Introduction
Strabismus is an ocular disease characterized by the
inability of both eyes of an individual to gaze at a target
simultaneously and the optic axis of both eyes is separated. It can
lead to binocular vision impairment, and is often accompanied by
amblyopia and loss of stereo vision. The incidence of strabismus in
children aged 3–6 years in eastern China is 5.65% (1). There is no ideal classification
method for strabismus in clinical practice, and its treatment
mainly relies on surgical correction. Dysfunction of the
extraocular muscles (EOMs) is an important cause of strabismus. In
particular, aberrant EOM development, EOM dystrophy (2) and abnormal position of the EOM
(3) can lead to the occurrence of
strabismus, while dysfunction of the EOM pulleys has also been
associated with the condition (4,5). The
eye movement system is underpinned by a complex neural network. The
structures in the nucleus of the brain, and the EOMs and nerves
that dominate them must all work perfectly and synchronously to
ensure normal visual function. For instance, the development of
Duane's retraction syndrome has been reported to be associated with
mechanical abnormalities of the external rectus muscle (6), aberrant innervation (7) and the absence of an abduction nucleus
(8). Neuronal activity in the
brain area that is involved in eye movements is also crucial. The
frontal eye field (FEF) participates in the control of eye movement
(4) and conjugate eye movement
(5). A previous study reported
that the gray matter volume of the FEF is increased in strabismus
patients (9). Therefore,
strabismus pathogenesis is directly associated with the EOM and
brain function and/or structural alterations.
Amblyopia is caused by abnormal visual experiences
(such as monocular strabismus, anisometropia, high ametropia and
form-deprivation myopia) during visual development, resulting in
decreased monocular and/or binocular best-corrected visual acuity
(VA), but no organic lesion is observed on eye examination. The
diagnostic criteria for amblyopia include a visual reference value
that is below the lower normal limit (which is 0.5 for 3–5-year-old
children, and 0.7 for children aged ≥6 years), or the best
corrected VA of two eyes differs by ≥0.2, then the eye with poorer
VA exhibits amblyopia (10). In
China, the prevalence of amblyopia in children is 2–3% (11). At present, early detection and
early treatment of amblyopia are important, with standard treatment
strategies including accurate glasses and concealment of the
dominant eye. According to its etiology, amblyopia can be divided
into the strabismus, anisometropic, ametropic and form-deprivation
types (12). The pathogenesis of
amblyopia is highly complex; the two theories currently recognized
involve abnormal binocular interaction and form deprivation
(13,14). Simultaneously, abnormal activities
in the associated brain regions have also been suggested to
contribute to the development of amblyopia. For instance, Wang
et al (15) reported that
activation of the intraparietal sulcus, FEF and motor sensitive
(V5) areas was reduced in patients with amblyopia. Thus, the
exploration of brain activity in patients with strabismus and
amblyopia (SA) is of practical significance.
Functional magnetic resonance imaging (fMRI)
(16) is the primary examination
technique used to locate and quantify functional brain areas. Its
main advantages over traditional MRI techniques are high spatial
resolution and the ability to reveal detailed microscopic
structural changes. fMRI technology includes diffusion weighted
imaging (DWI), diffusion tensor imaging (DTI), magnetic resonance
spectroscopy, and blood oxygen level-dependent (BOLD) fMRI. Among
these, DWI can reflect the diffusion movement and limitation of
water molecules in tissues and lesions, while DTI reveals the
anisotropy of diffusion movement towards water molecules, and can
therefore be used to analyze white matter fiber bundles. In
addition, BOLD fMRI uses the principle of blood oxygenation level
dependence, that is, inconsistencies in the local hemodynamics of
neurons following excitation, in order to reveal spontaneous
neuronal activity by quantifying alterations in blood oxygen level
signals. Thus, the study of brain cognitive activities, and the
localization and quantification of brain functional activity areas
is facilitated by fMRI. This technology has been widely used to
study SA. In addition to these aforementioned studies, the volume
of gray matter in the occipital eye field and parietal eye field
has been reported to be reduced in patients with strabismus
(17), while the functional
connectivity between the V1 and V2 regions was decreased in SA
monkeys (18). Furthermore,
multiple brain areas are reportedly dysfunctional in concomitant
strabismus (19).
Resting-state fMRI (rs-fMRI) analysis can be
conducted using various methods, among which regional homogeneity
(ReHo) is widely used. ReHo is a computational method based on
functional differentiation, which was first proposed by the Chinese
scholar Professor Yu-Feng Zang. This method can be used to analyze
the consistency of brain activity signals and provide information
about brain function (20,21). ReHo functions by assuming that the
hemodynamics of each voxel in a brain region with the same function
is approximately identical, and that the hemodynamics of the brain
region may change due to variations in function or task. Thus, the
level of consistency of BOLD signals can be represented by
evaluating the degree of hemodynamic consistency between voxels in
the region of interest and voxels that are adjacent to it
simultaneously, which can be expressed using a ReHo value that
reveals the consistency of spontaneous neuronal activity.
Therefore, changes in ReHo values indicate alterations in brain
hemodynamics, that is, changes in the synchrony of spontaneous
neuronal activity. An increase in ReHo indicates an increase in the
synchrony of spontaneous neuronal activity, whereas a decreased
ReHo value suggests reduced synchrony and disordered activity. This
method has been successfully applied to the investigation of the
etiology of various eye diseases, including concomitant strabismus
(19), optic neuritis (22), type 2 diabetes with retinopathy
(23), glaucoma (24), open-globe injury (25), late monocular blindness (26) and retinal detachment (27), as well as a number of neurogenic
diseases, such as sleep disorders (28) and Parkinson's disease (29).
In the present study, the brain activity of patients
with SA was investigated using the ReHo method, in order to confirm
alterations in brain function and explore the potential
pathophysiological mechanism. To the best of our knowledge, this is
the first study to examine SA using this method.
Subjects and methods
Subjects
A total of 16 patients with SA who were treated in
the Department of Ophthalmology of The First Affiliated Hospital of
Nanchang University (Nanchang, China) were enrolled into the
present study. The patients included 6 men and 10 women, among
which there were 11 cases with exotropia and 5 with esotropia. The
inclusion criteria were as follows: i) adults with an age of >18
years; ii) patients diagnosed with strabismus; and iii) >2-line
difference in the best-corrected VA (≥0.20 logMAR units) between
the amblyopic and the fellow eye, with central fixation. Patients
were excluded according to the following criteria: i) patients with
a history of previous ocular surgery, including intraocular and
extraocular surgery; ii) patients with evidence of other eye
diseases, such as cataract, glaucoma, optic neuritis, macular
degeneration, infection, inflammation, and ischemic disease; iii)
other diseases that may affect the experimental results, including
mental illness; and iv) alcoholism or drug addiction.
In addition, 16 healthy controls (HCs), including 6
men and 10 women, were matched with the patients in terms of age
and sex. All HCs met the following criteria: i) MRI examination
exhibited no abnormalities in the brain parenchyma; ii) no history
of eye disease, with best-corrected VA of ≤0 logMAR units; iii) no
psychiatric disease; and iv) able to undergo MRI examination (for
example, no implanted steel plate).
