Introduction
Mucosal epidermal keratinocytes release
proinflammatory cytokines during the fast innate immune response to
microbial infection (1–3). Interleukin-1α (IL-1α) is one of the
cytokines produced by oral keratinocytes (3–8). The
binding of IL-1α to its cell surface IL-1 receptor induces the
activation of nuclear factor-κB, c-Jun N-terminal kinase and
p38-mitogen activated protein kinase (MAPK) target gene
transcription (9,10). Functionally, pro-IL-1α has a
nuclear localization signal at its N-terminus and binds
HS-1-associated protein X-1 to allow for translocation into the
nucleus; it then binds transcription activators to directly affect
target gene transcription (9,10).
In response to IL-1α, the expression levels of antimicrobial
proteins/peptides, including calprotectin (a heterodimer complex of
S100A8/A9), defensin and adrenomedullin, are significantly
upregulated in epidermal keratinocytes (1–3,5-7). Calprotectin has
a variety of antimicrobial activities in keratinocytes and is
important in mucosal innate immunity (1–8). In
addition, tumor-suppressive roles of calprotectin in head and neck
squamous cell carcinoma (HNSCC) have been reported (11,12).
Mechanistically, calprotectin negatively regulates G2/M
cell cycle progression and growth in a protein phosphatase
2α-dependent manner in HNSCC (11,12).
The human S100A8 promoter has been well
characterized (13). Transcription
factors that can regulate the expression of human S100A8
include CAAT enhancer binding protein α (C/EBPα) (14), C/EBPβ (15) and hypoxia inducible factor-1
(16). However, several copies of
Ets transcription factor, E-Box and C/EBPβ consensus sequences have
been observed in the murine S100A8 promoter region (17).
Although IL-1α has been reported to increase the
expression of S100A8 in the HaCaT human keratinocyte cell
line (5,6), the mechanism underlying the effect of
IL-1α on human S100A8 in keratinocytes remains to be fully
elucidated. Determining the molecular mechanism underlying the
expression of S100A8 induced by IL-1α may provide a better
understanding of the roles of calprotectin during the infection of
mucosal epithelial cells. Therefore, to the best of our knowledge,
the results of the present study are the first to provide a
conceivable mechanism underlying the effects of human S100A8
induced by IL-1α in epidermal keratinocytes.
Materials and methods
Cell culture and IL-1α treatment
The human TR146 epithelial cancer cells (ATCC,
Manassas, VA, USA) were maintained in Dulbecco's modified Eagle's
medium (Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% FBS (Gemini Bio-Products, Sacramento, CA,
USA). The TR146 cells were incubated at 37°C in an incubator with
5% CO2. Recombinant IL-1α (Sino Biological, Inc.,
Beijing, China) was dissolved and cell treatment was then performed
as described previously (1).
Briefly, cells were seeded 105 per well into 24-well
plates. Following overnight incubation at 37°C, cells were washed
with Dulbecco's phosphate-buffered saline (DPBS), different
concentrations of IL-1α were added or bovine serum albumin (BSA;
vehicle, 50 µg/ml BSA in DPBS).
Reverse transcription-quantitative
polymerase chain reaction analysis (RT-qPCR) analysis
Total RNA was extracted using TRIzol reagent (Thermo
Fisher Scientific, Inc.). Reverse transcription was performed with
a FastQuant RT kit and gDNase (Tiangen Biotech Co., Ltd., Beijing,
P. R. China). RT-qPCR with SYBR Green I was then conducted. Primers
for the qPCR amplification of human S100A8 [primer pair
S100A8-1 forward (F)/S100A8-1reverse (R)] (1), and β-actin (ACTB; primer pair
ACTB-1F/ACTB-1R) (18) were used
(Table I). Each reaction was
performed in a 20 µl volume containing 1XSYBR qPCR MasterMix
(Fermentas; Thermo Scientific, Inc.), 50 nM of each primer and 1 µl
cDNA. The cycling conditions were: 2 min at 95°C, followed by 40
cycles at 95°C for 5 sec and 60°C for 30 sec. The mRNA expression
levels of human S100A8 were standardized to the mRNA
expression of β-actin. The RT-qPCR results were quantified using
the 2−ΔΔCq method (19).
 | Table I.Oligonucleotides used in the present
study. |
Table I.
Oligonucleotides used in the present
study.
