Introduction
Neurodegeneration is defined as a progressive loss
of structure and/or function of neurons that may lead to neuronal
cell death (1). Neurodegenerative
diseases, including amyotrophic lateral sclerosis, Parkinson's
disease (PD), Alzheimer's disease (AD) and Huntington's disease,
have been observed to be a result of neurodegenerative processes
(2,3). Accumulating evidence has demonstrated
the association between inflammatory responses and brain injury
(3). In diseases associated with
the central nervous system, the structure of the blood-brain
barrier is frequently impaired; consequently, lymphocytes are able
to enter the brain parenchyma through the blood-brain barrier
(4). These immune cells may
initiate various physiopathological reactions in the brain, and the
activation of certain signaling pathways is able to mediate the
elimination of various infectious agents (5). Metabolic disorders, including type II
diabetes and obesity, are the principal factors associated with
metabolic inflammation (6). A
previous study identified that metabolic diseases may mediate the
association between neurodegeneration and brain injury (7). Previous studies have identified that
the inflammatory response is associated with various
neurodegenerative pathways (8).
Furthermore, pro-inflammatory cytokines, including tumor necrosis
factor (TNF)-α, interleukin (IL)-1β and IL-6, have been reported to
serve important roles in the pathophysiology of depression
(9,10). The release of inflammatory
cytokines is primarily regulated by the NF-κB signaling pathway;
therefore, the NF-κB signaling pathway may be involved in the brain
injury response (11). Apoptotic
cell death, mediated by Caspase-3, has been identified to be
associated with brain injury; this apoptotic pathway is associated
with multiple pro-apoptotic and anti-apoptotic factors, including
Bcl-2, Bax and matrix metallopeptidase 9 (MMP-9) (12–14).
However, the role of these pathways in metabolic diseases and brain
injury remains unclear. Therefore, it is necessary to understand
the molecular mechanisms underlying the associations among
metabolic disorders, inflammatory response and brain injury. The
present study aimed to examine the roles of the NF-κB and Caspase-3
signaling pathways in the process of brain injury in high
fat-induced mouse models.
Carnosic acid (CA) is a benzenediol abietane
diterpene extracted from rosemary and common sage. A previous study
demonstrated that CA may be used as a preservative and antioxidant
in various products, including toothpaste, mouthwash and chewing
gum (15). In addition, CA has
been reported to exhibit anti-tumor effects on colon cancer, breast
cancer and skin tumor (16). CA
may affect multiple biological processes, including cell growth,
cell apoptosis, reactive oxygen species (ROS) release and
inflammatory response (17). CA
has also been demonstrated to be associated with ROS, which can
regulate the expression levels of antioxidant phase II enzymes,
including nicotinamide-adenine dinucleotide phosphate quinone
dehydrogenase 1, glutathione-S-transferase and uridine diphosphate
glucuronosyltransferase (18).
Therefore, CA may be able to increase the antioxidant ability of
cells and organisms.
CA is able to regulate the inflammatory response by
decreasing the expression levels of inflammatory mediators,
including TNF-α (19). In
addition, CA may inhibit activation of the NF-κB signaling pathway
by increasing the activity or the expression levels of various
molecular components including spleen-associated tyrosine kinase,
SRC proto-oncogene, non-receptor tyrosine kinase, PI3K, pyruvate
dehydrogenase kinase 1, Akt, IκB kinase (IKK) and NF-κB inhibitor α
(IκBα) (20). In addition, CA
serves important roles in neuroprotection. CA has been identified
to inhibit the synthesis of amyloid-β 1–42 in SH-SY5Y cells by
increasing the expression levels of the p53-dependent
metalloproteinase ADAM metallopeptidase domain 17 (21). In addition, CA is able to increase
the synthesis of glutathione, and to repress the JNK and p38
signaling pathways, via the nuclear factor, erythroid 2 like 2
pathway, which may inhibit 6-hydroxydopamine-induced apoptosis of
SH-SY5Y cells (22). These effects
suggest that administration of CA may represent a novel therapeutic
strategy to treat AD and PD. Since CA has been identified to
exhibit anti-inflammatory and anti-apoptotic functions in cancer
cells, the present study aimed to determine whether treatment with
CA is able to mediate the inflammatory response following injury,
thus facilitating the repair of the damaged tissue.
In the present study, mice fed a high-fat diet (HFD)
were used to establish animal models of brain injury, and the
effects of CA were investigated. Furthermore, the mRNA and protein
expression levels of factors involved in the NF-κB and Caspase-3
signaling pathways were examined.
Materials and methods
Animals
All animals were treated according to the guidelines
for the Care and Use of Laboratory Animals (23), and the study was approved by The
Committee on The Ethics of Animal Experiments of Xinjiang Medical
University (approval no. XM2017MD). In total, 40 male C57BL/6 mice
(weight, 18–22 g; age, 6 weeks) were purchased from The
Experimental Animal Center of Nanjing Medical University
(certificate of conformity no. SCXK JS 2016-0002). Mice were
acclimatized for 7 days prior to the start of the study. Mice were
provided access to drinking water and standard rodent chow ad
libitum. The temperature of housing environment was set as
22±2°C. The relative humidity was set at 60±10% under a 12-h
light/dark cycle. The mice were randomly divided into four groups:
i) Control mice (Con); ii) HFD mice (Veh); iii) HFD mice treated
with 10 mg/kg CA (CA-L); and iv) HFD mice treated with 20 mg/kg CA
(CA-H). The HFD protocol was performed according to a previous
study (24). CA was purchased from
Changsha Yaying Biotechnology Co., Ltd. CA solution was prepared
according to a previous study (18). Mice were treated with CA via gavage
for 9 weeks following a 6-week period of HFD. The caloric intake
was calculated by subtracting the fecal caloric excretion (fecal
caloric content × fecal excretion) from the dietary caloric intake
(diet caloric content × food intake). After 15 weeks, all mice were
fasted for 12 h. The blood of anesthetized mice was collected via
retro-orbital puncture. Subsequently, body and liver weights were
measured, and the brain, pancreas and whole liver tissues were
collected at 4°C. The tissues were frozen in liquid nitrogen and
stored at −80°C. For histology, the tissues were fixed in 10%
neutral buffered formalin (saturated aqueous formaldehyde from
Thermo Fisher Scientific, Inc., buffered to pH 6.8–7.2 with 100 mM
phosphate buffer) for 24 h at 25°C.
Biochemical analysis
According to the metabolic syndrome diagnostic
criteria provided by the World Health Organization (25), insulin resistance (IR) is required
to diagnose metabolic syndrome. In addition, to diagnose metabolic
syndrome, two other criteria among the following five are required:
Obesity (waist/hip ratio: >0.90 for males, >0.85 for females;
or body mass index >30 kg/m2), hyperglycemia,
dyslipidemia [triglycerides (TG): >150 mg/dl or high-density
lipoprotein cholesterol: <35 mg/dl for males, <39 mg/dl for
females], hypertension (≥140/90 mmHg) and microalbuminuria. In the
present study, IR, TG and hyperglycemia were selected to assess
metabolic syndrome in mouse models. After 15 weeks, blood was
collected by retro-orbital puncture method. Additionally, the serum
was obtained from blood samples via centrifugation with 2,000 RPM
(800 × g) for 15 min at 4°C. Then, the serum was transferred to a
5-ml centrifuge tube, which was placed on the glacial table. Serum
levels of TG (cat. no. A110-2-1) and total cholesterol (TC; cat.
no. A111-2-1) were tested with biochemical kits (Nanjing Jiancheng
Taihao Biotechnology Co., Ltd.). Glucose (cat. no. F006-1-1) and
insulin (cat. no. H203) levels were also measured using biochemical
kits (Nanjing Jiancheng Taihao Biotechnology Co., Ltd.).
ELISA measurement
After 15 weeks, the serum was obtained via
centrifugation of blood samples with 2,000 RPM (800 × g) for 15 min
at 4°C. ELISA kits, including TNF-α (cat. no. MTA00B), IL-1β (cat.
no. MLB00C) and IL-6 (cat. no. D6050) was used to identify the
serum concentrations of these inflammatory cytokines. The detailed
steps were followed according to the manufacturer's protocols
(R&D System, Inc.). The standard curve for each cytokine was
calculated; the concentration of the antigens was determined at 450
nm.
