Introduction
Although there has been great improvement in the
treatment of heart failure, ischemia/reperfusion (I/R) injury
remains a leading cause of mortality in Western countries (1). Numerous studies have indicated that
transplantation of mesenchymal stem cells (MSCs) represents a
potential tool for the repair and regeneration of cardiomyocytes,
thus restoring the function of the heart (2). However, the effects of MSCs are
limited because the majority of transplanted MSCs undergo cell
death soon after transplantation in the ischemic microenvironment
(3). Thus, great efforts have been
made to improve the survival of donor MSCs. The lack of cellular
growth factors and/or insufficient blood supply to the ischemic
region are considered to be the key factors contributing to the
high rate of MSC transplantation failure (4). Therefore, hypoxia and serum
deprivation (H/SD) conditions can be used to mimic the hostile
ischemic microenvironment (4). The
enhancement of MSC viability and survival under ischemic conditions
may be a strategy to improve the efficiency of MSC therapy.
Ulinastatin (UTI), a broad-spectrum protease
inhibitor that can be purified from human urine, has attracted
attention for its protective effects via immunomodulatory and
anti-inflammatory properties. For instance, it has been reported
that UTI protects human endothelial cells from oxidative damage by
suppressing the c-Jun N-terminal kinase/c-Jun signaling pathway
(5). UTI was also reported to
ameliorate I/R injury in the spinal cord (6). Furthermore, a recent study showed
that UTI protected against lipopolysaccharide-induced cardiac
microvascular endothelial cell dysfunction (7). However, the protective effects of UTI
on MSCs for regeneration have not been investigated yet. In the
current study, it was hypothesized that UTI may be able to repress
apoptosis induced by H/SD conditions and thereby improve the
survival of MSCs. To confirm this, the effects of UTI on the
H/SD-induced apoptosis of MSCs and the underlying molecular
mechanisms were investigated.
Materials and methods
Materials
UTI was purchased from Beijing Solarbio Science
& Technology Co., Ltd. (Beijing, China); antibodies for
caspase-3 (cat. no. 14220S), B-cell lymphoma (Bcl)-2 (cat. no.
15071S), induced myeloid leukemia cell differentiation protein
Mcl-1 (Mcl-1; cat. no. 94296S), Bcl-extra large (Bcl-xl; cat. no.
2762S), Bcl-associated protein X (Bax; cat. no. 2772S), activating
transcription factor 4 (ATF4; cat. no. 11815S), GAPDH (cat. no.
8884S), horseradish peroxidase-linked secondary anti-rabbit (cat.
no. 7074) and anti-mouse (cat. no. 7076) were obtained from Cell
Signaling Technology, Inc. (Danvers, MA, USA). C/EBP homologous
protein (CHOP; cat. no. ab11419), phospho-Akt (cat. no. ab38449),
total Akt (cat. no. ab85683), phospho-phosphoinositide 3-kinase
(PI3K; cat. no. ab182651), total-PI3K (cat. no. ab32089),
phospho-mammalian target of rapamycin (mTOR; ab109268) and
total-mTOR (cat. no. ab134903) were purchased from Abcam
(Cambridge, UK). The Annexin V-fluorescein isothiocyanate (FITC)
Apoptosis Detection Kit was purchased from BD Bioscience (Franklin
Lakes, NJ, USA). The Caspase-3 Activity Colorimetric Assay Kit was
purchased from Beyotime Institute of Biotechnology (Haimen, China).
LY294002 and wortmannin, which were used at a concentration of 20
µM, were purchased from Selleck Chemicals (Houston, TX, USA). Cells
were pretreated with LY294002 and wortmannin for 1 h at 37°C prior
to experimentation. All other chemicals were obtained from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Rapamycin was
purchased from Sigma-Aldrich (Merck KGaA), which was kept as 1 mM
stock at −80°C and used at 20 µM. Cells were pretreated with
rapamycin for 1 h at 37°C prior to experimentation.
Culture of MSCs
MSCs were isolated from the bone marrow of two male
2-week-old Sprague-Dawley rats (weighing 60–80 g) as described
earlier (8). The rats were
maintained under the following conditions: Temperature, 22±2°C;
humidity, 55±5%; 12-h light/dark cycle; free access to food and
water. All studies were performed under the approval of the
Institutional Animal Care and Use Committee of Ningbo Medical
Center Lihuili Eastern Hospital (Ningbo, China). Briefly, MSCs were
flushed from femurs and tibias with 10 ml Dulbecco's-modified
Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) containing 1% penicillin/streptomycin (HyClone;
GE Healthcare, Logan, UT, USA). The cells were centrifuged at 300 ×
g for 5 min at 4°C. The pellets were resuspended in 6 ml DMEM with
10% fetal bovine serum (HyClone; GE Healthcare) and 1%
penicillin/streptomycin and plated in a plastic flask at 37°C in a
humidified atmosphere containing 5% CO2. After 3 days,
the medium was replaced, and the cells were washed with PBS. The
medium was replaced every 3 days. At 8 days after seeding, the
cells became >70% confluent. Subsequently, the cells were
collected from the dishes and expanded at a 1:2 dilution. All
subsequent experiments were performed using MSCs at passages
3–5.
For the identification of the MSC immunophenotypic
characteristics, the cells were collected, washed with PBS
(HyClone; GE Healthcare) and labeled with the following antibodies:
Phycoerythrin (PE)-labeled anti-cluster of differentiation 45
(CD45; cat. no. 553091; BD Pharmingen; BD Biosciences), anti-CD105
(cat. no. 611314; BD Pharmingen; BD Biosciences), anti-CD90 (cat.
no. 55595; BD Pharmingen; BD Biosciences) and FITC-labeled-CD73
(cat. no. 561254; BD Pharmingen; BD Biosciences). Subsequently, the
cells were detected by flow cytometry and analyzed using FACSDiva
software (version 6.1.3; BD Biosciences).
Small interfering RNA (siRNA)
transfection
MSCs (4×105 cells/well) were seeded in
6-well plates. When cells reached ~70% confluence, MSCs were
transfected with 200 pM negative control siRNA
(5′-ACGGAACAGCGCACCGAGGCGAA-3′), siRNA against CHOP
(5′-CCAGGAAACGGAAACAGAGTT-3′) or ATF4 (5′-TCCCTCCATGTGTAAAGGA-3′;
Genepharm Inc., Sunnyvale, CA, USA) using Lipofectamine®
2000 (Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. A total of 24 h post-transfection, cells
were harvested and the effects of knockdown were investigated.
