Introduction
Thyroid stimulating hormone (TSH) is produced by the
anterior pituitary, and is used for the regulation of thyroid
hormone production, which subsequently controls metabolic activity
(1). TSH consists of an α-subunit
and a non-covalently bound β-subunit (TSHβ) (2). The former is also a subunit of
luteinizing hormone, follicle stimulating hormone and chorionic
gonadotropin. By contrast, TSHβ is unique and responsible for TSH
activity and specific immunogenicity (3).
The mouse TSHβ gene consists of five exons, with the
coding region located within exons 4 and 5 (4). The first functional alternatively
spliced variant of mouse TSHβ was reported by Vincent et al
(5) in 2009. The in-frame TSHβ
splice variant involves the retention of a portion of intron 4 and
the entire coding region of exon 5, but does not include exon 4. At
present, the only evidence of alternative exon splicing of the
mouse TSHβ gene involves exons 1, 2 and 3, all of which are located
outside of the TSHβ-coding region (6,7). To
the best of our knowledge, there is no evidence at present of
alternative splicing of TSHβ within the coding region itself, or of
splicing that affects the expression of the TSHβ protein. Previous
studies have demonstrated that the TSHβ splice variant transcript
is expressed in the mouse pituitary, thyroid, bone marrow, spleen,
small intestine, large intestine, kidney, liver, heart and adipose
tissues, whereas the full-length native TSHβ transcript is largely
restricted to the pituitary gland (8). These observations suggest that the
extra-pituitary TSHβ splice variant may be markedly different from
the full-length, native TSHβ. Various studies have focused on
establishing a role for the TSHβ splice variant in different
diseases. Previous studies have revealed that the extra-pituitary
TSHβ splice variant serves a vital role in pathological processes,
including autoimmunity, inflammation and bone remodeling (9,10).
Despite this, the fundamental physiological characteristics of the
TSHβ splice variant remain unknown, and an in-depth understanding
of its physiological effects is required.
The aim of the present study was to perform a series
of examinations to verify the following: i) Whether the TSHβ splice
variant has the potential to induce thyroid follicular cells to
synthesize thyroid hormone; and ii) whether the TSHβ splice variant
is regulated by the hypothalamus-pituitary-thyroid (HPT) axis,
similarly to the native form of TSHβ.
Materials and methods
Animals and sampling
C57BL/6 mice (6–8 weeks old, 50% male and 50%
female, weighing 20–26 g) were purchased from the Experimental
Animal Center of the Academy of Military Medical Sciences of China
(Beijing, China). The animals were housed in a
temperature-controlled room (21–23°C) at 40±10% relative humidity
under a 12:12-h light/dark cycle, with access to food and water
ad libitum. All procedures used were in accordance with the
Logistics University of Chinese People's Armed Police Force animal
welfare guidelines. The animal protocols were approved by the
Ethics Review Committee of the Logistics University of Chinese
People's Armed Police Force (Tianjin, China).
A total of 156 C57BL/6 mice were used in the present
study. Of these, 60 mice were randomly divided into the following
groups: Control (n=20), where mice were administered with an
intraperitoneal injection of saline; a 5 µg purified TSHβ splice
variant protein (see Generation of a TSHβ splice variant fusion
protein) intraperitoneal injection group (n=20); and a 10 µg
purified TSHβ splice variant protein intraperitoneal injection
group (n=20). The mice were sacrificed at 0.5, 1 and 4 h after
intraperitoneal injection. Blood samples were collected, and the
serum levels of free tri-iodothyronine (FT3) and free thyroxine
(FT4) were determined using a radioimmunoassay (RIA) performed by
the Radiology Institute of the Logistics University of Chinese
People's Armed Police Force (Tianjin, China). The
radioimmunodetection was repeated three times.
In another experiment, 60 mice were randomly divided
into the following three groups: Control group (n=20), where mice
were administered with an intraperitoneal injection of saline; a T3
group (n=20), where mice were administered with an intraperitoneal
injection of 2 mg/kg T3 (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany); and a thyroid-releasing hormone (TRH) group (n=20), where
mice were intraperitoneally injected with 0.05 mg/kg TRH (Nanjing
Peptide Biotech Ltd., Nanjing, China). The animals were sacrificed
at 1 and 4 h after injection. Whole blood samples, anti-coagulated
with 1% heparin sodium solution (0.1 ml; Lianxing Biotech Co.,
Ltd., Tianjin, China), were collected for the isolation of
peripheral blood leukocytes (PBLs). In addition, pituitary gland,
thyroid and spleen tissue samples were collected for the detection
of TSHβ expression. All assays were repeated three times.
Mouse PBLs
Mouse whole blood samples, anti-coagulated with 1%
heparin sodium solution, were collected. To process each blood
sample, 1:3 (v/v) red blood cell:lysis buffer (Yuanpinghao Biotech
Co., Ltd., Beijing, China) was added to the blood samples, and the
sample tubes were mixed and placed on ice for 5 min. This was
followed by centrifugation at 12,000 × g for 1 min at 4°C. The
clear, red supernatant was carefully removed, and the pellet that
remained contained the PBLs. Total protein was extracted and
quantified, and the levels of the TSHβ splice variant were detected
by western blot analysis.
Mouse thyroid follicular epithelial
cells
Previous studies have described methods to isolate
thyroid cells from mice (11–13).
To obtain follicular epithelial cells in the present study, 36
experimentally naïve mice were sacrificed. The animal protocols
were approved by the Ethics Review Committee of the Logistics
University of Chinese People's Armed Police Force. The fresh
thyroid tissues were first minced with crossed razor blades into
pieces <1.5 mm in diameter. The pieces were subsequently rinsed
twice with Ham's F12 nutrient mixture without bovine serum (Thermo
Fisher Scientific, Inc., Waltham, MA, USA), followed by
disaggregation using dispersing reagents [103 kU/l collagen I and
1.35 kU/l dispase in Earle's balanced salt solution; pH 7.4
(Sigma-Aldrich; Merck KGaA)] for 40 min at 37°C with agitation (100
oscillations/min). The samples were centrifuged at 200 × g for 10
min at room temperature. The supernatants were discarded, and the
cells were resuspended in Ham's F12 nutrient mixture (Thermo Fisher
Scientific, Inc.) supplemented with 10% fetal bovine serum (FBS,
Thermo Fisher Scientific, Inc.), 2 mM L-glutamine, 10 mg/l insulin,
10 nM hydrocortisone, 5 mg/l transferrin and 1 U/l bovine TSH (all
these elements were purchased from Sigma-Aldrich; Merck KGaA), 10
U/l penicillin and 100 mg/l streptomycin (Lianxing Biotech Co.,
Ltd.). Following gentle mechanical agitation, the disaggregated
cell suspensions were transferred into 24-well Corning tissue
culture plates (Corning Inc., Corning, NY, USA) and incubated in a
humidified 5% CO2 incubator. The medium was refreshed
every 3 days with decreasing concentrations of FBS (from 10 to 5%).
The mouse thyroid follicular epithelial cells were cultivated until
80% confluency, which was reached on day 7. On day 8, media was
removed and replaced with Ham's F12 nutrient mixture lacking bovine
TSH. Stimulation experiments were performed with the addition of
purified TSHβ splice variant protein at a concentration of 2 µg/ml
on day 9. Supernatants were collected for detecting the levels of
T3 and T4. Cells were harvested at 0, 0.5, 1 and 4 h following TSHβ
splice variant stimulation. Total protein was isolated from the
cells and quantified. The experiments were repeated three
times.
