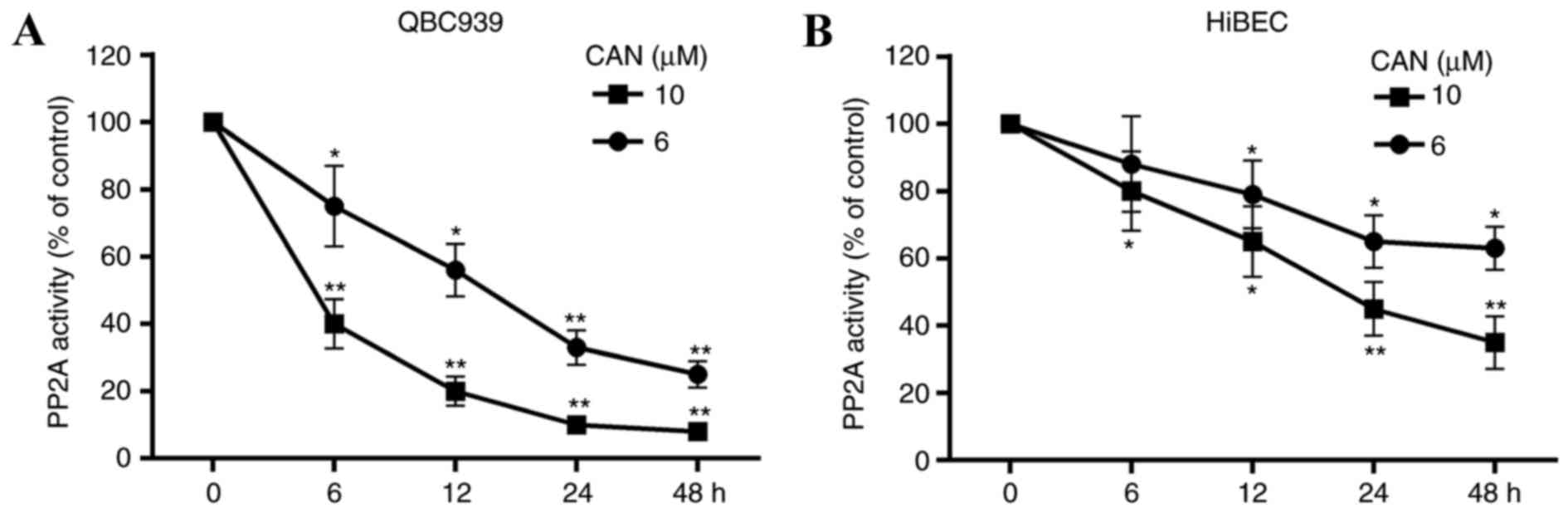
CAN inhibits the activity of PP2A
The effect of treatment with CAN (6 or 10 µM for 6,
12, 24 and 48 h) on the activity of PP2A in cells was determined.
As presented in Fig. 2, PP2A
activity was decreased in QBC939 cells and HiBECs following
treatment with CAN. Compared with the HiBECs, increased inhibition
of PP2A activity by CAN in QBC939 cells was observed. Additionally,
PP2A activity was significantly inhibited in HiBECs with CAN
treatment at 10 mM for 24 and 48 h (Fig. 2B).
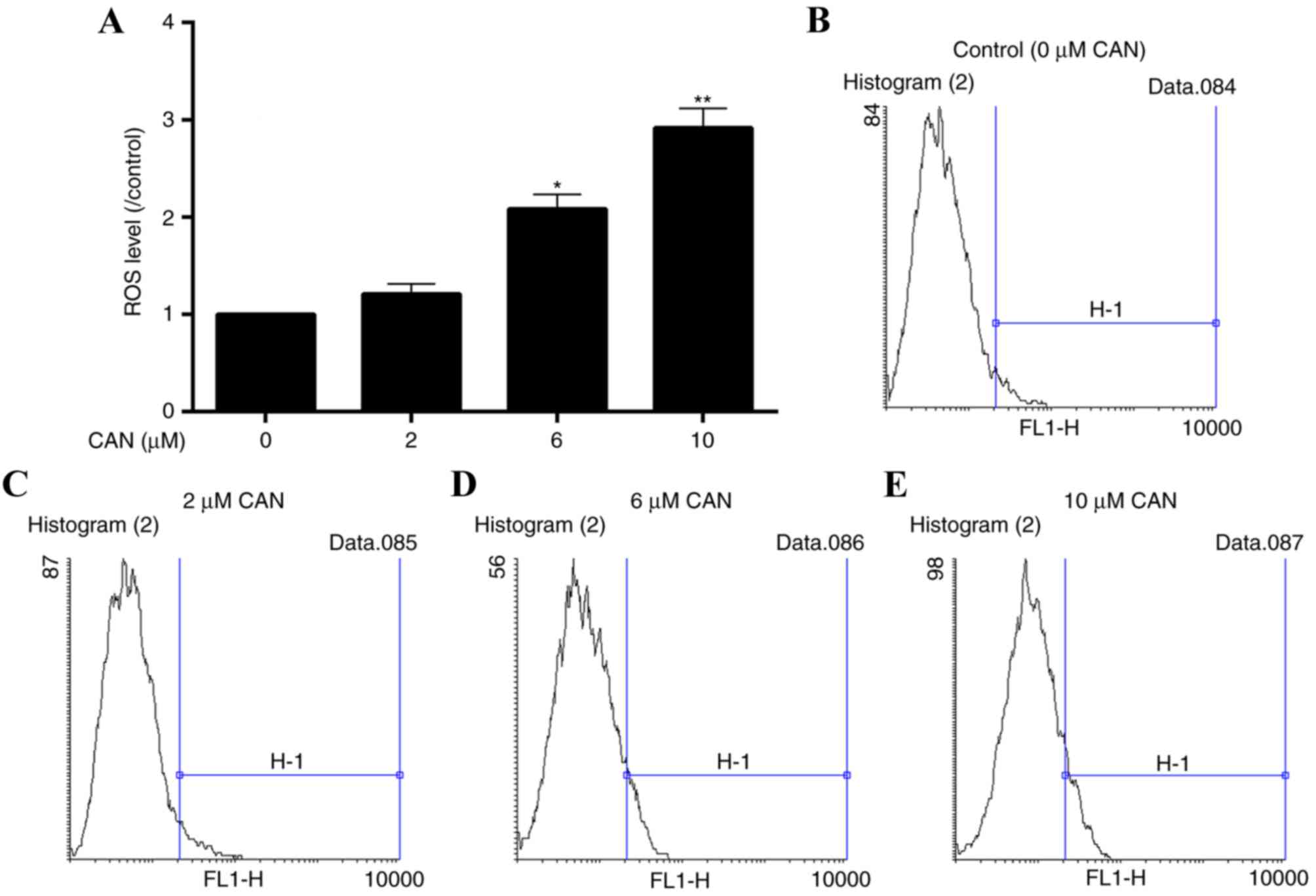
ROS levels in QBC939 are significantly
increased following treatment with CAN for 24 h
To assess the oxidative damage in QBC939 cells
induced by CAN, alterations in ROS levels in cells were determined.
