Introduction
Coronary heart disease poses a major health problem
worldwide. It is often caused by coronary atherosclerosis or by
vasospasm stenosis or obstruction. Although there has been
remarkable progress in basic and clinical research on coronary
heart disease, patient outcome is still not optimal. For example,
percutaneous coronary intervention (PCI) is an important
therapeutic strategy to treat coronary heart disease. However,
restenosis of the coronary arteries occurs in roughly 5–10% of
individuals after stent placement (1). Two important vascular smooth cell
processes-proliferation and migration-are the main factors that
drive restenotic vascular remodeling as well as atherosclerosis
after PCI (2). Therefore, there is
an urgent need to develop novel approaches to prevent and treat
coronary restenosis.
Resveratrol (3,5,4′-trihydroxy-trans-stilbene), a
non-flavonoid polyphenolic compound (3), is considered a phytoalexin because it
is produced by plants under stress conditions (4). Resveratrol has been shown to exert
several beneficial effects in the treatment of cardiovascular
diseases, including hypertension, atherosclerosis, ischemic heart
diseases, heart failure, arrhythmia, and stroke (5). Resveratrol inhibits vascular smooth
muscle cell (VSMC) proliferation (6–9) and
neointimal hyperplasia after arterial injury (10,11),
but the mechanism is not fully understood. The present study
investigated the mechanism underlying resveratrol's inhibitory
effects on VSMC proliferation and migration and neointimal
hyperplasia in a carotid balloon injurymodel in rats.
More specifically, the present study was designed to
gain better insight into resveratrol's potential effects on the
Ca2+/calmodulin-dependent protein kinase II and histone
deacetylases 4 (CaMKII-HDAC4) pathway. CaMKIIis a multimeric
enzyme, and its activity is regulated by Ca2+/calmodulin
(CaM) binding, which activates its protein kinase activity and
promotes intrasubunitautophosphorylation (12). CaMKII is strongly expressed in
VSMCs (13) and is critical for
VSMC proliferation (14),
migration (15), and neointima
proliferation after vascular injury (16,17).
Interestingly, resveratrol prevents diabetes-induced retinal
neuronal cell death via downregulation of CaMKII (18). It also reduces the expression and
phosphorylation of CaMKII induced by aortic banding in rats
(19).
Histone deacetylases (HDACs) play a central role in
the epigenetic regulation of gene expression (20). HDAC4 controls platelet derived
growth factor-BB (PDGF-BB)-mediated increases in VSMC proliferation
and migration (20). HDAC4
activity is regulated by CaMKII in various cell types (13,21).
Therefore, in the present study, we used in
vitro and in vivo models to test the hypothesis that
resveratrol's regulation of the CaMKII-HDAC4 pathway is critical
for its inhibitory effects on VSMCs. Our study provides a rationale
for the use of resveratrol to prevent and treat coronary
restenosis.
Materials and methods
Cell culture
The VSMC line A7r5 was purchased from the Cell Bank
of Chinese Academy of Sciences, Shanghai, China. The cells were
cultured in Dulbecco's modified Eagle's medium (DMEM) containing
10% fetal bovine serum (FBS) in a humidified atmosphere of 5%
CO2 at 37°C. Upon reaching 50–60% confluence, the cells
were treated with angiotensin II (AngII) (1 µM) or resveratrol at
various concentrations (10, 25, and 50 µM) dissolved in dimethyl
sulfoxide (DMSO) alone or in combination. The concentrations used
in the present study were based on the previous report (8).
Cell growth and proliferation
assay
Cell growth was measured using the MTT assay. In
brief, cells (104 cells/well) were seeded and cultured
in 96-well plates overnight. After treatment with AngII or
resveratrol for indicated periods, the MTT solution (20 µl, 5
mg/ml) was added to each well and incubated at 37°C for 4 h; 150 µl
of DMSO was then added. The absorbance at 490 nm was measured with
a microplate reader. Our initial study showed that 50 µM
resveratrol induced the greatest inhibition of cell growth; thus,
this concentration was chosen for subsequent experiments.
The EdU incorporation assay was used to determine
the cell proliferation rate. The cells (104 cells/well)
were cultured in 96-well plates, and then incubated with 50 nM of
EdU for an additional 2 h at 37°C, fixed with 4% formaldehyde for
15 min at room temperature, and then treated with 0.5% Triton X-100
for 20 min at room temperature to permeabilize the cells. The cells
were washed three times with phosphate buffered saline (PBS) and
then incubated in a 1X Apollo reaction cocktail (100 µl/well) for
30 min. DNA was stained with 10 µg/ml Hoechst 33342 solution (100
µl/well) for 20 min and visualized with fluorescence microscopy.
Five fields were randomly selected from each sample image; the
EdU-positive cells were counted, and the relative positive ratio
was calculated.
Cell migration assay
Cell migration was analyzed using the Transwell
system (Corning, Inc., Corning, NY, USA), per the manufacturer's
instructions. In brief, cells (105/well) were seeded on
the upper chamber with serum-free DMEM containing resveratrol (10,
25, and 50 µM) or DMSO. DMEM supplemented with AngII (1 µM) was
added into the lower chamber. The apparatus was incubated for 8 h.
The cells that had not migrated were removed from the top of the
insert. Those cells that did migrate were fixed in methanol and
then stained with crystal violet (0.5%). The migrated cells were
manually counted using phase-contrast microscopy.
Western blot analysis
Total protein was isolated using a protein
extraction kit (Beyotime, Shanghai, China), according to the
manufacturer's instructions. Protein concentration was quantified
using the bicinchoninic acid protein assay (Beyotime). Equal
amounts of protein from all samples were separated using 10% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
then transferred to polyvinylidene difluoride (PVDF) membranes (EMD
Millipore, Billerica, MA, USA). The membranes were blocked with 5%
fat-free milk in Tris-buffered saline with Tween-20 (TBST) and then
incubatedwith primary antibodies at 4°C overnight and subsequently
incubated with secondary antibodies at room temperature for 1 h.
The protein bands were detected using an enhanced chemiluminescence
dependent detection system. The following dilutions were used for
the primary antibodies: Anti-CaMKII antibody (1:1,000; SAB
Biotherapeutics, Inc., Sioux Falls, SD, USA), anti-phosphorylated
(p)-CaMKIIantibody (1:1,000; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA), anti-HDAC4 antibody (1:1,000; Cell Signaling
Technology, Inc., Danvers, MA, USA), anti-p-HDAC4 antibody
(1:1,000; SAB Biotherapeutics, Inc.), anti-α-smooth muscle actin
(α-SMA) antibody (1:1,000; Abcam, Cambridge, MA, USA),
anti-proliferating cell nuclear antigen (PCNA) antibody (1:1,000;
Cell Signaling Technology, Inc.), anti-cyclin-dependent kinase-4
(CDK4) antibody (1:1,000; Abcam), and anti-β-actin antibody
(1:1,000; Cell Signaling Technology, Inc.).
