Introduction
An epidemiological survey demonstrated that the
prevalence of diabetes in adults ≥18 years old in China was ≤11.6%
(1). Diabetic neuropathy is one of
the three principal complications of diabetes, occurring earlier
and more frequently compared with other common complications,
including nephropathy and retinopathy (2). Overall, ≥50% of patients with
diabetes exhibit a certain degree of diabetic peripheral neuropathy
(DPN); the prevalence of DPN has been reported to be 16.2–28.9%
(3). Patients with diabetes and
DPN may exhibit alterations in sensation, movement or organ
function, depending on the type of nerves involved (4). Sensory neuropathy may cause numbness
to touch and vibration, reduced position sense associated with
poorer coordination and balance, and reduced sensitivity to pain
and alterations in temperature (5,6).
Motor neuropathy may cause impaired balance and coordination, and
most commonly, muscle weakness (5,6). The
detailed pathogenesis of peripheral nervous system dysfunction
associated with diabetes is unknown. Previous studies have
demonstrated that this pathogenesis may be associated with
long-term hyperglycemia, increased production of glycosylation end
products, alterations in microcirculation and neurotrophic factors,
oxidative stress and autoimmune disorders (7,8). In
addition, it has been reported that the polyol pathway is the
principal source of diabetes-induced oxidative stress in nerves
(9); however, there are no
clinically effective treatments for DPN. Insulin therapy may
regulate blood glucose; however, a number of patients with diabetes
still develop DPN. Therefore, understanding the pathogenesis of
neuropathy in patients with diabetes is necessary for the
development of effective treatments. Recently, the roles of
insulin-like growth factor 1 (IGF-1) in the prevention/treatment of
diabetes and associated complications have become a subject of
interest.
IGF-1 is a single-stranded polypeptide of 70 amino
acids and has been considered to be a multipotent neurotrophic
factor that is widely expressed in the body (10). A previous study has demonstrated
that IGF-1 ameliorated increased oxidative stress in the liver,
suggesting that IGF-1 may regulate mitochondrial function and
oxidative stress (11). In
addition, IGF-1 was observed to improve mitochondrial function
in vitro and in vivo (12). IGF-1 was reported to provide
trophic support for numerous neurons of the peripheral and central
nervous systems (13); DPN has
been proposed to be closely associated with IGF-1 (14). A recent study suggested that IGF-1
serves a role for neurotrophic function in the treatment of
diabetic neuropathy (15);
however, the molecular and cellular mechanisms underlying the
effects of IGF-1 on DPN require further investigation.
The present study aimed to determine the
neurotrophic effects of IGF-1 in a mouse model of diabetic
neuropathy. The potential underlying mechanisms were examined using
a mouse model of neuropathy in db/db mice. Additionally, the
present study investigated the peripheral drug-specific target of
IGF-1. The results of the present study may aid the development of
novel clinical therapeutic strategies and provide a basis for
future investigations into the pathogenesis of DPN.
Materials and methods
Ethical approval
All experimental procedures were approved by the
Ethics of Animal Experiments Committee of the Tongji University
School of Medicine (Shanghai, China). The present study was
conducted according to the Institutional Animal Care and Research
Advisory Committee of the Medical Department of Tongji
University.
Blood chemistry
The onset of diabetes was confirmed by measuring
fasting blood glucose levels. One drop of tail blood was analyzed
using a standard glucometer (Contour TS meter, Bayer AG,
Leverkusen, Germany) beginning from 8 weeks of age to 20 weeks of
age and repeated every 2 weeks to document the progression of
diabetes.
Animals
In total, 45 male C57 BKS db/db mice (cat. no.
N000180) weighing 50–60 g were purchased from Nanjing Biomedical
Research Institute of Nanjing University (Nanjing, China). In
total, 15 male C57 BKS db/m mice (cat. no. 201500531244) weighing
25–30 g were purchased from Shanghai Laboratory Animal Co., Ltd.
(Shanghai, China). The homozygous (db/db) mice were used as a model
of type 2 diabetes; whereas, heterozygous mice (db/m) served as
non-diabetic controls. Mice were housed in wire-bottomed cages in
22±1°C with a 12-h light/dark cycle. The mice were raised on a
commercial pellet diet (Jiangsu Province Collaborative Medicine
Bioengineering Co., Ltd, Jiangsu, China) and were provided with
access to tap water ad libitum. Mice were divided into four groups:
Db/m group (control; n=15), db/db group (diabetic model; n=15),
IGF-1-treated db/db mice (IGF-1 group; n=15) and the
IGF-1-picropodophyllin (IGF-1-PPP)-treated db/db group (n=15). The
weight and blood glucose of each mouse were measured every 2 weeks
from 8–20 weeks of age.
Animal treatments
Recombinant (r)IGF-1 was purchased from Sino
Biological Inc. (Beijing, China), which was dissolved in normal
saline and stored in aliquots at −80°C. PPP was purchased from
Selleck Chemicals (Shanghai, China) and freshly dissolved in
dimethyl sulfoxide at a concentration of 50 mM prior to use. The
IGF-1 group was intraperitoneally injected with rIGF-1 (0.4 mg/kg)
every day. The IGF-1-PPP-treated db/db group was intraperitoneally
injected with PPP (20 mg/kg) and rIGF-1 (0.4 mg/kg) every day from
8 weeks of age for ~12 weeks (16). Blood samples (0.8–1.2 ml) were
drawn from the abdominal aorta and anti-coagulated with 1 mg/ml
EDTA. Subsequently, samples were used to determine serum levels of
IGF-1 and IGF binding protein 3 (IGFBP-3). As described below,
sciatic nerves were analyzed to determine motor nerve conduction
velocity (MNCV) and morphology; the protein expression levels of
IGF-1, IGF-1 receptor (IGF-1R), c-Jun N-terminal kinase (JNK),
extracellular signal-regulated kinase (ERK) and p38 were
additionally investigated.
Behavioral studies
Hot plate method
At 6 weeks of age, mice were placed on a glass plate
within a transparent bottomless plastic chamber (20×10×20 cm) and
were allowed to acclimate for 30 min. Paw withdrawal latency (PWL)
was considered as an index of the nociceptive threshold. At the
beginning of the trials, the plantar surface of the mouse's paws
were exposed to a light beam of radiant heat (cat. no. BME-410C;
automatic thermal pain stimulator; Institute of Biomedical
Engineering, Chinese Academy of Medical Sciences, Tianjin, China).
Once the mouse lifted its paw, the light beam was turned off
immediately; the duration between exposure to the light beam to the
raising of the paw was recorded and denoted as the PWL. The hind
paws were tested alternately at intervals of 5 min. The cut-off
time for heat stimuli was 30 sec.
Von Frey method
The Von Frey method was conducted as previously
described (17). The 50% paw
withdrawal threshold (PWT) of mice was calculated as follows: A
Plexiglas cubicle was placed on a nylon mesh and the mouse was
placed in a bottomless glass box. The von Frey fibers (cat. no.
NC12775-99; North Coast Medical, Inc., Morgan Hill, CA, USA) were
used to stimulate the feet of the mouse's hind limbs for <4 sec
and the mice remained in the Plexiglass box for 30 min. Mice which
appeared to lift or lick their feet were regarded as exhibiting a
positive reaction, otherwise a negative score was reported.