Following incubation with CAN at 0, 2, 6 and 10 µM for 24 h, ROS
levels were elevated at all levels of treatment, compared with the
control (0 µM; Fig. 3).
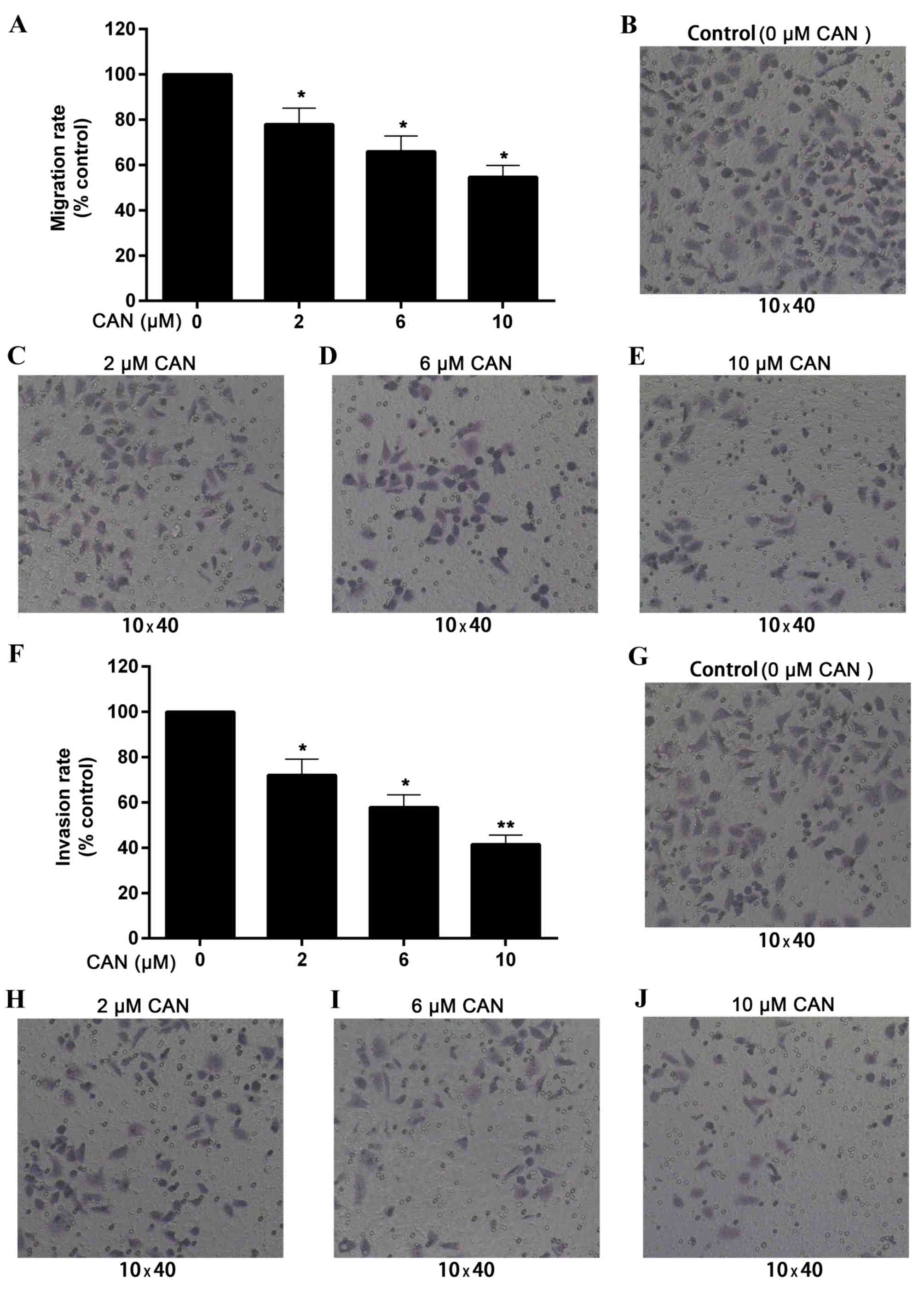
CAN effectively inhibits the migration
and invasion of QBC939
Transwell cell migration and invasion assays were
performed to assess the effect of CAN on the growth of QBC939. As
presented in Fig. 4A-E, QBC939
migration was reduced by CAN at 2, 6 and 10 µM for 24 h, compared
with the control (without treatment). The migration rate of QBC939
cells was decreased to 54.7% of that of the control following
treatment with 10 µM for 24 h.
Similarly, the invasion rate was significantly
reduced by treatment with CAN for 24 h and appeared to be
dose-dependent. As presented in Fig.
4F-J, the relative invasion rates in cells following treatment
with 2, 6 and 10 µM CAN for 24 h were 72.1, 57.8 and 41.5%,
respectively, compared with the control.
