Introduction
Astrocytes are one of the most abundant cell types
within the central nervous system (CNS) and play essential roles in
maintaining healthy brain function, including providing structural
support, regulating blood flow, modulating neuronal metabolism, and
maintaining the extracellular microenvironment (1,2).
They are also a critical structural and functional part of synapses
(3,4) and the neurovascular unit (5,6), and
communicate with neurons and endothelial cells (7,8),
contributing to angiogenesis, neurogenesis, and synaptic
plasticity. Additionally, astrocytes are primary responders to CNS
injury such as infection, trauma, ischemia and neurodegenerative
disease, where they either exert critical beneficial
neuroprotective and neurorestorative effects or play detrimental
roles by triggering glutamate excitotoxicity, inflammatory molecule
release and oxidative stress (9–11).
Thus, to develop successful clinical neuroprotective and
neurorestorative strategies, further investigation targeted on
astrocytes is need for a promising therapeutic target of
pharmacological and cell-based approaches.
Ginkgolide extracted from the Ginkgo biloba
leaves have been documented to possess a broad spectrum of
pharmacological properties, including neuroprotection, anticancer,
cardioprotection and stress alleviating, and potential benefits
against ischemic stroke, Alzheimer's disease (12,13)
and psychiatric disorders (14).
Ginkgolide B (GB) is a primary active monomer of ginkgolide that
inhibits the activity of platelet-activating factor (PAF) by
binding to its membrane receptor (15). BN 52021 has a protective effect
against myocardial ischemia/reperfusion (IR) dysfunction (16), cerebral ischemic damage and the
neurological deficits of mice after middle cerebral artery
occlusion (MCAO) (17). GB also
efficiently alleviates spinal cord injury by inhibiting STAT1
expression (18) and protects
human umbilical vein endothelial cells via pregnane X receptor
activation (19). GB treatment has
been shown to significantly decrease intracranial pressure (ICP)
and improve cerebral perfusion pressure (CPP) as well as reduce the
lactate/pyruvate ratio (LPR) in patients with non-traumatic severe
acute hemorrhagic stroke (20).
Therefore, GB appears to benefit tissue protection by different
mechanisms in several disorders.
Ginkgolide K (GK, C20H22O9 as shown in Fig. 1, with one more hydroxyls than GB),
a derivative compound of GB and isolated from the leaves of the
Ginkgo biloba, can markedly improve neural cytotoxicity and
shows neuroprotective effects. GK has been reported to exhibit
protective effects against glutamate cytotoxicity and
H2O2-induced cytotoxicity in PC12 cells
(21,22). GK also protects the heart against
endoplasmic reticulum stress by activating the inositol-requiring
enzyme 1a/X box-binding protein-1 pathway (23), and against acute ischemic stroke
caused by MCAO through antioxidative effects (24). To the best of our knowledge, there
has been no comparative study of GB and GK or a study examining the
role of GK on astrocytes exposed to oxygen-glucose deprivation
(OGD).
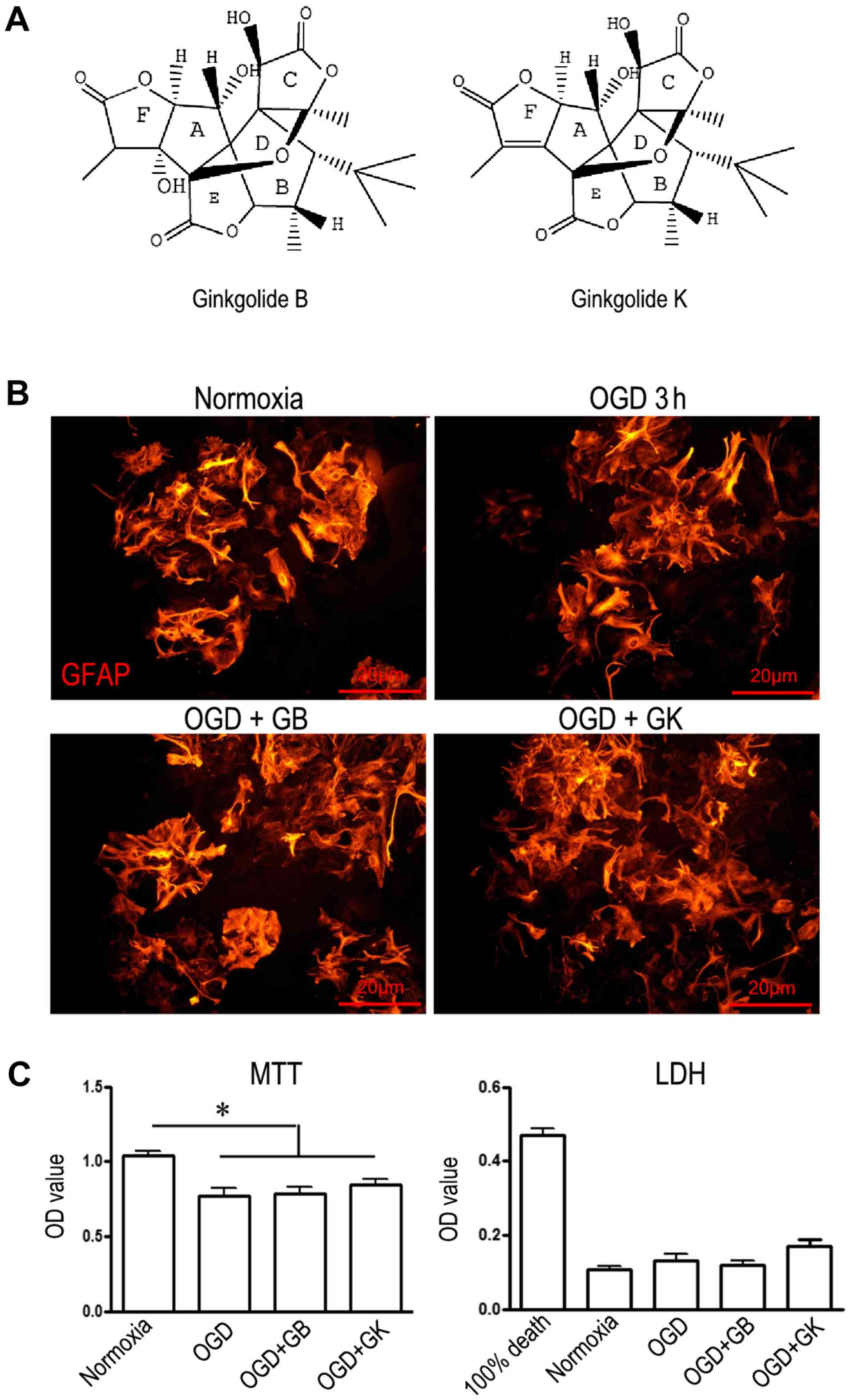 | Figure 1.Structure and cytotoxicity of GB and
GK. (A) The structure of GB and GK. (GB molecular formula:
C20H24O10; and GK molecular
formula: C20H22O9). (B)
Immunostaining of GFAP in primary cultured astrocytes (scale bars,
20 µm). (C) The viability and level of cell death of astrocytes
exposed to OGD or OGD+GB/GK. Cell viability was measured by MTT
assay, and cell death was measured by LDH. Quantitative results are
the mean ± standard error of the mean, and analyzed from three
independent experiments with similar results. *P<0.05, as
indicated. GB, ginkgolide B; GK, ginkgolide K; OGD, oxygen-glucose
deprivation; GFAP, glial fibrillary acidic protein; LDH, lactate
dehydrogenase; MTT,
3-(4,5-dimethylthazol-2-yl)-2,5-diphenyltetrazolium bromide. |
However, whether GK can also exert effects on
astrocytes, which are the most abundant cell population in the
brain, is unknown. In the present study, we used primary astrocytes
that were exposed to OGD for a cell model of ischemic stroke to
evaluate how GK influences astrocyte function to potentially affect
neuronal survival and recovery after ischemia.
Materials and methods
Ethical Statement
The present study was approved by the Ethics
Committee of Fudan University (Shanghai, China), based on the
recommendations established in the Guide for the National Science
Council of the Republic of China. The protocols were approved by
the Ethics Committee of Fudan University. The approval number from
the IRB is ‘20150572A259’. This manuscript was written in
accordance with the ARRIVE (Animal Research: Reporting in
vivo Experiments) guidelines. Pregnant C57/B6 female mice were
housed 1 per cage in a temperature-controlled room (22°C±1°C) under
a 12-hour dark/light cycle with free access to water and food in
the Animal House of Fudan University. Newborn mice (<24 h) were
sacrificed by decapitation, and the brains were removed.
