Introduction
Endometriosis (Ems) is a common benign gynecological
disease in women of child-bearing age. It indicates that
endometrial tissues (glands and mesenchyme) with growth function
are coated with endometrium in the uterine cavity (1). Additionally, endometrial tissues may
grow in other sites apart from the myometrium. Ems morbidity has
notably increased in recent years, with the major clinical symptoms
of infertility, chronic pelvic pain and dysmenorrhea (1). Ems frequently occurs in the ovary,
rectovaginal pouch and vesicouterine pouch (1). It is a benign disease, but certain of
its biological behaviors resemble those of malignancies (2). It is extremely invasive, and may lead
to extensive and severe adhesion. Additionally, Ems is regarded as
a benign tumor, which severely affects patient quality of life
(2).
microRNAs (miRNAs/miRs) are a class of endogenous
RNAs with regulatory functions in eukaryotes (3). They are ~22-23 nucleotides in length
(4). miRNAs are extensively
distributed in plants, animals and viruses, and negatively regulate
gene expression at the post-transcriptional level through
complementary pairing of mRNA (3).
Eventually, they may lead to mRNA degradation or translational
inhibition (5). As important
regulatory molecules, miRNAs are involved in a series of vital life
processes, such as virus defense, hematopoiesis, organogenesis,
cell proliferation, apoptosis, fat metabolism and tumorigenesis
(5).
The Wnt/β-catenin signaling pathway is a key pathway
regulating cell growth and proliferation (6). A previous study demonstrated that
such a pathway serves a vital role in the genesis, metastasis and
invasion of multiple tumors (6).
In addition, it may be involved in the adhesion, invasion and
angiogenesis of the ectopic endometrium (7). Wnt/β-catenin signaling pathway serves
a decisive role in endometrial gland formation and mesenchymal
development (7).
Transcription factor zinc-finger E-box binding
homeobox (ZEB)1 located in the short arm of human chromosome 10, is
also referred to as TCF8 or δEF1 (8). Recently, it was indicated that ZEB1
serves a crucial role in tumorigenesis (8) in different cancer types, including
breast cancer, prostate cancer, lung cancer and endometrial cancer.
Transcription factor ZEB-1 is one of the factors inducing Ems
(9). A previous study on the role
of ZEB1 in promoting tumor cell metastasis focused on its
inhibitory effect on E-cadherin (10). Cadherins are a class of
Ca2+-dependent transmembrane glycoproteins (10). E-cadherin marks epithelial cells
and its deletion may be observed in Ems (10). Mesenchymal cell markers, including
N-cadherin, are upregulated in Ems (11). Wang et al (12) demonstrated that miRNA-33b is able
to mediate the cellular apoptosis of endometrial cells. The present
study aimed to investigate the role and mechanism of miRNA-33b in
Ems.
Materials and methods
Animals and rat model
Female Sprague-Dawley rats (6–7 weeks old; 180–200
g; n=12) were housed at 22–24°C, 55–60% humidity with a 12-h/12-h
light/dark cycle, and were given a regular chow diet and water
ad libitum. The present study was approved by the Animal
Care and Use Committee of Affiliated Qilu Hospital of Shandong
University (Jinan, China). All rats were randomly assigned to
control (n=6) and Ems (n=6) groups. In the control group, rats were
anesthetized with 35 mg/kg pentobarbital sodium without
intervention. Rats were anesthetized with 35 mg/kg pentobarbital
sodium, and a vertical incision in the abdomen was made. The uterus
was removed and immediately washed with PBS, the endometrium was
cut into 0.5×0.5-cm sections and uterine segments were sutured onto
the peritoneum close to blood vessels. 0.5 µg/kg/day of Estradiol
benzoate (MedChem Express, Shanghai, China) was subcutaneously
injected for 3 days following the surgery. Following treatment with
Estradiol benzoate for 3 days, rats were sacrificed using
decollation under 35 mg/kg pentobarbital sodium.
Cell culture and cell
transfection
Endometrial stromal cells were separated from the
isolated endometrial tissues, and tissue was finely minced. Cells
were dispersed and incubated in Dulbecco's modified Eagle's medium
(DMEM)/F-12 (Gibco; Thermo Fisher Scientific, Inc.),
penicillin-streptomycin solution, and 2 mg/ml collagenase II for 1
h at 37°C. Subsequently, endometrial stromal cells were separated
using a 100 µm filter. Stromal cells were pelleted by
centrifugation at 200 × g for 10 min at 4°C and incubated with
DMEM/F-12 containing fetal bovine serum (10%, v/v; Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) at 37°C in a humidified atmosphere
containing 5% CO2. Then, transfection with 100 ng of
miR-33b (5′-GUGCAUUGCUGUUGCAUUGC-3′ and
5′-AAUGCAACAGCAAUGCACUU-3′), anti-miR-33b (forward,
5′-CCAAGGATCTCCAGGCTCGAA-3′ and reverse,
5′-TTCGAGCCTGGAGATCCTTGG-3′), small interfering (si)-ZEB1
(sc-38643; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and
negative mimics (forward, 5′-TTCTCCGAACGTGTCACGT-3′ and reverse,
5′-ACGTGACACGTTCGGAGAA-3′) was performed using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), according to the
manufacturer's protocol. Following transfection for 4 h, old medium
was removed and new DMEM/F-12 was added into cell. Next,
transfection of miR-33b or negative mimics was performed for 4 h.
For Wnt activation, 10 µM of SKL2001 (MedChemExpress, Shanghai,
China) was added to the cells for 72 h.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from frozen tissues or cells
using TRIzol® reagent (Life Technologies; Thermo Fisher
Scientific, Inc.). Total RNA (1 µg) was transcribed to first-strand
cDNA using an RT kit (Toyobo Life Science, Osaka, Japan). The
RT-qPCR protocol was performed using SYBR-Green PCR master mix
(Bio-Rad Laboratories, Hercules, CA, USA) and an Applied Biosystems
7300 Real-Time PCR System (Thermo Fisher Scientific, Inc.). The
primer sequences were: miR-33b forward, 5′-ATTCTTTCGAACTGTCTTGG-3′
and reverse, 5′-TCACCCTCGGCTGTCCTGACA-3′; U6 forward,
5′-AGTACCAGTCTGTTGCTGG-3′ and reverse, 5′-TAATAGACCCGGATGTCTGGT-3′.
The qPCR cycle was set to an initial 95°C for 10 min, followed by
40 cycles of 95°C for 25 sec, 60°C for 30 sec, and 72°C for 30 sec.
Gene expression levels were measured using the 2−∆∆Cq
method (13).
Gene microarray hybridization
Isolated RNA was reverse transcribed into cDNA and
hybridized to Affymetrix HG-U133 Plus 2.0 GeneChip arrays l
(Affymetrix, Santa Clara, CA, USA). Data were analyzed through
TargetScan version 7.1 (http://www.targetscan.org) and QIAGEN's Ingenuity
Pathway Analysis (IPA, QIAGEN, Redwood City, USA) (14).
