Introduction
Cardiovascular complications resulting from
atherosclerosis are the leading causes of morbidity and mortality
in patients with coronary heart disease (CHD) (1). Endothelial dysfunction is the first
step in the initiation of atherosclerosis and is caused by
endothelial injury and inflammation (2). The injured endothelial monolayer may
be regenerated by circulating bone marrow-derived endothelial
progenitor cells (EPC), which accelerate re-endothelialization and
limit the progression of the atherosclerotic lesions (3). EPCs are precursor cells with high
proliferation potential and capacity to differentiate into
endothelial cells (3). EPCs also
participate in physiological and pathological neovascularization
(3), making them attractive for
cell therapy targeting the regeneration of ischemic tissues
(4,5). Importantly, the numbers of
circulating EPCs are low in certain diseases, including coronary
artery disease (CAD) and diabetes (6–8). An
improved understanding of the mechanisms by which EPCs are
regulated may provide novel insights into therapeutic
neovascularization, but the exact mechanism leading to EPC
deficiency remains unknown.
A high level of circulating oxidized low-density
lipoproteins (oxLDLs) is an independent predictor for future
cardiac events (9–11). In addition, it has been
demonstrated that oxLDLs may be one of the factors affecting the
growth and bioactivity of EPCs. Indeed, Wang et al (12) indicated that oxLDLs decreased the
numbers and activity of EPCs. Wu et al (13) suggested that oxLDLs regulated the
number and function of EPCs through the p38 mitogen-activated
protein kinase (p38 MAPK) pathway. Tie et al (14) revealed that oxLDLs disrupt the
phosphoinositide 3-kinase/protein kinase B (PI3K/Akt) pathway in
EPCs, leading to apoptosis. Lin et al (15) suggested that the effects of oxLDLs
on EPCs were dose-dependent. Nevertheless, the underlying mechanism
of the action remains largely unknown.
Insulin-like growth factor-1 (IGF-1) and the IGF-1
receptor affect the differentiation and apoptosis of various cells
(16,17). IGF-1 levels decrease during aging
and are decreased in patients with CVD (18,19).
A low level of IGF-1 has been identified as an independent risk
factor for CVD (20,21). IGF-1 not only participates in
protecting the endothelium but also affects the number and function
of stem cells. Indeed, Urbanek et al (22) identified that IGF-1 improves the
proliferation of cardiac stem cells, resulting in improved
regeneration following heart infarction. Treatment of mice with
IGF-1 increases the number and function of EPCs (23). Agonists of the IGF-1 receptor
improve EPC function (24).
Furthermore, EPCs treated with IGF-1 exhibit increased expression
and activity of the endothelial nitric oxide synthase (eNOS)
(23).
Considering these data, it was hypothesized that
IGF-1 may protect EPCs from induction of ox-LDLs, and that the eNOS
axis is involved in this effect. Therefore, the present study aimed
to investigate whether IGF-1 protects EPCs from injury caused by
ox-LDLs via the eNOS/NO pathway in vitro. The results may
provide novel insights for the eventual use of EPCs to treat
patients with CVD.
Materials and methods
Preparation and oxidation of LDLs
Ethical approval was obtained by the Medical Ethics
Committee of The Second Xiangya Hospital (Changsha, China). Human
LDLs (d=1.019–1.063 g/ml) were isolated by sequential
ultracentrifugation (235,000 × g at 4°C for 24 h) of plasma from 20
normolipidemic subjects (10 males and 10 females from January to
July 2017) following overnight fasting, as described previously
(25). Informed consent was
obtained. The purity of the LDLs was assessed by agarose gel
electrophoresis and the protein concentration was determined by the
modified Lowry method (26). The
LDL particles were dialyzed by semi-permeable membrane (3500D) for
24 h with 0.01 M PBS (pH 7.4) at 4°C to remove EDTA, then oxidized
by exposure to CuSO4 (10 mM CuSO4, 24 h at
37°C) (27). EDTA was added and
the LDL particles were dialyzed by semi-permeable membrane (3500D)
for 24 h with PBS to terminate the oxidization at 4°C.
Thiobarbituric acid-reactive substances and agarose gel
electrophoretic mobility were determined. oxLDLs were sterilized by
passing through a 0.22-µm Millipore filter (SLGP033RB; Merck KGaA,
Darmstadt, Germany).
Isolation and culture of EPCs
EPCs were cultured as described previously (28,29).
Briefly, 40 ml peripheral blood from healthy volunteers [aged 18 to
33 years old (21.0±4.5 years)] who provided informed consent were
subjected to density gradient centrifugation (671 × g for 20 min at
room temperature) with Histopaque-1077 (10771; Sigma-Aldrich; Merck
KGaA) to isolate peripheral blood mononuclear cells (PBMCs).
Following purification and 3 washing steps, 10×106 PBMCs
per well were plated on fibronectin-coated 6-well plates. The cells
were cultured in endothelial basal medium-2 (EBM-2; cat. no.
CC3156; Clonetics; Lonza Group Ltd., Walkersville, MD, USA) with
single EGM-2MV aliquots containing 5% fetal bovine serum (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA), vascular
endothelial growth factor (VEGF), fibroblast growth factor-2,
epidermal growth factor, insulin-like growth factor and ascorbic
acid. After 4 days, non-adherent cells were removed by washing with
PBS. Fresh medium was added, and the culture was continued for 8
days. Non-adherent cells were removed again by washing with PBS and
the adherent cells were considered as EPCs and harvested for
subsequent experiments.