Drugs and reagents
GB and GK were extracted and separated from ginkgo
leaf by recrystallization and high-performance liquid
chromatography (HPLC) separation and had a purity >98%. GB and
GK were dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich, Merck
KGaA, Darmstadt, Germany) before the experiment. Dulbecco's
modified Eagle's medium (DMEM), fetal bovine serum, 100 U/ml
penicillin, and 100 µg/ml streptomycin were obtained from Gibco
(Thermo Fisher Scientific, Inc., Waltham, MA, USA). All other
reagents were from Sigma-Aldrich (Merck KGaA) unless otherwise
stated.
Primary astrocyte culture and
treatment
Primary cortical astrocytes were prepared from
newborn mice at postnatal 24 h as previously described with minor
modifications (25). Briefly,
meninges-free cortices were cut into small cubes (<1
mm3) and digested with 0.25% trypsin at 37°C for 15 min.
The suspension of tissue fragments was passed through a 40-µm cell
strainer and allowed pre-adherence for 1 h to remove contamination
from fibroblasts. The supernatant containing unattached cells was
transferred to 25 or 75-cm2 flasks (Corning, Inc.,
Corning, NY, USA) and cultured in an incubator with 5%
CO2 at 37°C. The culture medium was changed with
complete DMEM every 3–4 days. When the cultures reached 80–90%
confluence, cells were sub-cultured for another 7 days before they
were used. The purity of astrocytes in cultures was determined by
staining for astrocytic marker glial fibrillary acidic protein
(GFAP). More than 95% cells showed GFAP immunoreactivity in the
cultures.
For OGD, cells were exposed to 95% nitrogen and 5%
CO2 maintained by constant gas flow at 37°C in all
experiments (Forma Anaerobic System; Thermo Fisher Scientific,
Inc.). Oxygen tension was maintained at 1–2% during the duration of
the test. The media used in these experiments consisted of
sugar-free culture medium containing salts, 10% fetal bovine serum,
100 U/ml penicillin, and 100 µg/ml streptomycin. Control groups
were grown under standard culture conditions (5% CO2 and
95% oxygen).
Astrocytes were exposed to OGD for 3 h followed by
re-oxygenation for 24 h. Before re-oxygenation, cells were washed
in PBS, and the medium was replaced with complete DMEM containing
glucose and 10% fetal bovine serum. At the same time, GB and GK (30
µg/ml, dissolved in DMSO) were added to different cell cultures
(ratio of GB/GK: medium=1:1,000). The same volume of DMSO was added
as a control.
In the preliminary experiments, we used the
5H-SY5Y/A53T cells to observe the effect of GK with different
concentrations (2, 10, 30 and 50 ug/ml). The results showed that
only high concentrations (30 and 50 ug/ml) of GK can promote
autophagy to degrade alpha-synuclein and induce the BDNF in the
5H-SY5Y/A53T cells. Therefore, in this study, we used 30 ug/ml of
GK.
Morphological observation
For astrocyte morphology observation, astrocytes
were washed in PBS, fixed with 4% paraformaldehyde and stained with
the anti-GFAP antibody. The cells were observed under a
fluorescence microscope (BX60; Olympus Imaging America Inc., Center
Valley, PA, USA).
Cell viability
The cell viability was measured using an
3-(4,5-dimethylthazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. Briefly, a total of 10 µl MTT (5 mg/ml in PBS, BDH
Chemicals) was added to each 96-well containing 200 µl medium
before the conduction of incubation at 37°C for 4 h. The reaction
was stopped by the addition of 100 µl DMSO. The optical density
(OD) was measured at 570 nm by a Synergy H1 microplate reader
(BioTek Instruments, Inc., Winooski, VT, USA), and the results were
expressed as an OD value.
LDH release
Cell cytotoxicity was quantitatively assessed by
measuring the activity of LDH released from the damaged cells into
the culture medium. At the end of culture, the supernatants that
contained detached cells were centrifuged at 2000 rpm for 10 min.
The supernatant was then used for the LDH activity assay. The
enzyme was quantified by using an assay kit according to the
manufacturer's protocol. The absorbance of the samples was read at
440 nm using a microplate reader (Synergy H1; BioTek Instruments,
Inc.). The LDH release was expressed as a percentage of
experimental vs. 100% damaged cells.
Enzyme-linked immunosorbent assay (ELISA). The
quantity of interleukin (IL)-1β, IL-6, IL-10, TNF-α, and PAF
released by astrocytes was measured by ELISA. Supernatants from
different cultures were collected and centrifuged at 2000 rpm for
10 min to remove cell debris. The concentrations of IL-1β, IL-6,
IL-10, TNF-α and PAF in supernatants were measured using commercial
immunoassay kits (IL-1β, IL-6, IL-10, and TNF-α from R&D System
and PAF; Cloud-Clone Corp., Houston, TX, USA) following the
manufacturer's instructions and quantified by reference to standard
curves.
Western blot analysis
Protein from astrocytes was extracted on ice with
RIPA buffer plus protease inhibitor and phosphatase inhibitor
cocktail (both Thermo Fisher Scientific Inc.). Protein
concentration was determined by BCA Protein Assay (Thermo Fisher
Scientific Inc.). Protein extracts (30 µg) were separated by
SDS-PAGE and transferred onto nitrocellulose membranes
(AmershamProtran 0.2 NC; GE Healthcare Life Sciences, Chicago, IL,
USA). The membranes were then incubated with anti-p-NF-κB/p65 (Cell
Signaling Technology, Inc., Danvers, MA, USA), anti-Nrf2, anti-heme
oxygenase-1 (HO-1; both Abcam, Cambridge, MA, USA), anti-Nlrp3,
anti-PI3K, anti-p-Akt (all Cell Signaling Technology, Inc.),
anti-WNT-1 (Abgent Inc., San Diego, CA, USA), anti-Fzd1 (R&D
Systems, Inc., Minneapolis, MN, USA), anti-β-catenin (Cell
Signaling Technology, Inc.), anti-BDNF (Abcam), anti-GDNF (Santa
Cruz Biotechnology, Inc., Dallas, TX, USA), and anti-β-actin (Cell
Signaling Technology, Inc.) antibodies overnight at 4°C. Bands were
visualized by HRP-conjugated secondary antibodies and
chemiluminescence (ECL) kit (EMD Millipore, Billerica, MA, USA)
under a ChemiDoc XRS+ system (Bio-Rad Laboratories, Inc., Hercules,
CA, USA).
Immunocytochemistry
Cells were fixed for 20 min with 4%
paraformaldehyde, blocked with Triton X-100 containing blocking
buffer, and then incubated with antibodies against GFAP (Abcam) at
4°C overnight. After the cells were washed with PBS, the secondary
antibody Alexa Fluor 555 goat anti-rabbit IgG (Thermo Fisher
Scientific Inc.) was added. Immunoreactive cells were observed
under fluorescence microscopy (BX60; Olympus Imaging America Inc.)
by two investigators.
Statistical analysis
Data were presented as the mean ± standard error of
the mean. The raw data were analyzed by using one-way analysis of
variance with Tukey's post hoc test, using GraphPad Prism 5.0
package (GraphPad Software, Inc, La Jolla, CA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
The structure and cytotoxicity of GB
and GK
Fig. 1A illustrates
the structure of GB and GK. GB (molecular formula: C20H24O10) is
one of the diterpenes that occurs naturally in the leaves of the
Ginkgo biloba (25). GK
(molecular formula: C20H22O9) is a derivative compound of GB. The
morphology of astrocytes stained with GFAP was not abnormal after
treatment with OGD, OGD+GB or OGD+GK; however, the number of
GFAP-positive cells appeared to be reduced in astrocytes treated
with OGD alone (Fig. 1B). The
viability of astrocytes exposed to OGD for 3 h declined by 26.8%
compared with the viability of astrocytes exposed to normoxic
conditions (OD 0.77±0.05 vs. 1.039±0.03), while the viability of
OGD astrocytes treated with GB and GK for 24 h was not
significantly different from that of astrocytes exposed to OGD
alone (Fig. 1C). The LDH release
from OGD astrocytes with or without GB/GK treatment was similar to
that from cells exposed to normoxia (Fig. 1C). The results demonstrate that the
concentration of GB and GK used in this experimental did not
influence the viability or death of cultured primary
astrocytes.
Effect of GB and GK on PAF inhibition
in OGD astrocytes
As shown in Fig. 2,
PAF was xulated in cultured primary astrocytes treated with OGD for
3 h (P<0.05). Both GB and GK, as PAF antagonists, effectively
inhibited the level of PAF compared with OGD treatment alone
(P<0.05 for both).
