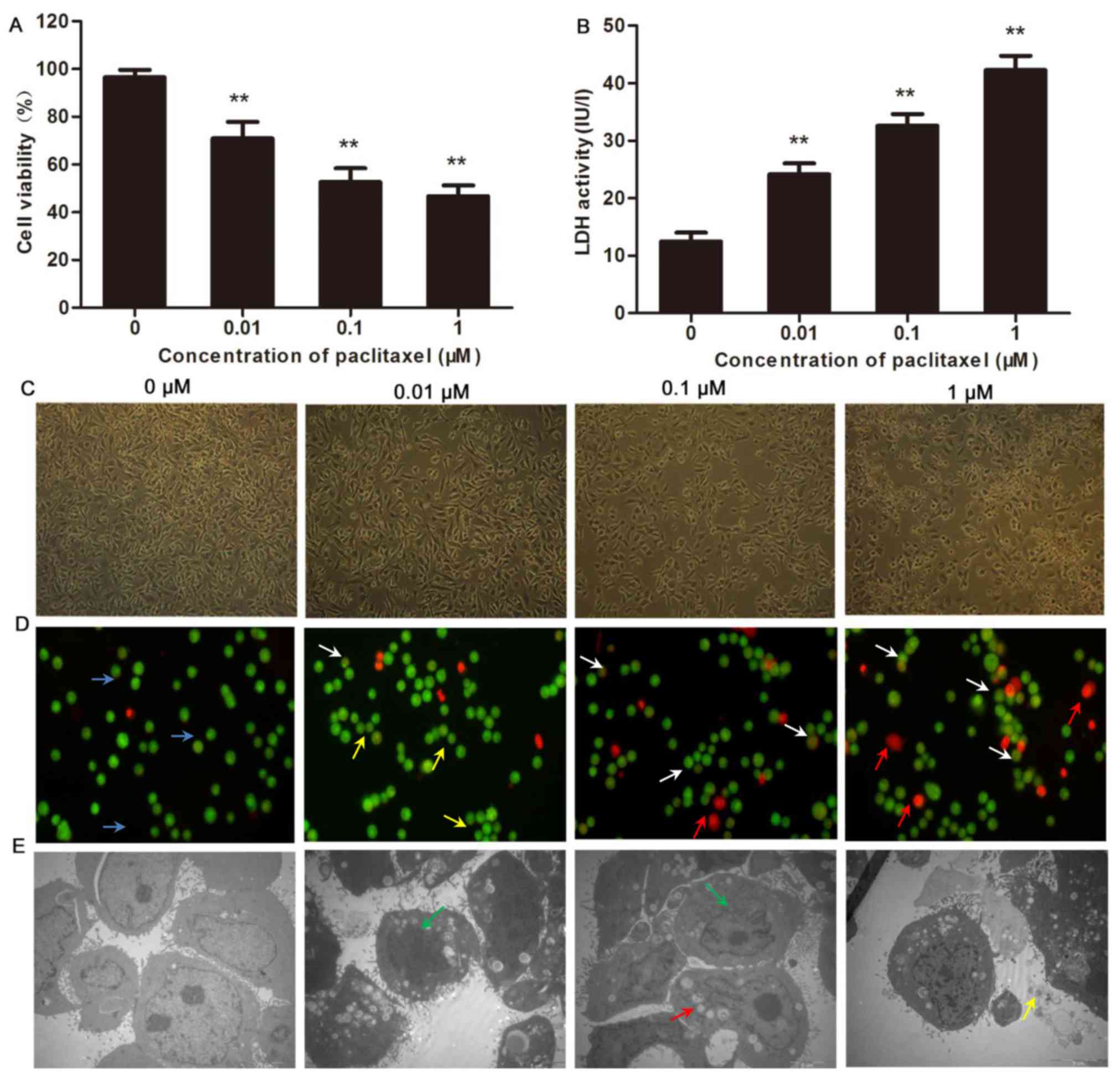
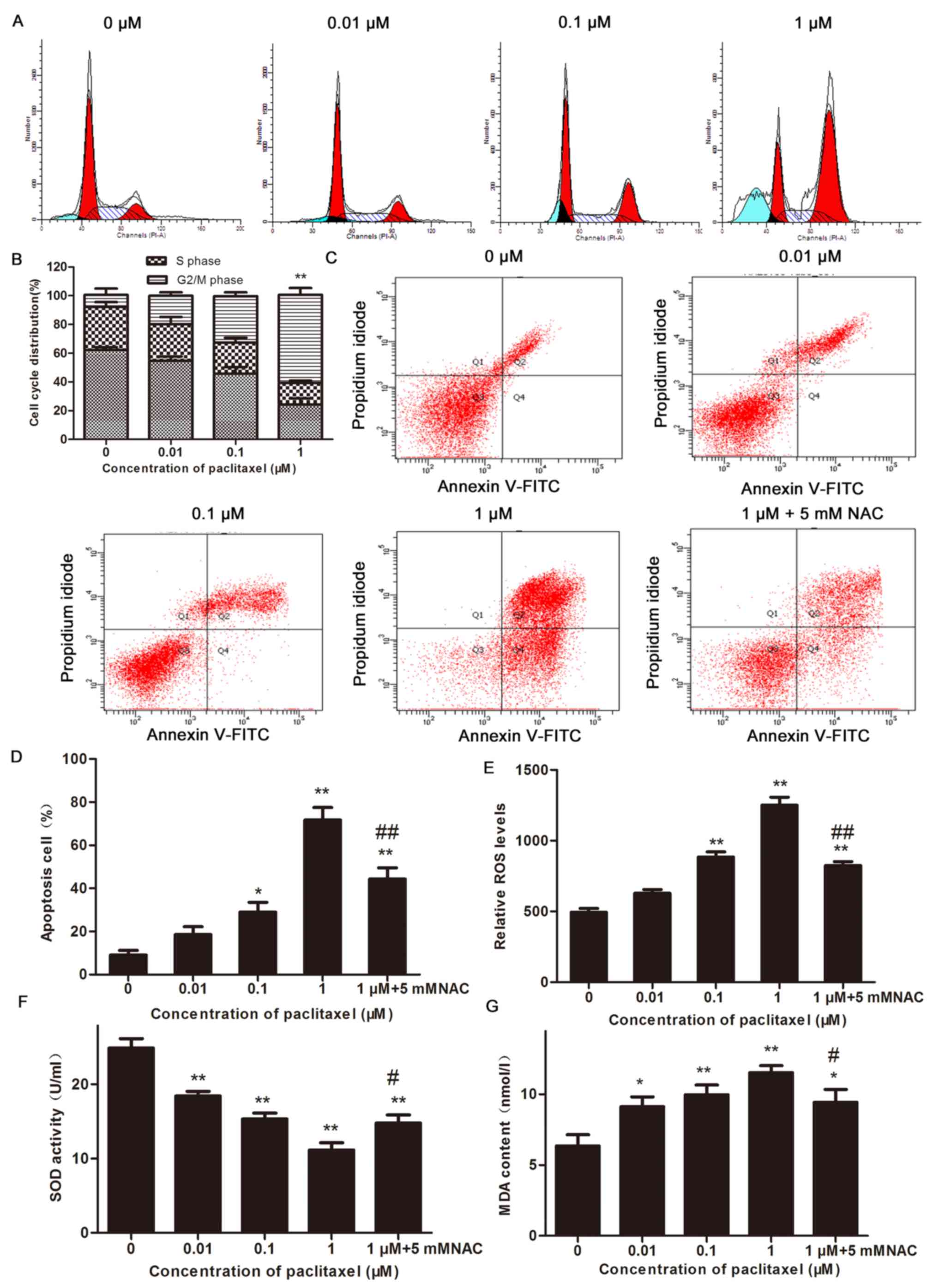
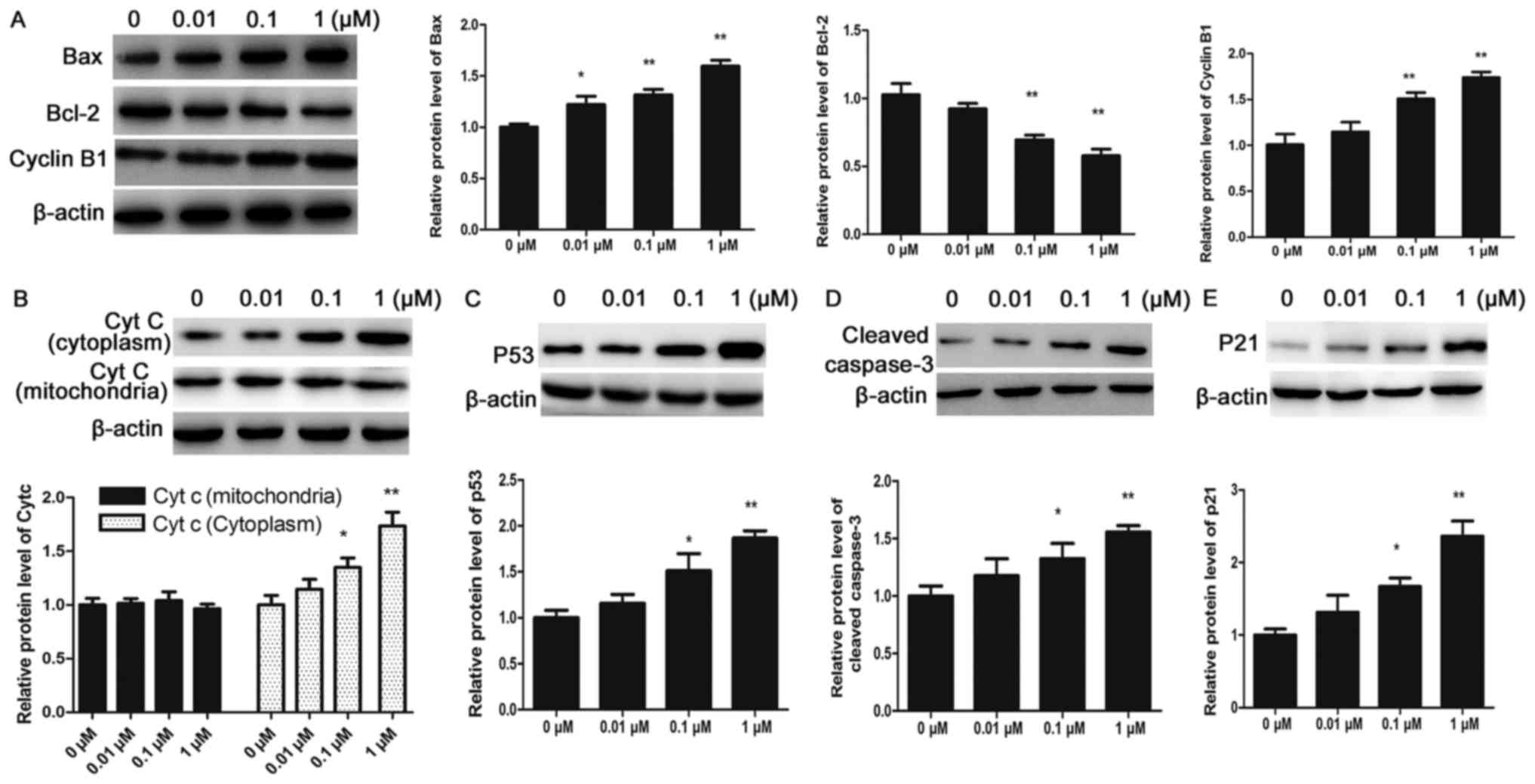
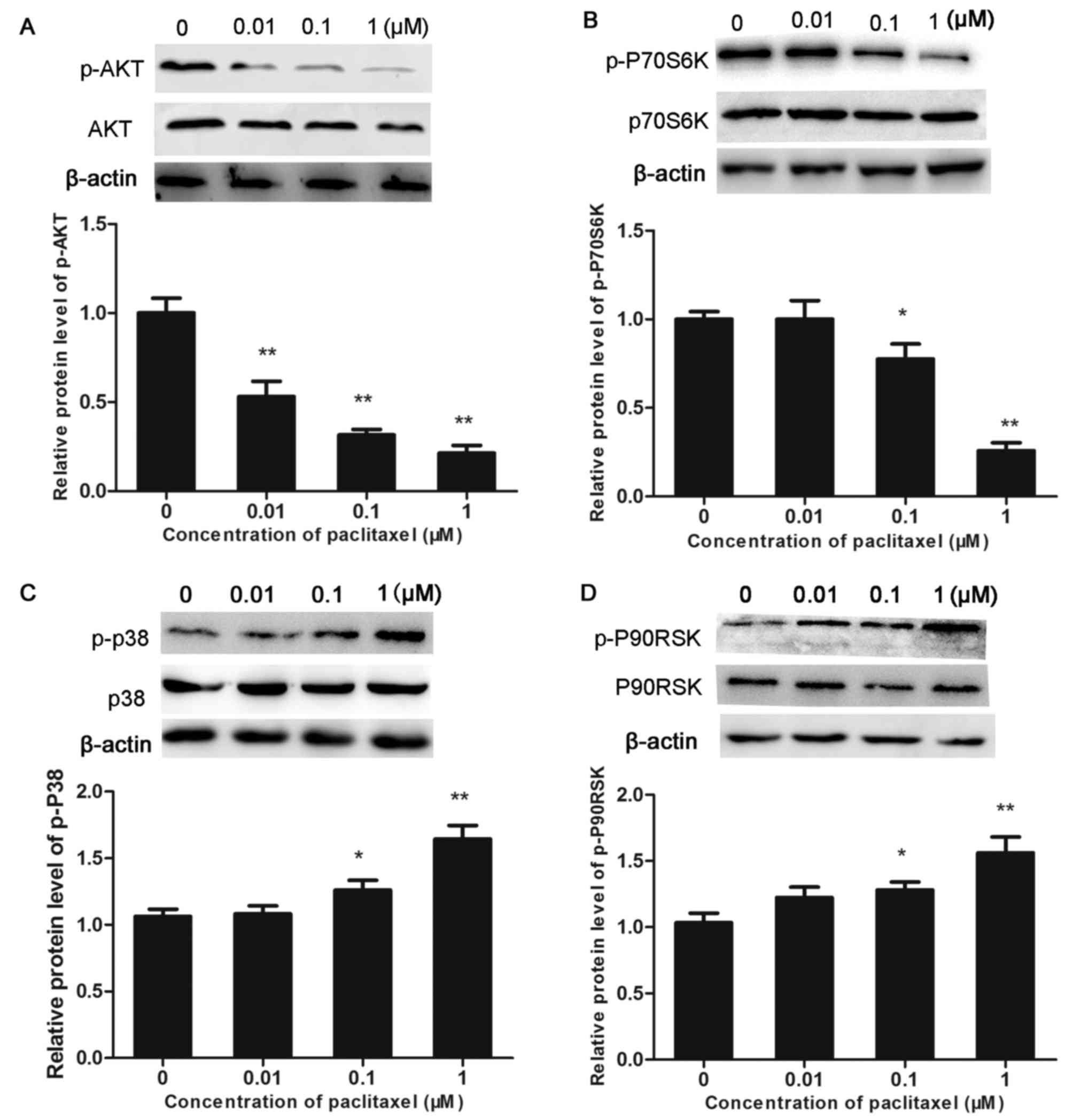
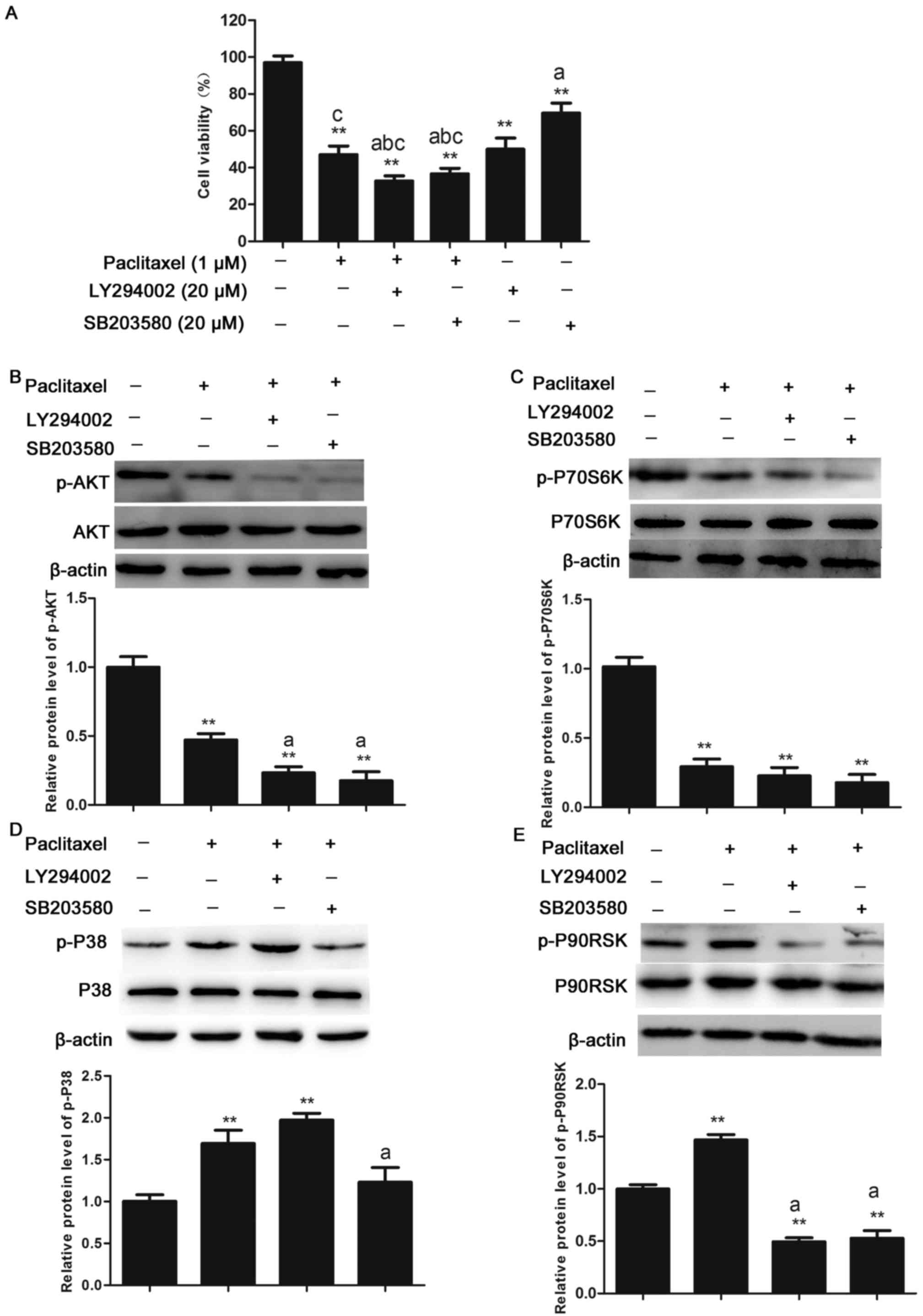