EPC characterization
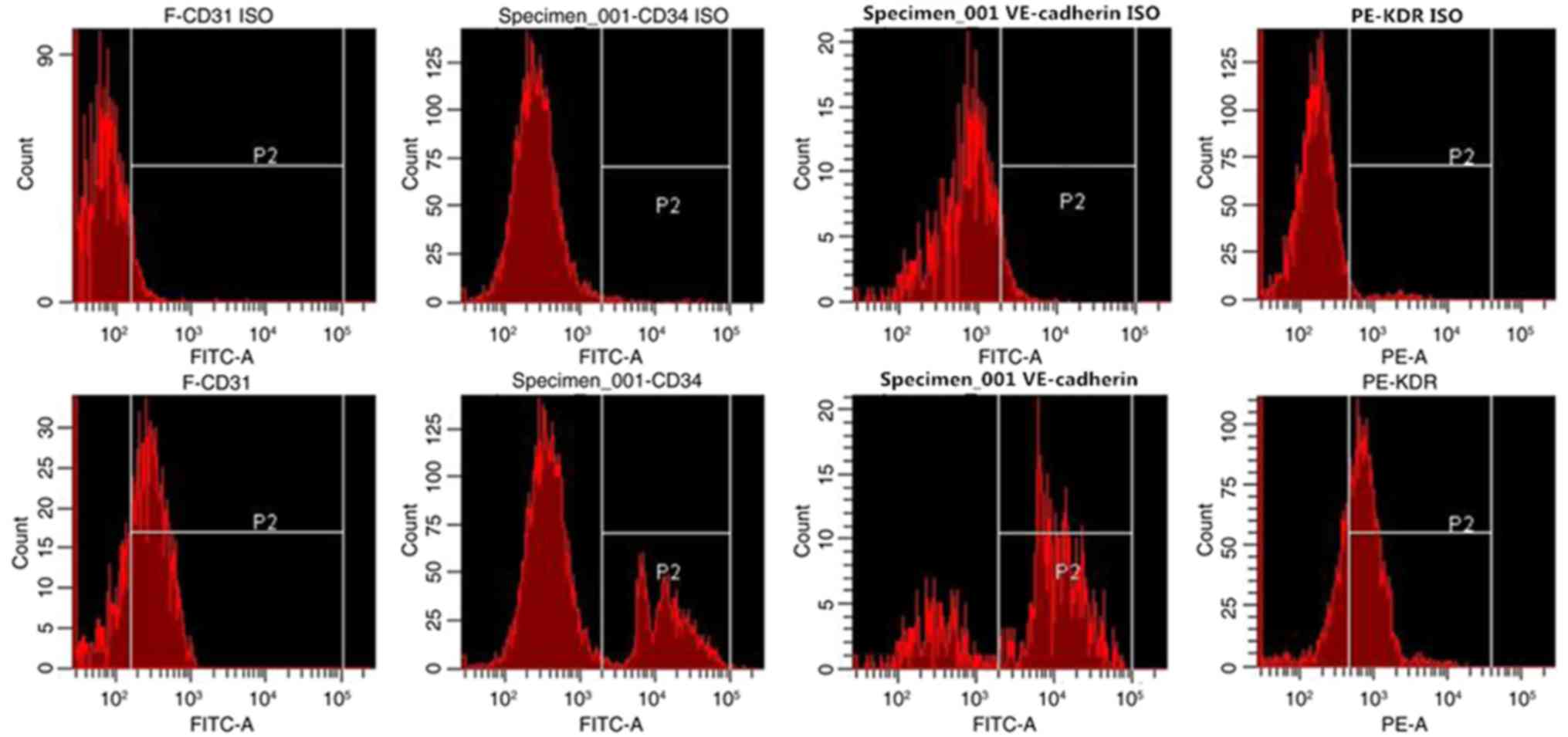
To confirm the endothelial phenotype, the expression
of endothelial protein markers was measured by flow cytometry (BD
Biosciences, Franklin Lakes, NJ, USA). EPCs were detached with 1 mM
EDTA in PBS and incubated for 15 min with human fluorescein
isothiocyanate (FITC)-conjugated kinase insert domain receptor
(KDR; cat. no. FAB357F-025; R&D Systems, Minneapolis, MN, USA),
anti-vascular endothelium cadherin (cat. no. sc9989; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA), phycoerythrin
(PE)-conjugated cluster of differentiation 31 (CD31; cat. no.
553373; BD Biosciences), or rat anti-mouse FITC-conjugated cluster
of differentiation 34 (CD34; DS-MB-03816; Raybiotech Life, Inc.,
Atlanta, GA, USA). For vascular endothelial cadherin (VE-cadherin)
analysis, cells were first incubated with mouse anti-human
VE-cadherin (cat. no. 555661; BD Biosciences; 1:1,000) for 30 min
at 4°C. Following washing with PBS twice for 5 min each, cells were
incubated with FITC-conjugated goat anti-mouse secondary antibody
(1:200) for 30 min at 4°C (cat. no. F9384: Sigma-Aldrich; Merck
KGaA). Mouse IgG1 isotype control antibody (cat. no. 555121; BD
Biosciences; 1:1,000) served as controls. Following incubation, the
cells were fixed with 1% paraformaldehyde for 15 min at 4°C and
quantitative analysis was performed on a FACScan flow cytometer (BD
Biosciences) and analyzed with CellQuest software (version 5.1; BD
Biosciences) with 20,000 cells/sample.
Treatment of EPCs
EPCs were treated without or with 100 mg/ml oxLDLs
for 24 h. EPCs in the IGF-1 group were pretreated with 0.1 or 0.5
µg/ml IGF-1 for 30 min prior to exposure to oxLDLs, as described
previously (30,31). An additional group of cells was
also pretreated with 100 µM nomega-nitro-L-arginine methyl ester
(L-NAME), an inhibitor of eNOS, for 60 min and then with 0.5 µg/ml
IGF-1 for 30 min prior to exposure to oxLDLs.
Apoptosis assay
Apoptosis was analyzed using an Annexin V/propidium
iodide kit (556547; BD Biosciences). Briefly, 100 µl
1×106/ml cells were incubated with 5 µl Annexin V-FITC
and 5 µl propidium iodide (PI) for 15 min at room temperature.
Following washing, the cells were diluted in 400 µl Annexin
V-binding buffer and immediately detected using a flow
cytometer.
Proliferation assay
Mitogenic activity was measured using a colorimetric
MTS assay (Cell-Titer 96® AQueous Non-radioactive Cell
Proliferation assay; cat. no. G1111; Promega Corporation, Madison,
WI, USA). EPCs were harvested and seeded on a 96-well plate
(1×104 cells per well) in 0.1 ml EBM-2 medium
supplemented with 0.5% bovine serum albumin (BSA; Gibco; Thermo
Fisher Scientific, Inc.) in the presence of human recombinant
vascular endothelial growth factor (100 ng/ml; cat. no. 293-VE-010;
R&D Systems). After 24 h the MTS/phenazine methosulfate
solution was added to each well for 3 h and the absorbance at 570
nm was measured using an ELISA plate reader (S5 Versa Analyzer,
Cellular Technology Ltd., Cleveland, OH, USA).