MNCV measurements
Sciatic NCV was measured using a diabetic peripheral
nerve injury-screening instrument (electromyography evoked
potential meter; cat. no. NDI-097; Shanghai Haishen Medical
Electronic Instrument Co., Ltd., Shanghai, China) as described in a
previous study (18), with minor
modifications at 8 weeks of age. Mice of the four groups were
anesthetized in a prone position. The electrodes of the stimulus
needle were positioned between the femur and the calcaneal
tubercle; the electrode of the recording needle was placed via the
site of the ankle of the sciatic nerve. The reference electrode was
positioned between the stimulation and recording electrodes, the
reference electrode was 1 cm from the recording electrode; all
three types of electrodes were needle electrodes. A stimulus was
applied with a single pulse square wave, 0.1 msec wave width,
1.5-times the intensity of the threshold. Each of the two stimuli
had an interval of >6 sec. The room temperature (20.0±0.5°C) was
strictly controlled and the animal body temperature was maintained
at 37°C. The distance between the stimulus electrode and the
recording electrode was measured. The MNCV was calculated from the
duration between the onset of the stimulation to the evoked
potential of the muscle.
Tissue preparation and morphological
observation
Mice were anesthetized with 40 mg/kg pentobarbital,
the abdominal cavities of the mice were surgically opened following
local disinfection. Blood samples (0.8–1.2 ml) were obtained from
the abdominal aorta, maintained at room temperature for 15 min
prior to centrifugation in an Eppendorf tube containing EDTA (1
mg/ml) and subsequently centrifuged for 20 min at 2,000 × g at 4°C.
The supernatant was stored in an Eppendorf tube and preserved at
−70°C for further use. The mice were sacrificed via cervical
dislocation. Subsequently, the sciatic nerve specimens were
collected. The majority of the specimens were frozen in liquid
nitrogen until further analysis, 2 mm long sections were fixed with
2.5% glutaraldehyde for 6 h at 4°C. Subsequently, the samples were
rinsed with 0.1 M phosphate buffer and treated with 1%
OsO4 solution (Alfa Aesar; Thermo Fisher Scientific,
Inc.) for 3 h, dehydrated with a graded series of ethanol (30, 50,
80 and 90%) and propylene oxide, and embedded in epoxy resin
(Shanghai Resin Factory Co., Ltd, Shanghai, China). Semi-thin
sections (0.8–1 µm) were stained with 1% toluidine blue (Sinopharm
Chemical Reagent Co., Ltd., Shanghai, China) for histopathology at
room temperature for 4 min. Suitable areas of ultrathin (80–90 nm)
nerve sections were selected for ultrastructural analysis. These
were sectioned using a diamond knife (cat. no. 706602; Leica
Microsystems GmbH, Wetzlar, Germany), mounted on a copper grid
(Shanghai Huake Experimental Devices and Materials Co., Ltd.,
Shanghai, China), and stained with uranyl acetate for 30 min and
lead citrate for 10 min at room temperature. Sections were analyzed
and images were captured under a transmission electron microscope
(Tecnai-12; FEI; Thermo Fisher Scientific, Inc.; magnification,
×6,000; five fields of view analyzed).
ELISA analysis
The blood samples that were collected from the mice
were analyzed. Blood samples from the abdominal aorta were
centrifuged for 20 min at 2,000 × g at 4°C. The serum was stored in
an Eppendorf tube and preserved at −70°C for further use. The serum
IGF-1 and IGFBP-3 expression levels were measured using ELISA kits
(cat. nos. DL-IGF1-Mu and DL-IGFBP3-Mu; Wuxi Donglin Sci & Tech
Development Co, Ltd., Nanjing, China), according to the
manufacturer's protocol.
Western blot analysis
Sections of the sciatic nerve at 20 weeks of age and
after 12 weeks of treatment from mice in each group were frozen in
liquid nitrogen and preserved at −80°C for further analysis.
Samples were homogenized three times for 15 sec, in ice-cold
radioimmunoprecipitation assay homogenization buffer (Merck KGaA,
Darmstadt, Germany) containing Protease Inhibitor Cocktail Set III
(cat. no. 539134; Thermo Fisher Scientific, Inc.) and Phosphatase
Inhibitor Cocktail Set V (cat. no. 524629; Thermo Fisher
Scientific, Inc.) using a PRO 200 homogenizer (PRO Scientific,
Inc., Oxford, CT, USA). The homogenate was centrifuged at 4°C for
30 min at 12,000 × g and the supernatant was analyzed to detect the
total protein concentration. The protein concentration was
determined using a protein assay kit (DC Protein Assay kit; Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Samples (40 µg) were
separated via 10% SDS-PAGE and transferred onto polyvinylidene
difluoride membranes with an electrophoresis blotting transfer
apparatus. The membranes were blocked with 5% milk powder for 1 h
at 4°C and subsequently incubated with primary antibodies overnight
at 4°C. The following day, following three washes with
Tris-buffered saline with 0.1% Tween-20, the membranes were
incubated with the second antibody at room temperature for 1 h.
Bands were visualized following exposure to an enhanced
chemiluminescence reagent (cat. no. 5978613; GE Healthcare Life
Sciences, Shanghai, China) according to the manufacturer's
protocol. Quantitative values were obtained from the densitometry
of the western blotting bands using Gel-Pro Analyzer software
(version 4.0; Media Cybernetics, Inc., Rockville, MD, USA).
Antibodies against IGF-1 (1:100; cat. no. AF791-SP) and IGF-1R
(1:100; cat. no. AF-305-SBD) were purchased from R&D Systems,
Inc. (Minneapolis, MN, USA), phosphorylated (p)-ERK (1:200; cat.
no. 612358) was purchased from BD Biosciences (Franklin Lakes, NJ,
USA) and total (t)-ERK (1:100; cat. no. 1051) was obtained from
Boster Biological Technology (Pleasanton, CA, USA). The GAPDH
antibody (1:200; cat. no. 2118) was purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA). Antibodies against p-p38
(1:100; cat. no. AM063), t-p38 (1:100; cat. no. AM065), p-JNK
(1:100; cat. no. AF1762) and t-JNK (1:100; cat. no. AF1048) were
purchased from Beyotime Institute of Biotechnology (Haimen, China).
Rabbit anti-mouse immunoglobulin G (IgG) secondary antibody
[horseradish peroxidase (HRP)-conjugated; 1:10,000; cat. no. 58802)
and mouse anti-rabbit IgG secondary antibody (HRP-conjugated;
1:10,000; cat. no. 5127) were obtained from Cell Signaling
Technology, Inc. Rabbit anti-goat IgG secondary antibody
(HRP-conjugated; 1:10,000; cat. no. HAF017) was purchased from
Bio-Techne, Minneapolis, MN, USA.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The sciatic nerves from experimental mice in each
group were frozen in liquid nitrogen. Total mRNA was isolated with
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) and subsequently reverse-transcribed using
the PrimeScript™ RT reagent kit (Takara Bio, Inc., Otsu,
Japan) according to the manufacturer's protocol. qPCR was performed
with SYBR Premix ExTaq (Takara Biotechnology Co., Ltd. Dalian,
China) and the following primers provided by Sangon Biotech Co.,
Ltd. (Shanghai, China): IGF-1R, forward,
5′-ATCCTGTGTTCTTCTATGTCC-3′ and reverse,
5′-CCAACCTGCTGTTATTTCTC-3′; and GAPDH forward,
5′-TCCTGCACCACCAACTGCTTAG-3′ and reverse,
5′-AGTGGCAGTGATGGCATGGACT-3′. The thermocycling conditions were:
95°C for ≥30 sec, 40 cycles at 95°C for 15 sec and 60°C for 60 sec
in a DNA iCycler apparatus (7900 HT Sequence Detection System, ABI,
USA). Genes were quantified using the 2−∆∆Cq method
(19) with GAPDH as the
housekeeping gene. For each set of reactions, the samples were
analyzed in triplicate.
