Introduction
Intervertebral disc degeneration (IDD) is one of the
major causes of lower back pain and spinal degenerative diseases.
Globally, the point prevalence of lower back pain was reported to
be 9.4%, which ranked the highest regarding the number of years
living with the condition (1).
Recently, the morbidity from spinal degenerative diseases has
rapidly increased with the aging of the population; at present,
therapies mainly focus on alleviating clinical symptoms rather than
restoring the underlying pathophysiological processes. Therefore,
further investigation into IDD is urgently required (2).
Previous studies demonstrated that nucleus pulposus
cell (NPC) apoptosis serves a significant role in the occurrence
and development of IDD (3–6). The apoptosis of NPCs initiates
degenerative cascades in the aging nucleus pulposus (NP), and leads
to structural and mechanical instability of the intervertebral disc
(7,8). Therefore, the anti-apoptotic
targeting of NPCs by molecular or cellular therapies in the
intervertebral disc via percutaneous puncture may delay or reverse
the process of IDD.
Noncoding RNAs, including microRNAs (miRNAs) and
long noncoding RNAs (lncRNAs), regulate gene expression in numerous
cells, and have been demonstrated to be highly involved in IDD
(9). In NPCs, lower levels of
miR-155 expression promoted Fas-mediated apoptosis by targeting
Fas-associated protein with death domain and caspase-3 (10), and decreased the amount of aggrecan
and collagen type II via matrix metalloproteinase (MMP)-16
upregulation (11). Additionally,
increasing evidence has suggested the important roles of lncRNAs in
IDD, which may serve as novel therapeutic targets for degenerative
spinal diseases (12,13). In addition, the sophisticated
crosstalk between miRNAs and lncRNAs also suggests their notable
functions of coordinating gene expression in a multitude of
processes (14); however,
investigations into the interactions between miRNAs and lncRNAs in
IDD are scarce.
The growth arrest-specific transcript 5 (GAS5) gene
was firstly isolated by Schneider et al (15) in growth-inhibited cells in 1988 and
was classified as a non-protein-coding multiple small nucleolar RNA
that serves a major growth-inhibiting role (16). Numerous studies reported that GAS5
was pivotal in promoting apoptosis and suppressing cell
proliferation in mammals (17–19);
however, the function of GAS5 in IDD remains unknown. In the
present study, lncRNAs targeted by miR-155 were screened and the
lncRNA GAS5 was predicted to be a target of miR-155 in NPCs.
Therefore, the functional mechanism of GAS5 was investigated to
reveal the effects of GAS5 on primary human NPC apoptosis.
Interestingly, GAS5 overexpression promoted NPC apoptosis via B
cell lymphoma 2 (Bcl-2) downregulation and caspase-3 upregulation.
The findings of the present study indicated that GAS5 may be a
novel therapeutic target for IDD due to its pro-apoptotic
effects.
Materials and methods
Cell culture
Intervertebral disc-derived primary human NPCs were
obtained from ScienCell Research Laboratories, Inc. (cat. no. 4800)
(20,21), and cultured in NPC medium (cat. no.
4801; both ScienCell Research Laboratories, Inc., San Diego, CA,
USA) at 37°C in a humidified atmosphere containing 5%
CO2. At ~80-90% confluence, cells were passaged at a
ratio of ~1:2-3.
Prediction of miR-155 targeting
lncRNAs by TargetScan
A total of three miRNA target gene prediction
software programs were compared, including TargetScan version 7.0
(http://www.targetscan.org/), PITA
version 6 (https://omictools.com/pita-tool) and PicTar
(https://pictar.mdc-berlin.de/); analysis
with TargetScan was selected for its high sensitivity and accuracy
in analyzing non-conserved domains (22,23).
The miRNAs targeting lncRNAs were screened based on the method of
conserved seed pairing via TargetScan analysis; however, during the
prediction of miRNA-targeted lncRNAs, the TargetScan Perl script
was run by replacing the mRNA sequences with lncRNA sequences. In
the present study, NPC apoptosis-associated lncRNAs were screened
by searching the lncRNAs targeted by miR-155. miR-155 expression is
decreased in the degenerative NP (10,11);
866 upregulated lncRNAs were confirmed as subjects for analysis in
the present study. Additionally, retrieval with TargetScan version
7.0, Ensembl Genome Browser (http://asia.ensembl.org/index.html), UCSC Genome
Browser (http://genome.ucsc.edu/), and miRBase
release 21 (http://www.mirbase.org/) further
confirmed the sequence of 631 lncRNAs used for bioinformatics
analysis.
Transfection of miR-155 mimics
The sequence of mature hsa-miR-155-5p (MIMAT0000646)
was retrieved from the miRBase database (http://www.mirbase.org/). Double-chain hsa-miR-155-5p
and double-chain negative control (NC) RNA were designed and
synthesized by Shanghai GenePharma Co., Ltd. (Shanghai, China). The
synthesized hsa-miR-155-5p mimics were: Sense,
5′-UUAAUGCUAAUCGUGAUAGGGGU-3′ and antisense,
5′-CCCUAUCACGAUUAGCAUUAAUU-3′. The double-chain NC RNA sequences
were: Sense, 5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense,
5′-ACGUGACACGUUCGGAGAATT-3′. The day prior to transfection, NPCs
were seeded into a 24-well plate at a density of
0.5×105/well in 500 µl NPC medium. At ~70% confluence,
NPCs were transfected with 1 µl double-chain hsa-miR-155-5p (20 µM)
and double-chain NC RNA (20 µM), respectively, using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). After 72 h of incubation at
37°C, the cells were assessed by reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) as described below.
Lentivirus-mediated hsa-miR-155
overexpression
Lentivirus-mediated miR-155 overexpression reagents
were ordered from Shanghai (Lingke) Biotechnology Co., Ltd.
(Shanghai, China), Packaging 293T cells were transfected with
plasmids comprised of pRsv-REV, pMDlg-pRRE, pMD2G and transfer
vector: pLenO-GTP. The NPCs transfected miR-155 overexpression
exhibited green fluorescence under fluorescence microscopy due to
the element of pLenO-GTP expressing green fluorescence protein.
Using fluorescence microscopy (magnification, ×40; Olympus
IX73-DP80; Olympus Corporation, Tokyo, Japan), the efficiency of
transduction was evaluated by calculating the percentage of green
fluorescent protein-positive NPCs in 10 fields of view near the
center of the culture vessel. The day prior to transduction, NPCs
were seeded into a 6-well plate at a density of
5.0×105/well in 2.5 ml NPC medium and incubated under a
humidified atmosphere with 5% CO2 at 37°C. At ~60%
confluence, NPCs were transduced with miR-155 or a NC at a
multiplicity of infection (MOI) of 80. After 96 h post-incubation,
total RNA was extracted and assessed via an lncRNA array as
described below. The efficiency of transduction was determined
successful when >95% at 96 h after transduction.
