Introduction
A number of previous studies have reported an in
vivo ambient glutamate concentration as high as 1–4 µM
(1–3). Given that the 50% effective
concentration (EC50) of the N-methyl-D-aspartate (NMDA)
receptor for glutamate was ~2 µM (4), this concentration range would have
significant effects on neuronal excitability. However, opposing
data indicated that the ambient glutamate concentration was lower,
within the nanomolar range, which was in better agreement with the
theoretical minimum concentration of glutamate (2 nM) (5).
Treatment with two different concentrations of
glutamate (175 and 250 µM) for 1 h led to different outcomes in rat
cortical cells, and cultures treated with 250 µM glutamate suffered
a loss in overall activity that was not observed in cultures
treated with 175 µM glutamate in rat cortical cells, serving as a
model of traumatic brain injury (6). Loss of calcium homeostasis is a key
mediator of glutamate-induced cell death, which is involved in
Alzheimer's disease (7) and other
age-associated neurodegenerative conditions, such as oxidative
stress (8) and cellular energy
deficits (9). It has been reported
that glutamate (8 mM) treatment alone caused a significant increase
(50%) in cell death compared with the control group in the HT22
mouse hippocampal cell line, whereas glutamate (1 mM) treatment
alone induced one fold increase (50%) in cell death compared with
the control group in primary cultures of rat cortical neuronal
cells (10), where astrocytes
could be involved in the neuroprotective effect. Certain studies
have demonstrated that astrocytes expressing glutamic acid
decarboxylase 67 (GAD67) protected primary neurons from the
toxicity of exposure to 300 µM glutamate for 10 min (11) or from serum glutamate (12). Specific compounds, such as
pyruvate, β-amyloid and ceftriaxone, act to protect neurons from
damage during brain ischemia via astrocytes (7–9).
However, co-cultures of neurons and astrocytes have been reported
to increase neuronal sensitivity to glutamate treatment for 24 h
(13).
According to these controversial results, the aim of
the present study was to investigate glutamate-induced rat cortical
neuronal toxicity in the presence or absence of astrocytes. The
study also further explored the protection of neurons by
astrocytes.
Materials and methods
Materials
Dulbecco's modified Eagle's medium (DMEM)/F12,
neurobasal medium, B27 supplement and fetal bovine serum (FBS) were
purchased from Thermo Fisher Scientific, Inc. (Gibco; Waltham, MA,
USA). Trypsin was purchased from Lonza Bioscience (Walkersville,
MD, USA). Penicillin and streptomycin were purchased from
Biological Industries (Beit Haemek, Israel). Poly-D-lysine,
monosodium glutamate, DAPI and DNase were obtained from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Mouse monoclonal
anti-neuronal nuclei (NeuN; GTX30773) antibody as a neuronal marker
was purchased from GeneTex, Inc. (Irvine, CA, USA), and mouse
monoclonal anti-glial fibrillary acidic protein (GFAP) as astrocyte
marker was purchased from BD Biosciences, Franklin Lakes, NJ, USA
(556328). 5-Fluorouracil and uridine were from TCI Development Co.,
Ltd. (Shanghai, China). Cell Counting kit-8 (CCK-8) was purchased
from Zoman Biotechnology Co., Ltd. (Beijing, China), while papain
was obtained from Acros Organics (Belgium). Cell culture plates,
flasks and inserts were purchased from Corning, Inc. (Corning, NY,
USA).
Animals
Newborn Wistar rats (0–1 day) were obtained to
culture cortical neurons, and newborn Wistar newborn rats (1–3
days) to culture cortical astrocytes (Hebei Medical University,
Shijiazhuang, China). The pregnant and neonatal rats (5.5~10 g)
were housed with standard chow and water ad libitum in an
ambient temperature of 22±2°C and kept under a 12/12 h light/dark
cycle with the lights on at 07:00 a.m. All animal care and
experimental procedures were performed in accordance with approved
guidelines of the National Institutes of Health for the Care and
Use of Laboratory Animals, and the guidelines were approved by the
Committee of Ethics on Animal Experiments of Hebei Medical
University. All efforts were made to minimize suffering and the
number of animals was used in the study.
Primary rat cortical neuronal
culture
Primary cultures of cortical neurons from newborn
Wistar rats (0–1 day) were obtained as described previously
(14). Briefly, the brains of
newborn Wistar rats (0–1 day) were removed subsequent to
decapitation, and the meninges were stripped away. The cortices of
these brains were dissected in ice-cold DMEM-F12 basal culture
media supplemented with 26% glucose. The tissues were treated with
2 mg/ml papain and 42 µg/ml DNase for 30 min in 37°C incubator, and
then gently dissociated by trituration in neuronal culture media
(97% neurobasal medium supplemented with 2% B27, 100 U/ml
penicillin and 100 µg/ml streptomycin). The digestion was
terminated by FBS at room temperature, and the cell suspension was
filtered prior to centrifugation (447 × g for 10 min room
temperature). Cells were centrifuged three times, washed with
Hanks' balanced salt solution (with 1.3 mM calcium and 0.5 mM
magnesium) and finally resuspended in neurobasal medium. Cells were
then seeded on 1 µg/ml poly-D-lysine-coated 24-well plates at a
density of 7×105 cells/well and maintained in neuronal
culture media in a humidified atmosphere with 5% CO2 at
37°C. Culture medium was replaced every 2–3 days to minimize
culture debris. Pure neuronal cultures were obtained by addition of
1 µmol/L 5-fluorouracil and uridine in the culture at day 3, which
was refreshed by neuronal culture media 2 days later. After 9–11
days, the purity of neurons was identified by NeuN and DAPI
fluorescent staining.
Primary rat cortical astrocyte
culture
Cerebral cortical astrocytes were prepared as
previously described, with slight modification (15). Briefly, cultured cortices were
obtained from Wistar newborn rats (1–3 days) and passed through a
nylon sieve (75-µm pore size) into DMEM/F12 supplemented with 10%
FBS, 100 U/ml penicillin and 100 µg/ml streptomycin. The cell
suspension was seeded in poly-D-lysine-coated flasks at 37°C in a
humidified atmosphere with 5% CO2. The medium was
refreshed the following day and changed every two days until the
cells covered 80–90% of the culture flask. At 9–11 days, these
primary cultures were purified by a physical method to eliminate
non-astrocytic cells. The flasks were shaken inside an incubator
for 12–18 h, detached cells were eliminated, and the medium was
renewed. Residuary cells were digested with 0.25% trypsin, seeded
into 24-well plates (8×105 cells/plate) and maintained
in DMEM/F12 culture media in a humidified atmosphere with 5%
CO2 at 37°C. Culture medium was replaced every 2–3 days
to minimize culture debris. Experiments were usually conducted on
the third day.