CAN-induced cell inhibition is
partially dependent on the IKKα/IκBα/NF-κB signaling pathway
PP2A may deactivate IKKα by dephosphorylation of its
active site, while IKKα is involved in the stimulation of the NF-κB
pathway (25). Therefore, the
activities of genes involved in the NF-κB signaling pathway in
QBC939 cells was determined. As presented in Fig. 5A, p-IKKα levels were increased
following treatment of cells with 2, 6, 10 µM CAN for 24 h,
compared with the control. However, inhibition of PP2A by CAN
exerted no apparent effects on the total IKKα levels. The
expression levels of p-IκBα, the target protein of p-IKKα, were
significantly elevated, and total IκBα levels were decreased when
PP2A was inhibited by CAN, compared with the control (Fig. 5B). The levels of p65 in the nucleus
was further determined. As illustrated in Fig. 5C, compared with the control, p65
levels were significantly increased in the nucleus following
treatment with CAN. The phosphorylation of p65 and total p65 levels
in cells treated with CAN (6 µM) was determined, and it was
demonstrated that CAN increased the expression levels of p-P65
protein and had no marked impact on the expression of total p65
protein in cells (Fig. 5D). The
dual-luciferase reporter gene assay revealed that CAN exerted light
stimulation of the transcriptional activity of NF-κB (p65; Fig. 5E). To test whether CAN-induced cell
inhibition is dependent on, partially dependent on or independent
of the NF-κB pathway, the effect of CAPE, a specific inhibitor of
p65, on nuclear p65 was further measured, and it was demonstrated
that CAPE reversed CAN-induced nuclear translocation of p65
(Fig. 5F) and partially weakened
the cytotoxicity of CAN (Fig.
5G).
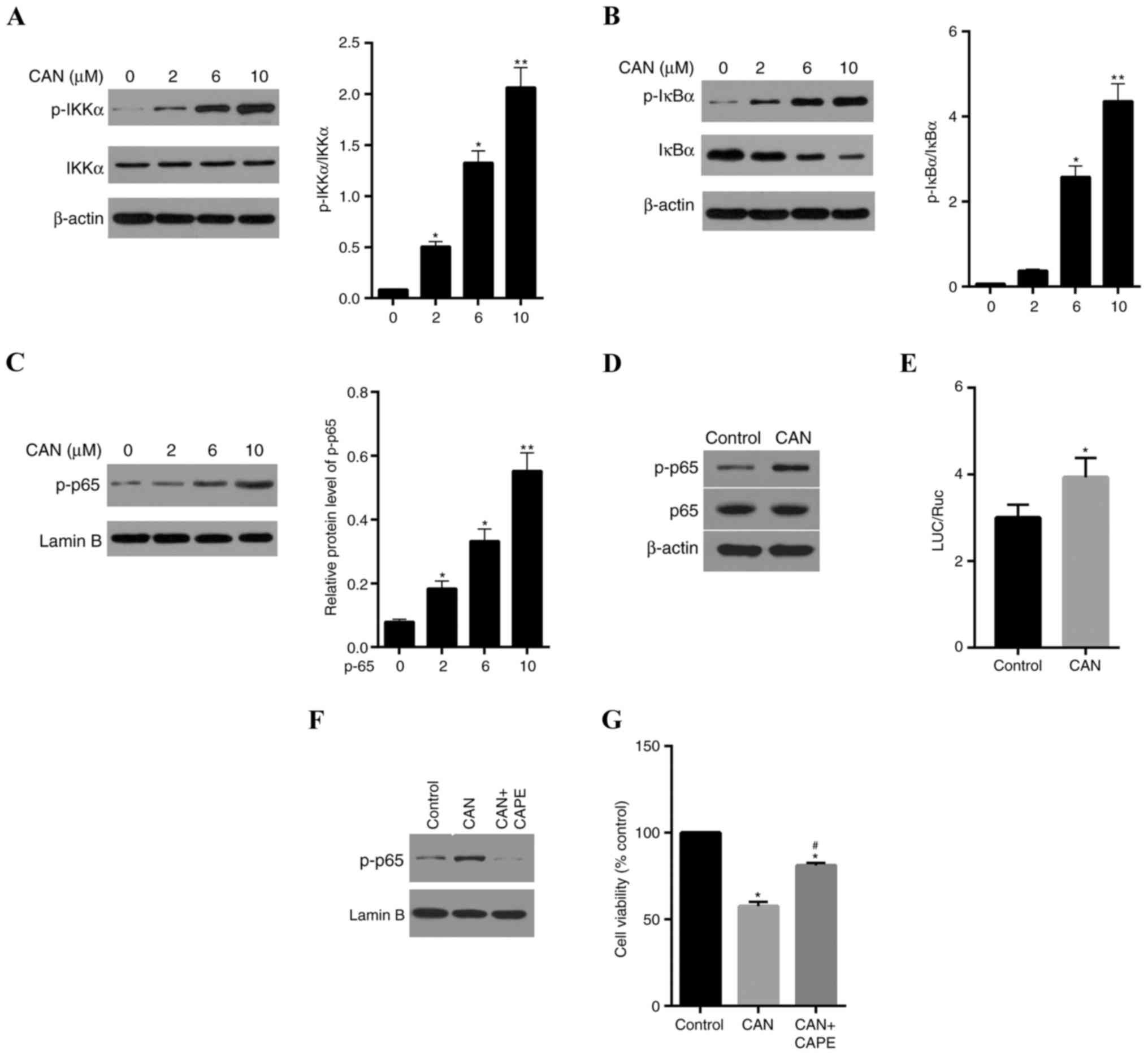 | Figure 5.CAN-induced proliferation inhibition
in QBC939 cells involves the activation of the NF-κB pathway. (A)
p-IKKα levels in QBC939 cells were elevated following treatment
with CAN, in a dose-dependent manner, compared with the control (0
µM). (B) p-IκBα and IκBα levels in cells were significantly
increased and decreased, respectively, compared with the control,
following treatment with CAN. (C) p-p65 levels in the nucleus were
increased by treatment with CAN. (D) CAN increased p-p65 levels,
although it had a weak impact on the total p65 protein expression.
(E) The dual-luciferase reporter gene assay indicated that CAN may
stimulate NF-κB(p65) transcriptional activity. (F) CAPE (1 µM), a
specific inhibitor of p65, reduced the CAN-induced nuclear
translocation of p-p65 and (G) partially decreased the cytotoxicity
of CAN. Data are expressed as the mean ± standard deviation.