Generation of a TSHβ splice variant
fusion protein
Total RNA was isolated from the mice PBLs using
TRIzol® reagent (Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. RNA was reverse
transcribed to synthesize cDNAs using a Reverse Transcription
System (Promega Corporation, Madison, WI, USA). Polymerase chain
reaction (PCR) amplification of the coding region of the TSHβ
splice variant was conducted in a total reaction volume of 20 µl
containing 4 µl cDNA. PCR was conducted as follows: Initial
denaturation at 95°C for 5 min, then 30 cycles of denaturation at
95°C for 30 sec, annealing at 60°C for 30 sec and extension at 72°C
for 30 sec, and a final extension step at 72°C for 5 min. The
primer sequences were as follows: Forward,
5′-ATCATGTTAAGATCTCTTTTCTTT-3′, reverse,
5′-AACCAGATTGCACTGCTATTGAA-3′. The coding region of the TSHβ splice
variant was subcloned into the pcDNA3.1/V5-His-TOPO vector (Thermo
Fisher Scientific, Inc.). Plasmid DNA was obtained using standard
methods. Chinese hamster ovary (CHO) cells (GF113, Shanghai Gefan
Biotechnology Co., Ltd.) were transfected with the generated
plasmid DNA using an Amaxa electroporator (Amaxa Biosystems; Lonza
Group Ltd., Basel, Switzerland) according to the manufacturer's
instructions. Briefly, CHO cells were cultivated in Ham's F12 media
and incubated at 37°C with a humidified atmosphere of 5%
CO2 until they reached 90% confluency, at which point
they were harvested by trypsinization (cells were incubated for 5
min at 37°C with 0.5 mg/ml trypsin). Cells (1×106) were
resuspended in 100 µl room-temperature Nucleofector®
Solution (82 µl of Nucleofector Solution plus 18 µl of supplement;
Amaxa Biosystems; Lonza Group Ltd.). The resulting cell suspension
was combined with 2 µg recombinant DNA, and the cell/DNA suspension
was transferred into an Amaxa certified cuvette, which was inserted
into the Nucleofector Cuvette Holder. The U-023 Nucleofector
program was performed, following which the cuvette was immediately
taken out of the holder, and 600 µl of pre-equilibrated Ham's F12
medium was then added. Cells were gently transferred into 6-well
plates (final volume 1.5 ml media/well). Transfected cells were
incubated in humidified 37°C/5% CO2 incubator. Stable
transfected cells were selected by continuous culture in medium
containing 1.2 mg/ml neomycin. A total of 1×107 cells
were used to purify His-tagged recombinant proteins with the Ni-NTA
Fast Start kit (Qiagen China Co., Ltd., Shanghai, China). The
concentration of recombinant protein was determined using a
Coomassie Plus-200 Protein assay (Pierce; Thermo Fisher Scientific,
Inc.), and aliquots were stored at −80°C.
Western blotting
Total proteins from mouse thyroid follicular
epithelial cells, and mouse PBLs, and pituitary gland, thyroid and
spleen tissues were extracted using a ReadyPrep™ Protein Extraction
kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Protein
concentrations were determined using Coomassie Protein Assay
reagent (Thermo Fisher Scientific, Inc.), according to the
manufacturer's instructions. The protein samples were subsequently
denatured in SDS sample buffer (125 mM Tris-HCl, pH 6.8, 50%
glycerol, 2% SDS, 5% b-mercaptoethanol and 0.01% bromophenol blue)
at 100°C for 10 min. Equal amounts of protein (30 µg/lane)
separated by 18% SDS-PAGE were transferred to a polyvinylidene
fluoride membrane at 15 V for 25 min using Trans-Blot SD (Bio-Rad
Laboratories, Inc.). The membranes were blocked with 5% non-fat
milk in TBS and Tween 20 (TBST; 25 mM Tris-base, 138 mM NaCl, 2.7
mM KCl, 0.2% Tween-20 and deionized water; pH 7.4) for 2 h at room
temperature. The membranes were immunoblotted with primary
antibodies overnight at 4°C. The primary antibodies included
anti-TSHβ antibody (1:500; cat. no. sc-7813), anti-sodium-iodide
symporter (NIS) antibody (1:200; cat. no. sc-514487),
anti-thyroperoxidase (TPO) antibody (1:200; cat. no. sc-376876; all
purchased from Santa Cruz Biotechnology, Inc., Dallas, TX, USA),
anti-thyroglobulin (TG) antibody (1:200; cat. no. ab-187378; Abcam,
Cambridge, UK) and anti-GAPDH antibody (1:800; cat. no. BM1623;
Boster Biological Technology, Pleasanton, CA, USA). The membranes
were subsequently washed with TBST, and incubated donkey anti-goat
horseradish peroxidase (HRP)-conjugated secondary antibody (cat.
no. sc-2020; Santa Cruz Biotechnology, Inc.) and goat anti-mouse
HRP-conjugated secondary antibody (cat. no. ZB-2305; dilution,
1:5,000; OriGene Technologies, Inc., Rockville, MD, USA) for 2 h at
room temperature. Following a final wash in TBST, the
immunoreactive TSHβ splice variant bands were detected using an
enhanced chemiluminescence system (Immobilon™ Western
Chemiluminescent HRP Substrate; Merck KGaA), and the images were
acquired using the Kodak Medical X-ray processor (Kodak, Rochester,
NY, USA). Band densities were quantified using Image-Pro Plus v6.0
software (Media Cybernetics, Inc., Rockville, MD, USA).