Primary rat cortical co-culture of
neurons and astrocytes
Neuron-astrocyte co-cultures were generated by
plating pure neurons in the wells of a 6-well plate
(1×106 cells/well) and pure astrocytes (8×105
cells/well) in the culture strip inserts of the plates with
neuronal culture media for 2 days. Samples were kept in an
incubator with a humidified atmosphere and 5% CO2 at
37°C. An Olympus inverted phase contrast microscope (Olympus
Corporation, Tokyo, Japan) was used to capture images of cell
density, morphology and spreading on the multilayer surfaces.
Immunofluorescence analysis
Cortical neurons and astrocytes were rinsed in PBS,
and fixed with 4% paraformaldehyde for >2 h. Subsequent to
washing in PBS, cells were incubated for 20 min in 0.4% Triton
X-100 in PBS at 37°C, further washed three times with PBS, blocked
with 10% goat serum at 37°C for 1 h and then washed for a further
three times with PBS. Next, neurons were incubated overnight at 4°C
with anti-neuron-specific nuclear protein NeuN monoclonal antibody
(dilution, 1:150 in PBS), followed by five washes with PBS for 5
min each. The neurons were subsequently incubated with a
488-conjugated goat anti-mouse secondary antibody (072031806;
Kirkegaard & Perry Laboratories Inc., Gaithersburg, MD, USA;
dilution, 1:200 in PBS) for 1 h at 37°C and washed for a further
five times in PBS. Similarly, astrocytes were incubated with mouse
anti-GFAP monoclonal antibody (BD Biosciences; dilution, 1:200 in
10% goat serum), washed five times in PBS, and then incubated with
a KPL 488-conjugated goat anti-mouse secondary antibody
(Gaithersburg, MD, USA; dilution 1:200 in PBS) for 1 h at 37°C,
followed by further washing for five times in PBS. Subsequently,
neurons or astrocytes were incubated with 0.005% DAPI for 10 min at
room temperature and further washed five times in PBS. Images were
collected on a Nikon Eclipse Ti-E inverted fluorescence microscope
(Nikon Corporation, Tokyo, Japan), and then processed and
visualized using NIS-Elements D (version 4.50; Nikon Corporation)
to assess NeuN/DAPI and GFAP/DAPI staining. High-resolution images
were created using Photoshop CS2 (Adobe, Inc., Mountain View, CA,
USA).
Glutamate-induced exitotoxicity in rat
cortical neurons and/or astrocytes
Monosodium glutamate was dissoluted with water, and
the stock solution of 2,000 mM was stored at −20°C. Rat cortical
neurons alone were exposed to 10, 25, 50, 100, 200, 500 and 1,000
µM glutamate and 10 µM glycine (there is glycine combining site in
glutamate NMDA receptors, and glutamate-induced effect can be
increased in the presence of glycine) for 15, 30 and 60 min in
Mg2+-free Locke's buffer (154 mM NaCl, 5.6 mM KCl, 3.6
mM NaHCO3, 1.3 mM CaCl2, 5.6 mM D-glucose and
5 mM HEPES; pH 7.4) (16). In
addition, co-cultures of rat cortical neurons and astrocytes were
exposed to 10, 100, 200, 500, 1,000 and 2,000 µM glutamate and 10
µM glycine for 15, 30 and 60 min in Mg2+-free Locke's
buffer. Rat cortical astrocytes alone were exposed to 500, 1,000
and 2,000 µM glutamate for 6, 12 and 24 h in DMEM-F12 basal medium.
Next, glutamate was washed out thoroughly, and replaced with
neurobasal culture media or DMEM-F12 complete medium in a
humidified atmosphere with 5% CO2 at 37°C. After 24 h, a
CCK-8 assay was used to assess the cell viability.
Cell viability
The cell viability of cultured rat cortical neurons,
astrocytes, and the co-culture of neurons and astrocytes was
measured 24 h after glutamate treatment by CCK-8 assay. Briefly,
CCK-8 solution (10% final concentration) was added to cultured
neurons and/or astrocytes, and incubated for 4 h. The optical
densityof each sample was measured using BioTek Synergy 2 (BioTek
Instruments, Inc., Winooski, VT, USA) at 450 nm. Quantitative
dose-toxicity examination was performed in the rat cortical neurons
alone, astrocytes alone and the co-culture of neurons and
astrocytes, and the half-maximal inhibitory concentration
(IC50) was measured.
Statistical analysis
All data are expressed as the mean ± standard error
of the mean. Each n value represents data from one culture well.
Unless otherwise indicated, an n value of 8–12 culture wells was
used for each group in each experiment, with repetitions conducted
using at least 3 independent dissections for each experiment.
Differences between group means were analyzed with one-way analysis
of variance, followed by the Bonferroni posthoc test. The
differences were considered as statistically significant when the
P-value was <0.05. Nonlinear regression analysis and
IC50 estimates for dose-response data were performed
with GraphPad Prism software (version 6.0; GraphPad Software, Inc.,
La Jolla, CA, USA).