*P<0.05, **P<0.01 vs. control; #P<0.05,
CAN+CAPE vs. CAN. CAN, cantharidin; CAPE, caffeic acid phenethyl
ester; NF-κB, nuclear factor-κB; IKK, inhibitor of NF-κB kinase;
LUC, luciferase; p, phosphorylated; IκBα, NF-κB inhibitor α; Ruc,
Renilla luciferase. |
CAN-induced stimulation of the
IKKα/IκBα/NF-κB pathway is specifically involved in the inhibition
of PP2A
To confirm the inhibition of PP2A on the
IKKα/IκBα/NF-κB pathway in QBC939 cells, the effects of OA, a
specific PP2A inhibitor, on cells were determined. The results
indicated that the IKKα/IκBα/NF-κB pathway was activated by OA
(Fig. 6A) and PP2A activity and
cell viability were inhibited in cells treated with OA (Fig. 6B and C). The role of PP2A in
CAN-induced cell inhibition was further confirmed using DES, a
specific activator of PP2A. As presented in Fig. 6D and E, PP2A activity and cell
viability was increased in a dose-dependent manner.
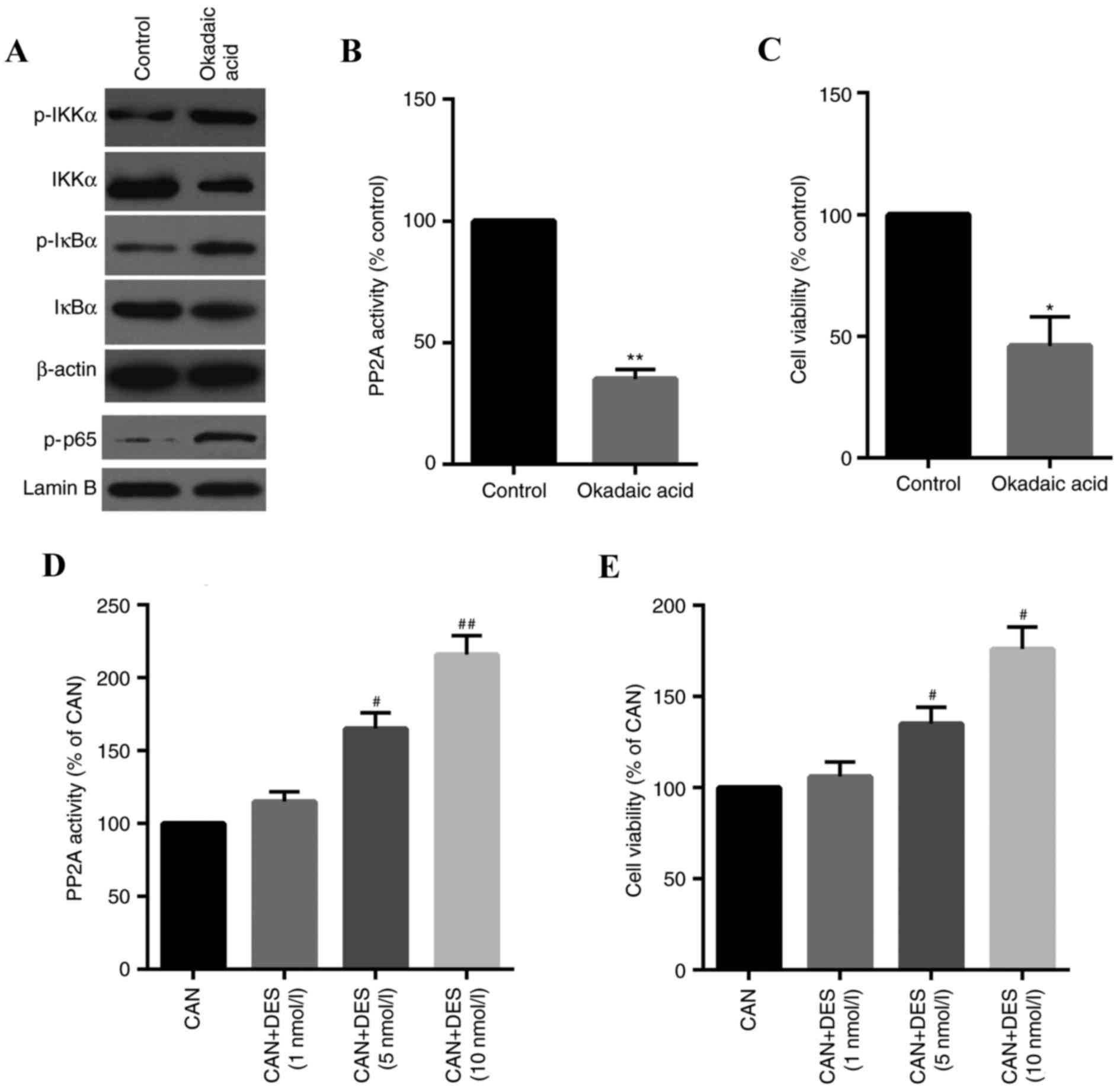 | Figure 6.Specific inhibition of PP2A is
involved in the CAN-induced proliferation of QBC939 cells. (A) The
phosphorylation of IKKα/IκBα/NF-κB-associated proteins was
increased by OA (1 nM), as measured by western blotting. (B) PP2A
activity and (C) cell viability were suppressed in cells treated
with OA. (D) PP2A activity and (E) cell viability were upregulated
in a dose-dependent manner in cells treated with DES (10 nM), a
specific activator of PP2A. Data are expressed as the mean ±
standard deviation. *P<0.05, **P<0.01 vs. control;
#P<0.05, ##P<0.01 vs. CAN. CAN,
cantharidin; DES, D-erythro-sphingosine; NF-κB, nuclear factor-κB;
IKK, inhibitor of NF-κB kinase; IκBα, NF-κB inhibitor α; OA,
okadaic acid; p, phosphorylated; PP2A, serine/threonine-protein
phosphatase 2A. |
Migration and invasion-associated
genes are significantly altered at the translational and
transcriptional levels following treatment with CAN
The expression of genes associated with migration
and invasion, including MMP2, MMP9, TIMP-1 and TIMP-2, was examined
(Fig. 7). The results demonstrated
that the mRNA and protein expression levels of MMP2 and MMP9 were
significantly downregulated when cells were treated with CAN
compared with the control. By contrast, the expression levels of
TIMP-1 and TIMP-2 mRNA and protein in cells were increased in a
dose-dependent manner following treatment with CAN, compared with
the control. The dual-luciferase reporter gene assay revealed that
the transcriptional activities were downregulated for MMP2 and MMP9
and were upregulated for TIMP-1 and TIMP-2in cells treated with CAN
compared with the control (Fig.