Statistical analysis
SPSS software version 18.0 for Windows (SPSS, Inc.,
Chicago, IL, USA) was used for statistical analysis. Data are
expressed as the mean ± standard deviation and analyzed for
statistical significance by one-way analysis of variance, followed
by Tukey's post-hoc test. Experiments were repeated three times.
P<0.05 was considered to indicate a statistically significant
difference.
Results
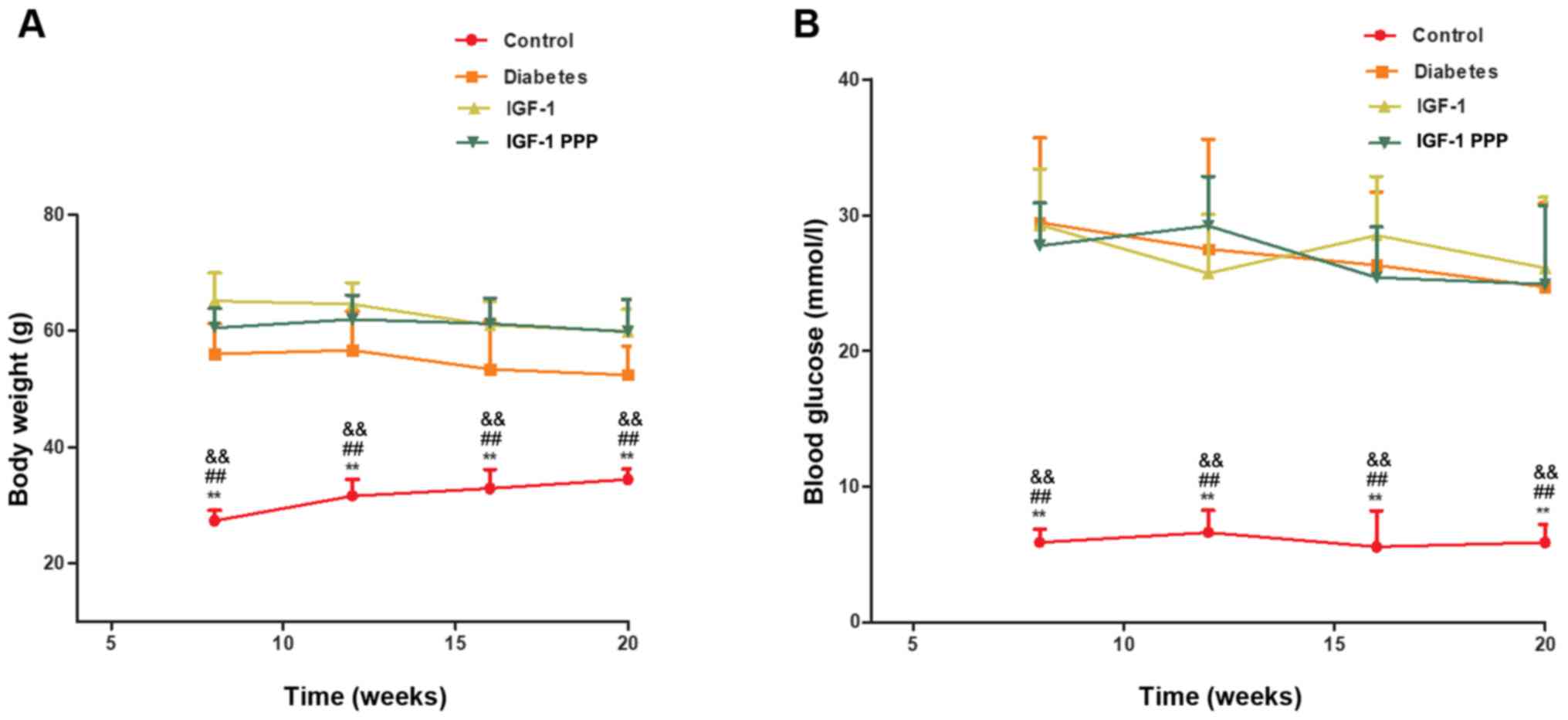
Body weight and blood glucose of
mice
No significant differences were observed in body
weight (Fig. 1A) and fasting blood
glucose levels (FBG; Fig. 1B)
between the diabetes, IGF-1 and IGF-1-PPP groups. Over time, the
weight of the diabetes, IGF-1 and IGF-1-PPP groups decreased;
however, were still significantly increased compared with the
control at different time points (P<0.01). For the control
group, although the weight of control group was increased, the
differences were not significant at different time points
(P>0.05). For the diabetes, IGF-1 and IGF-1-PPP groups, although
these three groups exhibited weight loss, the differences were not
significant at different time points (P>0.05).
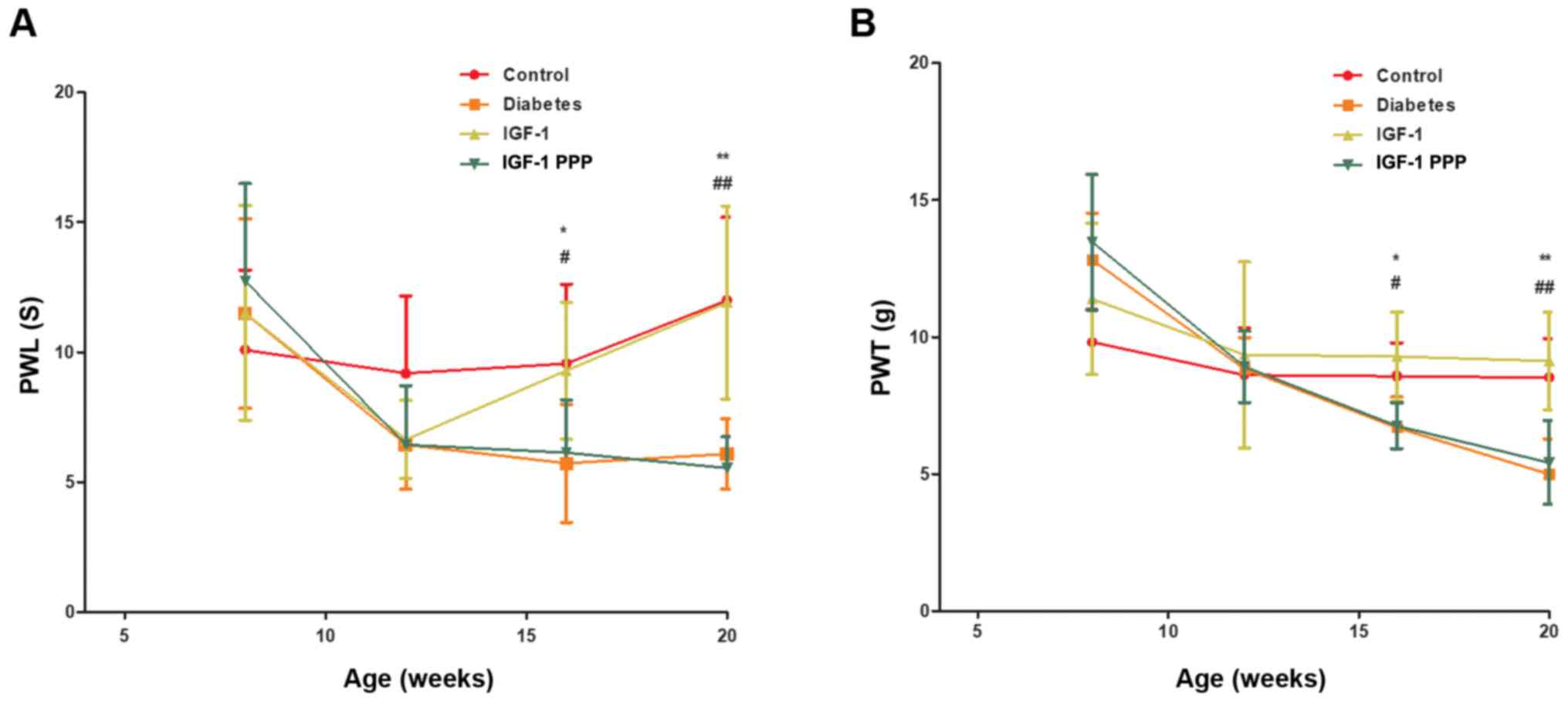
Effects of IGF-1 and the IGF-1R
antagonism PPP on behavior
Compared with the control and IGF-1 groups, the PWL
values of the diabetes and IGF-1-PPP groups were markedly extended
after 12 weeks of intervention. The maximum pain threshold was
increased by 6.7±1.1 sec (n=8–10; Fig.