Cell cycle analysis
Cells were cultured in DMEM without serum for 24 h
to induce synchronization in G0-phase of the cell cycle. DMEM
containing serum and resveratrol was then added to the cells to
stimulate cell cycle progression. 48 h later, the cells were
trypsinized, collected, and centrifuged at 1,250 × g for 5 min. To
stain DNA, propidium iodide (PI) (50 µg/ml) was added at 37°C for
30 min; flow cytometry was used to analyze cell cycle.
Rat carotid artery balloon injury and
treatment
All the experimental procedures involving animals
were approved by the Animal Care and Use Committee of South China
Agricultural University. All the animals were fed with a standard
diet of rat chow and housed in a well-controlled environment
(21–26°C; 40–70% humidity; 12/12-h light/dark cycle). Eighteen male
Sprague-Dawley rats (weighing 400–450 g) were randomly divided into
three groups: Sham, injury, and resveratrol-treated groups (n=6 for
each group). The rats were anesthetized by an intraperitoneal
injection of pentobarbital (30 mg/kg) before operation. Balloon
injury of the left common carotid artery was done as previously
published (22). Briefly, after an
intravenous injection of heparin sodium (100 U/kg), the left
common, external, and internal carotid arteries were exposed.
Microvascular clips were used to temporarily block blood flow in
the common and internal carotid arteries. We partially clipped the
external carotid artery with microscissors at ~2 mm distal to the
carotid bifurcation. A balloon angioplasty catheter (diameter=1.25
mm; length=10 mm; Boston Scientific, Marlborough, MA, USA) was
placed in the common carotid artery by way of the external carotid
artery. The balloon was then inflated to create moderate resistance
and pulled back and forth three times. After the catheter was
removed, the external carotid branch was ligated and blood flow in
the common and internal carotid arteries was restored. The same
surgical procedure was applied to the sham group, but without
balloon insertion. The rats in the resveratrol group were treated
with 2 ml resveratrol (50 mg/kg/day) by gavage for 4 weeks. The
sham and injury control groups were treated with the same volume of
physiological saline.
Morphometric analysis
Rats were anesthetized with pentobarbital (200
mg/kg) prior to sacrifice. We carefully dissected the injured
carotid arteries and then fixed them in 4% paraformaldehyde. Three
cross-sections, 6-µm in thickness, were cut from each sample at the
approximate middle of the injured artery, stained with hematoxylin
and eosin (H&E), and observed under a microscope. The internal
and external elastic lamina, lumen, neointimal, and medial areas
were analyzed by Image-Pro Plus 6.0 software, and the ratio of
intima/media areas for each sample was calculated.
Statistical analysis
Data are expressed as mean ± standard error of the
mean. We used one-way analysis of variance (ANOVA) to analyze
differences between groups, with Tukey post hoc test when
necessary. P<0.05 was considered to indicate a statistically
significant difference. All data and statistical analyses were
performed using the SPSS Statistical software (version 17.0; SPSS,
Inc., Chicago, IL, USA).
Results
Resveratrol inhibits AngII-induced
cell proliferation
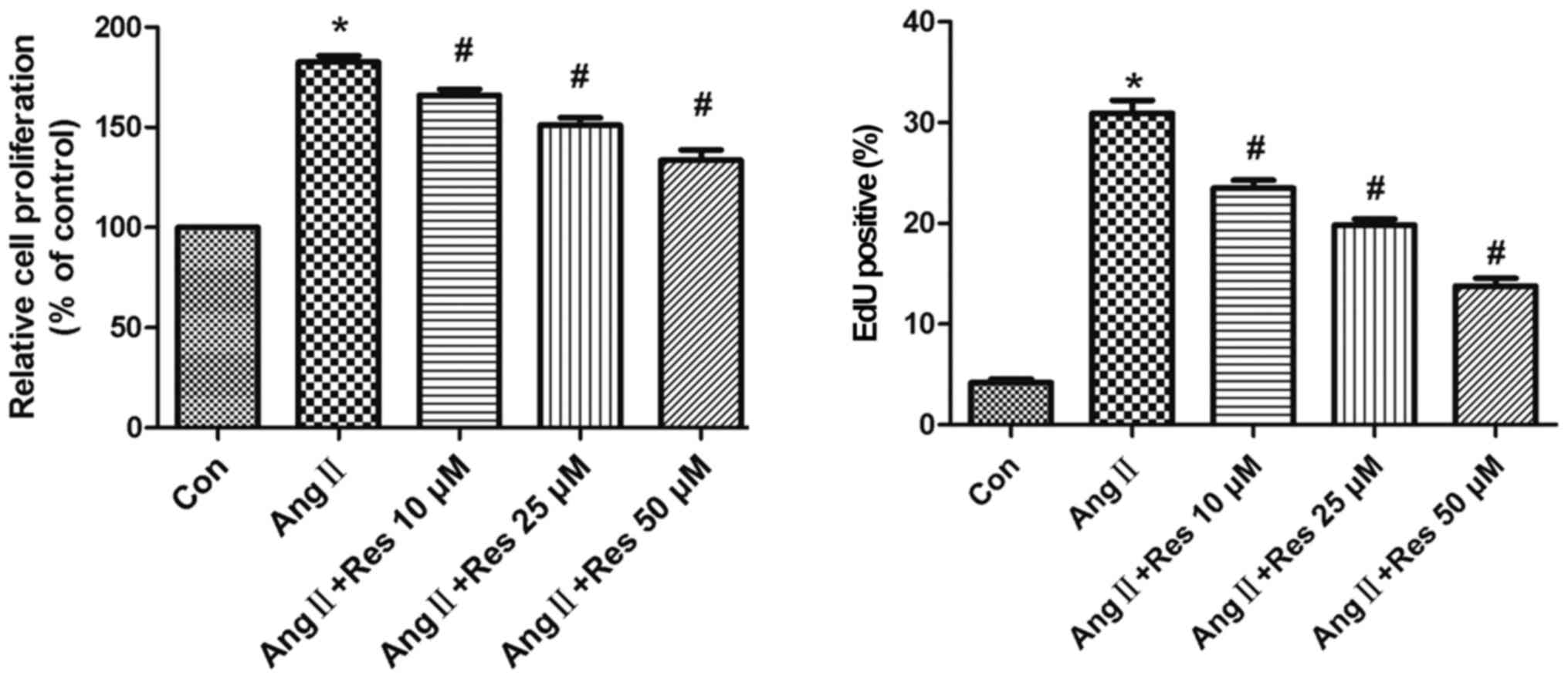
To assess the inhibitory effects of resveratrol on
AngII-stimulated cell proliferation, serum-starved A7r5 cells were
pre-treated with various concentrations of resveratrol for 30 min
and then incubated with AngII (1 µM) for 24 h. As shown in Fig. 1, the results from the MTT assay
indicated that AngII induced growth of A7r5 cells, which was
significantly prevented by resveratrol treatment in a
concentration-dependent manner. The results from the EdU
incorporation assay confirmed that AngII significantly increased
the amount of DNA, an index of increased cell proliferation.