7C-F).
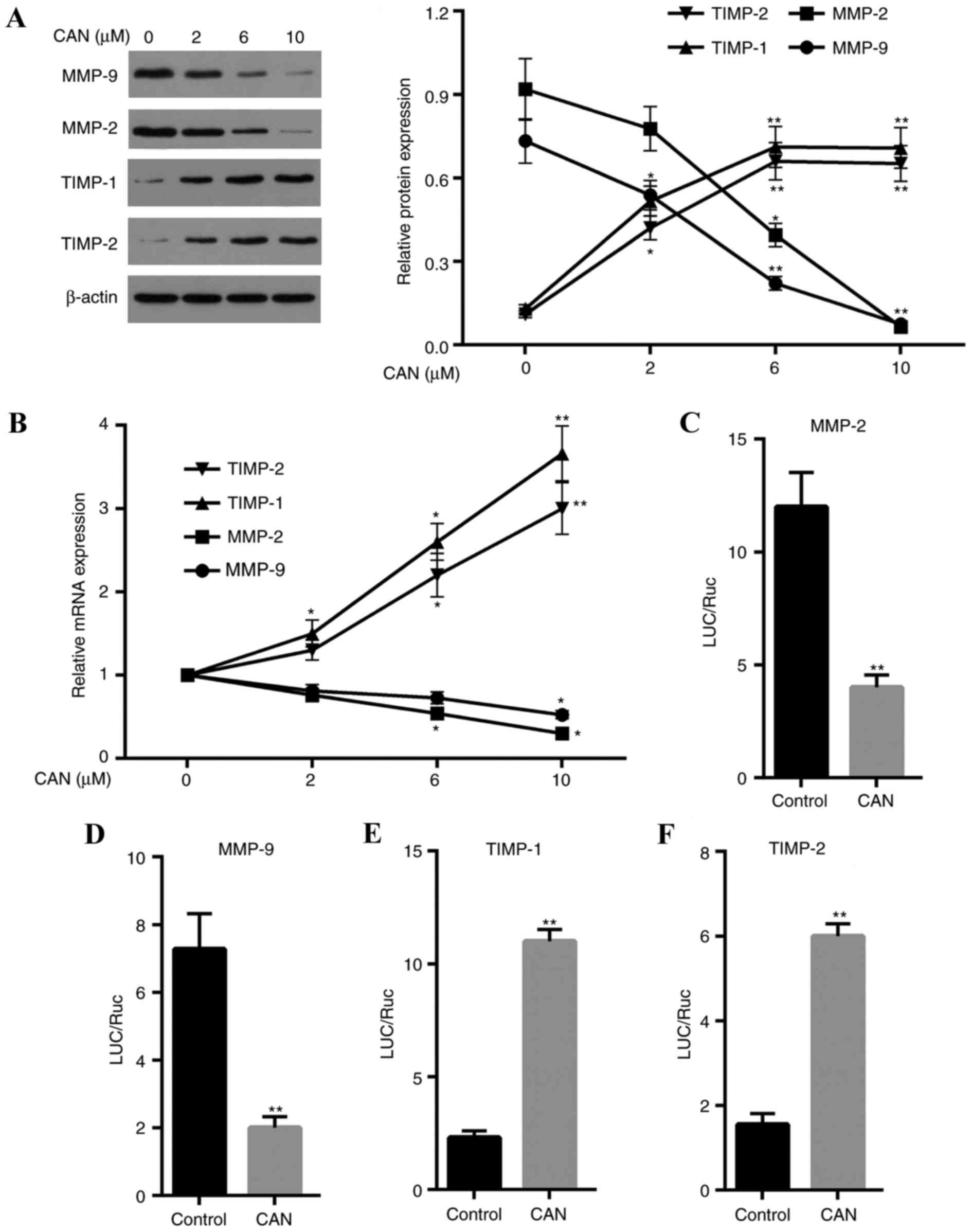 | Figure 7.CAN inhibits the migration and
invasion of QBC939 cells through upregulation of TIMP-1 and TIMP-2,
and downregulation of MMP2 and MMP9. (A) MMP2 and MMP9 protein
expression levels were decreased in QBC939 cells following
treatment with CAN, whereas TIMP-1 and TIMP-2 protein expression
levels were increased in QBC939 cells following treatment with CAN.
(B) QBC939 cells treated with CAN exhibited reduced levels of MMP2
and MMP9 mRNA, while expression levels of TIMP-1 and TIMP-2 mRNA
were increased. (C) The dual-luciferase reporter gene assay
demonstrated that the transcriptional activities were decreased for
MMP2 and (D) MMP9, and increased for (E) TIMP-1 and (F) TIMP-2, in
cells treated with CAN. Data are expressed as the mean ± standard
deviation. *P<0.05, **P<0.05 vs. control. CAN, cantharidin;
LUC, luciferase; TIMP, metalloproteinase inhibitor; MMP2, 72 kDA
type IV collagenase; MMP9, matrix metalloproteinase 9; Ruc,
Renilla luciferase. |
Discussion
Cholangiocarcinoma is a type of cancer with a high
mortality rate, characterized by low survival rates, and is hardly
diagnosed in the early phases (7,9). CAN
is a product extracted from traditional Chinese medicine, serving
an important role in the treatment of a variety of types of
advanced cancer (18,22). In the present study, the action of
CAN on cholangiocarcinoma QBC939 cells was determined.