Human lncRNA plus mRNA array
Total RNA was extracted using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) from
lentivirus-mediated miR-155 overexpression NPCs and NC groups. The
purity and concentration, as determined by the optical density (OD)
260/OD280 ratio, was ≥1.90 with total RNA ≥1 µg per sample, which
was assessed with a NanoDrop 1000 device (NanoDrop Technologies;
Thermo Fisher Scientific, Pittsburgh, PA, USA). Denaturing
formaldehyde agarose gel electrophoresis (percentage of 1.2%)
demonstrated clear RNA bands, with 28S to 18S exceeding 2 bands and
good RNA integrity (data not shown). Then, lncRNA array detection
was performed by Beijing Capitalbio Technology Co., Ltd. (Beijing,
China).
The Agilent human lncRNA + mRNA Array v4.0 (Agilent
Technologies, Inc., Santa Clara, CA, USA) was used to detect
lncRNAs in the present model system. Cy5 and Cy3 were used to label
the molecule types on the same array at 37°C for 1.5 h and 70°C for
5 min, respectively. The Agilent array contained probes capable of
detecting ~41,000 human lncRNAs and 34,000 human mRNAs, in a 4×180
K format. The array also contained 4,974 internal Agilent control
probes. The experiments were performed according to the
manufacturer's protocols and conducted twice. The fluorescent
signals of Cy3 and Cy5 were obtained, and differentially expressed
mRNAs and lncRNAs were screened, at a fold change cutoff of 2 and
P<0.05.
Lentivirus-mediated GAS5
overexpression
Lentivirus-mediated GAS5 overexpression reagents
were ordered from Lingke Biotechnology Co., Ltd., by using
packaging 293T cells transfected with plasmids comprised of
pRsv-REV, pMDlg-pRRE, pMD2G and transfer vector: pLenO-GTP. The
NPCs with GAS5 overexpression exhibited green fluorescence for the
element of pLenO-GTP expressing green fluorescence protein. NPCs
were seeded into a 6-well plate at a density of
5.0×105/well in 2.5 ml NPC medium and incubated for 24 h
under a humidified atmosphere with 5% CO2 at 37°C. At
~60% confluence, NPCs were transduced with GAS5 (NR_002578.2;
http://www.ncbi.nlm.nih.gov/nuccore/NR_002578.2)
or NC (empty vector) at an MOI of 80. After 96 h post-incubation,
GAS5 expression was assessed by RT-qPCR.
RT-qPCR
Total RNA was extracted from NPCs using TRIzol
reagent; the purity and quantity of RNA were assessed using a
NanoDrop 1000 system. RT was performed on an ABI Veriti gradient
PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.)
using a PrimeScript™ RT-PCR kit (Takara Bio, Inc., Otsu,
Japan), the reaction conditions were: 30°C for 10 min, 42°C for 30
min and 72°C for 15 min. The following primers were used:
Hsa-miR-155-5p forward, 5′-GGGGGTAATGCTAATCGTGAT-3′ and reverse,
5′-GTGCGTGTCGTGGAGTCG-3′; U6 forward,
5′-GCTTCGGCAGCACATATACTAAAAT-3′ and reverse,
5′-CGCTTCACGAATTTGCGTGTCAT-3′; lncRNA GAS5 sense,
5′-GCTTACTGCTTGAAAGGGTCT-3′ and antisense,
5′-CACTGGGAGGCTGAGGAT-3′; β-actin sense,
5′-GCACCACACCTTCTACAATGAG-3′ and antisense,
5′-ACAGCCTGGATAGCAACGT-3′. qPCR was performed on a Light Cycler 480
II RT-PCR system (Roche Diagnostics, Indianapolis, IN, USA) with
SYBR-Green I (Takara Bio, Inc.). The reaction conditions were: 95°C
for 30 sec, 95°C for 5 sec and 60°C for 30 sec (40 cycles). Each
experiment was repeated three times, the relative mRNA expression
levels were evaluated by the 2−ΔΔCq method (24).
Cell apoptosis detection by flow
cytometry
NPCs exhibited green fluorescence following
lentiviral transduction, Annexin V-allophycocyanin (eBioscience;
Thermo Fisher Scientific, Inc.) was used for cytomembrane staining
(NPCs were stained for 25 min at room temperature without light),
and propidium iodide (eBioscience; Thermo Fisher Scientific, Inc.)
was used for nuclear staining (NPCs were stained for 5 min at room
temperature). Subsequently, the transfection efficiency and
apoptosis of NPCs were assessed using a CytoFLEX flow cytometer
(Beckman Coulter, Inc., Brea, Ca, USA). Data were analyzed via the
FlowJo 7.6.1 software (FlowJo LLC, Ashland, OR, USA). NPCs stained
with Annexin V-allophycocyanin without propidium iodide were
considered as early apoptotic NPCs, and the early apoptosis rate
was determined by calculating the percentage of NPCs in the lower
right quadrant of the scatter diagram.
Western blotting
Total protein from NPCs was extracted following
lysis with radioimmunoprecipitation assay buffer (Thermo Fisher
Scientific, Inc.), and quantified with a Bicinchoninic Acid protein
assay. Then, 40 µg total protein from NPCs were loaded and
separated by 15% SDS-PAGE and transferred onto polyvinylidene
difluoride membranes (Merck KGaA, Darmstadt, Germany). Following
blocking with 5% bovine serum albumin for 2 h at room temperature,
rabbit anti-human caspase-3, rabbit anti-human Bcl-2 and mouse
anti-human β-actin polyclonal antibodies (cat. nos. 9665, 2870 and
3700; 1:1,000; Cell Signaling Technology, Inc., Danvers, MA, USA)
were added respectively overnight at 4°C. Then, membranes were
washed with tris-buffered saline with 0.1% Tween-20 and incubated
with anti-rabbit secondary antibodies conjugated to horseradish
peroxidase (cat. no. 7074; 1:1,000) for rabbit anti-human caspase-3
and rabbit anti-human Bcl-2 antibodies and anti-mouse secondary
antibodies conjugated to horseradish peroxidase for mouse
anti-human β-actin antibody (cat. no. 7076; 1:1,000; both Cell
Signaling Technology, Inc.). Finally, the membranes were developed
with an enhanced chemiluminescence reagent (Pierce™ ECL
Western Blotting Substrate, Pierce; Thermo Fisher Scientific,
Inc.); protein bands were imaged under a FluorChem FC2 visualizer
(Alpha Innotech, San Leandro, CA, USA).
Statistical analysis
SSPSS 19.0 (IBM Corp., Armonk, NY, USA) was used for
data analysis. Data were presented as the mean ± standard deviation
from three independent experiments performed in triplicate.