Hematoxylin and eosin (H&E)
staining
Endometriosis tissue was collected and fixed with 4%
paraformaldehyde for 24 h. The colonic sections of 4 µm were cut
from formalin-fixed, paraffin-embedded tissue blocks. Tissue
samples were stained with HE assay for 15 min and evaluated under
the light microscope (Olympus BX51).
Cell viability and apoptosis
MTT (5 mg/ml; Sigma Aldrich; Merck KGaA) was added
to the cells for incubation at 37°C for 4 h. Subsequently, the old
medium was removed and dimethyl sulfoxide was added to the cells
for 20 min at 37°C. The absorbance was measured by
spectrophotometry with a microplate reader (model 680; Bio-Rad
Laboratories, Inc.) at 490 nm. Endometrial stromal cells were
washed with PBS and resuspended in 100 µl 1X binding buffer
(BestBio, Shanghai, China) and incubated with Annexin V-fluorescein
isothiocyanate (FITC; BestBio) and propidium iodide (PI; BestBio)
for 15 min at room temperature in the dark. The apoptosis rate was
analyzed using a flow cytometer (C6; Beckman Coulter Inc., Brea,
CA, USA) analyzed using Image Lab version 3.0 (Bio-Rad
Laboratories, Inc.).
Lactate dehydrogenase (LDH)
activity
LDH activity levels were measured using LDH activity
kits (C0016; Beyotime Institute of Biotechnology, Haimen, China).
The absorbance was measured by spectrophotometry with a microplate
reader (model 680; Bio-Rad Laboratories, Inc.) at 450 nm.
Caspase 3/9 activity assay
Endometrial stromal cells were lysed with lysis
buffer (radioimmunoprecipitation assay buffer) containing a
protease inhibitor cocktail (phenylmethanesulfonyl fluoride) and
EDTA at 4°C for 30 min. Cells were centrifuged at 12,000 × g for 10
min at 4°C. Subsequently, the concentration of total protein was
determined using a bicinchoninic acid (BCA) assay, and 10 µg/lane
total protein was used to analyze caspase 3/9 activity levels using
caspase 3/9 activity kits (C1115 and C1158, Beyotime Institute of
Biotechnology). The absorbance was measured by spectrophotometry
with a microplate reader (model 680; Bio-Rad Laboratories) at 405
nm.
Western blot analysis
Endometrial stromal cells were lysed with lysis
buffer (radioimmunoprecipitation assay buffer) containing protease
inhibitor cocktail (phenylmethanesulfonyl fluoride) and EDTA at 4°C
for 30 min. Cells were centrifuged at 12,000 × g for 10 min at 4°C.
Subsequently, the concentration of total protein was determined
using the BCA assay, and 40 µg/lane total protein was subjected to
10–12% SDS-PAGE and subsequently transferred onto a polyvinylidene
difluoride membrane (Bio-Rad Laboratories, Inc.). The membrane was
blocked with 5% non-fat milk in TBS containing 0.1% Tween-20 for 1
h at 37°C, and subsequently incubated with antibodies against Wnt
(sc-376029; 1:1,000), β-catenin (sc-65480; 1:1,000), ZEB1
(sc-515797; 1:1,000), apoptosis regulator BAX (Bax; sc-20067;
1:1;000) and GAPDH (sc-51631; 1:5,000; all from Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) overnight at 4°C. Following
this, membranes were incubated with anti-rabbit immunoglobulin G
secondary antibody (sc-2004; 1:5,000; Santa Cruz Biotechnology,
Inc.) at 37°C for 1 h and were developed using an enhanced
chemiluminescence kit (GE Healthcare Bio-Sciences, Pittsburgh, PA,
USA). Protein levels were quantified using Bio-Rad Laboratories,
Inc. Quantity One software (version 3.0).
Statistical analysis
All data are expressed as the mean ± standard error
using SPSS version 17.0 (SPSS, Inc., Chicago, IL, USA). Statistical
differences were measured using one way analysis of variance with
Bonferroni's correction for multiple comparisons. P<0.05 was
considered to indicate a statistically significant difference.
Results
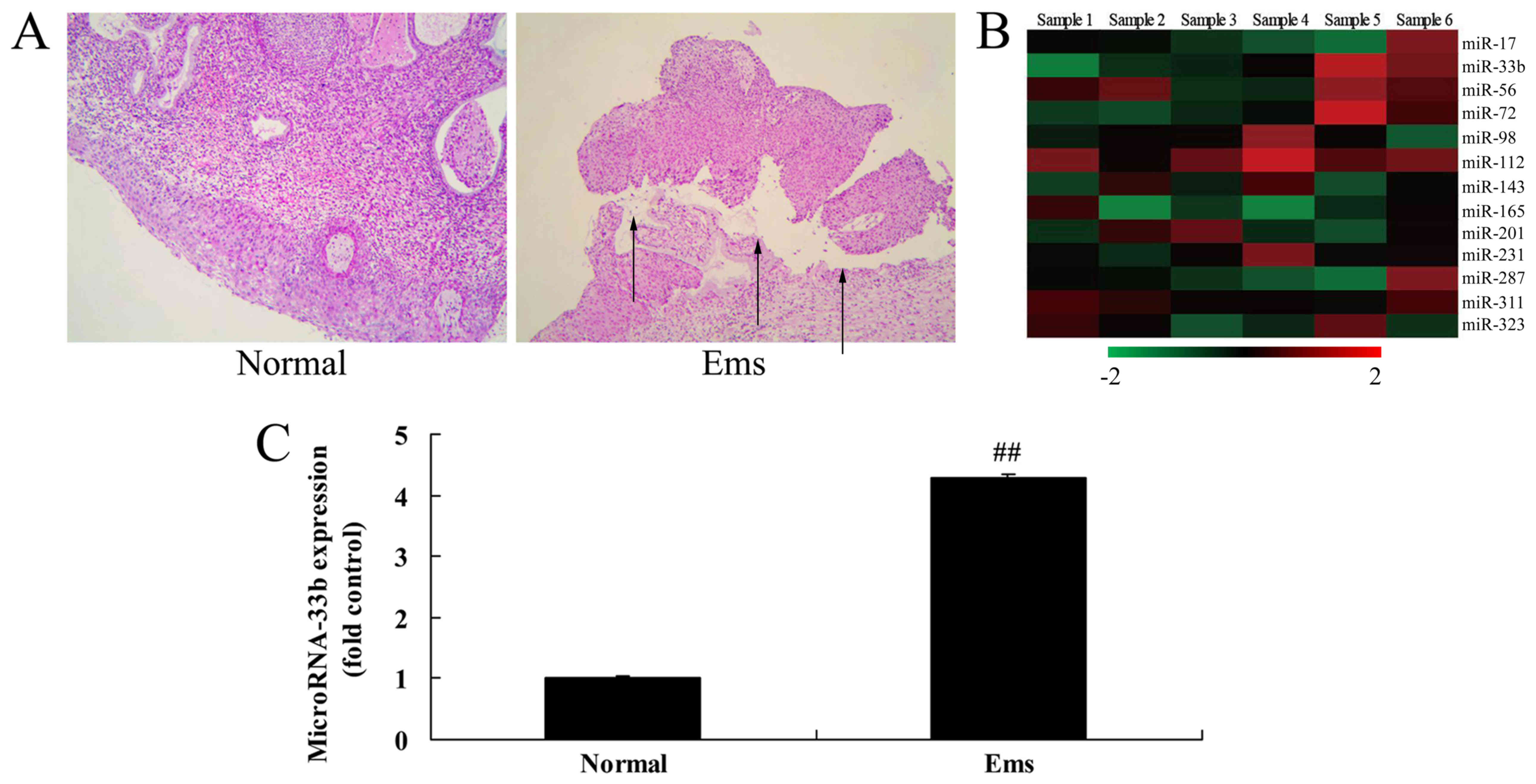
miR-33b expression in Ems
Firstly, H&E staining suggested that Ems was
successfully induced in the Ems group compared with the control
group (Fig. 1A). As demonstrated
in Fig. 1B and C, miR-33b
expression was upregulated by 4.29±0.05 fold in the Ems rat model
compared with the control group.