Following treatment with CAN, the cell viability of
QBC939 cells, Hucc-t1 cells and HiBECs was decreased, although it
was demonstrated that HiBECs were less sensitive to CAN compared
with QBC939 and Hucc-t1 cells. The activity of PP2A in cells
treated with CAN was determined. The results confirmed that CAN is
a strong and specific inhibitor of PP2A in cholangiocarcinoma
cells, since PP2A activity in HiBECs was increased compared with
that in QBC939 cells. These results revealed that CAN exhibited
higher efficiency in inhibiting QBC939 viability, and lower
cytotoxicity was observed in normal HiBECs. Similarly, a number of
reports have demonstrated that CAN and its derivatives have a
selective inhibitory effect on cancer cells compared with normal
cells (18,22,26).
In addition, CAN derivatives, including synthetic cantharidin
analogue, have decreased cytotoxicity in normal cells, supporting
their application in the treatment of a variety of types of cancer
(27).
ROS are the principal stimulator of cytotoxicity and
an important factor causing oxidative damage (28,29).
In the present study, ROS levels were increased in a dose-dependent
manner in cells treated with CAN. An efficient method of inhibiting
the development of cancer is to reduce the migration and invasion
of cancer cells (30). In the
present study, the effect of CAN on the migration and invasion
rates of QBC939 cells was determined. The results revealed that the
migration and invasion rates were significantly decreased in a
dose-dependent manner. Similarly, other studies proved that CAN has
a marked inhibitory effect on the migration and invasion of cancer
cells, including pancreatic cancer cells and liver cancer cells
(18,31,32).
These findings suggested that the inhibitory effect of CAN on
cancer may involve inhibition of the migration and invasion of
cancer cells.
PP2A is a pivotal protein phosphatase in cells and
is involved in the phosphorylation of protein kinases, including
IKK, glycogen synthase kinase-3β, protein kinase A, protein kinase
C and RAC-α serine/threonine-protein kinase (AKT) (17,33).
In the present study, the IKKα/IκBα/NF-κB pathway was examined
following treatment with CAN, and it was revealed that p-IKKα and
p-IκBα levels were significantly elevated following treatment with
CAN. However, IKKα levels were not significantly affected following
treatment with CAN. By contrast, total IκBα levels were decreased
following treatment with CAN. Other investigations revealed that
CAN was a dose-dependent inhibitor of PP2A, resulting in a similar
elevation in the phosphorylation of IKKα, AKT and IκBα, thereby
regulating the following pathway (18,26,32).
The results of the present study confirmed the role of PP2A in the
CAN-induced elevation in the phosphorylation of IKKα, AKT and IκBα
using a specific activator (DES) of PP2A. Furthermore, in the
present study, the IKKα/IκBα/NF-κB pathway was activated and
confirmed by the observation of an increased level of p65 in the
nucleus and increased phosphorylation levels of p65 in the cells.
Numerous studies have demonstrated that activation of the NF-κB
pathway may enhance the transcription of a number of genes
associated with cell proliferation (14,15,34).
However, by contrast, a number of investigations additionally
revealed that apoptosis was stimulated by the NF-κB pathway
(35). These contradictions may be
explained by differences in the cell treatments (35). The results of the present study
revealed that CAN inhibited the growth of QBC939 cells by reducing
migration and invasion, which was additionally observed in other
studies (18,32). Notably, the present study
demonstrated that CAN-induced cell inhibition is partially
dependent on the activation of the IKKα/IκBα/NF-κB pathway, which
was confirmed by inhibition of the nuclear translocation of p65
using an inhibitor (CAPE) of the NF-κB pathway. In addition to this
inhibitor, other inhibitors require further investigation to
confirm the role of the NF-κB pathway in CAN-induced cell
inhibition. Furthermore, in order to fully understand the molecular
mechanisms of CAN in cells, further investigation is required.
MMPs are zinc ion-dependent proteases, involved in
cell migration and invasion (36,37).
Among them, MMP2 and MMP9 are able to degrade the extracellular
matrix (ECM) by hydrolyzing collagen IV, through which they promote
the migration and invasion of cancer cells into other tissues
(37,38). The results demonstrated that the
mRNA and protein expression levels of MMP2 and MMP9 were decreased
following treatment of the cells with CAN, which may lead to a
decrease in ECM degradation, and inhibition of cell migration and
invasion. Notably, studies revealed that NF-κB p65, the subunit of
NF-κB, directly activates the expression of MMP2 and MMP9 via
interaction with their DNA binding sites (39–41).
Previous studies demonstrated that the NF-κB p65 level has a
positive association with MMP2 and MMP9 (37,40,42).
In addition, studies have reported that activation of the NF-κB
pathway promotes tumor cell invasion and migration (43,44).
Notably, the results demonstrated that there is a negative
association between NF-κB p65, and MMP2 and MMP9, suggesting the
inhibitory effects of the NF-κB pathway on cell invasion and
migration. TIMPs are the specific inhibitor of MMPs (45–47).
Therefore, the expression of MMP2 and MMP9 expression may be
implicated in the balance between TIMP-1 and TIMP-2 and NF-κB p65
expression. However, the mechanisms underlying these interactions
remain unclear and require further investigation.
In conclusion, following treatment with CAN, cell
migration and invasion in the cholangiocarcinoma cell line QBC939
was inhibited. However, the MTT assay revealed that the
cytotoxicity of CAN in QBC939 cells was higher compared with
HiBECs. The inhibitory effect of CAN on QBC939 cells may partially
involve the IKKα/IκBα/NF-κB signaling pathway, and interactions
with TIMP-1, TIMP-2, MMP2 and MMP9 proteins.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Public
Technology Application Research Program of the Science Technology
Department of Zhejiang Province (grant no. 2017C33045).
