Introduction
Epithelial cell adhesion molecule (EpCAM) is
expressed by a majority of epithelial tissues and is involved in
cell signaling, proliferation, differentiation and migration. EpCAM
is deregulated in epithelial malignancies and is abundantly
expressed in human carcinomas of different origins (1,2). In
addition to its role in cell adhesion, EpCAM acts as signaling
molecule with tumor growth promoting functions. EpCAM is a part of
the molecular network of oncogenic receptors and is considered to
be a promising target for anti-cancer therapy (2). In addition, as a marker of aggressive
ovarian cancer and an important suppressor of anti-tumor immunity,
EpCAM represents an attractive target for specific immune-based
therapies (1). In human epithelial
ovarian cancer, EpCAM is overexpressed consistently across all
histological subtypes (3,4). High-throughput genomic analysis of
genetic fingerprints of primary and metastatic ovarian carcinomas
demonstrates that EpCAM is one of the top differentially expressed
genes in all tested epithelial ovarian cancer types (5,6).
Treating breast cancer cell lines with EpCAM small interfering RNA
resulted in a decrease in the rates of cell proliferation,
migration and invasion; thus, these data provide compelling
evidence for a direct onco- and metastatogenic role of the EpCAM
protein in breast cancer (7).
Ovarian cancer progression and chemotherapy
resistance depends to a great extent on the cancer
microenvironment. Ovarian cancer cells have the ability to alter
the composition of the microenvironment to affect host cells
(8). The immune system serves an
important role in ovarian cancer progression and may also be
involved in the development of drug resistance via cytokine and
chemokine signaling pathways (9–11).
The key cytokines in this process appear to be interleukin 6 (IL-6)
and interleukin 8 (CXCL8/IL-8). Increased levels of these cytokines
have been identified in ascites fluid from patients with ovarian
cancer (8,12). Bonneau et al (13) demonstrated that alterations in IL-8
expression in ovarian tumor cells are correlated with tumor
chemoresistance and overall survival, and proposed the IL-8 level
as a predictor of poor prognosis in patients with ovarian cancer.
IL-8 also induced epithelial-mesenchymal transition in ovarian or
breast cancer cells, suggesting its potential role in enhancing
ovarian cancer cell metastasis (14,15).
However, an increased serum IL-6 level is additionally associated
with poor prognosis in patients with early and advanced stage
ovarian cancer. IL-6 promotes tumor cell migration and attachment,
augmenting cancer invasiveness (16). IL-8 and IL-6 are involved in the
activation of numerous cellular pathways responsible for
proliferation, metastasis or tumor cell survival. Consequently, the
present study evaluated the impact of IL-6 and IL-8 on the
expression of EpCAM.
Materials and methods
Materials
All reagents necessary for cell cultures (RPMI-1640,
bovine serum, L-glutamine and penicillin-streptomycin) were
purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany)
unless indicated otherwise.
Cell culture
The human A2780 ovarian cancer cell line was
obtained from the European Collection of Authenticated Cell
Cultures (Salisbury, UK). A2780 cells were cultured in RPMI 1640
medium, supplemented with L-glutamine, penicillin-streptomycin (10
U/ml; 100 µg/ml) and 10% fetal bovine serum, in a humidified
atmosphere of 95% air and 5% CO2 at 37°C.
Cell proliferation assay following
inhibition by anti-EPCAM antibodies
The effect of inhibiting EpCAM on the proliferation
of the A2780 cells was determined using an MTT assay. The cells
were cultured at a density of 5×103 cells/well in
96-well cell culture plates (Nunc™
MicroWell™; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) for 24 h. The cells were treated with various concentrations
of anti-EPCAM antibody (1 and 10 ng/ml; Abcam, Cambridge, UK; cat.
no. ab85987) and were incubated for 30 and 60 min, and 48 h at
37°C. The viability of the treated cells was assessed by MTT assay.
Tetrazolium dye (Merck KGaA) was dissolved in PBS with
Ca2+ and Mg2+ (5 mg/ml; Merck KGaA) and 15 µl
of this solution was added to the cell culture. The amount of
formazan dye dissolved in 10% sodium dodecyl sulfate solution was
determined by quantifying its absorbance at 570 nm using the
FLUOstar Omega Microplate Reader (BMG Labtech GmbH, Ortenberg,
Germany). The proliferation rate (PR) was calculated via the
following equation: PR (%)=(absorbance of treatment
probe/absorbance of control probe) ×100.
