Introduction
Innate immunity is the first line of host defense
against viral infection. Upon viral infection, pattern recognition
receptors, including Toll-like receptors and RIG-I-like receptors
(RLRs), recognize viral RNAs, which initiate a series of signaling
cascades. RLRs consist of retinoic acid-inducible gene I (RIG-I)
and melanoma differentiation-associated protein 5 (MDA-5), which
are cytosolic helicases sensing viral RNA (1–5).
RIG-I and MDA5 are structurally similar, containing two N-terminal
caspase activation and recruitment domain (CARD) domains, a central
DEAD box helicase/ATPase domain with RNA helicase activity.
Although RIG-I and MDA5 recognize different viral RNAs, VISA (also
known as MAVS, IPS-1 and Cardif) is an essential adaptor located on
the outer membrane of mitochondria shared by RIG-I and MDA5. VISA
has an N-terminal CARD domain for interaction with RIG-I and MDA5,
a C-terminal transmembrane domain that inserts VISA on the outer
membrane of the mitochondria and a central proline-rich domain that
interacts with various downstream signaling proteins to transduce
antiviral signals (6–11). Once RLRs recognize viral RNA,
activated RIG-I and MDA5 translocate to mitochondria and bind to
VISA. Activation of VISA at the mitochondria acts as a platform for
the recruitment of various signaling molecules, including
tank-binding kinase 1 (TBK1), IκB kinase (IKK)ε, IKKα, IKKβ, NF-κB
essential modulator and other signaling proteins to form a VISA
signalosome in the RLR-VISA signaling pathway. This results in the
phosphorylation and activation of IFN regulatory factor (IRF)3/IRF7
by TBK1/IKKε and nuclear factor (NF)-κB through the IKK complex,
inducing IFN and proinflammatory cytokine expression (8–13).
Although a number of interacting partners have been
described in association with VISA (12), further investigation is required of
the subtle regulatory mechanisms of VISA in antiviral signaling
pathways through the identification of novel VISA interaction
partners and its underlying signaling mechanisms. A yeast
two-hybrid screen for VISA interacting partners identified HAUS
augmin-like complex subunit 8 (HAUS8) as a VISA interacting
partner. HAUS8 is a microtubule-associated protein essential for
maintaining spindle integrity and chromosomal stability (14). The present study demonstrated that
HAUS8 positively regulated the RLR-VISA dependent antiviral
signaling pathway by recruiting the VISA complex, leading to
promotion and activation of the transcription factors IRF3 and
NF-κB to facilitate the activation of the IFN-β promoter induced by
Sendai virus infection. Further study additionally revealed that
HAUS8 increased the polyubiquitination of VISA, RIG-I and TBK1.
Collectively, HAUS8 may positively regulate the RLR-VISA antiviral
signaling pathway.
Materials and methods
Cell culture and reagents
293T cells were cultured in Dulbecco's modified
Eagle's medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc.) at 37°C in a 5% CO2 incubator. Sendai
virus was provided by Dr Hong-Bing Shu (Wuhan University, Wuhan,
China). The mouse anti-Flag and anti-hemagglutinin (HA) monoclonal
antibodies were obtained from Sigma-Aldrich; Merck KGaA (Darmstadt,
Germany). The rabbit antibody against IRF3 (cat no. sc-9082) and
the goat antibody against actin (cat no. sc-1616) were purchased
from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). The
dual-luciferase reporter assay system was obtained from Promega
Corporation (Madison, WI, USA).
Constructs
CMV promoter-based mammalian expression plasmids for
human Flag-tagged RIG-I, Flag-tagged N terminal of RIG-I (RIG-I-N;
1–284), Flag-tagged VISA, Flag-tagged TBK1, Flag-tagged
TNF-receptor-associated factor (TRAF)3 and Flag-tagged IRF3-5D,
HA-tagged ubiquitin, IFN-β promoter luciferase reporter plasmid,
ISRE luciferase reporter construct and NF-κB luciferase reporter
construct (8,15) were provided by Dr. Hong-Bing Shu.
Human HAUS8 and mammalian expression vector pRK-5 (also provided by
Dr. Hong-Bing Shu) with an N-terminal HA or Flag fusion tag were
digested using the restriction enzymes Sal I and Not I (New England
Biolabs, Inc., Ipswich, MA, USA), and constructed using ligase (New
England Biolabs, Inc.) according to the manufacturer's
instructions. An empty expression vector pRK-5 was used as a
negative control in HAUS8 overexpression associated experiments.
The human HAUS8 RNA interference (RNAi) constructs were generated
using the pSuper. Retro vector (OligoEngine, Seattle, WA, USA),
following protocols recommended by the manufacturer. A pSuper.
Retro RNAi plasmid targeting green fluorescent protein (GFP) was
used as a control in HAUS8 knockdown associated experiments. The
target sequences for the human HAUS8 constructs were: small
interfering (si)#1, 5′-CCTGGATCTCTCTGCTATT-3′; si#2,
5′-CCGGATTTATCTGAAGCAA-3′; si#3, 5′-CCGTAAAGATGGAGAACAA-3′; and the
target sequence used for GFP was 5′-GCAAGCTGACCCTGAAGTT-3′
(provided by Dr. Hong-Bing Shu).
Yeast two-hybrid screen and
dual-luciferase reporter assay
Yeast strain AH109, purchased from (Clontech
Laboratories, Inc., Mountainview, CA, USA), was transfected with
plasmids containing 1 µg human VISA (pGBT9-VISA; Clontech
Laboratories, Inc.) and 100 µg human 293T (pACT2; Clontech
Laboratories, Inc.) using the lithium acetate/single-stranded
carrier DNA/polyethylene glycol method (16). Positive clones were screened for
via nutritional defects (Ser, Thr and His), and analyzed via
sequencing (BGI, Shenzhen, China). 293T cells (~2.5×105)
were plated in 24-well plates and transfected, using the calcium
phosphate method (17), with
plasmids carrying an IFN-β promoter, ISRE or NF-κB luciferase
reporter gene (firefly luciferase; 100 ng/well) and pRL-TK
(Renilla luciferase plasmid; 50 ng/well) together with a
doses of pRK5-HAUS8 (0.05, 0.1, 0.2 and 0.4 µg), GFP-siRNA or
HAUS8-specific siRNAs (0.5 µg), pRK5-RIG-I-N (1–284), pRK5-VISA,
pRK5-TBK1 or pRK5-IRF3-5D [(in the presence or absence of HAUS8,
(0.5 µg per plasmid)]. The same amount of total DNA in each
transfection was normalized by added corresponding empty control
plasmid or siGFP. A total of 12 h post-transfection, cells were
infected with (+) or without (−) Sendai virus for 12 h in 37°C
incubator as previously described (1,8), at
a multiplicity of infection of 5 plaque-forming units/cell, and
cells were then collected and lysed using 1X passive lysis buffer
included in the Dual-Luciferase® Reporter Assay System
(cat. no. E1980; Promega Corporation). Subsequently, luciferase
activity was measured with the dual-luciferase reporter assay
system (Promega Corporation) with a GloMax™ 20/20
Luminometer (Promega Corporation) according to the manufacturer's
protocols. Data was normalized by the ratio of firefly luciferase
activity to Renilla luciferase activity, and the experiments
were repeated three times.
Immunoprecipitation
293T cells (~6×106) were seeded in 100 mm
dishes and transfected with pRK5-Flag-RIG-I (8 µg), pRK5-Flag-VISA
(8 µg), pRK5-Flag-TBK1 (8 µg), pRK5-HA-HAUS8 (8 µg), empty control
plasmids (8 µg) or pRK5-HA-ubiquitin (8 µg; in the presence or
absence of HAUS8) using the calcium phosphate method (8,15).