Histopathological examination
Histopathological evaluation was performed on the
liver, pancreas and brain tissues of mice. Samples were fixed with
10% formalin buffer for 24 h at 25°C. Subsequently, the pretreated
samples were embedded in paraffin and sliced (3–5 µm). H&E
staining was performed according to the previous method (26). Briefly, samples were stained with
hematoxylin for 10 min and with eosin for 1 min (both at room
temperature) to establish the diagnosis areas. After H&E
staining, histopathological alterations were observed using a light
microscope. For each sample, three randomly selected microscopic
fields of view were used to calculate the pathological scores. The
microscopic fields were scored blindly using a scale from 0
(normal) to 5 (highly destructive pathology). The mean of the three
values was used to calculate an overall pathological score, as
previously described (27).
Additionally, immunohistochemical staining was
performed on 3–5-µm thick paraffin-embedded tissue sections.
Sections were deparaffinized in xylene, hydrated using a graded
alcohol series, and washed with TBS for 10 min and distilled water
for a further 10 min. Endogenous peroxidase activity was blocked
with 3% v/v H2O2 in water for 5 min. Antigen
retrieval was performed for all antibodies by placement of the
sections in citrate buffer and heating in a microwave oven for 15
min. Sections were treated with 25 ml blocking buffer for 1 h at
room temperature. The treated sections were separately incubated
with primary antibodies for TNF-α (1:200; cat. no. 11948) and IL-1β
(1:400; cat. no. 12703) at 4°C overnight (both Cell Signaling
Technology, Inc.). Then, all treated sections were incubated with
the biotin-conjugated secondary antibody (1:800; cat. no. ab6720;
Abcam) for 30 min at room temperature. The standard
streptavidin-biotin-peroxidase complex method was performed using
an LSAB System Universal kit (Dako; Agilent Technologies, Inc.) for
10 min, 3,3′-diaminobenzidine solution was used as a chromogen for
5 min, and all sections were counterstained with Mayer's
haematoxylin for 1 min and mounted; all reactions were performed at
room temperature. The species were observed under a light
microscope (Eclipse 80i; Nikon Corporation). The percentage of
IL-1β- and TNF-α-positive cells was calculated using the inForm
cell analysis software (version 2.0.4743.16069; PerkinElmer, Inc.).
The histopathological examination method was performed as
previously described (28).
Western blot analysis
Total protein from different groups was extracted
using the T-PER Tissue Protein Extraction Reagent kit (Thermo
Fisher Scientific, Inc.). Protein concentration was determined
using a bicinchoninic protein assay kit (Thermo Fisher Scientific,
Inc.). Proteins (50 µg/lane) were separated by 10% SDS-PAGE.
Subsequently, proteins were transferred to PVDF membranes, which
were blocked with TBS containing 0.05% Tween-20 (TBS-T),
supplemented with 5% skim milk (Sigma-Aldrich; Merck KGaA) at room
temperature for 2 h on a rotating shaker. Membranes were
subsequently washed with TBS-T. The primary antibodies used were as
follows: IL-1β (1:100; cat. no. PA1351; Boster Biological
Technology), IL-18 (1:500; cat. no. RP1017; Boster Biological
Technology), TNF-α (1:500; cat. no. RP1000; Boster Biological
Technology), IKKα (1:1,000; cat. no. 11930; Cell Signaling
Technology, Inc.) and phosphorylated (p)-IKKα (1:1,000; cat. no.
2697; Cell Signaling Technology, Inc.), IκBα (1:500; cat. no. 4814;
Cell Signaling Technology, Inc.) and p-IκBα (1:500; cat. no. 9246;
Cell Signaling Technology, Inc.), NF-κB (1:1,000; cat. no. 8242;
Cell Signaling Technology, Inc.) and p-NF-κB (1:1,000; cat. no.
3033; Cell Signaling Technology, Inc.), glial fibrillary acidic
protein (GFAP; 1:2,000; cat. no. 3670; Cell Signaling Technology,
Inc.), neuronal nuclei (Neu-N; 1:1,500; cat. no. 24307; Cell
Signaling Technology, Inc.), ionized calcium-binding adapter
molecule 1 (Iba-1; 1:2,000; cat. no. ab15690; Abcam), Bax (1:1,000;
cat. no. ab32503; Abcam), Bcl-2 (1:1,500; cat. no. ab182858;
Abcam), MMP-9 (1:100; cat. no. AF909; R&D Systems), Caspase-3
(1:400; cat. no. ab13585; Abcam), and GAPDH (1:1,000; cat. no.
ab8245; Abcam). The antibodies were diluted in TBS-T and were
incubated with the membranes at 4°C overnight. Subsequently, the
membranes were washed with TBS-T followed by incubation with
ImmPRESS® HRP Universal Antibody Polymer Detection Kit
(1:2,000; cat. no. MP-7500-15; Maravai LifeSciences) for 1 h at
room temperature. The protein bands were detected using an ECL
western blot detection kit (Thermo Fisher Scientific, Inc.).
Western blot bands were observed with an ECL Western Blotting
Analysis system (cat. no. RPN2108; GE Healthcare Life Sciences) and
exposed to X-ray films (Kodak). Image Studio Lite Western Blot
Analysis Software version 3.1 (LI-COR Biosciences) was chosen to
perform pixel quantification of the images.
Reverse transcription-quantitative PCR
(RT-qPCR) analysis
Total RNA was isolated from individual mouse brains
using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). The total RNA was used to evaluate the relative
mRNA expression levels of IL-1β, IL-18, TNF-α, Bax, Bcl-2, MMP-9,
Caspase-3 and GAPDH. The reverse transcribed cDNA was synthesized
using the SuperScript First-Strand Synthesis kit (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. The primers used for qPCR are listed in Table I. Primer sequences were verified
with NCBI primer blast tool to avoid non-specific annealing
(https://www.ncbi.nlm.nih.gov/tools/primer-blast/).
All primers were obtained from Sangon Biotech Co., Ltd. Reactions
were performed in a total volume of 20 µl, and consisted of 10 µl
2X SYBR Green PCR master mix (cat. no. 4309155; Applied Biosystems;
Thermo Fisher Scientific, Inc.), 1 µl forward primer (10 pmol), 1
µl reverse primer (10 pmol), 1 µl cDNA template and 7 µl double
distilled water. The thermocycling conditions were as follows: 94°C
for 3 min, then 40 cycles of 95°C for 15 sec and 60°C for 25 sec.
BioRad iCycler iQ detection system was used in this study (Bio-Rad
Laboratories, Inc.). GAPDH was used as the reference gene.
Normalization and fold change for each gene were calculated using
the 2−∆∆Cq method (29).
 | Table I.Primers used in the reverse
transcription-quantitative PCR analysis. |
Table I.
Primers used in the reverse
transcription-quantitative PCR analysis.
| Gene symbol | Primer sequences
(5′→3′) |
|---|
| GAPDH | F:
CATTCAAGACCGGACAGAGG |
|
| R:
ACATACTCAGCACCAGCATCACC |
| IL-6 | F:
GAACCGGCACCTGACACC |
|
| R:
CACGACTTCGTCACCGGTAA |
| TNF-α | F:
AGCACAAAGAGAGTGTCGC |
|
| R:
AGTCGTCCAGTGTGTGTA |
| IL-1β | F:
GAGTGATAGACAGCAAGCC |
|
| R:
GGCCGTCAATGTATGTTGGTG |
| IL-18 | F:
GCAGCAGGTGAGTGGGCAGT |
|
| R:
ACTGTCGCCTGGTTCTCTGTGC |
| Bax | F:
CAGTTAGGAGACGACAG |
|
| R:
AGCGTCGCTGGATGTGTGA |
| MMP-9 | F:
CACCTTCTTGTCGACCGCCTA |
|
| R:
TCCGCGTCTGTTCGGCAT |
| Bcl-2 | F:
TCCTGGGACTCTTCTTATTTACCA |
|
| R:
TTGCCTGCTAAAGGCAATTACC |
| Caspase-3 | F:
GAGCAAGCAAGATTTACTCGA |
|
| R:
AGCCAGCTACATGGATCTAAA |
Statistical analysis
All experiments were repeated three times. Data are
presented as the means ± SEM. Treated cells, tissues and the
corresponding controls were compared using GraphPad Prism (version
6.0; GraphPad Software, Inc.). Multiple groups were compared using
one-way ANOVA. Differences between groups were calculated using the
Student-Newman-Keuls post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
The effects of CA on metabolic
syndrome
Metabolic disease has previously been reported to be
associated with neurodegenerative disease via the inflammatory
response (30). A previous study
suggested that HFD could induce metabolic diseases in rodents,
causing type II diabetes characterized by IR (31). In the present study, animal models
of metabolic syndrome were established by feeding mice a HFD. Mouse
body weight in the Veh group was significantly higher than in the
Con group (Fig. 1A; P<0.01).