EpCAM expression
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
A2780 cells were seeded into Petri dishes
(3×105 cells/ml; volume, 5 ml). After 24 h, the cells
were washed with PBS with Ca2+ and Mg2+ and
incubated at 37°C for 24 h in medium containing various
concentrations of IL-6 and IL-8 (1, 10 and 100 ng/ml). The total
RNA was extracted using a High Pure RNA Isolation kit (Roche
Diagnostics GmbH, Mannheim, Germany), according to the
manufacturer's protocol. The extracted RNA was diluted in DNase and
RNase-free water. The quality and quantity of isolated RNA was
measured using a NanoDrop® spectrophotometer (Thermo
Fisher Scientific, Inc., Wilmington, DE, USA). Total RNA (2 µg) was
reverse transcribed using High-Capacity cDNA Reverse Transcriptase
[per reaction: 2 µl 10× RT Buffer; 0,8 µl 25× dNTP Mix (100 nM); 2
µl 10× RT Random Primers; 1 µl MultiScribe Reverse Transcriptase; 1
µl RNase Inhibitor; 3.2 µl nuclease-free water; Thermo Fisher
Scientific, Inc.] in a final volume of 20 µl, under the following
temperature conditions: 25°C for 10 min, 37°C for 120 min and 85°C
for 5 min. Subsequently, EpCAM expression was quantified 1 µl of
the resulting cDNA solution (100 ng) using EpCAM specific
TaqMan® Gene Expression assay (Assay ID, Hs00901885_m1;
Thermo Fisher Scientific, Inc.) under the following temperature
condition: 50°C for 2 min; 95°C for 30 sec; 40 cycles at 95°C for 3
sec and 60°C for 30 sec. The relative expression was calculated