H/SD treatment
The harvested MSCs were cultured in 6-well
(1×105 cells/well) or 96-well plates (1×104
cells/well) (Corning Incorporated, Corning, NY, USA) for subsequent
experiments. To induce MSC apoptosis by exposing them to H/SD
conditions, the normal medium was replaced with glucose- and
serum-free medium. Subsequently, the MSCs were placed in an
oxygen-free incubator for 12 h. MSCs cultured under normal
conditions were used as controls.
MTT assay
Cell viability was assayed using the MTT assay as
described previously (9). Briefly,
cells were seeded in 96-well plates with a density of
2×104/well. When reaching ~80% confluence, the cells
were exposed to H/SD treatment with or without UTI (100, 200 and
400 U/ml). After 12 h, 25 µl MTT (5 mg/ml; Sigma-Aldrich; Merck
KGaA) was added to the cultured media, and the cells were incubated
for another 4 h. The medium was then removed, and 200 µl dimethyl
sufoxide was added to each well. The absorbance of the solutions
was measured using a microplate reader (BioTek China, Beijing,
China) at 495 nm. The relative cell viability was measured by
comparison with cells cultured under normal conditions. The
calculated cell viability percentages from the three parallel
experiments were averaged for each set of experimental
conditions.
Apoptosis analysis
Cellular apoptosis was detected using an Annexin
V/propidium iodide (PI) Apoptosis Detection kit (BD Pharmingen; BD
Biosciences) according to the manufacturer's protocol. Briefly,
1×105 cells were harvested and washed in ice-cold PBS,
then cells were resuspended in 300 µl binding buffer and incubated
with 5 µl Annexin V-FITC solution for 30 min in the dark at 4°C,
followed by further incubation with 5 µl PI for 5 min at room
temperature. Apoptotic cells were then determined using a flow
cytometer (FACSCalibur; BD Biosciences). The results were analyzed
by the FlowJo 10.4 (FlowJo LLC, Ashland, OR USA).
Western blot analysis
Cells were collected and lysed in
radioimmunoprecipitation buffer (Beyotime Institute of
Biotechnology). Equal amounts of lysates (20 µg) were resolved by
SDS-PAGE on 12% gels and transferred to polyvinylidene fluoride
(PVDF) membranes. PVDF membranes were incubated with primary
antibodies at a dilution of 1:1,000 overnight at 4°C. Subsequently,
the membranes were washed three times with TBS with Tween20 and
incubated for 1 h at room temperature with the corresponding
horseradish peroxidase-conjugated secondary antibodies (1:10,000;
anti-mouse cat. no. 7076, or anti-rabbit cat. no. 7074; Cell
Signaling Technology, Inc.). The signals were visualized using an
enhanced chemiluminescence reagent (Pierce; Thermo Fisher
Scientific, Inc.). The density of the protein bands was analyzed
using ImageJ 1.41 software (National Institutes of Health,
Bethesda, MD, USA).
Caspase-3 activity assay
The caspase-3 activity was assayed using the
Caspase-3 Activity Colorimetric Assay kit (Beyotime Institute of
Biotechnology) according to the manufacturer's protocol. In brief,
cellular lysates (150 µg) were incubated with 50 µl reaction buffer
containing 10 mM dithiothreitol for 1 h at 37°C in the substrate
DEVD-pNA (200 µM) in a total volume of 100 µl. The pNA light
emission following cleavage from the substrate was quantified using
a microplate reader (BioTek China) at 405 nm.
Statistical analysis
Statistical analysis was performed using SPSS 19.0
software (SPSS, Inc., Chicago, IL, USA). Experiments were repeated
three times and data are expressed as the mean ± standard
deviation. Differences among groups were tested by one-way analysis
of variance, followed by Tukey's test. P<0.05 was considered to
indicate a statistically significant difference.
Results
UTI promotes the viability of MSCs
under H/SD conditions
MSC characteristics were confirmed by flow
cytometric analysis, which showed that the cells were negative for
CD45, and positive for CD90, CD103 and CD73 markers (data not
shown). To investigate the protective effects of UTI, cell
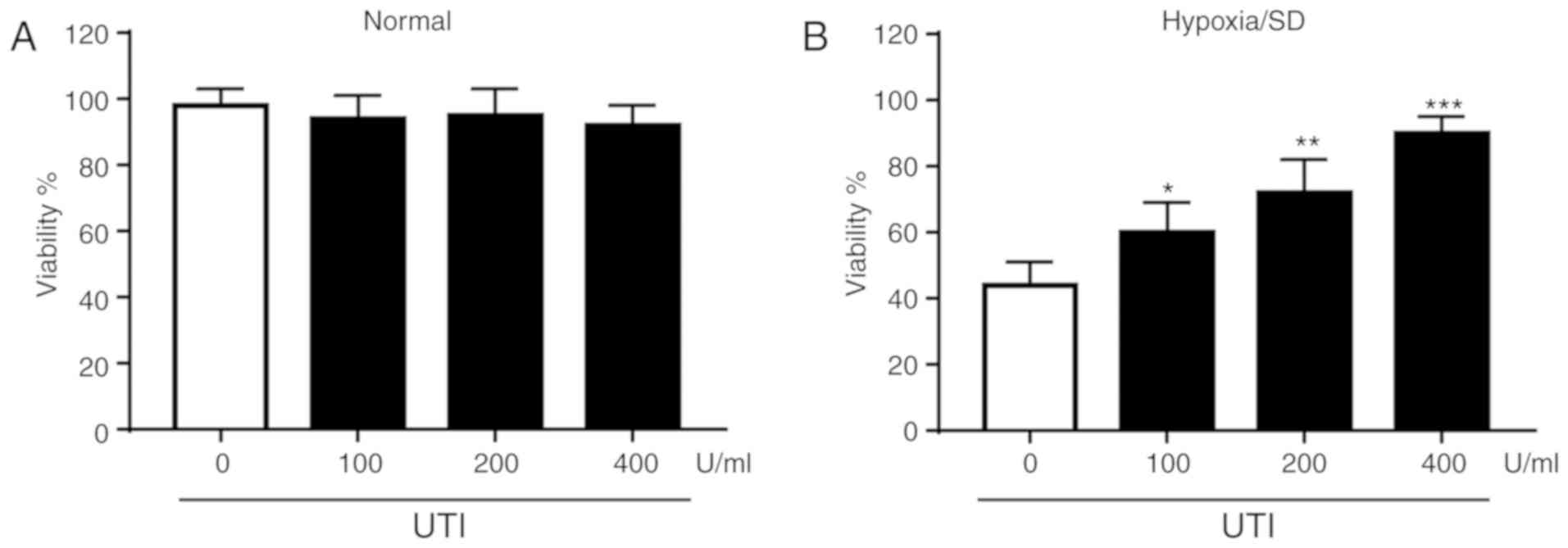
viability was initially examined. As shown in Fig. 1A, UTI exhibited little cytotoxicity
on MSCs under normal culture conditions. Cells were treated with
increasing concentrations of UTI (100, 200 and 400 U/ml) when
exposed to H/SD for 12 h (Fig.
