Introduction
Exercise, eccentric contractions, acute trauma and
disease are all causal mechanisms of skeletal muscle injury
(1–5). Although the type of injury differs,
the general injury and repair mechanisms are similar (1–5).
When skeletal muscle is injured, it undergoes sequential phases of
degeneration, inflammation, regeneration and fibrosis (1–5). For
the repair and regeneration of skeletal muscle, it is important to
induce the proliferation and differentiation of skeletal myoblasts
and to inhibit the apoptosis of myoblasts (2). Therefore, anti-inflammatory and
anti-apoptotic treatments are important for skeletal muscle
regeneration and to promote healing following the occurrence of
injury (1,4–6).
Baicalin is a flavonoid glycoside extracted from
Scutellaria baicalensis, which is used in traditional
Chinese medicine, has been reported to possess significant
anti-inflammatory and anti-apoptotic properties, and is widely used
in the treatment of injury and inflammatory diseases (7–12).
For example, Lin et al (7,8)
reported that baicalin inhibited H2O2-induced
cell cytotoxicity in a human renal proximal tubular epithelial cell
line, and attenuated renal ischemia-reperfusion injury by
suppressing inflammation and apoptosis. A study by Cao et al
(9) demonstrated that baicalin
attenuated global cerebral ischemia/reperfusion injury in gerbils
through anti-oxidative and anti-apoptotic pathways. In addition,
Zhu et al (10) reported
that baicalin increased survival in a murine model of polymicrobial
sepsis through inhibiting the inflammatory response and lymphocyte
apoptosis, and Xiping et al (11) demonstrated that baicalin offered
protection to the thymus rats with severe acute pancreatitis. Our
previous study also demonstrated that baicalin significantly
inhibited oxidative stress damage induced by
H2O2 and decreased cell apoptosis in endplate
chondrocytes, which may provide potential therapeutic benefits for
patients with osteoarthritis (13).
Therefore, in order to further investigate the
underlying role and mechanism of baicalin in injured skeletal
muscle, the present study focused on
H2O2-stimulated C2C12 myoblasts in
vitro to investigate the role of baicalin on cell apoptosis,
and established an animal model of injured skeletal muscle to
observe the influence of baicalin on the uptake of
β-2-[18F]-fluoro-2-deoxy-D-glucose (18F-FDG) in lesions
in vivo via small animal positron emission tomography (PET)
imaging.
Materials and methods
Cell viability
C2C12 mouse myoblast cells (ATCC, cat. no. CRL-1772)
were grown in 96-well plates (BD Falcon; BD Biosciences, San Jose,
CA, USA) at a density of ~1×105/ml (100 µl/well) in
Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc.), supplemented with 10% fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and
streptomycin (Gibco; Thermo Fisher Scientific, Inc.). The C2C12
myoblasts were then washed with PBS buffer (Gibco; Thermo Fisher
Scientific, Inc.) and incubated with medium containing different
concentrations of H2O2 (0, 50, 100, 150, 200,
300, 500, 600, 800 and 1,000 µM; Lingfeng, Shanghai, China) at 37°C
for 4 h, respectively. Untreated cells were referred to as the
normal control. The cell viability of C2C12 myoblasts was
determined using a cell counting kit-8 (CCK-8) assay (Dojindo
Molecular Technologies, Inc., Kumamoto, Japan). The C2C12 myoblasts
were subsequently incubated with H2O2 (500
µM) under the co-existence of baicalin at various treatment time
points (−1, 0, 1 and 2 h) at 37°C for 4 h, respectively. In
addition, to judge the optimal incubation time, the appropriate
treated concentration of baicalin on C2C12 myoblasts was also
investigated. The C2C12 myoblasts were pretreated with baicalin at
different concentrations (5, 15, 25, 50, 100, 150, 200, 250 and 300
µM) for 1 h, and were then incubated with
H2O2 (500 µM) at 37°C for 4 h, respectively.