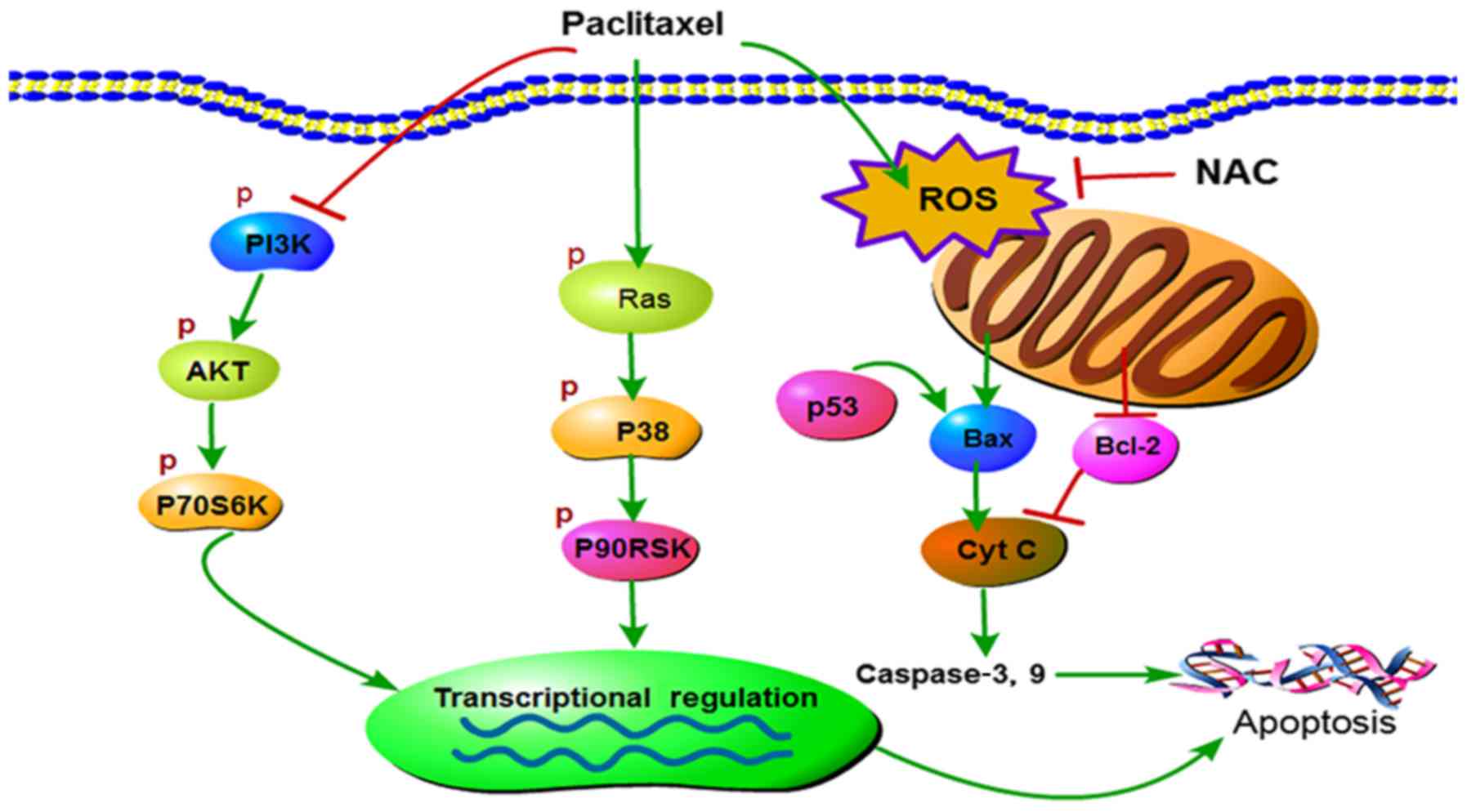
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sorenmo K: Canine mammary gland tumors.
Vet Clin North Am Small Anim Pract. 33:573–596. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vail DM and MacEwen EG: Spontaneously
occurring tumors of companion animals as models for human cancer.
Cancer Invest. 18:781–792. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chauhan A, Sharma MM and Kumar K:
Evaluation of surgical outcomes of oncoplasty breast surgery in
locally advanced breast cancer and comparison with conventional
breast conservation surgery. Indian J Surg Oncol. 7:413–419. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shamsi M and Islamian Pirayesh J: Breast
cancer: Early diagnosis and effective treatment by drug delivery
tracing. Nucl Med Rev Cent East Eur. 20:45–48. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jeong JY, Kim KS, Moon JS, Song JA, Choi
SH, Kim KI, Kim TH and An HJ: Targeted inhibition of phosphatidyl
inositol-3-kinase p110β, but not p110α, enhances apoptosis and
sensitivity to paclitaxel in chemoresistant ovarian cancers.