1B). The data revealed that H/SD reduced cell viability and UTI
protected MSCs against H/SD injury in a dose-dependent manner.
UTI protects MSCs from apoptosis under
H/SD conditions
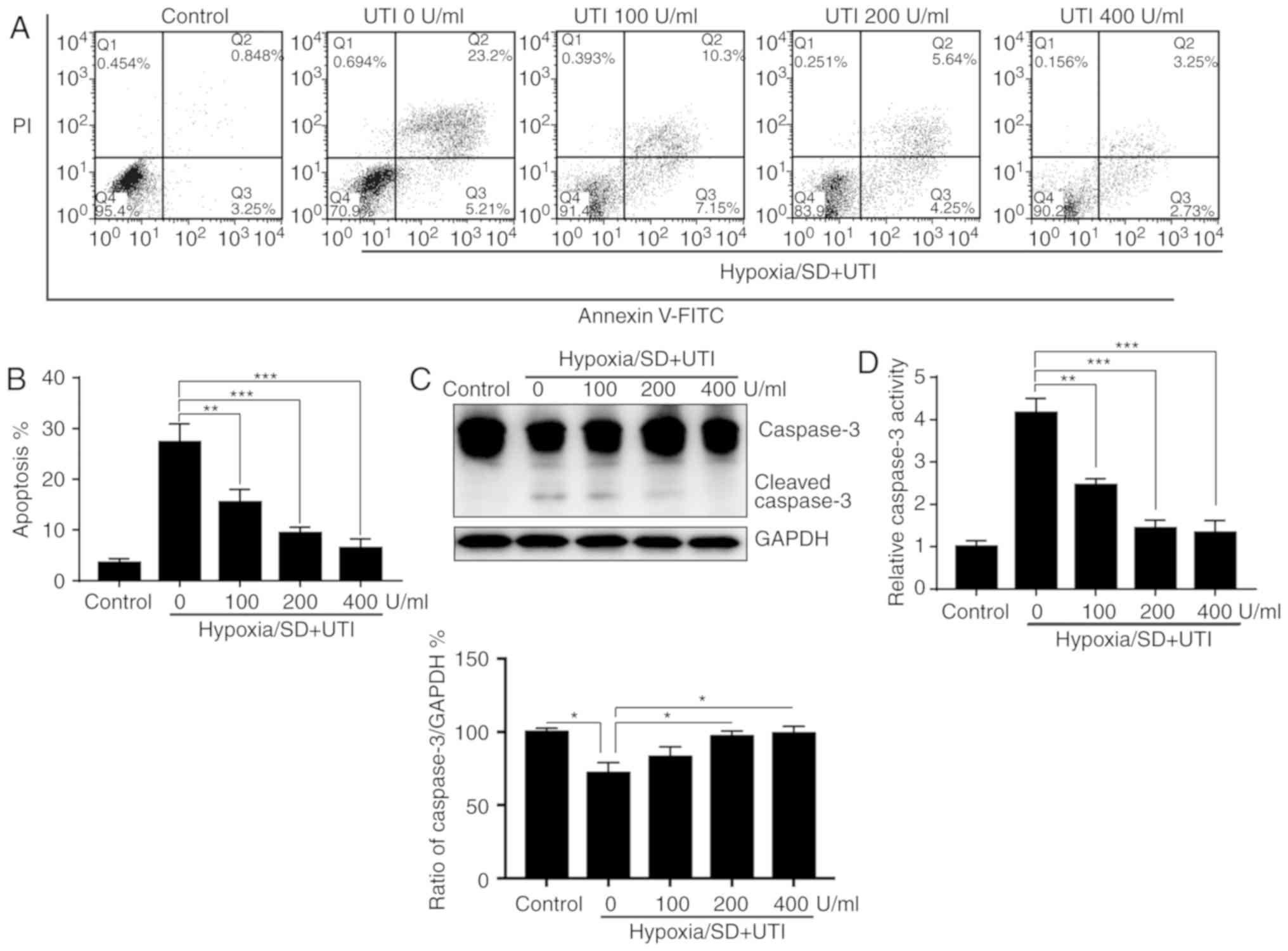
To further analyze the cytoprotective effects of
UTI, the apoptosis rate of the MSCs was assayed by Annexin V/PI
staining and flow cytometry. As indicated in Fig. 2A and B, treatment with UTI reduced
the apoptosis induced by H/SD in a dose-dependent manner.
Subsequently, the levels of cleaved caspase-3 were evaluated by
western blotting. Cleaved caspase-3 was notably inhibited by UTI in
a dose-dependent manner (Fig. 2C).
Furthermore, caspase-3 activity assays revealed that activation of
caspase-3 induced by H/SD was also decreased by UTI in a
dose-dependent manner (Fig. 2D).
Taken together, these findings demonstrated that UTI protects MSCs
against apoptosis under H/SD conditions.
UTI treatments modulate Bcl-2 family
protein expression levels
The process of apoptosis is regulated by Bcl-2
family proteins, which can be divided into anti-apoptotic and
pro-apoptotic members (10).
Therefore, whether Bcl-2 family members were affected by UTI was
investigated. As indicated in Fig.
3, anti-apoptotic Bcl-2, Mcl-1 and Bcl-xl were decreased, while
pro-apoptotic Bax was upregulated in MSCs exposed to H/SD
conditions. Treatment with UTI reversed the effects of H/SD on
Bcl-2 family proteins (Fig. 3).
These findings suggested that UTI exerts a protective effect
against H/SD-induced apoptosis via modulation of Bcl-2 family
proteins in MSCs.