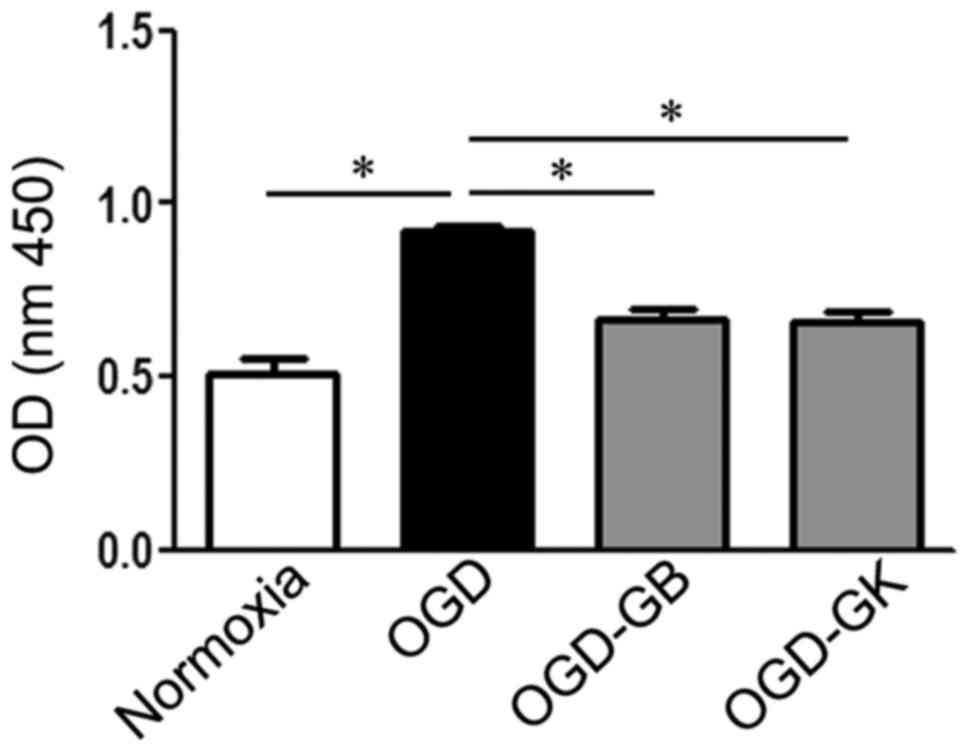 | Figure 2.Effect of GB and GK on PAF inhibition
in OGD astrocytes. Primary astrocytes were exposed to OGD for 3 h.
At the beginning of re-oxygenation, GB and GK (30 ug/ml, dissolved
in DMSO) were added. The same volume of DMSO was added as a
control. Following 24 h, the supernatants were collected for the
PAF assay. Quantitative results are the mean ± standard error of
the mean, and analyzed from three independent experiments with
similar results. *P<0.05, as indicated. GB, ginkgolide B; GK,
ginkgolide K; OGD, oxygen-glucose deprivation; PAF,
platelet-activating factor; OD, optical density; DMSO, dimethyl
sulfoxide. |
Effect of GB and GK on the
anti-inflammatory and antioxidant capacity of OGD astrocytes
In this study, both GB and GK inhibited the
expression of p-NF-κB/p65 (Fig.
3A) (P<0.001 for both), but neither changed Nlrp3 expression
in OGD astrocytes (Fig. 3A)
(P>0.05). Compared to OGD astrocytes and GB-treated OGD
astrocytes, OGD astrocytes treated with GK released less IL-6 and
TNF-α and produced more IL-10 (Fig.
3B) (P<0.001 and P<0.01 for IL-6, respectively,
P<0.001 for TNF-α, and P<0.05 for IL-10). These results show
that both GB and GK suppressed the expression of p-NF-κB/p65 and
that GK more effectively inhibited the production of inflammatory
IL-6 and TNF-α in OGD astrocytes. We measured antioxidative Nrf2
and HO-1 expression by western blot analysis. As shown in Fig. 4, although GB increased the
expression of Nrf2, GK more efficiently induced the expression of
Nrf2 (Fig. 4) (P<0.05 vs. OGD
astrocytes treated with GB). Simultaneously, HO-1 expression was
significantly elevated in OGD astrocytes treated with GK (Fig. 4) (P<0.05). These results
indicate that GK efficiently induced the expression of
antioxidative Nrf2 and HO-1 in OGD astrocytes.
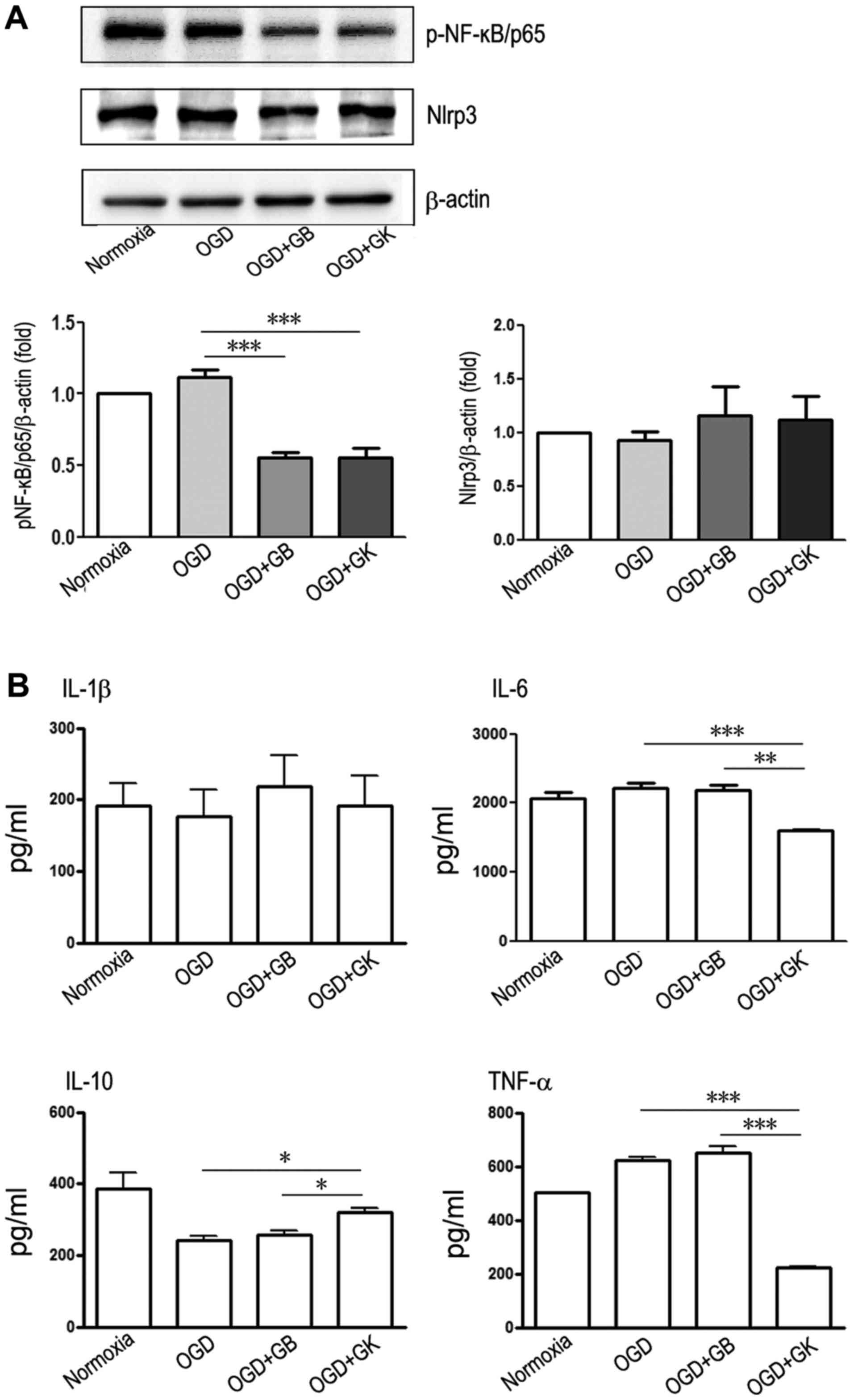 | Figure 3.Anti-inflammatory effects of GB and
GK in OGD astrocytes. Primary astrocytes were exposed to OGD for 3
h. At the beginning of re-oxygenation, GB and GK (30 µg/ml,
dissolved in DMSO) were added. The same volume of DMSO was added as
a control. Following 24 h, the supernatants were collected for
ELISA assay, and the cells were collected for western blotting. (A)
The protein expression of p-NF-κB/p65 and Nlrp3 by western blot,
and (B) the concentrations of IL-1β, IL-6, IL-10 and TNF-α by