The same amount of total DNA in each transfection was normalized by
added corresponding empty control plasmid. A total of 12 h
post-transfection, cells were treated with or without Sendai virus
for 12 h at 37°C. A total of 22 h subsequent to transfection, cells
were collected and lysed with lysis buffer containing 20 mM Tris
(pH 7.5), 150 mM NaCl, 1% Triton, 1 mM EDTA, 10 µg/ml aprotinin, 10
µg/ml leupeptin and 1 mM phenylmethylsulfonyl fluoride (PMSF). For
each immunoprecipitation, 0.5 ml lysate was incubated with the
appropriate amount of indicated antibody [anti-HA (1:1,500; cat.
no. H3663; Sigma-Aldrich; Merck KGaA), anti-RIG-I, VISA, or TBK1
(1:1,000; cat. no. 8348; Cell Signaling Technology] and ~30 µl
protein G/A-Sepharose beads in 20% ethanol (GE Healthcare, Chicago,
IL, USA) overnight at 4°C. The Sepharose beads were washed three
times with 1 ml lysis buffer containing 500 mM NaCl, and the
precipitates were fractionated by 10% SDS/PAGE.
Western blot
Cells were lysed using the aforementioned lysis
buffer for 1 h at 4°C as described above. Protein concentration was
determined using a bicinchoninic acid protein assay kit (Tiangen
Biotech Co., Ltd., Beijing, China). Proteins samples (10 µg) were
separated using 10% SDS-PAGE, transferred onto a nitrocellulose
membrane (EMD Millipore, Billerica, MA, USA) and then blocked using
5% non-fat milk for 1 h at room temperature. Membranes were then
incubated with anti-Flag or anti-HA antibodies (1:4,000; cat. no.
H3663; Sigma-Aldrich; Merck KGaA), anti-IRF3 (1:2,000; cat. no.
sc-9082; Santa Cruz Biotechnology, Inc.), anti-P65 or anti-p-P65
(1:2,000; cat. no. 9936; Cell Signaling Technology, Inc.) and
anti-β actin (1:2,000; cat. no. sc-8432; Santa Cruz Biotechnology,
Inc.) overnight at 4°C. Membranes were then incubated with the
following secondary antibodies for 1 h at 4°C: Horseradish
peroxidase (HRP)-conjugated goat anti-mouse IgG Ab (1:5,000; cat.
no. 1706516; Bio-Rad Laboratories, Inc., Hercules, CA, USA) or
HRP-conjugated anti-rabbit IgG Ab (1:5,000; cat. no. 7074; Cell
Signaling Technology, Inc.). Proteins were visualized using the
enhanced chemiluminescent reagent in the Gel Doc XR Gel
Documentation System (Bio-Rad Laboratories, Inc.) according to the
manufacturer's instructions. The bands were analyzed using Image
Lab software (version 5.1; Bio-Rad Laboratories, Inc.).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted using an Eastep®
Super total RNA Extraction Kit (Promega Corporation) according to
the manufacturer's protocol, and cDNA was synthesized using an
GoScript™ Reverse Transcription System (Promega Corporation). An
Eastep® qPCR Master Mix kit (Promega Corporation) was
used for real-time fluorescent qPCR assays. PCR thermocycling
conditions were as follows: 50°C for 2 min and 95°C for 10 min;
followed by 40 cycles of denaturation at 95°C for 15 sec, annealing
at 60°C for 1 min; and a final extension at 72°C for 5 min.
Expression levels of genes of interest were normalized to the
expression of β-actin. Relative gene expression data was analyzed
using the 2−ΔΔCq method (18). The primers used for qPCR were as
follows: Human (h)HAUS8 forward, 5′-AAGAGTTCAAGGTGGAAGAGTGA-3′;
hHAUS8 reverse, 5′-TCAGACATCTTCCCTCGGGT-3′; hIFN-β forward,
5′-CTAACTGCAACCTTTCGAAGC-3′; hIFN-β reverse,
5′-GGAAAGAGCTGTAGTGGAGAAG-3′; hβ-actin forward,
5′-GTCGTCGACAACGGCTCCGGCATG-3′; and hβ-actin reverse,
5′-ATTGTAGAAGGTGTGGTGCCAGAT-3′.
Native PAGE assay
293T cells (~1×106) were seeded in 6-well
plates and transfected with the indicated plasmids
(pRK5-Flag-HAUS8, empty vectors, GFP-RNAi or #3 HAUS8 RNAi
plasmids; 2.5 µg each per well). A total of 12 h post-transfection,
cells were infected with (+) or without (−) Sendai virus for 12 h
at 37°C, and followed by collecting and lysing cells using native
lysis buffer for 1 h at 4°C, which was contained 50 mM Tris (pH
8.0), 150 mM NaCl, 1% NP-40, 1 mM EDTA, 1 mM PMSF and 0.5% Sodium
deoxycholate (19). The cell
lysates in native PAGE sample buffer [62.5 mM Tris-Cl (pH 6.8), 15%
glycerol and 1% deoxycholate] were separated on a native PAGE and
then immunoblotted with an anti-IRF3 antibody (19,20).
Statistical analysis
All data presented in the histograms are presented
as the mean ± standard deviation of at least three independent
experiments. The Student's t-test was performed to compare the
statistical significance of differences between two groups, and
one-way analysis of variance with Tukey's post hoc analysis was
used for multiple comparisons (n≥3). These statistical analyses
were performed using GraphPad Prism software (version 5.0; GraphPad
Software, Inc., La Jolla, CA, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Identification of HAUS8 as a positive
regulator of virus-mediated RLR-VISA signaling
As VISA serves a key role in the antiviral signaling
pathway, the present study attempted to further elucidate the
function and underlying mechanism of VISA by looking for novel VISA
interaction proteins. A systematic search for VISA interacting
proteins in a yeast two-hybrid screen (8,21)
was performed, and HAUS8 was identified to be one of the positive
clones by gene sequencing analysis (data not shown). Further
co-immunoprecipitation and immunoblot analyses were performed to
verify whether HAUS8 functions in the RLR-VISA signaling pathway.
293T human kidney cells were transfected with empty vector or
HA-HAUS8 expression vector, and plasmids for Flag-tagged RIG-I,
VISA and TBK. A total of 12 h post-transfection, the cells were
infected with (+) or without (−) Sendai virus, followed by
co-immunoprecipitation with anti-HA beads and western blot analysis
with anti-Flag antibodies. The data revealed that HAUS8 interacted
with RIG-I, VISA and TBK1. The association between HAUS8 and the
three signaling molecules was augmented following infection with
Sendai virus, and HAUS8 was obviously associated with RIG-I upon
Sendai virus infection (Fig. 1A).
These results suggested that HAUS8 may serve a role in the RLR-VISA
signaling pathway by associating with the VISA signalosome.