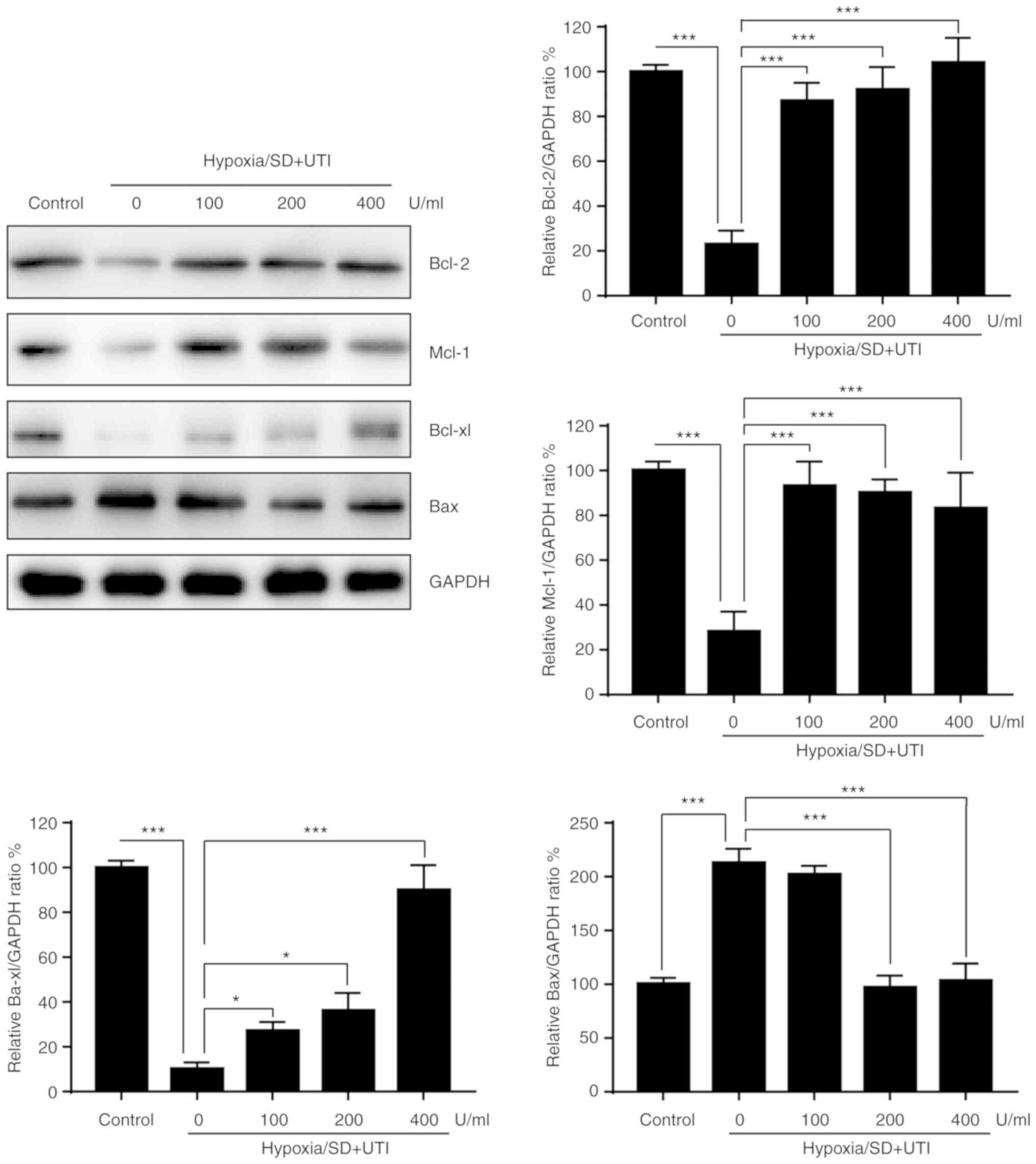 | Figure 3.Effects of UTI on the Bcl-2 family
proteins. MSCs were treated with various doses of UTI under
hypoxia/SD conditions for 12 h. Subsequently, cellular lysates were
subjected to western blot analysis with the indicated antibodies,
and the results were quantified. The data are presented as the mean
± standard deviation (n=3). *P<0.05; ***P<0.001, vs. the
group without UTI. MSC, mesenchymal stem cell; SD, serum
deprivation; UTI, ulinastatin; Bcl, B-cell lymphoma; Bax,
Bcl-associated X protein; Mcl-1, induced myeloid leukemia cell
differentiation protein Mcl-1; Bcl-xl, Bcl-extra large. |
UTI protects MSCs from H/SD-induced
apoptosis via inhibition of endoplasmic reticulum (ER) stress
Previous studies indicated that ATF4 and CHOP are
vital players in ER stress that are involved in apoptosis induced
by H/SD (11). To determine
whether ER stress is involved in the protective effect of UTI, ATF4
and CHOP expression levels were examined by western blotting. As
shown in Fig. 4A, H/SD induced the
upregulation of ATF4/CHOP, and treatment with UTI reversed the
effect of H/SD on ATF4/CHOP expression levels. To further confirm
the role of ER stress in H/SD-induced apoptosis of MSCs, siRNA was
performed to knock down CHOP and ATF4 expression levels (Fig. 4B). As indicated in Fig. 4C, silencing of CHOP or ATF4
protects MSCs from apoptosis induced by H/SD. Taken together, these
results suggested that UTI protects MSCs from H/SD-induced
apoptosis, at least partially, by inhibiting ER stress.