2A) compared with the control group; the difference was
statistically significant (P<0.05). Compared with the control
and IGF-1 groups, the PWT of the diabetes and IGF-1-PPP groups
significantly decreased after 12 weeks of intervention (P<0.05).
The maximum pain threshold was decreased by 3.2±0.4 g (n=8–10;
Fig. 2B) compared with the control
group.
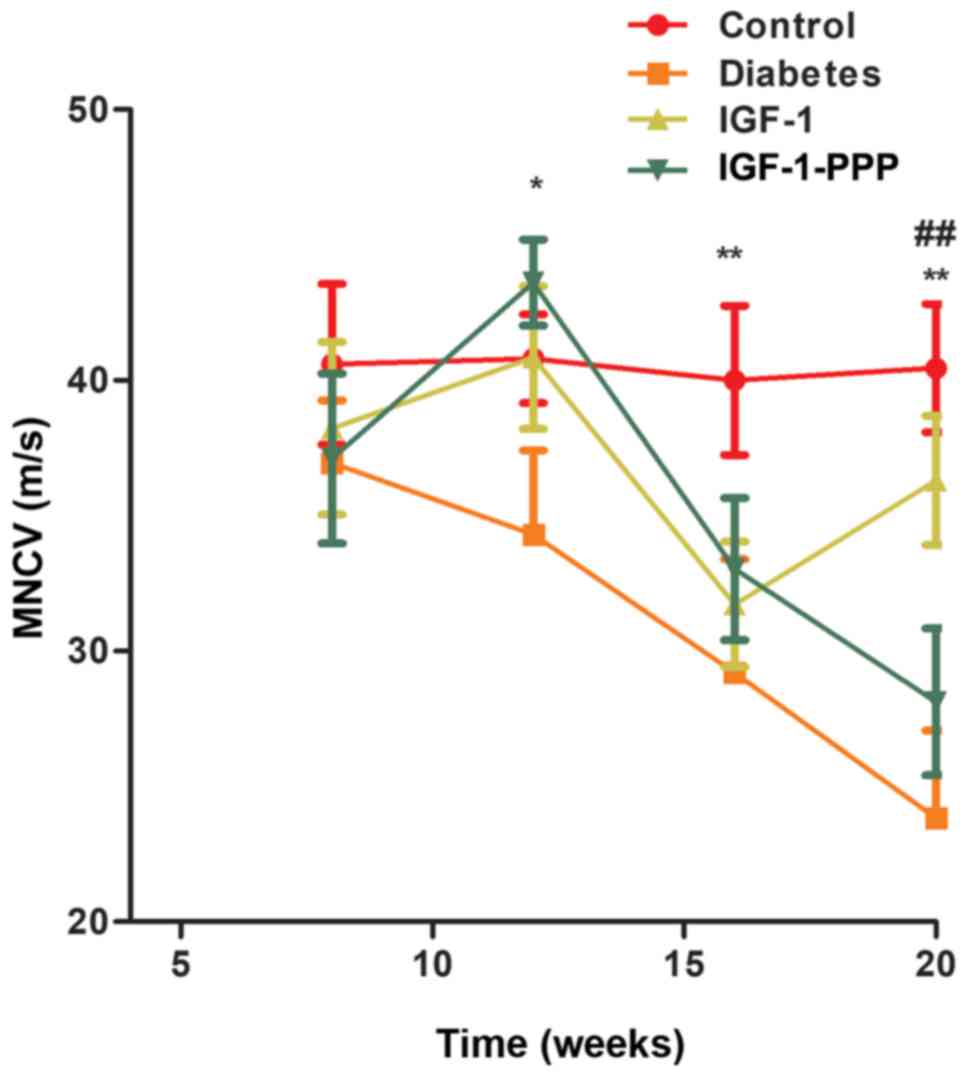
Effects of IGF-1 and IGF-1R antagonism
on MNCV
No significant differences were observed in the
rates of conduction in sciatic nerves between the four groups at 8
weeks of age prior to treatment (P>0.05). Following 12 weeks of
treatment (20 weeks old), the sciatic MNCV in the diabetes, IGF-1
and IGF-1-PPP groups was significantly lower compared with the
control group at 12 weeks of age (P<0.05). Additionally,
following 12 weeks of treatment, the sciatic MNCV in the diabetes
and IGF-1-PPP groups was significantly lower compared with the
control group; however, the levels of the MNCV of the IGF-1 group
significantly increased at 20 weeks of age and were similar to the
control group (P<0.01; Fig.
3).
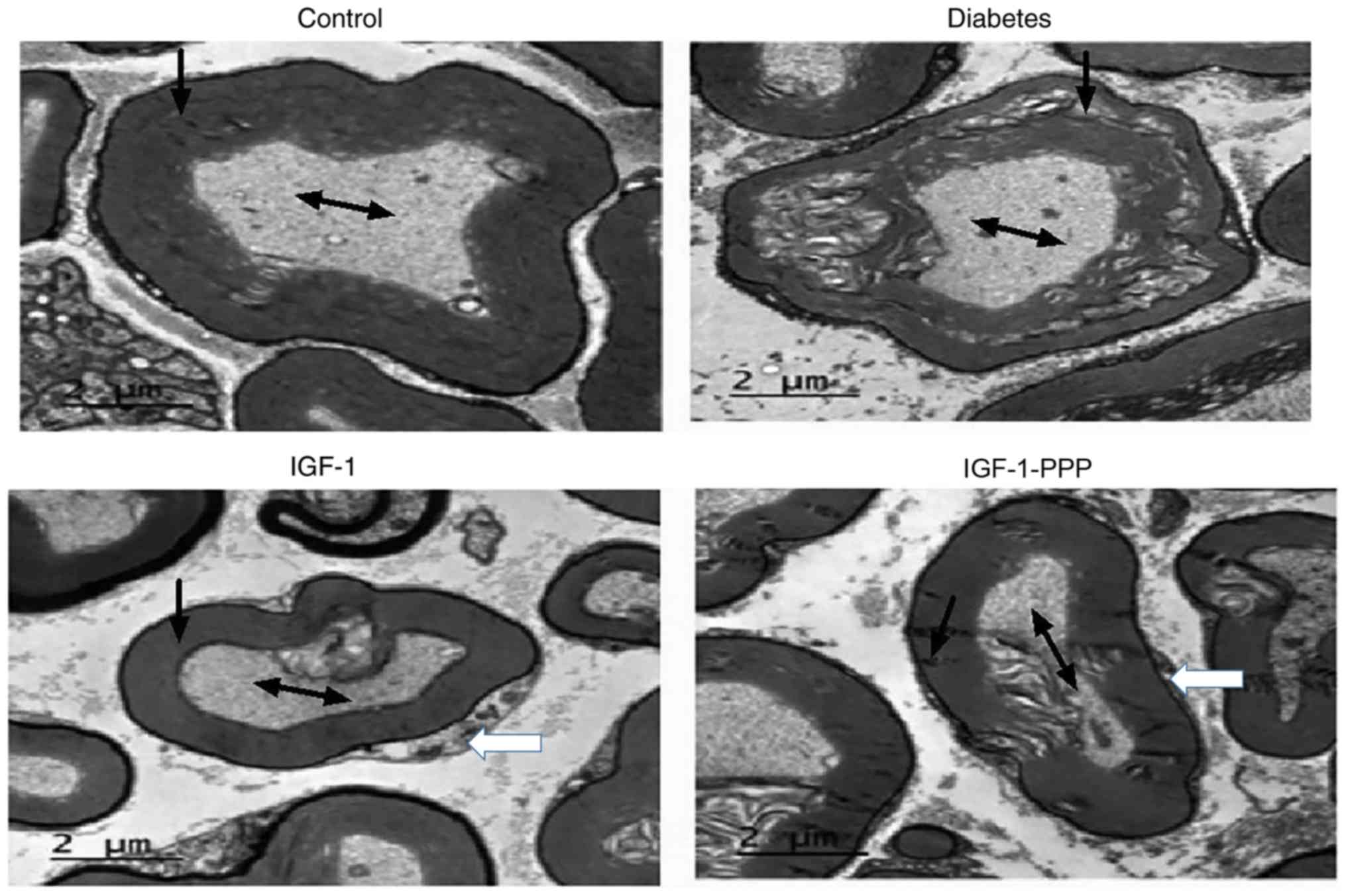
Effects of IGF-1 and IGF-1R antagonism
on sciatic nerve morphology
Following 12 weeks of treatment, the sciatic nerve
axis of the control group was relatively complete and the myelin
sheath exhibited regular formation at 20 weeks of age under a
transmission electron microscope. The myelin sheath in the sciatic
nerve of the diabetes group exhibited irregular vacuoles and
separation of the intima from the myelin sheath. Following rIGF-1
intervention, however, the sciatic nerves of the diabetes group
revealed signs of recovery, which manifested as an increase in
Schwann cells indicated by the white arrows, an intact myelin
sheath and notable reduced number of vacuoles. The recovery of
impaired sciatic nerves was attenuated following the administration
of the IGF-1R antagonist, PPP (Fig.