Comparisons between groups were conducted using an Independent
Samples t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Overexpression of miR-155 in NPCs
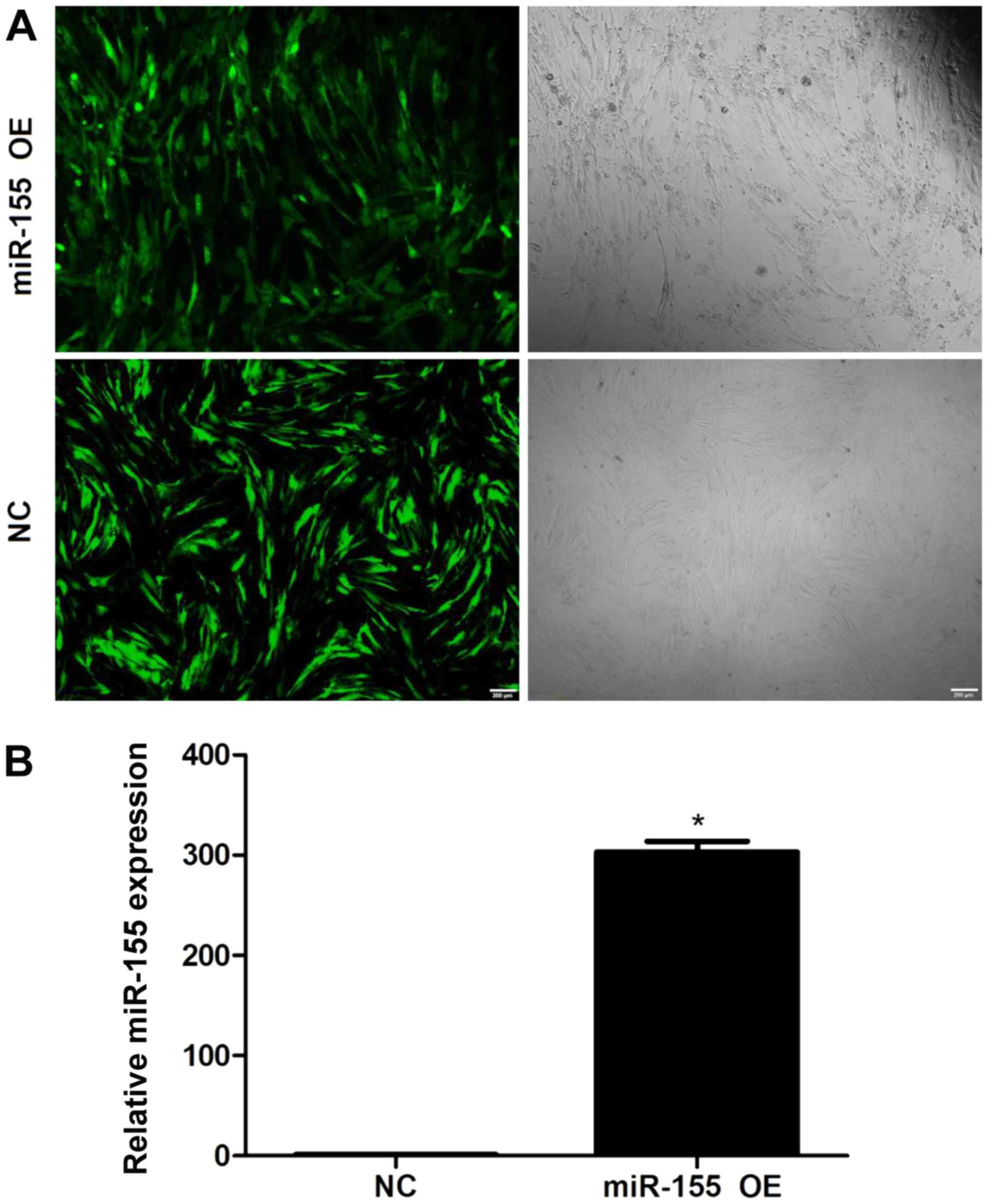
NPCs were transduced with lentiviral vectors
(Fig. 1). Under fluorescence
microscopy, the transduction efficiencies were determined to be
>95% (Fig. 1A). In addition,
miR-155 expression levels in lentiviral-transduced NPCs were
assessed by RT-qPCR; significantly higher miR-155 expression levels
were detected in NPCs transduced with miR-155 compared with the
control group (Fig. 1B). These
findings indicated a highly efficient and successful overexpression
of miR-155 in transduced NPCs.
Prediction of miR-155 targeted
lncRNAs
TargetScan data analysis revealed that among the 631
upregulated lncRNAs with defined sequences, there were 148
differentially expressed lncRNAs with 178 hsa-miR-155-5p binding
sites in total (data not shown). Among these, NR_002819 with 8,707
nucleotides, which included 6 hsa-miR-155-5p binding sites;
ENST00000511037 and NR_027451 contained 1801 and 5343 nucleotides,
respectively, which included 5 hsa-miR-155-5p binding sites. In
addition, there were 19 lncRNAs with 2 hsa-miR-155-5p binding sites
and 126 lncRNAs containing 1 hsa-miR-155-5p binding site.
Furthermore, NR_002578 was observed to contain a hsa-miR-155-5p
binding site and was also predicted to be a target of miR-155.
Additionally, a literature review indicated that NR_002578 is an
lncRNA transcribed from GAS5 and is highly expressed in NPCs of the
degenerative intervertebral disc (12), with a tumor suppressor role in a
variety of tumors, promoting tumor cell apoptosis (19).
miR-155 overexpression inhibits GAS5
expression in NPCs
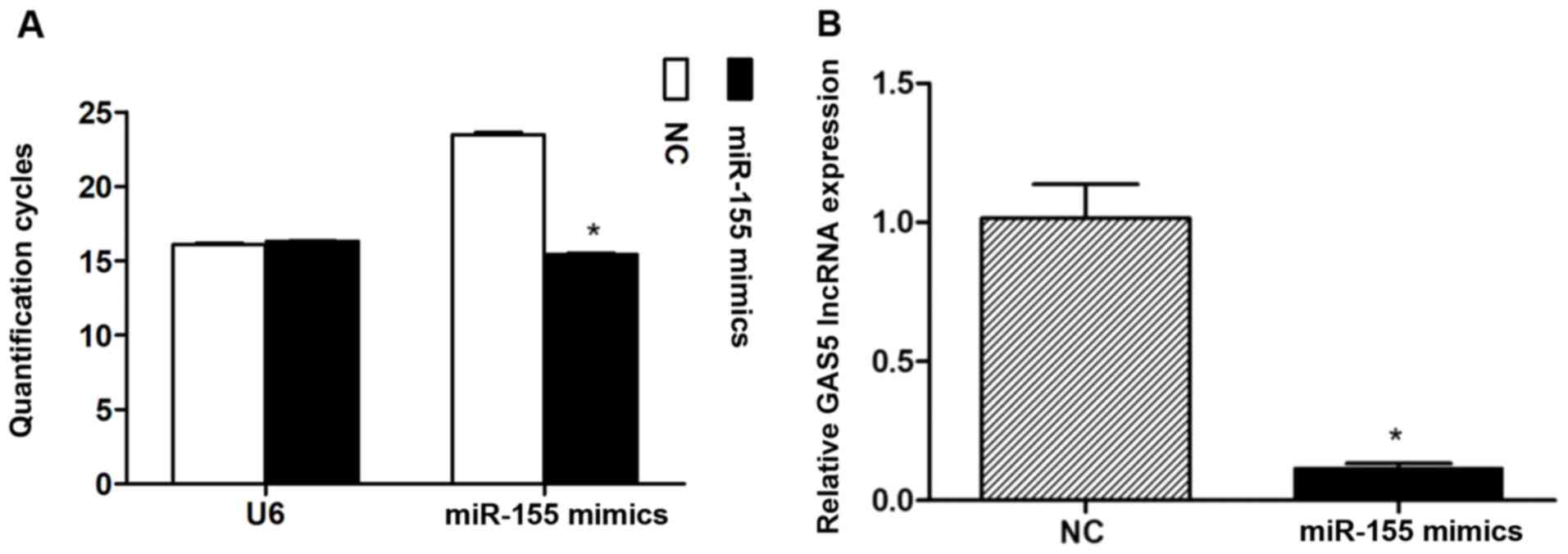
After ~72 h following miR-155 mimics transfection
into NPCs (Fig. 2A), the overall
GAS5 expression levels were determined. The results revealed
significantly lower expression levels of GAS5 compared with the NC
group (Fig. 2B).