ELISA. Semi-quantitative results are presented as the mean ±
standard error of the mean, and analyzed from three independent
experiments with similar results. *P<0.05, **P<0.01 and
***P<0.001, as indicated. GB, ginkgolide B; GK, ginkgolide K;
OGD, oxygen-glucose deprivation; p-, phosphorylated; NF-κB, nuclear
factor-κB; Nlrp3, NLR family pyrin domain containing 3; IL-,
interleukin; TNF-α, tumor necrosis factor-α; DMSO, dimethyl
sulfoxide. |
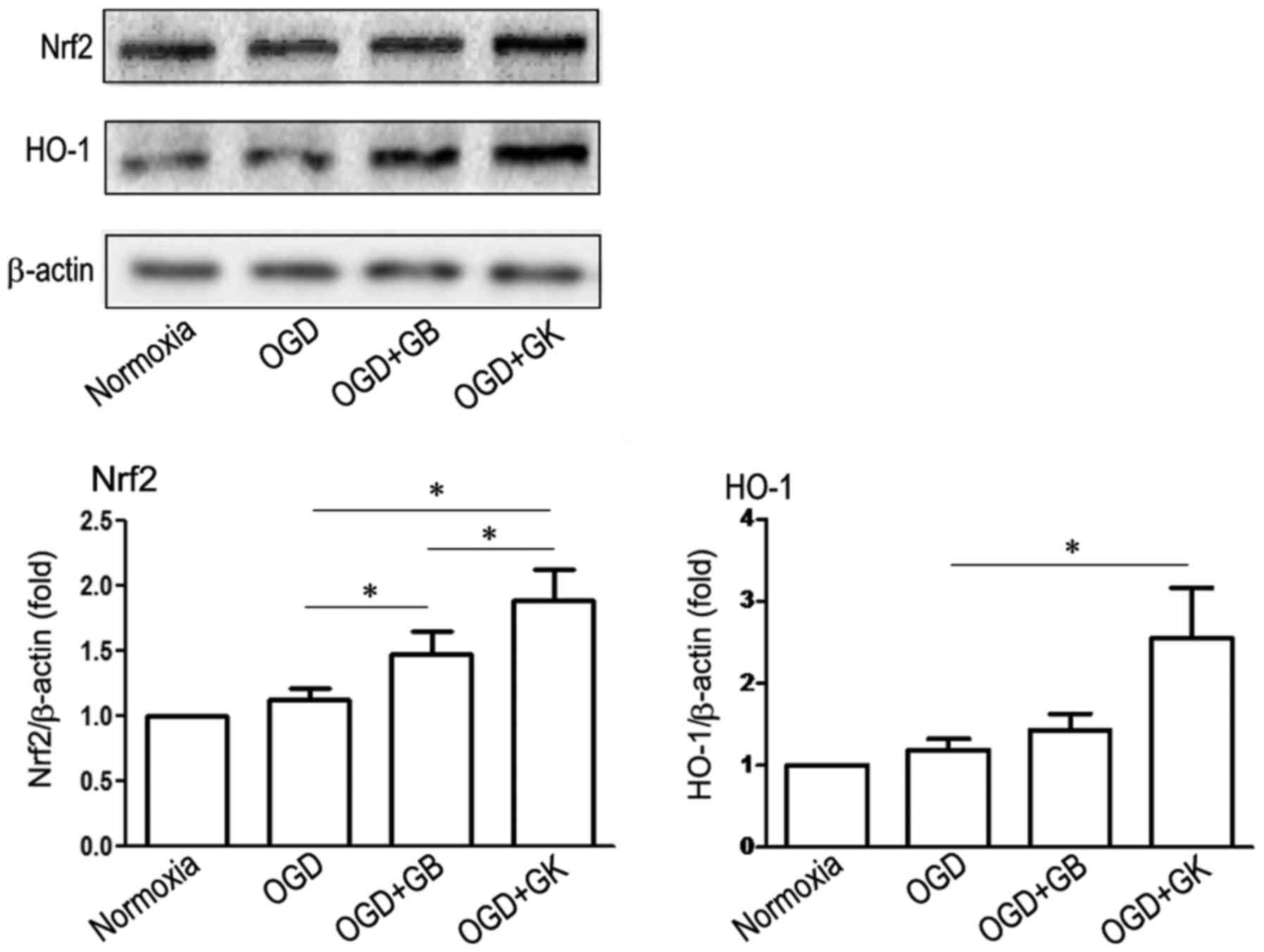 | Figure 4.Antioxidative effects of GB and GK in
OGD astrocytes. Primary astrocytes were exposed to OGD for 3 h. At
the beginning of re-oxygenation, GB and GK (30 µg/ml, dissolved in
DMSO) were added. The same volume of DMSO was added as a control.
Following 24 h, the cells were collected to measure the expression
of Nrf2 and HO-1 by western blotting. Semi-quantitative results are
the mean ± standard error of the mean, and analyzed from three
independent experiments with similar results. *P<0.05, as
indicated. GB, ginkgolide B; GK, ginkgolide K; OGD, oxygen-glucose
deprivation; Nrf2, nuclear factor-erythroid 2-related factor 2;
DMSO, dimethyl sulfoxide; HO-1, heme oxygenase-1. |
We measured antioxidative Nrf2 and HO-1 expression
by Western blot. As shown in Fig.
4, although GB increased the expression of Nrf2, GK more
efficiently induced the expression of Nrf2 (Fig. 4) (P<0.05 vs. OGD astrocytes
treated with GB). Simultaneously, HO-1 expression was significantly
elevated in OGD astrocytes treated with GK (Fig. 4) (P<0.05). These results
indicate that GK efficiently induced the expression of
antioxidative Nrf2 and HO-1 in OGD astrocytes.
Effect of GB and GK on PI3K/Akt
pathway of OGD-astrocytes
Phosphorylated PI3K/Akt pathway serves an
anti-inflammatory role through suppressing the phosphorylation of
downstream NF-κB (26). Besides,
the activation of Nrf2 can be modulated via PI3K/Akt pathway
(27). We compared the role of GB
and GK on PI3K/Akt pathway of OGD-astrocytes. As shown in Fig. 5, after OGD-astrocytes cells were
treated with GB, the expression of PI3K and p-Akt was inhibited
compared with OGD-astrocytes alone (for PI3K P<0.05, and for
p-AKT P=0.084), although the latter did not reach statistical
significance. However, the treatment of GK on OGD-astrocytes showed
higher expression of PI3K and p-AKT compared with GB (Fig. 5) (P<0.05, respectively).
