Introduction
Gastric cancer is the third leading cause of
cancer-associated mortality worldwide (1–3).
Although the diagnosis and treatment of gastric cancer has improved
over the past few decades, new cases and estimated mortalities are
increasing every year (4). The
development of gastric cancer is a complex process. Several studies
have investigated the mechanism of gastric cancer and numerous
therapeutic targets have been explored; however, effective
therapeutic targets have not yet been identified (3,5).
Aberrant activation of epithelial-mesenchymal transition (EMT) is a
crucial process in gastric carcinogenesis (5,6).
Typically, levels of E-cadherin, N-cadherin, twist family BHLH
transcription factor 1 (TWIST) and snail family transcriptional
repressor 1 (SNAIL) are detected to evaluate the EMT process.
Forkhead box (FOX)K2 is a member of the FOX transcription factor
family; FOXK2 is a vital protein that is phosphorylated by the
cyclin-dependent kinase (CDK) complex (7). FOXK2 regulates numerous genes
involved in cell adhesion, motility, metabolism, apoptosis and
tumorigenesis (8,9). Previous research has demonstrated
that the overexpression of FOXK2 suppresses EMT in non-small cell
lung cancer, through inhibition of N-cadherin and β-catenin
expression (10).
Gene expression is regulated by transcription and
relies on transcription factors to enable or disable gene
expression. It has been reported that FOXK2 recruits distinct
corepressor complexes, including proteins such as SIN3
transcription regulator family member A, RE1 silencing
transcription factor, nuclear receptor corepressor 2 and histone
deacetylases, which are important for gene transcription (11,12).
A recent report demonstrated that FOXK2 interacts with polycomb
complex molecules and recruits tumor suppressor proteins to modify
the structure of chromatin (13).
FOXK2 is involved in several signaling pathways,
including the mechanistic target of rapamycin, Wnt and AKT
serine/threonine kinase 1 pathways. These signaling pathways
regulate cell proliferation and death by affecting the expression
of important genes via the transcriptional repressor FOXK2
(10,14). FOXK2 not only affects
tumorigenesis, but also tumor drug resistance. A previous study
demonstrated that FOXK2 improves MCF-7 cell susceptibility to
paclitaxel by regulating cyclin B1 (12). However, the function and mechanism
of FOXK2 in gastric cancer remains largely unknown. In the present
study, the function of FOXK2 in the development and progression of
gastric cancer was investigated.
Materials and methods
Patients and tissue specimens
The present study was approved by the Ethics
Committee of Tianjin Nankai Hospital (Tianjin, China). Clinical
data were collected between July 2016 and July 2017, and a total of
150 patients were recruited to the study from Tianjin Nankai
Hospital. Written informed consent was obtained from all patients.
Clinical information, including age, sex, differentiation grade and
tumor size, was obtained (Table
I). Tissue samples of gastric cancer and para-tumor tissue were
excised from the patients with gastric cancer during resection.
Tissue samples were fixed immediately in 4% neutral-buffered
formalin at room temperature for 24 h and subsequently processed to
prepare paraffin-embedded sections (1×1×0.5 cm).
 | Table I.Association between FOXK2 expression
and gastric cancer clinicopathological features. |
Table I.
Association between FOXK2 expression
and gastric cancer clinicopathological features.
|
|
| FOXK2
expression |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
feature | Patients (n) | Low (n) | High (n) | P-value |
|---|
| Age (years) |
|
|
| 0.705 |
|
<50 | 86 | 43 | 43 |
|
|
≥50 | 64 | 34 | 30 |
|
| Sex |
|
|
| 0.142 |
|
Male | 75 | 34 | 41 |
|
|
Female | 75 | 43 | 32 |
|
| Tumor size
(cm) |
|
|
| 0.002 |
|
<5 | 77 | 30 | 47 |
|
| ≥5 | 73 | 47 | 26 |
|
|
Differentiation |
|
|
| 0.042 |
|
Well | 22 | 9 | 13 |
|
|
Moderate | 102 | 49 | 53 |
|
|
Poor | 26 | 19 | 7 |
|
FOXK2 expression analysis in
datasets
FOXK2 expression data in gastric cancer were
collated from the Human Protein Atlas (15).
Immunohistochemical staining
Among the samples, 22 tumors were classified as well
differentiated, 102 as moderately differentiated and 26 as poorly
differentiated. Formalin-fixed tissue samples were prepared prior
to immunohistochemical staining. Samples were stained using the
avidin-biotin complex method. Primary antibodies specific for FOXK2
(1:500; cat. no. ab50946; Abcam, Cambridge, MA, USA) were incubated
with sections overnight at 4°C. The samples were then incubated
with a biotin-conjugated goat anti-rabbit immunoglobulin G
secondary antibody (1:100; cat. no. TA130016; OriGene Technologies,
Inc., Beijing, China) at 37°C for 1 h. The expression of FOXK2 was
detected by coloration with DAB, and the procedure was performed as
previously described (16).
Staining intensity was scored as follows with an inverted
microscope (Olympus Corporation, Tokyo, Japan): 0, negative; 1,
weakly positive; 2, moderately positive and 3, strongly positive.
The percentage of FOXK2-positive cells was therefore scored as 0
(0%), 1 (1–25%), 2 (26–50%) or 3 (>50%).
Cell culture, chemical reagents and
antibodies
The human gastric cancer cell line BGC-823 was
obtained from the China Academia Sinica Cell Repository (Shanghai,
China). Cells were cultured in Dulbecco's modified Eagle's medium
(DMEM; Hyclone; GE Healthcare Life Sciences, Logan, UT, USA)
supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) under 5% CO2 at
37°C. FOXK2 (cat. no. ab50946; Abcam), cleaved caspase-3 (cat. no.
ab2302; Abcam), E-cadherin (cat. no. ab15148; Abcam) and N-cadherin
(cat. no. ab18203; Abcam) antibodies were purchased from Abcam.
GAPDH antibody (cat. no. TA802519) was purchased from OriGene
Technologies, Inc.