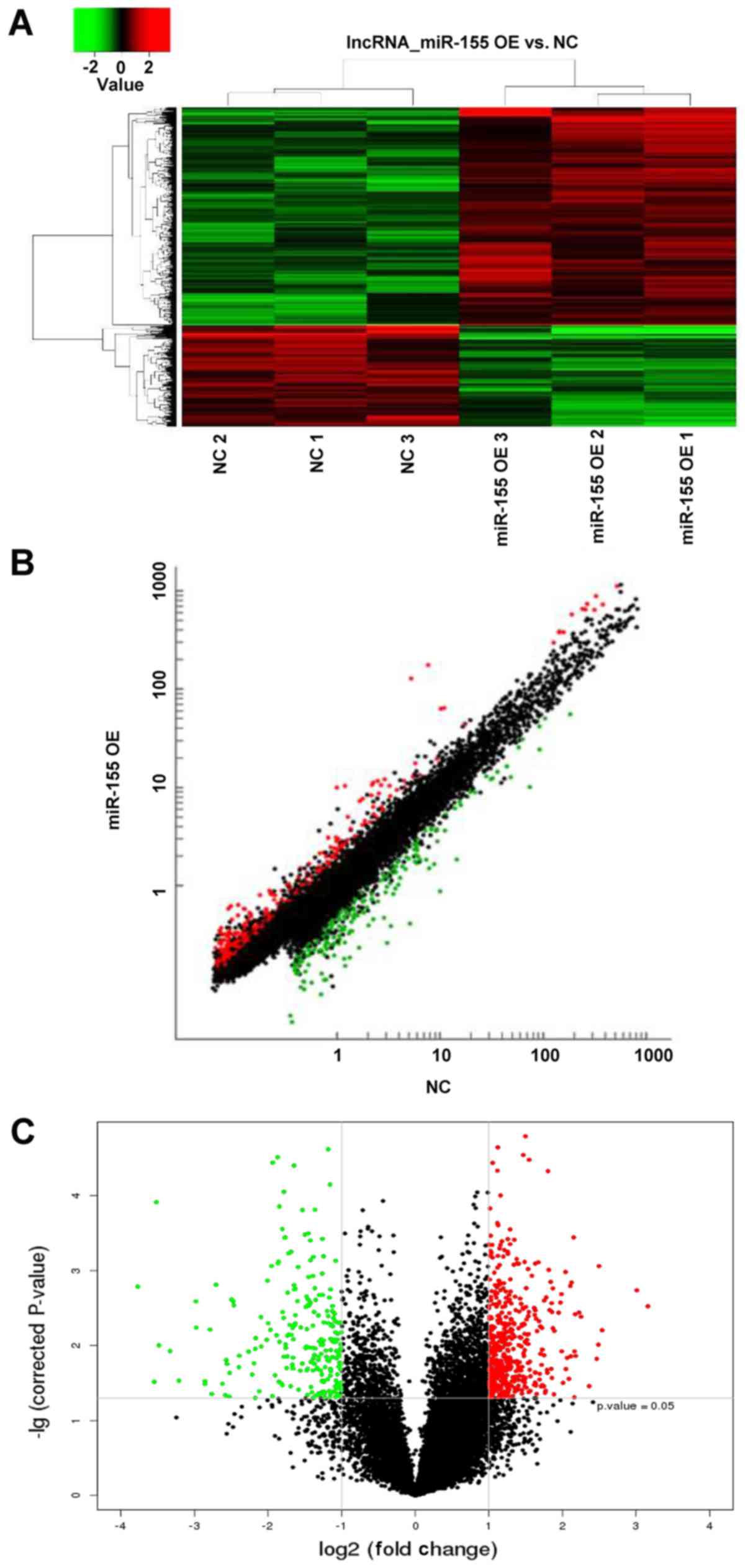
LncRNA array analysis in NPCs
overexpressing miR-155
LncRNA array analysis revealed 721 differentially
expressed lncRNAs following transduction with miR-155 lentiviruses,
492 upregulated and 229 downregulated lncRNAs, respectively
(Fig. 3). A total of 18 GAS5
transcripts were detected, and each individual transcript showed
downregulated expression (Table
I); however, the fold changes of each individual transcript did
not exhibit significance. The results suggested that different
overexpression mehtods using mimics or lentiviruses may affect GAS5
expression.
 | Table I.Expression of 29 transcripts of GAS5
lncRNA when overexpressing miR-155 in NPCs. |
Table I.