The mouse TSHβ gene consists of five exons and the
coding region of native TSHβ is located between exons 4 and 5. The
molecular weight of native TSHβ protein is 17 kDa. The TSHβ splice
variant consists of part of intron 4 and all of the coding region
of exon 5, and the molecular weight of the TSHβ splice variant
protein is 8 kDa (5). The
anti-TSHβ antibody specifically binds to the protein sequence
encoded by exon 5, and therefore detects both forms of the TSHβ
protein.
Statistical analysis
All data are presented as the mean ± standard error.
Comparisons between groups were performed using one-way analysis of
variance, followed by a least significant difference post-hoc test.
The statistical analyses were performed using SPSS software
(v.13.0; SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Increased serum FT3 and FT4 levels are
induced by TSHβ splice variant
Mice were injected with the purified TSHβ splice
variant protein at different concentrations, and the serum levels
of FT3 and FT4 were evaluated by RIA at 0.5, 1 and 4 h
post-injection. As demonstrated in Fig. 1, serum levels of FT3 peaked at 1 h
following injection, and declined to a normal level at 4 h
post-injection. Serum levels of FT4 exhibited a similar trend
(Fig. 2). The serum levels of FT3
in the 10 µg purified TSHβ splice variant protein-treated group
were 1.56-fold higher than the the saline-injected control group at
1 h after injection. In addition, the serum levels of FT3 in the 5
µg purified TSHβ splice variant protein-treated group were
1.28-fold higher than the saline-injected control group at 1 h
post-injection. Furthermore, the serum levels of FT4 in the 10 µg
purified TSHβ splice variant protein-injected group were 2.19-fold
higher than the saline-injected control group at 1 h
post-injection, while the FT4 levels of the 5 µg-injected group
were 2.05 fold higher relative to the control group. In summary,
the results demonstrated that both serum FT3 and FT4 levels were
significantly increased by TSHβ, and in a dose-dependent manner,
compared with the saline-injected groups of mice.
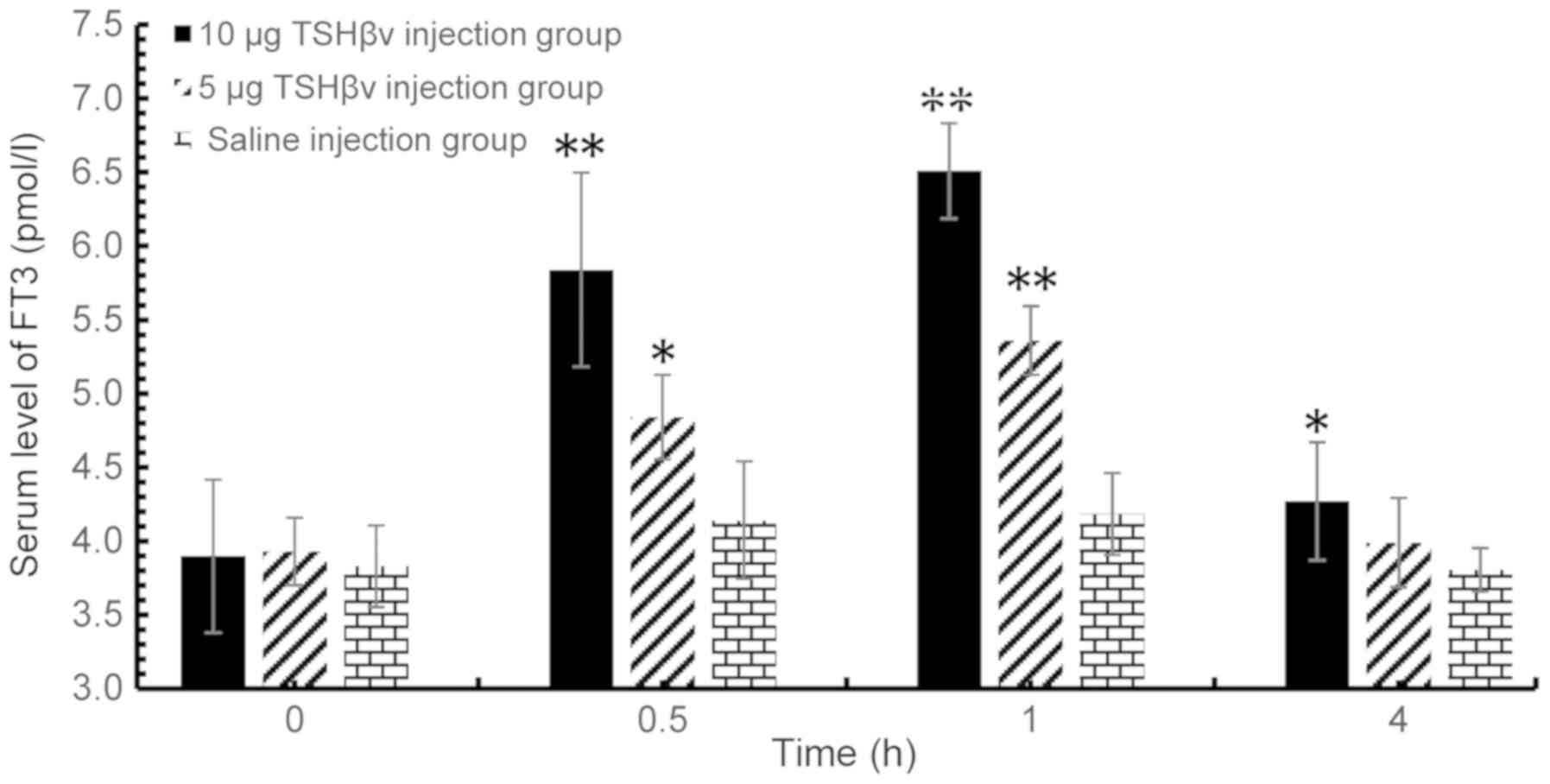 | Figure 1.Altered serum FT3 levels among the
control group, 5 and 10 µg TSHβv injection groups at 0.5, 1 and 4 h
following injection evaluated by radioimmunoassay. TSHβv-injected
(5 µg) vs. control animals: 0.5 h, P=0.037; 1 h, P<0.001; and 4
h, P=0.362. TSHβv-injected (10 µg) vs. control animals: 0.5 h,
P<0.001; 1 h, P<0.001; and 4 h, P=0.032. *P<0.05 and
**P<0.001 vs. respective control. FT3, free tri-iodothyronine;
TSHβv, thyroid-stimulating hormone β splice variant. |
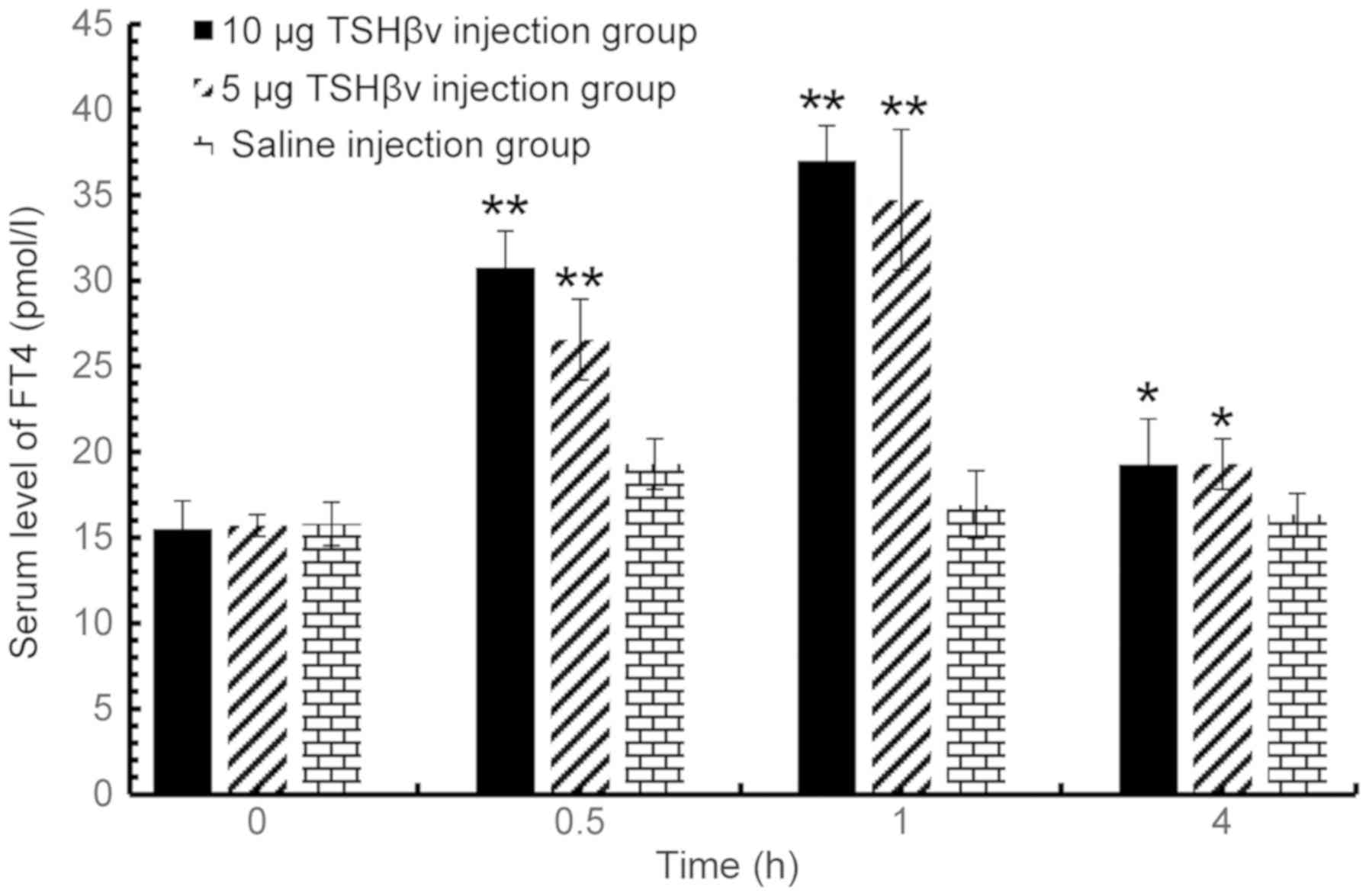 | Figure 2.Altered serum FT4 levels among the
control group, 5 and 10 µg TSHβv injection groups at 0.5, 1 and 4 h
following injection evaluated by radioimmunoassay. TSHβv-injected
(5 µg) and control animals: 0.5 h, P<0.001; 1 h, P<0.001; and
4 h, P=0.030. TSHβv-injected (10 µg) and control animals: 0.5 h,
P<0.001; 1 h, P<0.001; 4 h, P=0.031). *P<0.05 and
**P<0.001 vs. respective control. FT4, free thyroxine; TSHβv,
thyroid-stimulating hormone β splice variant. |
TSHβ splice variant induces thyroid
follicular cells to synthesize thyroid hormone
For in vitro analysis, mice thyroid
follicular cells first isolated and cultured. Following induction
with a recombinant TSHβ splice variant protein, the expression
levels of NIS, TPO and TG in mice thyroid follicular cells were
detected via western blot analysis. In addition, the levels of T3
and T4 in the supernatant were detected via RIA. As demonstrated in
Fig. 3A, T3 levels in the
supernatant peaked at 1 h post-induction, and sharply declined to a
normal level at 4 h following induction. The levels of T4 in the
supernatant similarly peaked at 1 h post-induction and then
declined; however, T4 levels in the induction group remained
significantly above control levels at 4 h following induction
(Fig. 3B). Overall, the in
vitro fluctuations in levels of the T3 and T4 appear to reflect
those observed in vivo, thus attesting to success of the
recombinant TSHβ splice variant. In addition, NIS protein
expression levels increased and peaked at 0.5 h post induction, and
gradually decreased to a normal level at 4 h following induction.
TG and TPO protein expression levels peaked at 0.5 h
post-induction, and slowly decreased thereafter; however, the
levels remained significantly increased at 4 h post-induction
compared with baseline expression prior to induction (Fig. 4). The expression levels of NIS in
the TSHβ splice variant protein-treated group were 2-fold higher
than in the control group at 0.5 h following treatment. TG
expression levels at 0.5 h post induction in the TSHβ splice
variant protein group were 1.85-fold higher than the control group
at the same time point. In addition, TPO expression levels in the
TSHβ splice variant protein-treated group were 1.92-fold higher
than the control group at 1 h post-induction.