using the 2−ΔΔCq method (17) using β-actin gene expression as a
reference.
Immunofluorescence
Cells were grown in 8-well cell culture slides
(Nunc™ MicroWell™) in RPMI 1640 with 10% FBS
and stimulated for 24 h with various concentrations of IL-6, IL-8
and a combination of IL-6/IL-8 (1, 10 and 100 ng/ml). Following
treatment, cells were fixed in 3.7% formaldehyde for 15 min at room
temperature and permeabilized in 0.1% Triton X-100 for 10 min at
room temperature. Following permeabilization, the cell culture
slides were blocked in 3% bovine serum albumin for 15 min at room
temperature, washed and incubated with mouse monoclonal anti-EpCAM
antibody (Abcam; cat. no. ab85987) at 10 µg/ml (1:100 dilution)
overnight at 4°C. Subsequently, the cells were incubated with
secondary antibody (donkey anti-mouse immunoglobulin G; Alexa Fluor
488; Abcam; cat. no. ab150105; 1:1,000 dilution) for 1.5 h at room
temperature. Fluorescence labeling was observed under a fluorescent
microscope (BX51; Olympus Corporation, Tokyo, Japan; magnification,
×400).
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 5 software (GraphPad Software, Inc., La Jolla, CA, USA).
Multiple comparisons were performed using one-way analysis of
variance followed by Tukey's post hoc test. Data are presented as
the mean ± standard deviation of three replicates. All statistical
tests were two-sided and P<0.05 was considered to indicate a
statistically significant difference.