| Primer name | Oligonucleotide
sequence (5′-3′)a |
|---|
| RT-qPCR
analysis |
|
|
S100A8-1F |
GGGCATCATGTTGACCGAGC |
|
S100A8-1R |
GTAACTCAGCTACTCTTTGTGGCTT |
|
ACTB-1F |
GACGACATGGAGAAAATCTG |
|
ACTB-1R |
ATGATCTGGGTCATCTTCTC |
| Plasmid
construction |
|
|
PA8-1 |
CTAGCTAGCAGGGACTGAGCCCTTTCCTGTAAACATG |
|
PA8-2 |
GAAGATCTGTCCAGCCTAGGAGACAATGTGCC |
|
PA8-3 |
GCAGGGCTGAGAGGCAGCTCC |
|
pGL3-S |
AGATCTGCGATCTAAGTAAGCTTGGCATTC |
|
DPA8-1 |
CCCGGACATGGGAAAAGCTCAG |
|
DPA8-2 |
GGTGGGGAGAGGATTTGTTCCTCC |
|
DPA8-3 |
CTCCATCTCCCAGGGCATGGTC |
|
DPA8-4 |
TGCGGTCTTTGGACCCTTTGAAAC |
|
DPA8-5 |
AAGCAAGTGGATGCCAGCAGC |
|
DPA8-6 |
TCTGATGGCCTGAAGCTGTGGG |
|
pGL3-AS |
GGCTAGCACGCGTAAGAGCTCGGTAC |
|
DDA8-1 |
CCAGCAGCCCAGAAAAAGAGCC |
|
DDA8-2 |
CTACCTGCTTTTTCCTTCTGGGCAC |
|
DDA8-3 |
TGCCTTCCTCTTTCCGCTTCTCC |
|
DDA8-4 |
TCCCCACCCAAAATTTTCATTCTGC |
|
DDA8-5 |
CAACTCTGGCAGGGAGAAGCTGTC |
| EMSA assays |
|
|
EP1s |
TCCCCACCCAAAATTTTCATTCTGC |
|
EP1as |
GCAGAATGAAAATTTTGGGTGGGGA |
|
EP2s |
CTGCACAGTGATTGCCACATTCACC |
|
EP2as |
GGTGAATGTGGCAATCACTGTGCAG |
|
EP3s |
CATTCACCTGGTTGAGAAACCAGAGAC |
|
EP3as |
GTCTCTGGTTTCTCAACCAGGTGAATG |
| ChIP assay |
|
|
Ch-1 |
TGCCTTCCTCTTTCCGCTTC |
|
Ch-2 |
CAGCTGCCCACAGCTTCAG |
|
Ch-3 |
GTACATGATGTGGGAAGGAG |
|
Ch-4 |
ACCTAGTGATGTGGACATTAC |
| Mutagenesis of
C/EBPβ binding sitesa |
|
|
M3-1 |
GCCACATTCACCTGGTTGAGCCTTCAGAGACTGTAGCAACTC |
|
M3-2 |
GAGTTGCTACAGTCTCTGAAGGCTCAACCAGGTGAATGTGGC |
Plasmid construction
A PCR fragment (primer pair PA8-1/PA8-2) was
amplified from human blood genomic DNA (Promega Corporation,
Madison, WI, USA) using Herculase® II Fusion DNA
Polymerase (Agilent Technologies, Inc., Santa Clara, CA, USA),
which was then ligated into pGL3-basic (Promega Corporation) via
the NheI and BglII restriction sites, to generate the
pGL3(−3096/+246) construct. The cycling conditions were as follows:
2 min at 94°C, followed by 36 cycles at 94°C for 20 sec, 63°C for
30 sec and 72°C for 90 sec, followed by a final extension at 72°C
for 3 min. A 7,906-bp fragment (PA8-3/pGL3-S) was amplified from
pGL3 (−3096/+246) and this fragment was then self-ligated to
generate the pGL3(−3096/-1) construct. To generate S100A8
promoter 5′deletion mutants, fragments of 6,697 bp
(DPA8-1/pGL3-AS), 5,739 bp (DPA8-2/pGL3-AS), 5,488 bp
(DPA8-3/pGL3-AS), 5,229 bp (DPA8-4/pGL3-AS), 5,053 bp
(DPA8-5/pGL3-AS) and 4,882 bp (DPA8-6/pGL3-AS) were amplified from
pGL3 (−3096/-1), respectively (Table
I). The products were purified and self-ligated to generate
pGL3(−1887/-1), pGL3(−929/-1), pGL3(−678/-1), pGL3(−419/-1),
pGL3(−243/-1) and pGL3 (−72/-1), respectively. Similarly, fragments
of 5,067 bp (DDA8-1/pGL3-AS), 5,042 bp (DDA8-2/pGL3-AS), 5,003 bp
(DDA8-3/pGL3-AS), 4,975 bp (DDA8-4/pGL3-AS) and 4,921 bp
(DDA8-5/pGL3-AS) were amplified from the pGL3 (−419/-1) construct
(Table I), and these the fragments
were purified and self-ligated to generate pGL3(−257/-1),
pGL3(−232/-1), pGL3(−193/-1), pGL3(−165/-1) and pGL3(−111/-1),
respectively. The cycling conditions for amplification of
self-ligated fragments were: 2 min at 94°C, followed by 36 cycles
at 94°C for 20 sec, 63°C for 30 sec and 72°C for 4 min, followed by
a final extension at 72°C for 5 min. Primer pairs (M3-1/M3-2) and
pGL3(−257/-1) were used for amplification to generate the
pGL3(−257/-1)-M3 constructs using the QuikChange XL site-directed
mutagenesis kit (Stratagene; Agilent Technologies, Inc.; Table I). All plasmid constructs were
confirmed by automated sequencing (Sangon Biotech Co., Ltd.,
Shanghai, China).
RNA interference
The C/EBPβ small interfering (si)-RNA sequence
(5′-UUGGCCACUUCCAUGGGUCUAAAGG-3′), as described previously
(20), was synthesized by Sangon
Biotech Co., Ltd. C/EBPβ was silenced by transfecting the cells
with 25 nM C/EBPβ siRNA using HiperFect transfection reagent
(Qiagen, Inc., Valencia, CA, USA), following which the cells were
collected for protein expression analysis. Non-specific siRNA
(Sangon Biotech Co., Ltd.) was used as the negative control.
Transfection and dual luciferase
assay
The TR146 cells were grown to 60–80% confluency,
following which the cells were transfected with a firefly
luciferase construct and a Renilla luciferase construct,
pRL-TK (20:1 ratio), using lipofectamine 3000™ (Thermo
Fisher Scientific, Inc.). The luciferase activities were measured
after 40 h (Promega Corporation). The luciferase activity was
normalized to Renilla luciferase activity.
Electrophoretic mobility shift assays
(EMSA)
The EMSA was conducted as previously described
(21). Briefly, 5′-biotin-labeled
single-strand probes were synthesized by Sangon Biotech Co., Ltd.
Double-stranded oligonucleotide probes were prepared by diluting
equimolar quantities of complementary oligonucleotides in 1X STE
buffer (100 mM NaCl, 50 mM Tris-HCl and 1 mM EDTA, pH 8.0),
incubated at 95°C for 3 min, and then slowly cooled to room
temperature. Nuclear extracts from the TR146 cells were extracted
using a nuclear extraction kit (Biyuntian, Shanghai, China). The
EMSA reaction mixtures were incubated on ice for 30 min with or
without unlabeled competitor, prior to adding end-labeled
oligonucleotides for 20 min on ice. For the competitive assays, a
200-fold molar excess of cold oligonucleotides was added to the
binding reaction prior to the addition of the hot-labeled
oligonucleotides. The binding reactions were analyzed by
transferring the reactants to positively charged nylon membranes
(cat. no. 11209299001, Roche Diagnostics, Indianapolis, IN, USA).