4).
Effects of IGF-1 and IGF-1R antagonism
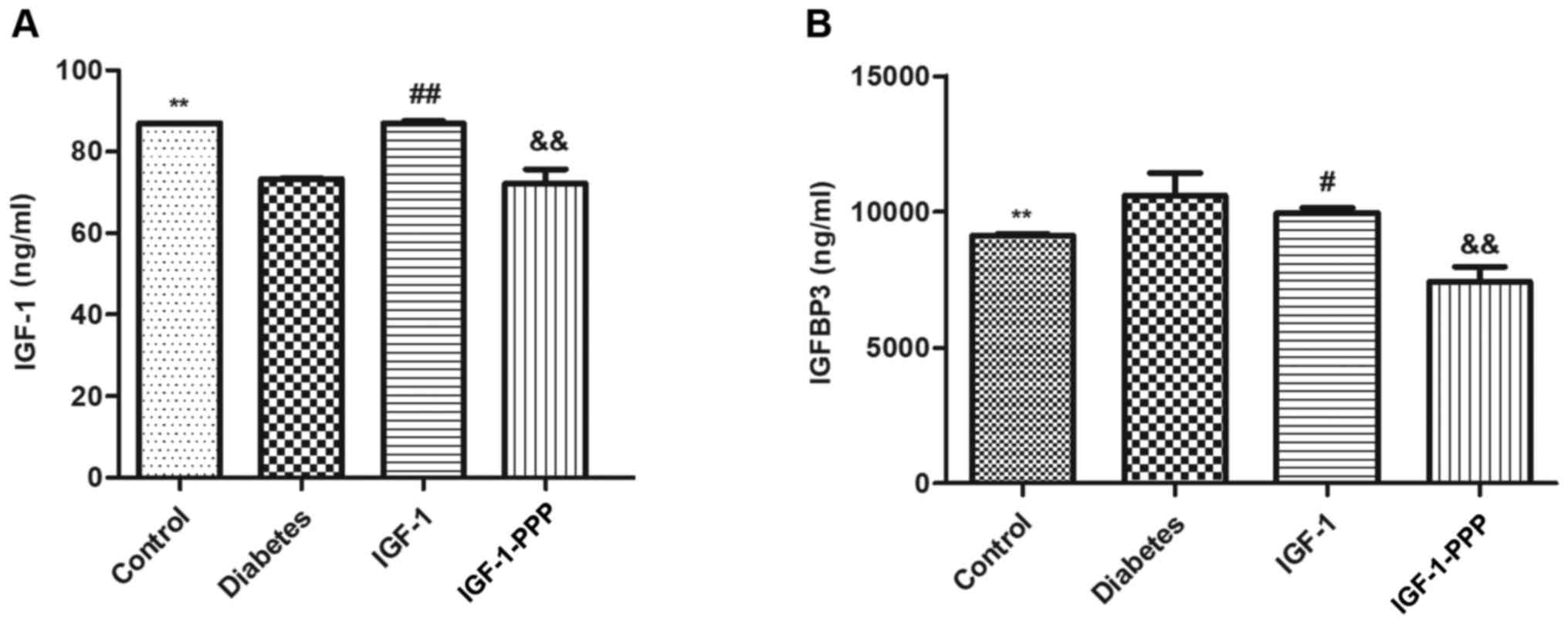
on IGF-1 and IGFBP-3 protein expression levels
The concentration of serum IGF-1 in the diabetes
group at 20 weeks of age was significantly lower compared with the
control group (P<0.05). Following 12 weeks of intervention with
IGF-1, the concentration of serum IGF-1 in the IGF-1 group
increased compared with the diabetes group, and the IGF-1-PPP group
significantly decreased compared with the IGF-1 group (Fig. 5A).
In addition, the serum concentration of IGFBP-3 in
the diabetes group at 20 weeks of age was significantly higher
compared with the control group (P<0.05). Following 12 weeks of
intervention with IGF-1, the serum concentration of IGFBP-3 in the
IGF-1 group was decreased compared with the diabetes group, and
decreased further in the IGF-1-PPP group compared with the IGF-1
group (Fig. 5B).
Effects of IGF-1 and IGF-1R antagonism
on IGF-1R, JNK, ERK and p38
No significant alterations were observed in the
protein expression levels of t-JNK, t-ERK and t-p38 in the sciatic
nerves of each group. (P>0.05; Fig.
6) The protein expression levels of p-JNK and p-ERK in the
sciatic nerves of the diabetes group were significantly lower
compared with the control group. The difference was statistically
significant (P<0.01). Compared with the diabetes group, the
expression levels of p-JNK and p-ERK in the IGF-1 group were
significantly increased (P<0.01). Compared with the IGF-1 group,
the expression levels of p-JNK and p-ERK in the IGF-1-PPP group
were significantly decreased (P<0.01; Fig. 6).