Results
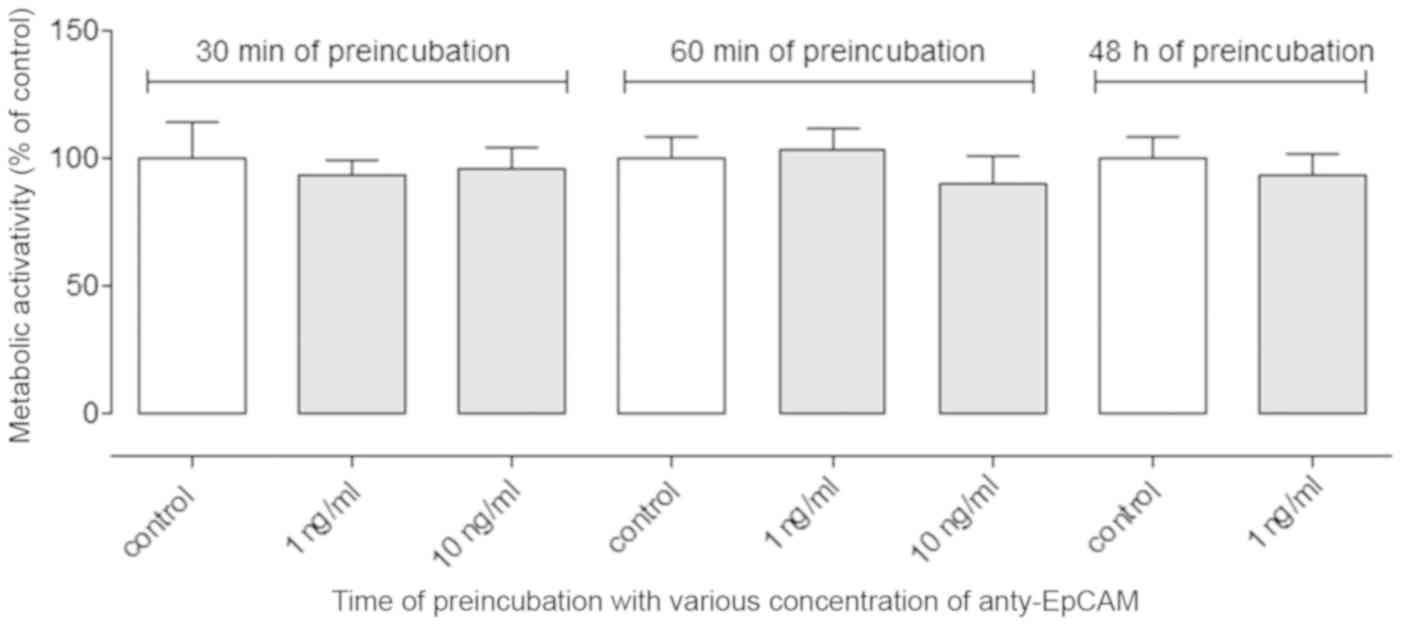
Blocking EpCAM with anti-EpCAM
antibody does not affect cellular proliferation
EpCAM molecules expressed by the A2780 cell line
were blocked by the addition of anti-EpCAM antibody and cell
proliferation, expressed as their metabolic activity, was measured
by MTT assay. However, no difference was observed between the
control and treated cell proliferation rates after 30 min, 60 min
and 48 h of incubation with anti-EpCAM antibody, regardless of the
antibody concentration (1 or 10 ng/ml; Fig. 1).
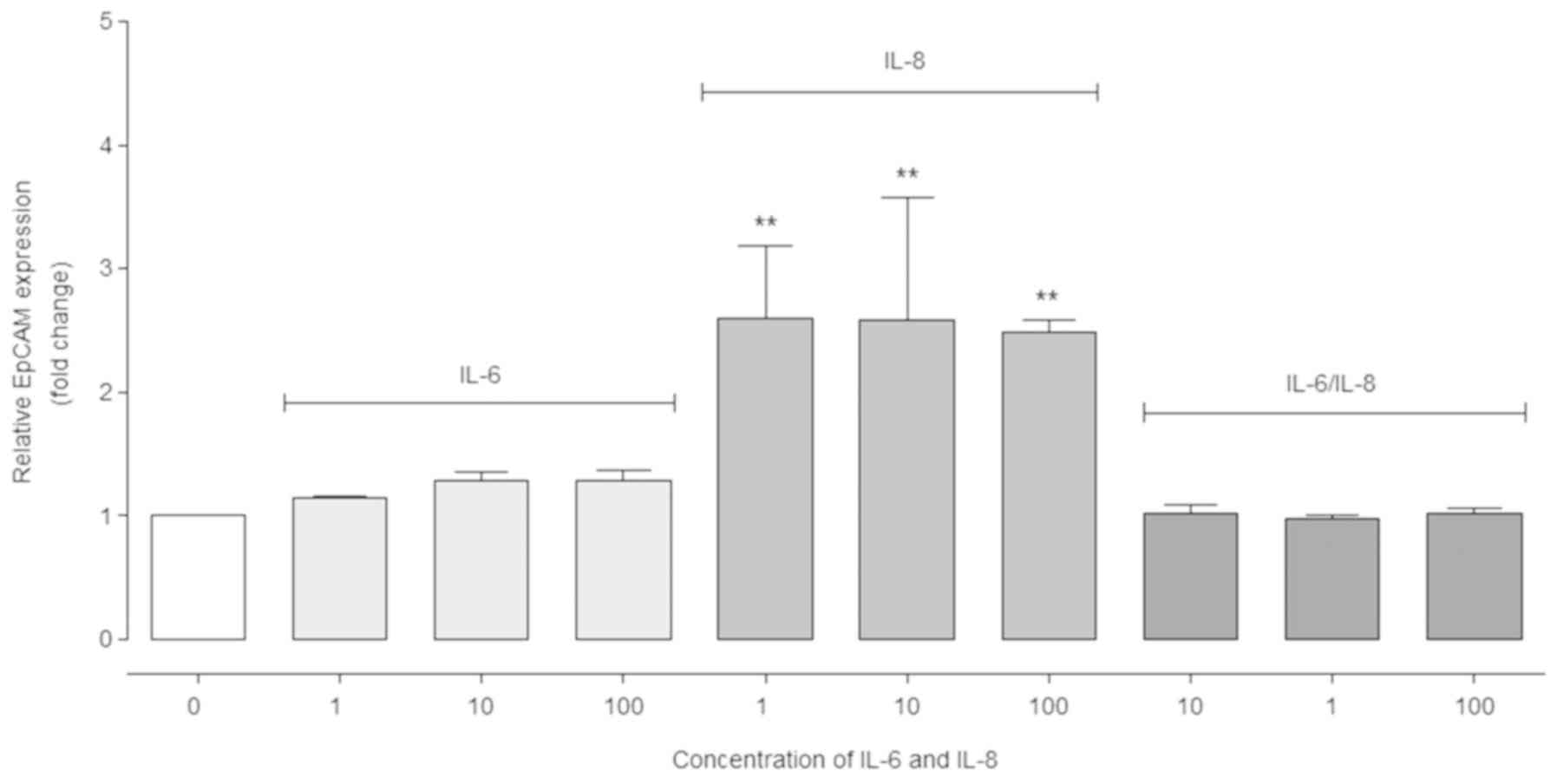
IL-8 has a positive effect on EpCAM
gene expression
RT-qPCR analysis demonstrated that the relative
EpCAM expression level increased significantly in the cells
stimulated with IL-8 compared with the control cells (P<0.01;
Fig. 2). On the contrary, in cells
treated with IL-6 (10 and 100 ng/ml) EpCAM expression did not
increase significantly. Moreover, in cells incubated with the
IL-6/IL-8 combination, no significant differences in expression
were observed (Fig. 2).
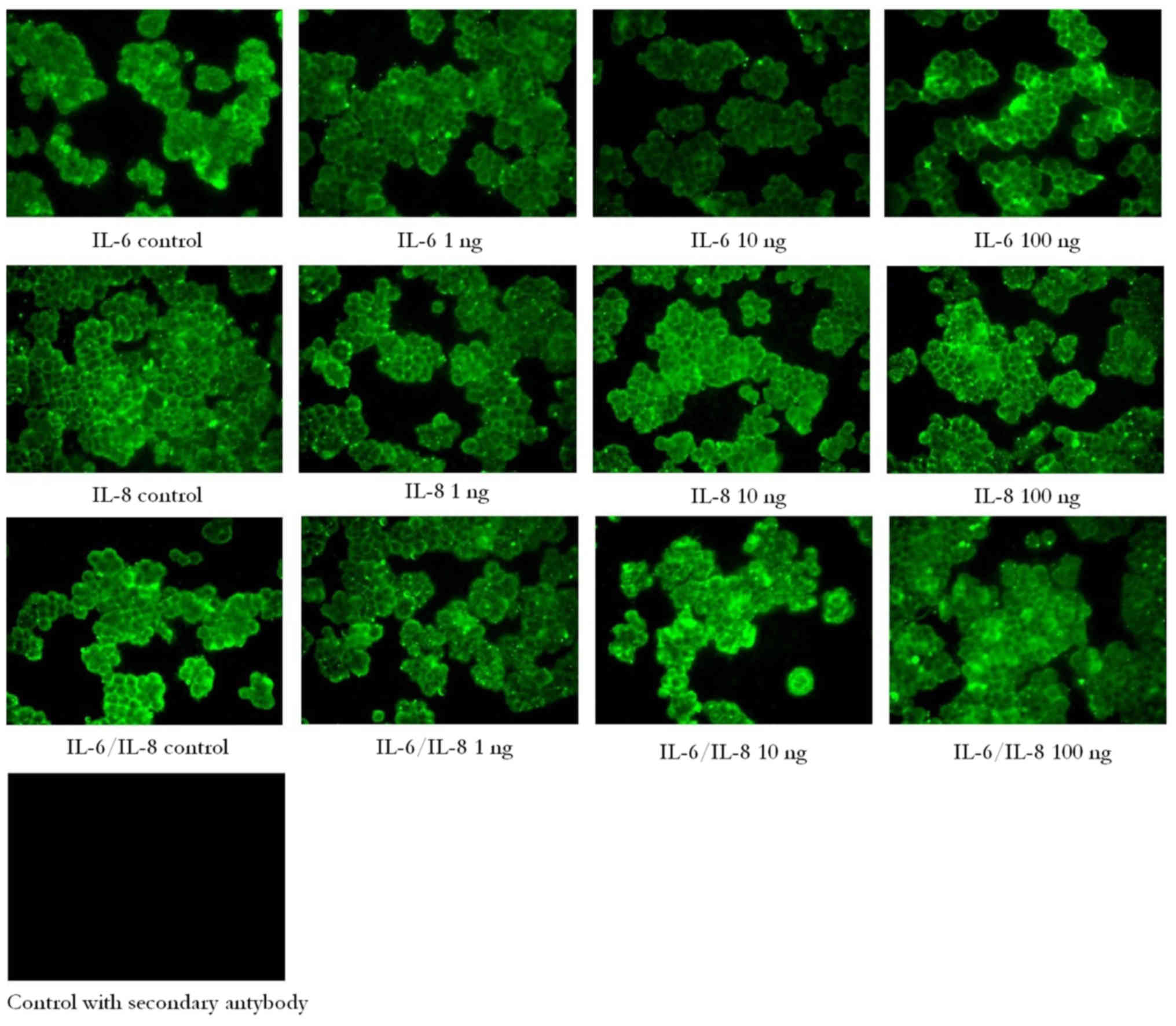
To assess the cellular localization of the EpCAM in
ovarian cancer cells, immunofluorescence analysis was performed in
the A2780 ovarian cancer cell line. As hypothesized, EpCAM
expression was detected at the cell membrane. However, no marked
differences in fluorescence were observed between cells treated
with IL-6, IL-8 or a combination of IL-6/IL-8 (Fig. 3).