Transfection
For transient cell transfection, gastric cancer
cells (1.5×106 cells/well) were seeded in 6-well plates
and cultured overnight, and subsequently transfected with FOXK2
plasmids encoding human FOXK2 or empty vector. The FOXK2
overexpression plasmid (plasmid no. S57120) and empty vector were
purchased from GenScript (Nanjing, China). FOXK2 small interfering
(si)RNA (si-FOXK2; 50 nM; sense, 5′-GAGTTCGAGTATCTGATGA-3 and
antisense, 5′-GCGAACACGTACACTGTCT-3′) and negative control siRNA
(50 nM; cat. no. siN05815122147-1-5) were purchased from Guangzhou
RiboBio Co., Ltd. (Guangzhou, China) and transfected with
X-tremeGENE™ 9 DNA transfection reagent (cat. no. 06365787001;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and X-tremeGENE™
siRNA transfection reagent (cat. no. 04476093001; Sigma-Aldrich;
Merck KGaA). Plasmid transfection (1 µg/well) was performed with
Lipofectamine® 3000 (cat. no. L300001; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. Cells
were collected and seeded for assays 48 h post-transfection.
Western blot analysis
Antibodies against FOXK2, cleaved caspase-3,
N-cadherin, E-cadherin and GAPDH were used for western blot
analysis, as described previously (14). The cells were washed with PBS three
times and protein was extracted in radioimmunoprecipitation assay
lysis buffer (Beijing Solarbio Science & Technology Co., Ltd.,
Beijing, China) with 1% phenylmethanesulfonyl fluoride. Protein
concentration was determined with a bicinchoninic acid protein
assay. Proteins (40 µg/lane) were separated by 10% SDS-PAGE. The
separated proteins were transferred to polyvinylidene fluoride
membranes and blocked in 5% bovine serum albumin (Beijing Solarbio
Science & Technology Co., Ltd.) at 37°C for 1 h. Following
this, membranes were incubated with primary antibodies against
FOXK2, cleaved caspase-3, N-cadherin, E-cadherin and GAPDH (1:1,000
dilution) at 4°C for 12 h, and subsequent incubation with
horseradish peroxidase-conjugated secondary antibody (1:2,000
dilution; cat. no. ZDR-5306; OriGene Technologies, Inc., Beijing,
China) at room temperature for 1 h. Protein expression was
visualized using with an enhanced chemiluminescence kit (cat. no.
WBKLS0500; Merck KGaA). Bands were analyzed with ImageJ software
(version 1.51j8; National Institutes of Health, Bethesda, MD,
USA).
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated with TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. Equal amounts of RNA were converted
into cDNA with a PrimeScript RT reagent kit (Promega Corporation,
Madison, WI, USA) according to manufacturer's protocol. Relative
expression levels of FOXK2 were determined by PCR with a
GoTaq® Real-Time PCR system (Promega Corporation). The
primer sequences for FOXK2 were as follows: Forward,
5′-AAGAACGGGGTATTCGTGGAC-3′ and reverse, 5′-CTCGGGAACCTGAATGTGC-3′.
The reference gene was GAPDH, and the primer sequences for GAPDH
were as follows: Forward, 5′-ACAACTTTGGTATCGTGGAAGG-3′ and reverse,
5′-GCCATCACGCCACAGTTTC-3′. The PCR conditions were as follows: 30
cycles of 94°C for 30 sec, 56°C for 30 sec, 72°C for 90 sec, and a
final extension at 72°C for 5 min. Quantification was performed
using the 2−∆∆Cq method (17).
Colony formation and Cell Counting Kit (CCK)-8
assays. Tumor cells transfected with si-FOXK2 or FOXK2 plasmid were
cultured at 2,000 cells/well in 6-well plates. The cells were
allowed to grow for 14 days, and the medium was changed every 3
days. Colonies were subsequently fixed with 4% paraformaldehyde for
1 h at room temperature, stained with 0.5% crystal violet for 10
min at room temperature and counted under an inverted microscope
(Olympus Corporation).
For assessment of cell proliferation, a CCK-8 assay
(Dojindo Molecular Technologies, Inc., Kumamoto, Japan) was
performed according to the manufacturer's protocol. To determine
the effect of FOXK2 on cell proliferation, tumor cells were
transfected with si-FOXK2 or FOXK2 plasmid, seeded into a 96-well
plate at a density of 2×103 cells in 100 µl culture
medium containing 10% FBS and cultured overnight. All experiments
were performed in triplicate. The medium was subsequently replaced
with 100 µl fresh medium containing 10% CCK-8 reagent, and cells
were incubated for 3.5 h at 37°C. At 0, 24, 48, 72, 96 and 120 h,
absorbance was measured at 450 nm using an ELx800 microplate reader
(BioTek Instruments, Inc., Winooski, VT, USA).
To determine the effect of FOXK2 on cell
proliferation, cells were also assessed with a colony formation
assay. Tumor cells transfected with si-FOXK2 or FOXK2 plasmid were
seeded into each well of a 12-well plate at a density of
5×104 cells in 2 ml culture medium containing 10% FBS,
and were cultured overnight. All experiments were performed in
triplicate. The cells were incubated at 37°C. At 0, 24, 48, 72, 96
and 120 h, the cells were counted following trypsin enzyme
digestion.
Apoptosis assay
Annexin V/propidium iodide (PI) staining was
performed to quantify cell apoptosis. Transfected cells
(8×106) in the logarithmic growth phase were collected
and subjected to Annexin V/PI staining using an Annexin
V-fluorescein isothiocyanate (FITC) Apoptosis Detection kit
(BioVision, Inc., Milpitas, CA, USA) according to the
manufacturer's protocol. The resulting fluorescence was measured by
flow cytometry using a FACS flow cytometer (BD Biosciences, San
Jose, CA, USA). Obtained data were analyzed via BD Cell Quest Pro™
software (version 5.1; BD Biosciences).