Expression of 29 transcripts of GAS5
lncRNA when overexpressing miR-155 in NPCs.
| Name | Transcript ID | Probe | Expression | P-value | Fold change |
|---|
| GAS5-001 |
ENST00000450589 | –a | – | – | – |
| GAS5-002 |
ENST00000431268 | p28959 | Downregulated | 0.38 | 1.12 |
| GAS5-003 |
ENST00000448718 | – | – | – | – |
| GAS5-004 |
ENST00000436656 | p33995_v4 | Downregulated | 0.26 | 1.17 |
| GAS5-005 |
ENST00000458220 | p547 | Downregulated | – | – |
| GAS5-006 |
ENST00000421068 | p545 | Downregulated | 0.76 | 1.08 |
| GAS5-007 |
ENST00000456293 | p543 | Downregulated | 0.43 | 1.15 |
| GAS5-008 |
ENST00000449289 | – | – | – | – |
| GAS5-009 |
ENST00000455838 | p541 | Downregulated | 0.45 | 1.13 |
| GAS5-010 |
ENST00000449589 | – | – | – | – |
| GAS5-011 |
ENST00000443799 | p544 | Downregulated | 0.66 | 1.08 |
| GAS5-012 |
ENST00000416952 | p542 | Downregulated | 0.49 | 1.12 |
| GAS5-013 |
ENST00000452197 | – | – | – | – |
| GAS5-014 |
ENST00000454068 | p33998_v4 | Downregulated | 0.19 | 1.36 |
| GAS5-015 |
ENST00000451607 | p34000_v4 | Downregulated | 0.09 | 1.35 |
| GAS5-016 |
ENST00000432536 | p34001_v4 | Downregulated | 0.20 | 1.31 |
| GAS5-017 |
ENST00000436285 | – | – | – | – |
| GAS5-018 |
ENST00000442067 | p540 | Downregulated | 0.07 | 1.53 |
| GAS5-019 |
ENST00000422183 | p33997_v4 | Downregulated | 0.30 | 1.24 |
| GAS5-020 |
ENST00000425771 | p546 | Downregulated | 0.19 | 1.27 |
| GAS5-021 |
ENST00000422008 | p539 | Downregulated | 0.02 | 1.77 |
| GAS5-022 |
ENST00000412059 | – | – | – | – |
| GAS5-023 |
ENST00000422207 | – | – | – | – |
| GAS5-024 |
ENST00000434796 | p33999_v4 | Downregulated | 0.27 | 1.21 |
| GAS5-025 |
ENST00000444470 | – | – | – | – |
| GAS5-026 |
ENST00000454813 | p538 | Downregulated | 0.36 | 1.13 |
| GAS5-027 |
ENST00000430245 | p33996_v4 | Downregulated | 0.32 | 1.18 |
| GAS5-028 |
ENST00000414075 | – | – | – | – |
| GAS5-029 |
ENST00000456812 | – | – | – | – |
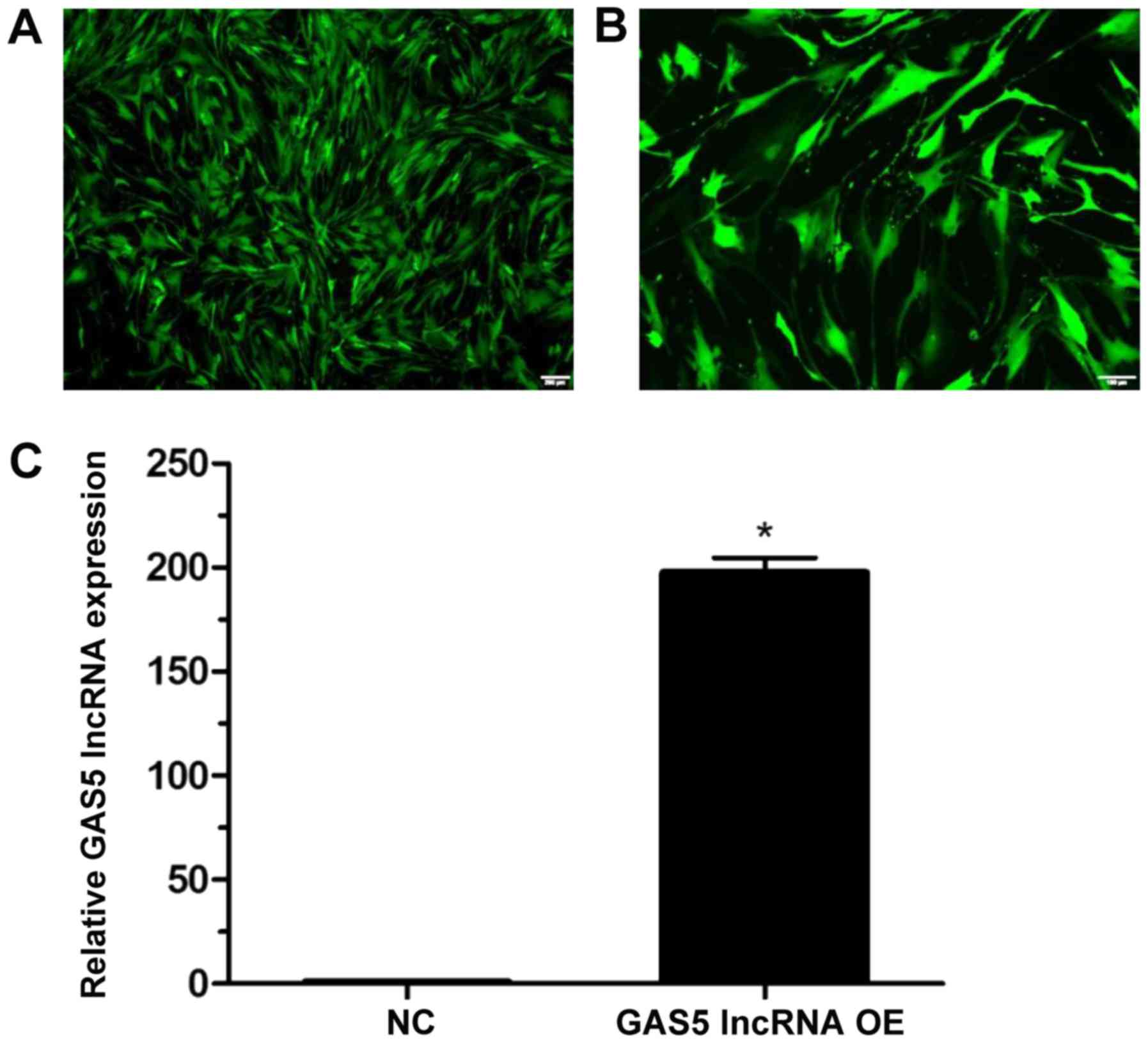
Effects of GAS5 overexpression on cell
apoptosis and apoptosis-associated proteins in NPCs
The transduction efficiency was determined to be 95%
at 96 h post-transduction (Fig.
4A), and classic bleb-like protuberances were observed during
cell growth (Fig. 4B). At 96 h
following lentiviral transduction, GAS5 expression levels were
significantly increased compared with the NC group (P<0.05;
Fig. 4C).