The present study was approved by the Medical Ethics
Committee of the First Affiliated Hospital of Nanchang University
and conformed to all the principles required by the Declaration of
Helsinki. Prior to signing informed consent, detailed information
on the study was provided to participants, including the purpose of
the research and possible risks to subjects.
MRI parameters
All subjects were scanned with a 3-Tesla magnetic
resonance scanner (Trio; Siemens AG, Munich, Germany). All
participants were requested to stay awake, keep their eyes closed
and relax their body until the end of the scan. Conventional T1
weighted image (T1WI) scans were collected, as well as T2WI
structural magnetic resonance data for the exclusion of brain
structural lesions, 3D high-resolution T1WI volume image data and
rs-fMRI data. Among them, 176 3D high-resolution T1WI volume images
were acquired by the T1-weighted 3D spoiled gradient sequence
(30). The specific scanning
parameters used were as follows: repetition time (TR), 1,900 msec;
echo time (TE), 2.26 msec; thickness, 1.0 mm; gap, 0.5 mm;
acquisition matrix, 256×256; flip angle, 9°; field-of-view (FOV),
250×250 mm. Furthermore, a total of 240 functional images were
acquired using the gradient-recalled echo-planar imaging sequence
(31), according to the following
specific scanning parameters: TR, 2,000 msec; TE, 30 msec;
thickness, 4.0 mm; gap, 1.2 mm; acquisition matrix, 64×64; flip
angle, 90°; and FOV, 220×220 mm. The scanning times for the two
sequences were 5 and 10 min, respectively.
fMRI data processing
Initially, the MRICRO software package (http://www.MRIcro.com) was used to examine and screen
the acquired brain data. Next, the SPM8 software package
(http://www.fil.ion.ucl.ac.uk/spm) on the
Matlab R2012b platform (MathWorks, Natick, MA, USA) was applied to
preprocess the two sets of data in this experiment, using the data
processing software package DPARSF (http://rfmri.org/DPARSF). The main steps of the
analysis were as follows: first, the data format was converted. In
order to remove the interference of the unstable magnetic field,
the data of the first 10 time points were then eliminated. In order
to remove the impact caused by different data acquisition time,
time correction was subsequently performed on the collected data.
Next, head movement correction was performed; head movement was
considered to be too large and the data was eliminated for one case
that had a maximum head movement in three directions of >2 mm
and a maximum rotation angle of >2°. Subsequently, spatial
standardization of the functional image was conducted, which was
necessary since each subject has a certain difference in brain
structure and volume. For this standardization, the functional
image was registered to the standard space of the Montreal
Neuroscience Institute, and all voxels were resampled to a size of
3×3×3 mm to obtain a more accurate brain area. The next step
involved removal of linear drift in order to eliminate the linear
chemotactic effect of the subject in the process of adapting to the
scanning environment. Finally, filtering was performed by
collecting data in the frequency range of 0.01–0.08 Hz to remove
the influence of high-frequency physiological noise, such as
breathing and heartbeat.
ReHo calculation and image
processing
REST software (http://sourceforge.net/projects/testing-fmri) was used
to calculate ReHo values for fMRI data without smooth
preprocessing. ReHo images were derived from the Kendall
consistency coefficient, which was obtained using the DPABI toolkit
software (http://rfmri.org/DPABI) to calculate the
time series consistency of each voxel in the brain and 26 voxels
adjacent to it. Next, the image was normalized, and the Fisher r to
Z transformation was performed. Finally, a 6×6×6 mm full-width was
applied at half-maximum Gaussian smooth kernel to perform Gaussian
smoothing of the ReHo image in order to improve the signal-to-noise
ratio.
Statistical analysis
Statistical evaluation of differences in variables,
such as demographics and visual measurements, between patients and
normal controls were conducted in SPSS version 20.0 software (IBM
Corp., Armonk, NY, USA) using two-sample t-tests. Differences in
ReHo values between SA and HC subjects were evaluated using
two-sample t-tests in REST software (State Key Laboratory of
Cognitive Neuroscience and Learning, Beijing Normal University,
Beijing, China). At the voxel level, the statistical threshold was
set to P<0.05, and for multiple comparisons using Gaussian
random field theory voxels, thresholds of P<0.01 and cluster
size of >40 voxels (AlphaSim-corrected) were employed. Using
differences in ReHo values in the same brain regions, receiver
operating characteristic (ROC) curves were generated to analyze and
identify SA patients and HCs. Pearson correlation analysis was also
used to evaluate the correlation between ReHo values and clinical
features of patients with altered brain regions, including the
association between disease duration and ReHo values. Correlations
and differences between SA and HC subjects were considered to be
statistically significant at P<0.05.
Results
Demographics and visual
measurements
No significant differences in age (P=0.615) or
best-corrected VA of the fellow eye (P=0.185) were detected between
the two groups. By contrast, the differences observed between the
two groups in the best-corrected VA of the amblyopic eye were
statistically significant (P<0.001; Table I).
 | Table I.Demographics and clinical
measurements of SA and HC groups. |
Table I.
Demographics and clinical
measurements of SA and HC groups.