Transwell invasion assay
The ability of cells to invade was assessed using
Matrigel-coated Transwell membranes (BD Biosciences). After 30 min
incubation at 37°C, the Matrigel solidified and served as an
extracellular matrix for tumor cell invasion analysis. The upper
chamber contained ~5×104 cells in 200 µl DMEM without
serum. The lower chamber contained DMEM with 10% FBS. The cells
were then incubated for 48 h at 37°C in an atmosphere containing 5%
CO2. Cell invasion to the underside of the
Matrigel-coated membrane was subsequently fixed with 4%
paraformaldehyde for 10 min at room temperature, stained with 0.5%
crystal violet for 10 min at room temperature followed by imaging
and counting under an inverted microscope at ×200 magnification
(Olympus Corporation). The results are expressed as the average
number of invasive cells per field.
Wound-healing assay
BGC-823 cells were transfected with si-FOXK2 or
FOXK2 plasmid at 37°C for 48 h, seeded into 6-well plates
(5×106 cells/well), cultured in serum-free medium and a
straight wound was created using a pipette tip. Cells were further
incubated at 37°C in an atmosphere containing 5% CO2 for
24 h. Images of wound healing were captured under an inverted
microscope at ×100 magnification (Olympus Corporation, Tokyo,
Japan). The percentage of wound closure was the rate of migration
distance compare to the control group. Results were analyzed with
ImageJ software (version 1.51j8; National Institutes of
Health).
Statistical analysis
Data are presented as the means ± standard deviation
of three independent experiments. Statistical analyses were
performed in SPSS version 16.0 (SPSS, Inc., Chicago, IL, USA). Two
groups were compared using Student's t-test, whereas multiple
groups were compared using one-way analysis of variance followed by
the Student-Newman-Keuls method. Survival curves were plotted using
the Kaplan-Meier method, and the differences between the survival
curves were examined by the log-rank test. Cox proportional hazards
models were used to identify factors with an independent influence
on survival (18–20). P<0.05 was considered to indicate
a statistically significant difference.
Results
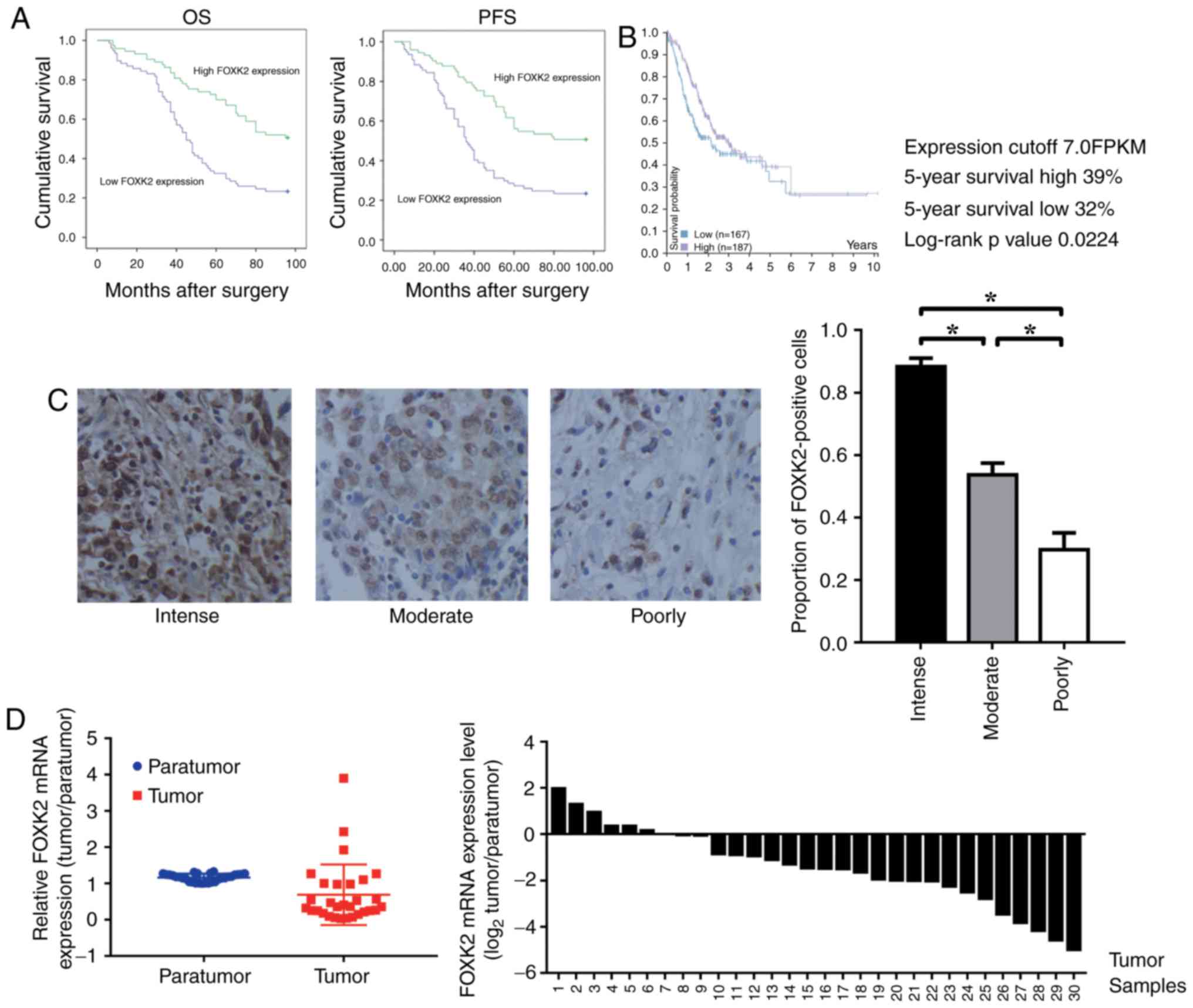
FOXK2 is downregulated in gastric
cancer, and high FOXK2 expression indicates a good prognosis
A total of 150 patients were examined (male:female,
1:1), and the associations between FOXK2 expression and
clinicopathological characteristics were investigated. Among the
samples, 22 tumors were well differentiated, 102 cases were
moderately differentiated and 26 were poorly differentiated. It was
demonstrated that the expression of FOXK2 was positively correlated
with tumor differentiation (P<0.05; Table I). There was no significant
association between FOXK2 expression with sex and age (P>0.05;
Table I). Cox regression analyses
revealed a significant association between overall survival and
tumor size, FOXK2 expression (P<0.001; Table II). The prognostic value of FOXK2
expression with regards to the OS and PFS of patients was
determined by survival analysis. The results revealed that high
FOXK2 expression was associated with an improved prognosis
(Fig. 1A).
 | Table II.Univariate and multivariate analyses
of prognostic parameters in patients with gastric cancer in terms
of overall survival. |
Table II.