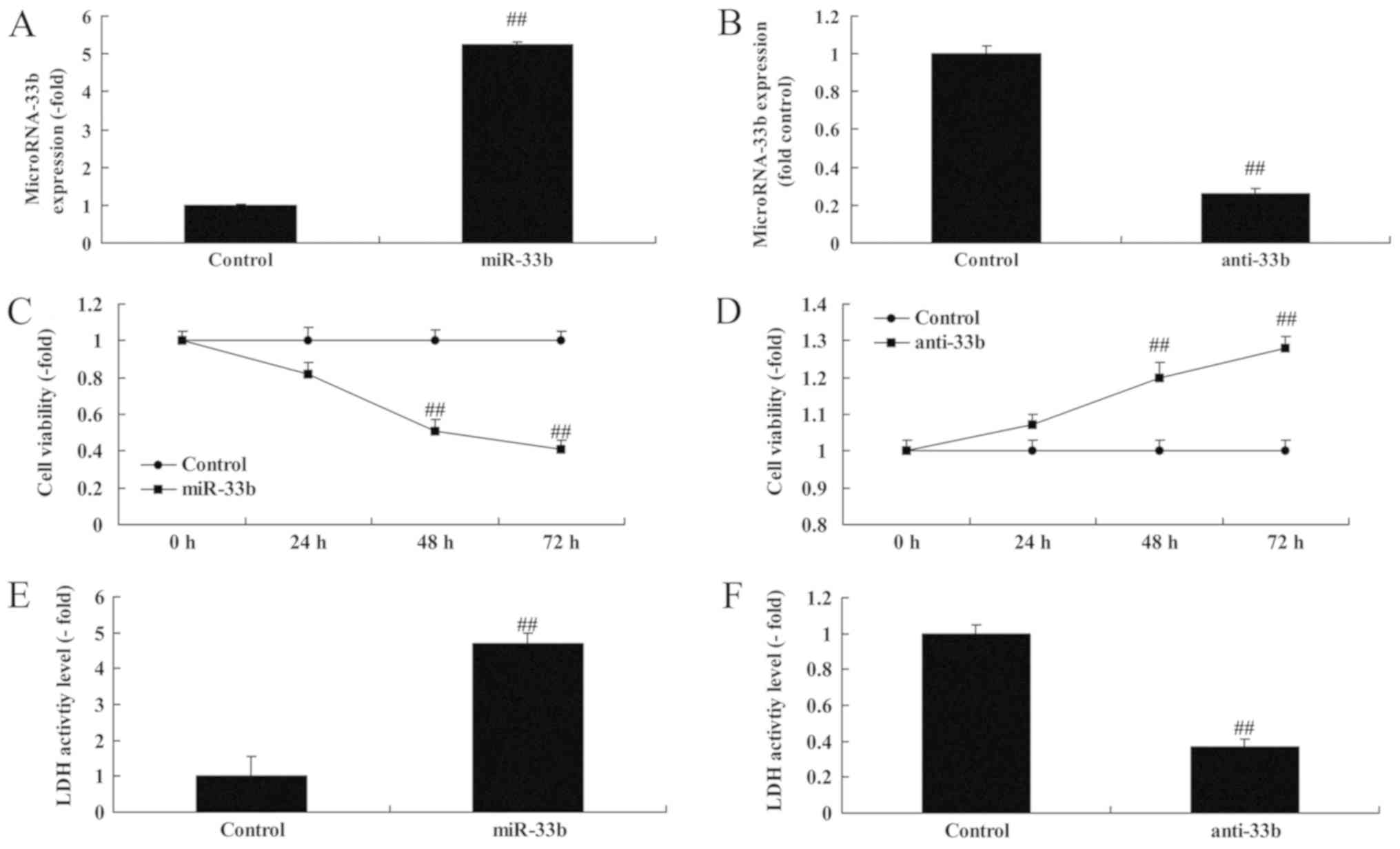
Effect of miR-33b on cell growth in
Ems
Notably, miR-33b expression was investigated using
miR-33b mimics or anti miR-33b mimics in vitro. Fig. 2A and B indicate that miR-33b
expression was upregulated by 5.26±0.04 fold or downregulated by
0.26±0.03 fold in the in vitro Ems model compared with the
control group. Overexpression of miR-33b suppressed cell viability
by 0.82±0.06 fold (48 h) or 0.51±0.06 fold (72 h), and enhanced the
lactate dehydrogenase (LDH) activity of Ems by 4.70±0.29 fold;
additionally, downregulation of miR-33b promoted cell viability by
1.23±0.06 fold (48 h) or 1.28±0.03 fold (72 h), and decreased the
LDH activity of Ems by 0.37±0.03 fold (Fig. 2C-F).