For the supershift assay, the nuclear extracts containing 5 µg
protein were incubated with 500 ng C/EBPα (D-5, cat. no. sc-365318;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA) or C/EBPβ
antibodies (cat. no. 23431-1-AP; Wuhan Sanying Biotechnology,
Wuhan, China) on ice for 30 min prior to adding end-labeled
oligonucleotides for 20 min on ice. The samples were
electrophoresed on a 5% non-denaturing polyacrylamide gel in 0.5X
Tris-borate-EDTA buffer. Detection was conducted using Lightshift
electrophoretic mobility shift reagent (Pierce; Thermo Fisher
Scientific, Inc.).
Chromatin immunoprecipitation (ChIP)
assays
ChIP assays were performed using the Magna
ChIP™ A/G kit (EMD Millipore, Billerica, MA, USA)
according to the manufacturer's protocol. Antibodies against C/EBPβ
(cat. no. 23431-1-AP; Wuhan Sanying Biotechnology) were combined
with protein A/G magnetic beads and were then incubated for 4 h at
4°C and rotated. Normal mouse IgG (Santa Cruz Biotechnology, Inc.)
was used as the negative control. Elution of the protein/DNA
complexes and reverse cross-links of the protein/DNA complexes to
free DNA were then performed. For amplification of the
S100A8 promoter (−193/-45), the Ch-1/Ch-2 primer pairs
(Table I) were used. Serving as
the control for the absence of C/EBPβ binding sites, the Ch-3/Ch-4
primer pairs (Table I) were used
to amplify the upstream fragment of human S100A8
(−2861/-2723). The thermocycling conditions were as follows: 2 min
at 94°C, followed by 32 cycles at 94°C for 20 sec, 59°C for 30 sec,
and 72°C for 30 sec, and then a final extension at 72°C for 3 min.
The PCR products were detected by 1.5% agarose gel
electrophoresis.
Western blot analysis
The cells were washed with Dulbecco's
phosphate-buffered saline (Gibco; Thermo Fisher Scientific, Inc.)
and were then extracted using mammalian cell lysate buffer
(Biyuntian). The cell extracts were centrifuged at 12,000 × g for 5
min at 4°C and the supernatants were collected. The protein
concentrations were determined using a bicinchoninic acid protein
concentration detection kit (Biyuntian). The cell extracts (20 µg
protein) were separated by 12% DS-PAGE, transferred onto 0.22-µM
nitrocellulose membranes, and incubated overnight at 4°C with
rabbit anti-myeloid-related protein-8 (an alias of S100A8; cat. no.
ab196689, Abcam, Cambridge, MA, USA) or mouse anti-β-actin (cat.
no. TA-09, OriGene Technologies, Inc., Beijing, China) at 1:2,000
dilution. The membranes were washed and then incubated 2 h at room
temperature with horseradish peroxidase-conjugated goat anti-rabbit
antibodies or goat anti-mouse antibodies (cat. nos. EM35111-01 and
EM35110-01, EMAR Biotechnology, Beijing, China) at 1:3,000
dilution. The immunoreactions were visualized using
Clarity™ Western ECL substrate (Bio-Rad Laboratories,
Inc., Hercules, CA, USA) and exposed to Amersham Hyperfilm ECL film
(Amersham; GE Healthcare Bio-Sciences, Pittsburgh, PA, USA). The
protein bands were evaluated using Quantity One software (version
4.6.5, Bio-Rad Laboratories, Inc.).
Bioinformatics analysis and
statistical analysis
TRANSFAC 7 (http://gene-regulation.com/pub/databases.html) online
prediction software was used to analyze transcription factor
binding for S100A8 promoter region. Between three and six
independent experiments were conducted in the present study.
Statistical analysis was performed with SPSS version 19.0 (IBM
Corp., Armonk, NY, USA). Comparisons between two groups were
performed with Student's t-test, and multiple comparisons were
conducted with one-way analysis of variance followed by
Bonferroni's post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
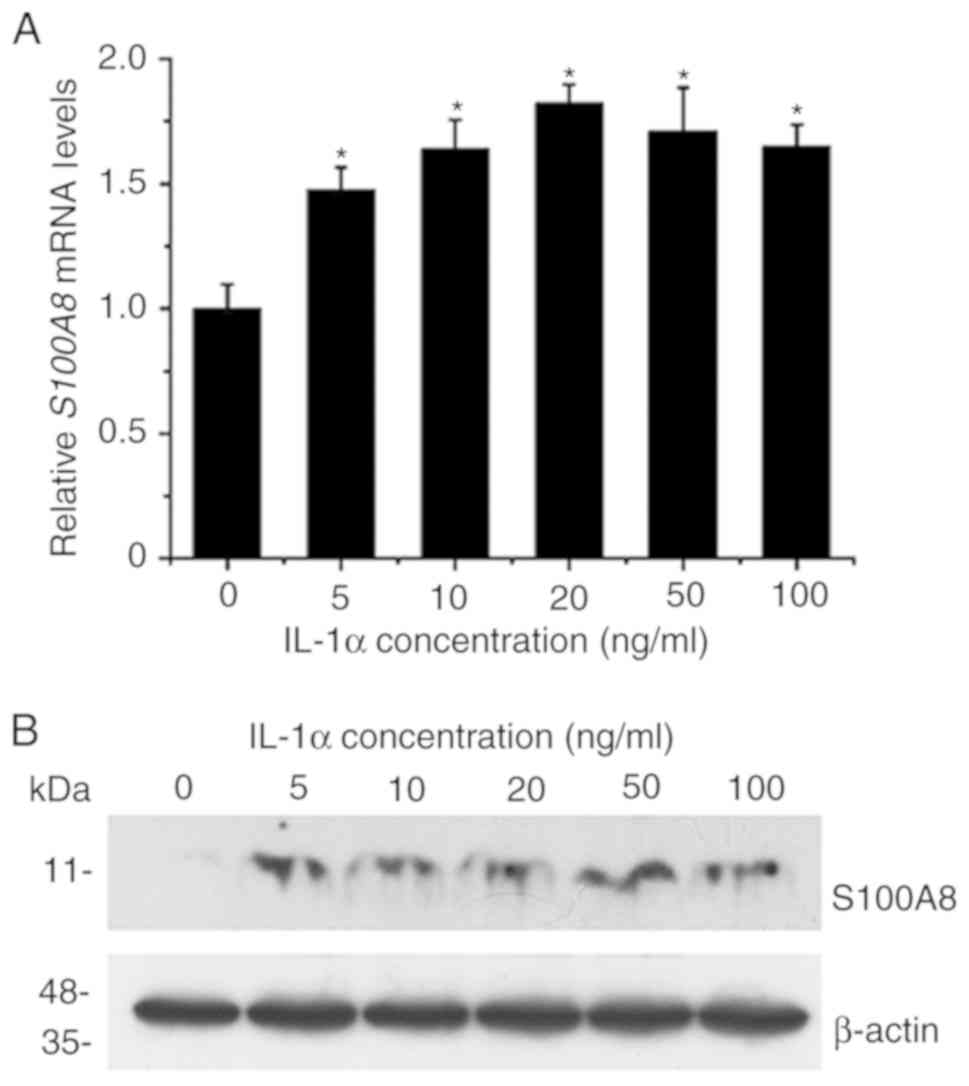
IL-1α activates the expression of
S100A8 in TR146 epithelial cells
The effect of IL-1α on the expression of
S100A8 in human TR146 epithelial cells was investigated by
RT-qPCR and western blot analyses. The results revealed that
treatment with various concentrations (5–100 ng/ml) of
IL-1αsignificantly upregulated the expression levels of
S100A8 at the mRNA level (Fig.
