Introduction
Hematopoietic stem cell transplants (HSCT) is an
effective therapy in the treatment of hematological malignancies
such as leukemias and lymphomas (1). The regimens required for transplant
produce profound immune deficiency in the early period after
transplantation (2). Conditioning
regimens include: Myeloablative (MAC) and reduced intensity
conditioning (RIC). MACs include concomitant or single use of
alkylating agents, such as cyclophosphamide (Cy) and busulfan (Bu),
while RICs are mainly performed with fludarabine (Flu) or low doses
of total body irradiation (TBI) (3,4).
Choice of conditioning depends on patient's age, underlying
disease, relevant comorbidities and type of donor. These regimens
are related to several risks such as infections, graft-vs.-host
disease (GVHD) and post-transplant lymphoproliferative disorder
(PTLD) (5). GVHD occurs in
approximately 40–90% of transplanted patients (6). ATG seems to be effective in GVHD
prophylaxis, and is related to reduced rates of relapses and
infections in adults who undergo bone marrow (BM) or peripheral
blood stem cells (PBSC) transplant (7).
Rates of mortality after HSCT often reach up to
50.0% (5). Some studies describe
that intensive conditioning regimens are associated with reduction
of tumor relapses, although it might simultaneously increase the
transplant-related mortality rates, including the mortality of
infections (4).
Epstein-Barr virus (EBV) is a ubiquitous Human
Herpes virus and infects 50–89% of children and remains latent, in
memory B cells, of ~90% of adults (8). Viral infections are known to be a
major cause of morbidity and mortality in patients undergoing HSCT
and Herpes virus are known to be among the most common viral
infections in these patients (5).
The iatrogenic suppression of T-cell with the immunosuppression of
the transplant regimens, allows the proliferation of infected B
cells (9). EBV is one of the most
important viruses in transplanted patients, and monitoring of EBV
DNA in peripheral blood is routinely performed in several
transplant centers, since these patients have a higher-risk of
developing complications (9–13).
This study aims to analyze the impact of the
different characteristics of allogeneic-HSCT (aHSCT) patients and
correlate with the development of EBV infection.
Materials and methods
Type of study and study
participant
A prospective follow-up study was performed with 40
consecutive patients who underwent aHSCT at the Bone Marrow
Transplant Service of Portuguese Oncology Institute of
Porto (IPO Porto; Porto, Portugal) between January and December
of 2015. Cases were selected randomly from the cohort of patients
undergoing aHSCT. The study was approved by the local Ethical
Committee and did not interfere with the routine procedures decided
by clinicians. Clinical data was collected from individual clinical
records and stored in a database with unique codification.
Sample processing
EBV detection is not routinely requested for all
HSCT patients in our institution (only for high-risk), and
therefore we have used samples collected during routine procedures
for patients monitoring selected at 6 different times: D+30, +60,
+90, +120, +150 and +180 days after transplant. Samples were
collected in EDTA-containing tubes and stored prior to
processing.
Sample processing was performed at the Virology
Service of IPO Porto. DNA was extracted by MagNA Pure Compact
Nucleic Acid Isolation kit I (Roche, Germany). DNA/RNA quality
was assessed by measuring the absorbance at 260/280 nm with
NanoDrop 1000 Spectrophotometer v3.7 (Thermo Fischer Scientific,
Inc., Wilmington, MA, USA).
EBV detection
EBV detection was performed with a real-time PCR
protocol targeting EBV polymerase gene (EBV POL) as previously
reported (14). Amplification was
performed with the ABI PRISM 7300 Sequencer Detection System
(Applied Biosystems, Foster City, CA, USA) and results were
obtained by measuring the geometric increase of probe fluorescence
during amplification and samples were considered positive when the
exponential curve exceeded the cycle threshold line. All
amplifications used positive and negative controls: As negative
control, we used double distilled water in replacement of template
DNA; and as positive control we have used samples from the External
Quality Control panel for EBV used at the Virology Service. Results
were independently analyzed by two of the authors and 10% of all
samples were randomly selected and re-submitted to amplification to
confirm the results.
Statistical analysis
Statistical analysis was performed with
IBM® SPSS Statistics 20 software (IBM Corp., Armonk, NY,
USA) for Mac. Chi-square or Fisher's exact test with a 5%
significance level were used to estimate odds ratio (OR) and the
corresponding 95% confidence intervals (CIs) as a measure of
association between the categorical variables and the risk of EBV
infection. Cox proportional hazard models were used to assess the
risk factors associated EBV infection. Kaplan-Meier with log-Rank
test was used to calculate the association between EBV infection
and post-transplant survival (OS).
Results
Clinical characteristics
This study included 40 patients, 27 males (67.5%)
and 13 females (32.5%), with ages between 1 and 63 years-old (mean
32.2±1.5, median 35 years old) who underwent aHSCT at our
institution between January and December of 2015.