Apoptosis. 18:509–520. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
McGuire WP, Rowinsky EK, Rosenshein NB,
Grumbine FC, Ettinger DS, Armstrong DK and Donehower RC: Taxol: A
unique antineoplastic agent with significant activity in advanced
ovarian epithelial neoplasms. Ann Intern Med. 111:273–279. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Quispe-Soto ET and Calaf GM: Effect of
curcumin and paclitaxel on breast carcinogenesis. Int J Oncol.
49:2569–2577. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Holmes FA, Walters RS, Theriault RL,
Forman AD, Newton LK, Raber MN, Buzdar AU, Frye DK and Hortobagyi
GN: Phase II trial of taxol, an active drug in the treatment of
metastatic breast cancer. J Natl Cancer Inst. 83:1797–1805. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang C, Wang R, Zhou K, Wang S, Wang J,
Shi H, Dou Y, Yang D, Chang L, Shi X, et al: JD enhances the
anti-tumour effects of low-dose paclitaxel on gastric cancer MKN45
cells both in vitro and in vivo. Cancer Chemoth Pharm. 78:971–982.
2016. View Article : Google Scholar
|
|
11
|
McGrogan BT, Gilmartin B, Carney DN and
McCann A: Taxanes, microtubules and chemoresistant breast cancer.
Biochim Biophys Acta. 1785:96–132. 2008.PubMed/NCBI
|
|
12
|
Hu J, Zhang NA, Wang R, Huang F and Li G:
Paclitaxel induces apoptosis and reduces proliferation by targeting
epidermal growth factor receptor signaling pathway in oral cavity
squamous cell carcinoma. Oncol Lett. 10:2378–2384. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Donaldson KL, Goolsby GL, Kiener PA and
Wahl AF: Activation of p34cdc2 coincident with taxol-induced
apoptosis. Cell Growth Differ. 5:1041–1050. 1994.PubMed/NCBI
|
|
14
|
Schiff PB, Fant J and Horwitz SB:
Promotion of microtubule assembly in vitro by taxol. Nature.
277:665–667. 1979. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ren L, Li Z, Dai C, Zhao D, Wang Y, Ma C
and Liu C: Chrysophanol inhibits proliferation and induces
apoptosis through NF-κB/cyclin D1 and NF-κB/Bcl-2 signaling cascade
in breast cancer cell lines. Mol Med Rep. 17:4376–4382.
2018.PubMed/NCBI
|
|
16
|
Kim GM, Kim S, Park HS, Kim JY, Nam S,
Park S, Kim SI, Kim D and Sohn J: Chemotherapy-induced irreversible
alopecia in early breast cancer patients. Breast Cancer Res Treat.
163:527–533. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Okano J and Rustgi AK: Paclitaxel induces
prolonged activation of the Ras/MEK/ERK pathway independently of
activating the programmed cell death machinery. J Biol Chem.
276:19555–19564. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu R, Sato N, Yanai K, Akiyoshi T, Nagai
S, Wada J, Koga K, Mibu R, Nakamura M and Katano M: Enhancement of
paclitaxel-induced apoptosis by inhibition of mitogen-activated
protein kinase pathway in colon cancer cells. Anticancer Res.
29:261–270. 2009.PubMed/NCBI
|
|
19
|
Tserga A, Chatziandreou I, Michalopoulos
NV, Patsouris E and Saetta AA: Mutation of genes of the PI3K/AKT
pathway in breast cancer supports their potential importance as
biomarker for breast cancer aggressiveness. Virchows Arch.