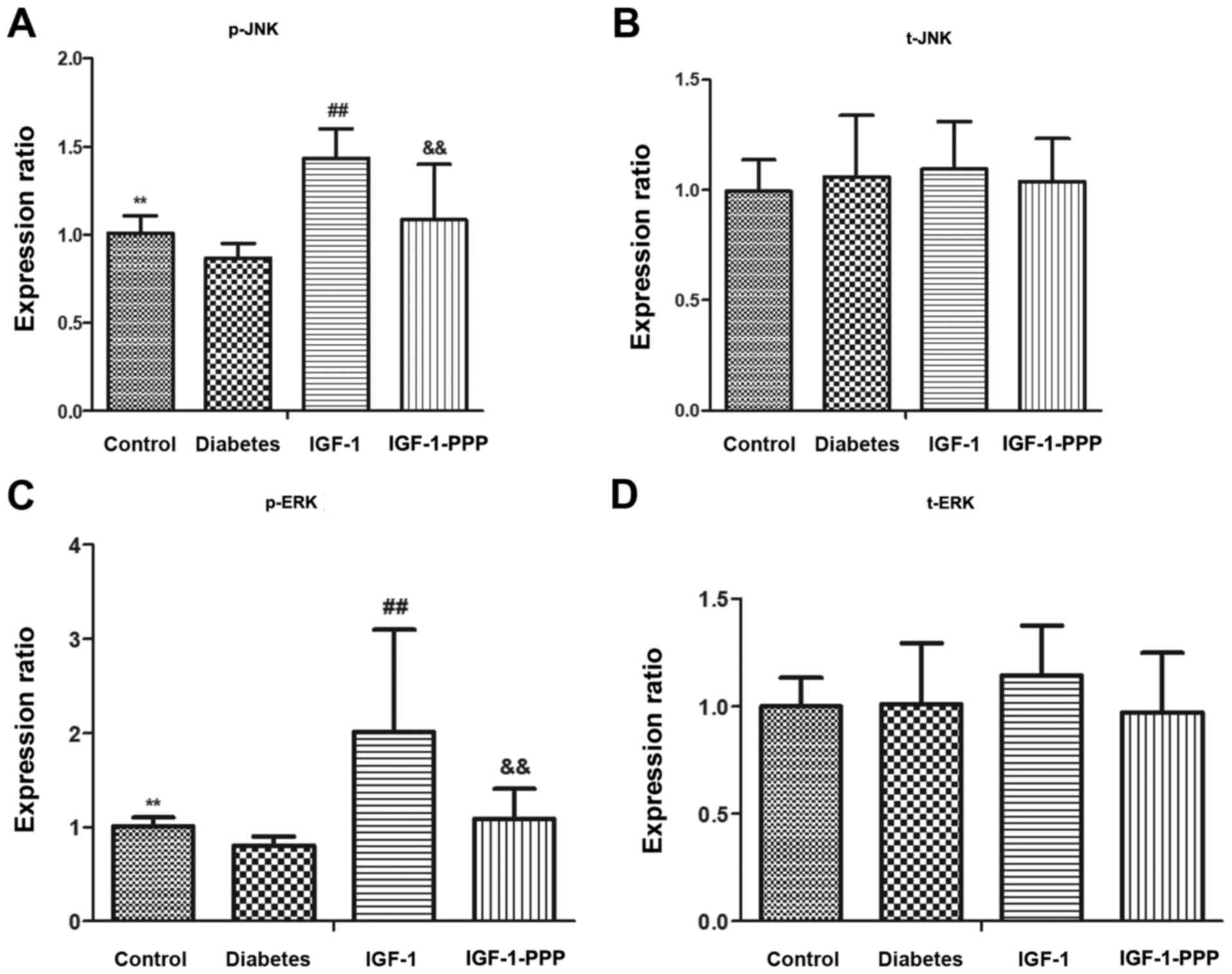 | Figure 6.Comparison of JNK, ERK and p38
expression and phosphorylation, and IGF-1R expression. Protein
expression levels of (A and B) JNK, (C and D) ERK, (E and F) p38
and (G) IGF-1R in sciatic nerves of the 4 groups at 20 weeks of age
were detected by western blot analysis and (H) semi-quantification
was performed. The data are presented as the mean ± standard
deviation. **P<0.01, control group vs. diabetes group;
##P<0.01, IGF-1 group vs. diabetes group;
&&P<0.01, IGF-1-PPP group vs. IGF-1 group. p,
phosphorylated; t, total; JNK, c-Jun N-terminal kinase; ERK,
extracellular signal-regulated kinase; IGF-1R, insulin-like growth
factor 1 receptor; PPP, picropodophyllin. |
The phosphorylation levels of p38 were relatively
high in the control group compared with the other three groups;
however, the difference was not statistically significant. No
significant differences were observed between the other three
groups (Fig. 6).
The expression levels of IGF-1R protein in the
sciatic nerves of the diabetes group were significantly lower
compared with the control group (P<0.01). Additionally, compared
with the diabetes group, the IGF-1R protein expression levels in
IGF-1 group were significantly increased (P<0.05). Conversely,
the IGF-1R protein expression levels in the IGF-1-PPP group were
decreased compared with the IGF-1 group; the difference was not
statistically significant (P>0.05; Fig. 6).
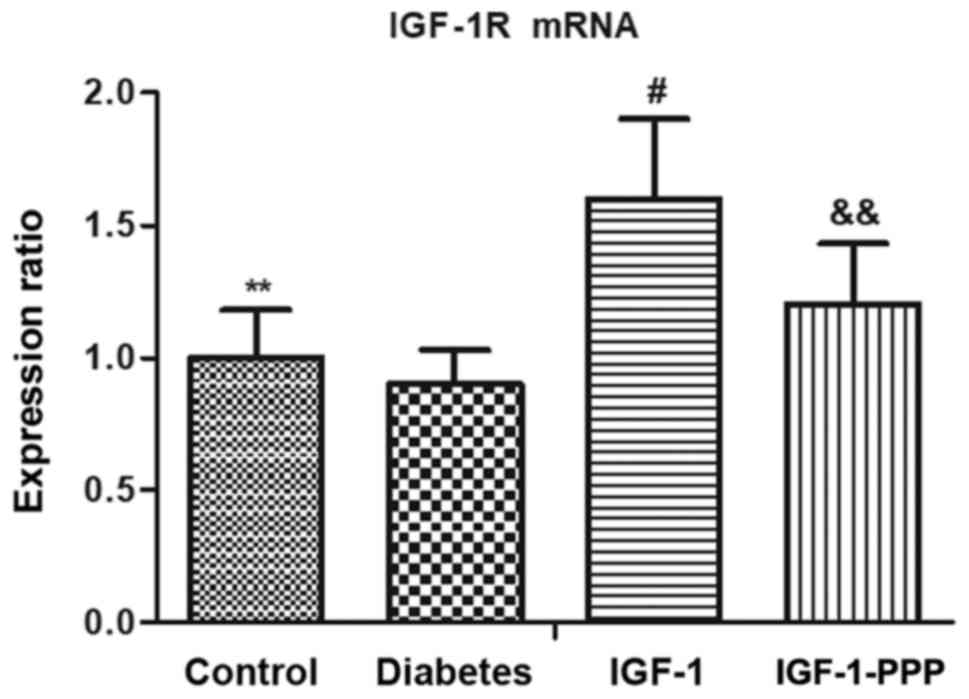
Effects of IGF-1 and IGF-1R antagonism
on IGF-1R mRNA expression
The mRNA expression levels of IGF-1R were observed
to be significantly decreased in the sciatic nerve of the diabetes
group compared with the control (Fig.
7). Conversely, a significant increase in the expression levels
of IGF-1R in the sciatic nerve of the IGF-1 group was detected
compared with the diabetes group (Fig.
7). Treatment with PPP induced a significant decrease in the
expression levels of IGF-1R compared with the IGF-1 group (Fig. 7).