Table I
demonstrates the characteristics of all patients. Briefly, patients
were submitted to aHSCT due to different hematological
malignancies, including aplastic anemia (n=3), acute leukemia
(n=23), chronic leukemia (n=2), non-Hodgkin lymphoma (n=1),
multiple myeloma (n=1), myelodysplastic/myeloproliferative syndrome
(n=7) and others, including primary immunodeficiency, myelofibrosis
and severe combined immunodeficiency (n=3). Off the 40 patients
submitted to aHSCT only one was being transplanted for the second
time. When evaluating the donor-receptor relation, 21 patients had
unrelated donors (52.5%) and the remaining 19 had related donors
(47.5%). Regarding the HLA-match, only one patient received a graft
from a HLA-mismatched donor. The source of cells for transplant was
mainly from peripheral blood (82.0%), while the remaining were from
BM (13.0%) and umbilical cord blood (5.0%). MAC conditioning was
used in 24 (60.0%) of our patients, with Bu and cyclophosphamide,
as well as ATG which was used in 14 of these patients. Reduced
intensity regimens were used in 16 patients with 6 of them
receiving ATG. Patients with unrelated donors, except in one case,
received ATG as part of GVHD prophylaxis. Prophylaxis for GVHD was
performed for all patients (data not available for 2 patients).
Acute GVHD (aGVHD) was observed in 21 patients, all of them with
grade 2 or higher; while chronic GVHD (cGVHD) was present in 5
patients, 4 with evolution from aGVHD and only one with de
novo cGVHD.
 | Table I.Clinical characteristics of
patients. |
Table I.
Clinical characteristics of
patients.
|
Characteristics | Number (%) |
|---|
| Age, median (range)
years old | 32.2 (1–63) |
| Sex, n (%) |
|
|
Male | 27 (67.5) |
|
Female | 13 (32.5) |
| Underlying disease,
n (%) |
|
|
Aplastic anemia | 3 (7.5) |
| Acute
leucemia | 23 (57.5) |
| Chronic
leucemia | 2 (5.0) |
|
Non-Hodgkin lymphoma | 1 (2.5) |
|
Multiple myeloma | 1 (2.5) |
|
Myelodysplastic/myeloproliferative
syndrome | 7 (17.5) |
|
Others | 3 (7.5) |
| Conditioning
regimen, n (%) |
|
|
BuCy | 4 (10.0) |
|
BuCy2 | 20 (50.0) |
| Cy | 1 (2.5) |
|
FluBu | 10 (25.0) |
|
FluCy | 3 (7.5) |
|
FluMelf | 1 (2.5) |
| ATG, n (%) |
|
|
Yes | 20 (50.0) |
| No | 20 (50.0) |
| Type of donor, n
(%) |
|
|
Related | 19 (47.5) |
|
Mismatched/unrelated | 21 (52.5) |
| Source of cells, n
(%) |
|
|
PBSC | 33 (82.5) |
| BM | 5 (12.5) |
|
UCB | 2 (5.0) |
EBV and CMV serological status was described as: IgM
positive and IgG negative/positive (active infection); IgM negative
and IgG positive (past-infection); and IgM negative and IgG
negative (susceptible). In our study, we found 3 patients were
susceptible for EBV-primary infection, 1 patient had an active
EBV-infection and 7 patients were susceptible of CMV-primary
infection (data not shown).
EBV infection
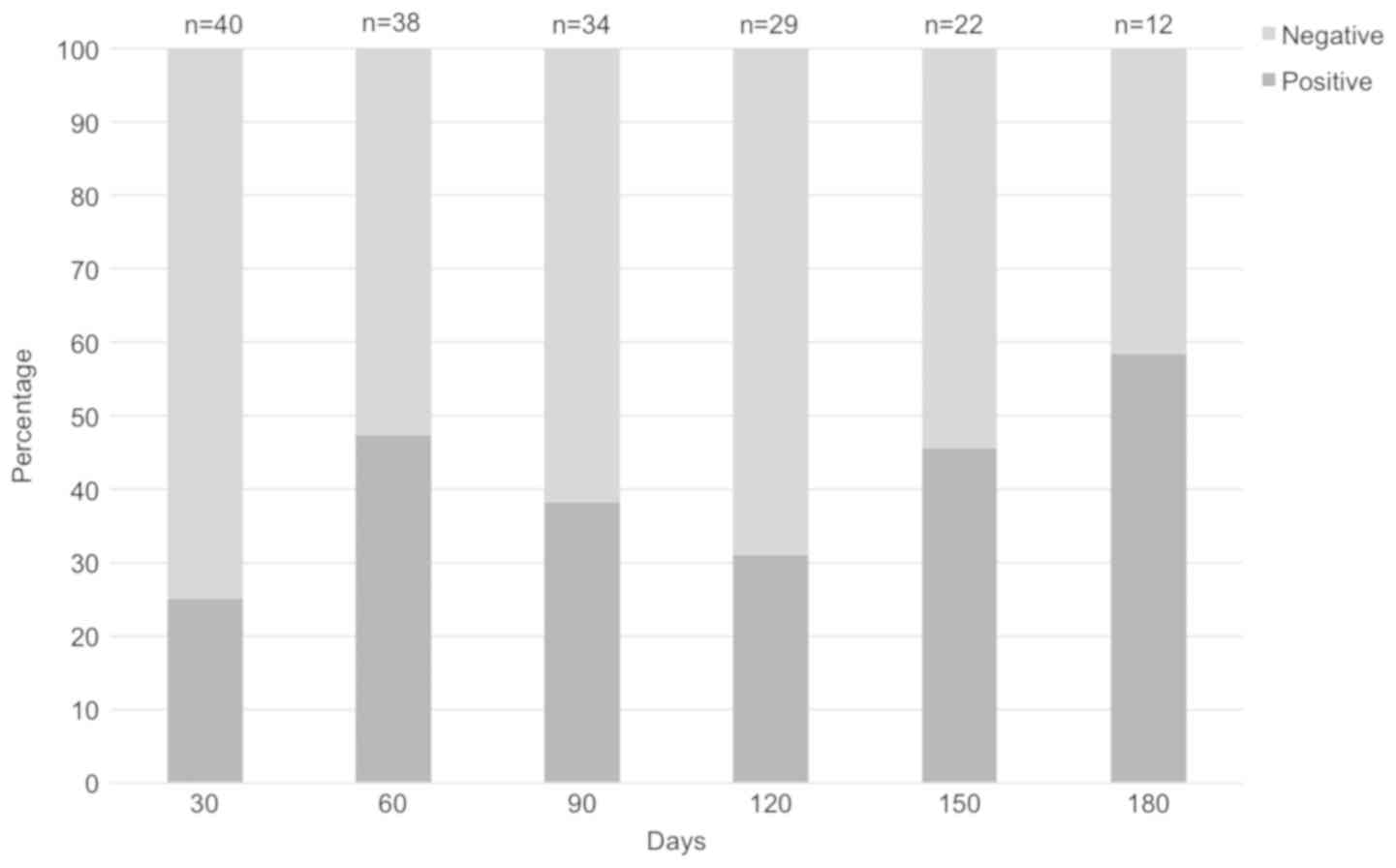
The overall data reveal that 70% of patients were at
least once positive during the follow-up period. Regarding the
specific times of sample collection in our study, results showed
the presence of EBV in 10/40 (25%) at D+30, 18/38 (47%) at D+60,
13/34 (38%) at D+90, 9/29 (31%) at D+120, 10/22 (45%) at D+150, and
7/12 (58%) at D+180 (Fig. 1).