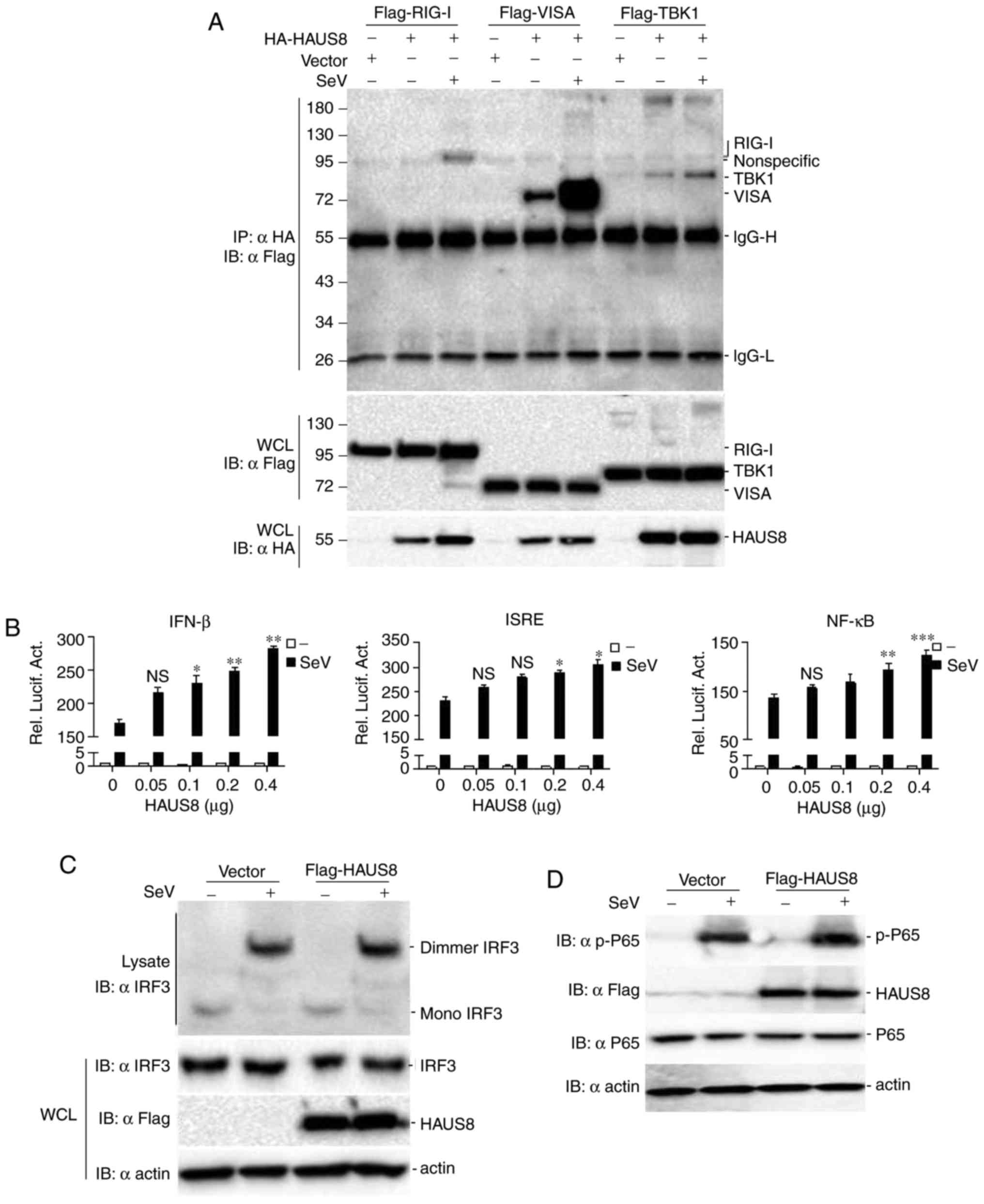 | Figure 1.Identification of HAUS8 as a positive
regulator of virus-mediated RLR-VISA signaling. (A) HAUS8 was
associated with the RLR-VISA signaling complex. 293T human kidney
cells (~6×106) seeded in 100-mm dishes were transfected
with the indicated plasmids [pRK5-Flag-RIG-I, pRK5-Flag-VISA,
pRK5-Flag-TBK1, pRK5-HA-HAUS8 or empty control plasmids (8 µg
each)]. Following 12 h of transfection, cells were infected with or
without Sendai virus for 10 h in 37°C, then cells were harvested
and lysed, and co-immunoprecipitation was performed with anti-HA.
Immunoblot analysis was performed with anti-Flag antibodies (upper
panels). Expression levels of the proteins were analyzed by
immunoblot analysis of the lysates with anti-HA and anti-Flag
antibodies (lower panels). (B) HAUS8 activates the IFN-β promoter,
ISRE and NF-κB. 293T cells (~2.5×105) seeded in 24-well
dishes were transfected with plasmids carrying an IFN-β promoter,
ISRE or NF-κB luciferase reporter gene (firefly luciferase; 100
ng/well) and pRL-TK (Renilla luciferase plasmid; 50
ng/well), together with an empty vector or the indicated dose of
the HAUS8 plasmid (0.05, 0.1, 0.2 and 0.4 µg). Following 12 h of
transfection, cells were infected with or without Sendai virus for
12 h at 37°C. Cells were harvested for luciferase assay. Data were
normalized by the ratio of firefly luciferase activity to
Renilla luciferase activity. HAUS8 enhanced the activation
of IRF3 and NF-κB induced by Sendai virus. 293T cells
(~1×106) seeded in 6-well dishes were transfected with
empty vector or HAUS8 plasmids (2.5 µg/well). Following 12 h of
transfection, cells were infected with (+) or without (−) Sendai
virus for 12 h at 37°C. Subsequently, cells were collected and
lysed, and analyzed for (C) IRF3 dimerization by native gel
electrophoresis or (D) P65 phosphorylation by SDS-PAGE. Error bars
indicate standard deviations. *P<0.05; **P<0.01;
***P<0.001; ns, no significant difference. HAUS8, HAUS augmin
like complex subunit 8; RLR-VISA, RIG-I-like receptors-virus
induced signaling adapter; HA, hemagglutinin; IFN-β, interferon-β;
ISRE, interferon stimulated response element; NF-κB, nuclear
factor-κB; IRF, interferon regulatory factor; WCL, whole cell
lysate. |
To examine whether HAUS8 may be involved in the
regulation of RLR-VISA mediated IFN induction, reporter assays were
performed. The results showed that HAUS8 itself had no effect on
activation of the IFN-β promoter, ISRE and NF-κB. However, HAUS8
potentiated Sendai virus, a negative sense single-stranded RNA
virus recognized by RIG-I (2), and
induced activation of the IFN-β promoter, ISRE and NF-κB in a
dose-dependent manner (Fig. 1B).
As activation of the IFN-β promoter requires coordinated and
cooperative activation of IRF3 and NF-κB (22–25),
the present study further determined whether HAUS8 potentiated
virus-induced activation of transcriptional factor IRF3 and NF-κB.
Consistently, overexpression of HAUS8 further increased the IRF3
dimerization and P-65 phosphorylation caused by SeV infection
(Fig. 1C and D). NF-κB activation
was measured by P65 phosphorylation, and IRF3 activation was
measured by its dimerization. These results suggested that HAUS8
potentiated virus-mediated RLR-VISA dependent IFN induction.
Knockdown of HAUS8 inhibits IFN-β and
antiviral responses
As overexpression of HAUS8 potentiated
virus-mediated activation of NF-κB and IRF3, leading to activation
of the IFN-β promoter, it was next determined whether endogenous
HAUS8 is required for virus-mediated IFN-β induction by specific
knockdown of HAUS8 endogenous expression.
To test this, the present study generated three
siRNA expression RNAi plasmids (pSuper-HAUS8-RNAi#1-3) that
targeted HAUS8 mRNA, and their effects on the knockdown of HAUS8
expression were determined by western blotting (Fig. 2A, left) and RT-qPCR (Fig. 2A, right). The present study
co-transfected a Flag-HAUS8 plasmid with an siRNA control or
HAUS8-specific siRNAs into 293T cells, and transfected TRAF3 as the
internal control in western blotting experiments. All three siRNAs
partially inhibited the expression of transfected HAUS8 (Fig. 2A). To determine whether HAUS8
serves a role in the virus-mediated activation of NF-κB, IRF3 and
the IFN-β promoter, the HAUS8 RNAi vectors were transfected into
293T cells and reporter assays were performed. As presented in
Fig. 2B, knockdown of HAUS8
expression inhibited Sendai virus-induced ISRE, NF-κB and IFN-β
promoter activation. The degree of inhibition was associated with
the efficiency of knockdown of HAUS8 expression by each RNAi
vector. The HAUS8-RNAi-#3 construct was selected for all additional
experiments described below as it demonstrated the greatest
knockdown of HAUS8. Transfection with HAUS8-RNAi-#3 resulted in
lower virus-mediated IFN-β production at the mRNA level by RT-qPCR
analysis (Fig. 2C).