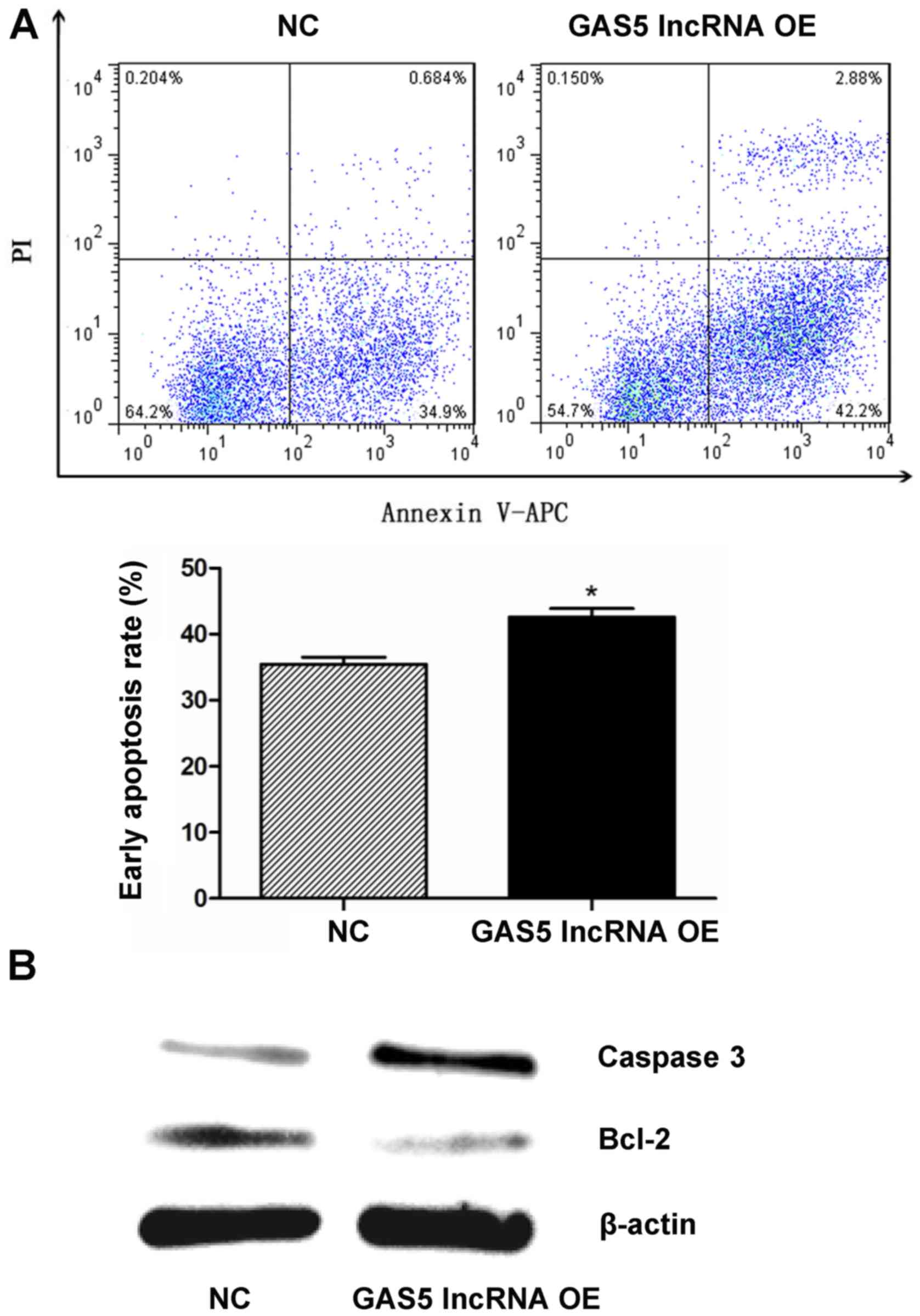
As detected by flow cytometry, the early apoptosis
rates were 35.4±1.86 and 42.6±2.23% in the NC and GAS5
overexpression groups, respectively, which indicated that GAS5
overexpression induced early apoptosis of NPCs (P<0.05; Fig. 5A). The expression levels of the two
apoptosis-associated proteins, caspase-3 and Bcl-2, which were
determined by western blotting, were notably upregulated and
downregulated, respectively, when GAS5 was overexpressed in NPCs
(Fig. 5B).
Discussion
Numerous lncRNAs are differentially expressed in NP
tissues, which suggests the important roles of lncRNAs in the
development of IDD (12,13). Previous studies have preliminarily
investigated several functional mechanisms of lncRNA GAS5, mostly
in neoplastic and osteoarthritis degenerative diseases. Song et
al (25) reported that GAS5
overexpression increased the expression levels of MMP-2, MMP-3,
MMP-9, MMP-13 and A disintegrin and metalloproteinase with
thrombospondin motifs-4 in cartilage cells, further inducing
apoptosis and inhibiting autophagy. These effects may be inhibited
by miR-21, which specifically targets GAS5. Li et al
(26) demonstrated that GAS5
ameliorated LPS-induced inflammatory injury in ATDC5 chondrocytes
by inhibiting the nuclear factor-κB and Notch signaling pathways.
Additionally, the roles of GAS5 lncRNA in tumor growth inhibition
were investigated in several studies. Guo et al (27) reported that GAS5 significantly
increased the expression of phosphatase and tensin homolog via
miR-103 inhibition, promoting the apoptosis of endometrial cancer
cells. Zhao et al (28)
also indicated that GAS5 inhibited glioma cell growth by directly
targeting and inhibiting miR-222; however, to the best of our
knowledge, no studies on cartilage tumors have been conducted.
In the present study, GAS5 was predicted as a target
of miR-155 by lncRNA-miRNA regulatory network analysis and the
overall expression of GAS5 was observed to be significantly reduced
following the transfection of NPCs with miR-155 mimics.
Furthermore, array analysis revealed that each detected GAS5
transcript exhibited a downregulated expression profile following
NPC transduction with miR-155 lentiviruses. These findings
indicated a negative regulatory effect of miR-155 on GAS5
expression in NPCs; however, the underlying regulatory mechanism
requires further investigation. For example, a dual-luciferase
reporter gene assay may demonstrate the interaction between GAS5
with miR-155 and an RNA pull-down assay may detect the target
proteins of GAS5; such investigations may clarify the functions of
GAS5.
Studies on a variety of cancers demonstrated that
GAS5 was a tumor suppressor, and inhibited proliferation and
promoted apoptosis; GAS5 was proposed to be involved in numerous
key regulatory pathways associated cell survival (29–31).
Therefore, the function of GAS5 in determining cell survival or
death has attracted increasing attention, particularly in cancer
research (19). Consistently, a
similar effect was observed in NPCs, as GAS5 overexpression
promoted NPC apoptosis in vitro.