The analysis of EBV DNA positivity (at least once
positive) and its association with clinicopathological
characteristics of patients is shown in Table II. The analysis according to sex
seem to show that post-transplant EBV infection is more frequent in
females (OR=8.33, P=0.033). Despite not statistically significant,
we have found that EBV infection is more frequent in patients with
unrelated donors (OR=3.12, P=0.112), engrafted with PBSC (OR=2.00,
P=0.414), submitted to MAC conditioning regimen (OR=2.96, P=0.121),
using ATG in the conditioning regimen (OR=2.91, P=0.135), and that
developed aGVHD (OR=3.09, P=0.112). Despite we observe an increased
prevalence of EBV in older patients, there was no statistically
significant association regarding age (child vs. adults, or median
age) (P>0.050). EBV serostatus prior to transplant does not seem
to be related with the development of infection during the
post-transplant period (data not shown).
 | Table II.Analysis of EBV infection among
allogeneic hematological stem cell transplant recipients. |
Table II.
Analysis of EBV infection among
allogeneic hematological stem cell transplant recipients.
| Variable | EBV infection n
(%) | P-value | OR (95% CI) |
|---|
| Sex |
|
|
|
| Male
(n=27) | 16 (59.3) | 0.033 | 8.33
(0.93–100) |
| Female
(n=13) | 12 (92.3) |
|
|
| Stem cell
source |
|
|
|
| Cord
blood or bone marrow (n=7) | 4 (57.1) | 0.414 | 2.00
(0.37–11.1) |
|
Peripheral blood (n=33) | 24 (72.7) |
|
|
| Conditioning
regimen |
|
|
|
| Reduced
intensity (n=16) | 9 (56.3) | 0.121 | 2.96
(0.73–11.9) |
|
Myeloablative (n=24) | 19 (79.2) |
|
|
| ATG |
|
|
|
| With
(n=19) | 11 (57.9) | 0.135 | 2.91
(0.70–12.1) |
| Without
(n=20) | 16 (80.0) |
|
|
| Donor |
|
|
|
| Related
(n=19) | 11 (57.9) | 0.112 | 3.12
(0.75–12.5) |
|
Unrelated (n=21) | 17 (81.0) |
|
|
| Acute GVHD |
|
|
|
| Absent
(n=19) | 11 (57.9) | 0.170 | 3.09
(0.75–12.8) |
| Present
(n=21) | 17 (81.0) |
|
|
| Age |
|
|
|
| <20
years old (n=14) | 8 (57.1) | 0.173 | 2.50
(0.62–10.1) |
| ≥20
years old (n=26) | 20 (76.9) |
|
|
| <35
years old (n=20) | 13 (65.0) | 0.366 | 1.61
(0.41–6.34) |
| ≥35
years old (n=20) | 15 (75.0) |
|
|
The analysis of EBV infection at the different times
during the follow-up period revealed different associations with
clinicopathological data, despite not all with statistical
significance. At D+60, EBV infection was associated with
transplants from unrelated donors (OR=3.9, P=0.058), with MAC
conditioning (OR=4.3, P=0.052) and use of ATG (OR=3.6, P=0.099),
although, with no statistical significance; at D+90, development of
GVHD was related a higher risk of infection (OR=6.7, P=0.032); and
finally, at D+150, EBV infection was associated with unrelated
donors (OR=8.0, P=0.043), use of ATG (OR=12.0, P=0.030) and
development of GVHD (OR=5.6, P=0.099). There was no significant
association between age and EBV infection in different times (data
not shown). The Cox regression analysis considering patients sex,
type of donor, conditioning regimen, use of ATG and development of
aGVHD, revealed an association of unrelated donor with EBV
infection at D+150 (HR=8.8, P=0.030).