| Parameter | SA | HC | t-value | P-value |
|---|
| Male/female | 6/10 | 6/10 | – | >0.99 |
| Age (years) | 24.50±5.91 | 24.94±5.23 | −0.222 |
0.615 |
| Handedness | 16 R | 16 R | – | >0.99 |
| Disease duration
(years) | 18.19±9.85 | – | – | – |
|
Esotropia/exotropia | 5/11 | – | – | – |
| Spherical
equivalent refractive error (diopters) |
1.22±0.56 |
1.25±0.67 | −0.365 |
0.741 |
|
| (−2.75–1.75) | (−2.75–2.00) |
|
|
| Angle of strabismus
(PD) |
26.25±12.71 | – | – | – |
| Best-corrected
VA |
|
Amblyopic eye |
0.77±0.53 | −0.05±0.08 |
6.149 |
<0.001 |
| Fellow
eye |
−0.03±0.09 | −0.01±0.07 | −0.651 |
0.185 |
ReHo differences
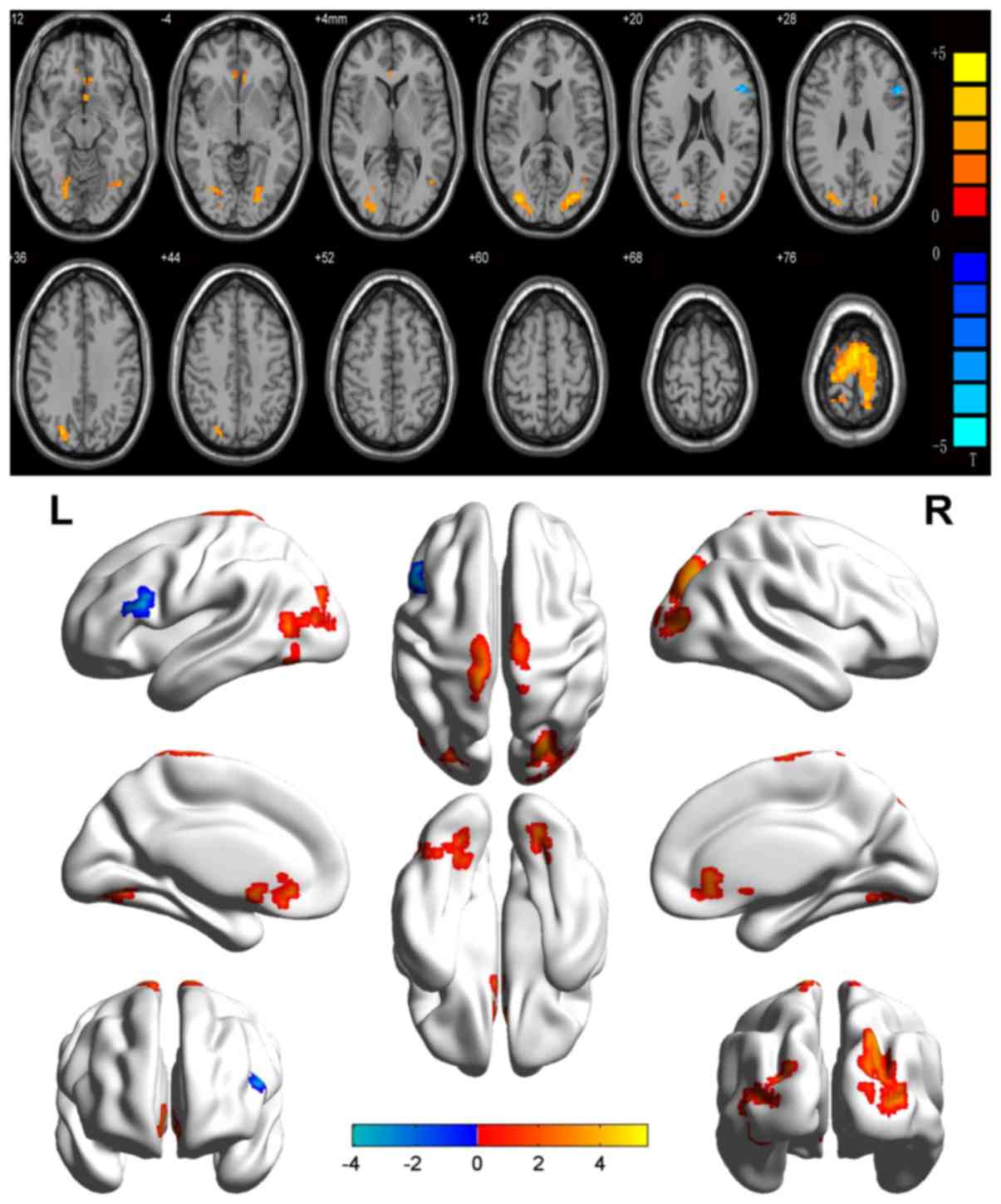
In the SA group, ReHo values were significantly
increased relative to the HCs in the following brain regions: left
lingual gyrus (LLG), right middle occipital gyrus and right
precuneus (RMOG/RP), bilateral anterior cingulate (BAC), left
middle occipital gyrus (LMOG) and bilateral precentral gyrus (BPG)
[Fig. 1 (red shading) and Table II)]. By contrast, the ReHo values
of the left inferior frontal gyrus (LIFG) were markedly reduced in
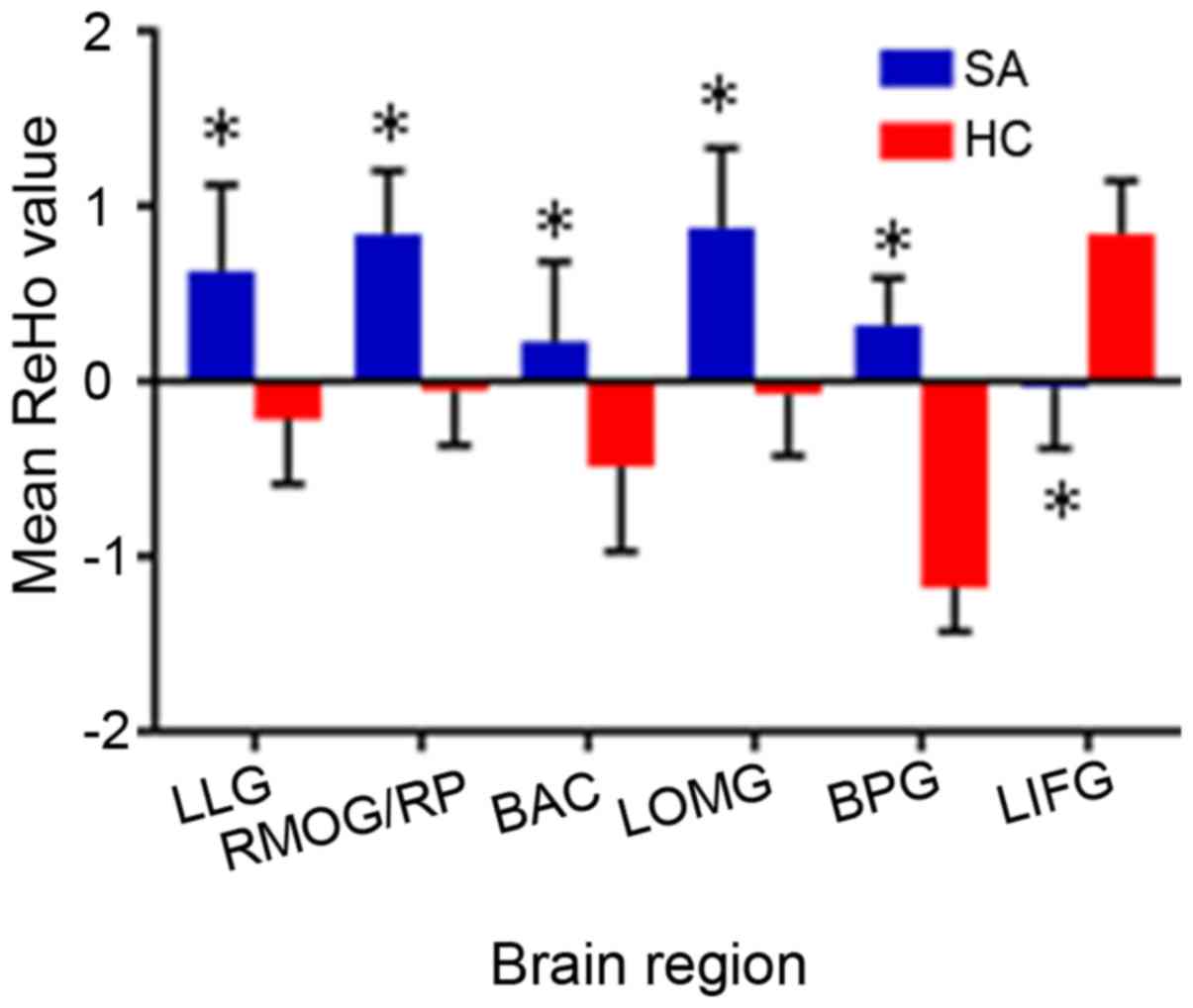
SA patients as compared with those in HCs [Fig. 1 (blue shading) and Table II]. The mean ReHo values in the
two groups are presented in Fig.