Discussion
EpCAM overexpression in ovarian carcinoma,
particularly in recurrent, highly metastatic, and
chemotherapy-resistant ovarian cancer (18), suggests the importance of EpCAM
expression for cancer growth and metastasis. Crucial evidence for a
tumor promoting role of EpCAM overexpression was provided by Munz
et al (19,20), who demonstrated upregulation of MYC
proto-oncogene, bHLH transcription factor and the tumor-promoting
protein epidermal-type fatty acid-binding protein by EpCAM
expression. Indeed, knockdown of EpCAM resulted in suppressed
proliferation and enhanced chemo- and radiosensitivity (21,22).
Positive expression of EpCAM is associated with
human epithelial ovarian tumor stage and differentiation, and lymph
node metastasis (23). Increased
expression of EpCAM also contributes to increased viability of
cancer cells in vitro and resistance to platinium-based
chemotherapy in patients with ovarian cancer. Finally, increased
expression of EpCAM is associated with a poor prognosis in patients
with ovarian cancer (24).
Therefore, EpCAM has begun to be a promising therapeutic target in
a number of antibody-based clinical trials. In the 2009, the
European Medicines Agency approved the use of an anti-EpCAM
antibody, catumaxomab, for the intraperitoneal treatment of
malignant ascites; however, in June 2017 the European Commission
withdrew the marketing authorization for catumaxomab in the
European Union (25,26).
In the present study, no effect of EpCAM inhibition
on the proliferation of the A2780 cell line was observed, despite
the presence of EpCAM molecules on the cell membrane. This
discrepancy may have been caused by differences in the cells used,
antibodies and/or time of treatment. This discrepancy may also be
associated with differences in the tumor microenvironment in
vivo and in vitro. Zheng et al (27) did not observe any effect of
anti-EpCAM antibodies on the proliferation and induction of
apoptosis in K562 and HL60 cells in vitro, even though the
same antibodies inhibited the growth of EpCAM-overexpressing solid
tumors and subcutaneously transplanted A549 tumors in
vivo.
The tumor microenvironment may have a marked
influence on the viability, proliferation and metastasis of ovarian
cancer cells (16). The lack of
expression of IL-6 and IL-8 protein, coupled with the expression of
IL-6 and IL-8 receptor proteins in A2780 cells, suggests a
paracrine mechanism of IL-6 and IL-8 responsivity (28,29).
Therefore, it was speculated that IL-6 and IL-8, as a part of the
tumor microenvironment, may influence EpCAM expression. Indeed, in
the present study, IL8 was demonstrated to increase the EpCAM
expression at the mRNA level, whereas treatment with IL-6 did not.
Notably, co-treatment with IL-6 and IL-8 did not stimulate EpCAM
expression. It may be hypothesized that src-homology 2
domain-containing phosphatase 2 (SHP-2) protein, a putative
negative modulator of IL-6 signaling, may also modulate IL-8
signaling under conditions of co-stimulation. SHP-2 serves an
important role in the control of proliferation, differentiation and
survival of different cells (30,31).
SHP-2 counteracts IL6-induced gene expression and the Janus kinase
(JAK)-signal transducer and activator of transcription (STAT)
pathway (32). Fischer et
al (33) reported that
impaired function of SHP-2 may lead to enhanced IL-6 signaling.