Discussion
DPN is one of the principal chronic complications
associated with diabetes, with high morbidity rates among patients
with diabetes. DPN reduces quality of life due to pain, sensory
loss, gait instability, fall-associated injuries, foot ulceration
and amputation, and the causative mechanisms of DPN remain unclear
(20,21). To the best of our knowledge, there
is currently no effective therapy for the treatment of DPN. IGF-1
has been reported to be closely associated with DPN (14). A previous study evaluated the
association of three diabetic complications on the serum free IGF-I
and IGFBP-3 expression levels in patients with type 1 diabetes
(22). It was demonstrated that
patients with type 1 diabetes mellitus exhibited lower serum free
IGF and IGFBP-3 expression compared with healthy individuals
(22). Neuropathy was reported to
be associated with a significant reduction in serum free IGF-1
(22). It has been proposed that
in a streptozotocin (STZ)-treated mouse model of type 1 diabetes
mellitus, mice (C57BL/6 N) developed hypoalgesia, which was
associated with the decline in serum IGF-1 expression levels
(23). The sensory complications
of DPN in the mice model of type 1 diabetes mellitus may be
corrected by restoring circulating IGF-1 expression levels to
normal via a tail-vein injection of a recombinant adeno-associated
virus, AAV8-smIGF-1 (23). The
partial reversal of established DPN suggested a role for IGF-1 in
the pathogenesis and therapy of this condition. Investigation into
the role of IGF-1 may aid the development of clinical treatments
for DPN (24). Furthermore, an
STZ-treated mouse (ICR mouse) model of DPN demonstrated that an
intrathecal injection of a recombinant adeno-associated virus,
rAAV2/1, regulated the expression of IGF-1 via the protein kinase B
(Akt)/phosphatidylinositol 3-kinase (PI3K) signaling pathway to
promote the expression of vascular endothelial growth factor and
the regeneration of nerves, thus improving motor and sensory
neuropathy (25).
The aim of the present study was to evaluate the
protective effects of IGF-1 in alleviating the peripheral
neuropathy symptoms typically associated with diabetes. For this
purpose, a mouse model of type 2 diabetic neuropathy was
established and behavioral analysis was performed in the present
study. Existing literature regarding the effects of
experimentally-induced diabetes on pain thresholds in rodent models
remains controversial (26). In
the present study, alterations in algesia were determined as PWL
and PWT. A previous study demonstrated that the mice developed
hyperalgesia at 10 weeks of age (26). However, in the present study, the
mice developed hyperalgesia until 16 weeks of age. PWL and PWT are
more suitable for measuring the pain threshold of acute pain, and
the duration of diabetes of 16–20 weeks in the mice is a sufficient
time to produce chronic pain, different from the previous
short-term mice study (26).
Furthermore, decreases in MNCV in the diabetes group were observed
in the present study. Therefore, abnormal electrophysiologic
parameters, particularly those of motor nerves, may be considered
as an early indicator of DPN. In addition, typical structural
abnormalities were observed, including demyelination and vacuolar
alterations; however, following treatment with IGF-1, the sciatic
nerves exhibited indications of recovery. This may due to the
expression of IGF-1R on Schwann cells; activation of this receptor
has been revealed to promote myelination (27). Furthermore, as one of the growth
factors expressed by Schwann cells, IGF-1 is reported to be
involved in the metabolism of neuronal myelin, suggesting that
IGF-1 may be involved in the onset and development of DPN (28).
Additionally, it was reported that reduced serum
IGF-1 was associated with alterations in electrophysiologic and
structural parameters in the diabetes group. Therefore, the present
study investigated the effects of IGF-1 on DPN following an
intraperitoneal injection of IGF-1. Following treatment, the serum
concentration IGF-1 in the IGF-1 group was increased, which was
consistent with the observed improvements of sciatic nerve
morphology.
The results of the present study suggested that
treatment with IGF-1 may benefit DPN-induced db/db mice. In
addition, treatment with IGF-1 following the onset of diabetes may
reverse neuronal deficits, as observed in the present study. This
was consistent with an earlier observation in a rat model, in which
IGF replacement therapy was proposed to reverse or prevent diabetic
neuropathy, independent of hyperglycemia or weight loss (29). The present study additionally
demonstrated that IGF-1 may be a potential therapeutic agent for
diabetic neuropathy; and similarly, a previous study reported that
rIGF-1 may restore sensory and motor NCVs in IGF-I deficient mice
(30). These results suggested
that the effects of IGF-1 as a treatment for DPN may occur via the
activation of IGF-1R.
IGF-1 targets the extracellular binding site of
IGF-1R and activates the downstream intracellular protein insulin
receptor substrate (IRS-1) via phosphorylation of the D
transmembrane subunit of IGF-1R (31). IRS-1 transduces the signal of IGF-1
via two downstream cascades, the Akt/PI3K and mitogen-activated
protein kinase (MAPK) signaling pathways (31). The association between the Akt and
MAPK signaling pathways has been reported, and their equilibrium is
important for maintaining the physiological function of normal
cells (32). Previous studies have
demonstrated that IGF-1 signaling is mostly dependent on the
Akt/PI3K pathway, to serve a pro-apoptotic role in neurons
(33,34); however, further investigation into
the association between the MAPK signaling pathway and IGF-1 is
required. The MAPK family of proteins constitutes a set of serine
and threonine protein kinases with several subfamilies. Among these
proteins are three important members, which maintain the normal
physiological function of cells, ERK, JNK and p38 (35). Therefore, it is important to
investigate the role of MAPK signaling in DPN. To gain insight into
the respective roles served by the MAPK signaling pathway, via
western blotting, the present study revealed that the role of
IGF-1, which offers maintenance support for the sciatic nerve
(36), may be associated with
increased expression levels of p-JNK and p-ERK, not p-p38. As only
preliminary signaling studies on the sciatic nerve of animals have
been conducted, further investigations of ion channels in
vitro is required to understand the protective effect of IGF-1
against neuropathy in diabetic mice and its potential underlying
mechanisms.
In conclusion, the present results suggested that,
in a diabetic animal model, IGF-1 may be a highly effective
therapeutic modality for treating diabetic peripheral neuropathy.
The effects of IGF-1, which may provide trophic support for the
sciatic nerve, may be associated with the increased phosphorylation
levels of JNK and ERK, not p38. These neurotrophic effects of IGF-1
may be attenuated following the administration of an IGF-1R
antagonist. Although at present, systemic administration of free
IGF-1 in patients has restricted therapeutic potential due to its
instability in during circulation and side effects, in the future,
IGF-1, provided by either an agonist or by agents that may serve to
upregulate IGF-1, may provide therapeutic benefits in DPN.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Shanghai
Nature Science Fund projects (grant no. 14ZR1434000; Shanghai,
China) and the Fund of Science and Technology Department of Pudong
New Area (grant no. PKJ2015-Y19; Shanghai, China).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HW conceived and designed the experiments. ZT, FC
and JL performed the experiments. HW, ZT and HZ analyzed the data.
JT and HL acquired the data. YZ and BF interpreted the data. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
All experimental procedures were approved by the
Ethics of Animal Experiments Committee of the Tongji University
School of Medicine. The present study was conducted according to
internationally recognized guidelines on animal welfare, in
addition to the regulations regarding animal welfare in Shanghai,
and the Guidelines of the Chinese Council on Animal Care.