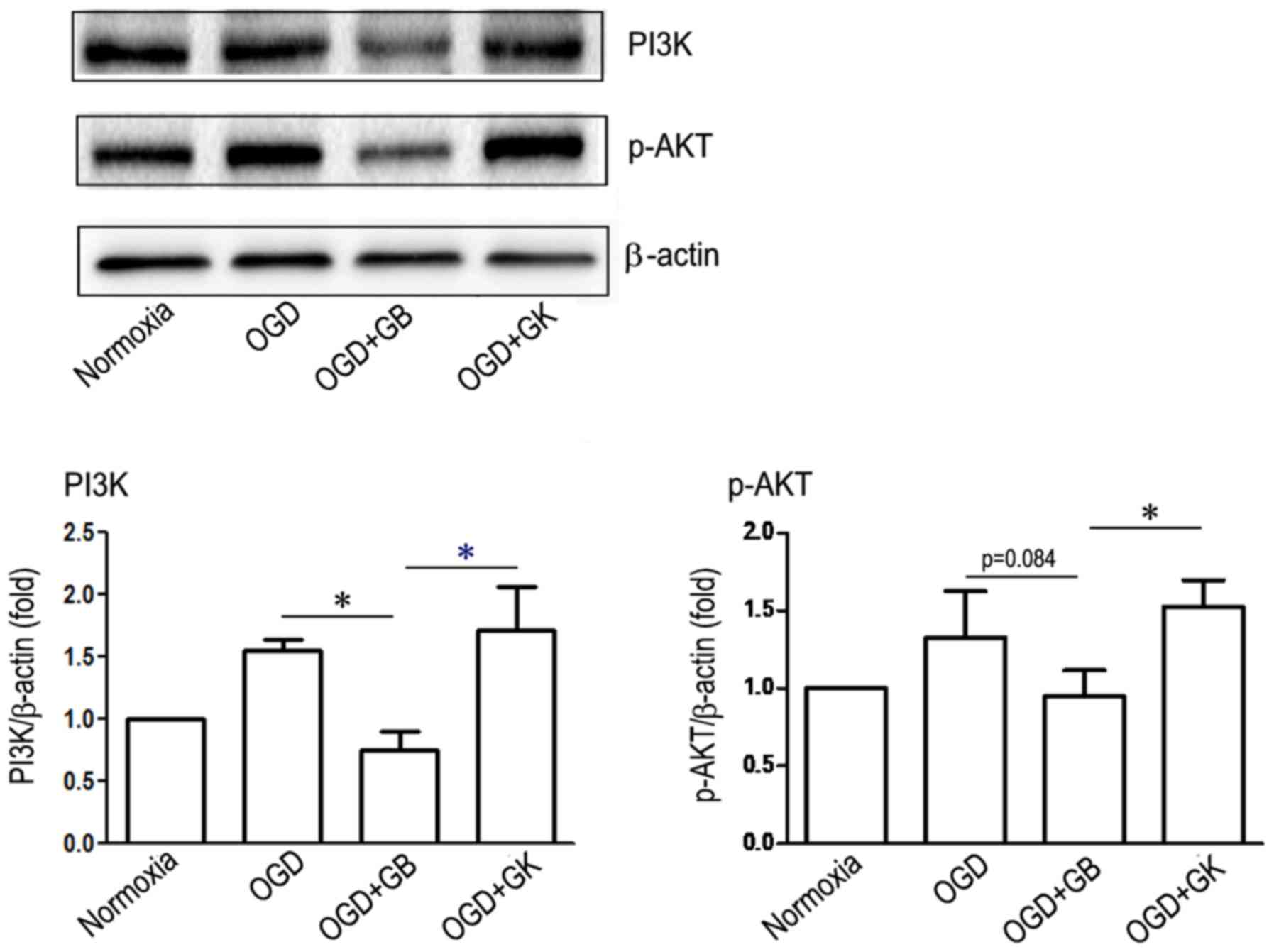 | Figure 5.Effect of GB and GK on the PI3K-AKT
signaling pathway in OGD astrocytes. Primary astrocytes were
exposed to OGD for 3 h. At the beginning of re-oxygenation, GB and
GK (30 µg/ml, dissolved in DMSO) were added. The same volume of
DMSO was added as a control. Following 24 h, the cells were
collected to measure the expression of PI3K and p-AKT by western
blotting. Semi-quantitative results are the presented as the mean ±
standard error of the mean, and analyzed from three independent
experiments with similar results. *P<0.05, as indicated. GB,
ginkgolide B; GK, ginkgolide K; OGD, oxygen-glucose deprivation;
PI3K, phosphoinositide 3-kinase; AKT, protein kinase B; p-,
phosphorylated; DMSO, dimethyl sulfoxide. |
Effect of GB and GK on the
WNT-1-Fzd1-β-catenin pathway in OGD astrocytes
The secreted Wnt1 ligand binds to its corresponding
receptor, Fzd1, which activates the downstream WNT pathway that
regulates neuronal proliferation and differentiation (28) and promotes angiogenesis (29). Compared with OGD treatment alone
and OGD treatment with GB, GK treatment induced the expression of
WNT-1 (Fig. 6) (P<0.05 and
P<0.01, respectively), but did not enhance the expression of
either Fzd1 or β-catenin in OGD astrocytes (Fig. 6).
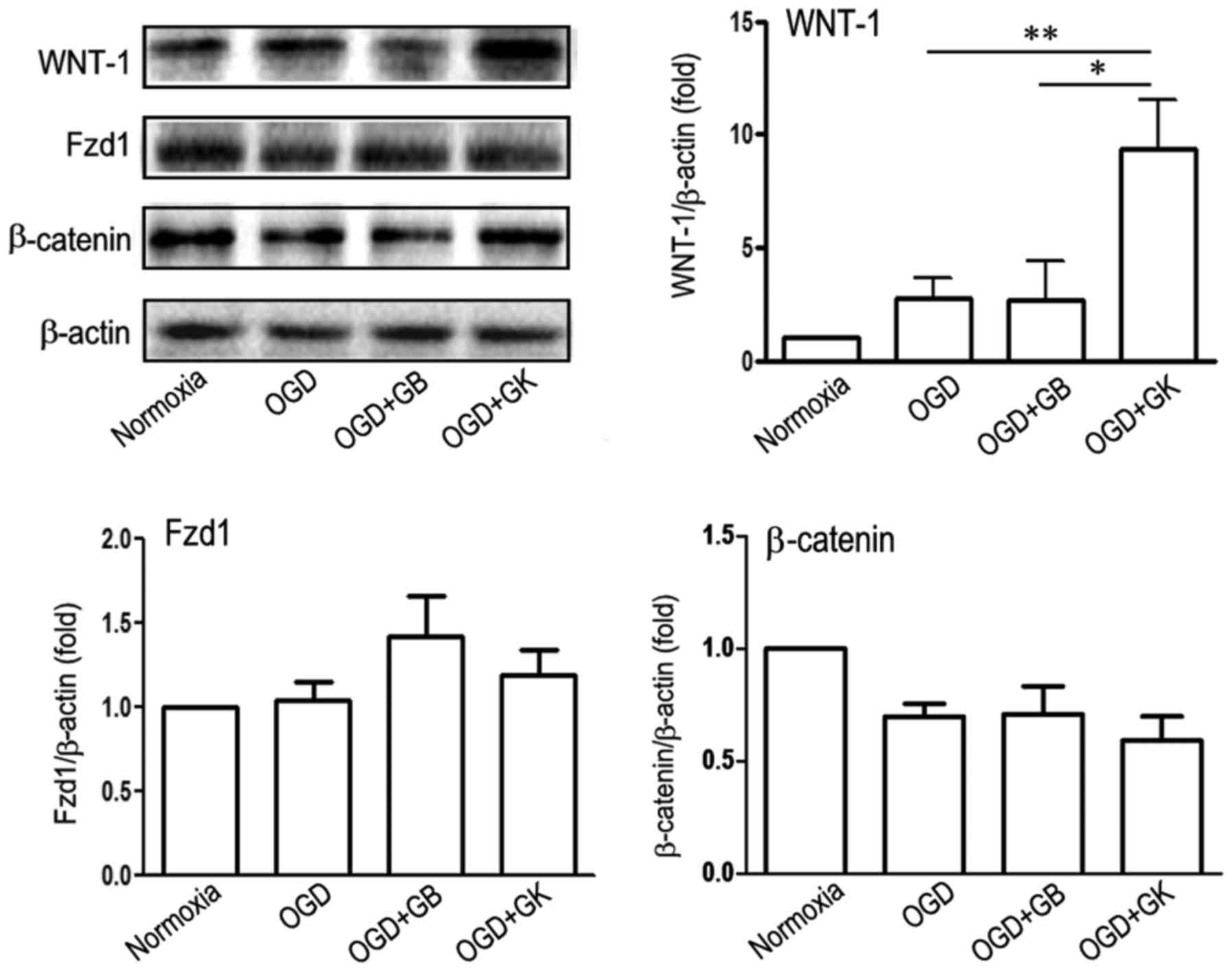 | Figure 6.Effect of GB and GK on the
WNT-1/Fzd1/β-catenin signaling pathway in OGD astrocytes. Primary
astrocytes were exposed to OGD for 3 h. At the beginning of
re-oxygenation, GB and GK (30 µg/ml, dissolved in DMSO) were added.
The same volume of DMSO was added as a control. Following 24 h, the
cells were collected to measure the expression of WNT-1, Fzd1 and
β-catenin by western blotting. Semi-quantitative results are the
presented as the mean ± standard error of the mean, and analyzed
from three independent experiments with similar results. *P<0.05
and **P<0.01, as indicated. GB, ginkgolide B; GK, ginkgolide K;
OGD, oxygen-glucose deprivation; WNT-1, Wnt family member 1; Fzd1,
frizzled class receptor 1; DMSO, dimethyl sulfoxide. |
The PI3K/Akt pathway mediates BDNF effects (30), while BDNF promotes the growth of
neurons in vitro through crosstalk with the WNT/β-catenin
pathway (31). As shown in
Fig. 7, GK treatment induced the
expression of BDNF (Fig. 6)
(P<0.05, respectively) but not GDNF compared with OGD treatment
alone and OGD treatment with GB.