2. The ReHo values in the altered brain areas of SA patients
were not significantly associated with any of the clinical features
evaluated in the present study (Table III).
 | Table II.Brain areas with significantly
different ReHo values between groups. |
Table II.
Brain areas with significantly
different ReHo values between groups.
| A, SA>HC |
|---|
|
|---|
|
|
|
|
| MNI
coordinates |
|
|
|---|
|
|
|
|
|
|
|
|
|---|
| Condition | Left/right | Brain regions | BA | X | Y | Z | Peak voxels | t-value |
|---|
| 1 | Left | Lingual gyrus | 19 | −36 | −69 | −9 | 86 |
3.6987 |
| 2 | Right | Middle occipital
gyrus/precuneus | 19 | 33 | −84 | 12 | 256 |
4.7141 |
| 3 | Bilateral | Anterior
cingulate | – | 6 | 30 | −6 | 74 |
3.8637 |
| 4 | Left | Middle occipital
gyrus | 19 | −27 | −87 | 9 | 116 |
4.5112 |
| 5 | Bilateral | Precentral
gyrus | 6 | 0 | −6 | 78 | 339 |
5.5492 |
|
| B,
SA<HC |
|
|
|
|
|
| MNI
coordinates |
|
|
|
|
|
|
|
|
|
|
|
Condition |
Left/right | Brain
regions | BA | X | Y | Z | Peak
voxels | t-value |
|
| 1 | Left | Inferior frontal
gyrus | 9 | −51 | 18 | 24 | 69 | −4.0693 |
 | Table III.Pearson correlations analysis. |
Table III.
Pearson correlations analysis.
| Brain regions | ReHo value (mean ±
SD) | Duration (years)
(mean ± SD) | r-value | P-value |
|---|
| Left lingual
gyrus | 0.6316±0.4937 | 18.05±9.55 | −0.343 | 0.196 |
| Right middle
occipital gyrus/precuneus | 0.8429±0.3633 |
| −0.254 | 0.342 |
| Bilateral anterior
cingulate | 0.2272±0.4586 |
| 0.063 | 0.817 |
| Left middle
occipital gyrus | 0.8784±0.4560 |
| −0.342 | 0.195 |
| Bilateral
precentral gyrus | 0.3208±0.2700 |
| −0.360 | 0.171 |
| Left inferior
frontal gyrus | −0.0320±0.3511 |
| −0.497 | 0.050 |
ROC curve analysis
By computational analysis, it was observed that the
ReHo values of altered brain regions may be useful diagnostic
indicators for SA (Table II). To
verify these findings, ROC curves were generated to analyze the
ReHo values in brain regions exhibiting apparent differences in SA
patients. Area under the curve (AUC) values of 0.7–0.9 indicates
that the disease can be diagnosed more accurately. The individual
AUCs of ReHo values in the different regions were as follows: LLG,
AUC=0.934 (P<0.001); RMOG/RP, AUC=0.965 (P<0.001); BAC,
AUC=0.902 (P<0.001); LMOG, AUC=0.938 (P<0.001); BPG,
AUC=0.922 (P<0.001); and LIFG, AUC=0.938 (P<0.001; Fig. 3). Taken together, these findings
suggest that the ReHo values of altered brain regions may serve as
diagnostic indicators for SA.
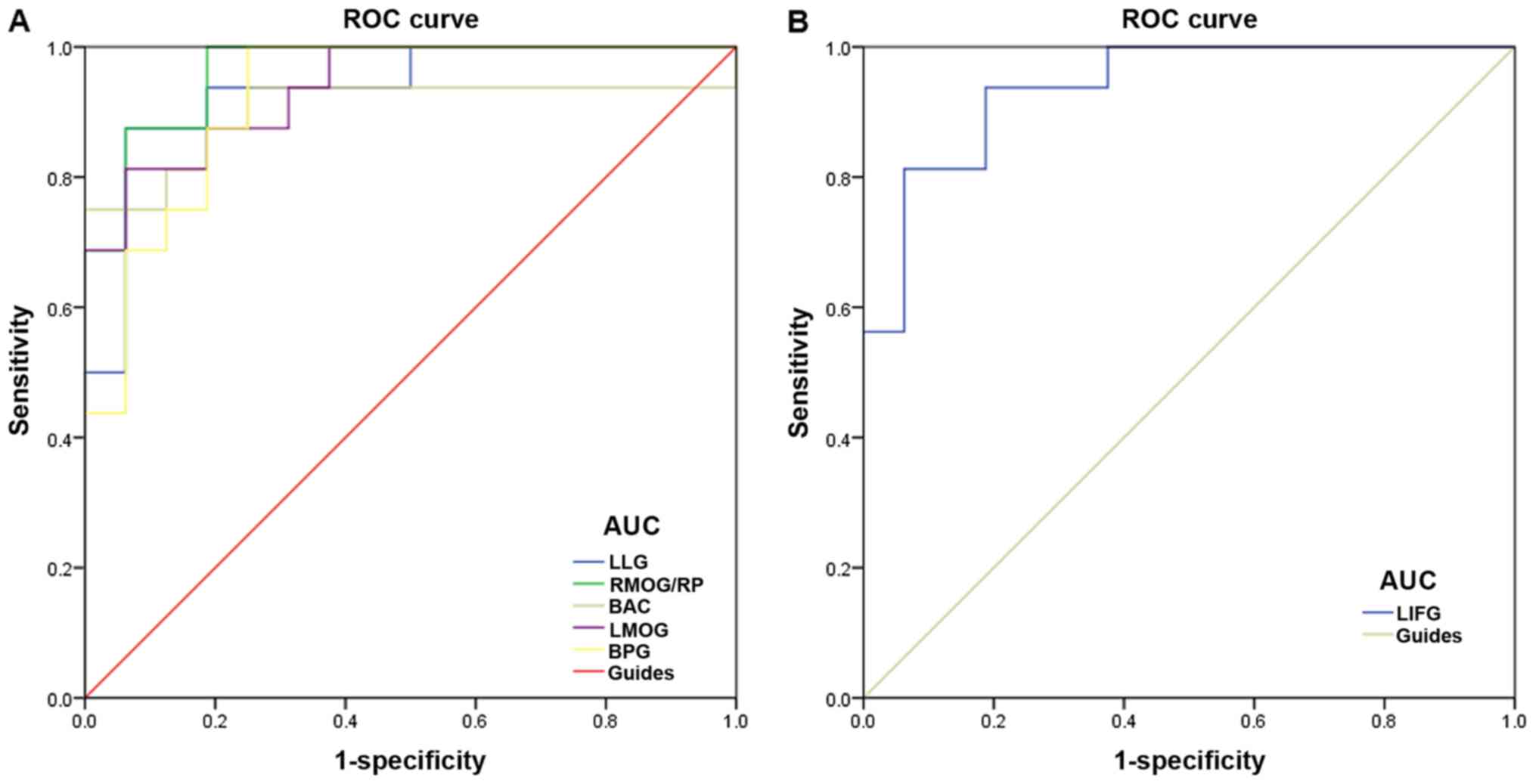 | Figure 3.ROC curve analysis of the ReHo values
for altered brain regions in the SA group. (A) The area under the
ROC curve was 0.934 for LLG (P<0.001; 95% CI, 0.847–1.000),
0.965 for RMOG/RP (P<0.001; 95% CI, 0.911–1.000), 0.902 for BAC
(P<0.001; 95% CI, 0.777–1.000), 0.938 for LMOG (P<0.001; 95%
CI, 0.860–1.000) and 0.922 for BPG (P<0.001; 95% CI,
0.830–1.000). (B) Area under the ROC curve for LIFG was 0.938
(P<0.001; 95% CI: 0.859–1.000). ROC, receiver operating
characteristic; ReHo, regional homogeneity; SA, strabismus and
amblyopia; LLG, left lingual gyrus; RMOG, right middle occipital
gyrus; RP, right precuneus; BAC, bilateral anterior cingulate;
LMOG, left middle occipital gyrus; BPG, bilateral precentral gyrus;
LIFG, left inferior temporal gyrus. |
Discussion
rs-fMRI is easier to implement in patients than