Univariate and multivariate analyses
of prognostic parameters in patients with gastric cancer in terms
of overall survival.
| Parameter | Univariate log-rank
test (P-value) | Cox multivariate
analysis (P-value) | Risk |
|---|
| Age (<50 vs. ≥50
years) |
0.153 | – | – |
| Sex (male vs.
female) |
0.095 | – | – |
| Differentiation
(well, moderate, poor) | – | <0.001 | 2.637 |
| Tumor size (<4
vs. ≥4 cm) | <0.001 |
0.008 | 1.840 |
| Forkhead box K2
expression (low vs. high) | <0.001 |
0.006 | 0.545 |
Kaplan-Meier survival analysis was performed to
analyze the association between FOXK2 mRNA expression and patient
survival. Data on gastric cancer were obtained from the Pathology
Atlas in The Human Protein Atlas (http://www.proteinatlas.org/). The results revealed
that a high level of FOXK2 expression was associated with improved
prognosis (P=0.0224; Fig. 1B).
FOXK2 expression was reduced in patients with
high-grade gastric cancer. The proportion of FOXK2-positive cells
in the intense, moderately and poorly differentiated gastric cancer
tissues was 88.3±4.7, 53.7±6.5 and 29.7±9.5%, respectively
(P<0.05; Fig. 1C). Compared
with the para-tumor tissue, the relative mRNA expression levels of
FOXK2 were significantly lower in the tumor tissue (Fig. 1D).
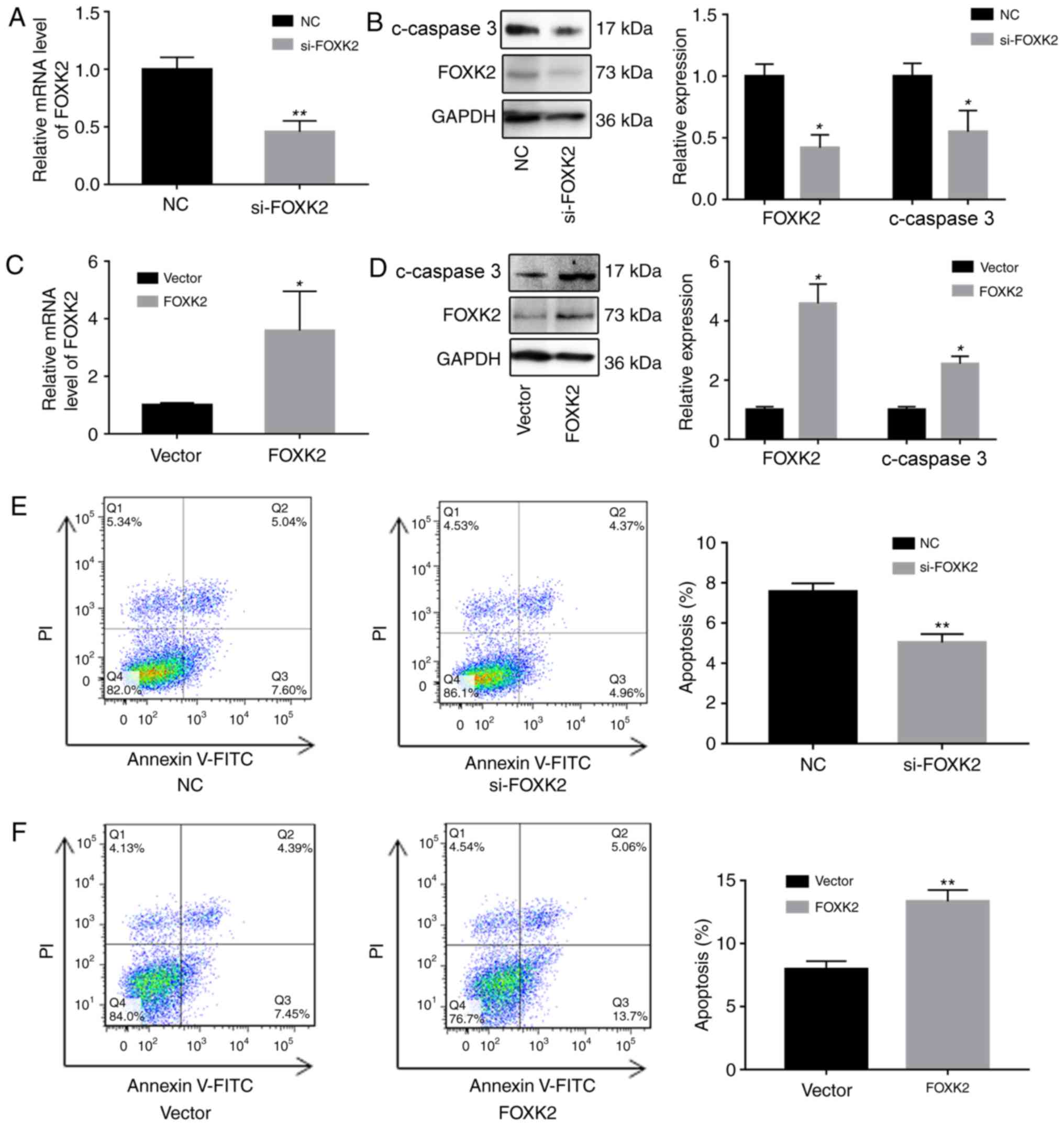
FOXK2 regulates gastric cancer cell
apoptosis
To investigate the functions of FOXK2, FOXK2 was
downregulated in BGC-823 cells using siRNA, and FOXK2 was
upregulated using a FOXK2 plasmid. Alterations in the expression of
FOXK2 were verified by RT-qPCR and western blotting. Western blot
analysis revealed that increased FOXK2 expression reduced the
upregulation of cleaved caspase-3 (Fig. 2A-D).