Immunofluorescence
Cells were suspended in 20 µl PBS and incubated with
10 µg/ml 1,19-dioctadecyl-3,3,3939-tetramethylindocar-bocyanine
perchlorate (Dil)-acetylated LDLs (ac-LDL) for 4 h at 37°C.
Following washing with PBS, the cells were fixed with 2%
paraformaldehyde for 10 min at room temperature and incubated with
FITC-Ulex europaeus agglutinin 1 (UEA-1; 50 µg/ml) for 1 h at 4°C.
The fluorescence signals were observed using an inverted
fluorescence microscope (magnification, ×200; Nikon Corporation,
Tokyo, Japan).
Measurement of nitric oxide (NO)
level
NO is an unstable product. Following metabolism, it
transforms to nitrate and nitrite rapidly. In addition, it is
difficult to measure NO directly. In the present study, NO
production in EPCs were measured by a colorimetric assay kit (cat.
no. A012; Nanjing Jiancheng Bioengineering Institute, Nanjing,
China) using a nitrate reductase method according to the
manufacturer's protocol. Absorbance was measured at 550 nm by a
spectrophotometer. The NO concentration was expressed as
µmol/l.
Western blot analysis
EPCs were washed and incubated in 75 µl lysis buffer
at 4°C for 40 min, as described previously (32). The nuclear and cytosolic fractions
were separated by a commercially available kit (NE-PRE Nuclear and
Cytoplasmic Extraction Reagents) according to the protocol of the
manufacturer (cat. no. 78833; Pierce Chemical Co., Dallas, TX,
USA), as described previously (33). Proteins (30–50 µg/lane) measured by
a bicinchoninc acid Protein Assay kit (Beyotime Institute of
Biotechnology, Nanjing, China) were loaded on 10% SDS-PAGE gels and
blotted on polyvinylidene difluoride (PVDF) membranes. Then, PVDF
membranes were incubated with 1% BSA at room temperature for 1 h.
Western blot analysis was then performed using antibodies against
eNOS (1:500; mouse monoclonal anti-eNOS antibody; cat. no. 612706;
BD Biosciences) at 4°C overnight. Following washing with TBST (0.1%
Tween-20) for 3 times (5 min each), the PVDF membranes were
incubated with a horseradish peroxidase-conjugated donkey
anti-mouse secondary antibody (cat. no. SA00001-8; ProteinTech
Group, Inc., Chicago, IL, USA; 1:10,000) for 1 h at room
temperature. Finally, following washing with TBST, the
autoradiographs were scanned and semi-quantitatively analyzed to
calculate the protein ratio.
Statistical analysis
SPSS 22.0 statistical software (IBM Corp., Armonk,
NY, USA) was used for data analysis. All data are presented as mean
± standard deviation. Statistical analyses were performed using
one-way analysis of variance followed by a Least Significant
Difference test. P<0.05 was considered to indicate a
statistically significant difference.
Results
LDL oxidation
The levels of thiobarbituric acid-reactive
substances were 2.13±1.59 and 24.4±8.31 nmol/mg protein in native
LDLs and oxLDLs, respectively. Compared with native LDLs, oxLDLs
indicated a 1.4±0.4 fold increase in electrophoretic mobility on
agarose gels.
Isolation and identification of
EPC
Flow cytometry was used to identify the endothelial
phenotype of the EPCs. After 8 days of culture, the expression
rates of KDR, VE-cadherin, CD34, and CD31 in the attached cells
were 68.8±7.5, 73.9±6.3, 25.4±9.1 and 77.1±7.2%, respectively
(Fig. 1). After 8 days in culture,
the attached cells took up Dil-acLDL and bound FITC-UEA-1 (Fig. 2A). Cells that were positive for
these 2 factors simultaneously were considered EPCs. They
constituted up to 90% of all attached cells. These results indicate
that EPCs were successfully isolated from PBMCs.
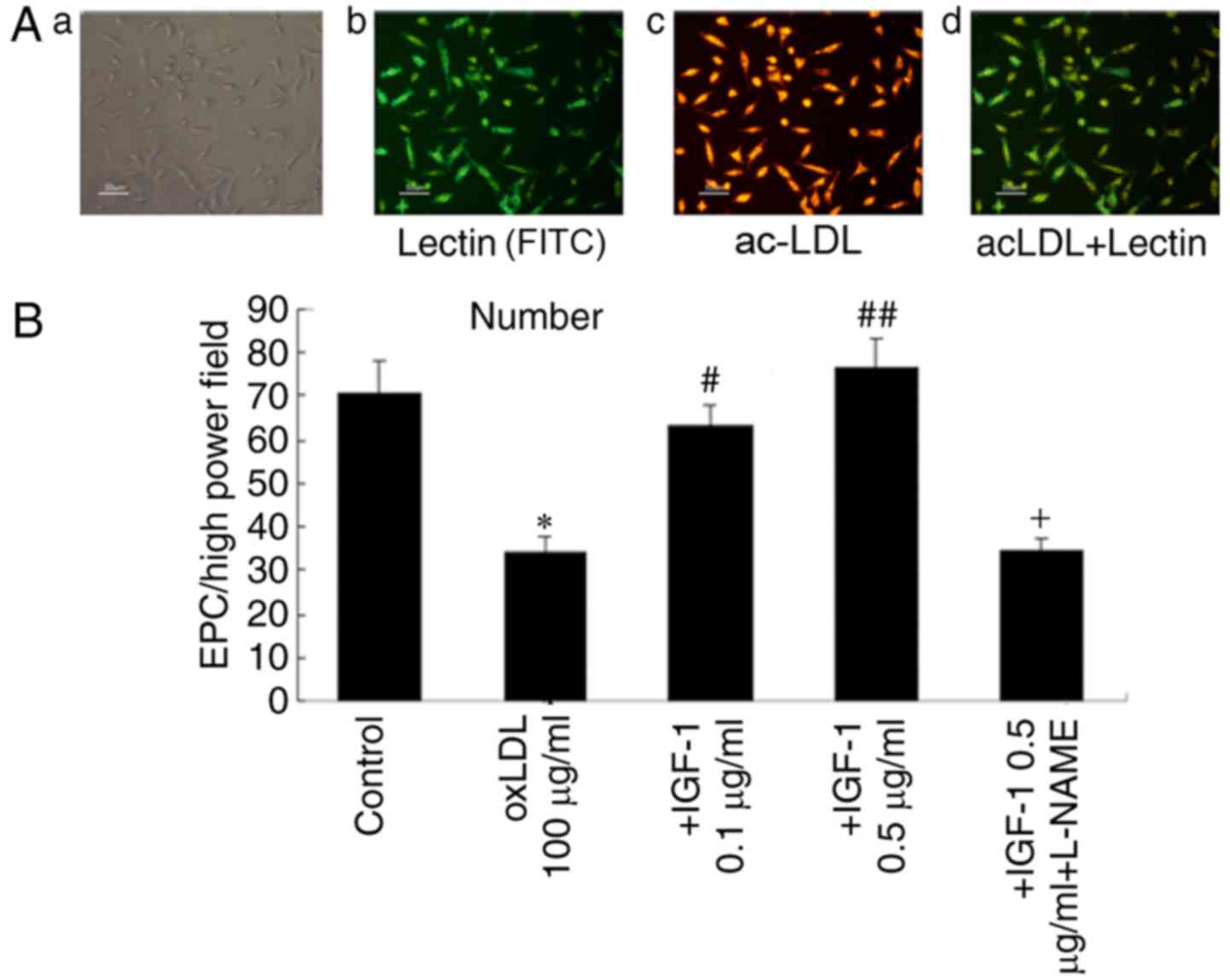 | Figure 2.Identification and the number of
EPCs. (A-a) Identification of the EPCs by immunofluorescence.