Availability of data and materials
All data generated and/or analyzed during this study
are included in this published article
Authors' contributions
HZ wrote the main manuscript. HZ, JX and SW
performed the experiments. HZ and JX designed the study. HZ, SW and
JP performed data analysis. JX, SW and JP contributed to manuscript
revisions and all authors reviewed the manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mittal PK, Moreno CC, Kalb B, Mittal A,
Camacho JC, Maddu K, Kitajima HD, Quigley BC, Kokabi N and Small
WC: Primary biliary tract malignancies: MRI spectrum and mimics
with histopathological correlation. Abdom Imaging. 40:1520–1557.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang W, Wang X, Zheng W, Li K, Liu H and
Sun Y: Genetic and epigenetic alterations are involved in the
regulation of TPM1 in cholangiocarcinoma. Int J Oncol. 50:3402017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim HJ, Kim CY, Hur YH, Koh YS, Kim JC,
Kim HJ and Cho CK: The prognostic factors for survival after
curative resection of distal cholangiocarcinoma: Perineural
invasion and lymphovascular invasion. Surgery Today. 44:1879–1886.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Horie Y, Akamizu H, Nishimura Y, Maeda N,
Kawasaki H, Kimura O, Hirooka Y, Hamazoe R, Kaibara N and Ohta Y:
Intrahepatic cholangiocarcinoma with a long-term survival of 12
years after surgical resection: Report of a case and review of the
literature. Hepatogastroenterology. 42:506–509. 1995.PubMed/NCBI
|
|
5
|
Yap AQ, Chen CL, Yong CC, Kuo FY, Wang SH,
Lin CC, Liu YW, Lin TL, Li WF, Millan CA and Wang CC:
Clinicopathological factors impact the survival outcome following
the resection of combined hepatocellular carcinoma and
cholangiocarcinoma. Surg Oncol. 22:55–60. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sriputtha S, Khuntikeo N, Promthet S and
Kamsa-Ard S: Survival rate of intrahepatic cholangiocarcinoma
patients after surgical treatment in Thailand. Asian Pac J Cancer
Prev. 14:1107–1110. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Harnois DM, Que FG, Celli A, Larusso NF
and Gores GJ: Bcl-2 is overexpressed and alters the threshold for
apoptosis in a cholangiocarcinoma cell line. Hepatology.
26:884–890. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Subimerb C, Pinlaor S, Khuntikeo N,
Leelayuwat C, Morris A, McGrath MS and Wongkham S: Tissue invasive
macrophage density is correlated with prognosis in
cholangiocarcinoma. Mol Med Rep. 3:597–605. 2010.PubMed/NCBI
|
|
9
|
Boberg KM, Bergquist A, Mitchell S, Pares
A, Rosina F, Broomé U, Chapman R, Fausa O, Egeland T, Rocca G and
Schrumpf E: Cholangiocarcinoma in primary sclerosing cholangitis:
Risk factors and clinical presentation. Scand J Gastroenterol.
37:1205–1211. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Burak K, Angulo P, Pasha TM, Egan K, Petz
J and Lindor KD: Incidence and risk factors for cholangiocarcinoma
in primary sclerosing cholangitis. Am J Gastroenterol. 99:523–526.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Purandare NC, Patel II, Trevisan J, Bolger
N, Kelehan R, von Bünau G, Martin-Hirsch PL, Prendiville WJ and
Martin FL: Biospectroscopy insights into the multi-stage process of
cervical cancer development: Probing for spectral biomarkers in
cytology to distinguish grades. Analyst. 138:3909–3916. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
O'Reilly DA, Roberts JR, Cartmell MT,
Demaine AG and Kingsnorth AN: Heat shock factor-1 and nuclear
factor-kappaB are systemically activated in human acute
pancreatitis. JOP. 7:174–184. 2006.PubMed/NCBI
|
|
13
|
Orban Z, Mitsiades N, Burke TR Jr, Tsokos
M and Chrousos GP: Caffeic acid phenethyl ester induces leukocyte
apoptosis, modulates nuclear factor-kappa B and suppresses acute
inflammation. Neuroimmunomodulation. 7:99–105. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liacini A, Sylvester J, Li WQ, Huang W,
Dehnade F, Ahmad M and Zafarullah M: Induction of matrix
metalloproteinase-13 gene expression by TNF-alpha is mediated by
MAP kinases, AP-1, and NF-kappaB transcription factors in articular
chondrocytes. Exp Cell Res. 288:208–217. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Da Silva-Ferrada E, Torres-Ramos M, Aillet
F, Campagna M, Matute C, Rivas C, Rodríguez MS and Lang V: Role of
monoubiquitylation on the control of IκBα degradation and NF-κB
activity. PLoS One. 6:e253972011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schultze SM, Hemmings BA and Tschopp O:
PI3K/AKT, MAPK and AMPK signalling: Protein kinases in glucose
homeostasis. Expert Rev Mol Med. 14:e12012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Janssens V and Goris J: Protein
phosphatase 2A: A highly regulated family of serine/threonine
phosphatases implicated in cell growth and signaling. Biochem J.
353:417–439. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li W, Xie L, Chen Z, Zhu Y, Sun Y, Miao Y,
Xu Z and Han X: Cantharidin, a potent and selective PP2A inhibitor,
induces an oxidative stress-independent growth inhibition of
pancreatic cancer cells through G2/M cell-cycle arrest and
apoptosis. Cancer Sci. 101:1226–1233. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kadioglu O, Kermani NS, Kelter G,
Schumacher U, Fiebig HH, Greten HJ and Efferth T: Pharmacogenomics
of cantharidin in tumor cells. Biochem Pharmacol. 87:399–409. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Martin WJ, Walton M and Harper J: Resident
macrophages initiating and driving inflammation in a monosodium
urate monohydrate crystal-induced murine peritoneal model of acute
gout. Arthritis Rheum. 60:281–289. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Reddy SB and Patel T: Current approaches
to the diagnosis and treatment of cholangiocarcinoma. Curr
Gastroenterol Rep. 8:30–37. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shou LM, Zhang QY, Li W, Xie X, Chen K,
Lian L, Li ZY, Gong FR, Dai KS, Mao YX and Tao M: Cantharidin and
norcantharidin inhibit the ability of MCF-7 cells to adhere to
platelets via protein kinase C pathway-dependent; downregulation of
α2 integrin. Oncol Rep. 30:1059–1066. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Imig JD, Dimitropoulou C, Reddy DS, White
RE and Falck JR: Afferent arteriolar dilation to 11, 12-EET analogs
involves PP2A activity and Ca2+ -activated K + channels.