Conversely, high concentrations of CA could significantly reduce
body weight compared with the Veh group (P<0.05). The present
results suggested that CA induced body weight loss in a
dose-dependent manner. Similarly, the liver weight in different
groups was also measured. Liver weight increased significantly in
the Veh group compared with in the Con group (P<0.05). Moreover,
high CA was sufficient to significantly reduce the liver weight
compared with the Veh group (P<0.05). The present results
suggested that CA induced liver weight loss in a dose-dependent
manner (Fig. 1B). In addition, the
daily food intake in the Veh group was significantly lower than in
the Con group (P<0.05). However, the daily food intake in the
Veh group was restored following treatment with a high dosage of CA
(P<0.05; Fig. 1C). Moreover,
the daily caloric intake in the Veh group was significantly higher
compared with that in the Con group (P<0.01). This effect was
reversed by CA treatment (P<0.05; Fig. 1D). In addition, serum levels of TG,
TC and insulin were analyzed. The present results suggested that
TG, TC and insulin in the Veh group were significantly higher than
in the Con group (P<0.001; Fig.
1E-G). Notably, CA treatment was able to significantly decrease
the serum levels of TG, TC and insulin. In addition, the serum
glucose level in the Veh group was significantly higher than the
Con group (P<0.01). By contrast, serum glucose level was
decreased following CA administration (Fig. 1H). The present results suggested
that HFD could induce metabolic syndrome in mice, and the effects
of HFD were reversed by CA administration. The present results
indicated that CA may be used to treat metabolic syndrome.
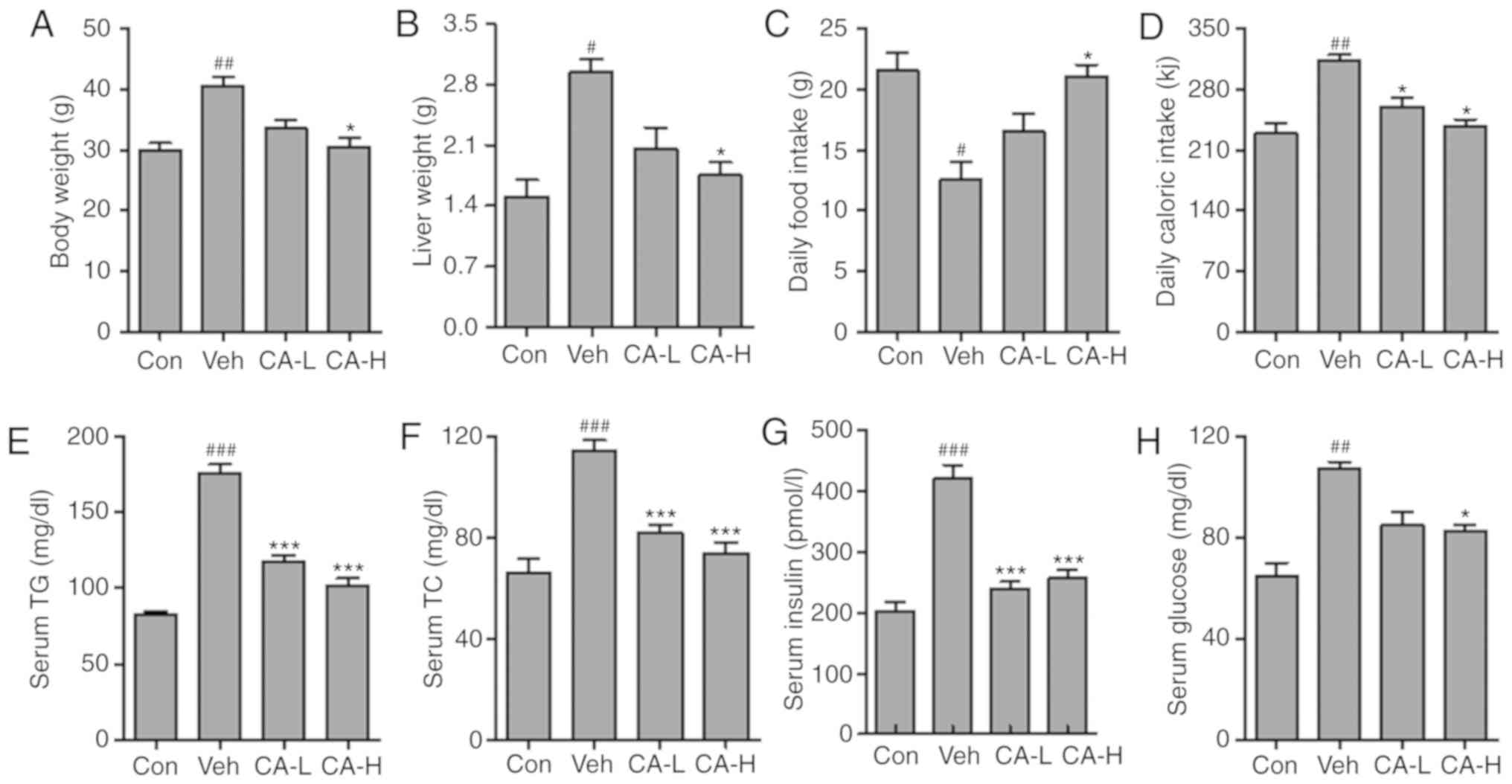 | Figure 1.Effects of CA on metabolic syndrome
in HFD mice. (A) Body weight, (B) liver weight, (C) daily food
intake and (D) daily caloric intake measured in the four
experimental groups. Serum levels of (E) TG, (F) TC, (G) insulin
and (H) glucose. Data are expressed as the means ± SEM. N=10/group.
#P<0.05, ##P<0.01 and
###P<0.001 vs. Con; *P<0.05 and ***P<0.001 vs.
Veh. CA, carnosic acid; CA-H, HFD mice treated with 20 mg/kg CA;
CA-L, HFD mice treated with 10 mg/kg CA; Con, Control group; HFD,
high-fat diet; TC, total cholesterol; TG, triglycerides; Veh, HFD
mice. |
CA reduces the inflammatory
response
In order to investigate whether CA could improve
metabolic disease-associated inflammatory response, the relative
expression levels of key factors associated with the inflammatory
response were examined. HFD induced the inflammatory response in
mouse liver tissue (Fig. 2A). The
pathological score in the Veh group (pathological score, 3.2) was
significantly higher than the Con group (pathological score, 0;
P<0.001). By contrast, mice in the CA-L and CA-H groups
exhibited a significant decrease in the pathological score
(pathological scores, 1 and 0.8, respectively) compared with the
Veh group (pathological score, 3.2; P<0.01). Moreover, pancreas
injury was observed in the Veh group (Fig. 2B; pathological score, 3.0).
Pancreas injury was significantly decreased following CA
administration (pathological scores, 0.8 and 0.5 in the CA-L and
CA-H groups, respectively). TNF-α and IL-1β are important
pro-inflammatory cytokines, with a role in various diseases
(32). Immunohistochemistry was
performed to investigate the protein expression levels of TNF-α and
IL-1β in mouse livers (Fig. 2C and
D). The protein expression levels of TNF-α and IL-1β were
significantly upregulated in the Veh group compared with in the Con
group (P<0.001). Treatment with CA was able to significantly
decrease the expression levels of both factors (P<0.001). The
present results suggested that CA effectively inhibited the
inflammatory response. Moreover, the serum levels of
pro-inflammatory cytokines, including IL-1β, IL-6 and TNF-α, were
investigated. The concentrations of IL-1β, IL-6 and TNF-α in the
Veh group were significantly higher than in the Con group
(P<0.001). The concentrations of these three pro-inflammatory
cytokines were significantly reduced following treatment with CA
(Fig. 2E-G). Collectively, the
present results suggested that HFD promoted metabolism-associated
inflammatory response in mice, and treatment with CA effectively
inhibited the secretion of pro-inflammatory cytokines in mice fed a
HFD.