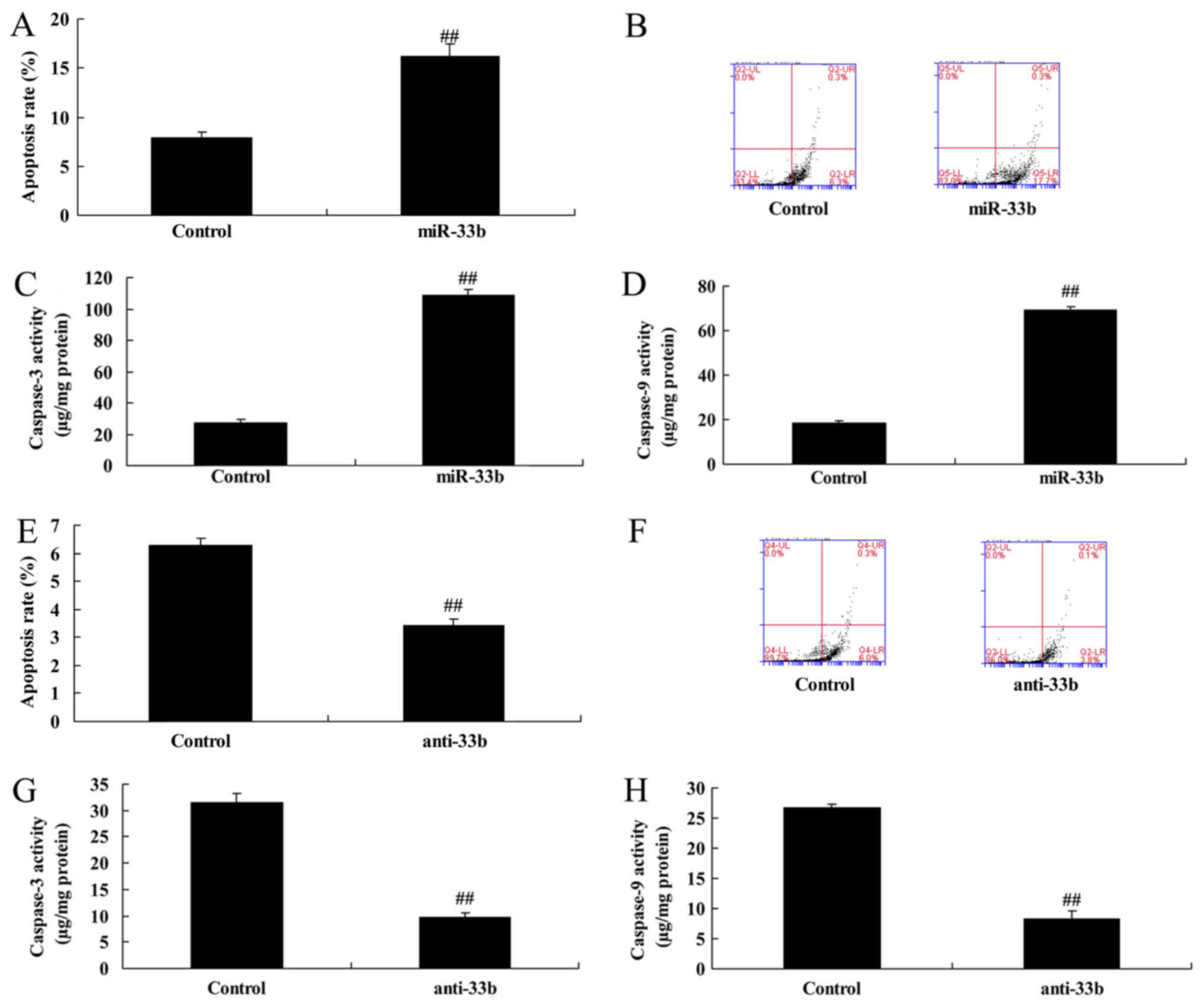
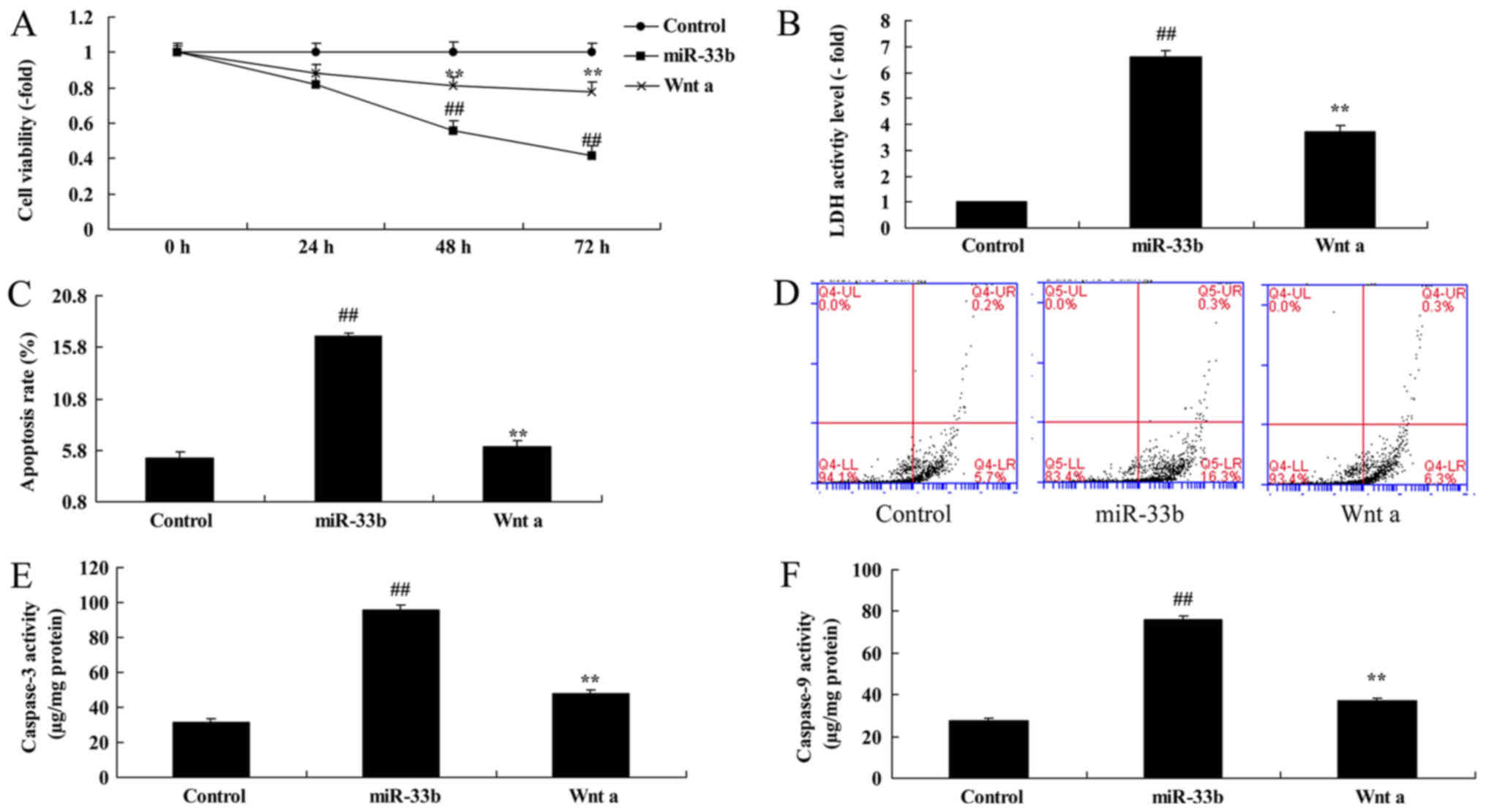
Effect of miR-33b on apoptosis in
Ems
Subsequently, it was demonstrated that
overexpression of miR-33b increased the apoptosis rate by
2.05±0.15-fold and promoted caspase-3 and 9 activity by 3.97±0.17
and 3.70±0.24-fold, respectively, in the Ems model compared with
the control group (Fig. 3A-D).