Microcirculation. 15:137–150. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tsuchiya Y, Osaki K, Kanamoto M, Nakao Y,
Takahashi E, Higuchi T and Kamata H: Distinct B subunits of PP2A
regulate the NF-κB signalling pathway through dephosphorylation of
IKKβ, IκBα and RelA. FEBS Lett. 591:4083–4094. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rauh R, Kahl S, Boechzelt H, Bauer R,
Kaina B and Efferth T: Molecular biology of cantharidin in cancer
cells. Chin Med. 2:82007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kok SH, Chui CH, Lam WS, Chen J, Lau FY,
Cheng GY, Wong RS, Lai PP, Leung TW, Tang JC and Chan AS: Apoptotic
activity of a novel synthetic cantharidin analogue on hepatoma cell
lines. Int J Mol Med. 17:945–949. 2006.PubMed/NCBI
|
|
28
|
Sharma V, Anderson D and Dhawan A: Zinc
oxide nanoparticles induce oxidative DNA damage and ROS-triggered
mitochondria mediated apoptosis in human liver cells (HepG2).
Apoptosis. 17:852–870. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang L, Li Y, Xing D and Gao C:
Characterization of mitochondrial dynamics and subcellular
localization of ROS reveal that HsfA2 alleviates oxidative damage
caused by heat stress in Arabidopsis. J Exp Bot. 60:2073–2091.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Deng G, Teng Y, Huang F, Nie W, Zhu L,
Huang W and Xu H: MicroRNA-101 inhibits the migration and invasion
of intrahepatic cholangiocarcinoma cells via direct suppression of
vascular endothelial growth factor-C. Mol Med Rep. 12:7079–7085.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
He TP, Mo LE and Liang NC: Inhibitory
effect of cantharidin on invasion and metastasis of highly
metastatic ovarian carcinoma cell line HO-8910PM. Ai Zheng.
24:443–447. 2005.(In Chinese). PubMed/NCBI
|
|
32
|
Hsiao YP, Tsai CH, Wu PP, Hsu SC, Liu HC,
Huang YP, Yang JH and Chung JG: Cantharidin induces G2/M phase
arrest by inhibition of Cdc25c and Cyclin A and triggers apoptosis
through reactive oxygen species and the mitochondria-dependent
pathways of A375.S2 human melanoma cells. Int J Oncol.
45:2393–2402. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Martin L, Magnaudeix A, Wilson CM, Yardin
C and Terro F: The new indirubin derivative inhibitors of glycogen
synthase kinase-3, 6-BIDECO and 6-BIMYEO, prevent tau
phosphorylation and apoptosis induced by the inhibition of protein
phosphatase-2A by okadaic acid in cultured neurons. J Neurosci Res.
89:1802–1811. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Makarov SS: NF-kappa B in rheumatoid
arthritis: A pivotal regulator of inflammation, hyperplasia, and
tissue destruction. Arthritis Res. 3:200–206. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mogi M, Ozeki N, Nakamura H and Togari A:
Dual roles for NF-kappaB activation in osteoblastic cells by serum
deprivation: Osteoblastic apoptosis and cell-cycle arrest. Bone.
35:507–516. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jovanović M, Stefanoska I, Radojcić L and
Vićovac L: Interleukin-8 (CXCL8) stimulates trophoblast cell
migration and invasion by increasing levels of matrix
metalloproteinase (MMP)2 and MMP9 and integrins alpha5 and beta1.
Reproduction. 139:789–798. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wu D, Pan H, Zhou Y, Zhou J, Fan Y and Qu
P: microRNA-133b downregulation and inhibition of cell
proliferation, migration and invasion by targeting matrix
metallopeptidase-9 in renal cell carcinoma. Mol Med Rep.
10:2491–2498. 2014. View Article : Google Scholar
|
|
38
|
Lo C, Lai TY, Yang JS, Yang JH, Ma YS,
Weng SW, Lin HY, Chen HY, Lin JG and Chung JG: Gallic acid inhibits
the migration and invasion of A375.S2 human melanoma cells through
the inhibition of matrix metalloproteinase-2 and Ras. Melanoma Res.
21:267–273. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Han YP, Tuan TL, Wu H, Hughes M and Garner
WL: TNF-alpha stimulates activation of pro-MMP2 in human skin
through NF-(kappa)B mediated induction of MT1-MMP. J Cell Sci.
114:131–139. 2001.PubMed/NCBI
|
|
40
|
Prakash M, Kale S, Ghosh I, Kundu GC and
Datta K: Hyaluronan-binding protein 1 (HABP1/p32/gC1qR) induces
melanoma cell migration and tumor growth by NF-kappa B dependent
MMP-2 activation through integrin α(v)β(3) interaction. Cell
Signal. 23:1563–1577. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen XJ, Wu MY, Li DH and You J: Apigenin
inhibits glioma cell growth through promoting microRNA-16 and
suppression of BCL-2 and nuclear factor-κB/MMP-9. Mol Med Rep.
14:2352–2358. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Karadimas SK, Klironomos G, Papachristou
DJ, Papanikolaou S, Papadaki E and Gatzounis G: Immunohistochemical
Profile of NF-κB/p50, NF-κB/p65, MMP-9, MMP-2, and u-PA in
experimental cervical spondylotic myelopathy. Spine (Phila Pa
1976). 38:4–10. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu X, Lin R, Zhao B, Guan R, Li T and Jin
R: Correlation between oxidative stress and the NF-κB signaling
pathway in the pulmonary tissues of obese asthmatic mice. Mol Med
Rep. 13:1127–1134. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wu Y and Zhou BP:
TNF-alpha/NF-kappaB/Snail pathway in cancer cell migration and
invasion. Br J Cancer. 102:639–644. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bourboulia D and Stetler-Stevenson WG:
Matrix metalloproteinases (MMPs) and tissue inhibitors of
metalloproteinases (TIMPs): Positive and negative regulators in
tumor cell adhesion. Semin Cancer Biol. 20:161–168. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bramhall SR, Neoptolemos JP, Stamp GW and
Lemoine NR: Imbalance of expression of matrix metalloproteinases
(MMPs) and tissue inhibitors of the matrix metalloproteinases
(TIMPs) in human pancreatic carcinoma. J Pathol. 182:347–355. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Nagase H and Brew K: Designing TIMP
(tissue inhibitor of metalloproteinases) variants that are
selective metalloproteinase inhibitors. Biochem Soc Symp.
70:201–212. 2003. View Article : Google Scholar
|