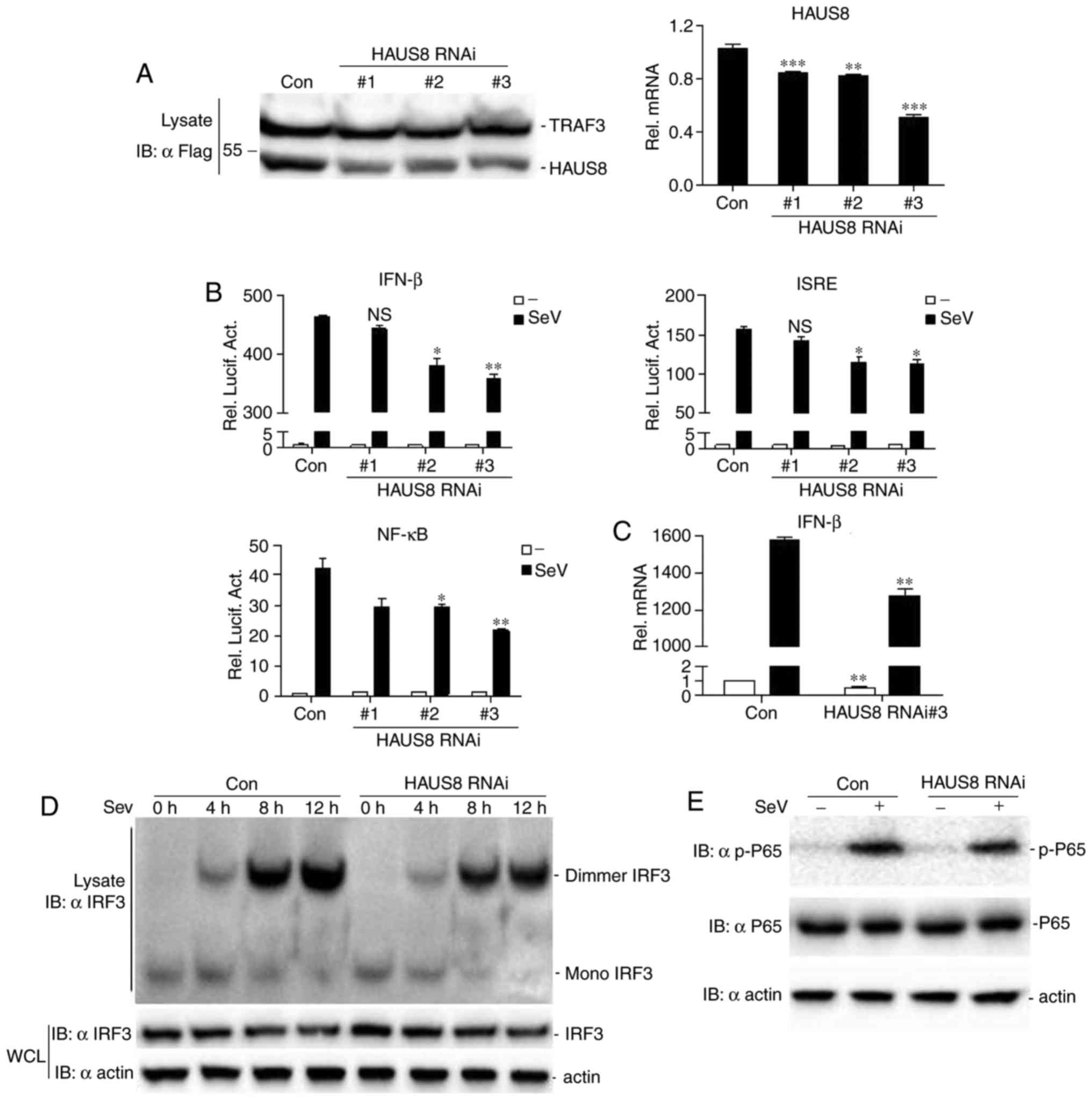 | Figure 2.Knockdown of HAUS8 inhibits IFN-β and
antiviral responses. (A) Effects of HAUS8 RNAi constructs on the
overexpression of HAUS8 by immunoblot analysis (left) or at the
mRNA level by RT-qPCR (right). 293T cells (~1×106) were
seeded in 6-well dishes and were transfected with Flag-HAUS8 (1.5
µg), Flag-TRAF3 (0.5 µg, as the internal control), together with
HAUS8-specific siRNA or control RNAi vector (2 µg). Following 24 h
of transfection, cells were lysed, and the lysates were analyzed by
western blotting with anti-Flag antibodies. 293T cells
(~1×106) seeded in 6-well dishes were transfected with
HAUS8-specific siRNA or control RNAi vector (3 µg). Following 24 h
of transfection, cells were lysed for extracting RNA plasmids and
RT-qPCR was performed. (B) Effect of HAUS8 RNAi constructs on
Sendai virus-induced IFN-β, ISRE or NF-κB activation was
determined. 293T cells (~2.5×105) seeded in 24-well
dishes were transfected with an IFN-β, ISRE or NF-κB luciferase
plasmid (firefly luciferase; 100 ng/well), pRL-TK (internal control
Renilla luciferase plasmid; 50 ng/well), together with
HAUS8-RNAi plasmids or control RNAi vector (0.5 µg). Following 12 h
of transfection, cells were infected with or without Sendai virus
for 12 h prior to the luciferase assays being performed. (C)
RT-qPCR analysis of IFN-β mRNA was performed. 293T cells
(~1×106) seeded in 6-well dishes were transfected with
control RNAi or #3 HAUS8 RNAi plasmids (3 µg), and 12 h subsequent
to transfection, cells were treated with or without Sendai virus
for 10 h prior to RT-qPCR being performed. Knockdown by HAUS8 RNAi
inhibited the activation of IRF3 and NF-κB induced by Sendai virus.
293T cells (~1×106) seeded in 6-well dishes were
transfected with control RNAi or #3 HAUS8 RNAi plasmids (2.5
µg/well). Following 12 h of transfection, the cells were infected
with or without Sendai virus for the indicated time periods for (D)
IRF3 activation detection, or 12 h for (E) NF-κB activation
detection. Error bars indicate the standard deviation. *P<0.05;
**P<0.01; ***P<0.001; ns, no significant difference; RT-qPCR,
reverse transcription-quantitative polymerase chain reaction;
HAUS8, HAUS augmin like complex subunit 8; IFN-β, interferon-β;
RNAi, RNA interference; siRNA, small interfering RNA; ISRE,
interferon stimulated response element; NF-κB, nuclear factor-κB;
SeV, Sendai virus; IRF, interferon regulatory factor; WCL, whole
cell lysate; con, control; TRAF, tumor necrosis factor
receptor-associated factor. |
Furthermore, control RNAi or HAUS8 RNAi were
transfected into 293T cells, then the cells were infected with (+)
or without (−) Sendai virus for different time periods to determine
IRF3 activation by measuring IRF3 dimerization. As presented in
Fig. 2D, HAUS8 knockdown decreased
the dimerization of endogenous IRF3 at different time points.