task-based fMRI, since patients are not required to perform
specific tasks during fMRI scans, thus reducing the potential
influence of confounding factors on the process (16). rs-fMRI can also provide more
functional information, helping to better understand the functional
mechanisms underlying specific diseases (16). The ReHo method has been
successfully applied in several ophthalmological and neurogenic
diseases, and has huge potential for further development (Table IV). To the best of our knowledge,
the present study is the first to evaluate resting-state brain
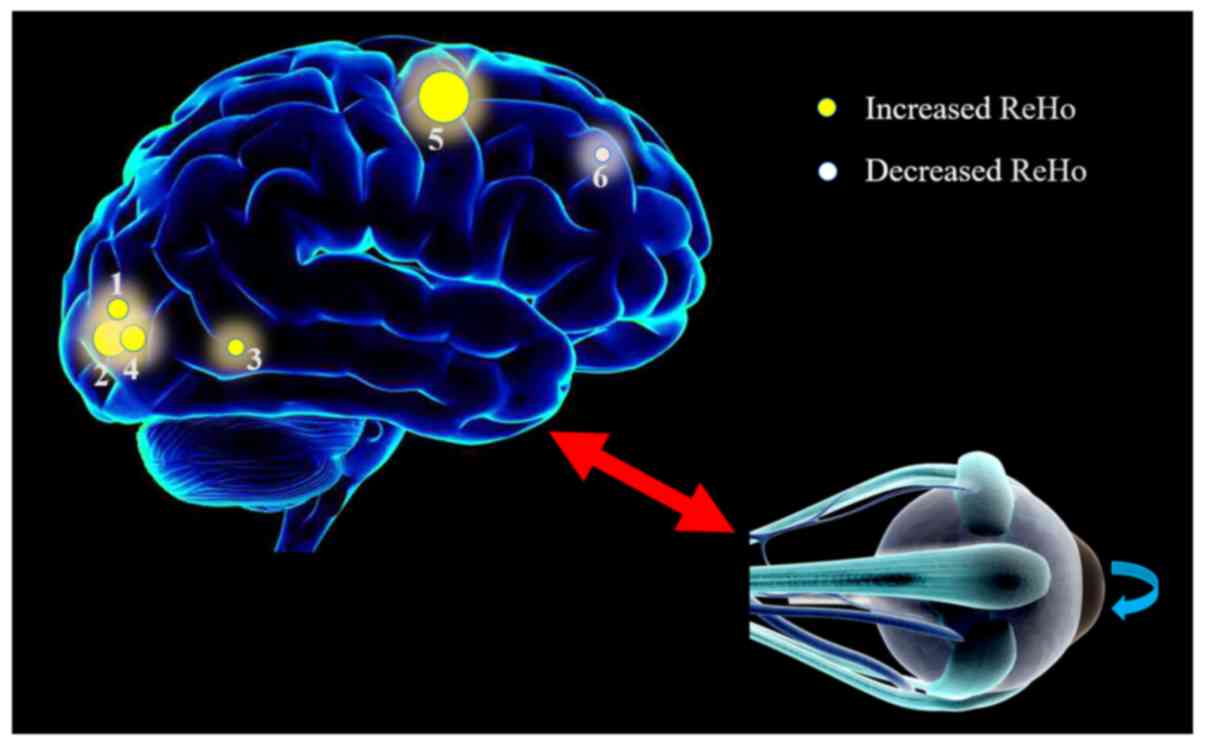
activity of patients with SA using the ReHo technique. Compared
with the HC individuals, patients with SA exhibited significantly
increased ReHo values in the LLG, RMOG/RP, BAC, LMOG and BPG areas,
while the ReHo value for the LIFG region was significantly lower
compared with that in HCs (Fig.
4).
 | Table IV.ReHo method applied in
ophthalmological and neurogenic diseases in the literature. |
Table IV.
ReHo method applied in
ophthalmological and neurogenic diseases in the literature.
| Author | Year | Disease | Refs. |
|---|
| Ophthalmological
diseases |
| Song
et al | 2014 | Glaucoma | (24) |
| Cui
et al | 2014 | Diabetic
retinopathy | (23) |
| Shao
et al | 2015 | Optic neuritis | (22) |
| Huang
et al | 2016 | Concomitant
strabismus | (19) |
| Huang
et al | 2016 | Open-globe
injury | (25) |
| Huang
et al | 2017 | Late monocular
blindness | (26) |
| Huang
et al | 2017 | Retinal
detachment | (27) |
| Neurogenic
diseases |
| Dai
et al | 2012 | Sleep
disorders | (28) |
| Li
et al | 2016 | Parkinson's
disease | (29) |
The occipital lobe is a key brain area for visual
processing, which controls eye movements and pupil accommodation
reflex activities associated with vision. The lingual gyrus is part
of the occipital lobe and an important component of the ventral
visual stream, which participates in processing information such as
shape, size, color, contour and object recognition. Thus, it is a
crucial brain area for visual judgment and visual attention
(32). Liang et al
(33) used the voxel-mirrored
homotopic connectivity (VMHC) method to analyze interhemispheric
functional connectivity changes in patients with anisometropic
amblyopia. They observed alterations in the lingual gyrus regions
of patients with strabismus, amblyopia and anisometropic amblyopia,
and the VMHC value of the lingual gyrus was found to be associated
with stereoacuity. Furthermore, Qi et al (34) used the surface-based morphological
measurement method and DTI to analyze changes in cortical thickness
and white matter integrity in children with amblyopia. These
researchers observed that the thickness of the lingual gyrus,
cuneus and occipital cortex, and the fractional anisotropy (FA)
values in the medial lingual cortex were all reduced. Yang et
al (35) demonstrated that
infants with esotropia exhibited high levels of activation in the
lingual gyrus. Huang et al (19) also reported that the ReHo value of
the lingual gyrus was increased in patients with concomitant
strabismus. Similarly, the present study identified differences in
the ReHo value of the LLG in adult patients with SA in comparison
with the controls. This can be explained by visual compensation in
patients with SA, while functional changes in the lingual gyrus may
also cause visual impairment.