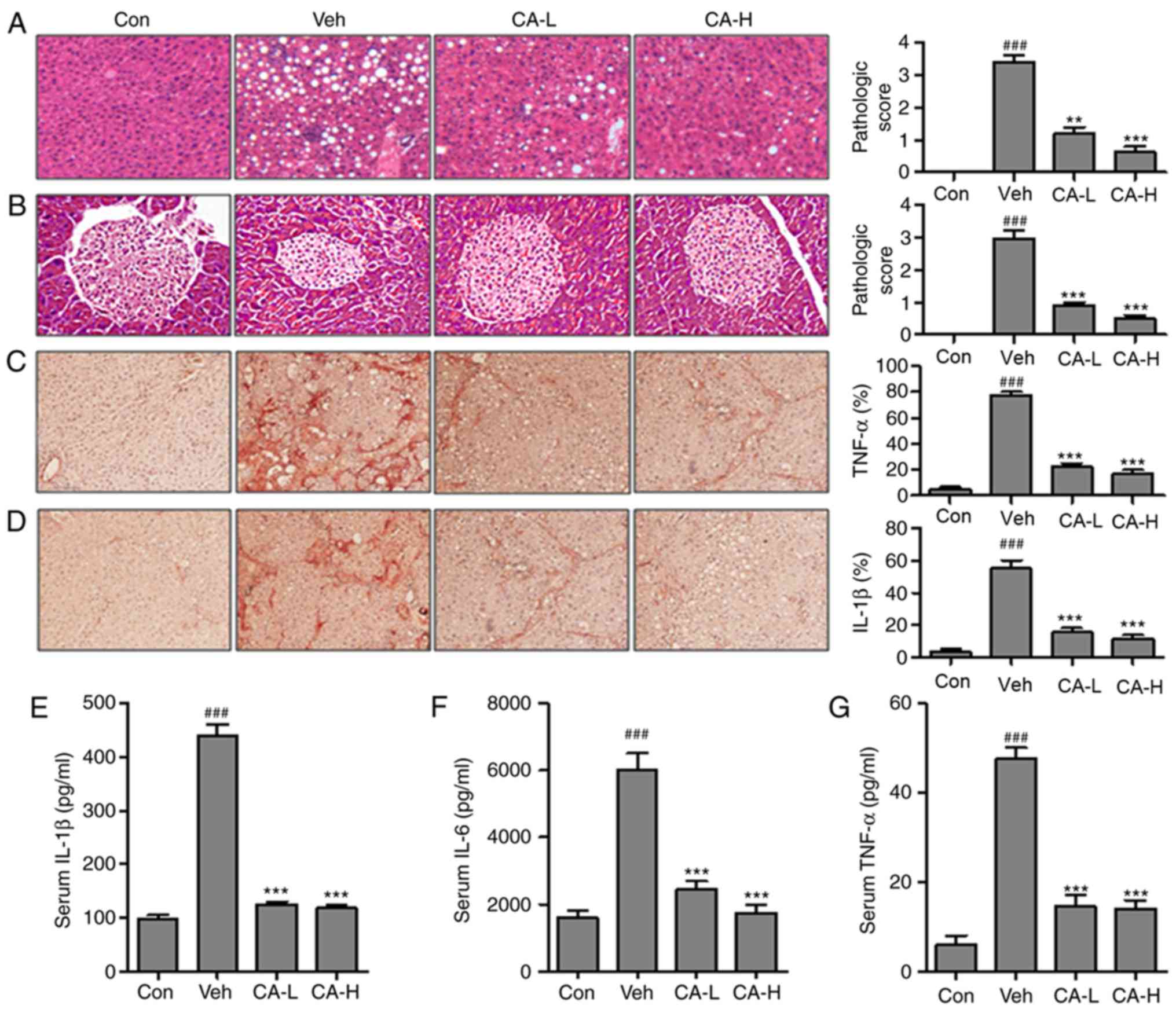 | Figure 2.CA decreases the inflammatory
response in HFD mice. Hematoxylin and eosin staining of (A) liver
and (B) pancreas indicated the pathological score in different
experimental groups (magnification, ×50). Immunohistochemical
analysis of (C) TNF-α and (D) IL-1β in the liver of HFD mice and
percentage of positive cells. Serum levels of (E) IL-1β, (F) IL-6
and (G) TNF-α. Data are expressed as the means ± SEM. n=10 in each
group. ###P<0.001 vs. Con; **P<0.01 and
***P<0.001 vs. Veh. CA, carnosic acid; CA-H, HFD mice treated
with 20 mg/kg CA; CA-L, HFD mice treated with 10 mg/kg CA; Con,
Control group; HFD, high-fat diet; IL, interleukin; TNF-α, tumor
necrosis factor-α; Veh, HFD mice. |
CA regulates the secretion of
pro-inflammatory cytokines in the brain
Central nervous system injury caused by metabolic
disease has been previously described (33). However, to the best of our
knowledge, no effective therapeutic strategies are currently
available. In the present study, brain injury was observed
following HFD, as indicated by high protein expression levels of
TNF-α and IL-1β in the brain (Fig.
3A). The present results suggested that HFD induced metabolic
syndrome and brain injury in mice. Additionally, CA was able to
inhibit the expression levels of TNF-α and IL-1β. Western blot
analysis was performed to examine the protein expression levels of
mature IL-1β and IL-18 in mouse brain tissues following different
treatments. The protein expression levels of mature IL-1β and IL-18
were significantly higher in the Veh group compared with in the Con
group (P<0.001). CA treatment decreased the protein expression
levels of mature IL-1β and IL-18 compared with in the Veh group
(P<0.001; Fig. 3B and C).
Furthermore, the protein expression levels of TNF-α were
upregulated in brain tissue collected from Veh mice (P<0.001).
Notably, the protein expression levels of TNF-α were reduced
following CA administration (P<0.001; Fig. 3B). In addition, RT-qPCR was used to
investigate the mRNA expression levels of pro-inflammatory
cytokines, including IL-1β, IL-6, IL-18 and TNF-α. The present
results suggested that IL-1β, IL-6, IL-18 and TNF-α were
upregulated in the Veh group compared with in the Con group
(P<0.001). Notably, the expression levels of these genes were
reduced following CA administration (P<0.001). The present qPCR
results were consistent with the aforementioned western blotting
results (Fig. 3C). Collectively,
the present results suggested that CA inhibited the secretion of
inflammatory cytokines in a mouse model of brain injury induced by
HFD.