Knockdown of HAUS8 decreased P65 phosphorylation induced by Sendai
virus (Fig. 2E). Together, these
data suggested that HAUS8 may be required for virus-mediated IRF3
or P65 activation and IFN-β promoter activation.
HAUS8 regulates antiviral signaling
via RLR-VISA dependent signaling downstream of VISA
signalosome
The effect of HAUS8 on RLR-VISA-induced IFN
signaling was investigated to determine whether HAUS8 acts upstream
or downstream of the VISA signalosome. In the luciferase reporter
assay, it was demonstrated that HAUS8 promoted VISA-, RIG-I- and
TBK1-mediated activation of ISRE, NF-κB and the IFN-β promoter
(Fig. 3A).
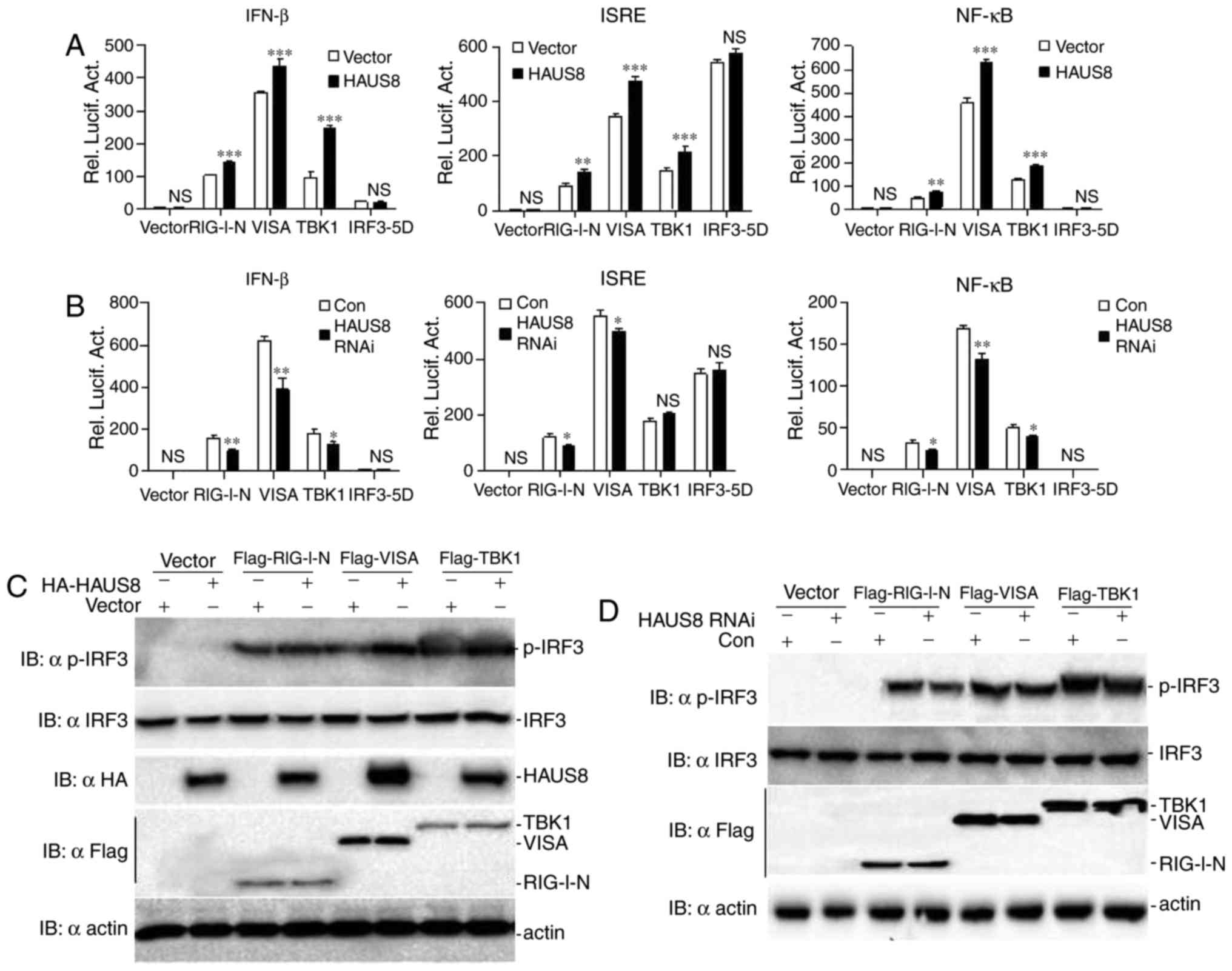 | Figure 3.HAUS8 regulates antiviral signaling
via RLR-VISA dependent signaling downstream of the VISA
signalosome. (A) HAUS8 augmented RIG-I-, VISA- and TBK1-mediated
activation of IFN-β promoter, ISRE or NF-κB. 293T cells
(~2.5×105) seeded in 24-well dishes were transfected
with plasmids carrying an IFN-β promoter, ISRE or NF-κB luciferase
reporter gene (firefly luciferase; 100 ng/well) and pRL-TK
(Renilla luciferase plasmid; 50 ng/well), together with
empty vector or HAUS8 expression vector (0.5 µg), and the indicated
expression vector [pRK5-RIG-I-N (1–284), pRK5-VISA, pRK5-TBK1 or
pRK5-IRF3-5D (0.5 µg each)]. Luciferase assays were performed 24 h
after transfection. (B) HAUS8 knockdown inhibited RIG-I-, VISA- and
TBK1-mediated activation of IFN-β promoter, ISRE or NF-κB. 293T
cells (~2.5×105) seeded in 24-well dishes were
transfected with plasmids carrying an IFN-β promoter, ISRE or NF-κB
luciferase reporter gene (firefly luciferase; 100 ng/well) and
pRL-TK (Renilla luciferase plasmid; 50 ng/well), together
with RNAi control or HAUS8 RNAi (0.5 µg), and the indicated
expression vector [pRK5-RIG-I-N (1–284), pRK5-VISA, pRK5-TBK1 or
pRK5-IRF3-5D (0.5 µg each]. Luciferase assays were performed 24 h
post-transfection. (C) HAUS8 positively regulated RIG-I-, VISA- and
TBK1-mediated activation of phosphorylated IRF3 and TBK1. 293T
cells (~1×106) seeded in 6-well dishes were transfected
with empty vector or HAUS8 expression vector (3 µg), together with
the indicated expression vector (2 µg). Following 24 h of
transfection, cells were harvested for detecting the
phosphorylation of IRF3 and TBK1. (D) HAUS8 knockdown inhibited
RIG-I-, VISA- and TBK1-mediated activation of phosphorylation IRF3
and TBK1. 293T cells (~1×106) seeded in 6-well dishes
were transfected with control RNAi or HAUS8 RNAi (3 µg), together
with the indicated expression vector (2 µg). Following 24 h of
transfection, cells were harvested for detecting the phosphorylated
IRF3 and TBK1. Error bars indicated standard deviation. *P<0.05;
**P<0.01; ***P<0.001; ns, no significant difference. HAUS8,
HAUS augmin like complex subunit 8; RLR-VISA, RIG-I-like
receptors-virus induced signaling adapter; HA, hemagglutinin;
IFN-β, interferon-β; TBK1, TANK-binding kinase 1; ISRE, interferon
stimulated response element; NF-κB, nuclear factor-κB; RIG1,
retinoic acid inducible gene 1; IRF, interferon regulatory factor;
con, control; rel. lucif. act., relative luciferase activity. |
In addition, the assay was performed to detect the
effect of HAUS8 knockdown on RLR-VISA dependent signaling. It was
found that HAUS8 knockdown markedly inhibited RIG-I-, VISA- and
TBK1-, although not IRF3-mediated activation of ISRE, NF-κB and
IFN-β promoter (Fig. 3B).