Adherent cells were observed by optical microscopy. (A-b)
FITC-lectin binding of EPCs. (A-c)
1,19-dioctadecyl-3,3,3939-tetramethylindocar-bocyanine perchlorate
(Dil)-labeled ac-LDLs uptake. (A-d) Double-positive cells were
identified as differentiating EPCs. (B) Effect of IGF-1 on the
numbers of EPCs following oxLDLs treatment. Treatment with 100
µg/ml oxLDL induced a decrease in EPC numbers. Pretreatment of EPCs
with IGF-1 inhibited the decrease induced by oxLDLs. L-NAME
inhibited these effects. Data are presented as mean ± standard
deviation. (n=6) *P<0.05 vs. control; #P<0.05 vs.
oxLDL (100 µg/ml); ##P<0.05 vs. +IGF-1 (0.1 µg/ml)
group; +P<0.05 vs. +IGF-1 (0.5 µg/ml) group. EPCs,
endothelial progenitor cells; Dil, ac-LDLs, acetylated low-density
lipoproteins; FITC, fluorescein isothiocyanate; IGF-1, insulin-like
growth factor-1; oxLDLs, oxidized low density lipoproteins; L-NAME,
nomega-nitro-L-arginine methyl ester. |
IGF-1 increases the number of EPCs
following oxLDL challenge
EPCs were characterized as adherent cells that were
doubly-positive for lectin and Di-LDL. The toxic effects of oxLDLs
were examined in EPCs; oxLDL significantly decreased the number of
EPCs. IGF-1 (0.1 or 0.5 µg/ml) significantly prevented the decrease
of EPCs caused by oxLDLs; the effect of 0.5 µg/ml IGF-1 was more
marked. When EPCs were incubated with L-NAME (100 µM), 0.5 µg/ml
IGF-1 and oxLDL for 24 h, L-NAMsE significantly decreased the
protective effect of IGF-1 against oxLDL (Fig. 2B). These results suggest that IGF-1
may protect EPCs against the toxic effects of oxLDL.
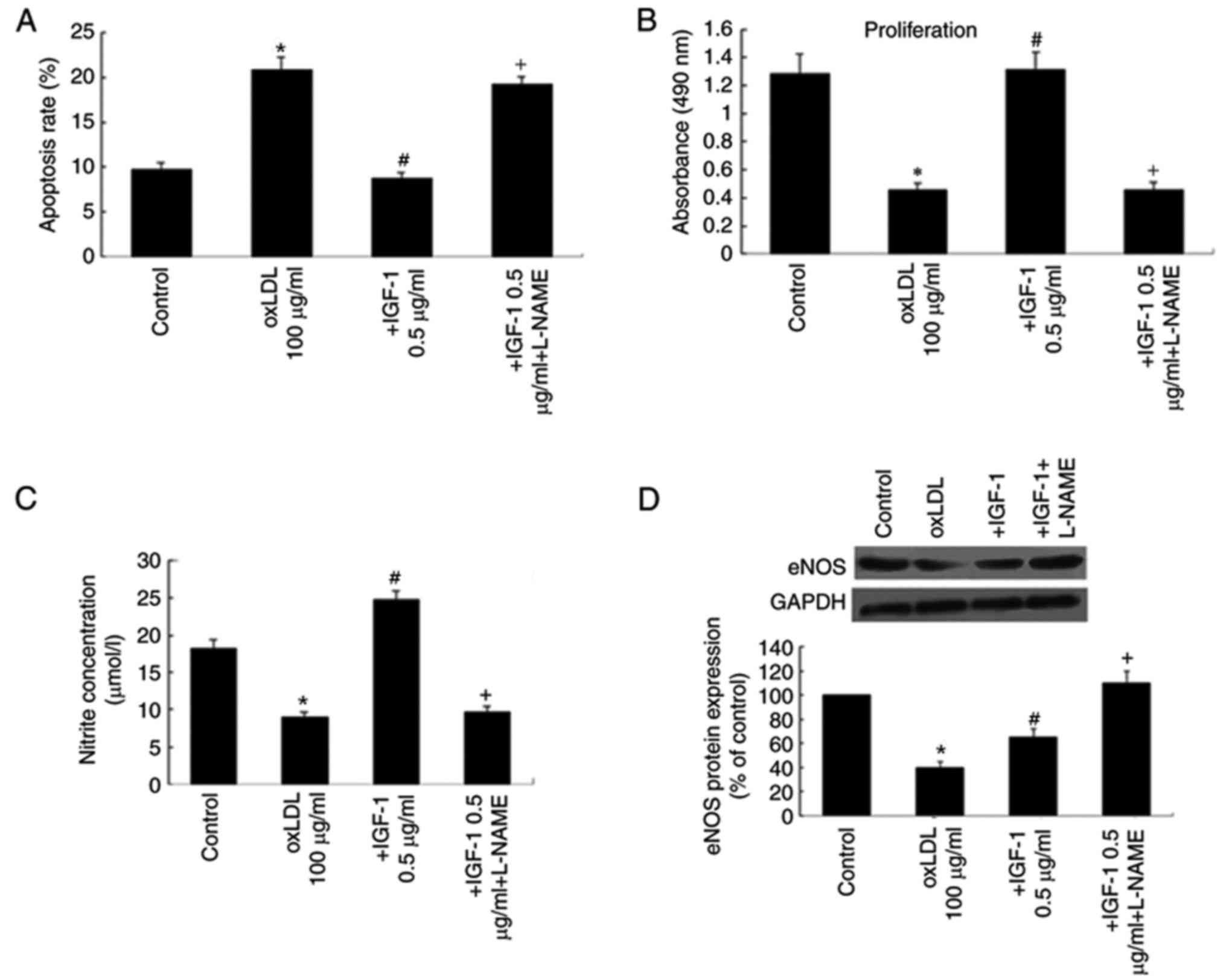
IGF-1 decreases apoptosis and
increases proliferation of EPCs following oxLDL challenge
The increase in the number of EPCs following IGF-1
treatment may be attributed to a combination of factors, including
inhibition of apoptosis and stimulation of proliferation.