Follow-up
None of these patients had clinical suspicion or
development PTLD during follow-up. Of the 40 patients included in
this study, 16 patients have deceased, 6 are alive with evidence of
disease and 18 are alive without evidence of disease. Cumulative
survival was evaluated by performing a Kaplan-Meier plot and
estimated survival time was approximately 476±58.7 days (data not
shown).
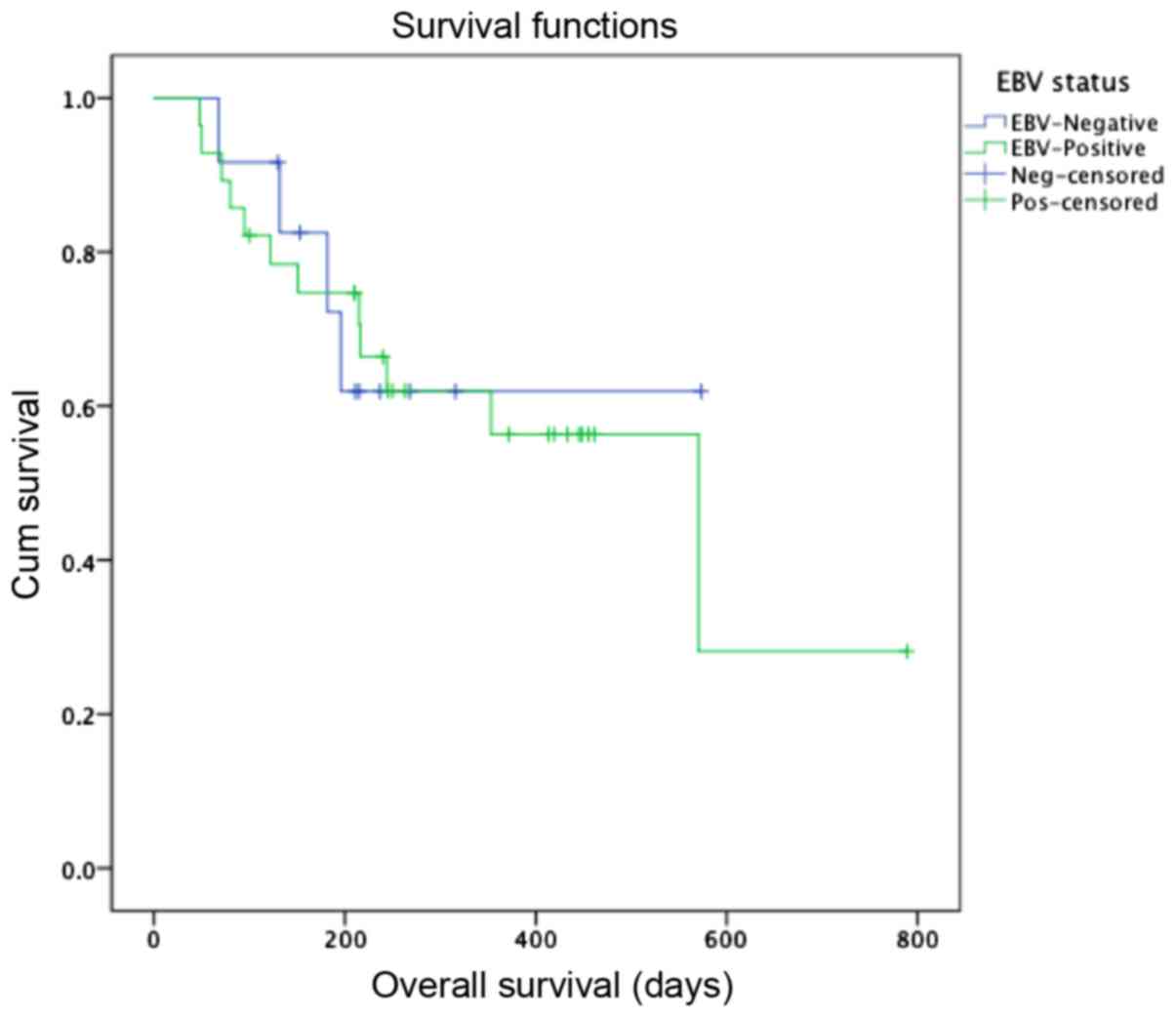
Analysis of EBV-infection impact on overall survival
(OS) is shown in Fig. 2. The
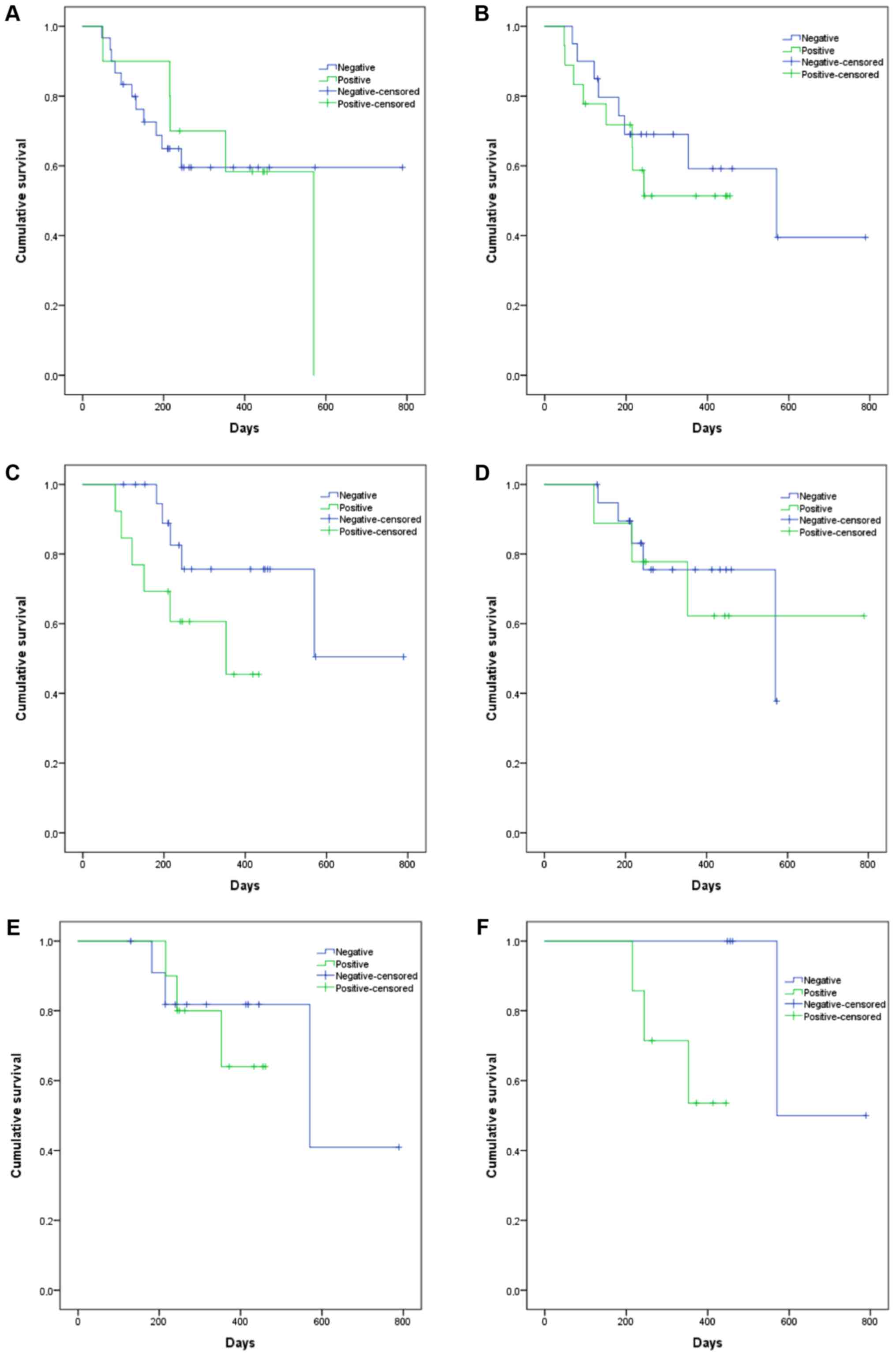
impact of EBV infectionon OS, in different times, is shown in
Fig. 3. Results suggest that EBV
positivity at D+90 days and D+180 may be associated with increased
mortality (P=0.095, 303.3 vs. 593.2 days and P=0.097, 367.0 vs.
679.5, respectively).
Discussion
aHSCT is an option for the treatment of
hematological malignancies and these patients are submitted to
pre-transplant treatments that reduce significantly the immune
system to avoid rejection of the graft (15). This immunosuppression is associated
with the occurrence of different events in the post-transplant
period, such as development of GVHD, infections and PTLDs (16). Viral infections are a major concern
in the subset of aHSCT, and while CMV infection has been
consistently associated with a significant morbidity/mortality
increase (17–19), EBV infection has been
underestimated in these patients since only a minority will suffer
from EBV-associated complications, such as PTLD (11,13,20).
The incidence of EBV DNAemia varies within
transplant centers, ranging from 0.1 to 63% (11,21,22).
In our study, we verified that 70.0% of our patients were positive
for EBV, at least once during the follow-up period, nevertheless
the prevalence of EBV-positive patients at a specific time ranged
from 25–58%. Dumas et al, monitored EBV viral load at least
once a week for 3 months and verified that EBV DNAemia occurred in