It was also demonstrated that HAUS8 increased VISA-,
RIG-I- and TBK1-mediated IRF3 activation (Fig. 3C), and HAUS8 knockdown decreased
RIG-I-, VISA- and TBK1-mediated IRF3 activation (Fig. 3D). Taken together, these data
suggested that HAUS8 may act as an active partner to regulate
virus-mediated RLR-VISA signaling by targeting downstream of the
VISA signalosome.
HAUS8 enhances the polyubiquitination
of VISA, RIG-I and TBK1
Tripartite motif-containing (TRIM) 25 is a ubiquitin
E3 ligase that serves a key role in virus-induced RIG-I activation,
by mediating the ubiquitination and oligomerization of RIG-1 and
its interaction with VISA (26). A
number of other E3 ligases, including ring finger protein 135
(27), TRIM4 (28) and mex-3 RNA binding family member C
(29) have additionally been
identified as important regulators in virus-induced IFN induction
by promoting K63-linked ubiquitination for RIG-I activation.
The post-translational modification and
ubiquitination of VISA, and its interacting partners, is a key
aspect of host cell regulation of antiviral signaling. Nedd4 family
interacting protein 1 (30),
proteasome subunit a type 7 (31),
TRIM25 (32) and other proteins
(12) have been reported to
enhance E3 ligase-mediated VISA degradation, leading to negative or
positive regulation of RLR-VISA dependent immune signaling.
It has been reported that the E3 ubiquitin ligases
ring finger protein 128 (33),
mindbomb E3 ubiquitin protein ligase (MIB)1, MIB2 (34) and E3 ubiquitin ligase Nrdp1
(35) positively activate TBK1 by
promoting its K63-linked polyubiquitination during viral infection,
and other proteins such as NLR family pyrin domain containing 4
(36) and TRAF-interacting protein
(37) was reported to negatively
regulate type I IFN signaling by targeting the kinase TBK1.
To determine whether HAUS8 regulates the
ubiquitination of VISA, RIG-I and TBK1, 293T cells were transfected
with the Flag-HAUS8 or empty vector as a control and HA-tagged
ubiquitin, and subsequently infected with or without Sendai virus.
A coimmunoprecipitation assay was performed using anti-VISA,
anti-RIG-I and anti TBK1 as immunoprecipitation antibodies. The
results revealed that HAUS8 markedly augmented the ubiquitination
of VISA, RIG-I and TBK1, and this ubiquitination increased more
following Sendai virus infection (Fig.
4A). Consistent with these results, HAUS8 knockdown attenuated
the ubiquitination of VISA, RIG-I and TBK1 under Sendai virus
infection (Fig. 4B). The results
suggested that HAUS8 positively regulates RLR-mediated antiviral
signaling by promoting the polyubiquitination of VISA, RIG-I and
TBK1 when RLR-VISA signaling is activated.
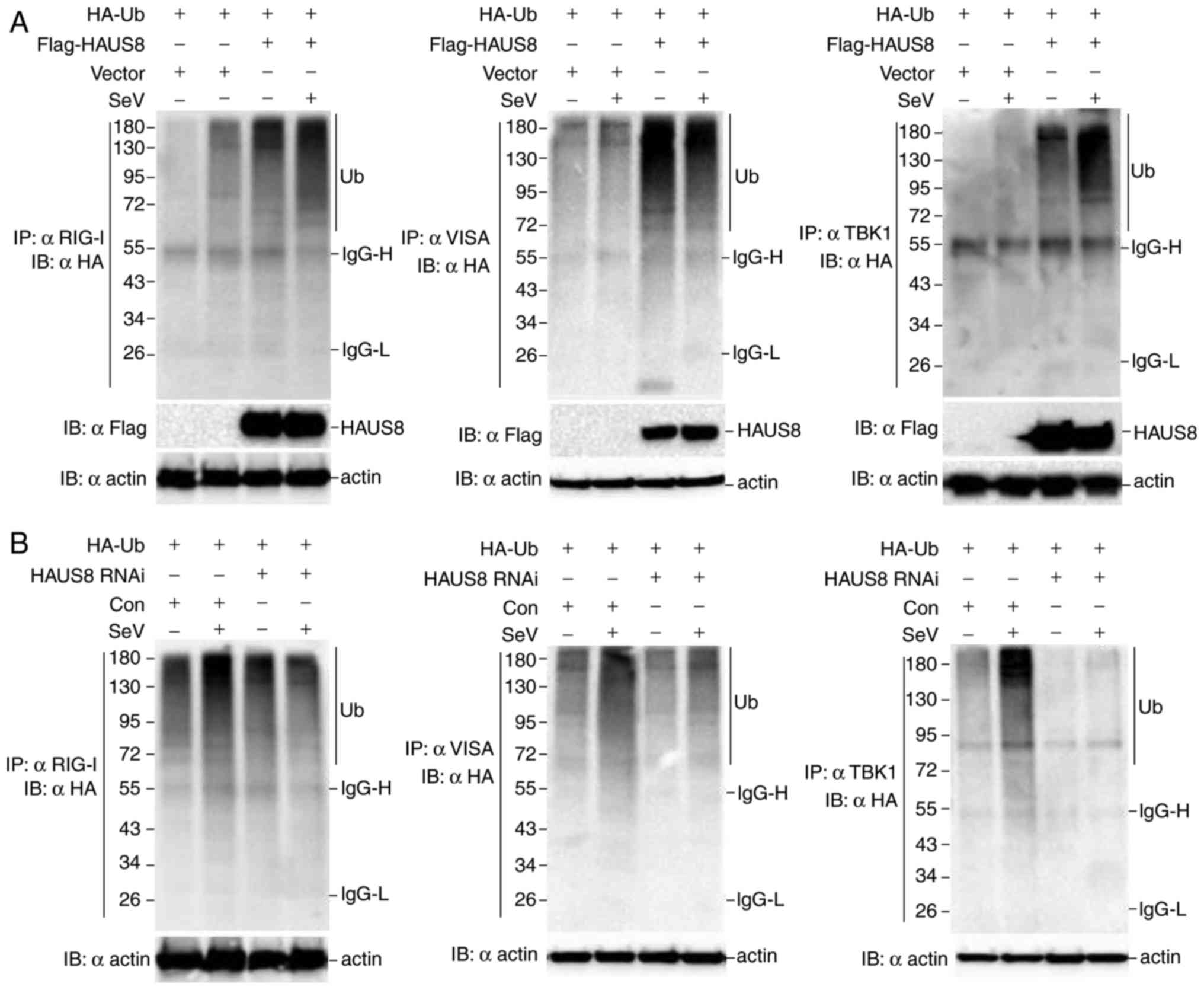 | Figure 4.HAUS8 enhances the polyubiquitination
of VISA, RIG-I and TBK1. (A) 293T cells (~6×106) seeded
in 100-mm dishes were transfected with empty vector or Flag-HAUS8
(8 µg), and HA-ubiquitin (8 µg). Following 12 h of transfection,
cells were infected with or without Sendai virus for 10 h;
immunoprecipitation was performed using anti-RIG-I, anti-VISA or
anti-TBK1 beads and immunoblotting analysis were performed with
anti-HA antibodies. (B) 293T cells (~6×106) seeded in
100-mm dishes were transfected with an RNAi control or HAUS8 RNAi
(8 µg) and HA-ubiquitin (8 µg). Following 12 h of transfection,
cells were infected with or without Sendai virus for 10 h;
immunoprecipitation was performed with anti-RIG-I, anti-VISA or
anti-TBK1 beads and immunoblotting analysis was performed with
anti-HA antibodies. HAUS8, HAUS augmin like complex subunit 8;
VISA, virus induced signaling adapter; HA, hemagglutinin; TBK1,
TANK-binding kinase 1; RIG1, retinoic acid inducible gene 1; RNAi,
RNA interference; Ub, ubiquitin; SeV, Sendai virus. |
Discussion
VISA serves vital roles in RLR signaling by
recruiting activated RIG-I and MDA5 and downstream signaling
proteins to form a protein complex assembly as a platform. Although
multiple partners for the VISA signalosome were identified to
regulate its function by diverse mechanisms, including the
spatiotemporal distribution, mitochondrial dynamics and
post-translational modifications, including phosphorylation and
ubiquitination (13,38), there remains a lack of
comprehensive understanding of the function of VISA. The present
study attempted to investigate how VISA fine-tunes RLR-mediated
antiviral signaling.