Furthermore, the results showed that resveratrol significantly
inhibited cell proliferation in a concentration-dependent manner.
These data suggest that resveratrol exerts a potent
anti-proliferative effect on AngII-induced cell proliferation.
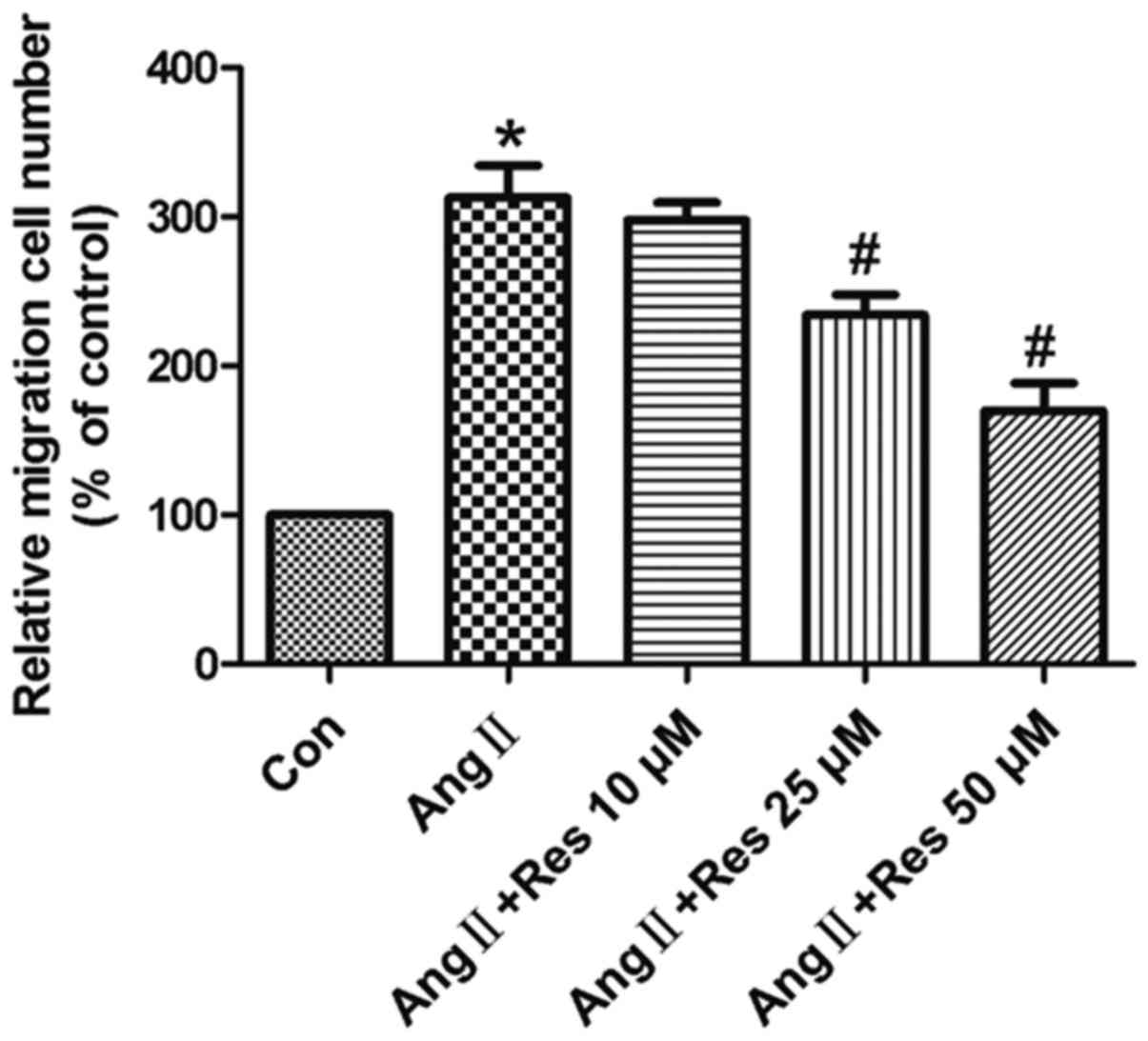
Resveratrol attenuates AngII-induced
cell migration
In the present study, cell migration was measured
using the Transwell chamber assay. As shown in Fig. 2, AngII stimulated cell migration,
compared to the unstimulated control group, and resveratrol
significantly inhibited migration in a concentration-dependent
manner.