approximately 14.0% of patients, which is significant lower than
what we describe (23). Hence,
these results show that there are significant differences amongst
transplant centers that may be explored considering the individual
characteristics of patients.
Literature refers that EBV infection is most common
within the first 100 days post-transplant, in high-risk patients
(24,25). In our study, we describe that EBV
infection varied throughout the follow-up period, with a mean of
65.6±39.6 days (range, 27–183). According to the guidelines for
diagnosis and monitoring of EBV DNAemia, the monitoring should be
performed by quantitative PCR and monitorization must start at the
first month post-transplant, and should be performed at least
during 4 months after transplantation, with a weekly frequency
(11,12). Despite this, many authors discuss
the cost-effectiveness of EBV monitoring once-a-week and therefore
many studies are required to show what would be the best time and
interval of monitoring. Moreover, EBV viral load has been having
different input data since the cutoff viral loads for treatment are
variable and the development of international standardization of
EBV viral load management is yet to be defined (12,13,26).
Gulley and Tang, affirm that routine monitoring of EBV infection is
viable in PTLD prevention, although further studies must be done to
correlate specific viral loads in the identification of high-risk
patients (27). As defined by
published guidelines, rituximab therapy is used prophylactically
before or shortly after transplant to reduce the risk of EBV
DNAemia and PTLD development in high-risk patients, such as
patients with EBV-seropositive donors (11). In these cases, viral load is also
informative, when there is a withdrawal of immunosuppression, and
rituximab administration, to verify if the treatment is being
successful (27).