1A). The maximal induction effects were observed with 10–50
ng/ml IL-1α. The results also indicated that the inductive effects
on the expression of S100A8 induced by various
concentrations (5–100 ng/ml) of IL-1α were detected at the protein
level (Fig. 1B). Taken together,
these results suggested that IL-1α significantly induced the
expression of S100A8 in human TR146 epithelial cells through
a mechanism associated with transcriptional regulation.
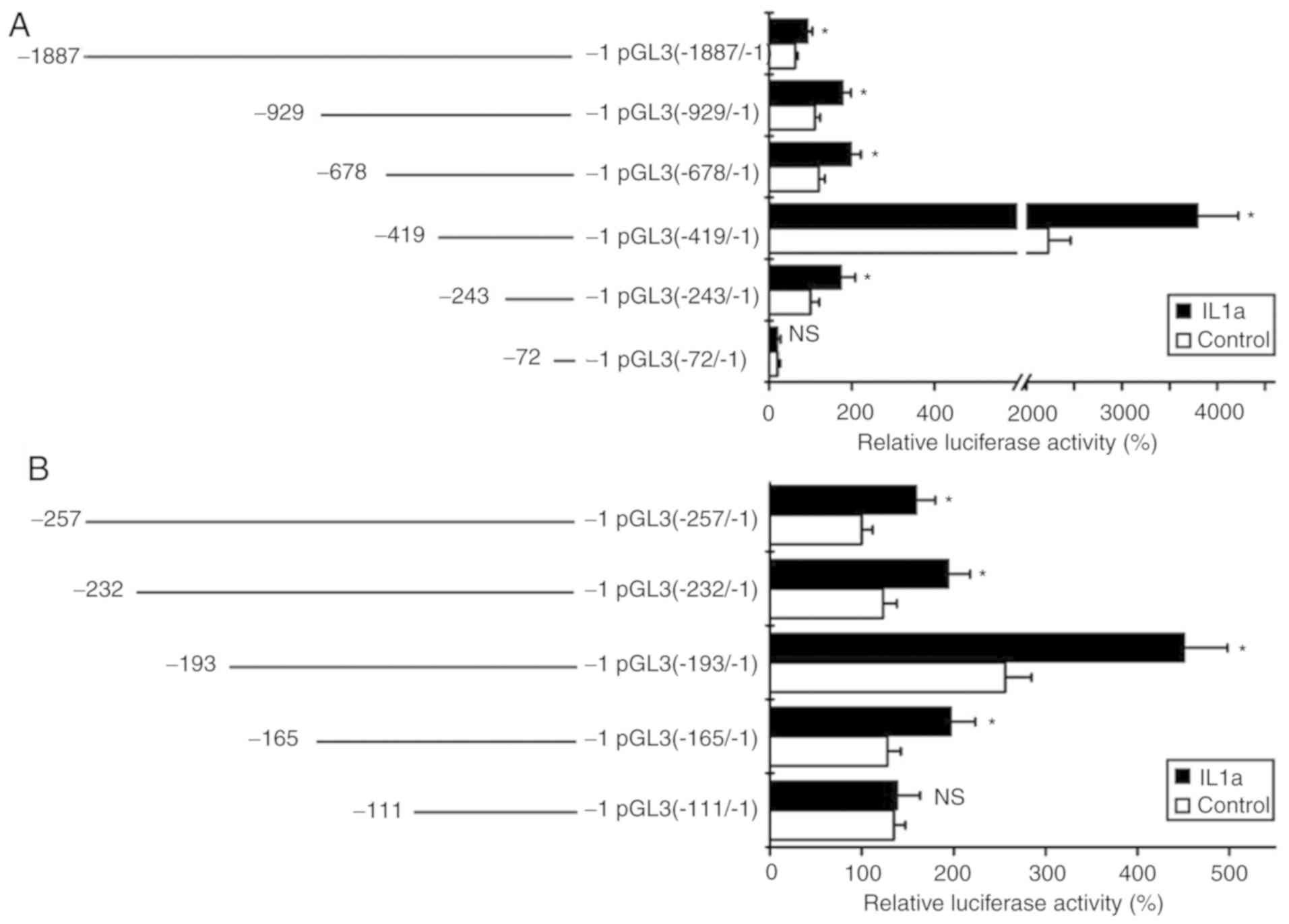
Promoter region of −165/-111 is
responsible for the upregulation of S100A8 by IL-1α treatment
To localize the promoter region that is responsible
for the upregulation of S100A8 induced by IL-1α treatment, a
series of promoter fragments of S100A8 were cloned into a
luciferase reporter gene vector, pGL3-basic, to generate several
deletion mutants. Following the transfection of these constructs
into TR146 cells, the cells were treated with IL-1α, and were then
collected for luciferase activity assays. The results revealed that
IL-1α treatment significantly enhanced promoter activity following
transfection with the pGL3 (−1887/-1), pGL3 (−929/-1), pGL3
(−678/-1), pGL3 (−419/-1) and pGL3 (−243/-1) constructs, but not
with the pGL3 (−72/-1) construct (Fig.