The three main apoptotic pathways (mitochondrial,
death receptor and endoplasmic reticulum stress pathways) are
involved in different stages of IDD development (32). Among them, the mitochondrial
pathway serves a major role in moderate to severe stages of IDD
(33,34). The release of apoptosis-associated
molecules, including cytochrome c (Cyt c),
apoptosis-inducing factor, Endo G and Smac in mitochondria, is
initiated by stress and apoptosis signaling; Cyt c then
binds apoptotic protease activating factor-1 and caspase-9 zymogen
forming the apoptosome, and activates the caspase-3 cascade that
ultimately leads to apoptosis (35). Bcl-2 and caspase proteases are two
protein families with conserved evolution. Bcl-2 protein controls
mitochondrial integrity and inhibits Cyt c release,
therefore inhibiting apoptosis (35). In addition, caspases initiate and
execute the process of apoptosis; among them, caspases-3, 6, and 7
execute the process of apoptosis (35). For instance, in vitro
silencing of caspase-3 suppressed the apoptosis of NPCs induced by
mechanical overload, further inhibiting IDD (36). In the present study, the effect of
GAS5 on NPC apoptosis was reported, and the underlying mechanism
was investigated. As aforementioned, GAS5 overexpression
downregulated the expression of Bcl-2 and upregulated that of
caspase-3, therefore promoting NPC apoptosis. Of note, GAS5
involvement in mitochondrial apoptosis has been previously
reported; by directly targeting miR-222, GAS5 could indirectly
inhibit Bcl-2 expression in glioma cells (28). In addition, GAS5 promoted the
apoptosis of ovarian cancer cells via caspase-3 and caspase-9
upregulation (37). Additionally,
the oncogenesis of some cartilage tumors, including chondrosarcoma
and chondroblastic osteosarcoma, has exhibited a high correlation
with the mitochondrial pathway of apoptosis (38–40).
Future studies on these tumors are required to further verify the
function of GAS5 in mitochondrial apoptosis, providing that GAS5,
as a tumor suppressor, may inhibit tumor growth and
proliferation.
Biomolecular therapy, cell therapy,
tissue-engineered construction and annulus fibrosus repair are
novel strategies for treating IDD (41). Using biomolecules, including
recombinant genes, proteins or platelet-rich plasma, to adjust
cellular metabolism and extracellular matrix regeneration, early
degenerated disks with sufficient viable NPCs can be treated to
mitigate the progression of disc degeneration (42). Midstage degeneration, characterized
by fewer active cells, can be treated with cell implantations,
including stem cells or allogeneic NPCs to meet the demand of the
disk for active NPCs and reverse the degenerative process (43,44).
Accordingly, with a thorough understanding of GAS5 functions,
ultrasound- or computed tomography-guided injection of
GAS5-silenced NPCs, or lentivirus-mediated GAS5 knockdown may
improve the precision and efficiency of IDD treatment.
There were several limitations of the present study.
Firstly, GAS5 overexpression in degenerative NP, though
demonstrated in a previous microarray study (12), requires further clinical
investigation. In addition, RNA sample extraction in severely
degenerated NP is yet to be resolved. The downregulation of GAS5
may have more clinical value in the treatment of IDD, while the
present study only conducted overexpression. Additionally, the
inter-regulatory dependence of the mechanism investigated in the
present study requires further study.
In conclusion, GAS5 overexpression resulted in
increased apoptosis in primary NPCs from the human intervertebral
disc via Bcl-2 downregulation and caspase-3 upregulation. GAS5,
predicted to be a target of miR-155 by TargetScan analysis, was
proposed to be downregulated by miR-155 overexpression. The
findings of the present study may provide novel insight into the
role of GAS5 in IDD pathogenesis and contribute to developments in
the treatment of IDD via biomolecular or cellular therapies.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Nature Science Foundation of China (grant no. 81672203).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YW, QS and HS made substantial contributions to the
design of the present study. YW, QS and XH performed the majority
of the experiments, data collection, statistical analysis, and
wrote the manuscript. ZC, FZ, KW and GH analyzed the data and
revised the manuscript. All authors reviewed the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interest.
References
|
1
|
Hoy D, March L, Brooks P, Blyth F, Woolf
A, Bain C, Williams G, Smith E, Vos T, Barendregt J, et al: The
global burden of low back pain: Estimates from the global burden of
disease 2010 study. Ann Rheum Dis. 73:968–974. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vo NV, Hartman RA, Patil PR, Risbud MV,
Kletsas D, Iatridis JC, Hoyland JA, Le Maitre CL, Sowa GA and Kang
JD: Molecular mechanisms of biological aging in intervertebral
discs. J Orthop Res. 34:1289–1306. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Luoma K, Riihimäki H, Luukkonen R,
Raininko R, Viikari-Juntura E and Lamminen A: Low back pain in
relation to lumbar disc degeneration. Spine (Phila Pa 1976).
25:487–492. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Le Maitre CL, Pockert A, Buttle DJ,
Freemont AJ and Hoyland JA: Matrix synthesis and degradation in
human intervertebral disc degeneration. Biochem Soc Trans.
35:652–655. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu Z, Ma C, Shen J, Wang D, Hao J and Hu
Z: SDF-1/CXCR4 axis induces apoptosis of human degenerative nucleus
pulposus cells via the NF-κB pathway. Mol Med Rep. 14:783–789.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shen J, Fang J, Hao J, Zhong X, Wang D,
Ren H and Hu Z: SIRT1 inhibits the catabolic effect of IL-1β
through TLR2/SIRT1/NF-κB pathway in human degenerative nucleus
pulposus cells. Pain physician. 19:E215–E226. 2016.PubMed/NCBI
|
|
7
|
Freemont AJ: The cellular pathobiology of
the degenerate intervertebral disc and discogenic back pain.
Rheumatology (Oxford). 48:5–10. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Urban JP and Roberts S: Degeneration of
the intervertebral disc. Arthritis Res Ther. 5:120–130. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li Z, Yu X, Shen J, Chan MT and Wu WK:
MicroRNA in intervertebral disc degeneration. Cell Prolif.
48:278–283. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang HQ, Yu XD, Liu ZH, Cheng X, Samartzis
D, Jia LT, Wu SX, Huang J, Chen J and Luo ZJ: Deregulated miR-155
promotes Fas-mediated apoptosis in human intervertebral disc
degeneration by targeting FADD and caspase-3. J Pathol.
225:232–242. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang WL, Chen YF, Meng HZ, Du JJ, Luan
GN, Wang HQ, Yang MW and Luo ZJ: Role of miR-155 in the regulation
of MMP-16 expression in intervertebral disc degeneration. J Orthop
Res. 35:1323–1334. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wan ZY, Song F, Sun Z, Chen YF, Zhang WL,
Samartzis D, Ma CJ, Che L, Liu X, Ali MA, et al: Aberrantly
expressed long noncoding RNAs in human intervertebral disc
degeneration: A microarray related study. Arthritis Res Ther.
16:4652014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen Y, Ni H, Zhao Y, Chen K, Li M, Li C,
Zhu X and Fu Q: Potential role of lncRNAs in contributing to
pathogenesis of intervertebral disc degeneration based on
microarray data. Med Sci Monit. 21:3449–3458. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Furió-Tarí P, Tarazona S, Gabaldón T,
Enright AJ and Conesa A: SpongeScan: A web for detecting microRNA
binding elements in lncRNA sequences. Nucleic Acids Res.
44:W176–W180. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schneider C, King RM and Philipson L:
Genes specifically expressed at growth arrest of mammalian cells.