Results
Immunofluorescence characterization of
neurons and astrocytes
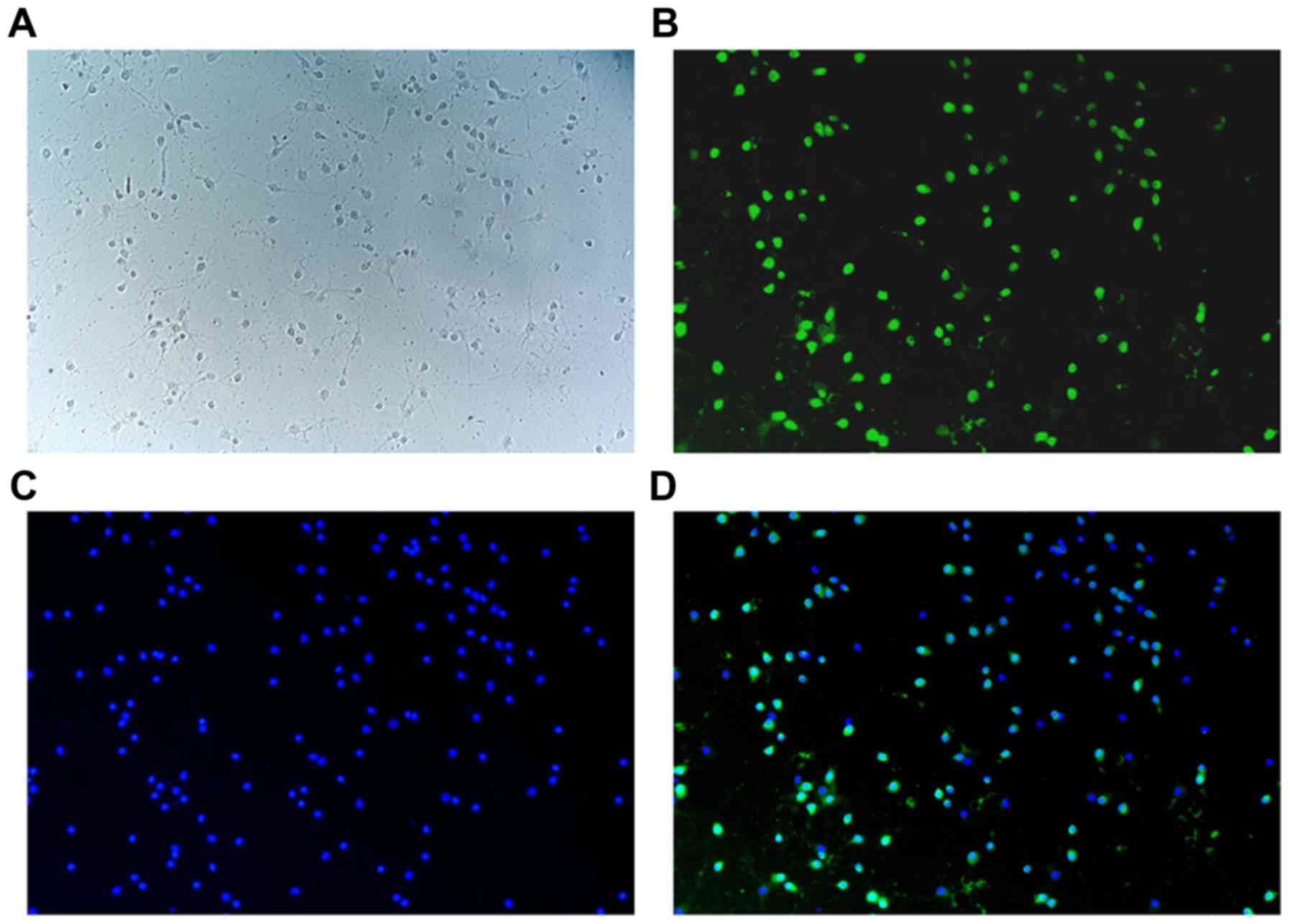
NeuN-immunofluorescence analysis indicated that the
purity of primary neurons was >95%, and dispersed neurons with
rare clumping were detected (Fig.
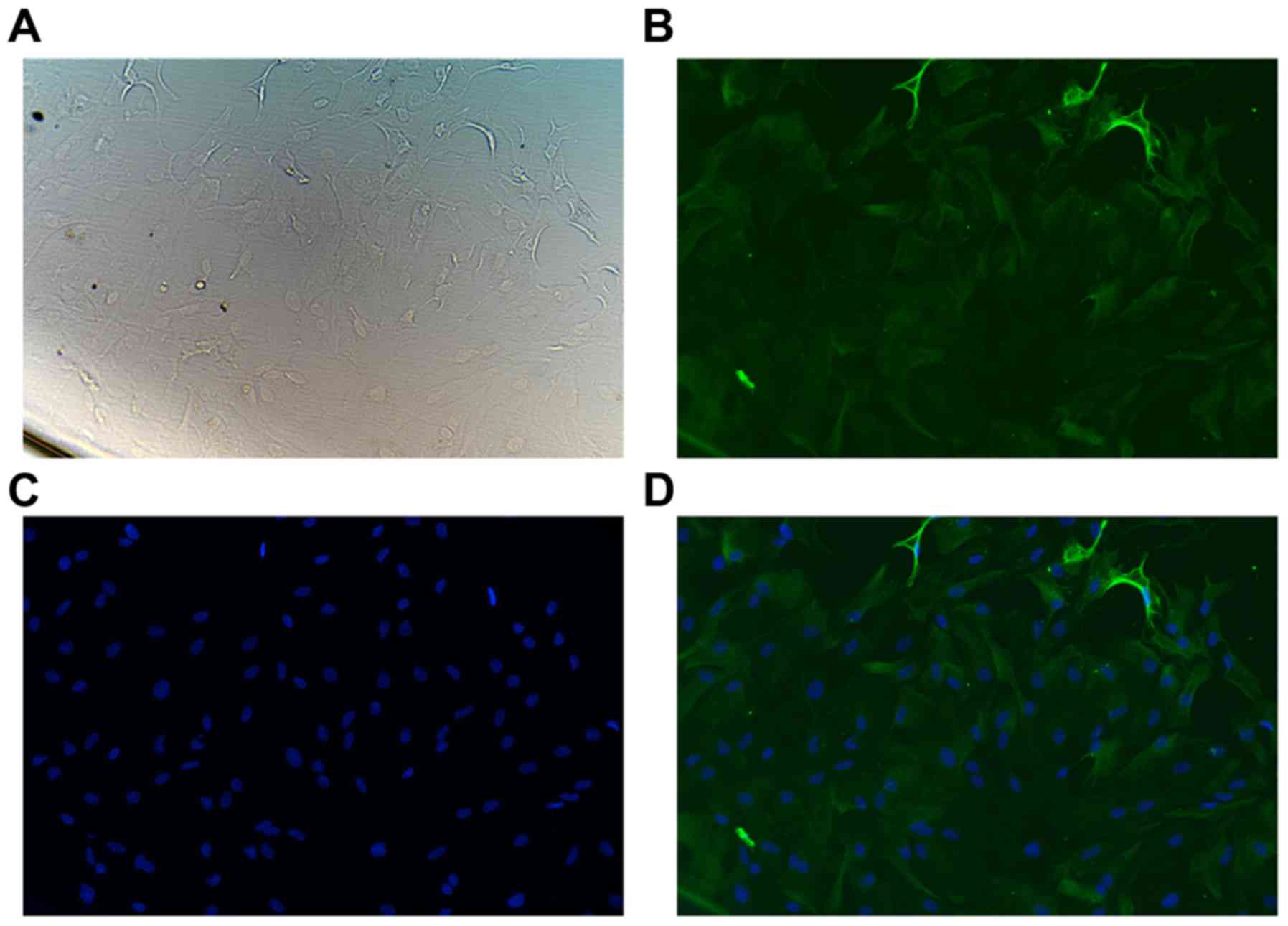
1). In addition, GFAP-immunofluorescence analysis revealed that
the purity of primary flat polygonal astrocytes was >95%
(Fig. 2; data not shown).