Another protein that regulates IL-6 signaling pathway is suppressor
of cytokine signaling 3 (SOCS3). SHP-2 and SOCS3 counteract each
other during IL-6-dependent gene activation (32). Mammic and Ghorpade (34) demonstrated that the SHP-2 protein
is involved, directly or indirectly, in the modulation of
extracellular signal-regulated kinase (ERK)/mitogen-activated
protein kinase (MAPK) leading to CXCL8 production. In addition,
SHP-2 was demonstrated to be involved in the regulation of the
expression and release of IL-8 (30). It is therefore possible that SHP-2
may be involved in the regulation of gene expression associated
with the signaling pathways of IL6 and IL-8.
Stimulation of the IL-6 receptor leads to activation
of the JAK2/STAT3 signaling pathway, which stimulates downstream
pathways involving ERK or phosphatidyl-inositol-3-kinase
(PI3K)/RAC-α serine/threonine-protein kinase (AKT) kinases
(35). Notably, IL-8/C-X-C
chemokine receptor type 1/2 (CXCR1/2) signaling promotes the
activation of similar primary effectors, e.g. PI3K/AKT, AKT, JAK2
and ERK (36). Although the two
interleukins activate receptors that share certain signaling
pathways, the primary targets of activation appear to differ. IL-6
receptor signaling primarily stimulates the STAT3 transcription
factor pathway, whereas IL-8/CXCR1/2 signaling primarily activates
PI3K or phospholipase C, promoting the activation of Akt and
protein kinase C. Since EpCAM expression has been demonstrated to
be stimulated by the RAF/mitogen-activated protein kinase kinase
(MEK)/ERK1/2 signaling pathway (4), which is not a principal pathway
activated by IL-6 or IL-8, stimulation of EpCAM expression by these
interleukins may depend on the fine-tuning of secondary signaling
pathways. A putative candidate appears to be an SHP-2 protein that
is involved in the modulation of the RAF/MEK/ERK1/2 signaling
pathway stimulated by IL-6, but not IL-8 or epidermal growth factor
receptor (37). The importance of
the involvement of SHP-2 in cytokine signaling remains unclear.
However, certain evidence suggests that the protein negatively
modulates leukemia inhibitory factor-mediated signaling (37) and is a negative effector in the
cytotoxic activity of interferons, likely due to downregulation of
JAK/STAT activity (38). Thus,
also assuming negative modulation of IL-6 signaling, SHP-2 protein
activated by IL-6 is also likely to attenuate the RAF/MEK/ERK1/2
signaling pathway activated by IL-8. However, further investigation
is necessary to confirm this hypothesis.
In conclusion, the present study demonstrated that
IL-8, but not IL-6, acts as an upregulator of EpCAM expression in
ovarian cancer cells. It is noteworthy that co-stimulation with
IL-8 and IL-6 abrogates IL-8-mediated EpCAM upregulation. The
mechanism of this crosstalk remains unknown.
Acknowledgements
Not applicable.
Funding
The present study was funded by the National Science
Centre (grant no. DEC-2011/01/D/NZ7/04688).
Availability of data and materials
The analyzed datasets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
LKS conceived and designed the experiments. LKS, SP,
KS and MC performed the experiments. LKS, BD, MK, BJJ, MMK, SP, MZD
and DFT analyzed the data. LKS and SP contributed
reagents/materials/analysis tools. LKS, SP, BD, MC, MMK, DFT and
MZD wrote the manuscript. BJJ, BD and MK critically reviewed the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AKT
|
RAC-α serine/threonine-protein
kinase
|
|
EpCAM
|
epithelial cell adhesion molecule
|
|
ERK
|
extracellular signal-regulated
kinase
|
|
IL-6
|
interleukin 6
|
|
IL-8/CXCL8
|
interleukin 8
|
|
JAK2
|
Janus kinase 2
|
|
MEK
|
mitogen-activated protein kinase
kinase
|
|
PI3K
|
phosphatidyl-inositol-3-kinase
|
|
SHP-2
|
src-homology 2 domain-containing
phosphatase 2
|
|
STAT3
|
signal transducer and activator of
transcription 3
|
References
|
1
|
Spizzo G, Went P, Dirnhofer S, Obrist P,
Moch H, Baeuerle PA, Mueller-Holzner E, Marth C, Gastl G and Zeimet
AG: Overexpression of epithelial cell adhesion molecule (Ep-CAM) is
an independent prognostic marker for reduced survival of patients
with epithelial ovarian cancer. Gynecol Oncol. 103:483–488. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Massoner P, Thomm T, Mack B, Untergasser
G, Martowicz A, Bobowski K, Klocker H, Gires O and Puhr M: EpCAM is
overexpressed in local and metastatic prostate cancer, suppressed