Patient consent to participate
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Xu Y, Wang L, He J, Bi Y, Li M, Wang T,
Wang L, Jiang Y, Dai M, Lu J, et al: Prevalence and control of
diabetes in Chinese adults. JAMA. 310:948–959. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Galuppo M, Giacoppo S, Bramanti P and
Mazzon E: Use of natural compounds in the management of diabetic
peripheral neuropathy. Molecules. 19:2877–2895. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bongaerts BW, Rathmann W, Kowall B, Herder
C, Stöckl D, Meisinger C and Ziegler D: Postchallenge hyperglycemia
is positively associated with diabetic polyneuropathy: The KORA F4
study. Diabetes Care. 35:1891–1893. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Duby JJ, Campbell RK, Setter SM, White JR
and Rasmussen KA: Diabetic neuropathy: An intensive review. Am J
Health Syst Pharm. 61:160–173; quiz 175–176. 2004.PubMed/NCBI
|
|
5
|
Morrison JF, Shehab S, Sheen R,
Dhanasekaran S, Shaffiullah M and Mensah-Brown E: Sensory and
autonomic nerve changes in the monosodium glutamate-treated rat: A
model of type II diabetes. Exp Physiol. 93:213–222. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Beiswenger KK, Calcutt NA and Mizisin AP:
Epidermal nerve fiber quantification in the assessment of diabetic
neuropathy. Acta Histochem. 110:351–362. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Grisold A, Callaghan BC and Feldman EL:
Mediators of diabetic neuropathy: Is hyperglycemia the only
culprit? Curr Opin Endocrinol Diabetes Obes. 24:103–111. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Román-Pintos LM, Villegas-Rivera G,
Rodríguez-Carrizalez AD, Miranda-Díaz AG and Cardona-Muñoz EG:
Diabetic polyneuropathy in Type 2 diabetes mellitus: Inflammation,
oxidative stress and mitochondrial function. J Diabetes Res.
2016:34256172016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chung SS, Ho EC, Lam KS and Chung SK:
Contribution of polyol pathway to diabetes-induced oxidative
stress. J Am Soc Nephrol. 14(8 Suppl 3): S233–S236. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yakar S, Pennisi P, Wu Y, Zhao H and
LeRoith D: Clinical relevance of systemic and local IGF-I. Endocr
Dev. 9:11–16. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hao CN, Geng YJ, Li F, Yang T, Su DF, Duan
JL and Li Y: Insulin-like growth factor-1 receptor activation
prevents hydrogen peroxide-induced oxidative stress, mitochondrial
dysfunction and apoptosis. Apoptosis. 16:1118–1127. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Puche JE, García-Fernández M, Muntané J,
Rioja J, González-Barón S and Castilla Cortazar I: Low doses of
insulin-like growth factor-I induce mitochondrial protection in
aging rats. Endocrinology. 149:2620–2627. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Carro E, Trejo JL, Núñez A and
Torres-Aleman I: Brain repair and neuroprotection by serum
insulin-like growth factor I. Mol Neurobiol. 27:153–162. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ishii DN: Implication of insulin-like
growth factors in the pathogenesis of diabetic neuropathy. Brain
Res Brain Res Rev. 20:47–67. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Papanas N and Ziegler D: Risk factors and
comorbidities in diabetic neuropathy: An update 2015. Rev Diabet
Stud. 12:48–62. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Menu E, Jernberg-Wiklund H, Stromberg T,
De Raeve H, Girnita L, Larsson O, Axelson M, Asosingh K, Nilsson K,
Van Camp B and Vanderkerken K: Inhibiting the IGF-1 receptor
tyrosine kinase with the cyclolignan PPP: An in vitro and in vivo
study in the 5T33MM mouse model. Blood. 107:655–660. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hargreaves K, Dubner R, Brown F, Flores C
and Joris J: A new and sensitive method for measuring thermal
nociception in cutaneous hyperalgesia. Pain. 32:77–88. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kanazawa Y, Takahashi-Fujigasaki J,
Ishizawa S, Takabayashi N, Ishibashi K, Matoba K, Kawanami D,
Yokota T, Tajima N and Utsunomiya K: The Rho-kinase inhibitor
fasudil restores normal motor nerve conduction velocity in diabetic
rats by assuring the proper localization of adhesion-related
molecules in myelinating Schwann cells. Exp Neurol. 247:438–446.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tesfaye S, Boulton AJ, Dyck PJ, Freeman R,
Horowitz M, Kempler P, Lauria G, Malik RA, Spallone V, Vinik A, et
al: Diabetic neuropathies: Update on definitions, diagnostic
criteria, estimation of severity and treatments. Diabetes Care.
33:2285–2293. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rathur HM and Boulton AJ: The neuropathic
diabetic foot. Nat Clin Pract Endocrinol Metab. 3:14–25. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Capoluongo E, Pitocco D, Santonocito C,
Concolino P, Santini SA, Manto A, Lulli P, Ghirlanda G, Zuppi C and
Ameglio F: Association between serum free IGF-I and IGFBP-3 levels
in type-I diabetes patients affected with associated autoimmune
diseases or diabetic complications. Eur Cytokine Netw. 17:167–174.
2006.PubMed/NCBI
|
|
23
|
Chu Q, Moreland R, Yew NS, Foley J,
Ziegler R and Scheule RK: Systemic insulin-like growth factor-1
reverses hypoalgesia and improves mobility in a mouse model of
diabetic peripheral neuropathy. Mol Ther. 16:1400–1481. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schmidt RE, Dorsey DA, Beaudet LN, Plurad
SB, Parvin CA and Miller MS: Insulin-like growth factor I reverses
experimental diabetic autonomic neuropathy. Am J Pathol.
155:1651–1660. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Homs J, Pagès G, Ariza L, Casas C, Chillón
M, Navarro X and Bosch A: Intrathecal administration of IGF-I by
AAVrh10 improves sensory and motor deficits in a mouse model of
diabetic neuropathy. Mol Ther Methods Clin Dev. 1:72014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
O'Brien PD, Sakowski SA and Feldman EL:
Mouse models of diabetic neuropathy. ILAR J. 54:259–272. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Russell JW, Cheng HL and Golovoy D:
Insulin-like growth factor-I promotes myelination of peripheral
sensory axons. J Neuropathol Exp Neurol. 59:575–584. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Delaney CL, Russell JW, Cheng HL and
Feldman EL: Insulin-like growth factor-I and over-expression of
Bcl-xL prevent glucose-mediated apoptosis in Schwann cells. J
Neuropathol Exp Neurol. 60:147–160. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhuang HX, Snyder CK, Pu SF and Ishii DN:
Insulin-like growth factors reverse or arrest diabetic neuropathy:
Effects on hyperalgesia and impaired nerve regeneration in rats.
Exp Neurol. 140:198–205. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gao WQ, Shinsky N, Ingle G, Beck K, Elias
KA and Powell-Braxton L: IGF-I deficient mice show reduced
peripheral nerve conduction velocities and decreased axonal
diameters and respond to exogenous IGF-I treatment. J Neurobiol.
39:142–152. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rabiee A, Krüger M, Ardenkjær-Larsen J,
Kahn CR and Emanuelli B: Distinct signalling properties of insulin
receptor substrate (IRS)-1 and IRS-2 in mediating insulin/IGF-1
action. Cell Signal. 47:1–15. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhou YL, Liu SQ, Yuan B and Lu N: The
expression of insulin-like growth factor-1 in senior patients with
diabetes and dementia. Exp Ther Med. 13:103–106. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pfäffle R, Kiess W and Klammt J:
Downstream insulin-like growth factor. Endocr Dev. 23:42–51. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Leinninger GM, Backus C, Uhler MD, Lentz
SI and Feldman EL: Phosphatidylinositol 3-kinase and Akt effectors
mediate insulin-like growth factor-I neuroprotection in dorsal root
ganglia neurons. FASEB J. 18:1544–1546. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Qi M and Elion EA: MAP kinase pathways. J
Cell Sci. 118:3569–3572. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mohammadi R and Saadati A: Influence of
insulin-like growth factor I on nerve regeneration using
allografts: A sciatic nervemodel. J Craniofac Surg. 25:1510–1515.
2014. View Article : Google Scholar : PubMed/NCBI
|