Glutamate-induced exitotoxicity of rat
cortical neurons in the absence of astrocytes
In the absence of astrocytes, the exposure of rat
cortical neurons to various glutamate concentrations (10, 25, 50,
100, 200, 500 and 1,000 µM) and 10 µM glycine for 15, 30 or 60 min,
respectively, resulted in a concentration-dependent decrease of the
neuronal survival rate compared with the control group
(Mg2+-free Locke's buffer without glutamate and
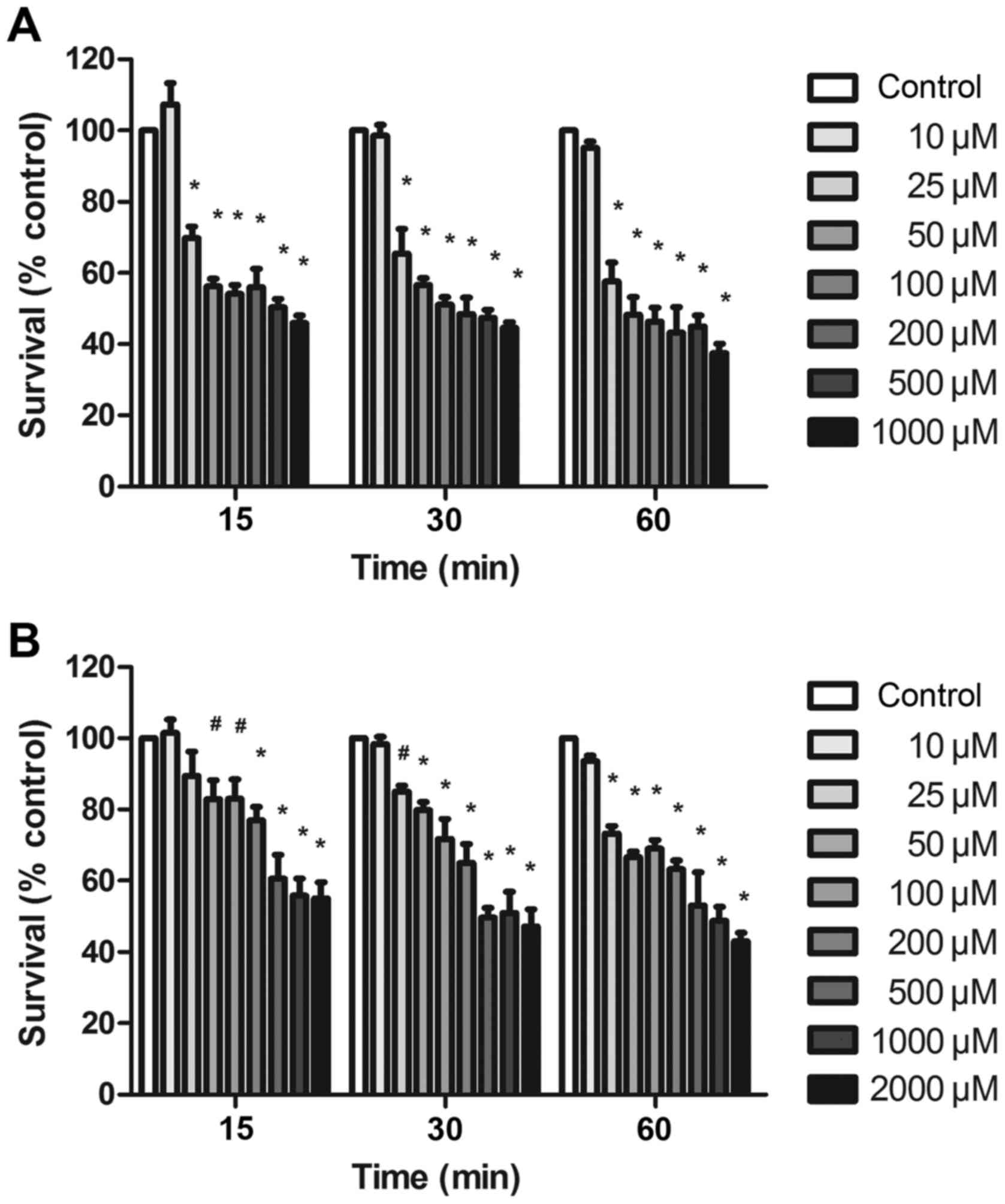
glycine). As shown in Fig. 3A,
treatment with 10 µM glutamate and 10 µM glycine had no evident
effect on neuronal survival at the three time points, while
exposure to 25 µM glutamate and 10 µM glycine had a significant
effect on neuronal survival, reducing the survival rate to
approximately 50–70% of the control value. The neuronal survival
rates of glutamate (25–1,000 µM) to control were reduced in a
concentration-dependent manner, but no significant difference was
observed between different glutamate concentrations (Fig. 3A). Furthermore, the IC50
of the survival rate in neurons exposed to glutamate for 15, 30 and
60 min compared with the control was 364.5, 258.5 and 138.3 µM,
respectively.
Glutamate-induced exitotoxicity of rat
cortical astrocytes alone
Astrocytes extracted from rat cerebral cortex were
exposed to different concentrations of glutamate for 6, 12 and 24
h. The astrocytic survival rates following expose to glutamate
(500, 1,000 and 2,000 µM) were not markedly changed compared with
the control group.