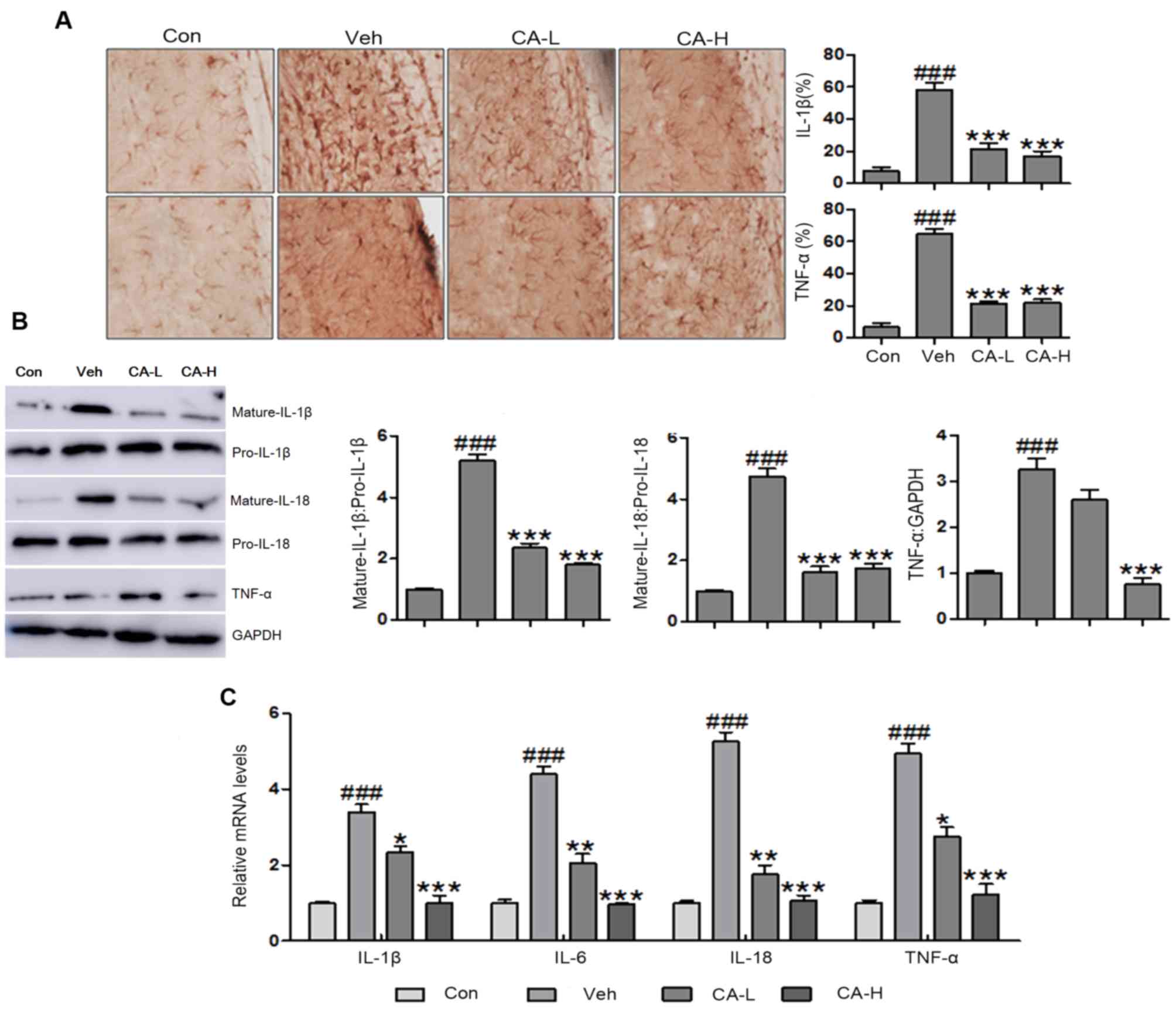 | Figure 3.Effects of CA on the secretion of
pro-inflammatory cytokines in the brain of HFD mice. (A)
Immunohistochemical analysis of IL-1β and TNF-α in brain tissues of
HFD mice (magnification, ×50). (B) Protein expression levels of
IL-1β, IL-18 and TNF-α in HFD mice, as assessed by western blot
analysis. (C) mRNA expression levels of pro-inflammatory cytokines
in the brain of HFD mice, as assessed by reverse
transcription-quantitative PCR analysis. Data are expressed as the
means ± SEM. n=10 in each group. ###P<0.001 vs. Con;
*P<0.05, **P<0.01 and ***P<0.001 vs. Veh. CA, carnosic
acid; CA-H, HFD mice treated with 20 mg/kg CA; CA-L, HFD mice
treated with 10 mg/kg CA; Con, Control group; HFD, high-fat diet;
IL, interleukin; TNF-α, tumor necrosis factor-α; Veh, HFD mice. |
CA improves brain injury by
suppressing the NF-κB signaling pathway
The NF-κB signaling pathway has been reported to be
associated with the secretion of pro-inflammatory cytokines
(34). The protein expression
levels of p-IKKα, p-IκBα and p-NF-κB in the Veh group were
increased compared with in the Con group (P<0.01; Fig. 4A). Notably, the protein expression
levels of p-IKKα, p-IκBα and p-NF-κB were significantly reduced
following CA administration (P<0.05). The present results
suggested that brain injury caused by HFD involved the NF-κB
signaling pathway, and CA was a negative regulator of the NF-κB
signaling pathway. As a biomarker of nerve injury, GFAP is
expressed in astrocytes (35). In
the present study, GFAP was highly expressed in mouse brain
tissues. High CA dosage decreased the expression levels of GFAP
(Fig. 4A). However, low CA dosage
did not affect the protein expression levels of GFAP. The present
data suggested that CA influenced the expression levels of GFAP in
a dose-dependent manner. Additionally, the protein expression
levels of Neu-N and Iba1 were decreased following treatment with CA
at low and high concentrations (Fig.
4B and C). Notably, the protein expression levels of Neu-N were
significantly upregulated in the Veh group compared with in the Con
group (P<0.001). Collectively, it was suggested that HFD caused
nerve injury by activating the NF-κB signaling pathway. In
addition, HFD increased the protein expression levels of GFAP,
Neu-N and Iba-1. Treatment with CA may attenuate brain injury by
decreasing the protein expression levels of GFAP, Neu-N, Iba-1 and
p-NF-κB.
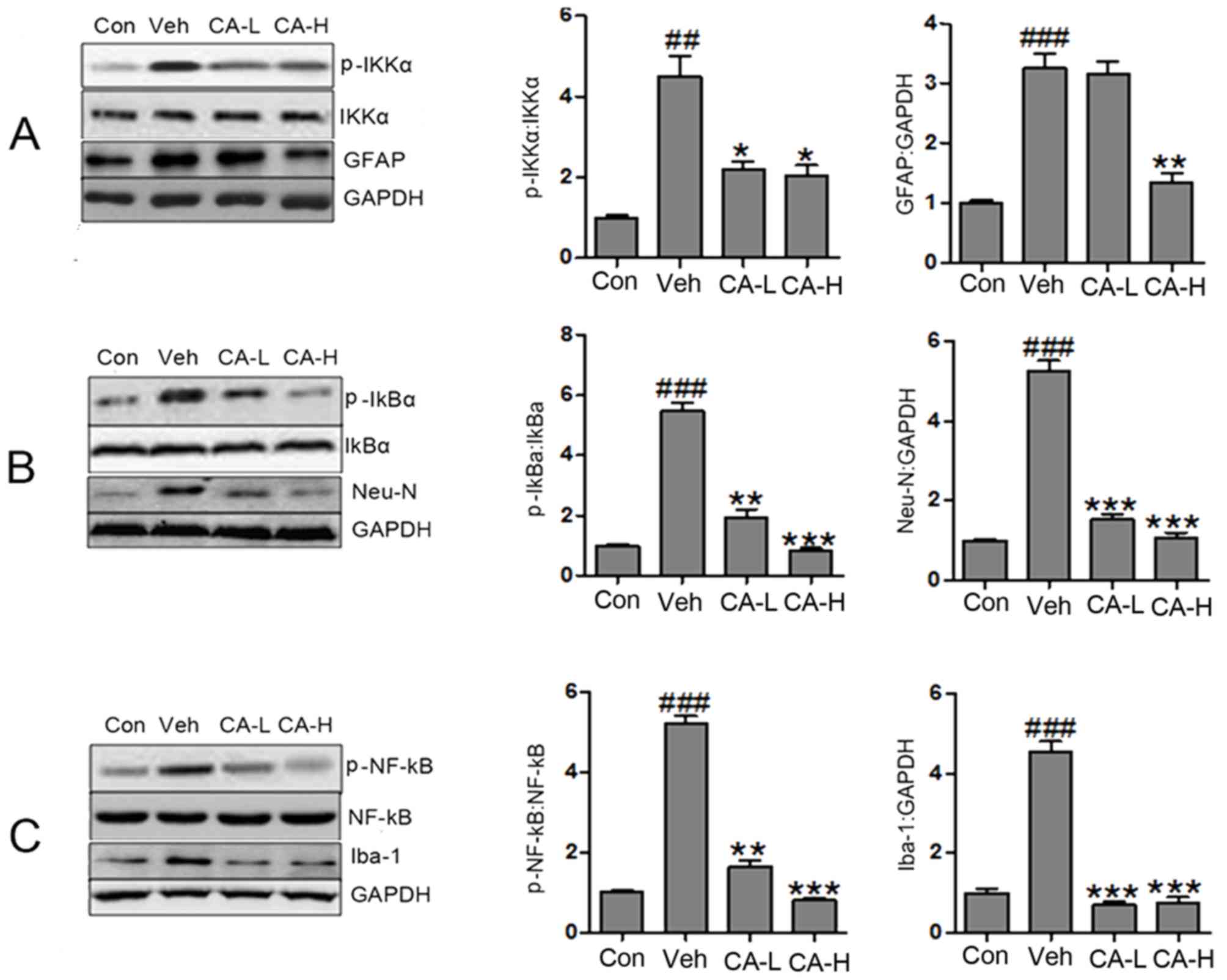 | Figure 4.CA attenuates brain injury in HFD
mice by inactivating the NF-κB signaling pathway. (A) Protein
expression levels of p-IKKα, IKKα, GFAP and GAPDH in different
groups, as assessed by western blot analysis. (B) Protein
expression levels of p-IκBα, IκBα, Neu-N and GAPDH in different
groups, as assessed by western blot analysis. (C) Protein
expression levels of p-NF-κB, NF-κB, Iba-1 and GAPDH in different
groups, as assessed by western blot analysis. Data are expressed as
the means ± SEM. n=10 in each group. ##P<0.01 and
###P<0.001 vs. Con; *P<0.05, **P<0.01 and
***P<0.001 vs. Veh. CA, carnosic acid; CA-H, HFD mice treated
with 20 mg/kg CA; CA-L, HFD mice treated with 10 mg/kg CA; Con,
Control group; GFAP, glial fibrillary acidic protein; HFD, high-fat
diet; IκBα, NF-κB inhibitor α; IKKα, IκB kinase α; Neu-N, neuronal
nuclei; Iba-1, ionized calcium-binding adapter molecule 1; p,
phosphorylated; Veh, HFD mice. |
CA attenuates brain injury by
decreasing caspase-3- associated apoptosis
Neurodegeneration has been suggested to be
associated with inflammatory response and cell apoptosis (36). Notably, Caspase-3 is an important
regulator of apoptosis (37). In
the present study, the Caspase-3 apoptotic pathway was
investigated. The pro-apoptotic factors Bax and MMP-9 were
significantly upregulated in mouse brain tissues from the Veh group
compared with in the Con group (P<0.01; Fig. 5A and B). CA treatment significantly
decreased the protein expression levels of both factors
(P<0.01). Additionally, the protein expression levels of
Caspase-3 were increased in the Veh group, but were reduced
following CA treatment (P<0.001; Fig. 5C). By contrast, Bcl-2, an
anti-apoptotic factor, was significantly downregulated in the Veh
group compared with in the Con group (P<0.05), and treatment
with CA was sufficient to upregulate the protein expression of
Bcl-2 in mouse brain (P<0.05; Fig.