469:35–43. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Son Y, Cheong YK, Kim NH, Chung HT, Kang
DG and Pae HO: Mitogen-activated protein kinases and reactive
oxygen species: How can ROS activate MAPK pathways? J Signal
Transduct. 2011:7926392011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang C, Lim W, Bazer FW and Song G:
Myricetin suppresses invasion and promotes cell death in human
placental choriocarcinoma cells through induction of oxidative
stress. Cancer Lett. 399:10–19. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Morales-Cano D, Calviño E, Rubio V,
Herráez A, Sancho P, Tejedor MC and Diez JC: Apoptosis induced by
paclitaxel via Bcl-2, Bax and caspases 3 and 9 activation in NB4
human leukaemia cells is not modulated by ERK inhibition. Exp
Toxicol Pathol. 65:1101–1108. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sakashita F, Osada S, Takemura M, Imai H,
Tomita H, Nonaka K, Takahashi T and Seishima M: The effect of p53
gene expression on the inhibition of cell proliferation by
paclitaxel. Cancer Chemother Pharmacol. 62:379–385. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Norbury CJ and Zhivotovsky B: DNA
damage-induced apoptosis. Oncogene. 23:2797–2808. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Festjens N, van Gurp M, van Loo G, Saelens
X and Vandenabeele P: Bcl-2 family members as sentinels of cellular
integrity and role of mitochondrial intermembrane space proteins in
apoptotic cell death. Acta Haematol. 111:7–27. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Anjum R and Blenis J: The RSK family of
kinases: Emerging roles in cellular signalling. Nat Rev Mol Cell
Biol. 9:747–758. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ban JO, Hwang CJ, Park MH, Hwang IK, Jeong
HS, Lee HP, Hyun BK, Kim JY, Youn HS, Ham YW, et al: Enhanced cell
growth inhibition by thiacremonone in paclitaxel-treated lung
cancer cells. Arch Pharm Res. 38:1351–1362. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xia RL, Lu Y, Zhu LN, Zhang SF, Zhao FK
and Fu CY: Different regulatory pathways are involved in the
proliferative inhibition of two types of leukemia cell lines
induced by paclitaxel. Oncol Rep. 30:1853–1859. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang J, Liu X, Bhalla K, Kim CN, Ibrado
AM, Cai J, Peng TI, Jones DP and Wang X: Prevention of apoptosis by
Bcl-2: Release of cytochrome c from mitochondria blocked. Science.
275:1129–1132. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nonaka M, Ikeda H, Fujisawa A, Uehara M
and Inokuchi T: Induction of apoptosis by paclitaxel in human oral
carcinoma cells. Int J Oral Maxillofac Surg. 35:649–652. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Suh DH, Kim MK, Kim HS, Chung HH and Song
YS: Mitochondrial permeability transition pore as a selective
target for anti-cancer therapy. Front Oncol. 3:412013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang M, Wang B, Gao J, Zhang Y, Xu W and
Tao L: Spinosad induces programmed cell death involves
mitochondrial dysfunction and cytochrome C release in Spodoptera
frugiperda Sf9 cells. Chemosphere. 169:155–161. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Azad N, Iyer AK, Wang L, Lu Y, Medan D,
Castranova V and Rojanasakul Y: Nitric oxide-mediated bcl-2
stabilization potentiates malignant transformation of human lung
epithelial cells. Am J Respir Cell Mol Biol. 42:578–585. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Meshkini A and Yazdanparast R: Involvement
of oxidative stress in taxol-induced apoptosis in chronic
myelogenous leukemia K562 cells. Exp Toxicol Pathol. 64:357–365.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Akiyama M, Sowa Y, Taniguchi T, Watanabe
M, Yogosawa S, Kitawaki J and Sakai T: Three combined treatment, a
Novel HDAC inhibitor OBP-801/YM753, 5-Fluorouracil and paclitaxel,
induces G2 phase arrest through the p38 pathway in human
ovarian cancer cells. Oncol Res. 25:1245–1252. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wu G, Qin XQ, Guo JJ, Li TY and Chen JH:
AKT/ERK activation is associated with gastric cancer cell
resistance to paclitaxel. Int J Clin Exp Pathol. 7:1449–1458.
2014.PubMed/NCBI
|
|
38
|
Lim W, Park S, Bazer FW and Song G:
Apigenin reduces survival of choriocarcinoma cells by inducing
apoptosis via the PI3K/AKT and ERK1/2 MAPK pathways. J Cell
Physiol. 231:2690–2699. 2016. View Article : Google Scholar : PubMed/NCBI
|