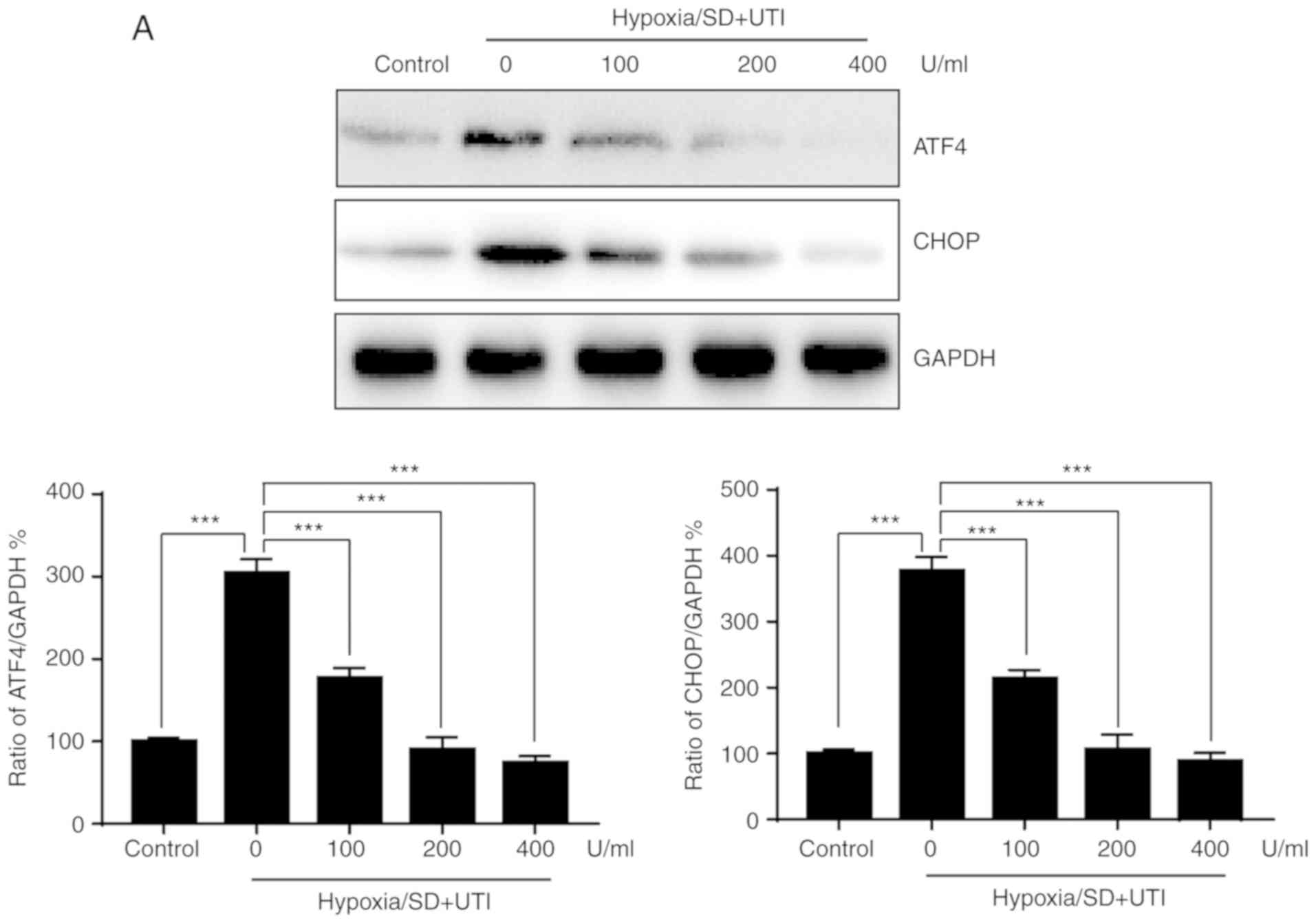 | Figure 4.UTI reduces apoptosis in MSCs by
inhibiting ER stress. MSCs were treated with various doses of UTI
under hypoxia/SD condiions for 12 h. (A) Total cellular lysates
were subjected to western blot analysis with the indicated
antibodies, and the results were quantified. (B) MSCs were
transfected with siRNA against CHOP or ATF4 for 24 h, and the
expression of CHOP or ATF4 was detected by western blotting. (C)
MSCs were transfected with siRNA against CHOP or ATF4 for 24 h,
then cells were cultured under hypoxia/SD conditions or not for
another 24 h, and apoptosis was quantified. The data are presented
as the mean ± standard deviation (n=3). **P<0.01; ***P<0.001.
MSC, mesenchymal stem cell; SD, serum deprivation; UTI,
ulinastatin; ATF4, activating transcription factor 4; CHOP, C/EBP
homologous protein; si, small interfering RNA; NC, normal
control. |
Inhibition of PI3K/Akt/mTOR could
interfere with the protective effects of UTI against apoptosis
induced by H/SD
The PI3K/Akt/mTOR signaling pathway serves a
critical role in cell survival (9). Thus, to examine whether the
PI3K/Akt/mTOR pathway is also involved in the anti-apoptotic
effects of UTI, its activation was investigated by western
blotting. As shown in Fig. 5A,
H/SD treatment reduced expression levels of phospho-Akt,
phospho-PI3K and phospho-mTOR compared with the control, whereas
these effects were reversed by UTI treatment. To verify that the
PI3K/Akt/mTOR signaling pathway was involved in UTI-mediated
anti-apoptotic effects, two PI3K inhibitors, wortmannin and
LY294002, were used. As shown in Fig.
5B, H/SD inhibited the expression of phospho-Akt, which can be
activated by UTI treatment. However, both wortmannin (20 µM) and
LY294002 (20 µM) fully inhibited the upregulation of phospho-Akt
induced by UTI (Fig. 5B).
Furthermore, the apoptotic cells were increased after wortmannin
and LY294002 treatment compared with the UTI group under H/SD
conditions (Fig. 5C).
Additionally, rapamycin, an mTOR inhibitor (20 µM), was applied to
investigate the role of mTOR in the protective effects of UTI. As
indicated in Fig. 5D, rapamycin
successfully inhibited the phosphorylation of mTOR. Additionally,
the apoptotic cells increased following rapamycin treatment
compared with the UTI group under H/SD conditions (Fig. 5E). Taken together, these findings
further confirmed that UTI exerts protective effects via activation
of the PI3K/Akt/mTOR pathway.