Therefore, the levels of apoptosis and proliferation of EPCs were
examined following oxLDL challenge and in response to IGF-1. The
results of the MTS assay demonstrated that treatment of EPCs with
IGF-1 significantly prevented EPC apoptosis and improved EPC
proliferation. These effects were significantly attenuated by
L-NAME (Fig. 3A and B).
IGF-1 increases NO generation and
upregulates eNOS protein
As the eNOS/NO axis may serve a role in the effects
of IGF-1 on EPCs, the effects of IGF-1 on the eNOS protein were
examined, and the effects of L-NAME, an eNOS inhibitor. NO
generation was decreased by treatment with 100 mg/ml oxLDLs. This
inhibitory effect of oxLDLs was prevented by the presence of 0.5
µg/ml IGF-1. Treatment with L-NAME significantly decreased NO
generation compared with the 0.5 µg/ml IGF-1 group (Fig. 3C). To verify the hypothesis that
IGF-1 protects EPCs against oxLDL through the eNOS pathway, eNOS
protein expression was assessed by western blot analysis.
Incubation of EPCs with 100 mg/ml oxLDL significantly suppressed
eNOS protein expression. Pretreatment with IGF-1 caused a partial
restoration of the downregulation of eNOS protein expression
induced by oxLDL (Fig. 3D).
Discussion
In the present study, EPCs were cultured from
circulating PBMCs. In agreement with previous studies (34–37),
the isolated EPCs expressed a number of endothelial-specific cell
surface markers including KDR, VE-cadherin, CD34, and CD31. They
also exhibited several endothelial properties, including the uptake
of Dil-acLDL and binding of FITC-UEA-1 (38,39).
IGF-1 alleviated the decrease in number of EPCs caused by oxLDLs,
reversed the increased apoptosis and decreased proliferation rates,
and increased the NO level. The protective effect of IGF-1 on EPCs
and NO production were abolished by L-NAME, a specific inhibitor of
eNOS. IGF-1 improved the decrease of eNOS induced by oxLDLs. These
results suggest that IGF-1 protects EPCs from dysfunction induced
by oxLDLs through a mechanism involving the eNOS/NO pathway.
Wu et al (13) suggested that oxLDL regulated the
number and function of EPCs through the p38 MAPK pathway. Tie et
al (14) indicated that oxLDL
disrupted the PI3K/Akt pathway in EPCs, leading to apoptosis. Lin
et al (15) demonstrated
that the effects of oxLDLs on EPCs were dose-dependent. Several
previous studies have indicated that IGF-1 protects endothelial
cells from oxLDL: Higashi et al (30) revealed that IGF-1 alleviated
oxLDL-induced oxidative stress and decreased cell senescence in
human aortic endothelial cells, and Wu et al (40) demonstrated that IGF-1 counteracted
the detrimental effects of oxLDL on the proliferation of EPCs.
Vascular lesions associated with the development of
atherosclerosis are partly repaired by endogenous EPCs via
NO-dependent mechanisms (41–43).
NO is considered to be a significant regulator of
neovascularization. Ma et al (44) revealed that oxLDLs decrease NO
generation; as EPC survival depends on NO production,
oxLDL-mediated decrease in NO production will lead to EPC death and
decreased proliferation (44). The
present study provided novel evidence indicating that IGF-1
increases proliferation and decreases apoptosis in EPCs induced by
oxLDL, and that this effect is inhibited by L-NAME, a known
inhibitor of eNOS. In agreement with these data, Bauersachs and
Thum (40) also indicated that
IGF-1 increases the bioavailability of NO in vivo,
supporting the present study.
In addition, EPC mobilization is dependent upon
eNOS; when eNOS is uncoupled, the mobilization and function of EPCs
are impaired (45). eNOS is also
necessary for EPC mobilization from the bone marrow (41). The results from the present study
suggested that IGF-1 pretreatment dose-dependently reversed the
decrease in eNOS expression caused by oxLDLs in EPCs. This suggests
that the protective effect of IGF-1 against oxLDLs is mediated, at
least in part, through the eNOS pathway. In agreement with this
conclusion, Thum et al (23) demonstrated that treatment of EPCs
with IGF-1 induced the expression and phosphorylation (ser1177) of
eNOS (23). In cultured
endothelial cells, IGF-1 increased NO production by eNOS through
Akt-dependent pathways (46). We
hypothesize that IGF-1 activates the IGF-1 receptor in EPCs. The
IGF-1 receptor interacts with a tyrosine kinase membrane receptor
that activates the PI3K/Akt signaling pathway (47,48),
facilitating eNOS expression and activity (49) and leading to the production of NO.
Nevertheless, this hypothetic mechanism requires additional study
for confirmation.
The data from the present study suggested a novel
property of IGF-1, namely an increase in EPC numbers associated
with increased proliferation and with decreased oxLDL-induced
apoptosis. Although the proportional contributions of angiogenesis
and vasculogenesis to neovascularization of adult tissue remain to
be determined, it is well established that EPCs participate in
repair following ischemic injury (5,7,38,42,50–53).