Glutamate-induced exitotoxicity of rat
cortical neurons in the presence of astrocytes
The exposure of rat cortical neurons to different
glutamate concentrations (10, 25, 50, 100, 200, 500, 1,000 and
2,000 µM) and 10 µM glycine in the presence of astrocytes for 15,
30 or 60 min, respectively, resulted in a concentration-dependent
decrease of the neuronal survival rate compared with the control
group. As shown in Fig. 3B,
treatment with 10 µM glutamate and 10 µM glycine had no evident
effect on neuronal survival at the three incubation times. By
contrast, treatment with 25 µM glutamate and 10 µM glycine resulted
in an evident tendency of decreased neuronal survival, and the
survival rate was reduced to approximately 70–80% of that of the
control group, with a significant statistical difference observed
at 30 and 60 min (P<0.01). In addition, the IC50 of
the neuronal survival rate in the neuron-astrocyte co-culture
exposed to glutamate for 15, 30 and 60 min compared with the
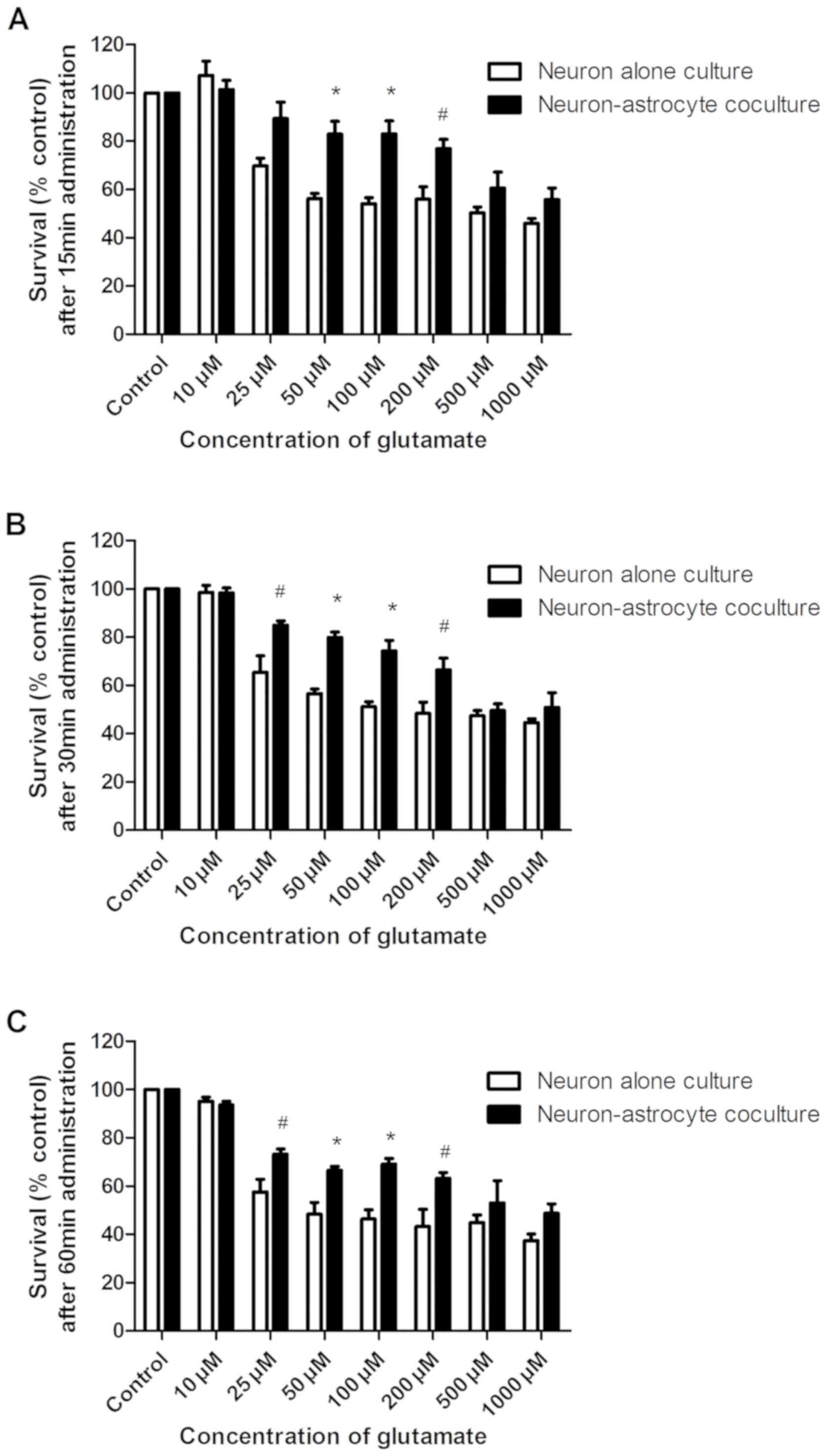
control was 1,935, 932.8 and 789.3 µM, respectively (Fig. 3B). In Fig. 4, the survival rate of neurons
following different glutamate concentrations at different time
periods was compared in neuron alone culture and neuron-astrocyte
coculture. As demonstrated in Fig.
4A, the survival rate of treatment with 50, 100 and 200 µM
glutamate and 10 µM glycine for 15 min in neuron-astrocyte
coculture was significantly increased compared with that in neuron
alone culture, while the survival rates of treatment with 25, 50,
100 and 200 µM glutamate and 10 µM glycine for 30 and 60 min in
neuron-astrocyte coculture was significantly increased compared
with that in neuron alone culture in Fig. 4B and C. Student's t-test was
conducted to determine statistically significant differences in the
results: #P<0.05 and *P<0.01 vs. the survival rate
of neurons in neuron alone culture. Furthermore, changes in the
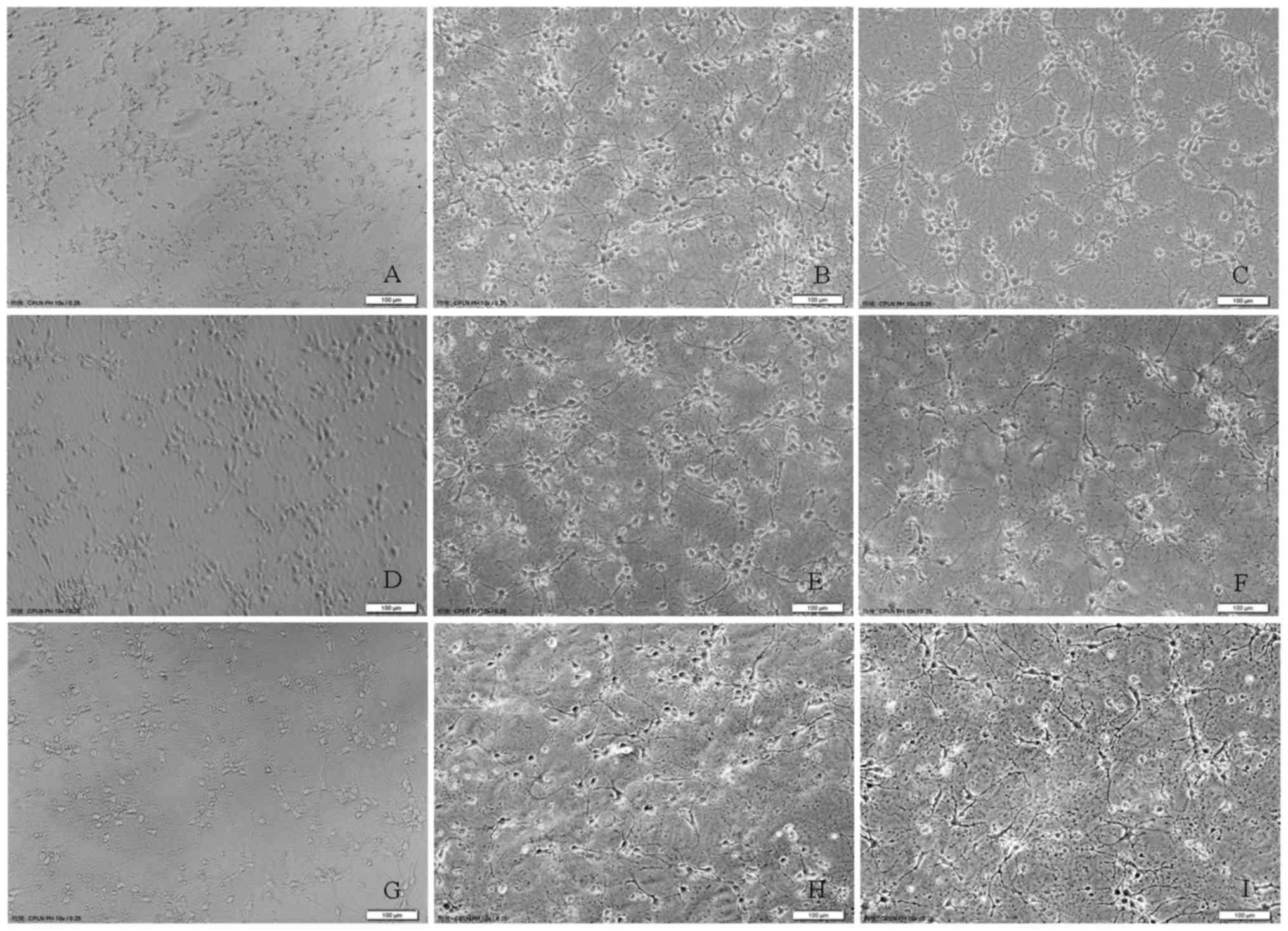
morphology of rat cortical neurons were observed at 15, 30 and 60
min following glutamate (200 and 1,000 µM) exposure in the presence
of astrocytes as compared with the control group (Fig. 5), proving the
concentration-dependent neurotoxicity of glutamate in the rat
cortical co-culture of neurons and astrocytes. All the above
results suggested that astrocytes enhanced the tolerance of rat
cortical neurons to glutamate excitotoxicity.
Discussion
In the present study, glutamate exposure of neurons
isolated from the cerebral cortex of newborn Wistar rats (0–1 day)
in the absence of astrocytes for 15, 30 and 60 min resulted in a
concentration-dependent neurotoxicity (IC50=364.5, 258.5
and 138.3 µM, respectively). These results were consistent with
previously reported findings that approximately 20±3% primary
hippocampal neurons of postnatal Wistar rats (0–1 day) were
destroyed in control cultures, while 40–50% neurons were destroyed