However, downregulation of miR-33b decreased the apoptosis rate by
0.54±0.04-fold (Fig. 3E and F) and
the caspase-3 and 9 activity by 0.39 ± 0.03 and 0.31 ± 0.05-fold,
respectively, in the Ems model (Fig.
3G and H).
Effect of miR-33b on Wnt/β-catenin in
Ems by ZEB1 expression
To analyze the mechanism of miR-33b in Ems, the
Wnt/β-catenin signaling pathway was investigated. It was
demonstrated that miR-33b was able to target the 3′UTR of ZEB1
(Fig. 4A). Subsequently,
overexpression of miR-33b suppressed ZEB1 protein expression by
0.34±0.04 fold, reduced Wnt and β-catenin protein expression by
0.44±0.06 and 0.32±0.12 fold, respectively, and induced Bax protein
expression by 2.56±0.13 fold in the in vitro Ems model
(Fig. 4B-F). Furthermore,
downregulation of miR-33b induced ZEB1 protein expression by
1.94±0.17 fold, reduced Wnt/β-catenin protein expression by
2.57±0.07 and 2.72±0.14 fold, respectively, and suppressed Bax
protein expression by 0.43±0.02 fold in the in vitro Ems
model compared with the control group (Fig. 4G-K).
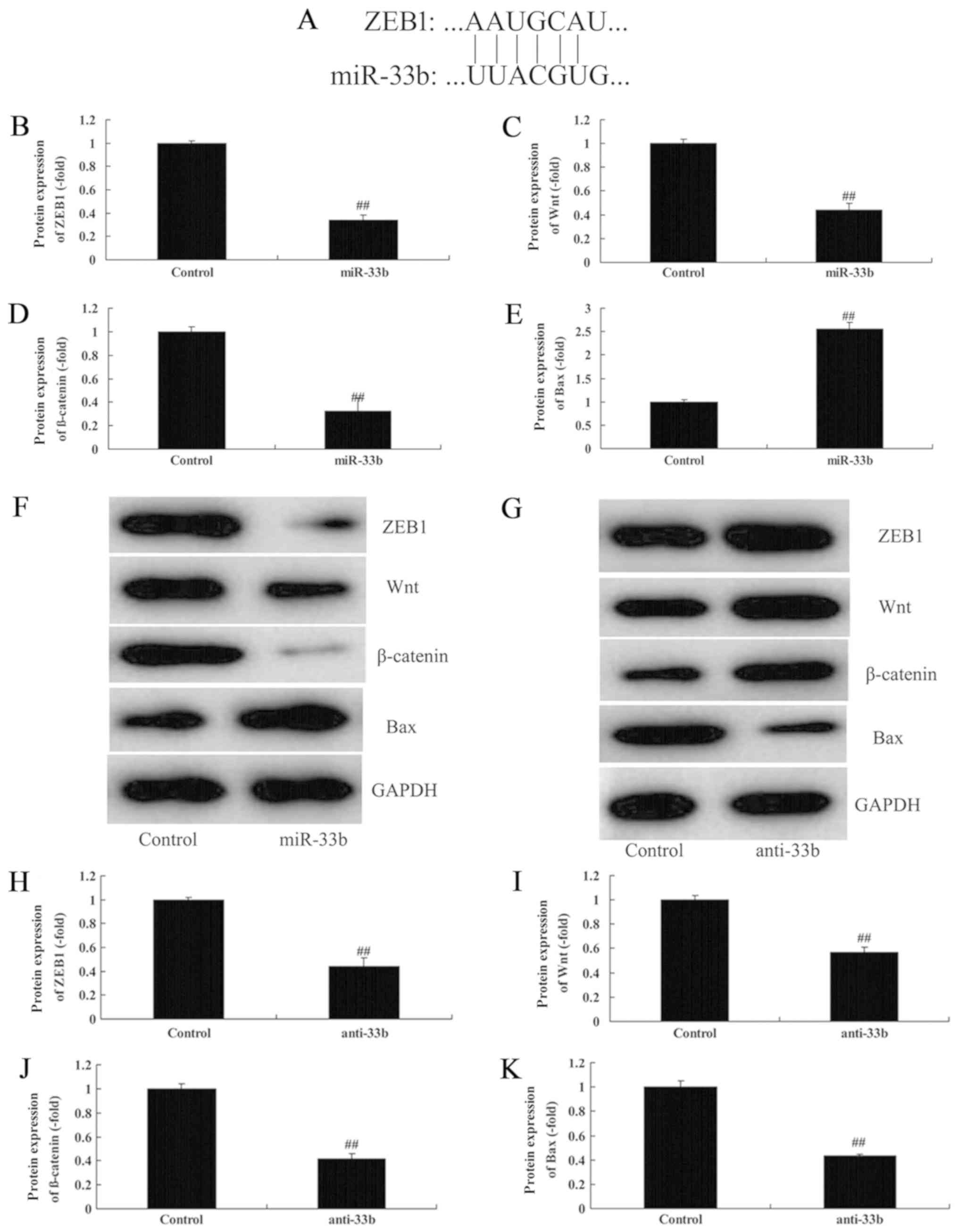 | Figure 4.Effect of miR-33b on Wnt/β-catenin in
Ems by ZEB1 expression. (A) miR-33b targets the 3′untranslated
region of ZEB1. Quantification of (B) ZEB1, (C) Wnt, (D) β-catenin
and (E) Bax protein expression levels, following overexpression of
miR-33b. (F) Representative western blotting image. (G) Western
blot analysis and quantification of (H) ZEB1, (I) Wnt, (J)
β-catenin and (K) Bax protein expression levels, following
downregulation of miR-33b. ##P<0.05 vs. control. Bax,
apoptosis regulator BAX; Ems, endometriosis; miR, microRNA; ZEB1,
zinc-finger E-box binding homeobox 1. |
ZEB1/Wnt/β-catenin influences the
effect of miR-33b in Ems
To examine the role of ZEB1/Wnt/β-catenin in the
effect of miR-33b on Ems, si-ZEB1 or Wnt agonist were
co-transfected with miR-33b mimics in Ems. As illustrated in
Fig. 5A-E, compared with the
miR-33b downregulation group, si-ZEB1 suppressed ZEB1 by 0.52±0.08
fold, decreased Wnt and β-catenin protein expression by 0.57±0.04
and 0.71±0.05 fold, respectively, and induced Bax protein
expression by 2.36±0.07 fold in the Ems model. Furthermore,
compared with the miR-33b overexpression group, the Wnt agonist
induced Wnt and β-catenin protein expression by 2.01±0.06 and
1.61±0.05 fold, respectively, and suppressed Bax protein expression
by 0.61±0.07-fold in the in vitro Ems model compared with
miR-33b (Fig. 5F-I).