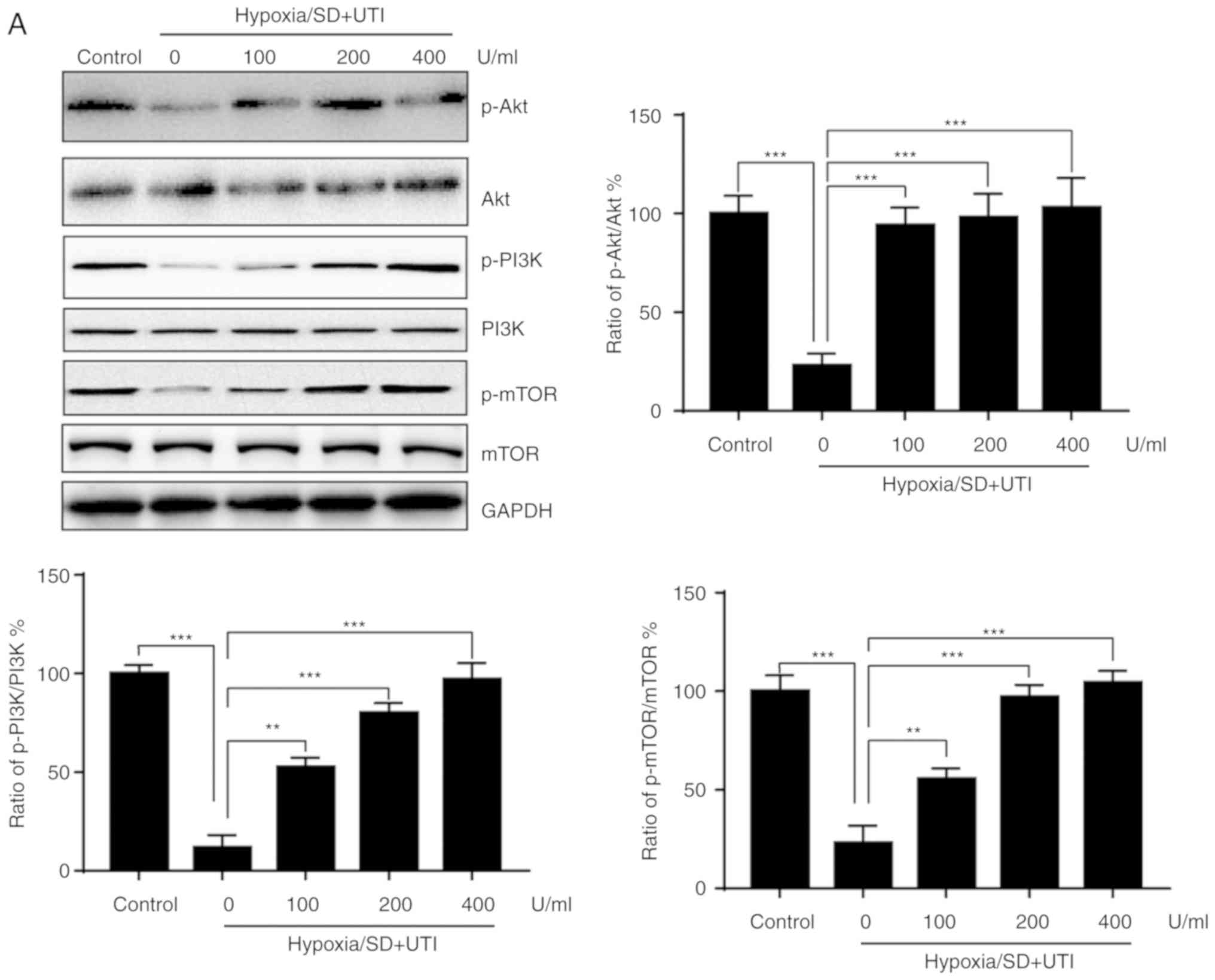 | Figure 5.UTI exerts anti-apoptotic effects via
activation of the PI3K/Akt pathway. (A) MSCs were treated with
various doses of UTI under hypoxia/SD conditions for 12 h. Total
cellular lysates were subjected to western blot analysis with the
indicated antibodies, and the results were semi-quantified. (B)
MSCs were exposed to hypoxia/SD conditions. In parallel
experiments, cells were treated with either UTI (400 U/ml),
wortmannin (20 µM) or LY294002 (20 µM). Total cellular lysates were
subjected to western blot analysis with the indicated antibodies,
and the results were quantified. (C) Cells were treated as above,
and then cellular apoptosis was measured. (D) MSCs were exposed to
hypoxia/SD conditions and were treated with either UTI (400 U/ml)
or UTI (400 U/ml) plus rapamycin (20 µM). Total cellular lysates
were subjected to western blot analysis with the indicated
antibodies, and the results were quantified. (E) Cells were treated
as above, and then cellular apoptosis was measured. The data are
presented as the mean ± standard deviation (n=3). **P<0.01;
***P<0.001. MSC, mesenchymal stem cell; SD, serum deprivation;
UTI, ulinastatin; p, phosphorylated; PI3K,
phosphatidylinositol-4,5-bisphosphate 3-kinase; mTOR, mammalian
target of rapamycin. |
Discussion
MSCs have exhibited great therapeutic potential due
to their ability to regenerate and repair the injured myocardium
through paracrine mechanisms following transplantation into an
ischemic or infarcted heart (12,13).
Since MSCs are easily obtained and expanded, they also represent
one of the cell therapy tools with the most potential for tissue
regeneration in various clinical diseases (14). However, the therapeutic application
of MSCs is limited by the fact that the majority of the implanted
MSCs do not survive transplantation, partially due to cell death
induced by the ischemic cardiac microenvironment (15). Hodgetts et al (16) reported that >90% of MSCs die
within 24 h of transplantation. Another study reported that ~21% of
MSCs survive after 4 h of transplantation and only 3.6% survive
after 7 days (17). Therefore,
great efforts have been made to prevent stem cell apoptosis to
improve their therapeutic potential. One available strategy to
enhance MSC survival is treatment with agents that can enhance the
viability of MSCs. For example, a previous study reported that
prostaglandin E improved MSC survival under H/SD conditions, which
is a commonly used model to mimic the ischemic environment in
vitro (18). Another study
reported that nicorandil protected MSCs from H/SD-induced apoptosis
(19). In the present study, UTI
inhibited the apoptosis of MSCs in a dose-dependent manner under
H/SD conditions. Furthermore, the anti-apoptotic effects of UTI
were revealed to be exerted via the modulation of Bcl-2 family
proteins, ER stress and PI3K/Akt signaling pathways. The present
study revealed the protective effects of UTI on MSCs under H/SD
conditions in vitro, and the mechanisms leading to
protection were investigated.
UTI is a multivalent Kunitz-type serine protease
inhibitor that can be isolated from human urine and blood (20). UTI has been reported to protect
organs against injury via inhibition of various proteases, such as
trypsin, chymotrypsin, neutrophil elastase and plasmin (21). Furthermore, UTI also possesses
therapeutic potential to treat inflammatory diseases and subsequent
organ damage with few side effects (22).
In the present study, UTI enhanced the proliferation
of MSCs in a dose-dependent manner. UTI also repressed the
apoptosis induced by H/SD and modulated the expression of Bcl-2
family of proteins. Apoptosis is a complex multistep process that
is subjected to regulation by various proteins. Apoptosis is
predominantly triggered via two pathways: The extrinsic and the
intrinsic/mitochondrial pathway (9). Deprivation of serum is a
well-established cause of apoptosis via activation of the
intrinsic/mitochondrial pathway (23). The intrinsic apoptotic pathway is
controlled by Bcl-2 family proteins (10). The effects of UTI on the inhibition
of caspase-3 and the regulation of Bcl-2 family proteins indicated
that UTI repressed apoptosis via blocking the
intrinsic/mitochondrial pathway. These findings were in line with a
previous study in which UTI enhanced the expression of Bcl-2, and
thereby, inhibited cell apoptosis in an animal model of hemorrhagic
shock (24).