It has been reported that the cytoskeletal system,
including microtubules and actin filaments, serves an important
role in antiviral signaling (39,40).
Focal adhesion kinase (FAK) is a protein tyrosine kinase that is
located at focal adhesions to connect the intracellular
cytoskeleton and the extracellular matrix. FAK interacts with VISA
in a viral infection-dependent manner and potentiates VISA-mediated
signaling; virus-induced NF-κB and IFN-β signaling in FAK-deficient
mouse embryonic fibroblasts was attenuated in a previous study, and
the overexpression of FAK in 293T cells enhanced the activation of
the NF-κB and IFN-β promoters in response to Sendai virus infection
(41). Rho guanine nucleotide
exchange factor 2 (GEF-H1) is a microtubule-associated guanine
nucleotide exchange factor for Rac and Rho GTPases. Overexpression
of GEF-H1 in 293T cells enhanced VISA-mediated IRF3 phosphorylation
and IFN-β promoter activation, and GEF-H1-deficient macrophages
exhibited significantly less IRF3 phosphorylation and IFN-β
promoter activation in response to VISA expression compared with
wild-type macrophages (42).
GEF-H1-deficient macrophages are markedly defective in the
induction of IFN-β upon viral infection or under treatment with
synthetic double stranded RNAs. Disruption of microtubule
polarization inhibited the activation of GEF-H1 and, consequently,
RLR signaling (42). It was
additionally reported that actin was translocated onto the
mitochondria, with faster kinetics compared with RIG-I, upon viral
infection (43). Collectively,
these previously published data indicate that, the host
cytoskeletal system have involved in the activation of the NF-κB
and IFN-β in innate immunity.
Consistent with previous findings (14,39,40),
a novel cytoskeleton protein HAUS8 was revealed to be involved in
the innate immune response against RNA viruses in the cytoplasm in
the present study. HAUS8 is a microtubule-associated protein and
serves a notable role in maintaining spindle integrity and
chromosome stability (14). In the
present study, it was demonstrated that HAUS8 is recruited into the
VISA complex by associating with the RIG-I/VISA/TBK1 components of
the RLR-VISA signaling pathway, and overexpression of HAUS8
augmented the activation of the transcription factors IRF3, NF-κB
and the IFN-β promoter induced by Sendai virus-mediated RLR-VISA
dependent antiviral signaling. Further investigation demonstrated
that HAUS8 upregulates the ubiquitination of VISA, RIG-I and TBK1.
The data suggested that HAUS8 acts as a positive regulator of
RLR-VISA signaling.
The data of the present study demonstrated that
HAUS8 associated with RIG-I only under viral infection, and the
association between HAUS8 and VISA or TBK1 increased upon virus
infection. The temporal and spatial associations between HAUS8 and
RIG-I, VISA or TBK1 indicated that HAUS8 may transfer from the
cytoplasm to the mitochondria and become a component of the
RLR-VISA signaling platform to serve an important role in the
redistribution of RIG-I or VISA.
HAUS8 knockdown may inhibit the ability of HAUS8 to
redistribute RIG-I and VISA, leading to impaired activation of
IRF3, NF-κB and the IFN-β promoter induced by Sendai virus. The
results suggested that HAUS8 may be required for the redistribution
of RIG-I and VISA in RLR-VISA signaling.
In conclusion, the present study demonstrated a
novel role of HAUS8, one of the subunits of augmin complex
associated with microtubule generation, in the regulation of the
RLR-VISA dependent signaling pathway, and may connect the
cytoskeleton and RLR-VISA antiviral signaling. Given that HAUS8 is
not an enzyme of the ubiquitination pathway, it was hypothesized
that HAUS8 may be acting as a physical scaffold and may recruit
certain ubiquitin-associated proteins to the VISA signalosome,
promoting the polyubiquitination of VISA, RIG-I and TBK1, leading
to activation of RLR-VISA signaling. Screening candidate E3
ubiquitin ligases that may work together with HAUS8 may help
elucidate a novel mechanism of RLR-VISA regulation, and this
requires further investigation.
Acknowledgements
The authors of the present study would like to thank
Dr Hong-Bing Shu (Medical Research Institute, Wuhan University,
Wuhan, China) for providing plasmids and other reagents.
Funding
This work was supported by grants from the National
Natural Science Foundation of China (grant nos. 31370876 and
31570876), the Natural Science Foundation of Jiangxi Province
(grant nos. 20143ACB20004 and 20161BAB204177), the Open Project
Program of Key Laboratory of Functional Small Organic Molecule,
Ministry of Education, and Jiangxi Normal University (grant no.
KLFS-KF-201407) and the Graduate Innovation Fund of Jiangxi Normal
University (grant no. YC2015-S141).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LX conceived the study. LX and TH performed the
analysis and wrote the manuscript. TH, TC and DW performed the
assays. All authors read and approved the manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yoneyama M, Kikuchi M, Natsukawa T,
Shinobu N, Imaizumi T, Miyagishi M, Taira K, Akira S and Fujita T:
The RNA helicase RIG-I has an essential function in double-stranded
RNA-induced innate antiviral responses. Nat Immunol. 5:730–737.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kato H, Sato S, Yoneyama M, Yamamoto M,
Uematsu S, Matsui K, Tsujimura T, Takeda K, Fujita T, Takeuchi O
and Akira S: Cell type-specific involvement of RIG-I in antiviral
response. Immunity. 23:19–28. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kato H, Takeuchi O, Sato S, Yoneyama M,
Yamamoto M, Matsui K, Uematsu S, Jung A, Kawai T, Ishii KJ, et al:
Differential roles of MDA5 and RIG-I helicases in the recognition
of RNA viruses. Nature. 441:101–105. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pichlmair A, Schulz O, Tan CP, Näslund TI,
Liljeström P, Weber F and Sousa Reis e C: RIG-I-mediated antiviral
responses to single-stranded RNA bearing 5′-phosphates. Science.
314:997–1001. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hornung V, Ellegast J, Kim S, Brzózka K,
Jung A, Kato H, Poeck H, Akira S, Conzelmann KK, Schlee M, et al:
5′-Triphosphate RNA is the ligand for RIG-I. Science. 314:994–997.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Takeuchi O and Akira S: Pattern
recognition receptors and inflammation. Cell. 140:805–820. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Goubau D, Deddouche S and Sousa Reis e C:
Cytosolic sensing of viruses. Immunity. 38:855–869. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu LG, Wang YY, Han KJ, Li LY, Zhai Z and
Shu HB: VISA is an adapter protein required for virus-triggered
IFN-beta signaling. Mol Cell. 19:727–740. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Seth RB, Sun L, Ea CK and Chen ZJ:
Identification and characterization of MAVS, a mitochondrial
antiviral signaling protein that activates NF-kappaB and IRF 3.