Therefore, increasing the number of circulating EPCs has been
demonstrated to improve neovascularization of ischemic hind limbs
(39,52), accelerate blood flow in diabetic
mice (53) and improve cardiac
function (51). At present,
treatment of mice with IGF-1 has been indicated to increase the
number of EPCs (54). IGF-1
normalization improves cardiovascular outcomes in patients with
growth hormone deficiency and low IGF-1 levels (55). Therefore, augmentation of
circulating EPC numbers by IGF-1 may contribute significantly to
the stimulation of neovascularization following tissue ischemia.
This may eventually be a novel therapeutic strategy in patients
with CAD.
Data from the present study and from Thum et
al (23) demonstrated that
IGF-1 increased the expression of eNOS in circulating EPCs and
exerted a protective effect on EPCs. The differences between the
present study and the study by Thum et al were as follows:
Firstly, in the present study, the EPCs were isolated from
peripheral blood of healthy young volunteers. However, circulating
EPCs from young volunteers (27.5±0.9 years) and elderly subjects
(74.1±0.9 years) were analyzed in the study by Thum et al
(23); secondly, flow cytometry
was used to identify the endothelial phenotype of the EPCs in the
present study. After 8 days of culture, the expression rates of
KDR, VE-cadherin, CD34, and CD31 in the attached cells were
68.8±7.5, 73.9±6.3, 25.4±9.1 and 77.1±7.2%, respectively.
Conversely, Thum et al (23) classified CD133+/VEGFR+ cells as
EPCs; thirdly, the present study indicated that IGF-1
dose-dependently increased the number of ox-LDLs injured EPCs.
However, Thum et al (23)
demonstrated that treatment of EPCs from elderly individuals with
IGF-1 improved function and attenuated cellular senescence;
finally, in the present study, IGF-1 was demonstrated to decrease
apoptosis of EPCs and improve EPCs proliferation following ox-LDL
challenge, potentially via the eNOS pathway, whereas Thum et
al (23) indicated that IGF-1
increased eNOS expression, phosphorylation and activity in EPCs in
a PI3K/Akt dependent manner.
The present study is not without limitations. The
different methods of preparation of oxLDLs have been demonstrated
to potentially yield different results (56) and only one method was used in the
present study; nevertheless, the CuSO4 method has been
revealed to produce oxLDLs that mimics those identified in advanced
plaques (56). In addition, the
different effectors and factors involved in NO production and eNOS
regulation were not assessed. Additional studies are required to
address this issue; future studies will involve establishing a
hyperlipidemic rat model and treatment with IGF-1 or L-NAME. The
number of circulating EPCs, EPCs function and the eNOS/NO axis will
then be measured to support the data of the present study.
In conclusion, IGF-1 increases the number of
oxLDLs-injured EPCs, potentially via the eNOS pathway. Increases in
EPC numbers may be beneficial for endothelial regeneration and
neovascularization, and for the inhibition of the development of
atherosclerosis. The results suggest that IGF-1 and the eNOS
pathway may be a therapeutic target for improving the prognosis of
CHD.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Project of
China Hunan Health and Family Planning Commission (grant no.
B20180147) and the Foundation of China Hunan Provincial Science and
Technology Department (grant no. 2017SK50115).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YGW conceived the study and designed the
experiments. HJW, GFL and LZX performed the experiments. YGW and
HJW analyzed the data and drafted the manuscript. All authors
reviewed and approved submission of the manuscript.
Ethical approval and consent to
participate
Ethical approval was awarded by the Medical Ethics
Committee of The Second Xiangya Hospital (approval no., S042).
Informed consent was gained from all participants.
Patient consent for publication
All volunteers approved publication of the
manuscript.
Competing interests
All authors declare that they have no competing
interests.
References
|
1
|
Lawton JS: Sex and gender differences in
coronary artery disease. Semin Thorac Cardiovasc Surg. 23:126–130.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Matsuzawa Y and Lerman A: Endothelial
dysfunction and coronary artery disease: Assessment, prognosis, and
treatment. Coron Artery Dis. 25:713–724. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Du F, Zhou J, Gong R, Huang X, Pansuria M,
Virtue A, Li X, Wang H and Yang XF: Endothelial progenitor cells in
atherosclerosis. Front Biosci. 17:2327–2349. 2012. View Article : Google Scholar :
|
|
4
|
Ii M: Bone marrow-derived endothelial
progenitor cells: Isolation and characterization for myocardial
repair. Methods Mol Biol. 660:9–27. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bauer SM, Goldstein LJ, Bauer RJ, Chen H,
Putt M and Velazquez OC: The bone marrow-derived endothelial
progenitor cell response is impaired in delayed wound healing from
ischemia. J Vasc Surg. 43:134–141. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vasa M, Fichtlscherer S, Aicher A, Adler
K, Urbich C, Martin H, Zeiher AM and Dimmeler S: Number and
migratory activity of circulating endothelial progenitor cells
inversely correlate with risk factors for coronary artery disease.
Circ Res. 89:E1–E7. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Loomans CJ, de Koning EJ, Staal FJ,
Rookmaaker MB, Verseyden C, de Boer HC, Verhaar MC, Braam B,
Rabelink TJ and van Zonneveld AJ: Endothelial progenitor cell
dysfunction: A novel concept in the pathogenesis of vascular
complications of type 1 diabetes. Diabetes. 53:195–199. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tepper OM, Galiano RD, Capla JM, Kalka C,
Gagne PJ, Jacobowitz GR, Levine JP and Gurtner GC: Human
endothelial progenitor cells from type II diabetics exhibit
impaired proliferation, adhesion, and incorporation into vascular
structures. Circulation. 106:2781–2786. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shimada K, Mokuno H, Matsunaga E, Miyazaki
T, Sumiyoshi K, Miyauchi K and Daida H: Circulating oxidized
low-density lipoprotein is an independent predictor for cardiac
event in patients with coronary artery disease. Atherosclerosis.