in 0.5 mM L-glutamate cultures for 30 min (17). In addition, in cultures exposed to
1 mM L-glutamate, up to 60% of the neurons died during this time
period of 30 min depending on the concentration of glutamate
(17). However, the results of the
present study were inconsistent with another study that addition of
glutamate (50 µM) to primary cortical neurons obtained from rat
embryos [embryonic day(E) 16–18] for 1 h resulted in a living rate
of neurons of ~20% in contrast to the control group (18). Furthermore, the apoptotic-like
death of cultured rat cortical neurons from 17-day-old
Sprague-Dawley rat embryos exposed to 50 µM glutamate for 15 min
was up to ~80% (19). Therefore,
the difference in glutamate-induced neurotoxicity between the
present study and previous studies may be a result of the culture
being obtained from newborn or fetal rats, and the neuron culture
of the fetal rats may be more sensitive to exposure to glutamate as
compared with that of the newborn rats.
Glutamate (10 mM) applied to the mouse hippocampal
HT22 cell line for 24 h has been reported to reduce the neuronal
cell survival by >80% (20).
HT22 cells do not express functional glutamate receptors;
therefore, glutamate toxicity was mediated by inhibiting the
cystine uptake (21). Furthermore,
another study demonstrated that cellular death was observed at ~6 h
after initiation of 1 mM glutamate exposure in primary cortical
neurons isolated from 18-day-old pregnant Sprague-Dawley rats, and
after ~8 h of exposure to 7–8 mM glutamate in HT22 cells (10). In addition, brief exposure to
glutamate was found to produce morphological changes in mature
(14–24 days in vitro) cortical neurons from fetal mouse
neocortex, beginning as quickly as 90 sec after exposure, followed
by widespread neuronal degeneration over the next hours, and a
quantitative dose-toxicity study suggested an EC50 of
50–100 µM for a 5-min exposure to glutamate (22). The reliable neurotoxicity produced
by exposure of cell cultures to 0.5 mM glutamate for 5 min was only
observed with mature cultures, whereas immature (5–7 days in
vitro) neurons and glia were unaffected by such glutamate
exposure, and partial effects were observed with cultures of
intermediate maturity (22).
Therefore, the difference of glutamate-induced neurotoxicity
between the present study and previous studies may also be due to
differences in primary neuron cultures and nervous cell lines,
genera of animals or degrees of maturity, which may depend on the
distribution density of glutamate receptors. Nervous cell lines
that do not express functional glutamate receptors may be more
resistant to glutamate-induced neurotoxicityin comparison with
primary neuronal culture, while the distribution of glutamate
receptors of immature (5–7 days in vitro) culture neurons
may be less than that of mature (14–24 days in vitro)
culture neurons.