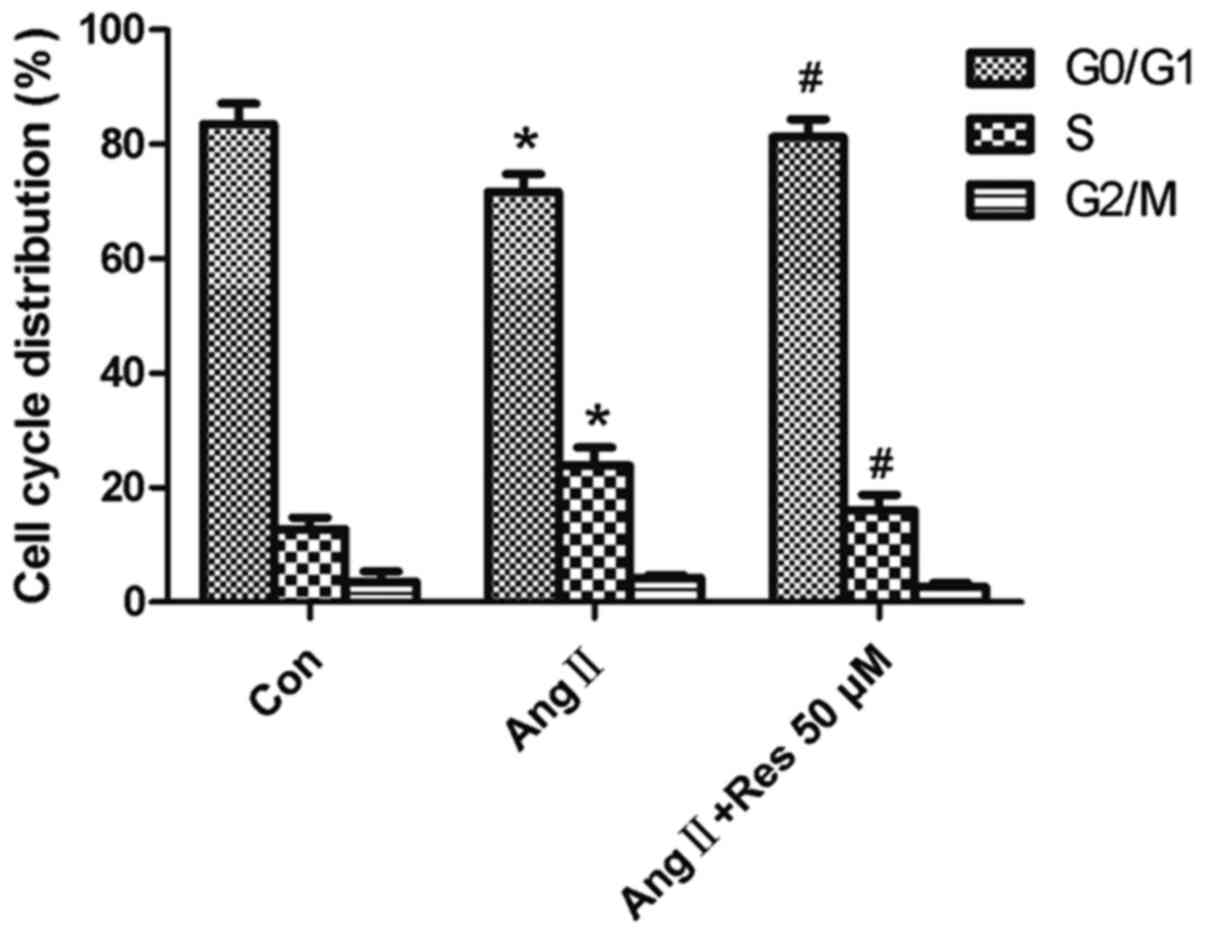
Resveratrol prevents AngII-induced
S-phase entry
As shown in Fig. 3,
pretreatment with resveratrol (50 µM) increased the number of cells
arrested in G0/G1, accompanied by a
concurrent decrease in the number of cells in S-phase, compared to
the AngII-treated group. These results indicate that resveratrol
inhibits AngII-induced G1-S progression. These results also suggest
that resveratrol may prevent AngII-induced S-phase entry in A7r5
cells as a mechanism for inhibiting cell growth, as observed
above.
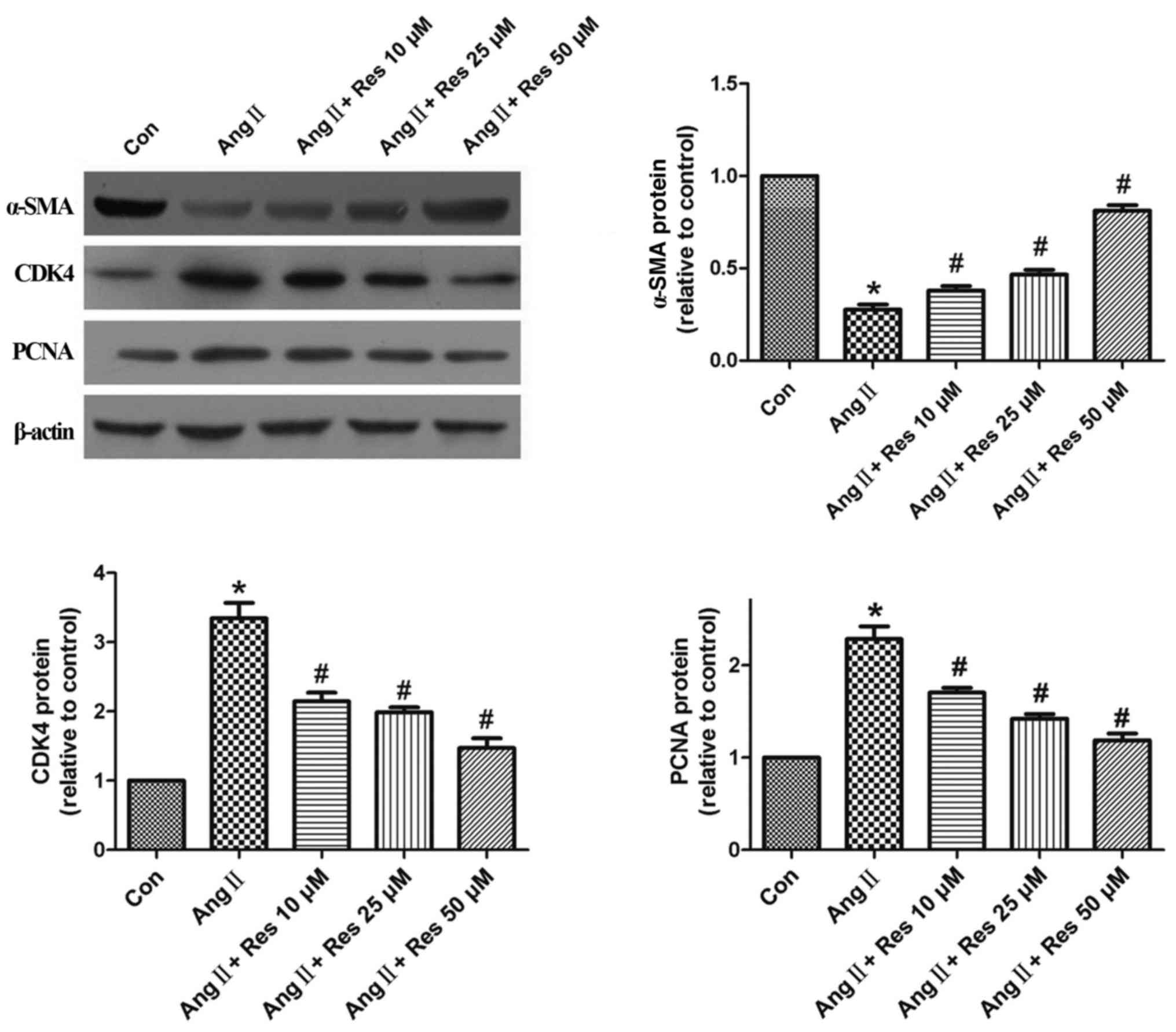
Resveratrol upregulates α-SMA and
suppresses CDK4 and PCNA protein expression
To investigate the underlying mechanism of
resveratrol's inhibitory effect on AngII-induced cell
proliferation, α-SMA, CDK4, and PCNA protein expression were
measured by western blot analysis. As shown in Fig. 4, stimulation with AngII (1 µM) for
24 h decreased α-SMA protein expression, compared to the control
group. Pretreatment with various concentrations of resveratrol for
30 min prior to AngII stimulation reversed this effect in a
concentration-dependent manner, compared to the AngII-stimulated
group. AngII-induced CDK4 and PCNA expression was also
significantly inhibited by resveratrol in a concentration-dependent
manner compared to the control.