Cell. 122:669–682. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Meylan E, Curran J, Hofmann K, Moradpour
D, Binder M, Bartenschlager R and Tschopp J: Cardif is an adaptor
protein in the RIG-I antiviral pathway and is targeted by hepatitis
C virus. Nature. 437:1167–1172. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kawai T, Takahashi K, Sato S, Coban C,
Kumar H, Kato H, Ishii KJ, Takeuchi O and Akira S: IPS-1, an
adaptor triggering RIG-I- and Mda5-mediated type I interferon
induction. Nat Immunol. 6:981–988. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jacobs JL and Coyne CB: Mechanisms of MAVS
regulation at the mitochondrial membrane. J Mol Biol.
425:5009–5019. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Belgnaoui SM, Paz S and Hiscott J:
Orchestrating the interferon antiviral response through the
mitochondrial antiviral signaling (MAVS) adapter. Curr Opin
Immunol. 23:564–572. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu G, Lin YT, Wei R, Chen Y, Shan Z and
Lee WH: Hice1, a novel microtubule-associated protein required for
maintenance of spindle integrity and chromosomal stability in human
cells. Mol Cell Biol. 28:3652–3662. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhong B, Yang Y, Li S, Wang YY, Li Y, Diao
F, Lei C, He X, Zhang L, Tien P and Shu HB: The adaptor protein
MITA links virus-sensing receptors to IRF3 transcription factor
activation. Immunity. 29:538–550. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gietz RD, Schiestl RH, Willems AR and
Woods RA: Studies on the transformation of intact yeast cells by
the LiAc/SS-DNA/PEG procedure. Yeast. 11:355–360. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chenuet S, Martinet D, Besuchet-Schmutz N,
Wicht M, Jaccard N, Bon AC, Derouazi M, Hacker DL, Beckmann JS and
Wurm FM: Calcium phosphate transfection generates mammalian
recombinant cell lines with higher specific productivity than
polyfection. Biotechnol Bioeng. 101:937–945. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Robitaille AC, Mariani MK, Fortin A and
Grandvaux N: A high resolution method to monitor
phosphorylation-dependent activation of IRF3. J Vis Exp: e53723.
2016. View Article : Google Scholar
|
|
20
|
Yamamoto M, Sato S, Hemmi H, Hoshino K,
Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K and
Akira S: Role of adaptor TRIF in the MyD88-independent toll-like
receptor signaling pathway. Science. 301:640–643. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fields S and Song O: A novel genetic
system to detect protein-protein interactions. Nature. 340:245–246.
1989. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Maniatis T, Falvo JV, Kim TH, Kim TK, Lin
CH, Parekh BS and Wathelet MG: Structure and function of the
interferon-beta enhanceosome. Cold Spring Harb Symp Quant Biol.
63:609–620. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Silverman N and Maniatis T: NF-kappaB
signaling pathways in mammalian and insect innate immunity. Genes
Dev. 15:2321–2342. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hiscott J, Grandvaux N, Sharma S, Tenoever
BR, Servant MJ and Lin R: Convergence of the NF-kappaB and
interferon signaling pathways in the regulation of antiviral
defense and apoptosis. Ann N Y Acad Sci. 1010:237–248. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yoneyama M, Suhara W and Fujita T: Control
of IRF-3 activation by phosphorylation. J Interferon Cytokine Res.
22:73–76. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gack MU, Shin YC, Joo CH, Urano T, Liang
C, Sun L, Takeuchi O, Akira S, Chen Z, Inoue S and Jung JU: TRIM25
RING-finger E3 ubiquitin ligase is essential for RIG-I-mediated
antiviral activity. Nature. 446:916–920. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Oshiumi H, Matsumoto M, Hatakeyama S and
Seya T: Riplet/RNF135, a RING finger protein, ubiquitinates RIG-I
to promote interferon-beta induction during the early phase of
viral infection. J Biol Chem. 284:807–817. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yan J, Li Q, Mao AP, Hu MM and Shu HB:
TRIM4 modulates type I interferon induction and cellular antiviral
response by targeting RIG-I for K63-linked ubiquitination. J Mol
Cell Biol. 6:154–163. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kuniyoshi K, Takeuchi O, Pandey S, Satoh
T, Iwasaki H, Akira S and Kawai T: Pivotal role of RNA-binding E3
ubiquitin ligase MEX3C in RIG-I-mediated antiviral innate immunity.
Proc Natl Acad Sci USA. 111:5646–5651. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang Y, Tong X and Ye X: Ndfip1 negatively
regulates RIG-I-dependent immune signaling by enhancing E3 ligase
Smurf1-mediated MAVS degradation. J Immunol. 189:5304–5313. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jia Y, Song T, Wei C, Ni C, Zheng Z, Xu Q,
Ma H, Li L, Zhang Y, He X, et al: Negative regulation of
MAVS-mediated innate immune response by PSMA7. J Immunol.
183:4241–4248. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Castanier C, Zemirli N, Portier A, Garcin
D, Bidère N, Vazquez A and Arnoult D: MAVS ubiquitination by the E3
ligase TRIM25 and degradation by the proteasome is involved in type
I interferon production after activation of the antiviral
RIG-I-like receptors. BMC Biol. 10:442012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Song G, Liu B, Li Z, Wu H, Wang P, Zhao K,
Jiang G, Zhang L and Gao C: E3 ubiquitin ligase RNF128 promotes
innate antiviral immunity through K63-linked ubiquitination of
TBK1. Nat Immunol. 17:1342–1351. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li S, Wang L, Berman M, Kong YY and Dorf
ME: Mapping a dynamic innate immunity protein interaction network
regulating type I interferon production. Immunity. 35:426–440.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang C, Chen T, Zhang J, Yang M, Li N, Xu
X and Cao X: The E3 ubiquitin ligase Nrdp1 ‘preferentially’
promotes TLR-mediated production of type I interferon. Nat Immunol.
10:744–752. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cui J, Li Y, Zhu L, Liu D, Songyang Z,
Wang HY and Wang RF: NLRP4 negatively regulates type I interferon
signaling by targeting the kinase TBK1 for degradation via the
ubiquitin ligase DTX4. Nat Immunol. 13:387–395. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang M, Wang L, Zhao X, Zhao K, Meng H,
Zhao W and Gao C: TRAF-interacting protein (TRIP) negatively
regulates IFN-β production and antiviral response by promoting
proteasomal degradation of TANK-binding kinase 1. J Exp Med.
209:1703–1711. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chiang C and Gack MU: Post-translational
control of intracellular pathogen sensing pathways. Trends Immunol.
38:39–52. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen C, Weisz OA, Stolz DB, Watkins SC and
Montelaro RC: Differential effects of actin cytoskeleton dynamics
on equine infectious anemia virus particle production. J Virol.
78:882–891. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sodeik B: Mechanisms of viral transport in
the cytoplasm. Trends Microbiol. 8:465–472. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bozym RA, Delorme-Axford E, Harris K,
Morosky S, Ikizler M, Dermody TS, Sarkar SN and Coyne CB: Focal
adhesion kinase is a component of antiviral RIG-I-like receptor
signaling. Cell Host Microbe. 11:153–166. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chiang HS, Zhao Y, Song JH, Liu S, Wang N,
Terhorst C, Sharpe AH, Basavappa M, Jeffrey KL and Reinecker HC:
GEF-H1 controls microtubule-dependent sensing of nucleic acids for
antiviral host defenses. Nat Immunol. 15:63–71. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ohman T, Rintahaka J, Kalkkinen N,
Matikainen S and Nyman TA: Actin and RIG-I/MAVS signaling
components translocate to mitochondria upon influenza A virus
infection of human primary macrophages. J Immunol. 182:5682–5692.
2009. View Article : Google Scholar : PubMed/NCBI
|


