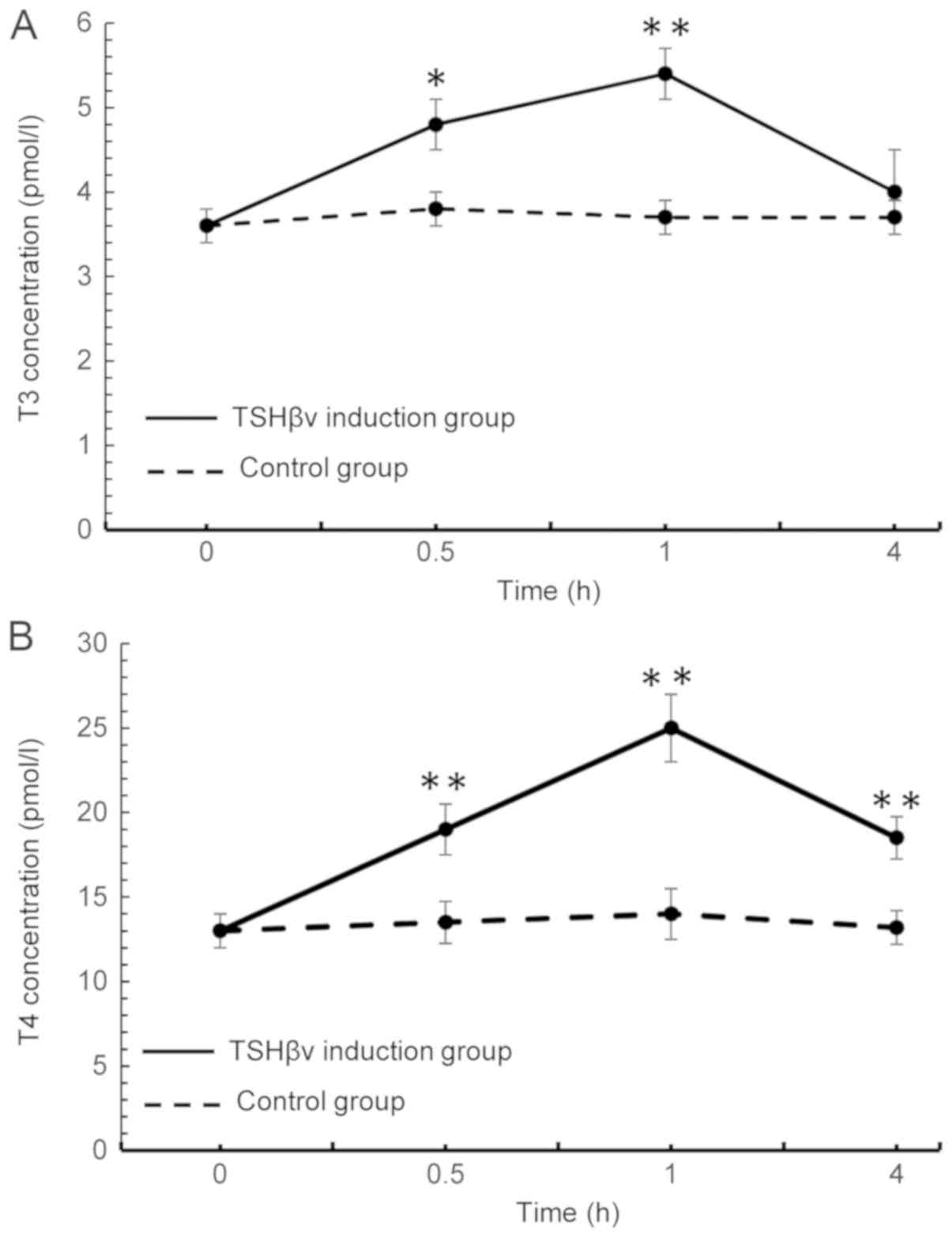 | Figure 3.Following induction of mouse thyroid
follicular cells with purified TSHβv protein, alterations in the
concentrations of (A) T3 and (B) T4 in the supernatant were
detected using a radioimmunoassay at 0, 0.5, 1 and 4 h following
induction. T3 levels, TSHβv-treated vs. control cells: 0.5 h,
P=0.006; 1 h, P<0.001; and 4 h, P=0.091. The T4 levels,
TSHβv-treated vs. control cells: 0.5 h, P<0.001; 1 h,
P<0.001; and 4 h, P<0.001. *P<0.01 and **P<0.001 vs.
respective control. T3, tri-iodothyronine; T4, thyroxine; TSHβv,
thyroid-stimulating hormone β splice variant. |
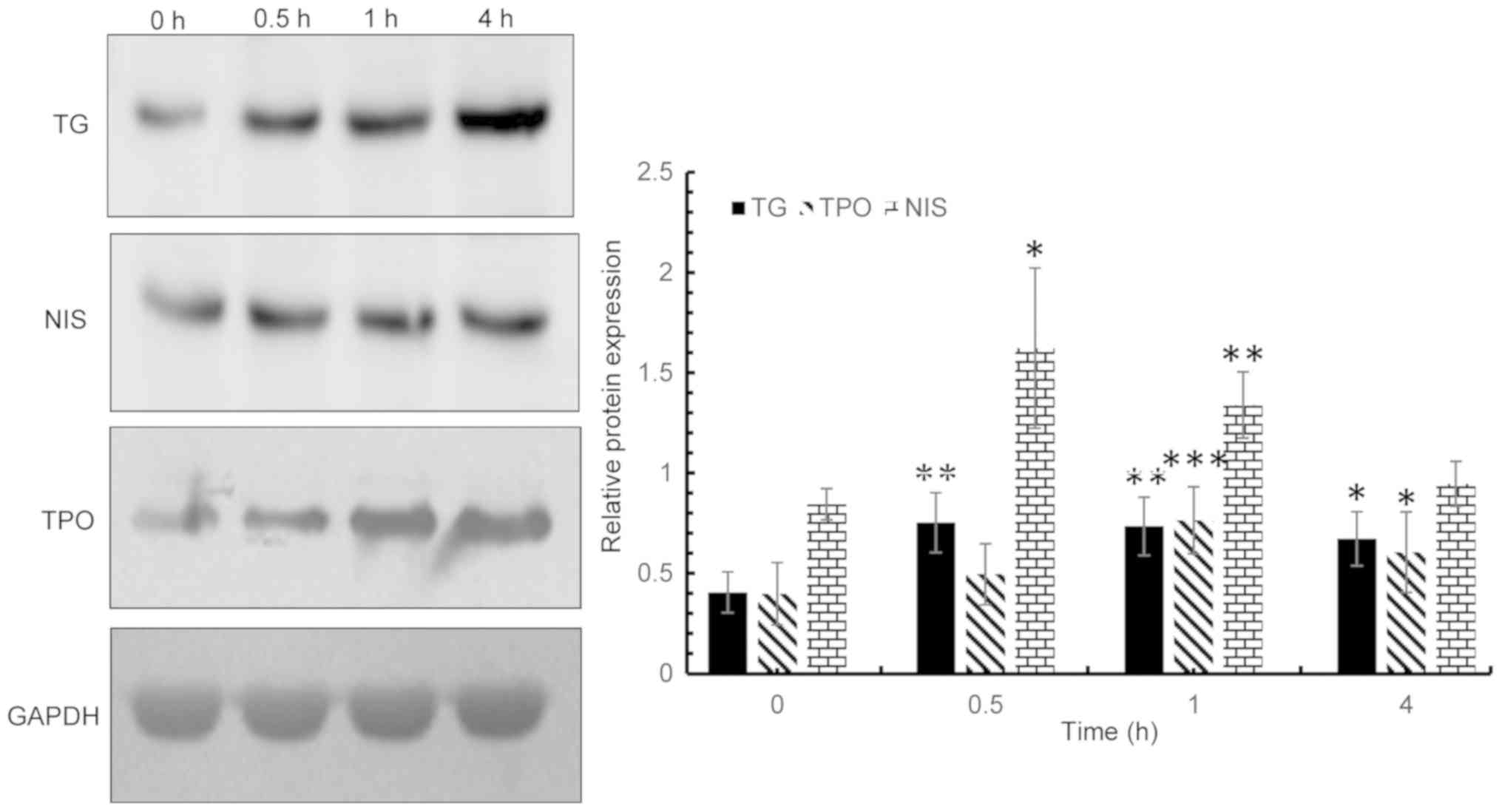 | Figure 4.Following induction of thyroid
follicular cells with purified thyroid-stimulating hormone β splice
variant protein, the expression levels of NIS, TG and TPO were
detected via western blot analysis at 0, 0.5, 1 and 4 h post
induction. NIS expression vs. 0 h: 0.5 h, P=0.016; 1 h, P=0.001;
and 4 h, P=0.367. TG expression vs. 0 h are: 0.5 h, P=0.004; 1 h,
P=0.003; and 4 h, P=0.015. TPO expression vs. 0 h are: 0.5 h,
P=0.092; 1 h, P<0.001; and 4 h, P=0.010. *P<0.05, **P<0.01
and ***P<0.001 vs. respective control. NIS, sodium iodide
symporter; TG, thyroglobulin; TPO, thyroperoxidase. |
TSHβ splice variant expression is not
regulated by the HPT axis
Mice were administered with T3 or TRH. The
expression levels of the TSHβ splice variant in pituitary, thyroid,
spleen tissues and PBLs were assessed by western blot analysis at 1
and 4 h following treatment. The native form and TSHβ splice
variant were detected in the pituitary (Figs. 5 and 6, respectively), as both forms are
expressed in the pituitary gland. The TSHβ splice variant was
detected in PBLs (Fig. 7), spleen
(Fig. 8) and thyroid (Fig. 9), as the native form of TSHβ is
known to not be expressed in these tissues (2–4). As
expected, the results indicated that the native form of TSHβ
expression was upregulated by TRH stimulation and downregulated by
T3 stimulation in the pituitary (Fig.