Untreated cells and cells treated with H2O2
only were referred to as the control groups. Cell viability was
determined using a 1:10 dilution of CCK-8 reagent and incubated at
37°C for 1 h. All the above data are presented as the mean of at
least three independent experimental repeats.
Cell apoptosis
The apoptosis of C2C12 myoblasts incubated in the
three groups (treated with 500 µM H2O2,
treated with 500 µM H2O2 + 100 µM baicalin
pre-treatment for 1 h, and the normal control) for 4 h was measured
using an Annexin V/PI assay kit (Nanjing KeyGen Biotech Co., Ltd.,
Nanjing, China) for flow cytometric analysis. Briefly, the cells
from each group were washed with PBS three times, harvested with
0.25% trypsin and centrifuged at 400 × g for 5 min at room
temperature. The supernatant was discarded, cells were resuspended
in 500 µl binding buffer and then immediately mixed with 5 µl
Annexin V-FITC and 5 µl propidium iodide and incubated for 10–15
min at room temperature. Finally, the mixture was analyzed using a
BD FACS Aria II flow cytometry (BD Biosciences). All data are
presented as the mean of at least three independent experimental
repeats.
Oxidative activity
The oxidative activity was assessed in the three
aforementioned groups following incubation with
H2O2 for 4 h. Following treatment, cells from
each of the groups were washed with PBS three times, harvested with
0.25% trypsin and centrifuged at 400 × g for 5 min at room
temperature. The supernatant was discarded, and the cells were
incubated with ROS working fluid (cat. no. KGT010-1; Nanjing KeyGen
Biotech Co., Ltd.) at 37°C for 15 min. The ROS working fluid was
then removed, fresh PBS was added, and detection was performed with
BD FACS Aria II flow cytometry (BD Biosciences) with an excitation
wavelength of 488 nm, an emission wavelength of 525 nm and the
channel of fluorescein isothiocyanate (FITC). Finally, the results
of flow cytometry were analyzed using Flowjo 10.1 software (Flowjo,
LLC, Ashland, OR, USA). All the above data are presented as the
mean of at least three independent experimental repeats.
In addition, the cells from each group were washed
with cold PBS three times, harvested with 0.25% trypsin, and
centrifuged at 400 × g for 5 min at room temperature. The
supernatant was discarded, and the cells were lysed with radio
immunoprecipitation assay (RIPA) buffer and centrifuged at 12,000 g
for 10 min at 4°C. Following this, 100 µl supernatant and 200 µl
malondialdehyde (MDA) working solution (cat. no. KGT003; Nanjing
KeyGen Biotech Co., Ltd.) were mixed and added to the 96-well
plate. The absorbance of each well was then measured at a
wavelength of 532 nm in an enzyme-linked immunometric meter
(FlexStation 3; Molecular Devices, LLC, Sunnyvale, CA, USA). All
the above data are presented as the mean of at least three
independent experimental repeats.
Mitochondrial function
Rhodamine 123 (cat. no. KGA217; Nanjing KeyGen
Biotech Co., Ltd.) were analyzed in above three groups following
the incubation with H2O2 for 4 h. Following
treatment, cells from each group were washed with PBS three times,
harvested with 0.25% trypsin, and centrifuged at 400 × g for 5 min
at room temperature. The supernatant was discarded, and the cells
was incubated with Rhodamine 123 working fluid at 37°C for 15 min.
The Rhodamine 123 working fluid was then removed, fresh PBS was
added, and detection was performed using BD FACS Aria II flow
cytometry with an excitation wavelength of 488 nm, an emission
wavelength of 525 nm and the channel of FITC. Finally, the results
of flow cytometry were analyzed using Flowjo 10.1 software. All the
above data are presented as the mean of at least three independent
experimental repeats.