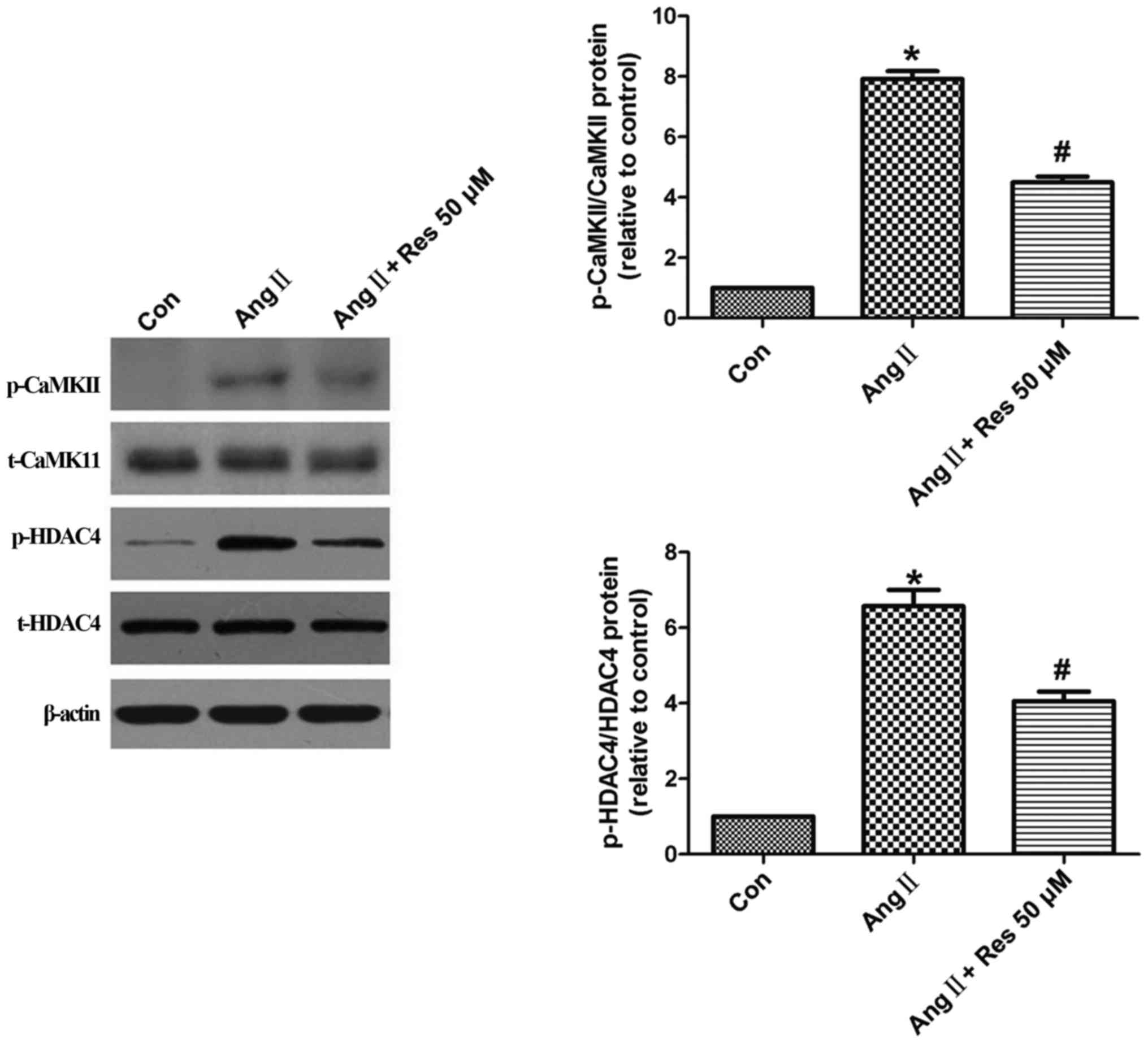
The CaMKII-HDAC4 pathway is involved
in resveratrol's inhibition of cell proliferation and
migration
To examine the potential role of the CaMKII-HDAC4
pathway in resveratrol's inhibition of AngII-induced proliferation
and migration, A7r5 cells were pretreated with resveratrol for 30
min and then stimulated with AngII (1 µM) for another 30 min. AngII
stimulation significantly increased p-CaMKII levels, which was
reversed by resveratrol (Fig. 5).
AngII similarly increased HDAC4 activation, which was also reversed
by resveratrol. No changes in total CaMKII or HDAC4 protein
expression were detected among the three groups.