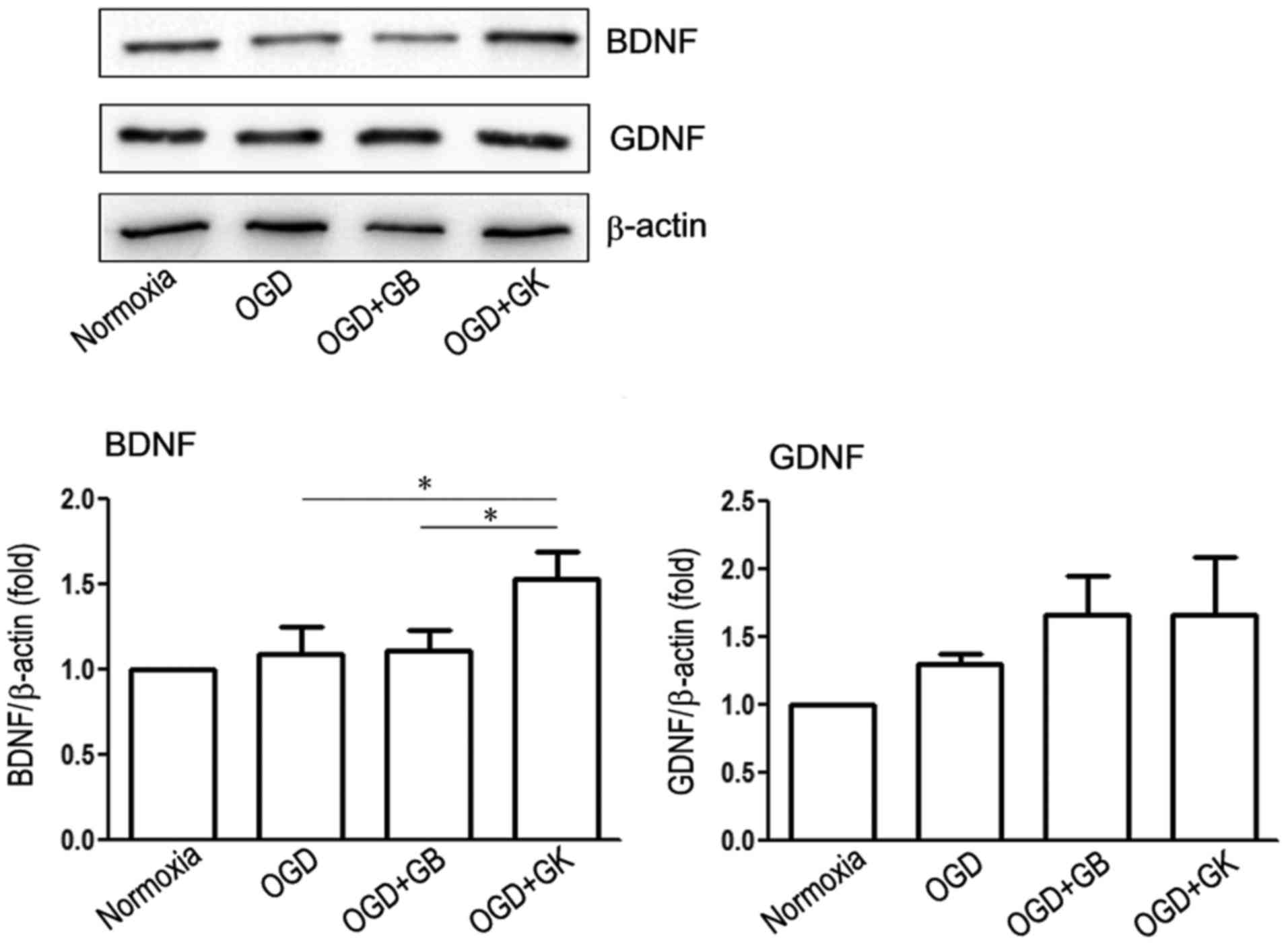 | Figure 7.Effect of GB and GK on neurotrophic
factors BDNF and GDNF in OGD astrocytes. Primary astrocytes were
exposed to OGD for 3 h. At the beginning of re-oxygenation, GB and
GK (30 µg/ml, dissolved in DMSO) were added. The same volume of
DMSO was added as a control. Following 24 h, the cells were
collected to measure the expression of BDNF and GDNF by western
blotting. Semi-quantitative results are presented as the mean ±
standard error of the mean, and analyzed from three independent
experiments with similar results. *P<0.05, as indicated. GB,
ginkgolide B; GK, ginkgolide K; OGD, oxygen-glucose deprivation;
BDNF, brain derived neurotrophic factor; GDNF, glial cell derived
neurotrophic factor; DMSO, dimethyl sulfoxide. |
Discussion
Astrocytes are the most numerous non-neuronal cell
type in the brain, stimulating neurite outgrowth and promoting
neuronal survival and regeneration through bi-directional
communication between astrocytes and neurons in the brain. The
recent discovery that astrocytes can differentiate into neurons
under some microenvironments has dramatically expanded our
knowledge of the regulation of astrocytes to neurons (32). In addition to providing support to
neurons, astrocytes can also secrete a series of pro-inflammatory
cytokines that modify the microenvironment (33,34),
which is crucial for the pathological processes of the brain
(35). In the present study, we
found that both GB and GK inhibited the expression of inflammatory
p-NF-κB/p65, and GK upregulated the expression of antioxidative
Nrf2 and HO-1 in OGD astrocytes, which could have a neuroprotective
effect through anti-inflammatory and anti-oxidative actions.
Interestingly, our study found that compared with GB, GK induced
astrocytic production of IL-10 and decreased IL-6 and TNF-α
expression. These results demonstrated that GK shows stronger
anti-inflammatory and antioxidant effects on OGD astrocytes.
Nrf2 signaling in astrocytes has been suggested as
an important therapeutic target for brain disorders (36). The activation of the Nrf2/HO-1 axis
plays a pivotal role in coordinating the antioxidant response and
maintaining redox homeostasis (27). The depletion of Nrf2 induced the
activation of NF-κB and the expression of TNF-α, IL-1β, IL-6 and
MMP9 resulting in more cell death in astrocytes after scratch
injury, suggesting that Nrf2 may be an important target for
anti-inflammation (37,38). The activation of NF-κB induced by
LPS was attenuated by various Nrf2 activators, such as sulforaphane
and curcumin (39), indicating
that depletion of Nrf2 induces augmentation of NF-κB activity and
the inflammatory response in the lung, brain, and intestine
(40). In this study, GK treatment
inhibited the expression of p-NF-κB/p65 and upregulated the Nrf2
pathway, indicating potential complicated crosstalk between NF-κB
and Nrf2, which may contribute to the improvement in the
inflammatory microenvironment of the brain and promote
neuroprotection and nerve regeneration. In this study, the
inhibition of NF-κB and TNF-α in OGD astrocytes treated with GK
might be related to the increased expression of IL-10 (41–43).
Additionally, a series of studies have demonstrated that the
activation and nuclear translocation of Nrf2 and the expression of
various antioxidant enzymes can be mediated via the PI3K/Akt
pathway (44,45).
PAF is a pleiotropic endogenous phospholipid that
mediates a diverse range of physiologic and pathologic processes.
PAF is produced by different cells, including mast cells,
basophils, neutrophils, eosinophils, fibroblasts, platelets,
endothelial cells, and even cardiac muscle cells (46). PAF is also synthesized in cultured
neurons following stimulation with neurotransmitters such as NMDA
and glutamic acid (47). However,
microglia has a chemotactic response to PAF, revealing that PAF is
one of the key mediators in neuron-microglia interactions (47). Additionally, endothelial cells were
found to produce PAF after stimulation by hypoxia or inflammatory
mediators (48). In this study,
PAF was also produced by astrocytes and was induced by OGD. Both GB
and GK inhibited the release of PAF from OGD astrocytes. Because
the PAF receptor is also expressed on neurons (49), PAF released from OGD astrocytes
also induces neuronal toxicity via a paracrine pathway. Reducing
PAF neurotoxic effects in the CNS with open new therapeutic
targets.
PAF has been reported to cause apoptosis in
enterocytes by inhibiting the PI3K/Akt signaling pathway (50). However, there is no reason to
believe that the GK-induced upregulation of PI3K/Akt expression is
related to PAF inhibition because GB also inhibited PAF but did not
increase the expression of PI3K/Akt. There is growing evidence to
indicate crosstalk between the Nrf2 and PI3K/Akt pathways in
response to oxidative insults (51,52).
Previous research has demonstrated that the PI3K/Akt pathway plays
a critical role in modulating Nrf2/HO-1 protein expression as an
upstream signaling molecule (53).
In our present study, a difference in PI3K/Akt expression was
observed between OGD astrocytes treated with GB and GK, and this
difference was possibly mediated by different modes of action.
Astrocytes have been suggested to maintain neuronal
activity and modulate neuronal networks. Thus, their structural
integrity and sustained function are essential for neuronal
viability (54). The Wnt/β-catenin
signaling pathway has been intensely studied as a critical
regulator of cell proliferation and cell fate during development,
including neural development (55,56).
Recently, the Wnt1-regulated frizzled-1/Fzd/β-catenin signaling
pathway was demonstrated to act as a candidate regulatory circuit
by controlling dopaminergic neuron-astrocyte crosstalk (57) or protecting cells from Aβ-oligomers
toxicity (58). Most
interestingly, we found that compared to GB, GK treatment obviously
induced the upregulation in WNT1 expression but did not influence
the expression of Fzd or β-catenin in OGD astrocytes. Secreted WNT1
molecules have been shown to be important not only for healthy
brain development but also for neurogenesis (59,60).