Tetramethylrhodamine methyl ester staining (JC-1;
cat. no. KGA602; Nanjing KeyGen Biotech Co., Ltd.) were analyzed in
the above three groups following incubation with
H2O2 for 4 h. Following treatment, the cells
from each group were washed with PBS three times, harvested with
0.25% trypsin, and centrifuged at 400 × g for 5 min at room
temperature. The supernatant was discarded, and the cells was
incubated with JC-1 working fluid at 37°C for 15 min. Then JC-1
working fluid was then removed and fresh PBS was added. The
analysis of JC-1 was detected with the channel of BD FACS Aria II
flow cytometry via P-phycoerythrin and analyzed using Flowjo 10.1
software. All the above data are presented as the mean of at least
three independent experimental repeats.
Mitochondrial apoptogenic factors
The protein levels of cytochrome c oxidase
(cyto-C) and apoptosis-inducing factor (AIF) from cytosolic
fractions were detected in the three experimental groups by western
blotting. The cells were collected and lysed by RIPA buffer for 15
min and centrifuged at 15,000 × g for 30 min at 4°C. The protein
concentrations were analyzed using a NanoDrop instrument, and 40 µg
protein from each sample were run on 10% salt-polyacrylamide gel
electrophoresis gels, and then transferred onto polyvinylidene
fluoride membranes. Following blocking with 5% nonfat dry milk, the
membranes were incubated overnight at 4°C with following primary
mouse monoclonal antibodies: Anti-cyto-C (cat. no. AC908; Beyotime
Institute of Biotechnology, Haimen, China], anti-AIF (cat. no.
AB32516; Abcam, Cambridge, UK) or anti-glyceraldehyde-3-phosphate
dehydrogenase (GAPDH; cat. no. AB181602; Abcam) at a dilution of
1:500. The membranes were washed and incubated in a 1:1,000
dilution of goat anti-mouse/rabbit HRP (cat. no.
115-035-003/111-035-003; Jackson ImmunoResearch Laboratories, Inc.,
West Grove, PA, USA) for 1 h at room temperature. The membranes
were then washed and visualized using Pierce™ ECL western blotting
substrate (Thermo Fisher Scientific, Inc.) followed by exposure to
Tanon 5200 (Tanon Science and Technology Co., Ltd.). Anti-cyto-C,
anti-AIF and GAPDH were detected as bands of approximately 15, 67
and 36 kDa, respectively. The data were normalized to the GAPDH
content of the same sample and analyzed using Image J v1.8.0
(National Institutes of Health, Bethesda, MD, USA). Similar to the
above methods, the levels of cyto-C and AIF from mitochondrial
fractions were also analyzed in the aforementioned three groups
using western blotting following incubation with
H2O2 for 24 h. The extracted proteins were
incubated with the following primary mouse monoclonal antibodies: A
1:1,000 dilution of anti-cyto-C, anti-AIF or anti-cyclooxygenase-4
(COX-4; cat. no. AB33958; Abcam) for 12 h at 4°C, followed by
incubation in a 1:1,000 dilution of goat anti-mouse/rabbit HRP
(Jackson ImmunoResearch Laboratories, Inc.) for 1 h at room
temperature. Anti-cyto-C, anti-AIF and COX-4 were detected as bands
of approximately 15, 67 and 17 kDa, respectively. Bands were
visualized according to the aforementioned procedure. Data were
normalized to the content of COX-4 in the same sample and analyzed
using Image J (National Institutes of Health). All the above data
are presented as the mean of at least three independent
experimental repeats.
Caspase proteins
The activation of caspase-3 and caspase-9 was
evaluated in the three experimental groups by western blot analysis
at the time point of 24 h. The extracted proteins were incubated
with 1:1,000 dilutions of the following primary mouse monoclonal
antibodies: Anti-caspase-3 (cat. no. AB2302; Abcam), anti-caspase-9
(cat. no. AB32539; Abcam) or anti-GAPDH for 12 h at 4°C, followed
by incubation in a 1:1,000 dilution of goat anti-mouse/rabbit HRP
(Jackson ImmunoResearch Laboratories, Inc.) for 1 h at room
temperature. Caspase-3 and caspase-9 were detected as bands of
approximately 17 and 46 kDa, respectively. Bands were visualized
according to the aforementioned procedure. The data were normalized
to the GAPDH content of the same sample and analyzed by ImageJ
(National Institutes of Health). The above data are presented as
the mean of at least three independent experimental repeats.