The effects of FOXK2 on apoptosis were subsequently
determined by western blot analysis and flow cytometry. The
percentage of apoptotic cells in the si-FOXK2 group (5.03±0.44%)
was significantly reduced compared with in the negative control
(NC) group (7.56±0.34%; P<0.01; Fig. 2E). Furthermore, the percentage of
apoptotic cells in the FOXK2 group (13.4±0.89%) was significantly
increased compared with in the vector group (7.9±0.64%; P<0.01;
Fig. 2F). Cells in the lower right
quadrant were early apoptotic cells, and the upper right quadrant
was designated as late apoptotic cells. These results indicated
that upregulation of FOXK2 expression induced early apoptosis,
whereas FOXK2 expression in gastric cancer cells had no effect on
late apoptosis.
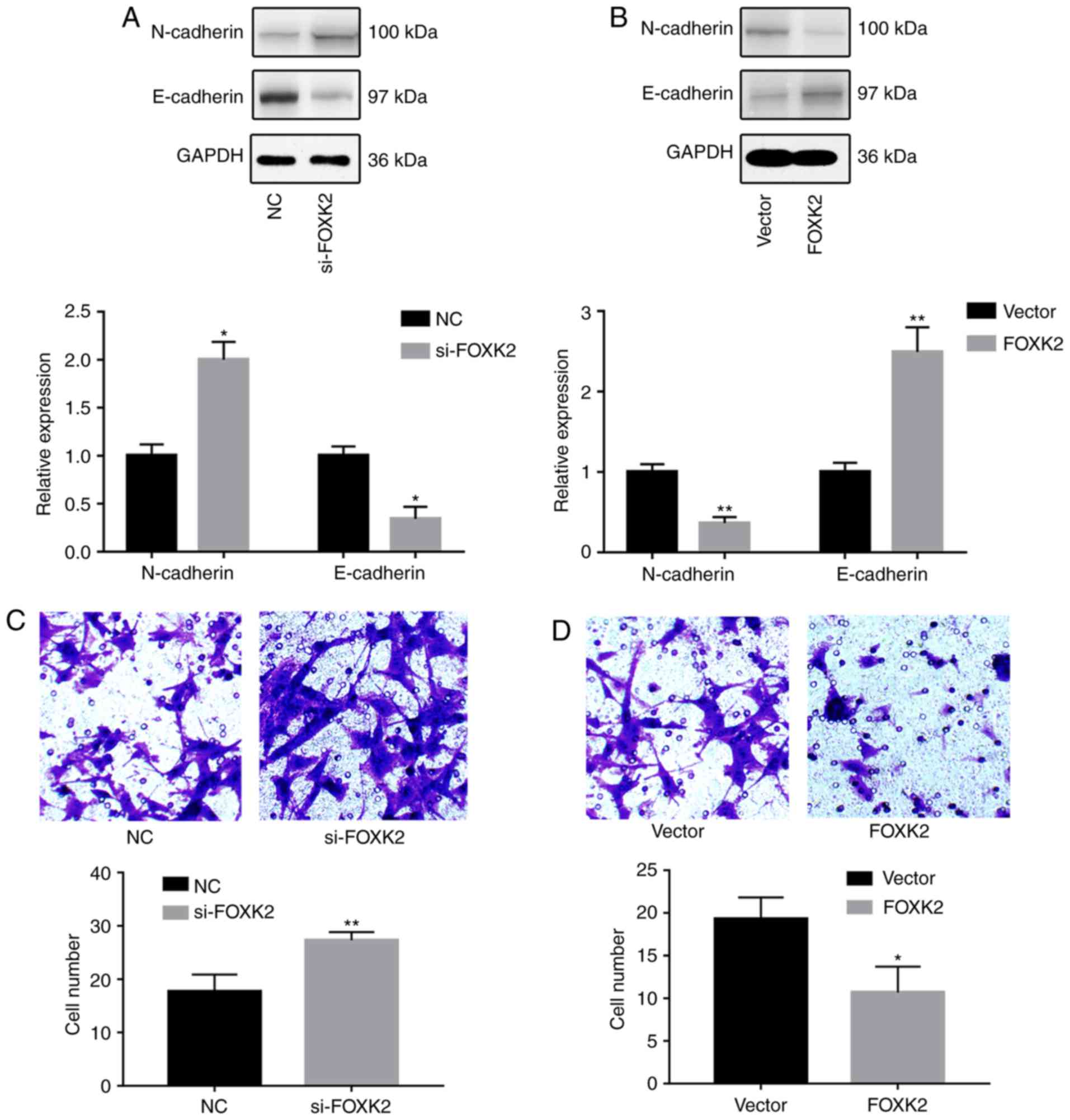
FOXK2 inhibits gastric cancer cell
invasion
To investigate the effects of FOXK2 on gastric
cancer cell migration and invasion, western blotting, Transwell and
wound-healing assays were conducted. Western blot analysis revealed
that FOXK2 knockdown increased the expression of N-cadherin and
decreased the expression of E-cadherin (P<0.05; Fig. 3A). Conversely, FOXK2 upregulation
decreased the expression of N-cadherin and increased the expression
of E-cadherin (P<0.01; Fig.