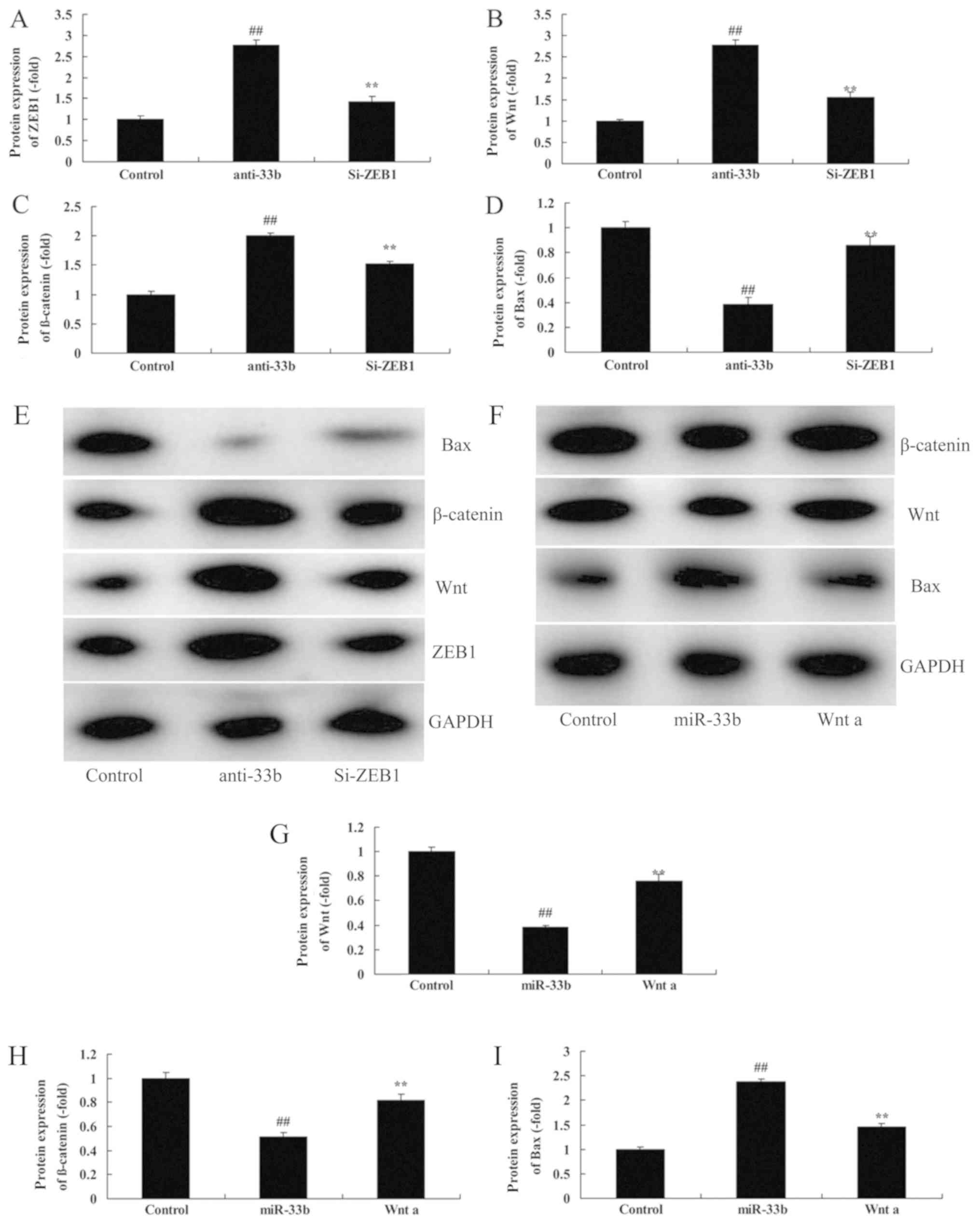 | Figure 5.ZEB1/Wnt/β-catenin mediates the effect
of miR-33b in Ems. Quantification of (A) Bax, (B) β-catenin, (C)
Wnt and (D) ZEB1 protein expression levels, following
downregulation of miR-33b. ##P<0.05 vs. control;
**P<0.05 vs. anti-33b. (E) Representative western blotting
image. (F) Western blot analysis and quantification of (G) Wnt, (H)
β-catenin, (I) and Bax protein expression levels, following
overexpression of miR-33b and treatment with a Wnt agonist.