174:343–347. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shimada K, Mokuno H, Matsunaga E, Miyazaki
T, Sumiyoshi K, Kume A, Miyauchi K and Daida H: Predictive value of
circulating oxidized LDL for cardiac events in type 2 diabetic
patients with coronary artery disease. Diabetes Care. 27:843–844.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gao S and Liu J: Association between
circulating oxidized low-density lipoprotein and atherosclerotic
cardiovascular disease. Chronic Dis Transl Med. 3:89–94. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang X, Chen J, Tao Q, Zhu J and Shang Y:
Effects of ox-LDL on number and activity of circulating endothelial
progenitor cells. Drug Chem Toxicol. 27:243–255. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu Y, Wang Q, Cheng L, Wang J and Lu G:
Effect of oxidized low-density lipoprotein on survival and function
of endothelial progenitor cell mediated by p38 signal pathway. J
Cardiovasc Pharmacol. 53:151–156. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tie G, Yan J, Yang Y, Park BD, Messina JA,
Raffai RL, Nowicki PT and Messina LM: Oxidized low-density
lipoprotein induces apoptosis in endothelial progenitor cells by
inactivating the phosphoinositide 3-kinase/Akt pathway. J Vasc Res.
47:519–530. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin FY, Tsao NW, Shih CM, Lin YW, Yeh JS,
Chen JW, Nakagami H, Morishita R, Sawamura T and Huang CY: The
biphasic effects of oxidized-low density lipoprotein on the
vasculogenic function of endothelial progenitor cells. PLoS One.
10:e01239712015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu XY, Song YH, Geng YJ, Lin QX, Shan ZX,
Lin SG and Li Y: Glucose induces apoptosis of cardiomyocytes via
microRNA-1 and IGF-1. Biochem Biophys Res Commun. 376:548–552.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vanamala J, Reddivari L, Radhakrishnan S
and Tarver C: Resveratrol suppresses IGF-1 induced human colon
cancer cell proliferation and elevates apoptosis via suppression of
IGF-1R/Wnt and activation of p53 signaling pathways. BMC Cancer.
10:2382010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yousefzadeh G, Masoomi M, Emadzadeh A,
Shahesmaeili A and Sheikhvatan M: The association of insulin-like
growth factor-1 with severity of coronary artery disease. J
Cardiovasc Med. 14:416–420. 2013. View Article : Google Scholar
|
|
19
|
Akturk IF, Yalcin AA, Biyik I, Caglar NT,
Isiksacan N, Sarikamis C, Uzun F, Celik O and Caglar IM: The role
of insulin-like growth factor-1 in development of coronary
no-reflow and severity of coronary artery disease in patients with
acute myocardial infarction. Postepy Kardiol Interwencyjnej.
10:12–17. 2014.PubMed/NCBI
|
|
20
|
Andreassen M, Raymond I, Kistorp C,
Hildebrandt P, Faber J and Kristensen LØ: IGF1 as predictor of all
cause mortality and cardiovascular disease in an elderly
population. Eur J Endocrinol. 160:25–31. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kaplan RC, Strickler HD, Rohan TE,
Muzumdar R and Brown DL: Insulin-like growth factors and coronary
heart disease. Cardiol Rev. 13:35–39. 2005.PubMed/NCBI
|
|
22
|
Urbanek K, Rota M, Cascapera S, Bearzi C,
Nascimbene A, De Angelis A, Hosoda T, Chimenti S, Baker M, Limana
F, et al: Cardiac stem cells possess growth factor-receptor systems
that after activation regenerate the infarcted myocardium,
improving ventricular function and long-term survival. Circ Res.
97:663–673. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Thum T, Hoeber S, Froese S, Klink I,
Stichtenoth DO, Galuppo P, Jakob M, Tsikas D, Anker SD,
Poole-Wilson PA, et al: Age-dependent impairment of endothelial
progenitor cells is corrected by growth-hormone-mediated increase
of insulin-like growth-factor-1. Circ Res. 100:434–443. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fleissner F and Thum T: The IGF-1 receptor
as a therapeutic target to improve endothelial progenitor cell
function. Mol Med. 14:235–237. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Senokuchi T, Matsumura T, Sakai M, Matsuo
T, Yano M, Kiritoshi S, Sonoda K, Kukidome D, Nishikawa T and Araki
E: Extracellular signal-regulated kinase and p38 mitogen-activated
protein kinase mediate macrophage proliferation induced by oxidized
low-density lipoprotein. Atherosclerosis. 176:233–245. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kannan Y, Sundaram K, Aluganti Narasimhulu
C, Parthasarathy S and Wewers MD: Oxidatively modified low density
lipoprotein (LDL) inhibits TLR2 and TLR4 cytokine responses in
human monocytes but not in macrophages. J Biol Chem.
287:23479–23488. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tie G, Yan J, Messina JA, Raffai RL and
Messina LM: Inhibition of p38 mitogen-activated protein kinase
enhances the apoptosis induced by oxidized low-density lipoprotein
in endothelial progenitor cells. J Vasc Res. 52:361–371. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Imanishi T, Hano T, Matsuo Y and Nishio I:
Oxidized low-density lipoprotein inhibits vascular endothelial
growth factor-induced endothelial progenitor cell differentiation.
Clin Exp Pharmacol Physiol. 30:665–670. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Imanishi T, Hano T and Nishio I:
Angiotensin II potentiates vascular endothelial growth
factor-induced proliferation and network formation of endothelial
progenitor cells. Hypertens Res. 27:101–108. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Higashi Y, Pandey A, Goodwin B and
Delafontaine P: Insulin-like growth factor-1 regulates glutathione
peroxidase expression and activity in vascular endothelial cells:
Implications for atheroprotective actions of insulin-like growth
factor-1. Biochim Biophys Acta. 1832:391–399. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ji S, Ma Q, Luo X and Peng J: Protective
effect of insulin-like growth factor-1 on vascular endothelial
function in hypercholesterolemia and the underlying mechanism.
Zhong Nan Da Xue Xue Bao Yi Xue Ban. 38:36–42. 2013.(In Chinese).