The cuneus is also located at the occipital lobe,
which forms part of the visual center, and is involved in the
processing of visual information in the retina-optic nerve-lateral
geniculate pathway. Research by Schraa-Tam et al (36) demonstrated that the cuneus is
involved in eye movement reflex that functions to stabilize the
image of the retina; therefore, dysfunction of the cuneus causes
eye movement disorders. The precuneus also forms an important part
of the default mode network (DMN) of the brain, which is associated
with cognition, memory, emotion and interaction regulation
(37,38). In a study of anisometropic
amblyopia, Liang et al (39) reported decreased amplitude of
low-frequency fluctuation values in the bilateral precuneus cortex
in adults with anisometropic amblyopia, and the degree of reduction
was associated with disease severity, suggesting that patients with
bilateral precuneus neurons exhibited weaker activity. In addition,
Huang et al (40) used DTI
technology to analyze changes in whole brain microstructure in
patients with strabismus. They found that the FA value of bilateral
precuneus was significantly increased, suggesting enhanced fiber
bundle integrity in this brain area. In the present study, the ReHo
value of the right precuneus of adult SA patients was observed to
be increased, which was similar to the results of Huang et
al (40), but contrary to the
findings presented by Liang et al (39). The differences observed among these
studies may be attributed to variations among samples, leading to
compensatory alterations to the precuneus. Simultaneously, lesions
of the precuneus may also contribute to the SA occurrence.
The middle occipital gyrus and the lingual gyrus are
in the V2 region, and the middle occipital gyrus is part of the
dorsal visual stream (DVS) in the visual center (41). The DVS functions to analyze spatial
information, such as location, direction, motion and action plan.
Chan et al (17) used the
voxel-based morphometry (VBM) method to analyze the volume of gray
matter in patients with concomitant strabismus, and observed that
the volume of gray matter in the strabismus group was decreased in
the occipital and parietal lobes relative to the HCs. In addition,
Yan et al (42) used DTI
and VBM techniques to study the white matter structure in 13
patients with concomitant exotropia, and reported that the DVS was
abnormal or impaired in these patients. By analyzing visual cortex
changes, Jia et al (43)
identified that patients with amblyopia exhibited significantly
reduced changes in activation in the V2 visual area. In the current
study, it was observed that ReHo values of the RMOG and LMOG in
adult patients with SA were increased, which was different from the
observations of previous studies. This may be due to differences in
the samples included. The increased ReHo values observed in the
present study can be attributed to visual compensation. According
to evidence from earlier studies, changes in the middle occipital
gyrus can be considered as one of the pathogenic factors in
patients with SA.
The cingulate gyrus is located between the medial
cingulate sulcus and the corpus callosum of the cerebral
hemispheres (44), and is an
important component of the limbic system and Papez circuits
(45), as well as the DMN brain
region, including the anterior cingulate gyrus, middle cingulate
gyrus and posterior cingulate gyrus. The anterior cingulate gyrus
has numerous established functions, including in emotion,
cognition, movement, visceral movement, maternal behavior and
social interactions. In addition, the association between the
cingulate gyrus and epilepsy is currently a hot research topic
(46,47). A number of studies have noted that
the anterior cingulate gyrus receives afferent neurons from the
thalamus (48); therefore, it can
be speculated that the cingulate gyrus may be associated with
visual function. Zhai et al (49) used fMRI technology to analyze the
efficacy of perceptual learning in the treatment of amblyopia, and
reported that the primary visual cortex, visual junction cortex and
right cingulate gyrus were significantly activated in patients
receiving this treatment, suggesting that the cingulate gyrus
contributes to the occurrence of amblyopia. In the present study,
the results revealed that the ReHo value of the BAC gyrus in adult
patients with SA was increased in comparison with that in the
controls. The current study findings are similar to those reported
by Zhai et al (49),
suggesting that treatment of patients promotes compensation and
that the cingulate gyrus is important in the occurrence of SA.
Functional recovery of the cingulate gyrus can serve a compensatory
role in treatment, which provides a further basis for the treatment
of SA.
The frontal lobe is the most complex part of the
brain. It mainly consists of four gyri, including the precentral,
superior frontal, middle frontal and inferior frontal gyri. Frontal
lobe lesions can cause several disorders, such as voluntary
movement, speech, cranial nerve, autonomic nervous function and
mental activity disorders. The precentral gyrus is also referred to
as the ‘cortical motor area’, which mainly accepts proprioception
from the skin, joints, tendons and skeletal muscles, and controls
the voluntary movement of the body. However, the frontal lobe is
also important to the integrity of visual function. Xiao et
al (50) used VBM analysis to
investigate changes in the visual cortex of amblyopic children, and
identified that the gray matter density of the middle frontal gyrus
was decreased. In a study of patients with strabismus, Ouyang et
al (51) observed that the
volumes of gray matter in the right posterior cingulate gyrus,
precentral gyrus and left cuneus were decreased, while the white
matter volume of the right precuneus and right anterior motor areas
of the patients were significantly decreased. Furthermore, Huang
et al (40) used DTI
technology to analyze alterations in the whole brain microstructure
in patients with strabismus, and reported that the mean diffusion
coefficient in the left middle frontal gyrus was significantly
decreased. In the current study, the data revealed that the ReHo
values were lower in the inferior frontal gyrus of SA patients as
compared with those in the HC group, which is consistent with the
observations of previous studies, suggesting that the inferior
frontal gyrus is important in the occurrence of SA. However, the
current study results indicated that the ReHo value of the BPG was
higher in SA patients in comparison with that in HCs, which is
contrary to the findings of previous studies. This is most likely a
result of differences in the study populations. The current study
results can be explained by a compensatory mechanism, while other
studies have concluded that their findings can be explained by
etiology. Nevertheless, all findings reveal that the frontal lobe
serves a vital role in visual processing and associated eye
movements.
ROC curve analysis in the present study demonstrated
the accuracy of the ReHo method for the diagnosis of patients.
Accuracy is perceived as excellent when AUC values are 0.7–0.9,
values between 0.5 and 0.7 are considered to indicate moderate
accuracy, while values <0.5 indicate low discrimination between
SA patients and HCs. The ROC curve values generated revealed that
the AUC for each brain region was >0.7, indicating that the ReHo
values of these changed brain regions exhibited a diagnostic
accuracy for the identification of SA. Taken together, it is
predicted that the ReHo method may be used for sensitive detection
of SA in the future.
The present study also has certain limitations. The
sample size was relatively small, and differences among samples may
have impacted the results. Furthermore, for certain individuals,
the length of the scanning time and small body movements may have
affected the scan results.