Animal models of injured skeletal
muscle induced by H2O2
All procedures were approved by the Animal Ethics
Committee at Shanghai East Hospital, Tongji University School of
Medicine (Shanghai, China). In total, five Female BALB/C mice of
6-8-weeks of age (18–20 g) were purchased from Shanghai SLAC
Laboratory Animal Co., Ltd. (Shanghai, China). Mice were kept in a
controlled environment (temperature range, 22–24°C; relative
humidity, 40–60%) with a 12 h light/dark cycle and had free access
to food and water. Mice were fasted for at least 24 h before the
experiment and anesthetized using isoflurane inhalation.
Subsequently, 500 µM H2O2 was intramuscularly
administered into the right hind leg. The left hind leg was
intramuscularly administered with 500 µM
H2O2, and the relevant group was pre-treated
with 100 µM baicalin for 1 h. At 3 days post-treatment, the animal
models were used for small animal PET imaging.
Small animal PET imaging
PET imaging was performed using a small animal PET
scanner (Siemens Inveon). Briefly, the mice (n=4) were injected
with ~3.7–5.55 MBq (100–150 µCi) of 18F-FDG through the
tail vein, and were anesthetized with 2% isoflurane for imaging
experiments at 1 h p.i. The PET images were reconstructed and
analyzed using the vendor-supplied software Inveon Research
Workspace (Preclinical Solutions, Siemens Healthcare Molecular
Imaging). Three-dimensional regions of interest (ROIs) were drawn
over the organs and tissues on decay-corrected whole-body PET
images. The results were divided by the injected dose, in order to
obtain an image ROI-derived percent injected dose per gram of
tissue (14).
Pathology
The BALB/C mice were sacrificed by cervical
dislocation following anesthetization with isoflurane. Bilateral
muscle lesions were separately fixed using 4% paraformaldehyde for
24 h and dehydrated using an ethanol series at room temperature.
Following fixation and dehydration, the muscle samples were
embedded in paraffin. Paraffin specimens were cut to a 5 µm
thickness and stained with hematoxylin and eosin (H&E) for 1 h
at room temperature. All stained samples were observed under a
light microscope (Leica Microsystems GmbH, Germany), and images
were acquired under the same conditions and displayed at the same
scale for comparison (magnification, ×100).
Statistical analysis
The quantitative data are expressed as the mean ±
SD. Statistical analysis was performed using one-way ANOVA for the
comparison of multiple groups and Student's t-test for two groups.
A 95% confidence level was selected, and P<0.05 was considered
to indicate a statistically significant difference.