2A). These results indicated that the promoter region
potentially responsible for the induced gene expression of
S100A8 by IL-1α may be located in the-243/-72 promoter
region. In order to further locate the associated promoter region
for the induced gene expression of S100A8 by IL-1α, a series
of deletion mutants were also constructed. The luciferase assays
showed that IL-1α treatment significantly induced promoter activity
for the pGL3 (−257/-1), pGL3 (−232/-1), pGL3 (−193/-1) and pGL3
(−165/-1) reporter gene constructs, but not for the pGL3 (−111/-1)
reporter gene construct (Fig. 2B).
Taken together, these results suggested that the −165/-111 promoter
region of S100A8 may be responsible for the inductive
effects of IL-1α.
IL-1α treatment induces the binding of
C/EBPβ to a specific site in the promoter region
As IL-1α can affect gene transcription via the
transcription factor C/EBPβ (22,23),
the present study analyzed the −165/-111 promoter region of human
S100A8 using online prediction software for transcription
factor binding, TRANSFAC 7 (http://gene-regulation.com/pub/databases.html)
(24). The results revealed that
there are three potential transcription factor C/EBPβ binding sites
in this region. Subsequently, three pairs of EMSA primers
(−166/-142 for E1; −145/-121 for E2 and −128/-102 for E3) were
designed to detect whether IL-1α treatment affects the combination
of transcription factors to these primers (Fig. 3A). No significant differences were
observed when comparing C/EBPβ binding and alterations in IL-1α
treatment with E1 primers (Fig.
3B). In addition, the EMSA results revealed no significant
difference in C/EBPβ binding following IL-1α treatment with the E2
primer (Fig. 3C). Notably, the
results demonstrated that the binding activity between the C/EBPβ
binding site (−113/-109) in E3 primer pairs and the transcription
factor C/EBPβ were significantly enhanced following IL-1α treatment
(Fig. 3D). In addition, the ChIP
assay verified the binding activity between C/EBPβ and the-193/-45
promoter region in vivo (Fig.
3E). These results suggested that the C/EBPβ binding site
(−113/-109) of the S100A8 gene promoter may be associated
with the upregulatory effect on the expression of S100A8
induced by IL-1α.
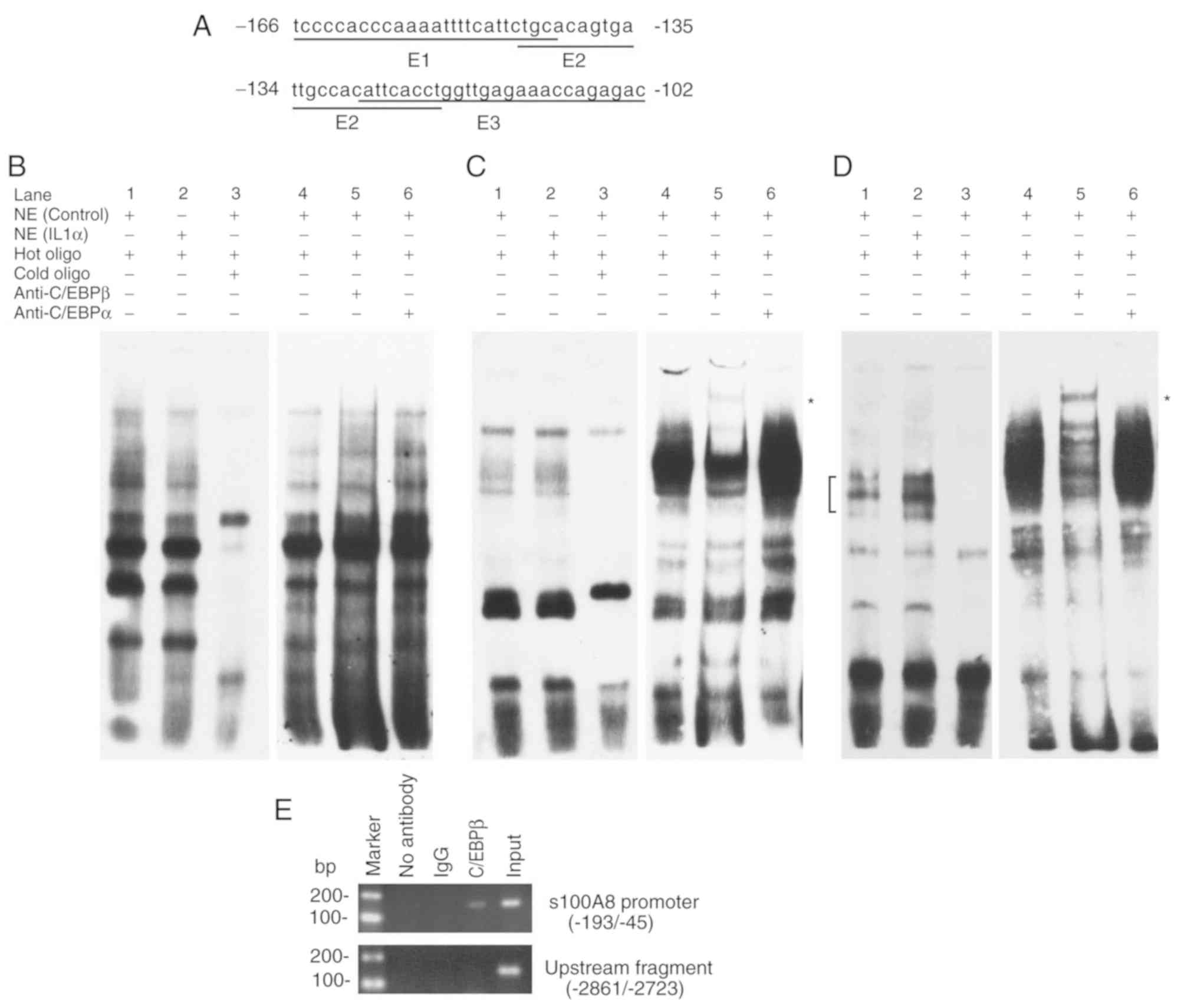 | Figure 3.Induction of C/EBPβ binding to the
specific site in the S100A8 promoter region by IL-1α. (A)
Oligonucleotide sequences used for EMSA analysis. Bold letters
indicate the potential binding sites for transcription factor
C/EBPβ. (B) Promoter region (−166/-142) sequence used for EMSA
analysis. (C) Promoter region (−145/-121) sequence used for EMSA
analysis. (D) Promoter region (−128/-102) sequence used for EMSA
analysis. (E) C/EBPβ binding to the S100A8 promoter in
vivo. Chromatin immunoprecipitation assays were performed using
DNA from TR146 cells and specific antibodies. Compared with the
competitive assay (left-hand panels of B-D), a longer exposure time
was performed for the supershift assay (right-hand panels of B-D).