by chemotherapy and modulated by MET-associated miRNA-200c/205. Br
J Cancer. 111:955–964. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Köbel M, Kolloger SE, Boyd N, McKinney S,
Mehl E, Palmer C, Leung S, Bowen NJ, Ionescu DN, Rajput A, et al:
Ovarian carcinoma subtypes are different diseases: Implications for
biomarker studies. PLoS Med. 5:e2322008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fan Q, Cheng JC, Qui X, Chang HM and Leung
PC: EpCAM is up-regulated by EGF via ERK1/2 signaling and
suppresses human epithelial ovarian cancer cell migration. Biochem
Biophys Res Commun. 457:256–261. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bignotti E, Tassi RA, Calza S, Ravaggi A,
Romani C, Rossi E, Falchetti M, Odicino FE, Pecorelli S and Santin
AD: Differential gene expression profiles between tumor biopsies
and short-term primary cultures of ovarian serous carcinomas:
Identification of novel molecular biomarkers for early diagnosis
and therapy. Gynecol Oncol. 103:405–416. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
English DP, Bellone S, Schwab CL, Roque
DM, Lopez S, Bortolomai I, Cocco E, Bonazzoli E, Chatterjee S,
Ratner E, et al: Solitomab, an epithelial cell adhesion
molecule/CD3 bispecific antibody (BiTE), is highly active against
primary chemotherapy-resistant ovarian cancer cell lines in vitro
and fresh tumor cells ex vivo. Cancer. 121:403–412. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Osta WA, Chen Y, Mikhitarian K, Mitas M,
Salem M, Hannun YA, Cole DJ and Gillanders WE: EpCAM is
overexpressed in breast cancer and is a potential target for breast
cancer gene therapy. Cancer Res. 64:5818–5824. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Thibault B, Castells M, Delord JP and
Couderc B: Ovarian cancer microenvironment: Implications for cancer
dissemination and chemoresistance acquisition. Cancer Metastasis
Rev. 33:17–39. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stronach EA, Cunnea P, Turner C, Guney T,
Aiyappa R, Jeyapalan S, de Sousa CH, Browne A, Magdy N, Studd JB,
et al: The role of interleukin-8 (IL-8) and IL-8 receptors in
platinum response in high grade serous ovarian carcinoma.
Oncotarget. 6:31593–31603. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Loose D and Van de Wiele C: The immune
system and cancer. Cancer Biother Radiopharm. 24:369–376. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kapka-Skrzypczak L, Popek S, Sawicki K,
Wolińska E, Czajka M and Skrzypczak M: Effect of IL-6 and IL-8 on
the expression of the complement activation inhibitors
MAC-inhibitory protein and decay-accelerating factor in ovarian
cancer A2780 cells. Oncol Lett. 12:1507–1512. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kulbe H, Chakravarty P, Leinster DA,
Charles KA, Kwong J, Thompson RG, Coward JI, Shioppa T, Robinson
SC, Gallagher WM, et al: A dynamic inflammatory cytokine network in
the human ovarian cancer microenvironment. Cancer Res. 72:66–75.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bonneau C, Rouzier R, Geyl C, Cortez A,
Castela M, Lis R, Daraï E and Touboul C: Predictive markers of
chemoresistance in advanced stages epithelial ovarian carcinoma.
Gynecol Oncol. 136:112–120. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yin J, Zeng F, Wu N, Kang K, Yang Z and
Yang H: Interleukin-8 promotes human ovarian cancer cell migration
by epithelial-mesenchymal transition induction in vitro. Clin
Transl Oncol. 17:365–370. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Matysiak M, Kapka-Skrzypczak L,
Jodłowska-Jędrych B and Kruszewski M: EMT promoting transcription
factors as prognostic markers in human breast cancer. Arch Gynecol
Obstet. 295:817–825. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Luo Z, Wang Q, Lau WB, Lau B, Xu L, Zhao
L, Yang H, Feng M, Xuan Y, Yang Y, et al: Tumor microenvironment:
The culprit for ovarian cancer metastasis? Cancer Lett.
377:174–182. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 26:402–408. 2001.
View Article : Google Scholar
|
|
18
|
Bellone S, Siegel ER, Cocco E, Cargnelutti
M, Silasi DA, Azodi M, Schwartz PE, Rutherford TJ, Pecorelli S and
Santin AD: Overexpression of epithelial cell adhesion molecule in
primary, metastatic, and recurrent/chemotherapy-resistant
epithelial ovarian cancer: Implications for epithelial cell
adhesion molecule-specific immunotherapy. Int J Gynecol Cancer.