5). However, T3 and TRH demonstrated no effect on the
expression of the TSHβ splice variant protein in the pituitary,
thyroid, spleen and PBLs.
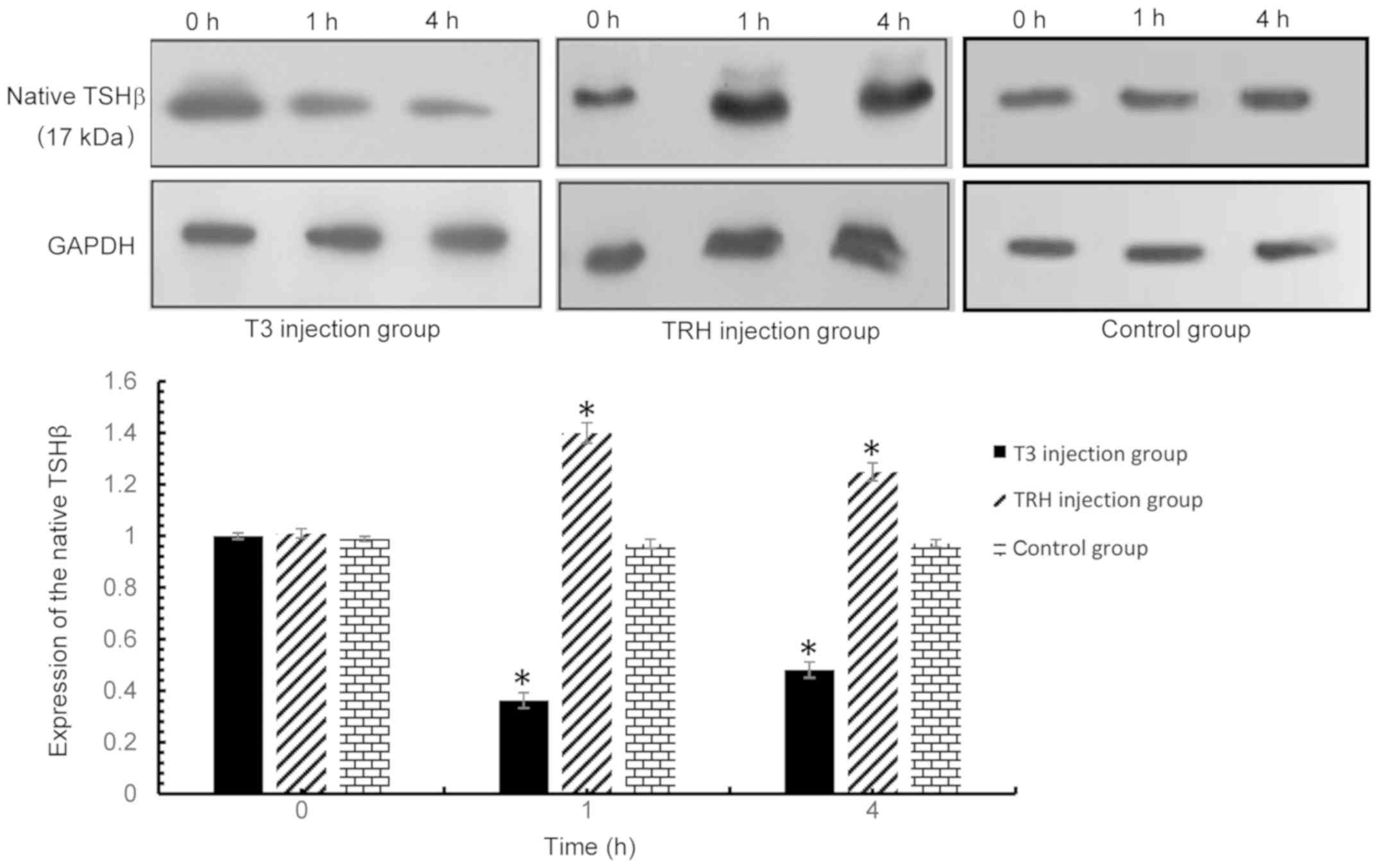 | Figure 5.Native TSHβ expression in the
pituitary was detected via western blot analysis at 0, 1 and 4 h
following injection with T3 or TRH. T3 injection group vs. control
group: 0 h, P=0.379; 1 h, P<0.001; and 4 h, P<0.001. TRH
injection group vs. control group: 0 h, P=0.107; 1 h, P<0.001;
and 4 h, P<0.001. *P<0.001 vs. respective control. TSHβ,
thyroid-stimulating hormone β; T3, tri-iodothyronine; TRH,
thyroid-releasing hormone. |
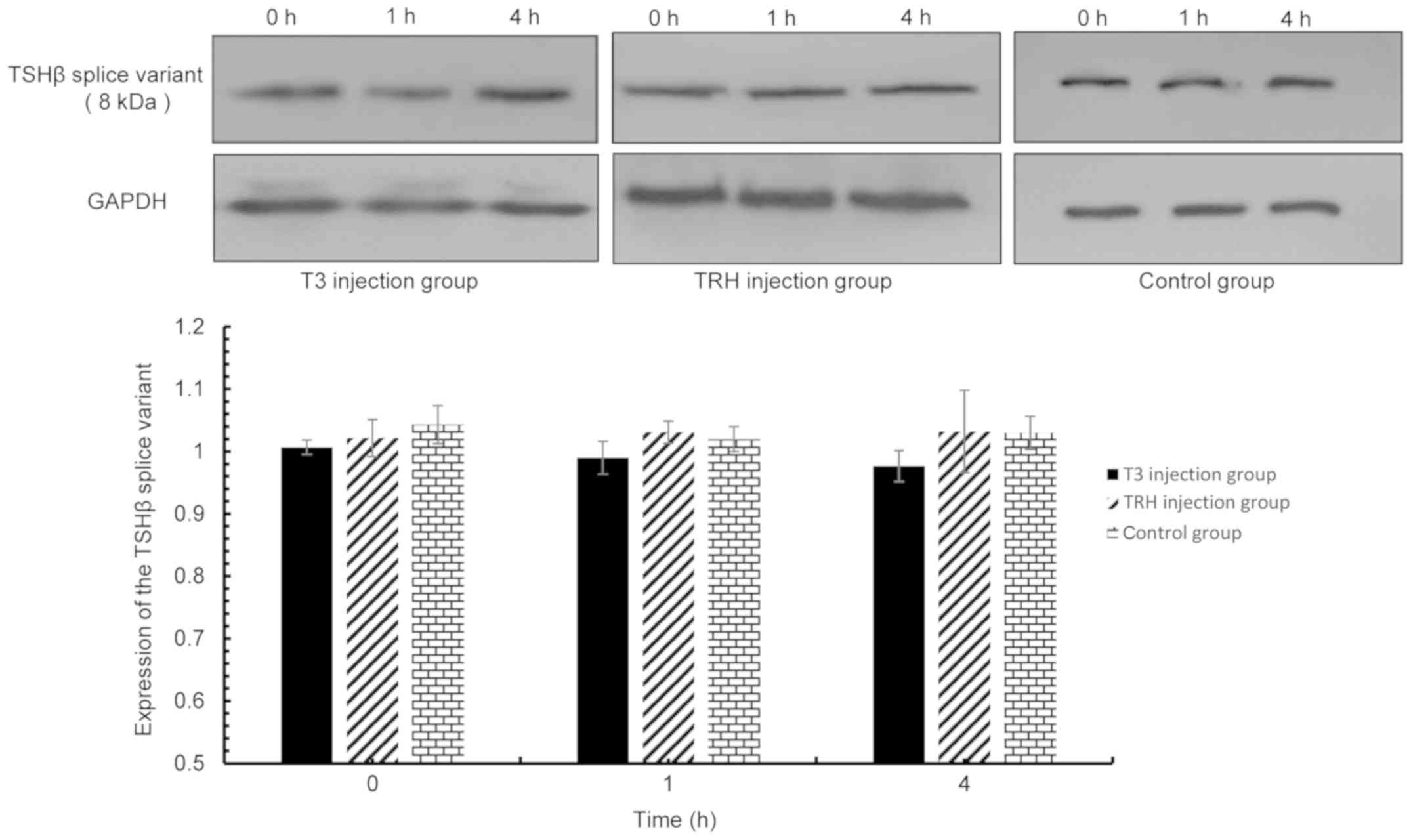 | Figure 6.TSHβ splice variant expression in the
pituitary was detected via western blot analysis at 0, 1 and 4 h
following injection with T3 and TRH. T3 injection group vs. control
group: 0 h, P=0.123; 1 h, P=0.804; 4 h, P=0.110. TRH injection
group vs. control group: 0 h, P=0.073; 1 h, P=0.754; and 4 h,
P=0.650. TSHβ, thyroid-stimulating hormone β; T3,
tri-iodothyronine; TRH, thyroid-releasing hormone. |
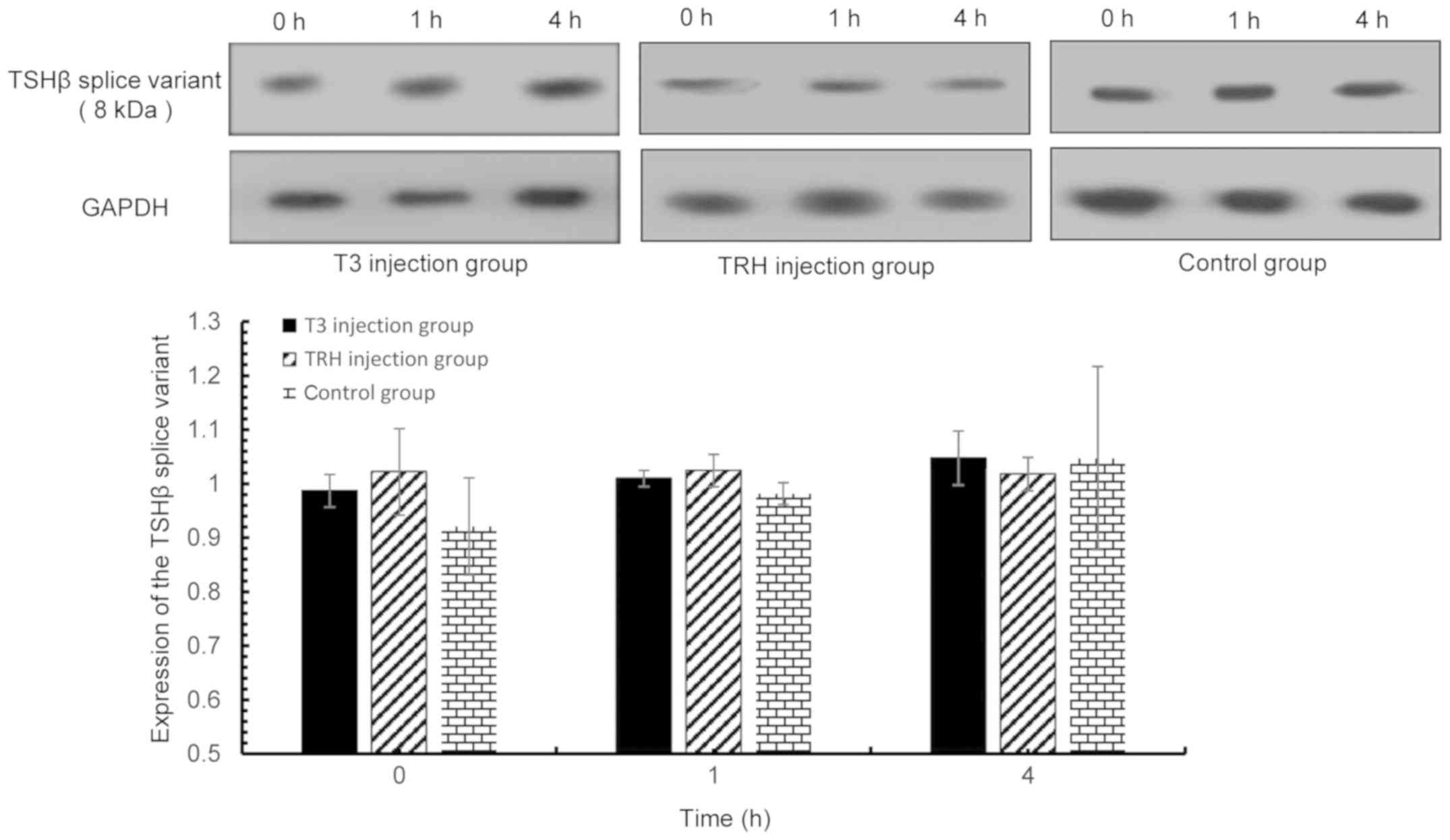 | Figure 7.TSHβ splice variant expression in PBLs
was detected via western blot analysis at 0, 1 and 4 h following T3
and TRH injection. T3 injection group vs. control group: 0 h,
P=0.323; 1 h, P=0.189; and 4 h, P=0.997. TRH injection group vs.
control group: 0 h, P=0.152; 1 h, P=0.064; and 4 h, P=0.740. TSHβ,
thyroid-stimulating hormone β; PBLs, peripheral blood leukocytes;
T3, tri-iodothyronine; TRH, thyroid-releasing hormone. |
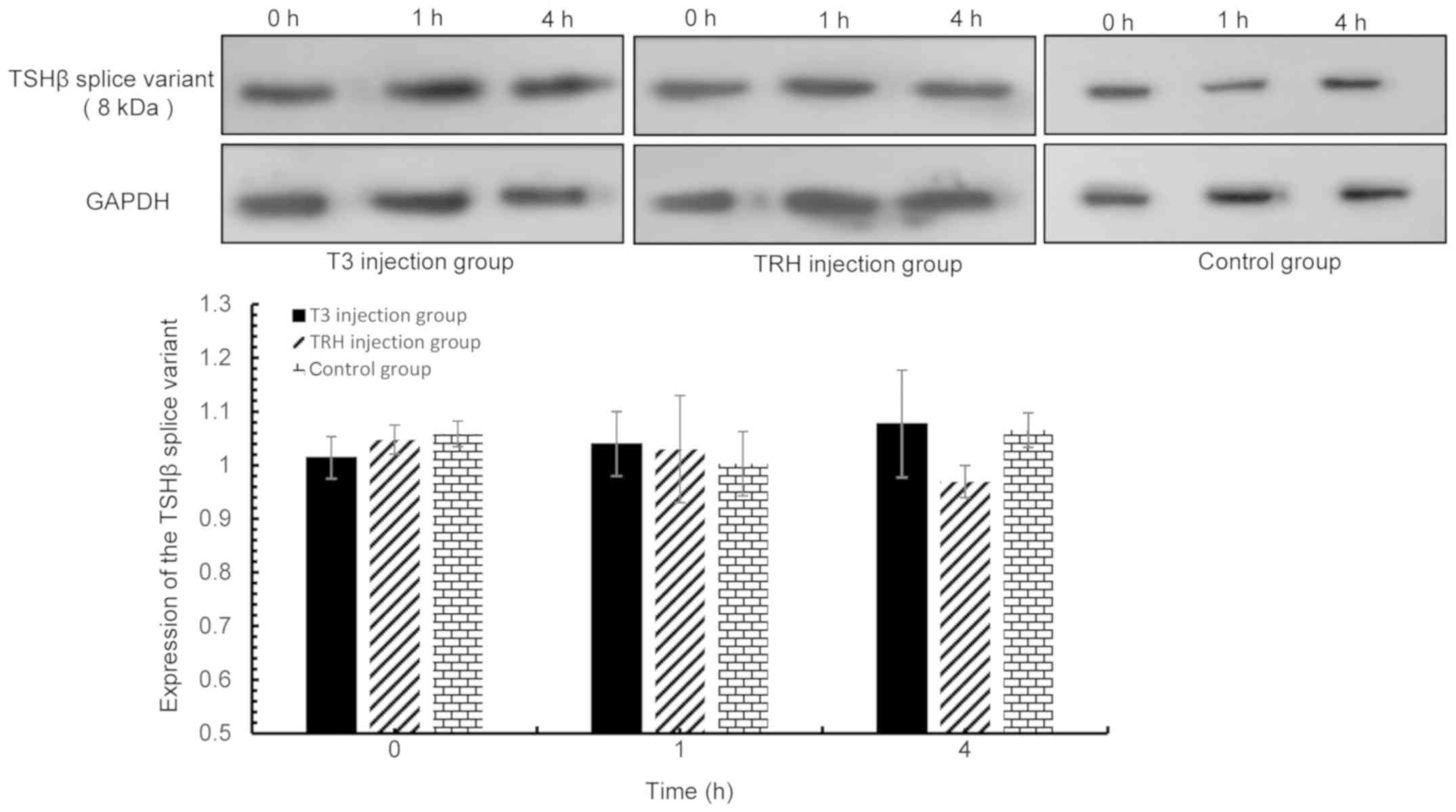 | Figure 8.TSHβ splice variant expression in the
spleen was detected via western blot analysis at 0, 1 and 4 h
following T3 and TRH injection. T3 injection group vs. control
group: 0 h, P=0.187; 1 h, P=0.603; and 4 h, P=0.829. TRH injection
group vs. control group: 0 h, P=0.692; 1 h, P=0.702; and 4 h,
P=0.114. TSHβ, thyroid-stimulating hormone β; T3,
tri-iodothyronine; TRH, thyroid-releasing hormone. |
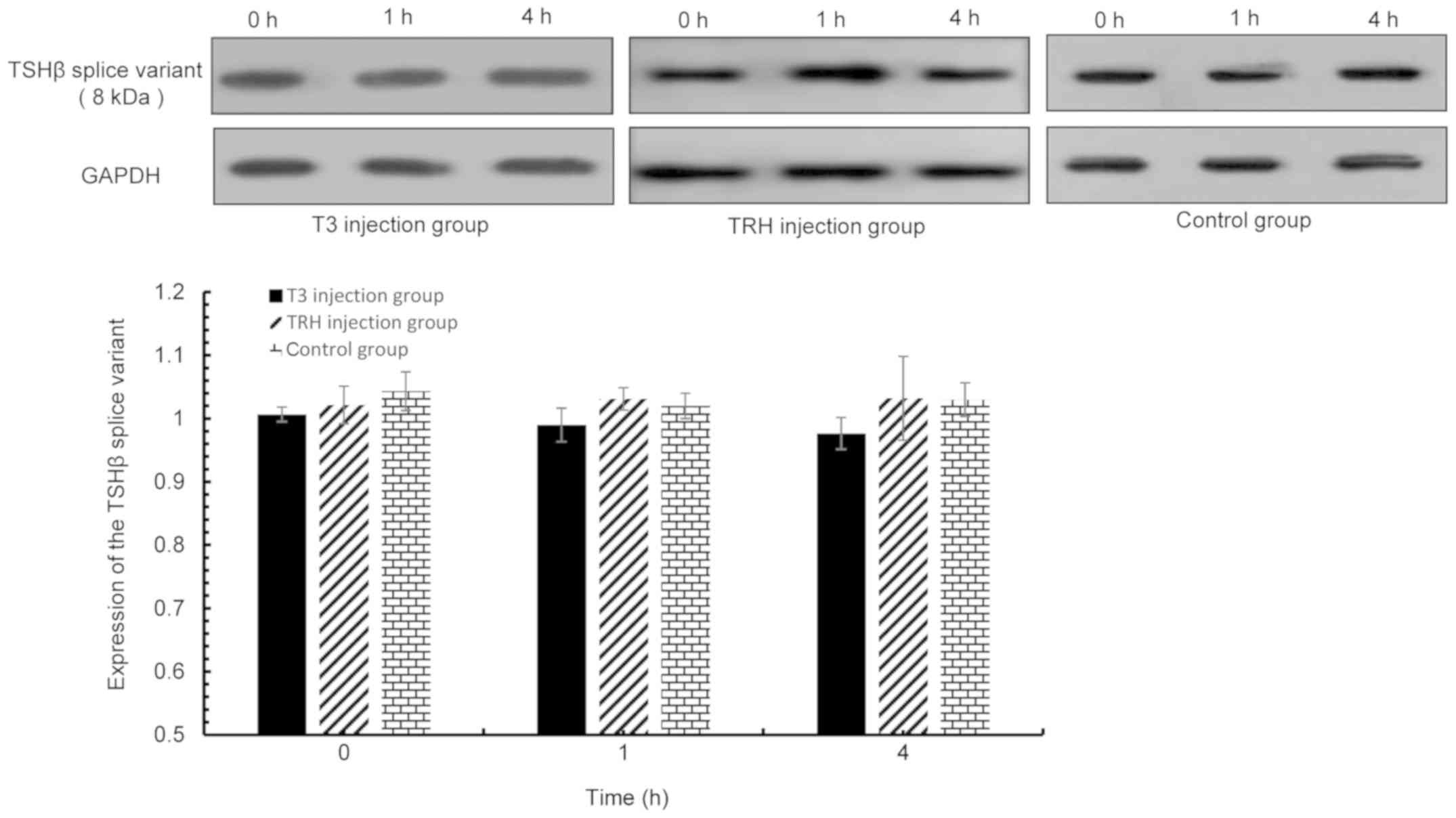 | Figure 9.TSHβ splice variant expression in the
thyroid was detected via western blot analysis at 0, 1 and 4 h
following T3 or TRH injection. T3 injection group vs. control
group: 0 h, P=0.12; 1 h, P=0.141; and 4 h, P=0.185. TRH injection
group vs. control group: 0 h, P=0.322; 1 h, P=0.553; and 4 h,
P=0.956. TSHβ, thyroid-stimulating hormone β; T3,
tri-iodothyronine; TRH, thyroid-releasing hormone. |