3B). Transwell assays were conducted in order to investigate
the effects of FOXK2 on gastric cancer invasion. The results
revealed that the invasion rate was increased by 54.7% in BGC-823
cells transfected with si-FOXK2 (P<0.01; Fig. 3C). The invasion rate was decreased
by 44.8% following FOXK2 plasmid transfection (P<0.05; Fig. 3D).
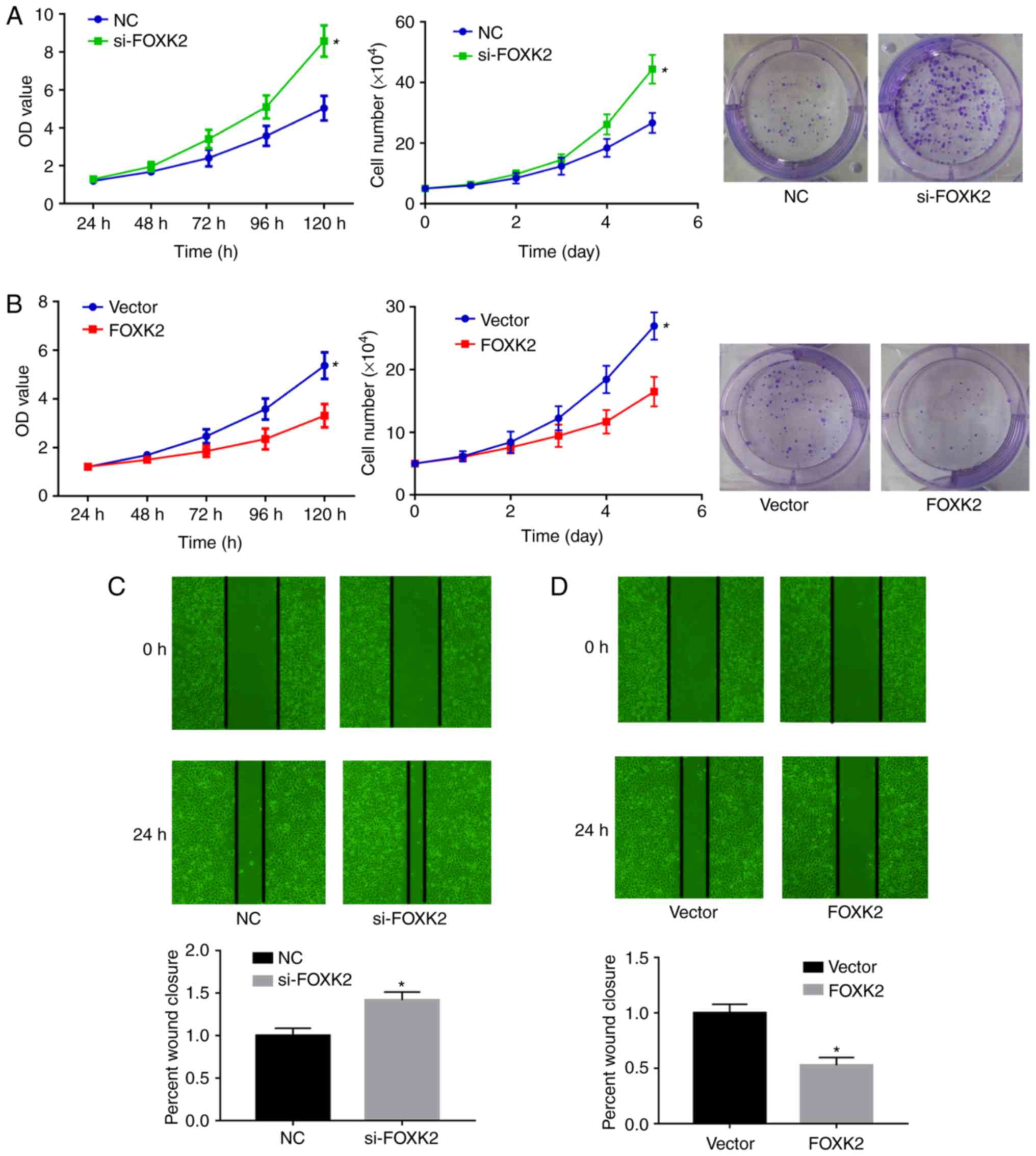
FOXK2 regulates gastric cancer cell proliferation
and migration. To clarify the role of FOXK2 in gastric cancer,
colony formation, CCK-8 and cell proliferation assays were
performed to examine BGC-823 cell proliferation. The expression
levels of FOXK2 in BGC-823 gastric cancer cells were downregulated
by si-FOXK2 and upregulated by FOXK2 plasmid transfection. Colony
formation, CCK-8 and cell proliferation assays demonstrated that
si-FOXK2 significantly increased the growth of gastric cancer cells
compared with the NC group (P<0.05; Fig. 4A), whereas FOXK2 plasmid
transfection inhibited BGC-23 cell growth compared with the vector
group (P<0.05; Fig. 4B).
Average colony numbers in the NC and si-FOXK2 groups were 24.7±5.7
and 46±3.2, respectively (P<0.05). Average colony numbers in the
vector and FOXK2 groups were 34±7.55 and 16.7±3.1, respectively
(P<0.05; Fig. 4A and B).
Wound-healing assays were conducted to investigate
the function of FOXK2 in gastric cancer cell migration. In BGC-823
cells, the migration rate was increased by 32% following si-FOXK2
transfection compared with the NC group (Fig. 4C). Migration rate was decreased by
47.3% following FOXK2 plasmid transfection (P<0.05). Taken
together, these results indicated that FOXK2 upregulation inhibited
the migration of gastric cancer cells (P<0.05; Fig. 4C and D).
Discussion
Gastric cancer has a poor prognosis and early
diagnosis is difficult. The most effective method of early
diagnosis is gastroscopy and pathological biopsy (21). Pathological diagnosis largely
depends on cell morphology, and the most commonly used marker
proteins are Ki-67 and B-cell lymphoma 2 (22,23).
Numerous studies have been conducted to identify novel therapeutic
targets, with limited success (24–26).