Lanes: 1, no IL-1α; 2, IL-1α; 3, 200-fold cold oligo; 4, no
antibody (negative control); 5, C/EBPβ antibody; 6, C/EBPα
antibody. Square brackets indicate the position of the DNA/protein
complex involving C/EBPβ. *Positions of supershift bands. At least
three experiments were performed. NE, nuclear extracts; Input,
sheared DNA prior to immunoprecipitation was used for
amplification; C/EBPβ, CCAAT/enhancer binding protein β; EMSA,
electrophoretic mobility shift assays. |
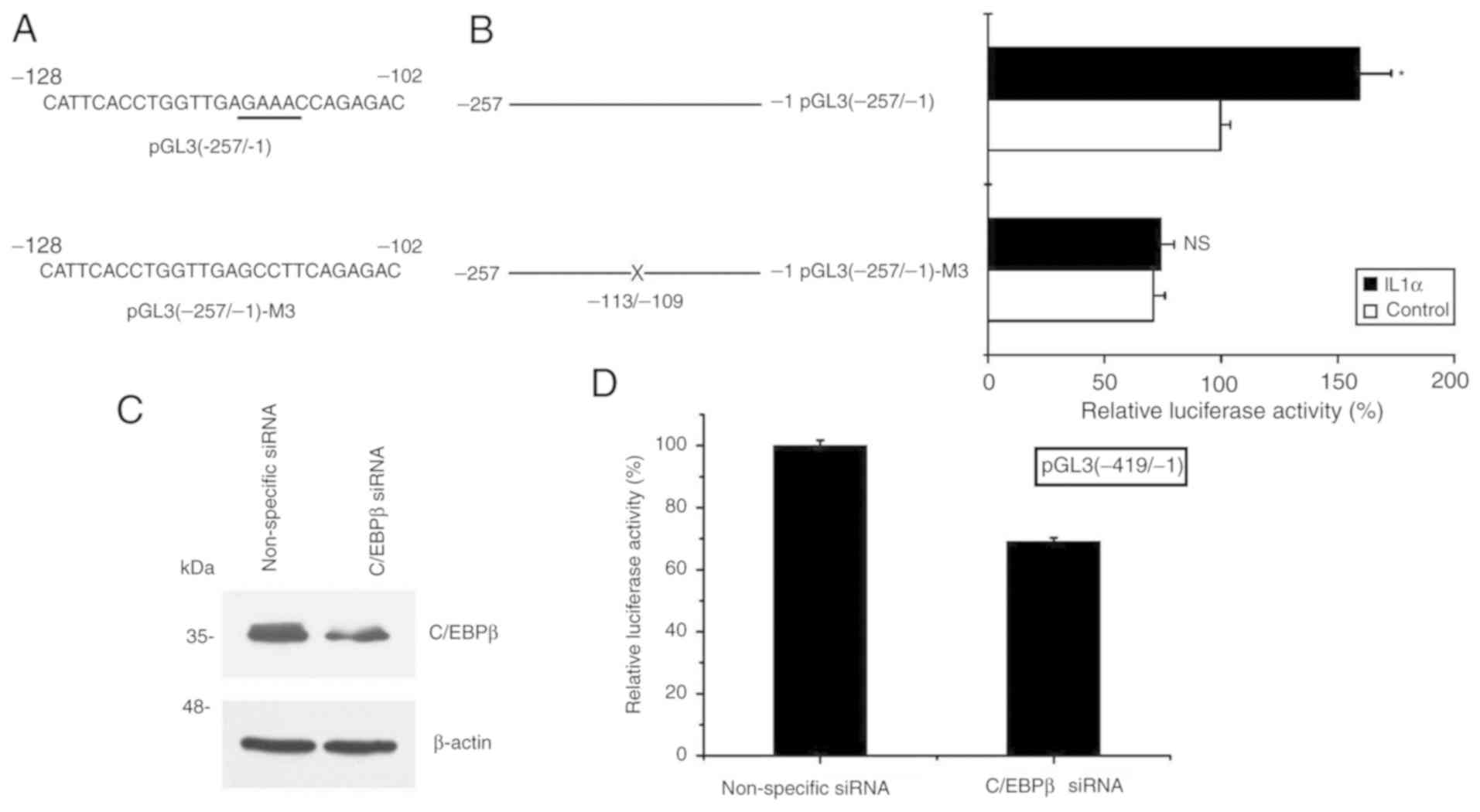
C/EBPβ is critical in the process of
S100A8 activation induced by IL-1α
Finally, to further elucidate the role of the
transcription factor C/EBPβ binding site (−113/-109) in the
S100A8 gene promoter, the pGL3 (−257/-1) reporter gene
vector was used as a template to generate the mutated C/EBPβ
binding site construct pGL3 (−257/-1)-M3 (mutation at the −113/-109
site) (Fig. 4A). Following
transfection with these constructs, the cells were collected
subsequent to IL-1α treatment, and the effect of IL-1α treatment on
S100A8 promoter activity was analyzed. The results
demonstrated that the inductive effect of IL-1α treatment was
attenuated following pGL3(−257/-1)-M3 transfection, whereas the
inductive effect remained following of pGL3(−257/-1) transfection
(Fig. 4B). By contrast, silencing
C/EBPβ significantly decreased S100A8 promoter activity
following pGL3(−419/-1) construct transfection (Fig. 4C and D). Taken together, these
results suggested that activation of the expression of
S100A8 induced by IL-1α in TR146 epithelial cells may
involve a mechanism associated with the increased binding activity
of C/EBPβ to a specific site (−113/-109) of the S100A8
promoter.