5D). In order to further investigate the expression levels of
these factors, RT-qPCR was performed to examine the mRNA expression
levels of Bax, MMP-9, Caspase-3 and Bcl-2 in different groups. The
qPCR results were consistent with the aforementioned protein
expression results (Fig. 5E).
Collectively, the present results suggested that CA attenuated
nerve injury caused by apoptosis in the mouse brain.
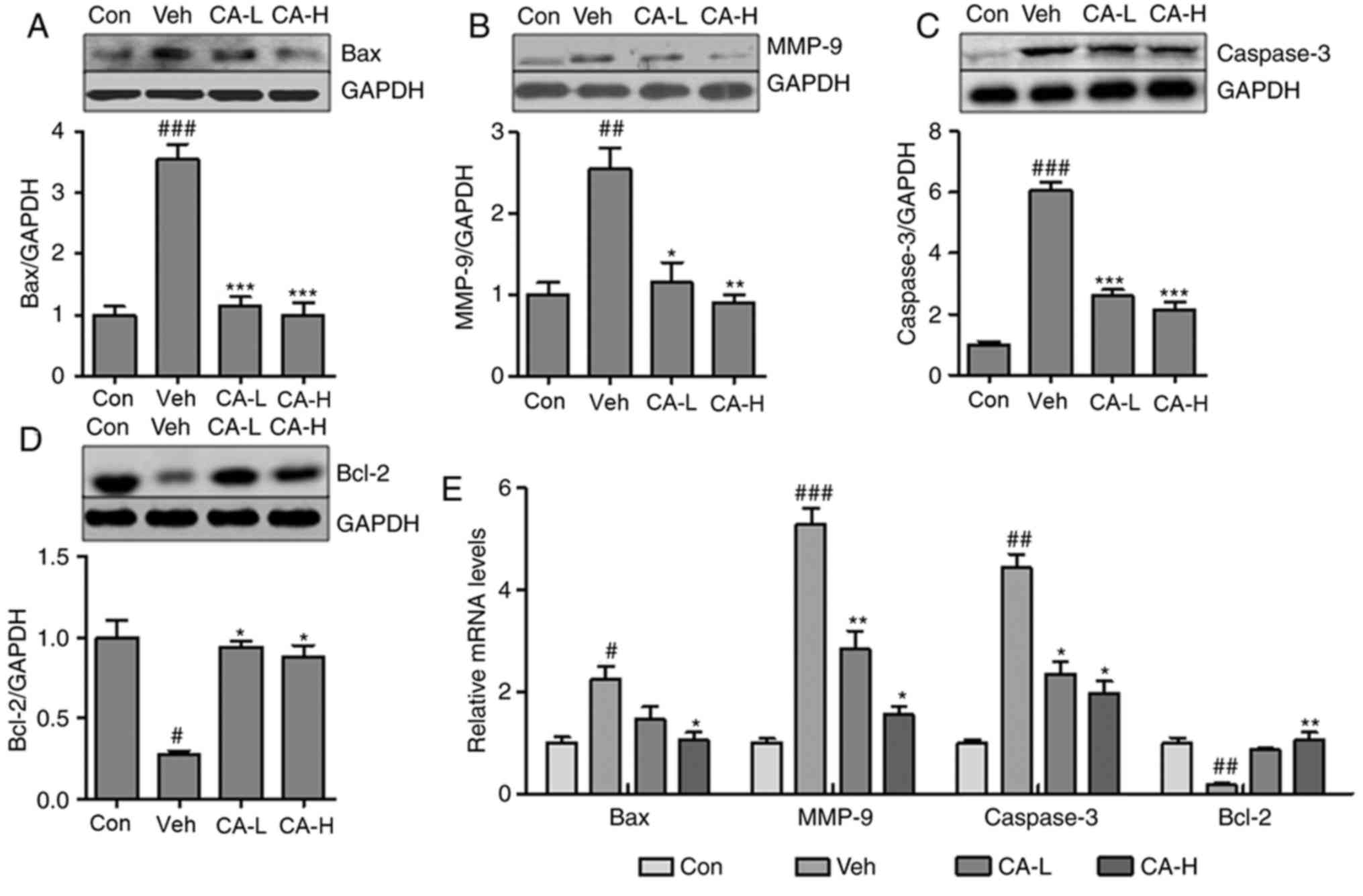 | Figure 5.CA alleviates brain injury by
suppressing Caspase-3-mediated apoptosis in HFD mice. Protein
expression levels of (A) Bax, (B) MMP-9, (C) Caspase-3 and (D)
Bcl-2 in the brain of HFD mice, as assessed by western blotting.
(E) mRNA expression levels of apoptotic factors in the brain of HFD
mice. Data are expressed as the means ± SEM. n=10 in each group.
#P<0.05, ##P<0.01 and
###P<0.001 vs. Con; *P<0.05, **P<0.01 and
***P<0.001 vs. Veh. CA, carnosic acid; CA-H, HFD mice treated
with 20 mg/kg CA; CA-L, HFD mice treated with 10 mg/kg CA; Con,
Control group; GFAP, glial fibrillary acidic protein; HFD, high-fat
diet; MMP-9, matrix metallopeptidase 9; Veh, HFD mice. |
Discussion
A previous study suggested that the incidence of
metabolic syndrome is increasing (38). Diabetes is one of the most common
metabolic diseases that lead to neurodegeneration (39). The mechanisms underlying
neurodegeneration caused by metabolic disease remain unclear.
Various therapeutic strategies have been used to treat patients
with brain injury caused by metabolic disorders (40). In the present study, the functions
of CA, an antioxidant compound extracted from Rosmarinus
officinalis L., were investigated in a mouse model of
HFD-induced metabolic syndrome (16). Previous studies have reported that
CA exhibits anti-cancer effects on colon cancer, acute myeloid
leukemia and skin cancer by serving as an anti-inflammatory,
antioxidant and antimicrobial agent (41–43).
However, the molecular mechanisms underlying the effects of CA,
which has previously been reported to alleviate brain injury,
remain poorly understood (44).
Therefore, in the present study, CA was used to investigate the
molecular mechanisms regulating neurodegeneration, inflammation and
apoptosis.
In the present study, HFD was found to cause
metabolic syndrome in mice, which exhibited higher body and liver
weights following HFD compared with in the Con group. The present
results are in line with a previous study (45). However, body weight and liver
weight were significantly decreased following CA administration.