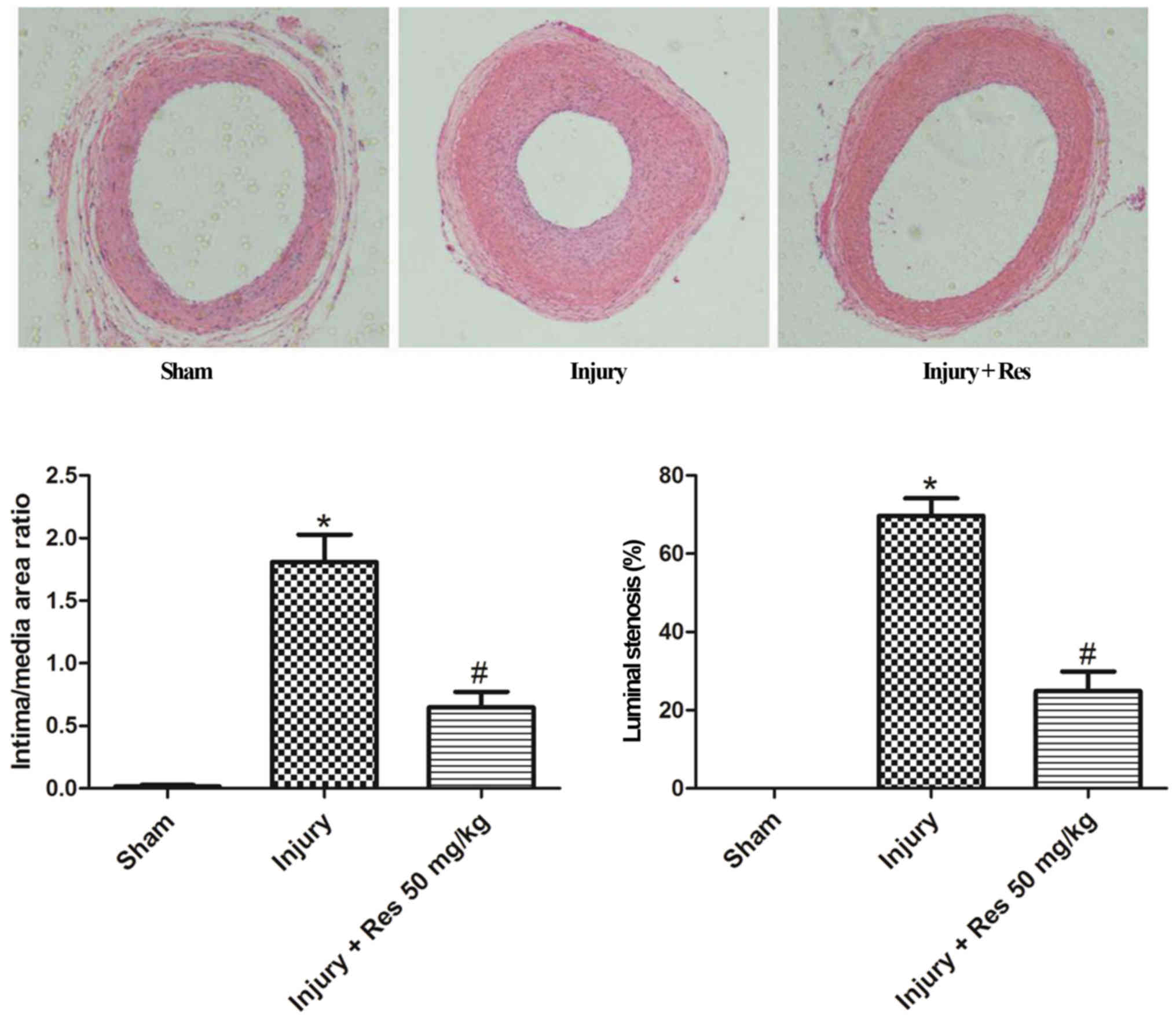
Resveratrol attenuates neointimal
hyperplasia in balloon-injured arteries
To investigate the effect of resveratrol on
neoinimal hyperplasia, rats were treated with resveratrol (50
mg/kg/day) by gastric gavage at 3 days before balloon injury and 28
days after balloon injury. As shown in Fig. 6, significant neointimal hyperplasia
was observed 28 days after balloon injury. The rats treated with
resveratrol showed a remarkable reduction in neointimal
hyperplasia, and the ratio of intima/media areas was significantly
decreased by over 64%, compared to the injured group.
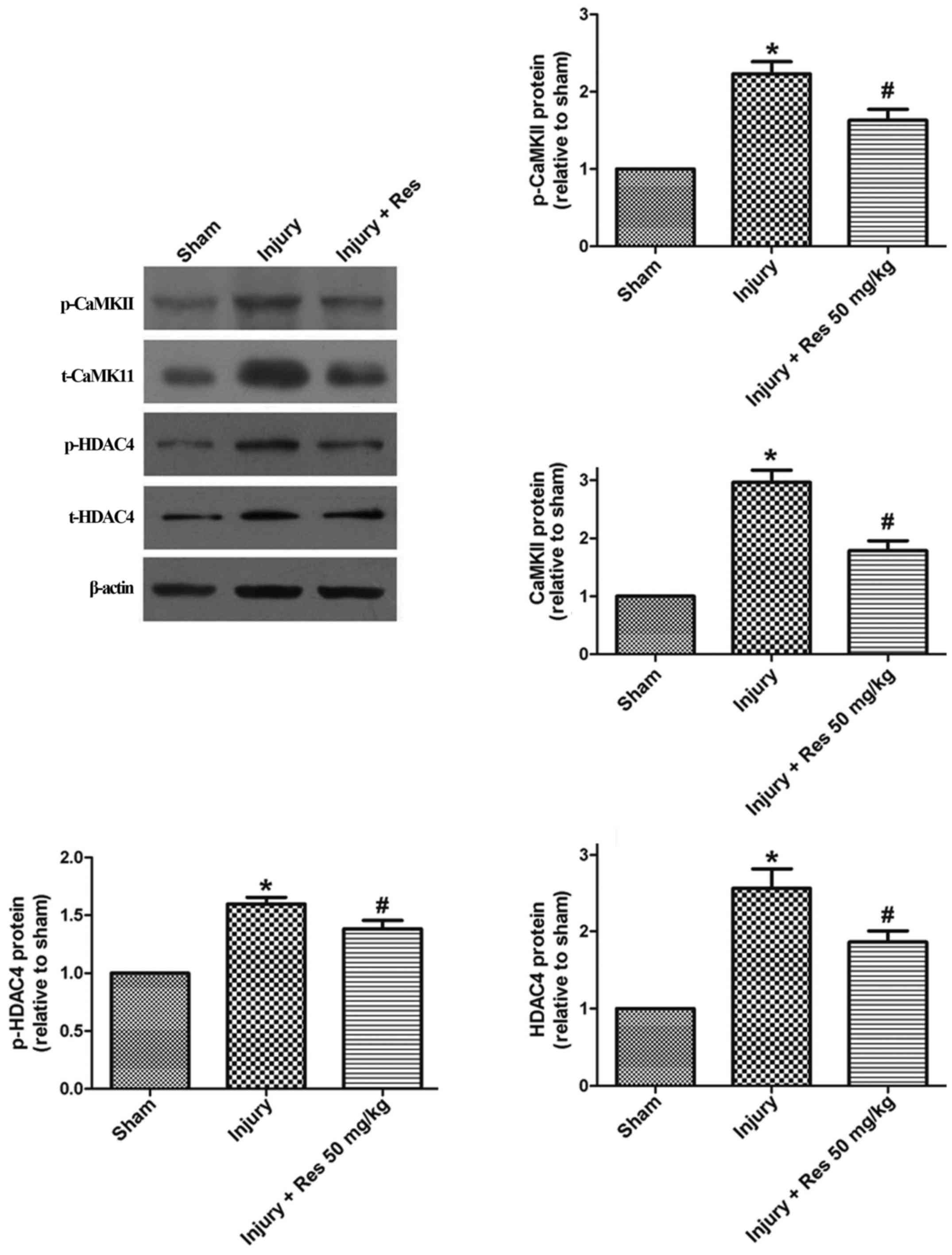
The CaMKII-HDAC4 pathway is involved
in the inhibitory effect of resveratrol on balloon injury
To explore the possible effect of resveratrol on the
CaMII-HDAC4 pathway after balloon injury, total CaMKII, p-CaMKII,
total HDAC4, and p-HDAC4 were measured in tissue samples using
western blot analysis. Total CaMKII, p-CaMKII, total HDAC4, and
pHDAC4 protein levels were all increased after balloon injury
compared to the sham group, and the effect on protein expression
was reversed by resveratrol treatment (Fig. 7).