Discussion
Due to the important roles of human S100A8 in
infectious diseases and tumors (1–8,11,12),
a number of studies have investigated the mechanism underlying the
transcriptional regulation of human S100A8 (15–17,25–27).
The upregulation of S100A8 by fibroblast growth factor-2 and
IL-1β, and its downregulation by transforming growth factor-β in
murine fibroblasts has been previously observed (25). Through activation of the protein
kinase A signaling pathway and subsequent stimulation of C/EBPβ
binding to the S100A8 promoter, prostaglandin E2 has been
reported to upregulate the expression of human S100A8
(15). Mechanistically, the
process of the induced expression of S100A8 by
glucocorticoids was positively regulated by protein kinase A and
negatively regulated by protein kinase C (26). Glucocorticoids increase the
transcription and mRNA half-life of human S100A8; the
upregulation process requires new protein synthesis, IL-10,
products of the cyclooxygenase-2 pathway, and both the
extracellular signal-regulated kinase (ERK)-1/2 and p38 MAPK
signaling pathways (26).
Furthermore, the expression of human S100A8 is induced by
polyinosinic:polycytidylic acid, a double strand RNA mimetic, and
its induction is dependent on the p38, ERK MAPK and protein kinase
R-dependent signaling pathways (27). Notably, the p38 MAPK signaling
pathway is critical in the process of tumor necrosis factor-α- and
IL-17A-induced expression of S100A8 in human keratinocytes
(28).
It has been demonstrated that IL-1α can affect gene
transcription by increasing C/EBPβ-dependent transcriptional
activity (22,23). A previous report also revealed that
IL-1α promotes the expression of stromal-derived factor-1 in
vascular smooth muscle cells by upregulating C/EBPβ in an inhibitor
of NF-κB kinase β signaling-dependent manner (29). An IL-1α-induced increase in the
binding of C/EBPβ to the 11β-hydroxysteroid dehydrogenase type 1 P2
promoter in human A549 epithelial cells has also been reported
(30). In the present study, the
results revealed that IL-1α treatment induced the expression of
S100A8 in TR146 epithelial cells, and the inductive effect
occurred at the transcriptional level. In addition, the activated
expression of S100A8 induced by IL-1α in TR146 epithelial
cells may involve a mechanism associated with increasing the
binding activity of C/EBPβ to a specific site (−113/-109) of the
S100A8 promoter. Overall, the results of the present study
are consistent with the findings reported in previous studies
(22,23,29,30).
Taken together, these similar findings support the hypothesis that
IL-1α increases the binding activity of the transcription factor
C/EBPβ to the promoter of specific genes and this may be a common
regulatory mechanism that affects target gene expression.
In conclusion, the present study provided novel
mechanistic insights into the transcriptional regulation of human
S100A8 in TR146 epithelial cells. However, the detailed
molecular mechanism requires further clarification.
Acknowledgements
Not applicable.
Funding
This study was supported by a grant from the Natural
Science Foundation of Guangxi (grant no. 2015GXNSFCA139008).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MQ, YZ, KZ and XZ made substantial contributions to
the conception and design of the study. YG and XZ analyzed the
results. MQ, YG and XZ drafted the manuscript. All the authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors confirm that they have no competing
interests.
References
|
1
|
Sorenson BS, Khammanivong A, Guenther BD,
Ross KF and Herzberg MC: IL-1 receptor regulates
S100A8/A9-dependent keratinocyte resistance to bacterial invasion.
Mucosal Immunol. 5:66–75. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zaia AA, Sappington KJ, Nisapakultorn K,
Chazin WJ, Dietrich EA, Ross KF and Herzberg MC: Subversion of
antimicrobial calprotectin (S100A8/S100A9 complex) in the cytoplasm
of TR146 epithelial cells after invasion by Listeria monocytogenes.
Mucosal Immunol. 2:43–53. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gaffen SL, Herzberg MC, Taubman MA and Van
Dyke TE: Recent advances in host defense mechanisms/therapies
against oral infectious diseases and consequences for systemic
disease. Adv Dent Res. 26:30–37. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lira-Junior R, Öztürk VÖ, Emingil G,
Bostanci N and Boström EA: Salivary and serum markers related to
innate immunity in generalized aggressive periodontitis. J
Periodontol. 88:1339–1347. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bando M, Hiroshima Y, Kataoka M, Shinohara
Y, Herzberg MC, Ross KF, Nagata T and Kido J: Interleukin-1alpha
regulates antimicrobial peptide expression in human keratinocytes.
Immunol Cell Biol. 85:532–537. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bando M, Hiroshima Y, Kataoka M, Herzberg
MC, Ross KF, Shinohara Y, Yamamoto T, Nagata T and Kido J:
Modulation of calprotectin in human keratinocytes by keratinocyte
growth factor and interleukin-1alpha. Immunol Cell Biol.