Discussion
TSH, secreted by the anterior pituitary, is known to
induce the production and secretion of thyroid hormones, T4 and T3
(1). It has since been established
that there are extra-pituitary sources of TSH, including the TSHβ
splice variant produced by cells of the immune system (8). Baliram et al (14) demonstrated that bone marrow-derived
macrophages preferentially produce the TSHβ splice variant. An
additional study indicated that the TSHβ splice variant was also
expressed in plasma cells of the thyroid of a patient with
Hashimoto's thyroiditis (15).
However, whether the production of extrapituitary TSHβ splice
variant promotes alterations in thyroid hormone synthesis, similar
to pituitary TSH, is yet to be elucidated. Previously published
in vivo and in vitro stimulation experiments have
been performed using isolated thyroid follicular epithelial cells.
Upon incubation of these cells in media containing the native form
of TSHβ, increased iodide transport activity, TG iodination,
protein synthesis and phospholipid synthesis were observed
(16). The results of the in
vivo and in vitro experiments performed in the present
study confirm that the TSHβ splice variant may also contribute to
the synthesis and secretion of thyroid hormones, which is similar
to the function of the native form of TSHβ. Therefore, the
immune-derived TSHβ splice variant may microregulate thyroid
hormone output via a paracrine pathway. This local regulatory
circuit is likely to serve as a physiologically efficient modulator
that conserves the energy-generating processes during and following
immune responses. In Hashimoto's thyroiditis, thyroid follicles are
destroyed by autoimmune attack induced by autoantibodies targeted
against thyroid peroxidase and/or thyroglobulin, resulting in
decreased T3 and T4 levels (17).
TSHβ-sensing (18–20) and TSHβ-producing (5,21)
leukocytes are trafficked to the thyroid and promote the synthesis
and secretion of thyroid hormones under immune stress conditions, a
defense response to maintain the energy-generating balance within
the body. As there are known TSHβ splice variants in human serum
(9), the TSHβ splice variant may
exert regulatory effects via telecrine signaling, in addition to
its paracrine signaling functions.
TSH synthesis in the anterior pituitary is
stimulated by TRH and inhibited by thyroid hormone in the HPT axis
(1). The HPT axis is a classical