Discussion
The major finding of this study is that resveratrol
attenuated AngII-induced VSMC proliferation and migration via
inhibiting CaMKII-HDAC4 signaling, which also resulted in decreased
neointimal formation in balloon-injured carotid arteries.
Changes in VSMC proliferation and migration are
largely reported to contribute to neointimal hyperplasia and
restenosis after angioplasty (23). It has been demonstrated that
environmental factors, such as PDGF, endothelin, transforming
growth factor, and inflammatory mediators, can induce synthetic
smooth muscle cell phenotypic differentiation (24). AngII is a well-documented activator
of VSMC proliferation and migration, which promotes atherosclerosis
and vessel stenosis (25–27). Here, we showed that resveratrol
inhibited AngII-induced proliferation and migration in A7r5 cells.
These results are consistent with previous studies that
demonstrated the anti-proliferative effects of resveratrol on VSMC
proliferation following serum (24) or PDGF-BB (28) stimulation.
Our results indicate that resveratrol's inhibitory
effect on AngII-induced cell proliferation might be associated with
its effects on α-SMA, PCNA, and CDK4 expression. α-SMA is a marker
of VSMC differentiation, PCNA is an indicator of cell
proliferation, and CDK4 plays a role in cell cycle regulation. The
Cyclin D/Cdk4 complex is integral for cell cycle progression from
G1 to S phase. Our results demonstrated that resveratrol increased
α-SMA expression and decreased PCNA and CDK4 expression in A7r5
cells stimulated by AngII, suggesting that resveratrol inhibits
cells proliferation through regulating α-SMA, PCNA, and CDK4.
The CaMKII-HDAC4 pathway plays an important role in
VSMC proliferation and migration. It has been shown that
resveratrol influences CaMKII activity in various vascular
diseases, such as diabetes-induced retinal neuronal cell death
(20) and aortic banding in rats
(21). In the present study, we
demonstrated that resveratrol inhibited AngII-induced
phosphorylation of CaMKII and HDAC4, suggesting that resveratrol
inhibits A7r5 proliferation, and migration maybe mediated by the
CaMKII-HDAC4 pathway.
In the present study, we attempted to confirm the
above in vitro results with in vivo experiments.
Neointimal hyperplasia is a common pathological process in vascular
diseases like atherosclerosis and restenosis, and is largely driven
by increased VSMC proliferation. We further demonstrated that
resveratrol decreased neointima formation and the ratio of intima
to media at 4 weeks after surgery. Meanwhile, resveratrol decreased
expression of total and phosphorylated CaMKII and HDAC4 in injured
arteries. In summary, our findings suggest that resveratrol may
attenuate neointimal hyperplasia via regulating the CaMKII-HDAC4
pathway.
Our study demonstrated that resveratrol inhibits
VSMC cell proliferation and migration in vitro, as well as
decreases neointimal hyperplasia in vivo through regulating
the CaMKII-HDAC4 pathway. The present study supports future
development of resveratrol as a therapeutic agent for the treatment
of proliferative vascular diseases, such as atherosclerosis and
restenosis after angioplasty.
Acknowledgements
The animal experimental procedures were performed at
the South China Agricultural University (Guangzhou, China). The
authors would like to thank the Animal Use and Care Committee of
South China Agricultural University for reviewing and approving the
experimental protocols and monitoring the study for animal welfare
issues. The authors would also like to thank the professional staff
in the animal center of the University for their excellent
assistance during the study.
Funding
The present study was supported by the Medical and
Health Science and Technology Project of Guangzhou (grant no.
20161A011072), the Science and Technology Planning Project Of
Guangdong Province (grant no. 2013B021800179), The Key Medical
Disciplines and Specialties Program of Guangzhou (2017–2019), The
Young Innovation Talents Project from The Department of Education
of Guangdong Province (grant no. 2016KQNCX130) and The National
Natural Science Foundation of China (grant no. 81570259).
Availability of data and materials
All the data generated or analyzed during this study
have been included in this published article. Original data can be
verified upon request.
Authors' contributions
XL and SL conceived and designed the experiments and
wrote the paper. XL, JZ, PM, CC, CL and QH performed the
experiments. BL and WO analyzed the data.
Ethics approval and consent to
participate
All of the experimental procedures involving animals
were approved by the Animal Care and Use Committee of South China
Agricultural University (Guangdong, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sturek M and Reddy HK: New tools for
prevention of restenosis could decrease the ‘oculo-stento’ reflex.
Cardiovasc Res. 53:292–293. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Han M, Wen JK, Zheng B, Cheng Y and Zhang
C: Serum deprivation results in redifferentiation of human
umbilical vascular smooth muscle cells. Am J Physiol Cell Physiol.
291:C50–C58. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Carrizzo A, Forte M, Damato A, Trimarco V,
Salzano F, Bartolo M, Maciag A, Puca AA and Vecchione C:
Antioxidant effects of resveratrol in cardiovascular, cerebral and
metabolic diseases. Food Chem Toxicol. 61:215–226. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dong HH and Ren HL: New progression in the
study of protective properties of resveratrol in anticardiovascular
disease. Bratisl Lek Listy. 105:225–229. 2004.PubMed/NCBI
|
|
5
|
Zordoky BN, Robertson IM and Dyck JR:
Preclinical and clinical evidence for the role of resveratrol in
the treatment of cardiovascular diseases. Biochim Biophys Acta.