In conclusion, the data of the present study
demonstrated that patients with SA exhibit abnormal spontaneous
activities in specific regions of the brain. These abnormal
spontaneous activities may be associated with the occurrence of SA
and are potentially associated with visual compensation. These
findings provide a basis for the study of the pathogenesis of SA
and indicate a potential direction for the development of
treatment.
Acknowledgements
Not applicable.
Funding
This study was supported by grants from the National
Natural Science Foundation of China (grant nos. 81660158, 81460092
and 81400372); Jiangxi Natural Science Foundation major projects
(grant no. 2016ACB21017); Jiangxi Province Key Research and
Development Projects (grant no. 20151BBG70223, 20181BBG70004);
Jiangxi Province Youth Science Foundation (grant nos.
20151BAB215016 and 20161BAB215198); Jiangxi Province Education
Department Key Projects (grant no. GJ160020); Jiangxi Province
Degree and Postgraduate Teaching Reform and Research Research
Project (grant no. JXYJC-2018-013); Spark Promotion Project for
Appropriate Health Technologies at Grass-roots Level in Jiangxi
Province (grant no. 20088003); Science and Technology Planning
Project of Jiangxi Provincial Health Planning Commission (grant no.
20175116); Traditional Chinese Medicine Science and Technology
Project of Jiangxi Provincial Health Planning Commission (grant no.
20150823).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YS, QHL and LY conceived and designed the study;
QHL, BL, QL, TS, WQS, PWZ, QY, YQS, YH and WFL performed the
experiments, and collected, analyzed and interpreted the data; QHL,
BL, QL and TS wrote the study; YS. LY and QHL reviewed and edited
the manuscript; all authors read and approved the manuscript.
Ethics approval and consent to
participate
All procedures performed in the present study
involving human participants were in accordance with the ethical
standards of the Ethical Committee of the First Affiliated Hospital
of Nanchang University (Nanchang, China), as well as with the 1964
Helsinki Declaration and its later amendments or comparable ethical
standards. Informed consent was obtained from all individual
participants included in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen X, Fu Z, Yu J, Ding H, Bai J, Chen J,
Gong Y, Zhu H, Yu R and Liu H: Prevalence of amblyopia and
strabismus in Eastern China: results from screening of preschool
children aged 36–72 months. Br J Ophthalmol. 100:515–519. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lewandowski KB: Strabismus as a possible
sign of subclinical muscular dystrophy predisposing to
rhabdomyolysis and myoglobinuria: a study of an affected family.
Can Anaesth Soc J. 29:372–376. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dickmann A, Petroni S, Salerni A, Parrilla
R, Savino G, Battendieri R, Perrotta V, Radini C and Balestrazzi E:
Effect of vertical transposition of the medial rectus muscle on
primary position alignment in infantile esotropia with A- or
V-pattern strabismus. J AAPOS. 15:14–16. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Clark RA: The role of extraocular muscle
pulleys in incomitant nonparalytic strabismus. Middle East Afr J
Ophthalmol. 22:279–285. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Oh SY, Clark RA, Velez F, Rosenbaum AL and
Demer JL: Incomitant strabismus associated with instability of
rectus pulleys. Invest Ophthalmol Vis Sci. 43:2169–2178.
2002.PubMed/NCBI
|
|
6
|
Kekunnaya R and Negalur M: Duane
retraction syndrome: causes, effects and management strategies.
Clin Ophthal (Auckland, NZ);. 11:19172017. View Article : Google Scholar
|
|
7
|
Breinin GM: In discussion of: De Gindersen
T, Zeavin B. Observations on the retraction syndrome of Duane. Arch
Ophthalmol. 55:5761956.
|
|
8
|
Parsa CF, Grant PE, Dillon WP Jr, du Lac S
and Hoyt WF: Absence of the abducens nerve in Duane syndrome
verified by magnetic resonance imaging. Am J Ophthalmol.
125:399–401. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lueder GT, Dunbar JA, Soltau JB, Lee BC
and McDermott M: Vertical strabismus resulting from an anomalous
extraocular muscle. J AAPOS. 2:126–128. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao K and Shi X: Learn new version of
Preferred Practice Pattern to further standardize the diagnosis and
treatment of amblyopia. Zhonghua Yan Ke Za Zhi. 50:481–484.
2014.(In Chinese). PubMed/NCBI
|
|
11
|
Jin H, Yi JL, Xie H, Xiao F, Wang WJ, Shu
XM, Xu YL, Chen SL and Ye WX: A study on visual development among
preschool children. Zhonghua yan ke za Zhi. 47:1102–1106.
2011.PubMed/NCBI
|
|
12
|
Avram E and Stănilă A: Functional
amblyopia. Oftalmologia. 57:3–8. 2013.(In Romanian). PubMed/NCBI
|
|
13
|
von Noorden GK: Abnormal Binocular
Interaction: Evidence in Humans. Strabismus and Amblyopia.
Lennerstrand G, von Noorden GK and Campos EC: Wenner-Gren Center
International Symposium Series, Palgrave Macmillan; London: pp.
275–284. 1988, View Article : Google Scholar
|
|
14
|
Sjöstrand JB: Form deprivation amblyopia-a
treatable cause of blindness. Acta Ophthalmol. 86:s2432010.
|
|
15
|
Wang H, Crewther SG, Liang M, Laycock R,
Yu T, Alexander B, Crewther DP, Wang J and Yin Z: Impaired
activation of visual attention network for motion salience is
accompanied by reduced functional connectivity between frontal eye
fields and visual cortex in Strabismic Amblyopia. Front Hum
Neurosci. 11:1952017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Turner R: Functional Magnetic Resonance
Imaging (fMRI). Runehov A.L.C..Oviedo L: Encyclopedia of Sciences
and Religions. 2013. pp. 352013
|
|
17
|
Chan ST, Tang KW, Lam KC, Chan LK, Mendola
JD and Kwong KK: Neuroanatomy of adult strabismus: A voxel-based
morphometric analysis of magnetic resonance structural scans.
Neuroimage. 22:986–994. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bi H, Zhang B, Tao X, Harwerth RS, Smith
EL III and Chino YM: Neuronal responses in visual area V2 (V2) of
macaque monkeys with strabismic amblyopia. Cereb Cortex.
21:2033–2045. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang X, Li SH, Zhou FQ, Zhang Y, Zhong
YL, Cai FQ, Shao Y and Zeng XJ: Altered intrinsic regional brain
spontaneous activity in patients with comitant strabismus: a
resting-state functional MRI study. Neuropsychiatr Dis Treat.
12:1303–1308. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zang Y, Jiang T, Lu Y, He Y and Tian L:
Regional homogeneity approach to fMRI data analysis. Neuroimage.
22:394–400. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tononi G, McIntosh AR, Russell DP and
Edelman GM: Functional clustering: Identifying strongly interactive
brain regions in neuroimaging data. Neuroimage. 7:133–149. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shao Y, Cai FQ, Zhong YL, Huang X, Zhang
Y, Hu PH, Pei CG, Zhou FQ and Zeng XJ: Altered intrinsic regional
spontaneous brain activity in patients with optic neuritis: a
resting-state functional magnetic resonance imaging study.