Moreover, high serum levels of TG and TC were induced in mice fed a
HFD. In the present study, CA was identified as a positive
regulator of lipid metabolism, being able to decrease the serum
levels of TG and TC. The present results suggested that CA may hold
the potential to treat metabolic diseases. In addition, HFD caused
an increase in the serum levels of insulin and glucose, and these
effects were reversed by CA treatment. The present results
suggested that treatment with CA was able to attenuate the
deleterious effects of HFD-induced metabolic syndrome.
Metabolic diseases are the primary cause of
metabolic-associated inflammation, which is associated with brain
injury (46). In the present
study, systematic inflammation caused by HFD increased the serum
levels of IL-β, IL-6 and TNF-α. In addition, the upregulation of
pro-inflammatory cytokines was observed in liver tissue. Notably,
treatment with CA downregulated the secretion of pro-inflammatory
cytokines in serum and tissue samples. The present results
suggested that CA was able to inhibit the inflammatory response, in
line with a previous study (20).
The NF-κB signaling pathway is involved in the inflammatory
response via p-IKKα and p-IκBα (47,48).
IKKα is regulated by the ubiquitination and degradation of IκBα,
which is mediated by the phosphorylation of this factor. Upon
degradation of IκBα, NF-κB can translocate into the nucleus and
bind to the κB sites, acting as a transcription factor and
promoting the transcription of its downstream genes. In addition,
the nuclear translocation of NF-κB can promote the secretion of
pro-inflammatory cytokines involved in tissue injury (49). In the present study, the protein
expression levels of IL-β, IL-6 and TNF-α in the brain of HFD mice
were higher than the Con group, suggesting that activation of the
inflammatory response may result in nerve injury. Notably, CA
treatment was sufficient to significantly reduce the expression
levels of multiple cytokines in the mouse brain. In addition, the
protein expression levels of multiple regulators of astrocyte and
microglia cell activation (50,51),
including GFAP, Iba-1 and Neu-N, were examined by western blot
analysis. The protein expression levels of these three factors are
associated with the inflammatory response, and GFAP, Iba-1 and
Neu-N are biomarkers of central nervous system injury (52). The present results suggested that
CA served multiple roles in a mouse model of metabolic syndrome,
mediating inactivation of the NF-κB signaling pathway,
downregulating the secretion of pro-inflammatory cytokines, and
decreasing the expression levels of GFAP, Iba-1 and Neu-N, leading
to a reduction in the inflammatory response and an attenuation of
brain injury.
A previous study demonstrated that apoptosis-induced
cell death is associated with brain injury (53). In the present study, western blot
analysis was performed to analyze the Caspase-3 signaling pathway,
which is associated with apoptosis (54). Additionally, the protein expression
levels of multiple pro-apoptotic factors, including Bax and MMP-9
(55), were investigated. The
present results suggested that CA decreased the protein expression
levels of Bax and MMP-9 in mouse brain. Similarly, the protein
expression levels of Caspase-3 were downregulated. By contrast, the
protein expression levels of Bcl-2 were upregulated following CA
administration. Therefore, the present results suggested that CA
could reduce brain injury by inhibiting apoptosis via the Caspase-3
signal pathway.
In conclusion, HFD induced metabolic syndrome and
activated the inflammatory response. Additionally, CA was
identified to regulate lipid metabolism. Moreover, CA alleviated
brain injury by decreasing inflammation and apoptosis through the
NF-κB and Caspase-3 signaling pathways, respectively. Collectively,
the present data suggested that CA may facilitate the development
of novel therapies aimed to treat metabolic syndrome.
Acknowledgements
Not applicable.
Funding
The present study was funded by The Natural Science
Foundation of Xinjiang Uygur Autonomous Region, China (grant no.
2016D01C152).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YoL and YZ designed and performed the experiments,
and drafted the manuscript. MH, YuL, YZ, and XC analyzed the data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The animal experiments were approved by The
Committee on The Ethics of Animal Experiments of Xinjiang Medical
University (approval no. XM2017MD).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Anandhan A, Essa MM and Manivasagam T:
Therapeutic attenuation of neuroinflammation and apoptosis by black
tea theaflavin in chronic MPTP/probenecid model of Parkinson's
disease. Neurotox Res. 23:166–173. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Barnum CJ and Tansey MG: Neuroinflammation
and non-motor symptoms: The dark passenger of Parkinson's disease?
Curr Neurol Neurosci Rep. 12:350–358. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Khan MM, Kempuraj D, Thangavel R and
Zaheer A: Protection of MPTP-induced neuroinflammation and
neurodegeneration by Pycnogenol. Neurochem Int. 62:379–388. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Obermeier B, Verma A and Ransohoff RM: The
blood-brain barrier. Handb Clin Neurol. 133:39–59. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fatima G, Das SK and Mahdi AA: Oxidative
stress and antioxidative parameters and metal ion content in
patients with fibromyalgia syndrome: Implications in the
pathogenesis of the disease. Clin Exp Rheumatol 31 (6 Suppl 79).
S128–S133. 2013.
|
|
6
|
Ghosh A, Kanthasamy A, Joseph J,
Anantharam V, Srivastava P, Dranka BP, Kalyanaraman B and
Kanthasamy AG: Anti-inflammatory and neuroprotective effects of an
orally active apocynin derivative in pre-clinical models of
Parkinson's disease. J Neuroinflammation. 9:2412012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yan J, Xu Y, Zhu C, Zhang L, Wu A, Yang Y,
Xiong Z, Deng C, Huang XF, Yenari MA, et al: Simvastatin prevents
dopaminergic neurodegeneration in experimental parkinsonian models:
The association with anti-inflammatory responses. PLoS One.
6:e209452011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen WW, Zhang X and Huang WJ: Role of
neuroinflammation in neurodegenerative diseases (Review). Mol Med
Rep. 13:3391–3396. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nizamutdinov D and Shapiro LA: Overview of
traumatic brain injury: An immunological context. Brain Sci.
7:E112017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mukandala G, Tynan R, Lanigan S and
O'Connor JJ: The effects of hypoxia and inflammation on synaptic
signaling in the CNS. Brain Sci. 6:E62016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ghosh A, Birngruber T, Sattler W, Kroath
T, Ratzer M, Sinner F and Pieber TR: Assessment of blood-brain
barrier function and the neuroinflammatory response in the rat
brain by using cerebral open flow microperfusion (cOFM). PLoS One.
9:e981432014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Czabotar PE, Lessene G, Strasser A and
Adams JM: Control of apoptosis by the BCL-2 protein family:
Implications for physiology and therapy. Nat Rev Mol Cell Biol.
15:49–63. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Koff JL, Ramachandiran S and
Bernal-Mizrachi L: A time to kill: Targeting apoptosis in cancer.
Int J Mol Sci. 16:2942–2955. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rajan TS, Giacoppo S, Trubiani O, Diomede
F, Piattelli A, Bramanti P and Mazzon E: Conditioned medium of
periodontal ligament mesenchymal stem cells exert anti-inflammatory
effects in lipopolysaccharide-activated mouse motoneurons. Exp Cell
Res. 349:152–161. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mengoni ES, Vichera G, Rigano LA,
Rodriguez Puebla ML, Galliano SR, Cafferata EE, Pivetta OH, Moreno
S and Vojnov AA: Suppression of COX-2, IL-1β and TNF-α expression
and leukocyte infiltration in inflamed skin by bioactive compounds
from Rosmarinus officinalis L. Fitoterapia. 8:414–421. 2011.
View Article : Google Scholar
|
|
16
|
Moore J, Yousef M and Tsiani E: Anticancer
effects of rosemary (Rosmarinus officinalis L.) extract and
rosemary extract polyphenols. Nutrients. 8:E7312016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jung KJ, Min KJ, Bae JH and Kwon TK:
Carnosic acid sensitized TRAIL-mediated apoptosis through
down-regulation of c-FLIP and Bcl-2 expression at the post
translational levels and CHOP-dependent up-regulation of DR5, Bim,
and PUMA expression in human carcinoma caki cells. Oncotarget.