In the present study, astrocytic toxicity was not
observed when astrocytes isolated from the rat cerebral cortex were
exposed to different concentrations of glutamate (500, 1,000 and
2,000 µM) for 6, 12 and 24 h (data not shown). This is consistent
with previous observations suggesting that cerebral astrocytes
isolated from the cerebral cortex of 1–3-day Wistar newborn rats
were resistant to injury by glutamate even in the presence of the
uptake inhibitor (23). The
morphology of astrocytes from fetal mouse neocortex appeared to be
completely unaffected by 0.5 mM glutamate for 5 min, and no
evidence of direct glutamate gliotoxicity was detected on glial
elements in mixed cortical cultures or in essentially pure glial
cultures prepared from postnatal animals (22). Furthermore, another study reported
that astrocytic toxicity was not induced by glutamate
concentrations <2 mM, and astrocytic death was not observed even
after a 24-h exposure to up to 100 µM glutamate in astrocytes alone
or co-cultured with neurons (13).
However, the glutamate gliotoxicity has been observed in pure glial
cultures prepared from chick retinal cells (24). In vivo experiments also
demonstrated the swelling of glia with glutamate neurotoxicity, but
the gliotoxicity was not observed upon treatment with low doses of
glutamate (25). Therefore,
combining these results with the findings of the present study,
whether glutamate can induce gliotoxicity may depend on various
factors, including the genera, maturity degree, concentration, and
in vivo or in vitro culture among others, although
this should be further explored.
The present study assessed the neuronal cytotoxic
effect of glutamate on rat cortical co-culture of neurons and
astrocytes, which were incubated with different concentrations of
glutamate (including 10, 25, 50, 100, 200, 500, 1,000 and 2,000 µM)
for 15, 30 and 60 min. For each concentration of glutamate, 10 µM
glycine was added to the medium, followed by assessment of cell
viability using the CCK-8 assay subsequent to the incubation
period. The IC50 values of the glutamate-induced
neuronal survival rate of the co-culture of neurons and astrocytes
for 15, 30 and 60 min were respectively 1,935, 932.8 and 789.3 µM,
indicating the time-dependent neurotoxicity of glutamate in the rat
cortical co-culture of neurons and astrocytes. By contrast, the
IC50 values of the glutamate-induced neuronal survival
rate in the neuron culture in the absence of astrocytes were 364.5,
258.5 and 138.3 µM for 15, 30 and 60 min incubation, respectively.
Compared with the culture without astrocytes, the IC50
of the glutamate-induced neuronal survival rate in the presence of
astrocytes was higher at each of the different incubation periods.
These findings were consistent with the observations of a previous
study, indicating that the EC50 of neuronal cytotoxicity
in pure neuron cultures isolated from albino ICR mice at E15
following exposure to glutamate for 15 min was ~5 µM, while that of
neuron and astrocyte co-cultures was ~100 µM (13). The EC50 of
glutamate-induced neurotoxicity (EC50=200 µM) in
neuron-astrocyte co-cultures isolated from the embryonic (E17-19)
and newborn rat cerebral cortex was greater compared with
neuron-enriched cultures (EC50=50 µM) (23). Furthermore, the survival rates of
treatment with 50, 100 and 200 µM glutamate and 10 µM glycine for
15, 30 and 60 min in neuron-astrocyte coculture were significantly
increased respectively compared with that in neuron alone culture.
However, the survival rates of treatment with 500 and 1,000 µM
glutamate and 10 µM glycine for 15, 30 and 60 min in
neuron-astrocyte coculture exhibited a tendency to increase without
significantly statistical differences, suggesting that astrocytes
can enhance the tolerance of rat cortical neurons to glutamate
excitotoxicity within a certain range.
Protection of PC12 cells against
H2O2 or serum deprivation by co-culture with
C8-GAD67 astrocytes has been reported in the presence of 1 mM
glutamate for 24 h, while the primary cortical neuron survival was
enhanced by co-culture with C8-GAD67 astrocytes, demonstrating that
GAD-expressing astrocytes induced an increase of antioxidant
activity, protecting neurons from various injuries (11). A previous study indicated that
excitotoxic glutamate exposure (500 µM glutamate and 10 µM glycine
for 10 min) by microperfusion in mixed primary cortical neuron
cultures from embryonic (E16) rats resulted in approximate 20–30%
neuronal loss after 1 h (26).
However, this excitotoxic glutamate exposure resulted in 87.0±1.6%
loss of cortical neurons after 24 h. The addition of MK-801 to the
incubation medium resulted in significant protection from the
glutamate-induced neuronal loss after 24 h, suggesting that chronic
toxic reaction to glutamate exposure may be receptor-dependent
(26). Another study reported that
reliable neurotoxicity produced by exposure of cell cultures
isolated from fetal mouse neocortex to 0.5 mM glutamate for 5 min
was only observed with mature cultures, while immature (5–7 days
in vitro) neurons and glia were unaffected by exposure to
0.5 mM glutamate for 5 min, and partial effects were observed with
cultures of intermediate maturity (22). Furthermore, the EC50 of
neuronal cytotoxicity in mouse pure neuron cultures exposed to
glutamate for 24 h was ~50 µM, while that of mouse neuron-astrocyte
co-cultures was ~5 µM (13). This
indicated that neuronal cytotoxicity was significantly increased in
the presence of astrocytes as compared with pure neuron cultures
for all glutamate concentrations for 24 h, and that astrocytes
increased neuronal sensitivity to chronic glutamate exposure (24 h)
as opposed to acute exposure (13).
Therefore, these aforementioned studies indicated
that astrocytes can protect acute glutamate-induced neuronal
toxicity possibly due to glutamate rapidly being taken up by
astrocytes. However, astrocytes can also aggravate chronic
glutamate-induced neuronal toxicity through increasing neuronal
sensitivity to chronic glutamate exposure, demonstrating that
chronic toxic reaction to glutamate exposure may be
receptor-dependent. Furthermore, glutamate-induced neuronal
toxicity may be affected by a variety of factors, including the
maturity of neurons and glia, and immature cells may be insensitive
to glutamate exposure. However, the roles and underlying mechanism
of astrocyte-induced protection against neuronal glutamate
excitotoxicity remains controversial, and further studies need to
be performed.
In conclusion, glutamate induced
concentration-dependent neurotoxicity in rat cortical neurons in
the presence or absence of astrocytes. The IC50 of
neuronal survival rate in the presence of astrocytes was higher in
comparison with that in the absence of astrocytes, suggesting that
astrocytes can enhance the tolerance of rat cortical neurons to
glutamate excitotoxicity.
Acknowledgements
Not applicable.
Funding
The authors acknowledge the support received for
this study from the Natural Science Foundationof China (grant no.
NSFC81402886), the Natural Science Foundation of Hebei Province
(grant no. H2016208071), and the Hebei Province Hundred Excellent
Innovative Talents Support Scheme (III) (grant no.