88:328–333. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hiroshima Y, Bando M, Kataoka M, Inagaki
Y, Herzberg MC, Ross KF, Hosoi K, Nagata T and Kido J: Regulation
of antimicrobial peptide expression in human gingival keratinocytes
by interleukin-1α. Arch Oral Biol. 56:761–767. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Silva EJ, Argyris PP, Zou X, Ross KF and
Herzberg MC: S100A8/A9 regulates MMP-2 expression and invasion and
migration by carcinoma cells. Int J Biochem Cell Biol. 55:279–287.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rider P, Carmi Y, Voronov E and Apte RN:
Interleukin-1α. Semin Immunol. 25:430–438. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Di Paolo NC and Shayakhmetov DM:
Interleukin 1α and the inflammatory process. Nat Immunol.
17:906–913. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Khammanivong A, Wang C, Sorenson BS, Ross
KF and Herzberg MC: S100A8/A9 (calprotectin) negatively regulates
G2/M cell cycle progression and growth of squamous cell carcinoma.
PLoS One. 8:e693952013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Khammanivong A, Sorenson BS, Ross KF,
Dickerson EB, Hasina R, Lingen MW and Herzberg MC: Involvement of
calprotectin (S100A8/A9) in molecular pathways associated with
HNSCC. Oncotarget. 7:14029–14047. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lagasse E and Clerc RG: Cloning and
expression of two human genes encoding calcium-binding proteins
that are regulated during myeloid differentiation. Mol Cell Biol.
8:2402–2410. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hayashi N, Kido J, Kido R, Wada C, Kataoka
M, Shinohara Y and Nagata T: Regulation of calprotectin expression
by interleukin-1alpha and transforming growth factor-beta in human
gingival keratinocytes. J Periodontal Res. 42:1–7. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Miao L, Grebhardt S, Shi J, Peipe I, Zhang
J and Mayer D: Prostaglandin E2 stimulates S100A8 expression by
activating protein kinase A and CCAAT/enhancer-binding-protein-beta
in prostate cancer cells. Int J Biochem Cell Biol. 44:1919–1928.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ahn GO, Seita J, Hong BJ, Kim YE, Bok S,
Lee CJ, Kim KS, Lee JC, Leeper NJ, Cooke JP, et al: Transcriptional
activation of hypoxia-inducible factor-1 (HIF-1) in myeloid cells
promotes angiogenesis through VEGF and S100A8. Proc Natl Acad Sci
USA. 111:2698–2703. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu K, Yen T and Geczy CL: Il-10
up-regulates macrophage expression of the S100 protein S100A8. J
Immunol. 166:6358–6366. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Singh BK, Sinha RA, Zhou J, Xie SY, You
SH, Gauthier K and Yen PM: FoxO1 deacetylation regulates thyroid
hormone-induced transcription of key hepatic gluconeogenic genes. J
Biol Chem. 288:30365–30372. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shaw AE, Reid SM, Ebert K, Hutchings GH,
Ferris NP and King DP: Implementation of a one-step real-time
RT-PCR protocol for diagnosis of foot-and-mouth disease. J Virol
Methods. 143:81–85. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Styner M, Meyer MB, Galior K, Case N, Xie
Z, Sen B, Thompson WR, Pike JW and Rubin J: Mechanical strain
downregulates C/EBPβ in MSC and decreases endoplasmic reticulum
stress. PLoS One. 7:e516132012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zou X, Gao Y, Ruvolo VR, Gardner TL,
Ruvolo PP and Brown RE: Human glycolipid transfer protein gene
(GLTP) expression is regulated by Sp1 and Sp3: Involvement of the
bioactive sphingolipid ceramide. J Biol Chem. 286:1301–1311. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Orjalo AV, Bhaumik D, Gengler BK, Scott GK
and Campisi J: Cell surface-bound IL-1alpha is an upstream
regulator of the senescence-associated IL-6/IL-8 cytokine network.
Proc Natl Acad Sci USA. 106:17031–17036. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bando M, Zou X, Hiroshima Y, Kataoka M,
Ross KF, Shinohara Y, Nagata T, Herzberg MC and Kido J: Mechanism
of interleukin-1α transcriptional regulation of S100A9 in a human
epidermal keratinocyte cell line. Biochim Biophys Acta 1829.
954–962. 2013.
|
|
24
|
Matys V, Fricke E, Geffers R, Gössling E,
Haubrock M, Hehl R, Hornischer K, Karas D, Kel AE, Kel-Margoulis
OV, et al: TRANSFAC: Transcriptional regulation, from patterns to
profiles. Nucleic Acids Res. 31:374–378. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rahimi F, Hsu K, Endoh Y and Geczy CL:
FGF-2, IL-1beta and TGF-beta regulate fibroblast expression of
S100A8. FEBS J. 272:2811–2827. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hsu K, Passey RJ, Endoh Y, Rahimi F,
Youssef P, Yen T and Geczy CL: Regulation of S100A8 by
glucocorticoids. J Immunol. 174:2318–2326. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Endoh Y, Chung YM, Clark IA, Geczy CL and
Hsu K: IL-10-dependent S100A8 gene induction in
monocytes/macrophages by double-stranded RNA. J Immunol.
182:2258–2268. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mose M, Kang Z, Raaby L, Iversen L and
Johansen C: TNFα- and IL-17A-mediated S100A8 expression is
regulated by p38 MAPK. Exp Dermatol. 22:476–481. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang B, Li W, Zheng Q, Qin T, Wang K, Li
J, Guo B, Yu Q, Wu Y, Gao Y, et al: Transforming growth factor
β-activated kinase 1 negatively regulates interleukin-1α-induced
stromal-derived factor-1 expression in vascular smooth muscle
cells. Biochem Biophys Res Commun. 463:130–136. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Esteves CL, Verma M, Róg-Zielińska E,
Kelly V, Sai S, Breton A, Donadeu FX, Seckl JR and Chapman KE:
Pro-inflammatory cytokine induction of 11β-hydroxysteroid
dehydrogenase type 1 in A549 cells requires phosphorylation of
C/EBPβ at Thr235. PLoS One. 8:e758742013. View Article : Google Scholar : PubMed/NCBI
|