##P<0.05 vs. control; **P<0.05 vs. miR-33b. Bax,
apoptosis regulator BAX; Ems, endometriosis; miR, microRNA; si,
small interfering; ZEB, zinc-finger E-box binding homeobox. |
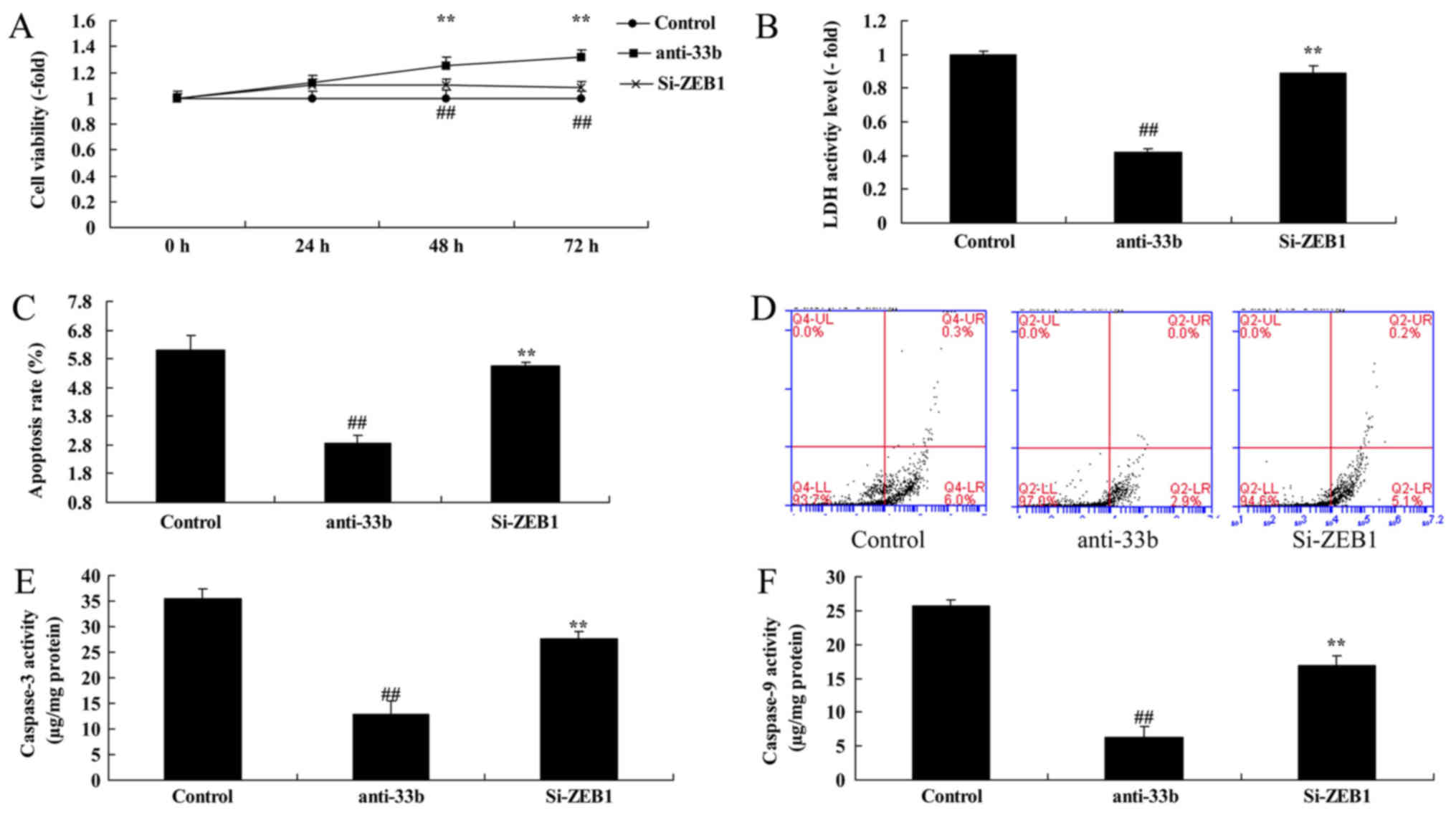
ZEB1 inhibition reduces the effect of
anti-miR-33b on cell viability in Ems
To gain insight into the function of miR-33b on Ems,
the effect of the ZEB1 inhibitor on the function of miR-33b in Ems
was analyzed. Compared with the miR-33b downregulation group, ZEB1
inhibition reduced cell viability by 0.81±0.05 (48 h) or
0.71±0.08-fold (72 h), increased LDH activity by 2.12±0.04 fold,
increased caspase-3 and 9 activity by 2.72±0.14 and 2.16±0.12-fold,
respectively, and apoptosis rate by 2.26±0.07 fold in the in
vitro Ems model (Fig. 6).
Wnt activation inhibits the effect of
miR-33b on cell viability in Ems
Furthermore, miR-33b mimics were co-transfected with
Wnt agonist in the in vitro Ems model. Compared with the
miR-33b overexpression group, Wnt activation inhibited cell growth
by 1.46±0.06 (48 h) or 1.86 ± 0.05 fold (72 h), LDH activity by
0.36±0.13 fold, caspase-3 and 9 activity by 0.39±0.03 and 0.48±0.12
fold, respectively, and apoptosis rate by 0.61±0.07 fold in the
in vitro Ems model following miR-33b (Fig. 7).
Discussion
Ems is a common gynecological disease, which has
similar features to malignancies, including cell growth, invasion,
distant metastasis and recurrence, affecting the quality of life of
patients (15). Ems is a benign
disease with malignant behavior. Its morbidity has exhibited an
increasing trend (15). However,
its pathogenesis remains unclear (15). miRNAs are endogenous RNAs with
regulatory functions discovered in eukaryotes and are able to
suppress gene expression at the transcriptional level (5). As important regulatory molecules,
miRNAs are involved in a series of vital life processes, including
antiviral defense, hematopoiesis, organogenesis, cell
proliferation, apoptosis, fat metabolism and tumorigenesis
(5). In the present study, it was
demonstrated that miR-33b expression was upregulated in an Ems rat
model. Wang and Ren (12)
demonstrated that miRNA-33b is able to mediate the apoptosis of
endometrial stromal cells. In the present study, miR-33b expression
was examined in a rat model, and miR-33b expression in vivo
may be analyzed in further studies.
A previous study has indicated that the
Wnt/β-catenin signaling pathway may induce epithelial-mesenchymal
transition (EMT) in epithelial cells (6). Research on the interaction between
β-catenin and estrogen is available. The Wnt/β-catenin signaling
pathway is highly conserved (16).
Additionally, a previous study has verified that the Wnt/β-catenin
signaling pathway serves a crucial role in ectopic endometrial cell
adhesion, invasion and angiogenesis (17). The intracellular accumulation of
β-catenin accounts for its principal mechanism of action (17). It activates its downstream target
genes, including vascular epithelial growth factor and matrix
metalloproteinases (MMPs), leading to abnormal cell proliferation,
differentiation and maturation (18). Of these genes, MMP-9 is able to
degrade extracellular matrix components, including type IV and type
V collagen and gelatin (19). In
addition, the Wnt/β-catenin signaling pathway may promote vascular
endothelial cell growth, thus leading to angiogenesis (6). Furthermore, the Wnt/β-catenin
signaling pathway is able to strengthen intercellular adhesion
through mutual activation with integrin and, as a result, it serves
a key role in ectopic adhesion, planting and the growth of
endometrial cells (6). The present
study demonstrated that overexpression of miR-33b may suppress ZEB1
protein expression and may reduce Wnt/β-catenin protein expression
in Ems in vitro. Wang et al (20) demonstrated that miRNA-33b inhibited
lung adenocarcinoma cell growth and invasion by suppressing the
Wnt/β-catenin/ZEB1 signaling pathway. These results are consistent
with the present ones, demonstrating that miRNA-33b may regulate
the ZEB1/Wnt/β-catenin signaling pathway to induce cell death in
Ems.