The identification of high-risk patients for EBV
infection is crucial for the correct clinical approach of
EBV-associated morbidity/mortality in aHSCT patients. Accessing
variables associated with transplant, and complications associated
with treatment, such as PTLD, include patients into two groups:
High-risk and low-risk. High risk patients have pre-transplant risk
factor such as T-cell depletion, EBV serology donor/recipient
mismatched, cord blood transplantation, HLA mismatch, Splenectomy
and second HSCT; and post-transplant risk factors as severe acute
or cGVHD and high or rising EBV DNAemia (11,13,25,28).
Our data showed that patients with unrelated and/or
mismatched donors were more prone to develop an EBV infection
post-transplant, and that using peripheral blood as source of stem
cells adds a higher risk for EBV infection. Studies report EBV
infections of 8.8% in MAC conditionings and 35.0% in RICs (29), 54.0% in T-cell depletion (30) and 65.0% in T-cell depletion
concomitant with ATG use (22).
Xuan et al showed that the use of ATG, HLA-mismatched,
unrelated donor and acute aGVHD were identified as risk factors for
EBV infection (4). These findings
are according those reporting that intensified conditioning
increase the incidence of EBV viremia and disease (4). In our study, myeloablation
conditioning and use of ATG demonstrated a 3-times higher risk of
developing EBV infection. ATG has been widely used to decrease the
incidence of GVHD (31), however,
because of T-cell depletion, ATG is also associated with relapse
(32). The optimal dose of ATG
depends on transplant characteristics, such as type of donor, and
may range from 2.5 to 30 mg/kg (33). ATG has beneficial effects in
preventing GVHD, although it delays immune reconstitution,
promoting an increased risk of EBV reactivation, and potentially
EBV-associated lymphoproliferative disease (34). Literature shows that higher doses
of ATG seem to be related with PTLD development (35). Indeed, at our institution, no more
than 10 mg/kg is used, and this may have contributed for the fact
that no PTLD was observed in this cohort. When addressing the
development of GVHD, we observed that patients who developed aGVHD,
had 3-times increased association with EBV infection. Furthermore,
we noticed that all patients with cGVHD, were positive for EBV at
least once in the post-transplant follow-up. As described
previously by Janeczko et al, GVHD is related to delayed
immune reconstitution, favoring infections in the early period
post-transplant. Moreover, viral infections are also associated
with delayed immune response and appear to be linked to the degree
of immunosuppression (36).
Review of EBV infection on different periods
post-transplant revealed that unrelated donor, myeloablation and
the use of ATG seem to be risk factors for EBV infection occurrence
at D+60; while GVHD is connected to EBV infection at D+90; and ATG,
unrelated donor and GVHD are related to EBV infection at day D+150.
These results seem to corroborate the literature regarding the
identification of high-risk markers for EBV infection. Furthermore,
our study reports that EBV positivity at D+90 days and D+180 might
be associated with increased mortality (P=0.095, 303.3 vs. 593.2
days and P=0.097, 367.0 vs. 679.5, respectively). We acknowledge
that EBV infection is not, by itself, responsible for higher
mortality rates, therefore further studies should be performed with
more patients.
EBV infection is still a major concern in the subset
of HSCT and these results showed that EBV routine monitoring is
useful in high-risk patients during the first months after
transplantation. In a previous study, the authors accessed the
importance of EBV monitoring in patients submitted to aHSCT,
regarding the development of PTLD (13). Although no patients of this study
developed PTLD, it is still important performing EBV monitoring
when several risk factors, are present. The implementation of
guidelines and standardization of EBV monitorization in HSCT
patients will contribute for the cost-effectiveness of this
monitorization reducing unnecessary morbidity/mortality associated
with EBV-infection.
Acknowledgements
The authors would like to acknowledge the support of
Mrs. Rute Silva from the Bone Marrow Transplant Service at IPO
Porto, who assisted the collection of clinic-pathological data for
the present study.
Funding
Joana Marinho-Dias received a grant for the
development of PhD from the Portuguese League Against Cancer (Liga
Portuguesa Contra o Cancro-Núcleo Regional do Norte) between April
and September 2016.
Availability of data and materials
The data obtained from the present study are part of
the institution clinical records of patients and are not publicly
available due to confidentiality but are available from the
corresponding author on reasonable request.
Authors' contributions
JM-D, RM and HS designed the study. CP-V, LL, RB, FC
and ACT collected and analyzed the clinical information of
patients. IB and RM collaborated in viral detection and
interpretation of results. JM-D and HS performed analysis of data,
the draft of the manuscript and its final version. All authors were
given the opportunity to revise the manuscript.
Ethics approval and consent to
participate
The present study was approved by The Ethics
Committee of Portuguese Oncology Institute of Porto and patients
provided informed consent for enrollment in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Howlader N, Noone AM, Krapcho M, Miller D,
Bishop K, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, et al:
SEER Cancer Statistics Review (CSR), 1975–2014. Nat Cancer Inst.
(Bethesda, MD). 2017.
|
|
2
|
Curtis RE, Travis LB, Rowlings PA, Socié
G, Kingma DW, Banks PM, Jaffe ES, Sale GE, Horowitz MM, Witherspoon
RP, et al: Risk of lymphoproliferative disorders after bone marrow
transplantation: A multi-institutional study. Blood. 94:2208–2216.