Neuropsychiatr Dis Treat. 11:3065–3073. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cui Y, Jiao Y, Chen YC, Wang K, Gao B, Wen
S, Ju S and Teng GJ: Altered spontaneous brain activity in type 2
diabetes: a resting-state functional MRI study. Diabetes.
63:749–760. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Song Y, Mu K, Wang J, Lin F, Chen Z, Yan
X, Hao Y, Zhu W and Zhang H: Altered spontaneous brain activity in
primary open angle glaucoma: a resting-state functional magnetic
resonance imaging study. PLoS One. 9:e894932014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang X, Li HJ, Ye L, Zhang Y, Wei R,
Zhong YL, Peng DC and Shao Y: Altered regional homogeneity in
patients with unilateral acute open-globe injury: a resting-state
functional MRI study. Neuropsychiatr Dis Treat. 12:1901–1906. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang X, Ye CL, Zhong YL, Ye L, Yang QC,
Li HJ, Jiang N, Peng DC and Shao Y: Altered regional homogeneity in
patients with late monocular blindness: a resting-state functional
MRI study. Neuroreport. 28:1085–1091. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang X, Li D, Li HJ, Zhong YL, Freeberg
S, Bao J, Zeng XJ and Shao Y: Abnormal regional spontaneous neural
activity in visual pathway in retinal detachment patients: a
resting-state functional MRI study. Neuropsychiatr Dis Treat.
13:2849–2854. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dai XJ, Gong HH, Wang YX, Zhou FQ, Min YJ,
Zhao F, Wang SY, Liu BX and Xiao XZ: Gender differences in brain
regional homogeneity of healthy subjects after normal sleep and
after sleep deprivation: a resting-state fMRI study. Sleep Med.
13:720–727. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li Y, Liang P, Jia X and Li K: Abnormal
regional homogeneity in Parkinson's disease: a resting state fMRI
study. Clin Radiol. 71:e28–e34. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Holmes D, Rettmann M and Robb R:
Visualization in Image-Guided Interventions. Image-Guided
Interventions. Peters T and Cleary K: Springer; Boston, MA: 2008,
View Article : Google Scholar
|
|
31
|
Harrison BJ and Pantelis C: Gradient-Echo
Images. Encyclopedia of Psychopharmacology. Stolerman IP: Springer;
Berlin, Heidelberg: 2010
|
|
32
|
Lee HW, Hong SB, Seo DW, Tae WS and Hong
SC: Mapping of functional organization in human visual cortex:
electrical cortical stimulation. Neurology. 54:849–854. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liang M, Xie B, Yang H, Yin X, Wang H, Yu
L, He S and Wang J: Altered interhemispheric functional
connectivity in patients with anisometropic and strabismic
amblyopia: a resting-state fMRI study. Neuroradiology. 59:517–524.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Qi S, Mu YF, Cui LB, Li R, Shi M, Liu Y,
Xu JQ, Zhang J, Yang J and Yin H: Association of optic radiation
integrity with cortical thickness in children with Anisometropic
Amblyopia. Neurosci Bull. 32:51–60. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang X, Zhang J, Lang L, Gong Q and Liu L:
Assessment of cortical dysfunction in infantile esotropia using
fMRI. Eur J Ophthalmol. 24:409–416. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Schraa-Tam CK, van der Lugt A, Smits M,
Frens MA, van Broekhoven PC and van der Geest JN: Differences
between smooth pursuit and optokinetic eye movements using limited
lifetime dot stimulation: a functional magnetic resonance imaging
study. Clin Physiol Funct Imaging. 29:245–254. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Uddin LQ: Salience processing and insular
cortical function and dysfunction. Nat Rev Neurosci. 16:55–61.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Weissman-Fogel I, Moayedi M, Taylor KS,
Pope G and Davis KD: Cognitive and default-mode resting state
networks: Do male and female brains “rest” differently? Hum Brain
Mapp. 31:1713–1726. 2010.PubMed/NCBI
|
|
39
|
Liang M, Xie B, Yang H, Yu L, Yin X, Wei L
and Wang J: Distinct patterns of spontaneous brain activity between
children and adults with anisometropic amblyopia: a resting-state
fMRI study. Graefes Arch Clin Exp Ophthalmol. 254:569–576. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Huang X, Li H-J, Zhang Y, Peng DC, Hu PH,
Zhong YL, Zhou FQ and Shao Y: Microstructural changes of the whole
brain in patients with comitant strabismus: evidence from a
diffusion tensor imaging study. Neuropsychiatr Dis Treat.
12:2007–2014. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wandell BA, Dumoulin SO and Brewer AA:
Visual field maps in human cortex. Neuron. 56:366–383. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yan X, Lin X, Wang Q, Zhang Y, Chen Y,
Song S and Jiang T: Doral visual pathway changes in patients with
comitant extropia. PLoS ONE 5 (6). e109312010. View Article : Google Scholar
|
|
43
|
Jia CH, Lu GM, Zhang ZQ, Wang Z, Huang W,
Ma F, Yin J, Huang ZP and Shao Q: Comparison of deficits in visual
cortex between anisometropic and strabismic amblyopia by fMRI
retinotopic mapping. Zhonghua Yi Xue Za Zhi. 90:1446–1452. 2010.(In
Chinese). PubMed/NCBI
|
|
44
|
San Pedro EC, Mountz JM, Ojha B, Khan AA,
Liu HG and Kuzniecky RI: Anterior cingulate gyrus epilepsy: the
role of ictal rCBF SPECT in seizure localization. Epilepsia.
41:594–600. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Devinsky O, Morrell M and Vogt BA:
Contribution of anterior cingulate cortex to behavior. Brain 118
(Pt 1). 279–306. 1995.
|
|
46
|
Braga AMDS, Fujisao EK, Verdade RC,
Paschoalato RP, Paschoalato RP, Yamashita S and Betting LE:
Investigation of the cingulate cortex in idiopathic generalized
epilepsy. Epilepsia. 56:1803–1811. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Alkawadri R, So NK, Van Ness PC and
Alexopoulos AV: Cingulate epilepsy: report of 3 electroclinical
subtypes with surgical outcomes. JAMA Neurol. 70:995–1002. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Unnwongse K, Wehner T and
Foldvary-Schaefer N: Mesial frontal lobe epilepsy. J Clin
Neurophysiol. 29:371–378. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhai J, Chen M, Liu L, Zhao X, Zhang H,
Luo X and Gao J: Perceptual learning treatment in patients with
anisometropic amblyopia: a neuroimaging study. Br J Ophthalmol.
97:1420–1424. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Xiao JX, Xie S, Ye JT, Liu HH, Gan XL,
Gong GL and Jiang XX: Detection of abnormal visual cortex in
children with amblyopia by voxel-based morphometry. Am J
Ophthalmol. 143:489–493. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ouyang J, Yang L, Huang X, Zhong YL, Hu
PH, Zhang Y, Pei CG and Shao Y: The atrophy of white and gray
matter volume in patients with comitant strabismus: evidence from a
voxel-based morphometry study. Mol Med Rep. 16:3276–3282. 2017.
View Article : Google Scholar : PubMed/NCBI
|