19:860–866. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Münz M, Kieu C, Mack B, Schmitt B, Zeidler
R and Gires O: The carcinoma-associated antigen EpCAM upregulates
c-myc and induces cell proliferation. Oncogene. 23:5748–5758. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Münz M, Zeidler R and Gires O: The
tumor-associated antigen EpCAM upregulates the fatty acid binding
protein E-FABP. Cancer Lett. 225:151–157. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ni J, Cozzi P, Hao J, Beretov J, Chang L,
Duan W, Shigdar S, Delprado W, Graham P, Bucci J, et al: Epithelial
cell adhesion molecule (EpCAM) is associated with prostate cancer
metastasis and chemo/radioresistance via the PI3K/Akt/mTOR
signaling pathway. Int J Biochem Cell Biol. 45:2736–2748. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li Y, Farmer RW, Yang Y and Martin RC:
Epithelial cell adhesion molecule in human hepatocellular carcinoma
cell lines: A target of chemoresistence. BMC Cancer. 16:2282016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zheng J, Zhao L, Wang Y, Zhao S and Cui M:
Clinicopathology of EpCAM and EGFR in human epithelial ovarian
carcinoma. Open Med (Wars). 12:39–44. 2017.PubMed/NCBI
|
|
24
|
Tayama S, Motohara T, Narantuya D, Li C,
Fujimoto K, Sakaguchi I, Tashiro H, Saya H, Nagano O and Katabuchi
H: The impact of EpCAM expression on response to chemotherapy and
clinical outcomes in patients with epithelial ovarian cancer.
Oncotarget. 8:44312–44325. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bokemeyer C: Catumaxomab-trifunctional
anti-EpCAM antibody used to treat malignant ascites. Expert Opin
Biol Ther. 10:1259–1269. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
https://www.ema.europa.eu/documents/public-statement/publicstatement-removab-withdrawal-marketing-authorisation-european-union_en.pdf20–11.
2018
|
|
27
|
Zheng X, Fan X, Fu B, Zheng M, Zhang A,
Zhong K, Yan J, Sun R, Tian Z and Wei H: EpCAM inhibition
sensitizes chemoresistant leukemia to immune surveillance. Cancer
Res. 77:482–493. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang Y, Guo XQ, Niu XL, Wu J, Zhu YQ and
Mao LQ: Relationship of IL-6 and IL-8 secretion in epithelial
ovarian cancer cell lines with their sensitivity to tamoxifen as
well as MAPK, Akt and estrogen receptor phosphorylation. Xi Bao Yu
Fen Zi Mian Yi Xue Za Zhi. 26:21–24. 2010.(In Chinese). PubMed/NCBI
|
|
29
|
Asschert JG, Vellenga E, Ruiters MH and de
Vries EG: Regulation of spontaneous and TNF/INF-induced IL-6
expression in two human ovarian-carcinoma cell lines. Int J Cancer.
82:244–249. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li FF, Shen J, Shen HJ, Zhang X, Cao R,
Zhang Y, Qui Q, Lin XX, Xie YC, Zhang LH, et al: Shp2 plays an
important role in acute cigarette smoke-mediated lung inflammation.
J Immunol. 189:3159–3167. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ke Y, Zhang EE, Hagihara K, Wu D, Pang Y,
Klein R, Curran T, Ranscht B and Feng GS: Deletion of Shp2 in the
brain leads to defective proliferation and differentiation in
neural stem cells and early postnatal lethality. Mol Cell Biol.
27:6706–6717. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lehmann U, Schmitz J, Weissenbach M,
Sobota RM, Hortner M, Friederichs K, Behrmann I, Tsiaris W, Sasaki
A, Schneider-Mergener J, et al: SHP2 and SOCS3 contribute to
Tyr-759-dependent attenuation of interleukin-6 signaling through
gp130. J Biol Chem. 278:661–671. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fischer P, Lehmann U, Sobota RM, Schmitz
J, Niemand C, Linnemann S, Haan S, Behrmann I, Yoshimura A,
Johnston JA, et al: The role of the inhibitors of interleukin-6
signal transduction SHP2 and SOCS3 for desensitization of
interleukin-6 signaling. Biochem J. 378:449–460. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mamik MK and Ghorpade A: Src homology-2
domain-containing protein tyrosine phosphatase (SHP) 2 and p38
regulate the expression of chemokine CXCL8 in human astrocytes.
PLoS One. 7:e455962012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Schafer ZT and Brugge JS: IL-6 involvement
in epithelial cancers. J Clin Invest. 117:3660–3663. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Waugh DJ and Wilson C: The interleukin-8
pathway in cancer. Clin Cancer Res. 14:6735–6741. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Feng GS: Shp-2 tyrosine phosphatase:
Signaling one cell or many. Exp Cell Res. 253:47–54. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
You M, Yu DH and Feng GS: Shp-2 tyrosine
phosphatase functions as a negative regulator of the
interferon-stimulated Jak/STAT pathway. Mol Cell Biol.
19:2416–2424. 1999. View Article : Google Scholar : PubMed/NCBI
|