1999.PubMed/NCBI
|
|
3
|
Juric MK, Ghimire S, Ogonek J, Weissinger
EM, Holler E, van Rood JJ, Oudshoorn M, Dickinson A and Greinix HT:
Milestones of hematopoietic stem cell transplantation-from first
human studies to current developments. Front Immunol. 7:4702016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xuan L, Huang F, Fan Z, Zhou H, Zhang X,
Yu G, Zhang Y, Liu C, Sun J and Liu Q: Effects of intensified
conditioning on Epstein-Barr virus and cytomegalovirus infections
in allogeneic hematopoietic stem cell transplantation for
hematological malignancies. J Hematol Oncol. 5:462012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fan J, Jing M, Yang M, Xu L, Liang H,
Huang Y, Yang R, Gui G, Wang H, Gong S, et al: Herpesvirus
infections in hematopoietic stem cell transplant recipients
seropositive for human cytomegalovirus before transplantation. Int
J Infect Dis. 46:89–93. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Funke VA, Moreira MC and Vigorito AC:
Acute and chronic Graft-versus-host disease after hematopoietic
stem cell transplantation. Rev Assoc Med Bras (1992). 62 Suppl
1:S44–S50. 2016. View Article : Google Scholar
|
|
7
|
Storek J, Mohty M and Boelens JJ: Rabbit
anti-T cell globulin in allogeneic hematopoietic cell
transplantation. Biol Blood Marrow Transplant. 21:959–970. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Styczynski J, Tridello G, Gil L, Ljungman
P, Hoek J, Iacobelli S, Ward KN, Cordonnier C, Einsele H, Socie G,
et al: Impact of donor Epstein-Barr virus serostatus on the
incidence of graft-versus-host disease in patients with acute
leukemia after hematopoietic stem-cell transplantation: A study
from the acute leukemia and infectious diseases working parties of
the European society for blood and marrow transplantation. J Clin
Oncol. 34:2212–2220. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Janani MK, Malathi J, Rela M, Farouk M,
Padmapriya J and Madhavan HN: Genotypic detection of Epstein Barr
virus in pediatric transplant recipients from India. Indian
Pediatr. 52:946–950. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Styczynski J, Einsele H, Gil L and
Ljungman P: Outcome of treatment of Epstein-Barr virus-related
post-transplant lymphoproliferative disorder in hematopoietic stem
cell recipients: A comprehensive review of reported cases. Transpl
Infect Dis. 11:383–392. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Styczynski J, van der Velden W, Fox CP,
Engelhard D, de la Camara R, Cordonnier C and Ljungman P: Sixth
European Conference on Infections in Leukemia, a joint venture of
the Infectious Diseases Working Party of the European Society of
Blood and Marrow Transplantation (EBMT-IDWP), the Infectious
Diseases Group of the European Organization for Research and
Treatment of Cancer (EORTC-IDG), the International
Immunocompromised Host Society (ICHS) and the European Leukemia Net
(ELN): Management of Epstein-Barr Virus infections and
post-transplant lymphoproliferative disorders in patients after
allogeneic hematopoietic stem cell transplantation: Sixth European
Conference on Infections in Leukemia (ECIL-6) guidelines.
Haematologica. 101:803–811. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Solano C, Mateo EM, Pérez A, Talaya A,
Terol MJ, Albert E, Giménez E, Vinuesa V, Piñana JL, Boluda JCH and
Navarro D: Epstein-Barr virus DNA load kinetics analysis in
allogeneic hematopoietic stem cell transplant recipients: Is it of
any clinical usefulness? J Clin Virol. 97:26–32. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Marinho-Dias J, Lobo J, Henrique H, et al:
Post-transplant lymphoproliferative disease in hematopoietic stem
cell transplant patients: A single center retrospective study
between 2005 and 2012. Mol Med Rep (In press). 2018. View Article : Google Scholar
|
|
14
|
Marinho-Dias J and Sousa H:
Cytomegalovirus infection and cervical cancer: From past doubts to
present questions. Acta Med Port. 26:154–160. 2013.PubMed/NCBI
|
|
15
|
Henig I and Zuckerman T: Hematopoietic
stem cell transplantation-50 years of evolution and future
perspectives. Rambam Maimonides Med J. 5:e00282014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Al-Mansour Z, Nelson BP and Evens AM:
Post-transplant lymphoproliferative disease (PTLD): Risk factors,
diagnosis, and current treatment strategies. Curr Hematol Malig
Rep. 8:173–183. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sousa H, Boutolleau D, Ribeiro J, Teixeira
AL, Pinho Vaz C, Campilho F, Branca R, Campos A Jr, Baldaque I and
Medeiros R: Cytomegalovirus infection in patients who underwent
allogeneic hematopoietic stem cell transplantation in Portugal: A
five-year retrospective review. Biol Blood Marrow Transplant.
20:1958–1967. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Campos AB, Ribeiro J, Boutolleau D and
Sousa H: Human cytomegalovirus antiviral drug resistance in
hematopoietic stem cell transplantation: Current state of the art.
Rev Med Virol. 26:161–182. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Campos AB, Ribeiro J, Pinho Vaz C,
Campilho F, Branca R, Campos A Jr, Baldaque I, Medeiros R,
Boutolleau D and Sousa H: Genotypic resistance of cytomegalovirus
to antivirals in hematopoietic stem cell transplant recipients from
Portugal: A retrospective study. Antiviral Res. 138:86–92. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Preiksaitis JK: New developments in the
diagnosis and management of posttransplantation lymphoproliferative
disorders in solid organ transplant recipients. Clin Infect Dis.