6:1556–1568. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tamaki Y, Tabuchi T, Takahashi T, Kosaka K
and Satoh T: Activated glutathione metabolism participates in
protective effects of carnosic acid against oxidative stress in
neuronal HT22 cells. Planta Medica. 76:683–688. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tsai CW, Liu KL, Lin YR and Kuo WC: The
mechanisms of carnosic acid attenuates tumor necrosis
factor-α-mediated inflammation and insulin resistance in 3T3-L1
adipocytes. Mol Nutr Food Res. 58:654–664. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Oh J, Yu T, Choi SJ, Yang Y, Baek HS, An
SA, Kwon LK, Kim J, Rho HS, Shin SS, et al: Syk/Src
pathway-targeted inhibition of skin inflammatory responses by
carnosic acid. Mediators Inflamm. 2012:7813752012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Meng P, Yoshida H, Matsumiya T, Imaizumi
T, Tanji K, Xing F, Hayakari R, Dempoya J, Tatsuta T,
Aizawa-Yashiro T, et al: Carnosic acid suppresses the production of
amyloid-β 1–42 by inducing the metalloprotease gene TACE/ADAM17 in
SH-SY5Y human neuroblastoma cells. Neurosci Res. 75:94–102. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen JH, Ou HP, Lin CY, Lin FJ, Wu CR,
Chang SW and Tsai CW: Carnosic acid prevents
6-hydroxydopamine-induced cell death in SH-SY5Y cells via mediation
of glutathione synthesis. Chem Res Toxicol. 25:1893–1901. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
National Research Council (US) Committee
for the Update of the Guide for the Care, Use of Laboratory
Animals, . Guide for the Care and Use of Laboratory Animals. 8th.
National Academies Press (US); Washington, DC: 2011
|
|
24
|
Zhao Y, Sedighi R, Wang P, Chen H, Zhu Y
and Sang S: Carnosic acid as a major bioactive component in
rosemary extract ameliorates high-fat-diet-induced obesity and
metabolic syndrome in mice. J Agric Food Chem. 63:4843–4852. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Alberti KG and Zimmet PZ: Definition,
diagnosis and classification of diabetes mellitus and its
complications. Part 1: Diagnosis and classification of diabetes
mellitus provisional report of a WHO consultation. Diabet Med.
15:539–553. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chan JK: The wonderful colors of the
hematoxylin-eosin stain in diagnostic surgical pathology. Int J
Surg Pathol. 22:12–32. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gouze E, Pawliuk R, Gouze JN, Pilapil C,
Fleet C, Palmer GD, Evans CH, Leboulch P and Ghivizzani SC:
Lentiviral-mediated gene delivery to synovium: Potent
intra-articular expression with amplification by inflammation. Mol
Ther. 7:460–466. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kramer AS, Latham B, Diepeveen LA, Mou L,
Laurent GJ, Elsegood C, Ochoa-Callejero L and Yeoh GC: InForm
software: A semi-automated research tool to identify presumptive
human hepatic progenitor cells, and other histological features of
pathological significance. Sci Rep. 8:34182018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yu W, Wu J, Cai F, Xiang J, Zha W, Fan D,
Guo S, Ming Z and Liu C: Curcumin alleviates diabetic
cardiomyopathy in experimental diabetic rats. PLoS One.
7:e5201320122012. View Article : Google Scholar
|
|
31
|
Klöting N and Blüher M: Adipocyte
dysfunction, inflammation and metabolic syndrome. Rev Nedcor Metab
Disord. 15:277–287. 2014. View Article : Google Scholar
|
|
32
|
Pozniak PD, White MK and Khalili K:
TNF-α/NF-κB signaling in the CNS: Possible connection to EPHB2. J
Neuroimmune Pharmacol. 9:133–141. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ricci G, Pirillo I, Tomassoni D, Sirignano
A and Grappasonni I: Metabolic syndrome, hypertension, and nervous
system injury: Epidemiological correlates. Clin Exp Hypertens.
39:8–16. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wan F and Lenardo MJ: The nuclear
signaling of NF-kappaB: Current knowledge, new insights, and future
perspectives. Cell Res. 20:24–33. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bramanti V, Tomassoni D, Avitabile M,
Amenta F and Avola R: Biomarkers of glial cell proliferation and
differentiation in culture. Front Biosci (Schol Ed). 2:558–570.
2010.PubMed/NCBI
|
|
36
|
Shalini S, Dorstyn L, Dawar S and Kumar S:
Old, new and emerging functions of caspases. Cell Death Differ.
22:526–539. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
D'Amelio M, Sheng M and Cecconi F:
Caspase-3 in the central nervous system: Beyond apoptosis. Trends
Neurosci. 35:700–709. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kaur J: A comprehensive review on
metabolic syndrome. Cardiol Res Pract. 2014:9431622014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bartzokis G: Alzheimer's disease as
homeostatic responses to age-related myelin breakdown. Neurobiol
Aging. 32:1341–1371. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
He Q, Liu J, Liang J, Liu X, Li W, Liu Z,
Ding Z and Tuo D: Towards improvements for penetrating the
blood-brain barrier-recent progress from a material and
pharmaceutical perspective. Cells. 7:E242018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Barni MV, Carlini MJ, Cafferata EG,
Puricelli L and Moreno S: Carnosic acid inhibits the proliferation
and migration capacity of human colorectal cancer cells. Oncol Rep.
27:1041–1048. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pesakhov S, Khanin M, Studzinski GP and
Danilenko M: Distinct combinatorial effects of the plant
polyphenols curcumin, carnosic acid, and silibinin on proliferation
and apoptosis in acute myeloid leukemia cells. Nutr Cancer.
62:811–824. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Park M, Han J, Lee CS, Soo BH, Lim KM and
Ha H: Carnosic acid, a phenolic diterpene from rosemary, prevents
UV-induced expression of matrix metalloproteinases in human skin
fibroblasts and keratinocytes. Exp Dermatol. 22:336–341. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Maynard ME, Underwood EL, Redell JB, Zhao
J, Kobori N, Hood KN, Moore AN and Dash PK: Carnosic acid improves
outcome after repetitive mild traumatic brain injury. J
Neurotrauma. Mar 26–2019.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lee YS, Cha BY, Saito K, Choi SS, Wang XX,
Choi BK, Yonezawa T, Teruya T, Nagai K and Woo JT: Effects of a
Citrus depressa Hayata (shiikuwavsa) extract on obesity in high-fat
diet-induced obese mice. Phytomedicine. 18:648–654. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Karelina K and Weil ZM: Neuroenergetics of
traumatic brain injury. Concussion. 1:CNC92015.PubMed/NCBI
|
|
47
|
Cao C, Zhu Y, Chen W, Li L, Qi Y, Wang X,
Zhao Y, Wan X and Chen X: IKKε knockout prevents high fat diet
induced arterial atherosclerosis and NF-κB signaling in mice. PLoS
One. 8:e649302013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Verhelst K, Verstrepen L, Carpentier I and
Beyaert R: IκB kinase ε (IKKε): A therapeutic target in
inflammation and cancer. Biochem Pharmacol. 85:873–880. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Liu T, Zhang L, Joo D and Sun SC: NF-κB
signaling in inflammation. Signal Transduct Target Ther.
2:170232017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Simpson JE, Ince PG, Lace G, Forster G,
Shaw PJ, Matthews F, Savva G, Brayne C and Wharton SB; MRC
Cognitive Function and Ageing Neuropathology Study Group, :
Astrocyte phenotype in relation to Alzheimer-type pathology in the
ageing brain. Neurobiol Aging. 31:578–590. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tremblay MÈ, Zettel ML, Ison JR, Allen PD
and Majewska AK: Effects of aging and sensory loss on glial cells
in mouse visual and auditory cortices. Glia. 60:541–558. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lyck L, Dalmau I, Chemnitz J, Finsen B and
Schrøder HD: Immunohistochemical markers for quantitative studies
of neurons and glia in human neocortex. J Histochem Cytochem.
56:201–221. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zlatic S, Comstra HS, Gokhale A, Petris MJ
and Faundez V: Molecular basis of neurodegeneration and
neurodevelopmental defects in Menkes disease. Neurobiol Dis.
81:154–161. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Simon DJ, Weimer RM, McLaughlin T, Kallop
D, Stanger K, Yang J, O'Leary DD, Hannoush RN and Tessier-Lavigne
M: A caspase cascade regulating developmental axon degeneration. J
Neurosci. 32:17540–17553. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Olsson M and Zhivotovsky B: Caspases and
cancer. Cell Death Differ. 18:1441–1449. 2011. View Article : Google Scholar : PubMed/NCBI
|



