Cell. 54:787–793. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Smith CM and Steitz JA: Classification of
gas5 as a multi-small-nucleolar-RNA (snoRNA) host gene and a member
of the 5′-terminal oligopyrimidine gene family reveals common
features of snoRNA host genes. Mol Cell Biol. 18:6897–6909. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mourtada-Maarabouni M, Hedge VL, Kirkham
L, Farzaneh F and Williams GT: Growth arrest in human T-cells is
controlled by the non-coding RNA growth-arrest-specific transcript
5 (GAS5). J Cell Sci. 121:939–946. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mourtada-Maarabouni M, Pickard MR, Hedge
VL, Farzaneh F and Williams GT: GAS5, a non-protein-coding RNA,
controls apoptosis and is downregulated in breast cancer. Oncogene.
28:195–208. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pickard MR and Williams GT: Molecular and
cellular mechanisms of action of tumour suppressor GAS5 LncRNA.
Genes (Basel). 6:484–499. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ehlicke F, Freimark D, Heil B, Dorresteijn
A and Czermak P: Intervertebral disc regeneration: Influence of
growth factors on differentiation of human mesenchymal stem cells
(hMSC). Int J Artif Organs. 33:244–252. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Foss BL, Maxwell TW and Deng Y:
Chondroprotective supplementation promotes the mechanical
properties of injectable scaffold for human nucleus pulposus tissue
engineering. J Mech Behav Biomed Mater. 29:56–67. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Friedman RC, Farh KK, Burge CB and Bartel
DP: Most mammalian mRNAs are conserved targets of microRNAs. Genome
Res. 19:92–105. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Song J, Ahn C, Chun CH and Jin EJ: A long
non-coding RNA, GAS5, plays a critical role in the regulation of
miR-21 during osteoarthritis. J Orthop Res. 32:1628–1635. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li F, Sun J, Huang S, Su G and Pi G:
LncRNA GAS5 overexpression reverses LPS-induced inflammatory injury
and apoptosis through up-regulating KLF2 expression in ATDC5
chondrocytes. Cell Physiol Biochem. 45:1241–1251. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guo C, Song WQ, Sun P, Jin L and Dai HY:
LncRNA-GAS5 induces PTEN expression through inhibiting miR-103 in
endometrial cancer cells. J Biomed Sci. 22:1002015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhao X, Wang P, Liu J, Zheng J, Liu Y,
Chen J and Xue Y: Gas5 exerts tumor-suppressive functions in human
glioma cells by targeting miR-222. Mol Ther. 23:1899–1911. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu Y, Lyu H, Liu H, Shi X, Song Y and Liu
B: Downregulation of the long noncoding RNA GAS5-AS1 contributes to
tumor metastasis in non-small cell lung cancer. Sci Rep.
6:310932016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ma C, Shi X, Zhu Q, Li Q, Liu Y, Yao Y and
Song Y: The growth arrest-specific transcript 5 (GAS5): A pivotal
tumor suppressor long noncoding RNA in human cancers. Tumour Biol.
37:1437–1444. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yu X and Li Z: Long non-coding RNA growth
arrest-specific transcript 5 in tumor biology. Oncol Lett.
10:1953–1958. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang H, Liu H, Zheng ZM, Zhang KB, Wang
TP, Sribastav SS, Liu WS and Liu T: Role of death receptor,
mitochondrial and endoplasmic reticulum pathways in different
stages of degenerative human lumbar disc. Apoptosis. 16:990–1003.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhao CQ, Zhang YH, Jiang SD, Jiang LS and
Dai LY: Both endoplasmic reticulum and mitochondria are involved in
disc cell apoptosis and intervertebral disc degeneration in rats.
Age (Dordr). 32:161–177. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gruber HE, Hoelscher GL, Bethea S and
Hanley EN Jr: Mitochondrial membrane potential and nuclear and gene
expression changes during human disc cell apoptosis: In vitro and
in vivo annulus findings. Spine (Phila Pa 1976). 40:876–882. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ding F, Shao ZW, Yang SH, Wu Q, Gao F and
Xiong LM: Role of mitochondrial pathway in compression-induced
apoptosis of nucleus pulposus cells. Apoptosis. 17:579–590. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yamada K, Sudo H, Iwasaki K, Sasaki N,
Higashi H, Kameda Y, Ito M, Takahata M, Abumi K, Minami A and
Iwasaki N: Caspase 3 silencing inhibits biomechanical
overload-induced intervertebral disk degeneration. Am J Pathol.
184:753–764. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gao J, Liu M, Zou Y, Mao M, Shen T, Zhang
C, Song S, Sun M, Zhang S, Wang B, et al: Long non-coding RNA
growth arrest-specific transcript 5 is involved in ovarian cancer
cell apoptosis through the mitochondria-mediated apoptosis pathway.
Oncol Rep. 34:3212–3221. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu JF, Chen CY, Chen HT, Chang CS and
Tang CH: BL-038, a benzofuran derivative, induces cell apoptosis in
human chondrosarcoma cells through reactive oxygen
species/mitochondrial dysfunction and the caspases dependent
pathway. Int J Mol Sci. 17(pii): E14912016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Aziz MNM, Hussin Y, Che Rahim NF, Nordin
N, Mohamad NE, Yeap SK, Yong CY, Masarudin MJ, Cheah YK, Abu N, et
al: Curcumin analog DK1 induces apoptosis in human osteosarcoma
cells in vitro through mitochondria-dependent signaling pathway.
Molecules. 23(pii): E752018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chou WH, Liu KL, Shih YL, Chuang YY, Chou
J, Lu HF, Jair HW, Lee MZ, Au MK and Chung JG: Ouabain induces
apoptotic cell death through caspase- and mitochondria-dependent
pathways in human osteosarcoma U-2 OS cells. Anticancer Res.
38:169–178. 2018.PubMed/NCBI
|
|
41
|
Moriguchi Y, Alimi M, Khair T, Manolarakis
G, Berlin C, Bonassar LJ and Härtl R: Biological treatment
approaches for degenerative disk disease: A literature review of in
vivo animal and clinical data. Global Spine J. 6:497–518. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Matta A, Karim MZ, Isenman DE and Erwin
WM: Molecular therapy for degenerative disc disease: Clues from
secretome analysis of the notochordal cell-rich nucleus pulposus.
Sci Rep. 7:456232017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang Z, Perez-Terzic CM, Smith J, Mauck
WD, Shelerud RA, Maus TP, Yang TH, Murad MH, Gou S, Terry MJ, et
al: Efficacy of intervertebral disc regeneration with stem cells-a
systematic review and meta-analysis of animal controlled trials.
Gene. 564:1–8. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang W, Deng G, Qiu Y, Huang X, Xi Y, Yu
J, Yang X and Ye X: Transplantation of allogenic nucleus pulposus
cells attenuates intervertebral disc degeneration by inhibiting
apoptosis and increasing migration. Int J Mol Med. 41:2553–2564.
2018.PubMed/NCBI
|