39:1016–1023. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Blaes AH, Cao Q, Wagner JE, Young JA,
Weisdorf DJ and Brunstein CG: Monitoring and preemptive rituximab
therapy for Epstein-Barr virus reactivation after antithymocyte
globulin containing nonmyeloablative conditioning for umbilical
cord blood transplantation. Biol Blood Marrow Transplant.
16:287–291. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
van Esser JW, van der Holt B, Meijer E,
Niesters HG, Trenschel R, Thijsen SF, van Loon AM, Frassoni F,
Bacigalupo A, Schaefer UW, et al: Epstein-Barr virus (EBV)
reactivation is a frequent event after allogeneic stem cell
transplantation (SCT) and quantitatively predicts
EBV-lymphoproliferative disease following T-cell-depleted SCT.
Blood. 98:972–978. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dumas PY, Ruggeri A, Robin M, Crotta A,
Abraham J, Forcade E, Bay JO, Michallet M, Bertrand Y, Socié G, et
al: Incidence and risk factors of EBV reactivation after unrelated
cord blood transplantation: A Eurocord and Société Française de
Greffe de Moelle-Therapie Cellulaire collaborative study. Bone
Marrow Transplant. 48:253–256. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Reddy N, Rezvani K, Barrett AJ and Savani
BN: Strategies to prevent EBV reactivation and posttransplant
lymphoproliferative disorders (PTLD) after allogeneic stem cell
transplantation in high-risk patients. Biol Blood Marrow
Transplant. 17:591–597. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Uhlin M, Wikell H, Sundin M, Blennow O,
Maeurer M, Ringden O, Winiarski J, Ljungman P, Remberger M and
Mattsson J: Risk factors for Epstein-Barr virus-related
post-transplant lymphoproliferative disease after allogeneic
hematopoietic stem cell transplantation. Haematologica. 99:346–352.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Marques HH, Shikanai-Yasuda MA, dAzevedo
LS, Caiaffa-Filho HH, Pierrotti LC, Aquino MZ, Lopes MH, Maluf NZ,
Campos SV and Costa SF: Management of post-transplant Epstein-Barr
virus-related lymphoproliferative disease in solid organ and
hematopoietic stem cell recipients. Rev Soc Bras Med Trop.
47:543–546. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gulley ML and Tang W: Using Epstein-Barr
viral load assays to diagnose, monitor, and prevent posttransplant
lymphoproliferative disorder. Clin Microbiol Rev. 23:350–366. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bar-Natan M and Nagler A: Epstein-Barr
virus-associated post-transplant lymphoproliferative disorder. Isr
Med Assoc J. 8:205–207. 2006.PubMed/NCBI
|
|
29
|
Cohen J, Gandhi M, Naik P, Cubitt D, Rao
K, Thaker U, Davies EG, Gaspar HB, Amrolia PJ and Veys P: Increased
incidence of EBV-related disease following paediatric stem cell
transplantation with reduced-intensity conditioning. Br J Haematol.
129:229–239. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
van Esser JW, Niesters HG, van der Holt B,
Meijer E, Osterhaus AD, Gratama JW, Verdonck LF, Löwenberg B and
Cornelissen JJ: Prevention of Epstein-Barr
virus-lymphoproliferative disease by molecular monitoring and
preemptive rituximab in high-risk patients after allogeneic stem
cell transplantation. Blood. 99:4364–4369. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dabas R, Lee R, Servito MT, Dharmani-Khan
P, Modi M, van Slyke T, Luider J, Durand C, Larratt L, Brandwein J,
et al: Antithymocyte globulin at clinically relevant concentrations
kills leukemic blasts. Biol Blood Marrow Transplant. 22:815–824.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Crocchiolo R, Esterni B, Castagna L, Fürst
S, El-Cheikh J, Devillier R, Granata A, Oudin C, Calmels B,
Chabannon C, et al: Two days of antithymocyte globulin are
associated with a reduced incidence of acute and chronic
graft-versus-host disease in reduced-intensity conditioning
transplantation for hematologic diseases. Cancer. 119:986–992.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bacigalupo A: ATG in allogeneic stem cell
transplantation: Standard of care in 2017? Point? Blood Adv.
1:569–572. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pidala J, Tomblyn M, Nishihori T, Ayala E,
Field T, Fernandez H, Perez L, Locke F, Alsina M, Ochoa JL, et al:
ATG prevents severe acute graft-versus-host disease in mismatched
unrelated donor hematopoietic cell transplantation. Biol Blood
Marrow Transplant. 17:1237–1244. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Podgorny PJ, Ugarte-Torres A, Liu Y,
Williamson TS, Russell JA and Storek J: High rabbit-antihuman
thymocyte globulin levels are associated with low likelihood of
graft-vs-host disease and high likelihood of posttransplant
lymphoproliferative disorder. Biol Blood Marrow Transplant.
16:915–926. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Janeczko M, Mielcarek M, Rybka B,
Ryczan-Krawczyk R, Noworolska-Sauren D and Kałwak K: Immune
recovery and the risk of CMV/EBV reactivation in children post
allogeneic haematopoietic stem cell transplantation. Cent Eur J
Immunol. 41:287–296. 2016. View Article : Google Scholar : PubMed/NCBI
|