1852:1155–1177. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ekshyyan VP, Hebert VY, Khandelwal A and
Dugas TR: Resveratrol inhibits rat aortic vascular smooth muscle
cell proliferation via estrogen receptor dependent nitric oxide
production. J Cardiovasc Pharmacol. 50:83–93. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brito PM, Devillard R, Nègre-Salvayre A,
Almeida LM, Dinis TC, Salvayre R and Augé N: Resveratrol inhibits
the mTOR mitogenic signaling evoked by oxidized LDL in smooth
muscle cells. Atherosclerosis. 205:126–134. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guo R, Li W, Liu B, Li S, Zhang B and Xu
Y: Resveratrol protects vascular smooth muscle cells against high
glucose-induced oxidative stress and cell proliferation in vitro.
Med Sci Monit Basic Res. 20:82–92. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang GX, Zhao XY, Meng ZX, Kern M,
Dietrich A, Chen Z, Cozacov Z, Zhou D, Okunade AL, Su X, et al: The
brown fat-enriched secreted factor Nrg4 preserves metabolic
homeostasis through attenuation of hepatic lipogenesis. Nat Med.
20:1436–1443. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang J, Chen J, Yang J, Xu CW, Pu P, Ding
JW and Jiang H: Resveratrol attenuates oxidative stress induced by
balloon injury in the rat carotid artery through actions on the
ERK1/2 and NF-kappa B pathway. Cell Physiol Biochem. 31:230–241.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Breen DM, Dolinsky VW, Zhang H, Ghanim H,
Guo J, Mroziewicz M, Tsiani EL, Bendeck MP, Dandona P, Dyck JR, et
al: Resveratrol inhibits neointimal formation after arterial injury
through an endothelial nitric oxide synthase-dependent mechanism.
Atherosclerosis. 222:375–381. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fujisawa H: Regulation of the activities
of multifunctional Ca2+/calmodulin-dependent protein kinases. J
Biochem. 129:193–199. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li H, Li W, Gupta AK, Mohler PJ, Anderson
ME and Grumbach IM: Calmodulin kinase II is required for
angiotensin II-mediated vascular smooth muscle hypertrophy. Am J
Physiol Heart Circ Physiol. 298:H688–H698. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
House SJ, Ginnan RG, Armstrong SE and
Singer HA: Calcium/calmodulin-dependent protein kinase II-delta
isoform regulation of vascular smooth muscle cell proliferation. Am
J Physiol Cell Physiol. 292:C2276–C2287. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mercure MZ, Ginnan R and Singer HA: CaM
kinase II delta2-dependent regulation of vascular smooth muscle
cell polarization and migration. Am J Physiol Cell Physiol.
294:C1465–C1475. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li W, Li H, Sanders PN, Mohler PJ, Backs
J, Olson EN, Anderson ME and Grumbach IM: The multifunctional
Ca2+/calmodulin-dependent kinase II delta (CaMKIIdelta) controls
neointima formation after carotid ligation and vascular smooth
muscle cell proliferation through cell cycle regulation by p21. J
Biol Chem. 286:7990–7999. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
House SJ and Singer HA: CaMKII-delta
isoform regulation of neointima formation after vascular injury.
Arterioscler Thromb Vasc Biol. 28:441–447. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim YH, Kim YS, Kang SS, Cho GJ and Choi
WS: Resveratrol inhibits neuronal apoptosis and elevated
Ca2+/calmodulin-dependent protein kinase II activity in diabetic
mouse retina. Diabetes. 59:1825–1835. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dong Q, Wu Z, Li X, Yan J, Zhao L, Yang C,
Lu J, Deng J and Chen M: Resveratrol ameliorates cardiac
dysfunction induced by pressure overload in rats via structural
protection and modulation of Ca(2+) cycling proteins. J Transl Med.
12:3232014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Usui T, Morita T, Okada M and Yamawaki H:
Histone deacetylase 4 controls neointimal hyperplasia via
stimulating proliferation and migration of vascular smooth muscle
cells. Hypertension. 63:397–403. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li C, Cai X, Sun H, Bai T, Zheng X, Zhou
XW, Chen X, Gill DL, Li J and Tang XD: The δA isoform of calmodulin
kinase II mediates pathological cardiac hypertrophy by interfering
with the HDAC4-MEF2 signaling pathway. Biochem Biophys Res Commun.
409:125–130. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Han M, Wen JK, Zheng B, Liu Z and Chen Y:
Blockade of integrin beta3-FAK signaling pathway activated by
osteopontin inhibits neointimal formation after balloon injury.
Cardiovasc Pathol. 16:283–290. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu C, Chen J, Zhang J, Hu X, Zhou X, Lu Z
and Jiang H: Naringenin inhibits angiotensin II-induced vascular
smooth muscle cells proliferation and migration and decreases
neointimal hyperplasia in balloon injured rat carotid arteries
through suppressing oxidative stress. Biol Pharm Bull.
36:1549–1555. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang J, Chen J, Xu C, Yang J, Guo Q, Hu Q
and Jiang H: Resveratrol inhibits phenotypic switching of
neointimal vascular smooth muscle cells after balloon injury
through blockade of Notch pathway. J Cardiovasc Pharmacol.
63:233–239. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Weber H, Taylor DS and Molloy CJ:
Angiotensin II induces delayed mitogenesis and cellular
proliferation in rat aortic smooth muscle cells. Correlation with
the expression of specific endogenous growth factors and reversal
by suramin. J Clin Invest. 93:788–798. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kohno M, Ohmori K, Nozaki S, Mizushige K,
Yasunari K, Kano H, Minami M and Yoshikawa J: Effects of valsartan
on angiotensin II-induced migration of human coronary artery smooth
muscle cells. Hypertens Res. 23:677–681. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dong X, Yu LG, Sun R, Cheng YN, Cao H,
Yang KM, Dong YN, Wu Y and Guo XL: Inhibition of PTEN expression
and activity by angiotensin II induces proliferation and migration
of vascular smooth muscle cells. J Cell Biochem. 114:174–182. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zghonda N, Yoshida S, Araki M, Kusunoki M,
Mliki A, Ghorbel A and Miyazaki H: Greater effectiveness of
ε-viniferin in red wine than its monomer resveratrol for inhibiting
vascular smooth muscle cell proliferation and migration. Biosci
Biotechnol Biochem. 75:1259–1267. 2011. View Article : Google Scholar : PubMed/NCBI
|