SLRC2017044).
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
L-NZ and W-BL designed the study; L-NZ, QW and L-ZL
performed the experiments; X-HX and JQ analyzed the data; and L-NZ
wrote the manuscript.
Ethics approval and consent to
participate
All experiments were performed with the approval of
the Ethics Committee of Hebei Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lerma J, Herranz AS, Herreras O, Abraira V
and Martín del Río R: In vivo determination of extracellular
concentration of amino acids in the rat hippocampus. A method based
on brain dialysis and computerized analysis. Brain Res.
384:145–155. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Baker DA, Xi ZX, Shen H, Swanson CJ and
Kalivas PW: The origin and neuronal function of in vivo nonsynaptic
glutamate. J Neurosci. 22:9134–9141. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nyitrai G, Kékesi KA and Juhász G:
Extracellular level of GABA and Glu: In vivo microdialysis-HPLC
measurements. Curr Top Med Chem. 6:935–940. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Patneau DK and Mayer ML:
Structure-activity relationships for amino acid transmitter
candidates acting at N-methyl-D-aspartate and quisqualate
receptors. J Neurosci. 10:2385–2399. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Levy LM, Attwell D, Hoover F, Ash JF,
Bjørås M and Danbolt NC: Inducible expression of the GLT-1
glutamate transporter in a CHO cell line selected for low
endogenous glutamate uptake. FEBS Lett. 422:339–342. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kutzing MK, Luo V and Firestein BL:
Measurement of synchronous activity by microelectrode arrays
uncovers differential effects of sublethal and lethal glutamate
concentrations on cortical neurons. Ann Biomed Eng. 39:2252–2262.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang J, An S, Hu W, Teng M, Wang X, Qu Y,
Liu Y, Yuan Y and Wang D: The neuroprotective properties of
hericium erinaceus in glutamate-damaged differentiated PC12 cells
and an Alzheimer's disease mouse model. Int J Mol Sci.
17:E18102016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cassano T, Pace L, Bedse G, Lavecchia AM,
De Marco F, Gaetani S and Serviddio G: Glutamate and mitochondria:
Two prominent players in the oxidative stress-induced
neurodegeneration. Curr Alzheimer Res. 13:185–197. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lu Z, Chouhan AK, Borycz JA, Lu Z, Rossano
AJ, Brain KL, Zhou Y, Meinertzhagen IA and Macleod GT:
High-probability neurotransmitter release sites represent an
energy-efficient design. Curr Biol. 26:2562–2571. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang Y and Bhavnani BR: Glutamate-induced
apoptosis in neuronal cells is mediated via caspase-dependent and
independent mechanisms involving calpain and caspase-3 proteases as
well as apoptosis inducing factor (AIF) and this process is
inhibited by equine estrogens. BMC Neurosci. 7:492006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lamigeon C, Bellier JP, Sacchettoni S,
Rujano M and Jacquemont B: Enhanced neuronal protection from
oxidative stress by coculture with glutamic acid
decarboxylase-expressing astrocytes. J Neurochem. 77:598–606. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ye ZC and Sontheimer H: Astrocytes protect
neurons from neurotoxic injury by serum glutamate. Glia.
22:237–248. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ha JS, Dho SH, Youm TH, Kwon KS and Park
SS: Astrocytic phospholipase A2 contributes to neuronal glutamate
toxicity. Brain Res 1590. 97–106. 2014. View Article : Google Scholar
|
|
14
|
Negishi T, Ishii Y, Kawamura S, Kuroda Y
and Yoshikawa Y: Cryopreservation of brain tissue for primary
culture. Exp Anim. 51:383–390. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Haseleu J, Anlauf E, Blaess S, Endl E and
Derouiche A: Studying subcellular detail in fixed astrocytes:
Dissociation of morphologically intact glial cells (DIMIGs). Front
Cell Neurosci. 7:542013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou L, Li F, Xu HB, Luo CX, Wu HY, Zhu
MM, Lu W, Ji X, Zhou QG and Zhu DY: Treatment of cerebral ischemia
by disrupting ischemia-induced interaction of nNOS with PSD-95. Nat
Med. 16:1439–1443. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun Y, März P, Otten U, Ge J and Rose-John
S: The effect of gp130 stimulation on glutamate-induced
excitotoxicity in primary hippocampal neurons. Biochem Biophys Res
Commun. 295:532–539. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bösel J, Gandor F, Harms C, Synowitz M,
Harms U, Djoufack PC, Megow D, Dirnagl U, Hörtnagl H, Fink KB and
Endres M: Neuroprotective effects of atorvastatin against
glutamate-induced excitotoxicity in primary cortical neurones. J
Neurochem. 92:1386–1398. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jiang Q, Gu Z and Zhang G: Activation,
involvement and nuclear translocation of c-Jun N-terminal protein
kinase 1 and 2 in glutamate-induced apoptosis in cultured rat
cortical neurons. Brain Res. 956:194–201. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Savaskan NE, Bräuer AU, Kühbacher M,
Eyüpoglu IY, Kyriakopoulos A, Ninnemann O, Behne D and Nitsch R:
Selenium deficiency increases susceptibility to glutamate-induced
excitotoxicity. FASEB J. 17:112–114. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Maher P and Davis JB: The role of
monoamine metabolism in oxidative glutamate toxicity. J Neurosci.
16:6394–6401. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Choi DW, Maulucci-Gedde M and Kriegstein
AR: Glutamate neurotoxicity in cortical cell culture. J Neurosci.
7:357–368. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Amin N and Pearce B: Glutamate toxicity in
neuron-enriched and neuron-astrocyte co-cultures: Effect of the
glutamate uptake inhibitor L-trans-pyrrolidine-2,4-dicarboxylate.
Neurochem Int. 30:271–276. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hyndman AG: The effects of glutamate and
kainate on cell proliferation in retinal cultures. Invest
Ophthalmol Vis Sci. 25:558–563. 1984.PubMed/NCBI
|
|
25
|
Olney JW: Glutamate-induced neuronal
necrosis in the infant mouse hypothalamus. An electron microscopic
study. J Neuropathol Exp Neurol. 30:75–90. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Churn SB, Sombati S, Taft WC and DeLorenzo
RJ: Excitotoxicity affects membrane potential and calmodulin kinase
II activity in cultured rat cortical neurons. Stroke. 24:271–278.
1993. View Article : Google Scholar : PubMed/NCBI
|