PubMed/NCBI
|
|
32
|
Dimmeler S, Aicher A, Vasa M, Mildner-Rihm
C, Adler K, Tiemann M, Rütten H, Fichtlscherer S, Martin H and
Zeiher AM: HMG-CoA reductase inhibitors (statins) increase
endothelial progenitor cells via the PI 3-kinase/Akt pathway. J
Clin Invest. 108:391–397. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Haendeler J, Hoffmann J, Diehl JF, Vasa M,
Spyridopoulos I, Zeiher AM and Dimmeler S: Antioxidants inhibit
nuclear export of telomerase reverse transcriptase and delay
replicative senescence of endothelial cells. Circ Res. 94:768–775.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Peng J, Liu B, Ma QL and Luo XJ:
Dysfunctional endothelial progenitor cells in cardiovascular
diseases: Role of NADPH oxidase. J Cardiovasc Pharmacol. 65:80–87.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Luo S, Xia W, Chen C, Robinson EA and Tao
J: Endothelial progenitor cells and hypertension: Current concepts
and future implications. Clin Sci. 130:2029–2042. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bianconi V, Sahebkar A, Kovanen P,
Bagaglia F, Ricciuti B, Calabro P, Patti G and Pirro M: Endothelial
and cardiac progenitor cells for cardiovascular repair: A
controversial paradigm in cell therapy. Pharmacol Ther.
181:156–168. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wils J, Favre J and Bellien J: Modulating
putative endothelial progenitor cells for the treatment of
endothelial dysfunction and cardiovascular complications in
diabetes. Pharmacol Ther. 170:98–115. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Asahara T, Murohara T, Sullivan A, Silver
M, van der Zee R, Li T, Witzenbichler B, Schatteman G and Isner JM:
Isolation of putative progenitor endothelial cells for
angiogenesis. Science. 275:964–967. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kalka C, Masuda H, Takahashi T, Kalka-Moll
WM, Silver M, Kearney M, Li T, Isner JM and Asahara T:
Transplantation of ex vivo expanded endothelial progenitor cells
for therapeutic neovascularization. Proc Natl Acad Sci USA.
97:3422–3427. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wu Y, Wang Q, Cheng L, Wang J, Sun X and
Lu S: IGF-1 reduces the apoptosis of endothelial progenitor cells
induced by oxidized low-density lipoprotein by the suppressing
caspase-3 activity. Cell Res. 18:S1592008. View Article : Google Scholar
|
|
41
|
Aicher A, Heeschen C, Mildner-Rihm C,
Urbich C, Ihling C, Technau-Ihling K, Zeiher AM and Dimmeler S:
Essential role of endothelial nitric oxide synthase for
mobilization of stem and progenitor cells. Nat Med. 9:1370–1376.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Friedrich EB, Walenta K, Scharlau J,
Nickenig G and Werner N: CD34/CD133+/VEGFR-2+
endothelial progenitor cell subpopulation with potent
vasoregenerative capacities. Circ Res. 98:e20–e25. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bauersachs J and Thum T: Endothelial
progenitor cell dysfunction: Mechanisms and therapeutic approaches.
Eur J Clin Invest. 37:603–606. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ma FX, Zhou B, Chen Z, Ren Q, Lu SH,
Sawamura T and Han ZC: Oxidized low density lipoprotein impairs
endothelial progenitor cells by regulation of endothelial nitric
oxide synthase. J Lipid Res. 47:1227–1237. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Thum T, Fraccarollo D, Schultheiss M,
Froese S, Galuppo P, Widder JD, Tsikas D, Ertl G and Bauersachs J:
Endothelial nitric oxide synthase uncoupling impairs endothelial
progenitor cell mobilization and function in diabetes. Diabetes.
56:666–674. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Michell BJ, Griffiths JE, Mitchelhill KI,
Rodriguez-Crespo I, Tiganis T, Bozinovski S, de Montellano PR, Kemp
BE and Pearson RB: The Akt kinase signals directly to endothelial
nitric oxide synthase. Curr Biol. 9:845–848. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Withers DJ, Burks DJ, Towery HH, Altamuro
SL, Flint CL and White MF: Irs-2 coordinates Igf-1
receptor-mediated beta-cell development and peripheral insulin
signalling. Nat Genet. 23:32–40. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
48
|
Isenovic ER, Meng Y, Divald A, Milivojevic
N and Sowers JR: Role of phosphatidylinositol 3-kinase/Akt pathway
in angiotensin II and insulin-like growth factor-1 modulation of
nitric oxide synthase in vascular smooth muscle cells. Endocrine.
19:287–292. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Dimmeler S, Fleming I, Fisslthaler B,
Hermann C, Busse R and Zeiher AM: Activation of nitric oxide
synthase in endothelial cells by Akt-dependent phosphorylation.
Nature. 399:601–605. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
50
|
Asahara T, Masuda H, Takahashi T, Kalka C,
Pastore C, Silver M, Kearne M, Magner M and Isner JM: Bone marrow
origin of endothelial progenitor cells responsible for postnatal
vasculogenesis in physiological and pathological
neovascularization. Circ Res. 85:221–228. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kocher AA, Schuster MD, Szabolcs MJ,
Takuma S, Burkhoff D, Wang J, Homma S, Edwards NM and Itescu S:
Neovascularization of ischemic myocardium by human
bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis,
reduces remodeling and improves cardiac function. Nat Med.
7:430–436. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
52
|
Murohara T, Ikeda H, Duan J, Shintani S,
Sasaki K, Eguchi H, Onitsuka I, Matsui K and Imaizumi T:
Transplanted cord blood-derived endothelial precursor cells augment
postnatal neovascularization. J Clin Invest. 105:1527–1536. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Schatteman GC, Hanlon HD, Jiao C, Dodds SG
and Christy BA: Blood-derived angioblasts accelerate blood-flow
restoration in diabetic mice. J Clin Invest. 106:571–578. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hou J, Peng X, Wang J, Zhang H, Xia J, Ge
Q, Wang X, Chen X and Wu X: Mesenchymal stem cells promote
endothelial progenitor cell proliferation by secreting insulinlike
growth factor1. Mol Med Rep. 16:1502–1508. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Thum T, Fleissner F, Klink I, Tsikas D,
Jakob M, Bauersachs J and Stichtenoth DO: Growth hormone treatment
improves markers of systemic nitric oxide bioavailability via
insulin-like growth factor-I. J Clin Endocrinol Metab.
92:4172–4179. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Higashi Y, Sukhanov S, Anwar A, Shai SY
and Delafontaine P: IGF-1, oxidative stress and atheroprotection.
Trends Endocrinol Metab. 21:245–254. 2010. View Article : Google Scholar : PubMed/NCBI
|