High ZEB1 expression has been demonstrated in
numerous malignancies, including lung, colorectal, prostate and
ovarian cancer (21). ZEB1 is able
to promote the malignant phenotype of Ems primarily by regulating
EMT (11). Similarly, high ZEB1
expression may be detected in breast cancer, and may regulate
uterine cell adhesion and polarity alterations (21). Furthermore, it may promote the
abnormal proliferation of uterine stem cells (22). A previous study demonstrated that
ZEB1 may regulate estrogen receptor-α silencing (22). A previous study indicated that ZEB1
may also be involved in epigenetic regulation during Ems (21). In the present study, it was
demonstrated that ZEB1 inhibition decreased the effect of
anti-miR-33b on cell growth in Ems. Wang et al (20) demonstrated that miRNA-33b inhibited
lung adenocarcinoma cell growth and invasion by suppressing
Wnt/β-catenin/ZEB1 signaling. Wang et al (12) reported that the
miR-33b/HMGA2/Twist1/ZEB1 axis serves a critical role in regulating
melanoma dissemination. The results of the present study suggested
that miRNA-33b/ZEB1/Wnt/β-catenin inhibited cell growth in an Ems
rat model. However, the downstream molecular pathway of
miRNA-33b/ZEB1/Wnt/β-catenin requires further study.
In conclusion, the present results demonstrated that
upregulation of miRNA-33b may promote Ems via Wnt/β-catenin by ZEB1
expression. Thus, restoration of miRNA-33b expression may be used
as a novel strategy for the treatment of Ems, although this
requires further investigation.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
SZ designed the experiment; HZ, GL and XS performed
the experiment; SZ and HZ analyzed the data; SZ wrote the
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Animal Care
and Use Committee of Affiliated Qilu Hospital of Shandong
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kang JL, Wang XX, Nie ML and Huang XH:
Efficacy of gonadotropin-releasing hormone agonist and an
extended-interval dosing regimen in the treatment of patients with
adenomyosis and endometriosis. Gynecol Obstet Invest. 69:73–77.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Soto E, Luu TH, Liu X, Magrina JF, Wasson
MN, Einarsson JI, Cohen SL and Falcone T: Laparoscopy vs. Robotic
Surgery for Endometriosis (LAROSE): A multicenter, randomized,
controlled trial. Fertil Steril. 107:996–1002 e1003. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nematian SE, Mamillapalli R, Kadakia TS,
Majidi Zolbin M, Moustafa S and Taylor HS: Systemic inflammation
induced by microRNAs: Endometriosis derived alterations in
circulating microRNA 125b-5p and let7b-5p regulate macrophage
cytokine production. J Clin Endocrinol Metab. 103:64–74. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shen L, Yang S, Huang W, Xu W, Wang Q,
Song Y and Liu Y: MicroRNA23a and microRNA23b deregulation
derepresses SF-1 and upregulates estrogen signaling in ovarian
endometriosis. J Clin Endocrinol Metab. 98:1575–1582. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wright KR, Mitchell B and Santanam N:
Redox regulation of microRNAs in endometriosis-associated pain.
Redox Biol. 12:956–966. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang L, Xiong W, Xiong Y, Liu H, Li N, Du
Y and Liu Y: Intracellular wnt/beta-catenin signaling underlying
17beta-estradiol-induced matrix metalloproteinase 9 expression in
human endometriosis. Biol Reprod. 94:702016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pazhohan A, Amidi F, Akbari-Asbagh F,
Seyedrezazadeh E, Farzadi L, Khodarahmin M, Mehdinejadiani S and
Sobhani A: The Wnt/β-catenin signaling in endometriosis, the
expression of total and active forms of β-catenin, total and
inactive forms of glycogen synthase kinase-3β, WNT7a and
DICKKOPF-1. Eur J Obstet Gynecol Reprod Biol. 220:1–5. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang P, Sun Y and Ma L: ZEB1: At the
crossroads of epithelial-mesenchymal transition, metastasis and
therapy resistance. Cell Cycle. 14:481–487. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schmalhofer O, Brabletz S and Brabletz T:
E-cadherin, beta-catenin, and ZEB1 in malignant progression of
cancer. Cancer Metastasis Rev. 28:151–166. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Browne G, Sayan AE and Tulchinsky E: ZEB
proteins link cell motility with cell cycle control and cell
survival in cancer. Cell Cycle. 9:886–891. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Eggers JC, Martino V, Reinbold R, Schäfer
SD, Kiesel L, Starzinski-Powitz A, Schüring AN, Kemper B, Greve B
and Götte M: microRNA miR-200b affects proliferation, invasiveness
and stemness of endometriotic cells by targeting ZEB1, ZEB2 and
KLF4. Reprod Biomed Online. 32:434–445. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang ZH, Zhang JL, Duan YL, Zhang QS, Li
GF and Zheng DL: MicroRNA-214 participates in the neuroprotective
effect of resveratrol via inhibiting α-synuclein expression in
MPTP-induced Parkinson's disease mouse. Biomed Pharmacother.
74:252–256. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Maegdefessel L, Spin JM, Raaz U, Eken SM,
Toh R, Azuma J, Adam M, Nakagami F, Heymann HM, Chernogubova E, et
al: miR-24 limits aortic vascular inflammation and murine abdominal
aneurysm development. Nat Commun. 5:52142014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nothnick WB: MicroRNAs and endometriosis:
Distinguishing drivers from passengers in disease pathogenesis.
Semin Reprod Med. 35:173–180. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Matsuzaki S and Darcha C: Involvement of
the Wnt/β-catenin signaling pathway in the cellular and molecular
mechanisms of fibrosis in endometriosis. PLoS One. 8:e768082013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tanwar PS, Lee HJ, Zhang L, Zukerberg LR,
Taketo MM, Rueda BR and Teixeira JM: Constitutive activation of
Beta-catenin in uterine stroma and smooth muscle leads to the
development of mesenchymal tumors in mice. Biol Reprod. 81:545–552.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang L, Xiong W, Xiong Y, Liu H and Liu
Y: 17 beta-Estradiol promotes vascular endothelial growth factor
expression via the Wnt/β-catenin pathway during the pathogenesis of
endometriosis. Mol Hum Reprod. 22:526–535. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Matsuzaki S and Darcha C: In vitro effects
of a small-molecule antagonist of the Tcf/ß-catenin complex on
endometrial and endometriotic cells of patients with endometriosis.
PLoS One. 8:e616902013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang S and Ren D: Allicin protects
traumatic spinal cord injury through regulating the HSP70/Akt/iNOS
pathway in mice. Mol Med Rep. 14:3086–3092. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Furuya M, Masuda H, Hara K, Uchida H, Sato
K, Sato S, Asada H, Maruyama T, Yoshimura Y, Katabuchi H, et al:
ZEB1 expression is a potential indicator of invasive endometriosis.
Acta Obstet Gynecol Scand. 96:1128–1135. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Panda H, Pelakh L, Chuang TD, Luo X,
Bukulmez O and Chegini N: Endometrial miR-200c is altered during
transformation into cancerous states and targets the expression of
ZEBs, VEGFA, FLT1, IKKbeta, KLF9, and FBLN5. Reprod Sci.
19:786–796. 2012. View Article : Google Scholar : PubMed/NCBI
|