In the present study, FOXK2 expression was evaluated
in gastric cancer tissues with different tumor grades. FOXK2
expression was downregulated in high-grade gastric cancer, compared
with in low-grade tissue. The results revealed that high FOXK2
expression indicated a better prognosis, thus indicating that FOXK2
may serve as a therapeutic target in gastric cancer and as a
prognostic marker for patients with gastric cancer at different
stages. In addition, the results demonstrated that FOXK2
overexpression reduced cell invasion, growth and proliferation. EMT
is a critical step in cancer metastasis (27,28);
during EMT, epithelial cells change phenotype and obtain the
characteristics of mesenchymal cells, gaining the ability to
migrate and contribute to tumor metastasis (29–31).
EMT is accompanied by an alteration in the expression of several
proteins, including N-cadherin, E-cadherin, TWIST, SNAIL and
β-catenin (16). FOXK2 has
previously been reported to act as a critical mediator of EMT in
certain tumors, such lung cancer (10). Notably, a study demonstrated that
overexpression of FOXK2 decreased the level of N-cadherin and
increased the level of E-cadherin (32), indicating that FOXK2 regulates the
EMT process and may act as the core protein during EMT. In
addition, a previous study revealed the relationship between FOXK2
and CDK; FOXK2 serves a crucial role in the cell cycle and is
phosphorylated in a cell cycle-dependent manner to inhibit cell
cycle progression (7).
Furthermore, FOXK2 expression is associated with tumor development,
as well as a poor prognosis and outcome (8). The CDK complex mediates the
phosphorylation of FOXK2, and Ser368 and Ser423 are the two sites
that control the activity of FOXK2 (7). FOXK2 regulates the phosphorylation of
CDK and arrests cells at the G2/M phase (6).
The functional mechanism by which FOXK2 inhibits
tumor growth remains unclear. It has been reported that FOXK2
regulates various signaling pathways, including Wnt. Furthermore,
FOXK2 acts as a core protein in the function of several oncogenes.
Nestal de Moraes et al (11) demonstrated that FOXK2 expression is
negatively correlated with enhancer of zeste homolog 2 (EZH2)
expression (10). EZH2 is
expressed at a high level in several malignancies, including
gastric and breast cancer (33).
In addition, it has been reported that the levels of EZH2 are
associated with tumor stage and prognosis (11); EZH2 is one protein through which
FOXK2 functions.
In conclusion, the findings of the present study
demonstrated that FOXK2 functions as a tumor suppressor in gastric
cancer. In support of this approach to cancer treatment, the
increasing availability of molecular diagnostic techniques may help
identify patients who are more likely to respond to related drugs,
such as paclitaxel (34). However,
the mechanism underlying the effects of FOXK2 on gastric cancer is
unclear, and the mechanism by which FOXK2 suppresses the growth of
gastric cancer cells requires further research.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Chinese National
Natural Science Foundation (grant no. 81671380), the Key Project of
Tianjin National Natural Science Foundation (grant no.
17JCZDJC35900), the Basic and Advanced Technology Research
Foundation (grant no. 152300410162) and the Science and Technology
Development Foundation from the Science and Technology Department
of Henan Province (grant no. 172102310103).
Availability of data and materials
The datasets generated and/or analyzed during the
current study available from the corresponding author on reasonable
request.
Authors' contributions
BW, HZ, XL, XW and DW designed the study. XL, XW and
WN performed the data collection and wrote the manuscript. BW and
HZ performed the data analysis. XL, XW and DW performed the in
vitro assays. BW, HZ, XL, XW and WN critically revised and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Tianjin Nankai Hospital (Tianjin, China) and all
patients provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lansdorpvogelaar I and Kuipers EJ:
Screening for gastric cancer in Western countries. Gut. 65:543–544.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Apicella M, Corso S and Giordano S:
Targeted therapies for gastric cancer: Failures and hopes from
clinical trials. Oncotarget. 8:57654–57669. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tsuji T, Ibaragi S and Hu GF:
Epithelial-mesenchymal transition and cell cooperativity in
metastasis. Cancer Res. 69:7135–7139. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Roussos ET, Keckesova Z, Haley JD, Epstein
DM, Weinberg RA and Condeelis JS: AACR special conference on
epithelial-mesenchymal transition and cancer progression and
treatment. Cancer Res. 70:7360–7364. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Marais A, Ji Z, Child ES, Krause E, Mann
DJ and Sharrocks AD: Cell Cycle-dependent regulation of the
forkhead transcription factor FOXK2 by CDK·cyclin complexes. J Biol
Chem. 285:35728–35739. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Qian Y, Xia S and Feng Z: Sox9 mediated
transcriptional activation of FOXK2 is critical for colorectal
cancer cells proliferation. Biochem Biophys Res Commun. 29:475–481.
2017. View Article : Google Scholar
|
|
8
|
Shi Z, Liu J, Yu X, Huang J, Shen S, Zhang
Y, Han R, Ge N and Yang Y: Loss of FOXF2 expression predicts poor
prognosis in hepatocellular carcinoma patients. Ann Surg Oncol.