Our results are consistent with the current opinion that astrocytes
in the adult brain, in addition to providing structural support for
neurons, also perform numerous functions that include forming
neuronal-glial-vascular units. Here, our results suggest that GK
treatment induced the expression of WNT1 in OGD astrocytes.
Since knocking down WNT1 in midbrain astrocytes
abolished DA neuroprotection, defining the Wnt1/Fzd-1/β-catenin
pathway as a novel astrocyte-neuron signaling system required for
survival and protection of adult midbrain DA neurons may be
necessary (57). BDNF is
postulated to be a direct target of the WNT pathway in glia. A
previous study showed that the WNT signaling pathway might directly
induce BDNF expression in Muller glia of the retina (61). In fact, BDNF and GDNF, as secretory
cytokines, should be able to determine the content in the
supernatant, but unlike other cytokines, the measurement of BDNF
and GDNF content in the supernatant is relatively complex and
unstable by ELISA method. In this study, we detected protein level
in cell extracts by Western blot. Our data do not exclude the
possibility that indirect regulation of BDNF expression by WNT
signaling may occur, possibly via WNT-dependent induction (62). In conclusion, this is the first
comparative study between the effects of GB and GK on OGD
astrocytes, demonstrating that these compounds have some different
biological effects. PAF expression was elevated in OGD astrocytes
and inhibited by both GB and GK treatment. Although both GB and GK
inhibited the expression of p-NF-κB/p65, GK showed stronger
anti-inflammatory and antioxidant effects in OGD astrocytes.
Compared to GB treatment, GK treatment resulted in a higher
expression of PI3K and p-Akt and induced upregulation of WNT1 and
BDNF expression, indicating that GK, as a natural plant compound,
may have more attractive prospects for clinical application in the
treatment of neurological disorders than GB.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 81371414),
and National Major Scientific and Technological Special Project for
Significant New Drugs Development (grant no. 2013ZX09402203).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LC, WX and B-GX designed the study. W-BY, Y-YZ and
B-GX performed the experiments. W-BY and B-GX wrote the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Fudan University, based on the recommendations
established in the Guide for the National Science Council of the
Republic of China (no. 20150572A259).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Seth P and Koul N: Astrocyte, the star
avatar: Redefined. J Biosci. 33:405–421. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Travis J: Glia: The brain's other cells.
Science. 266:970–972. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hahn J, Wang X and Margeta M: Astrocytes
increase the activity of synaptic GluN2B NMDA receptors. Front Cell
Neurosci. 9:1172015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Singh SK, Stogsdill JA, Pulimood NS,
Dingsdale H, Kim YH, Pilaz LJ, Kim IH, Manhaes AC, Rodrigues WS Jr,
Pamukcu A, et al: Astrocytes assemble thalamocortical synapses by
bridging NRX1α and NL1 via hevin. Cell. 164:183–196. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Filosa JA, Morrison HW, Iddings JA, Du W
and Kim KJ: Beyond neurovascular coupling, role of astrocytes in
the regulation of vascular tone. Neuroscience. 323:96–109. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xu L, Dan M, Shao A, Cheng X, Zhang C,
Yokel RA, Takemura T, Hanagata N, Niwa M and Watanabe D: Silver
nanoparticles induce tight junction disruption and astrocyte
neurotoxicity in a rat blood-brain barrier primary triple coculture
model. Int J Nanomedicine. 10:6105–6118. 2015.PubMed/NCBI
|
|
7
|
Giaume C and Oliet S: Introduction to the
special issue: Dynamic and metabolic interactions between
astrocytes and neurons. Neuroscience. 323:1–2. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tewari SG, Gottipati MK and Parpura V:
Mathematical modeling in neuroscience: Neuronal activity and its
modulation by astrocytes. Front Integr Neurosci. 10:32016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Capani F, Quarracino C, Caccuri R and Sica
RE: Astrocytes as the main players in primary degenerative
disorders of the human central nervous system. Front Aging
Neurosci. 8:452016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu Z and Chopp M: Astrocytes, therapeutic
targets for neuroprotection and neurorestoration in ischemic
stroke. Prog Neurobiol. 144:103–120. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Verkhratsky A, Steardo L, Parpura V and
Montana V: Translational potential of astrocytes in brain
disorders. Prog Neurobiol. 144:188–205. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ahlemeyer B and Krieglstein J:
Pharmacological studies supporting the therapeutic use of ginkgo
biloba extract for Alzheimer's disease. Pharmacopsychiatry. 1(Suppl
36): S8–S14. 2003.
|
|
13
|
Luo Y, Smith JV, Paramasivam V, Burdick A,
Curry KJ, Buford JP, Khan I, Netzer WJ, Xu H and Butko P:
Inhibition of amyloid-beta aggregation and caspase-3 activation by
the ginkgo biloba extract EGb761. Proc Natl Acad Sci USA.
99:12197–12202. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Curtis-Prior P, Vere D and Fray P:
Therapeutic value of ginkgo biloba in reducing symptoms of decline
in mental function. J Pharm Pharmacol. 51:535–541. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chung KF, Dent G, McCusker M, Guinot P,
Page CP and Barnes PJ: Effect of a ginkgolide mixture (BN 52063) in
antagonising skin and platelet responses to platelet activating
factor in man. Lancet. 1:248–251. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pei HX, Hua R, Guan CX and Fang X:
Ginkgolide B reduces the degradation of membrane phospholipids to
prevent ischemia/reperfusion myocardial injury in rats.
Pharmacology. 96:233–239. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shu ZM, Shu XD, Li HQ, Sun Y, Shan H, Sun
XY, Du RH, Lu M, Xiao M, Ding JH and Hu G: Ginkgolide B protects
against ischemic stroke via modulating microglia polarization in
mice. CNS Neurosci Ther. 22:729–739. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zheng JL, Li BS, Cao XC, Zhuo WK and Zhang
G: Alleviation of spinal cord injury by ginkgolide B via the
inhibition of STAT1 expression. Genet Mol Res. 15:42482016.
View Article : Google Scholar
|
|
19
|
Zhou T, You WT, Ma ZC, Liang QD, Tan HL,
Xiao CR, Tang XL, Zhang BL, Wang YG and Gao Y: Ginkgolide B
protects human umbilical vein endothelial cells against xenobiotic
injuries via PXR activation. Acta Pharmacol Sin. 37:177–186. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chi CL, Shen DF, Wang PJ, Li HL and Zhang
L: Effect of ginkgolide B on brain metabolism and tissue
oxygenation in severe haemorrhagic stroke. Int J Clin Exp Med.
8:3522–3529. 2015.PubMed/NCBI
|
|
21
|
Ma S, Liu H, Jiao H, Wang L, Chen L, Liang
J, Zhao M and Zhang X: Neuroprotective effect of ginkgolide K on
glutamate-induced cytotoxicity in PC 12 cells via inhibition of ROS
generation and Ca(2+) influx. Neurotoxicology. 33:59–69. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ma S, Liu X, Xun Q and Zhang X:
Neuroprotective effect of Ginkgolide K against H2O2-induced PC12
cell cytotoxicity by ameliorating mitochondrial dysfunction and
oxidative stress. Biol Pharm Bull. 37:217–225. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang S, Wang Z, Fan Q, Guo J, Galli G, Du
G, Wang X and Xiao W: Ginkgolide K protects the heart against
endoplasmic reticulum stress injury by activating the
inositol-requiring enzyme 1α/X box-binding protein-1 pathway. Br J
Pharmacol. 173:2402–2418. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ma S, Yin H, Chen L, Liu H, Zhao M and
Zhang X: Neuroprotective effect of ginkgolide K against acute
ischemic stroke on middle cerebral ischemia occlusion in rats. J
Nat Med. 66:25–31. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Maclennan KM, Darlington CL and Smith PF:
The CNS effects of ginkgo biloba extracts and ginkgolide B. Prog
Neurobiol. 67:235–257. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rao CY, Fu LY, Hu CL, Chen DX, Gan T, Wang
YC and Zhao XY: H2S mitigates severe acute pancreatitis through the
PI3K/AKT-NF-κB pathway in vivo. World J Gastroenterol.