The ER is an organelle involved in calcium
homeostasis, lipid biosynthesis and protein folding. Various
stimuli, such as ischemia, hypoxia, heat shock, and oxidative
stress, can lead to ER dysfunction, which is also termed ER stress
(25). It has been demonstrated
that ER stress serves an important role in the apoptosis of various
types of cells in models of ischemia in vivo and in
vitro (26). Furthermore, MSCs
are susceptible to apoptosis induced by ER stress, and blockage of
ER stress promoted the survival of MSCs under H/SD conditions
(27). In response to ER stress,
there is an upregulation of ER chaperones such as CHOP and ATF4
(28). In the present study, CHOP
and ATF4 expression levels were upregulated under H/SD conditions
and treatment with UTI could reverse this effect. These findings
indicated that the UTI inhibition of apoptosis of MSCs under H/SD
may be attributed to the blockage of ER stress.
PI3K/Akt/mTOR is a vital cell survival pathway, and
Akt phosphorylation enhances survival in various cell types
(29). Previous studies have
reported that activation of the Akt signaling pathway promoted the
survival of MSCs following transplantation into the ischemic
porcine heart (30). UTI has been
shown to inhibit apoptosis of cardiomyocytes via activation of the
PI3K/Akt signaling pathway, which serves a significant protective
role against MSC apoptosis (31).
The present results demonstrated that UTI activated the
PI3K/Akt/mTOR signaling pathway and that these protective effects
were clearly diminished by two PI3K inhibitors, wortmannin and
LY294002, and the mTOR inhibitor, rapamycin. These findings suggest
a critical role of the PI3K/Akt/mTOR signaling pathway in the
UTI-mediated protection of MSCs exposed to H/SD. These results
agree with previous studies suggesting that activation of
PI3K/Akt/mTOR enhances the survival of MSCs under H/SD conditions
(32). Currently, inhibition of
the PI3K/Akt/mTOR pathway is used as a therapeutic strategy against
various diseases, including human immunodeficiency virus and cancer
(33–36). The inhibition of PI3K/Akt/mTOR
signaling resulted in increased apoptosis of MSCs. Therefore, the
application of PI3K/Akt/mTOR inhibitors to MSCs should be
investigated further.
The present study has certain limitations. The H/SD
condition is an in vitro model that cannot fully mimic the
in vivo microenvironment. Further investigations are
required to unveil the cellular mechanisms involved in vitro
and in vivo. Additionally, UTI is a well-known regulator of
inflammatory processes that may also participate in the survival of
MSCs under H/SD conditions. Therefore, the role of inflammasomes in
MSC survival and its association with UTI needs to be
investigated.
Taken together, the present results provide
preliminary evidence indicating that UTI promotes MSC survival
under H/SD conditions. The pro-survival effects of UTI against
H/SD-induced apoptosis possibly relies on the modulation of the
Bcl-2 family of proteins, ER stress responses and PI3K/Akt
signaling pathways. Therefore, UTI may be a therapeutic agent with
the potential to optimize MSC therapy.
Acknowledgements
The authors would like to thank Dr Rui Yu (School of
Medicine, Ningbo University, Ningbo, Zhejiang, China) for their
critical comments on this manuscript.
Funding
The present study was supported by grants from the
Ningbo Natural Science Foundation of China (Grant no.
2015A610203).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
LS and ZD designed the research. LS, LY, PL and GY
performed the experiments. JC and DL analyzed the data. LS wrote
the manuscript. LS and ZD revised the manuscript.
Ethics approval and consent to
participate
All studies were performed under the approval of the
Institutional Animal Care and Use Committee of Ningbo Medical
Center Lihuili Eastern Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lopez AD, Mathers CD, Ezzati M, Jamison DT
and Murray CJ: Global and regional burden of disease and risk
factors, 2001: Systematic analysis of population health data.
Lancet. 367:1747–1757. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Strauer BE and Steinhoff G: 10 years of
intracoronary and intramyocardial bone marrow stem cell therapy of
the heart: From the methodological origin to clinical practice. J
Am Coll Cardiol. 58:1095–1104. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Menasché P: Current status and future
prospects for cell transplantation to prevent congestive heart
failure. Semin Thorac Cardiovasc Surg. 20:131–137. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhu W, Chen J, Cong X, Hu S and Chen X:
Hypoxia and serum deprivation-induced apoptosis in mesenchymal stem
cells. Stem Cells. 24:416–425. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li C, Ma D, Chen M, Zhang L, Zhang L,
Zhang J, Qu X and Wang C: Ulinastatin attenuates LPS-induced human
endothelial cells oxidative damage through suppressing JNK/c-Jun
signaling pathway. Biochem Biophys Res Commun. 474:572–578. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu B, Huang W, Xiao X, Xu Y, Ma S and Xia
Z: Neuroprotective effect of ulinastatin on spinal cord
ischemia-reperfusion injury in rabbits. Oxid Med Cell Longev 2015.
6248192015.
|
|
7
|
Yu Z, Rayile A, Zhang X, Li Y and Zhao Q:
Ulinastatin protects against lipopolysaccharide-induced cardiac
microvascular endothelial cell dysfunction via downregulation of
lncRNA MALAT1 and EZH2 in sepsis. Int J Mol Med. 39:1269–1276.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Makino S, Fukuda K, Miyoshi S, Konishi F,
Kodama H, Pan J, Sano M, Takahashi T, Hori S, Abe H, et al:
Cardiomyocytes can be generated from marrow stromal cells in vitro.
J Clin Invest. 103:697–705. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu R, Yu BX, Chen JF, Lv XY, Yan ZJ, Cheng
Y and Ma Q: Anti-tumor effects of Atractylenolide I on bladder
cancer cells. J Exp Clin Cancer Res. 35:402016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Garner TP, Lopez A, Reyna DE, Spitz AZ and
Gavathiotis E: Progress in targeting the BCL-2 family of proteins.
Curr Opin Chem Biol. 39:133–142. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Maekawa H and Inagi R: Stress signal
network between hypoxia and ER Stress in chronic kidney disease.