23:211–217. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen S, Jiang S, Hu F, Xu Y, Wang T and
Mei Q: Foxk2 inhibits non-small cell lung cancer
epithelial-mesenchymal transition and proliferation through the
repression of different key target genes. Oncol Rep. 37:2335–2347.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shan L, Zhou X, Liu X, Wang Y, Su D, Hou
Y, Yu N, Yang C, Liu B, Gao J, et al: FOXK2 elicits massive
transcription repression and suppresses the hypoxic response and
breast cancer carcinogenesis. Cancer Cell. 30:708–722. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nestal de Moraes G, Khongkow P, Gong C,
Yao S, Gomes AR, Ji Z, Kandola N, Delbue D, Man EP, Khoo US, et al:
Forkhead box K2 modulates epirubicin and paclitaxel sensitivity
through FOXO3a in breast cancer. Oncogenesis. 4:e1672015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen X, Ji Z, Webber A and Sharrocks AD:
Genome-wide binding studies reveal DNA binding specificity
mechanisms and functional interplay amongst Forkhead transcription
factors. Nucleic Acids Res. 44:1566–1578. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bowman CJ, Ayer DE and Dynlacht BD: Foxk
proteins repress the initiation of starvation-induced atrophy and
autophagy programs. Nat Cell Biol. 16:1202–1214. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shi Z, Qian X, Li L, Zhang J, Zhu S, Zhu
J, Chen L, Zhang K, Han L, Yu S, et al: Nuclear translocation of
β-catenin is essential for glioma cell survival. J Neuroimmune
Pharmacol. 7:892–903. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Uhlén M, Fagerberg L, Hallström BM,
Lindskog C, Oksvold P, Mardinoglu A, Sivertsson Å, Kampf C,
Sjöstedt E, Asplund A, et al: Proteomics. Tissue-based map of the
human proteome. Science. 347:12604192015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Choi IJ, Lee NR, Kim SG, Lee WS, Park SJ,
Kim JJ, Lee JH, Kwon JW, Park SH, You JH, et al: Short-term
outcomes of endoscopic submucosal dissection in patients with early
gastric cancer: A prospective multicenter cohort study. Gut Liver.
10:739–748. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guo JG, Guo CC, He ZQ, Cai XY and Mou YG:
High MMP-26 expression in glioma is correlated with poor clinical
outcome of patients. Oncol Lett. 16:2237–2242. 2018.PubMed/NCBI
|
|
19
|
Aoyama T, Yoshikawa T, Fujikawa H, Hayashi
T, Ogata T, Cho H, Yamada T, Hasegawa S, Tsuchida K, Yukawa N, et
al: Prognostic factors in stage IB gastric cancer. World J
Gastroenterol. 20:6580–6585. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Maounis NF, Dráberová E, Trakas N, Chorti
M, Riga D, Tzannis K, Kanakis M, Voralu K, Ellina E, Mahera E, et
al: Expression of γ-tubulin in non-small cell lung cancer and
effect on patient survival. Histol Histopathol.
180272018.PubMed/NCBI
|
|
21
|
Liu G, Xiong D, Zeng J, Chen B and Huang
Z: Clinicopathological and prognostic significance of Ki-67
immunohistochemical expression in gastric cancer: A systematic
review and meta-analysis. Onco Targets Ther. 10:4321–4328. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Min KW, Kim DH, Son BK, Kim DH, Kim EK,
Seo J, Ahn SB, Jo YJ, Park YS and Ha J: A High Ki67/BCL2 index
could predict lower disease-free and overall survival in
intestinal-type gastric cancer. Eur Surg Res. 58:158–168. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nishizawa T and Yahagi N: Long-term
outcomes of using endoscopic submucosal dissection to treat early
gastric cancer. Gut Liver. 12:119–124. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu H, Liu X, Gong P, Song W, Zhou M, Li Y,
Zhao Z and Fan H: Elevated TFAP4 regulates lncRNA TRERNA1 to
promote cell migration and invasion in gastric cancer. Oncol Rep.
40:923–931. 2018.PubMed/NCBI
|
|
25
|
Yuan J, Zeng J, Shuai C and Liu Y: TWSG1
is a novel tumor suppressor in gastric cancer. DNA Cell Biol.
37:574–583. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ji CD, Wang YX, Xiang DF, Liu Q, Zhou ZH,
Qian F, Yang L, Ren Y, Cui W, Xu SL, et al: Kir2.1 interaction with
Stk38 promotes invasion and metastasis of human gastric cancer by
enhancing MEKK2-MEK1/2-ERK1/2 signaling. Cancer Res. 78:3041–3053.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
George JT, Jolly MK, Xu J, Somarelli J and
Levine H: Survival outcomes in cancer patients predicted by a
partial EMT gene expression scoring metric. Cancer Res.
77:6415–6428. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
El-Sayed IY, Daher A, Destouches D, Firlej
V, Kostallari E, Maillé P, Huet E, Haidar-Ahmad N, Jenster G, de la
Taille A, et al: Extracellular vesicles released by
mesenchymal-like prostate carcinoma cells modulate EMT state of
recipient epithelial-like carcinoma cells through regulation of AR
signaling. Cancer Lett. 410:100–111. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Skrypek N, Goossens S, De Smedt E,
Vandamme N and Berx G: Epithelial-to-mesenchymal transition:
Epigenetic reprogramming driving cellular plasticity. Trends Genet.
33:943–959. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dominguez C, David JM and Palena C:
Epithelial-mesenchymal transition and inflammation at the site of
the primary tumor. Semin Cancer Biol. 47:177–184. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
da Silva SD, Alaoui-Jamali MA, Soares FA,
Carraro DM, Brentani HP, Hier M, Rogatto SR and Kowalski LP: TWIST1
is a molecular marker for a poor prognosis in oral cancer and
represents a potential therapeutic target. Cancer. 120:352–362.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sun Y, Xu K, He M, Fan G and Lu H:
Overexpression of Glypican 5 (GPC5) inhibits prostate cancer cell
proliferation and invasion via suppressing Sp1-mediated EMT and
activation of Wnt/β-catenin signaling. Oncol Res. Sep 6–2017.(Epub
ahead of print). View Article : Google Scholar :
|
|
33
|
Yamaguchi H, Du Y, Nakai K, Ding M, Chang
SS, Hsu JL, Yao J, Wei Y, Nie L, Jiao S, et al: EZH2 contributes to
the response to PARP inhibitors through its PARP-mediated poly-ADP
ribosylation in breast cancer. Oncogene. 37:208–217. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nestal de Moraes G, Ji Z, Fan LY, Yao S,
Zona S, Sharrocks AD and Lam EW: SUMOylation modulates
FOXK2-mediated paclitaxel sensitivity in breast cancer cells.
Oncogenesis. 7:292018. View Article : Google Scholar : PubMed/NCBI
|