neuroendocrine feedback system that was considered to be
functionally autonomous; however, an increasing amount of evidence
indicates that immune and neuroendocrine feedback systems may
interact (22). In addition to the
pituitary-thyroid circuit, there are other TSH-associated circuits
that regulate the immune-derived TSHβ splice variant, which may
function in extrathyroidal sites within the immune system, as
indicated by the ability of immune cells to produce this variant
(5). Present research is focused
on the mechanisms by which the expression of the TSHβ splice
variant is regulated by the immune system in disease states
(8,9,15,23).
However, it is known that the TSHβ splice variant, besides being
expressed in the pituitary, is also produced by immune cells,
including bone marrow-derived macrophages, plasma cells in the
thyroid and splenic leukocytes (10,14,15,23).
Native TSHβ is regulated by the HPT axis. However, to the best of
our knowledge, limited research has reported whether the HPT axis
is involved in the expression of the TSHβ splice variant.
Therefore, the HPT axis was selected as a research focus in the
present study in order to provide further insights into the
physiological regulation of the TSHβ splice variant, and to further
explore its potential pathological effects.
As TSHβ splice variant expression was detected in
the thyroid, spleen and PBLs, alterations in the expression of TSHβ
splice variant in these tissues and the pituitary were determined
in mice following injection with T3 or TRH. The results
demonstrated that only the native form of TSHβ expression was
altered in the pituitary. By contrast, the expression of TSHβ
splice variant remained unaltered in all evaluated tissues. There
is strong evidence to suggest that TSHβ splice variant expression
may be altered under immune stress. Vincent et al (5) reported that TSHβ splice variant
expression is increased in the thyroid following systemic virus
infection. In addition, Baliram et al (10) reported that in hyperthyroidism,
bone marrow resident macrophages exhibit the potential to induce
osteoprotective effects by overexpressing human TSHβ splice
variant, which may perform its local osteoprotective role via TSH
receptors on osteoblasts and osteoclasts. These results suggest
that the TSHβ splice variant may influence bone biology and serve
as a local osteoprotective resource for bone remodeling in disease
states and fracture repair.