21:4555–4563. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
de Oliveira MR, Ferreira GC and Schuck PF:
Protective effect of carnosic acid against paraquat-induced redox
impairment and mitochondrial dysfunction in SH-SY5Y cells: Role for
PI3K/Akt/Nrf2 pathway. Toxicol In Vitro. 32:41–54. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tang K, Yang J, Gao X, Wang C, Liu L,
Kitani H, Atsumi T and Jing N: Wnt-1 promotes neuronal
differentiation and inhibits gliogenesis in P19 cells. Biochem
Biophys Res Commun. 293:167–173. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Masckauchan TN, Shawbe CJ, Funahashi Y, Li
CM and Kitajewski J: Wnt/beta-catenin signaling induces
proliferation, survival and interleukin-8 in human endothelial
cells. Angiogenesis. 8:43–51. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu M, Kay JC, Shen S and Qiao LY:
Endogenous BDNF augments NMDA receptor phosphorylation in the
spinal cord via PLCgamma, PKC, and PI3K/Akt pathways during
colitis. J Neuroinflammation. 12:1512015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang JW, Ru J, Ma W, Gao Y, Liang Z, Liu
J, Guo JH and Li LY: BDNF promotes the growth of human neurons
through crosstalk with the Wnt/β-catenin signaling pathway via
GSK-3β. Neuropeptides. 54:35–46. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Magnusson JP, Goritz C, Tatarishvili J,
Dias DO, Smith EM, Lindvall O, Kokaia Z and Frisén J: A latent
neurogenic program in astrocytes regulated by Notch signaling in
the mouse. Science. 346:237–241. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rossi DJ, Brady JD and Mohr C: Astrocyte
metabolism and signaling during brain ischemia. Nat Neurosci.
10:1377–1386. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Choudhury GR and Ding S: Reactive
astrocytes and therapeutic potential in focal ischemic stroke.
Neurobiol Dis. 85:234–244. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Amantea D, Micieli G, Tassorelli C,
Cuartero MI, Ballesteros I, Certo M, Moro MA, Lizasoain I and
Bagetta G: Rational modulation of the innate immune system for
neuroprotection in ischemic stroke. Front Neurosci. 9:1472015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vargas MR and Johnson JA: The Nrf2-ARE
cytoprotective pathway in astrocytes. Expert Rev Mol Med.
11:e172009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cao X, Xiao H, Zhang Y, Zou L, Chu Y and
Chu X: 1, 5-Dicaffeoylquinic acid-mediated glutathione synthesis
through activation of Nrf2 protects against OGD/reperfusion-induced
oxidative stress in astrocytes. Brain Res. 1347:142–148. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pan H, Wang H, Zhu L, Mao L, Qiao L and Su
X: Depletion of Nrf2 enhances inflammation induced by oxyhemoglobin
in cultured mice astrocytes. Neurochem Res. 36:2434–2441. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jeong WS, Kim IW, Hu R and Kong AN:
Modulatory properties of various natural chemopreventive agents on
the activation of NF-kappaB signaling pathway. Pharm Res.
21:661–670. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jin W, Wang H, Yan W, Zhu L, Hu Z, Ding Y
and Tang K: Role of Nrf2 in protection against traumatic brain
injury in mice. J Neurotrauma. 26:131–139. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sondergaard JN, Poghosyan S, Hontelez S,
Louche P, Looman MW, Ansems M and Adema GJ: DC-SCRIPT regulates
IL-10 production in human dendritic cells by modulating NF-κBp65
activation. J Immunol. 195:1498–1505. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hovsepian E, Penas F, Siffo S, Mirkin GA
and Goren NB: IL-10 inhibits the NF-κB and ERK/MAPK-mediated
production of pro-inflammatory mediators by up-regulation of SOCS-3
in Trypanosoma cruzi-infected cardiomyocytes. PLoS One.
8:e794452013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang Y, Fan L, Meng X, Jiang F, Chen Q,
Zhang Z and Yan H: Transplantation of IL-10-transfected endothelial
progenitor cells improves retinal vascular repair via suppressing
inflammation in diabetic rats. Graefes Arch Clin Exp Ophthalmol.
254:1957–1965. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jang HJ, Hong EM, Kim M, Kim JH, Jang J,
Park SW, Byun HW, Koh DH, Choi MH, Kae SH and Lee J: Simvastatin
induces heme oxygenase-1 via NF-E2-related factor 2 (Nrf2)
activation through ERK and PI3K/Akt pathway in colon cancer.
Oncotarget. 7:46219–46229. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Xu L, He S, Yin P, Li D, Mei C, Yu X, Shi
Y, Jiang L and Liu F: Punicalagin induces Nrf2 translocation and
HO-1 expression via PI3K/Akt, protecting rat intestinal epithelial
cells from oxidative stress. Int J Hyperthermia. 32:465–473. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Palgan K and Bartuzi Z: Platelet
activating factor in allergies. Int J Immunopathol Pharmacol.
28:584–589. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Aihara M, Ishii S, Kume K and Shimizu T:
Interaction between neurone and microglia mediated by
platelet-activating factor. Genes Cells. 5:397–406. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Montrucchio G, Alloatti G and Camussi G:
Role of platelet-activating factor in cardiovascular
pathophysiology. Physiol Rev. 80:1669–1699. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Li Z, Shu Q, Li L, Ge M and Zhang Y:
Sequential expression of cyclooxygenase-2, glutamate receptor-2,
and platelet activating factor receptor in rat hippocampal neurons
after fluid percussion injury. Neural Regen Res. 9:978–985. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lu J, Caplan MS, Li D and Jilling T:
Polyunsaturated fatty acids block platelet-activating
factor-induced phosphatidylinositol 3 kinase/Akt-mediated apoptosis
in intestinal epithelial cells. Am J Physiol Gastrointest Liver
Physiol. 294:G1181–G1190. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Nakaso K, Yano H, Fukuhara Y, Takeshima T,
Wada-Isoe K and Nakashima K: PI3K is a key molecule in the
Nrf2-mediated regulation of antioxidative proteins by hemin in
human neuroblastoma cells. FEBS Lett. 546:181–184. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hwang YP and Jeong HG: Ginsenoside Rb1
protects against 6-hydroxydopamine-induced oxidative stress by
increasing heme oxygenase-1 expression through an estrogen
receptor-related PI3K/Akt/Nrf2-dependent pathway in human
dopaminergic cells. Toxicol Appl Pharmacol. 242:18–28. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Xu X, Li H, Hou X, Li D, He S, Wan C, Yin
P, Liu M, Liu F and Xu J: Punicalagin induces Nrf2/HO-1 expression
via upregulation of PI3K/AKT pathway and inhibits LPS-induced
oxidative stress in RAW264.7 macrophages. Mediators Inflamm.
2015:3802182015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Pekny M and Pekna M: Astrocyte
intermediate filaments in CNS pathologies and regeneration. J
Pathol. 204:428–437. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Machon O, Backman M, Machonova O, Kozmik
Z, Vacik T, Andersen L and Krauss S: A dynamic gradient of Wnt
signaling controls initiation of neurogenesis in the mammalian
cortex and cellular specification in the hippocampus. Dev Biol.
311:223–237. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhou CJ, Borello U, Rubenstein JL and
Pleasure SJ: Neuronal production and precursor proliferation
defects in the neocortex of mice with loss of function in the
canonical Wnt signaling pathway. Neuroscience. 142:1119–1131. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
L'Episcopo F, Serapide MF, Tirolo C, Testa
N, Caniglia S, Morale MC, Pluchino S and Marchetti B: A Wnt1
regulated Frizzled-1/β-Catenin signaling pathway as a candidate
regulatory circuit controlling mesencephalic dopaminergic
neuron-astrocyte crosstalk: Therapeutical relevance for neuron
survival and neuroprotection. Mol Neurodegener. 6:492011.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Chacon MA, Varela-Nallar L and Inestrosa
NC: Frizzled-1 is involved in the neuroprotective effect of Wnt3a
against Abeta oligomers. J Cell Physiol. 217:215–227. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Lie DC, Colamarino SA, Song HJ, Désiré L,
Mira H, Consiglio A, Lein ES, Jessberger S, Lansford H, Dearie AR
and Gage FH: Wnt signalling regulates adult hippocampal
neurogenesis. Nature. 437:1370–1375. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Kalani MY, Cheshier SH, Cord BJ, Bababeygy
SR, Vogel H, Weissman IL, Palmer TD and Nusse R: Wnt-mediated
self-renewal of neural stem/progenitor cells. Proc Natl Acad Sci
USA. 105:16970–16975. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Harada T, Harada C, Nakayama N, Okuyama S,
Yoshida K, Kohsaka S, Matsuda H and Wada K: Modification of
glial-neuronal cell interactions prevents photoreceptor apoptosis
during light-induced retinal degeneration. Neuron. 26:533–541.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Yi H, Hu J, Qian J and Hackam AS:
Expression of brain-derived neurotrophic factor is regulated by the
wnt signaling pathway. Neuroreport. 23:189–194. 2012. View Article : Google Scholar : PubMed/NCBI
|





