Front Physiol. 8:742017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shake JG, Gruber PJ, Baumgartner WA,
Senechal G, Meyers J, Redmond JM, Pittenger MF and Martin BJ:
Mesenchymal stem cell implantation in a swine myocardial infarct
model: Engraftment and functional effects. Ann Thorac Surg.
73:1919–1926. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Orlic D, Kajstura J, Chimenti S, Limana F,
Jakoniuk I, Quaini F, Nadal-Ginard B, Bodine DM, Leri A and Anversa
P: Mobilized bone marrow cells repair the infarcted heart,
improving function and survival. Proc Natl Acad Sci USA.
98:10344–10349. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Marofi F, Vahedi G, Biglari A,
Esmaeilzadeh A and Athari SS: Mesenchymal stromal/stem cells: A new
era in the cell-based targeted gene therapy of cancer. Front
Immunol. 8:17702017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hale SL, Dai W, Dow JS and Kloner RA:
Mesenchymal stem cell administration at coronary artery reperfusion
in the rat by two delivery routes: A quantitative assessment. Life
Sci. 83:511–515. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hodgetts SI, Beilharz MW, Scalzo AA and
Grounds MD: Why do cultured transplanted myoblasts die in vivo? DNA
quantification shows enhanced survival of donor male myoblasts in
host mice depleted of CD4+ and CD8+ cells or Nk1.1+ cells. Cell
Transplant. 9:489–502. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tang YL, Tang Y, Zhang YC, Qian K, Shen L
and Phillips MI: Improved graft mesenchymal stem cell survival in
ischemic heart with a hypoxia-regulated heme oxygenase-1 vector. J
Am Coll Cardiol. 46:1339–1350. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zeng K, Deng BP, Jiang HQ, Wang M, Hua P,
Zhang HW, Deng YB and Yang YQ: Prostaglandin E1 protects
bone marrow-derived mesenchymal stem cells against serum
deprivation-induced apoptosis. Mol Med Rep. 12:5723–5729. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang F, Cui J, Lv B and Yu B: Nicorandil
protects mesenchymal stem cells against hypoxia and serum
deprivation-induced apoptosis. Int J Mol Med. 36:415–423. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Inoue K and Takano H: Urinary trypsin
inhibitor as a therapeutic option for endotoxin-related
inflammatory disorders. Expert Opin Investig Drugs. 19:513–520.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Inoue K, Takano H, Yanagisawa R and
Yoshikawa T: Protective effects of urinary trypsin inhibitor on
systemic inflammatory response induced by lipopolysaccharide. J
Clin Biochem Nutr. 43:139–142. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Atal SS and Atal S: Ulinastatin-a newer
potential therapeutic option for multiple organ dysfunction
syndrome. J Basic Clin Physiol Pharmacol. 27:91–99. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bialik S, Cryns VL, Drincic A, Miyata S,
Wollowick AL, Srinivasan A and Kitsis RN: The mitochondrial
apoptotic pathway is activated by serum and glucose deprivation in
cardiac myocytes. Circ Res. 85:403–414. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen CC, Liu ZM, Wang HH, He W, Wang Y and
Wu WD: Effects of ulinastatin on renal ischemia-reperfusion injury
in rats. Acta Pharmacol Sin. 25:1334–1340. 2004.PubMed/NCBI
|
|
25
|
Merksamer PI, Trusina A and Papa FR:
Real-time redox measurements during endoplasmic reticulum stress
reveal interlinked protein folding functions. Cell. 135:933–947.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Badiola N, Penas C, Miñano-Molina A,
Barneda-Zahonero B, Fadó R, Sánchez-Opazo G, Comella JX, Sabriá J,
Zhu C, Blomgren K, et al: Induction of ER stress in response to
oxygen-glucose deprivation of cortical cultures involves the
activation of the PERK and IRE-1 pathways and of caspase-12. Cell
Death Dis. 2:e1492011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Motegi SI, Sekiguchi A, Uchiyama A, Uehara
A, Fujiwara C, Yamazaki S, Perera B, Nakamura H, Ogino S, Yokoyama
Y, et al: Protective effect of mesenchymal stem cells on the
pressure ulcer formation by the regulation of oxidative and
endoplasmic reticulum stress. Sci Rep. 7:171862017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sears TK and Angelastro JM: The
transcription factor ATF5: Role in cellular differentiation, stress
responses, and cancer. Oncotarget. 8:84595–84609. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Franke TF, Kaplan DR and Cantley LC: PI3K:
Downstream AKTion blocks apoptosis. Cell. 88:435–437. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lim SY, Kim YS, Ahn Y, Jeong MH, Hong MH,
Joo SY, Nam KI, Cho JG, Kang PM and Park JC: The effects of
mesenchymal stem cells transduced with Akt in a porcine myocardial
infarction model. Cardiovasc Res. 70:530–542. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen J, Crawford R, Chen C and Xiao Y: The
key regulatory roles of the PI3K/Akt signaling pathway in the
functionalities of mesenchymal stem cells and applications in
tissue regeneration. Tissue Eng Part B Rev. 19:516–528. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Guo Z, Li CS, Wang CM, Xie YJ and Wang AL:
CSE/H2S system protects mesenchymal stem cells from hypoxia and
serum deprivation-induced apoptosis via mitochondrial injury,
endoplasmic reticulum stress and PI3K/Akt activation pathways. Mol
Med Rep. 12:2128–2134. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Maksimovic-Ivanic D, Fagone P, McCubrey Y,
Bendtzen K, Mijatovic S and Nicoletti F: HIV-protease inhibitors
for the treatment of cancer: Repositioning HIV protease inhibitors
while developing more potent NO-hybridized derivatives? Int J
Cancer. 140:1713–1726. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nicoletti F, Fagone P, Meroni P, McCubrey
J and Bendtzen K: mTOR as a multifunctional therapeutic target in
HIV infection. Drug Discovery Today. 16:715–721. 2017. View Article : Google Scholar
|
|
35
|
Donia M, McCubrey JA, Bendtzen K and
Nicoletti F: Potential use of rapamycin in HIV infection. Br J Clin
Pharmacol. 70:784–793. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yu Y, Yu X, Ma J, Tong Y and Yao J:
Effects of NVP-BEZ235 on the proliferation, migration, apoptosis
and autophagy in HT-29 humancolorectal adenocarcinoma cells. Int J
Oncol. 49:285–293. 2016. View Article : Google Scholar : PubMed/NCBI
|