Our previous study demonstrated that the TSHβ splice
variant is expressed at significantly higher levels in thyroid
tissues of patients with Hashimoto's thyroiditis compared with
normal thyroid tissues (15). In
addition, the expression of the TSHβ splice variant was positively
associated with the degree of thyroid follicle damage in patients
with Hashimoto's thyroiditis (15). Montufar-Solis and Klein (23) reported that splenic leukocytes in
Listeria monocytogenes-infected mice migrate to the thyroid
and produce the intrathyroidal TSHβ splice variant. These published
observations and the results of the present study suggest that the
TSHβ splice variant may participate in the regulation of thyroid
hormone synthesis independently of the HPT axis. Moreover, there
may be a unique regulatory mechanism of the expression of the TSHβ
splice variant that may occur in certain disease states. The TSHβ
splice variant may also serve as a critical immunological regulator
during immune stress. The two putative nuclear factor-κB subunit
binding sites in intron 4 of mouse TSHβ are hypothesized to
regulate the TSHβ splice variant, and may be under the control of
immunologically-mediated transcription signals (22). Elucidating the precise mechanisms
underlying this regulation will require promoter activity and
binding assays.
In conclusion, thyroid hormones serve a major role
in metabolic function, and in response to stress and critical
illness (9,10,15,24,25).
The results of the present study suggest that the immune-derived
TSHβ splice variant may contribute to the higher levels of serum
thyroid hormones. The splice variant of TSHβ may contribute to this
increase via paracrine microregulation of thyroidal follicular
cells during advanced stages of infection or
inflammatory-associated disorders, and is independent of regulation
by the HPT axis; however, the mechanism of thyroid hormone
secretion stimulated by TSHβ splice variant has not been clarified.
Whether the TSHβ splice variant stimulates thyroid follicular cells
to produce thyroid hormone via the TSHβ receptor or via any other
pathways remains unknown. In the future, the authors of the present
study intend to produce a specific antibody against TSHβ splice
variant to answer these questions. The functional role of TSHβ
splice variant synthesis in the immune system also remains to be
determined. Nevertheless, the current study provides novel insights
into the biological features and role of TSHβ splice variant, and
these results may have implications in the current understanding of
immune-neuroendocrine interactions.
Acknowledgements
Not applicable.
Funding
The present study was supported by The National
Natural Science Foundation (grant nos. 81302577 and 81472140) and
Chinese People's Armed Police Force Foundation (grant no.
WHB201307).
Availability of data and materials
The datasets used and/or analyzed in the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
JM and CL conceived and designed the study; XL, ZZ,
TK, JL, RW and QD performed the experiments; CL and LL analyzed and
interpreted the data; CL completed the draft. All authors read and
approved the manuscript.
Ethics approval and consent to
participate
All procedures used were in accordance with the
Logistics University of Chinese People's Armed Police Force animal
welfare guidelines. The animal protocols were approved by the
Ethics Review Committee of the Logistics University of Chinese
People's Armed Police Force (Tianjin, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wang HC and Klein JR: Immune function of
thyroid stimulating hormone and receptor. Crit Rev Immunol.
21:323–337. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Szkudlinski MW, Fremont V, Ronin C and
Weintraub BD: Thyroid-stimulating hormone and thyroid-stimulating
hormone receptor structure-function relationships. Physiol Rev.
82:473–502. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang SH and Koenig RJ: A locally secreted
thyrotropin variant may regulate thyroid function in thyroid
inflammatory disorders. Thyroid. 19:5–6. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gordon DF, Wood WM and Ridgway EC:
Organization and nucleotide sequence of the gene encoding the
beta-subunit of murine thyrotropin. DNA. 7:17–26. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vincent BH, Montufar-Solis D, Teng BB,
Amendt BA, Schaefer J and Klein JR: Bone marrow cells produce a
novel TSHbeta splice variant that is upregulated in the thyroid
following systemic virus infection. Genes Immun. 10:18–26. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wood WM, Gordon DF and Ridgway EC:
Expression of the beta-subunit gene of murine thyrotropin results
in multiple messenger ribonucleic acid species which are generated
by alternative exon splicing. Mol Endocrinol. 1:875–883. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wolf O, Kourides IA and Gurr JA:
Expression of the gene for the beta subunit of mouse thyrotropin
results in multiple mRNAs differing in their 5′-untranslated
regions. J Biol Chem. 262:16596–16603. 1987.PubMed/NCBI
|
|
8
|
Klein JR: Biological impact of the TSHβ
splice variant in health and disease. Front Immunol. 5:1552014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu C, Li LY, Ying F, Xu C, Zang X and Gao
Z: A newly identified TSHβ splice variant is involved in the
pathology of Hashimoto's thyroiditis. Mol Biol Rep. 39:10019–10030.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Baliram R, Latif R, Morshed SA, Zaidi M
and Davies TF: T3 regulates a human macrophage-derived TSH-β splice
variant: Implications for human bone biology. Endocrinology.
157:3658–3667. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jeker LT, Hejazi M, Burek CL, Rose NR and
Caturegli P: Mouse thyroid primary culture. Biochem Biophys Res
Commun. 257:51l–5l5. 1999. View Article : Google Scholar
|
|
12
|
Ambesi-Impiombato FS, Parks LA and Coon
HG: Culture of hormone-dependent functional epithelial cells from
rat thyroids. Proc Natl Acad Sci USA. 77:3455–3459. 1980.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Curcio F, Ambesi-Impiombato FS, Perrella G
and Coon HG: Long term culture and functional characterization of
folicular cells from adult normal human thyroids. Proc Natl Acad
Sci USA. 91:9004–9008. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Baliram R, Chow A, Huber AK, Collier L,
Ali MR, Morshed SA, Latif R, Teixeira A, Merad M, Liu L, et al:
Thyroid and bone: Macrophage-derived TSH-β splice variant increases
murine osteoblastogenesis. Endocrinology. 154:4919–4926. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu CR, Miao J, Zhao ZK, Li LY, Liu YM,
Zhang YL, Li XH, Liu YQ, Gu YJ, Zhao Y and Luo JW: Functional human
TSHβ splice variant produced by plasma cell may be involved in the
immunologic injury of thyroid in the patient with Hashimoto's
thyroiditis. Mol Cell Endocrinol. 414:132–142. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Petitfrère E, Huet E, Sartelet H, Martiny
L, Legue O and Haye B: TSH-induced differentiated functions
correlate with enhancement of phosphotyrosine phosphatase activity
in thyroid cells. Effect of phorbol 12-myristate 13-acetate. J
Endocrinol. 169:603–611. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Burek CL and Rose NR: Autoimmune
thyroiditis and ROS. Autoimmun Rev. 7:530–537. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang HC, Dragoo J, Zhou Q and Klein JR: An
intrinsic thyrotropin-mediated pathway of TNF-alpha production by
bone marrow cells. Blood. 101:119–123. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bağriacik EU and Klein JR: The thyrotropin
(thyroid-stimulating hormone) receptor is expressed on murine
dendritic cells and on a subset of CD45RBhigh lymph node T cells:
Functional role for thyroid-stimulating hormone during immune
activation. J Immunol. 164:6158–6165. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Whetsell M, Bagriacik EU, Seetharamaiah
GS, Prabhakar BS and Klein JR: Neuroendocrine-induced synthesis of
bone marrow-derived cytokines with inflammatory immunomodulating
properties. Cell Immunol. 192:159–166. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schaefer JS and Klein JR: A novel thyroid
stimulating hormone beta-subunit isoform in human pituitary,
peripheral blood leukocytes, and thyroid. Gen Comp Endocrinol.
162:241–244. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schaefer JS and Klein JR: Immunological
regulation of metabolism-a novel quintessential role for the immune
system in health and disease. FASEB J. 25:29–34. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Montufar-Solis D and Klein JR: Splenic
leukocytes traffic to the thyroid and produce a novel TSHβ isoform
during acute listeria monocytogenes infection in mice. PLoS One.
11:e01461112016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Angelousi AG, Karageorgopoulos DE,
Kapaskelis AM and Falagas ME: Association between thyroid function
tests at baseline and the outcome of patients with sepsis or septic
shock: A systematic review. Eur J Endocrinol. 164:147–155. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Silva MH, Araujo MC, Diniz EM, Ceccon ME
and Carvalho WB: Thyroid abnormalities in term infants with fungal
sepsis. Rev Assoc Med Bras (1992). 62:561–567. 2016. View Article : Google Scholar : PubMed/NCBI
|