Results
Baicalin increases cell viability of
H2O2-stimulated c2c12 myoblasts
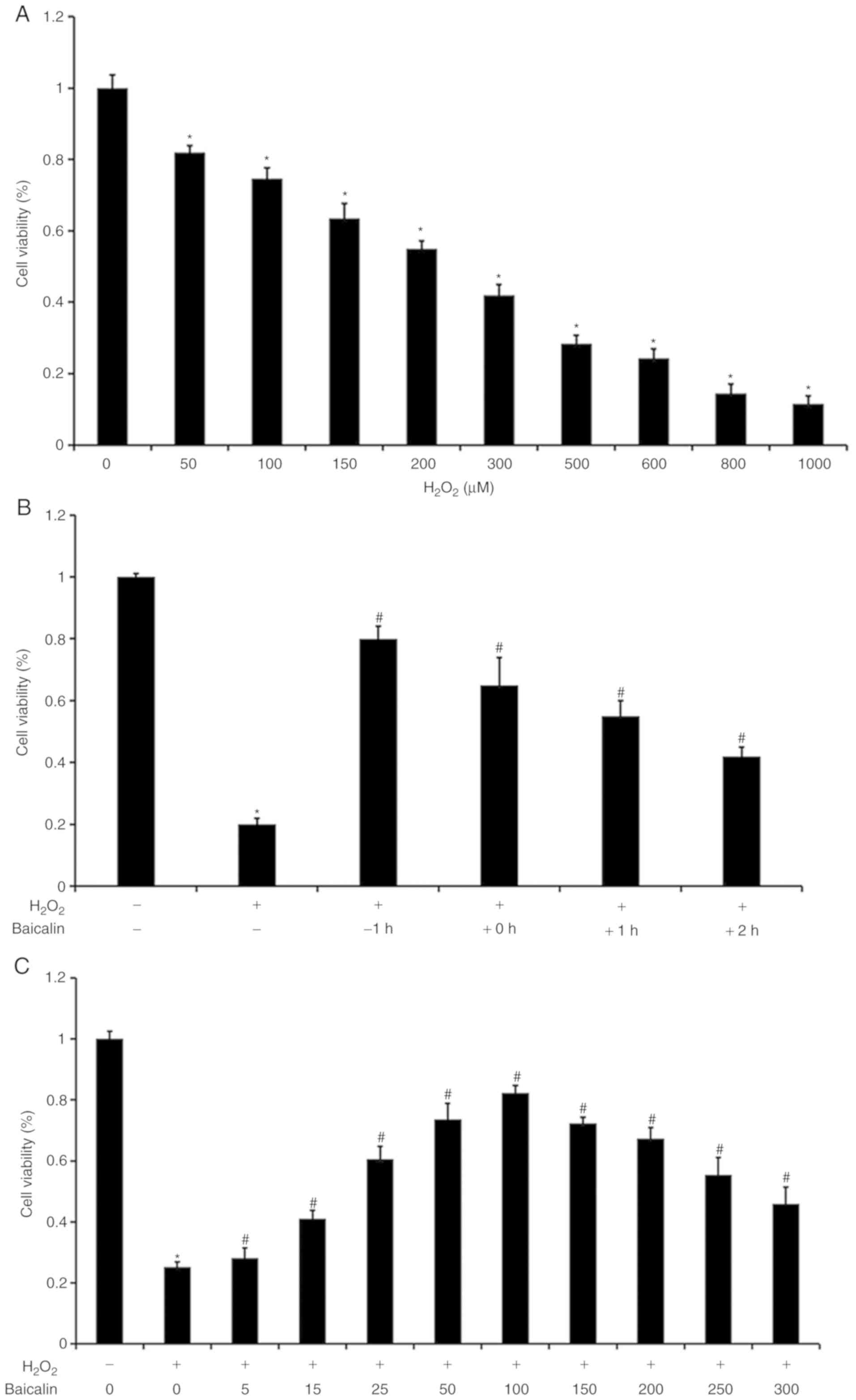
As illustrated in Fig.
1A, with increasing H2O2 concentrations,
the cell viability of the C2C12 myoblasts was gradually decreased.
The H2O2 concentration of 500 µM was selected
as an optimal dose for the subsequent experiments. As shown in
Fig. 1B, it was observed that cell
viability was highest when the C2C12 myoblasts under
H2O2 exposure were pre-treated with baicalin
for 1 h. Cell viability subsequently decreased with a gradual trend
when the C2C12 myoblasts were treated with baicalin no earlier than
the start of incubation with H2O2. As
baicalin concentrations increased, C2C12 myoblast viability was
initially gradually elevated, and then decreased under co-treatment
with 500 µM H2O2. Cell viability was highest
when the baicalin concentration reached 100 µM (Fig. 1C). Therefore, a baicalin
concentration of 100 µM was selected as the model dose for the
subsequent experiments.
Baicalin inhibits C2C12 myoblast
apoptosis induced by H2O2
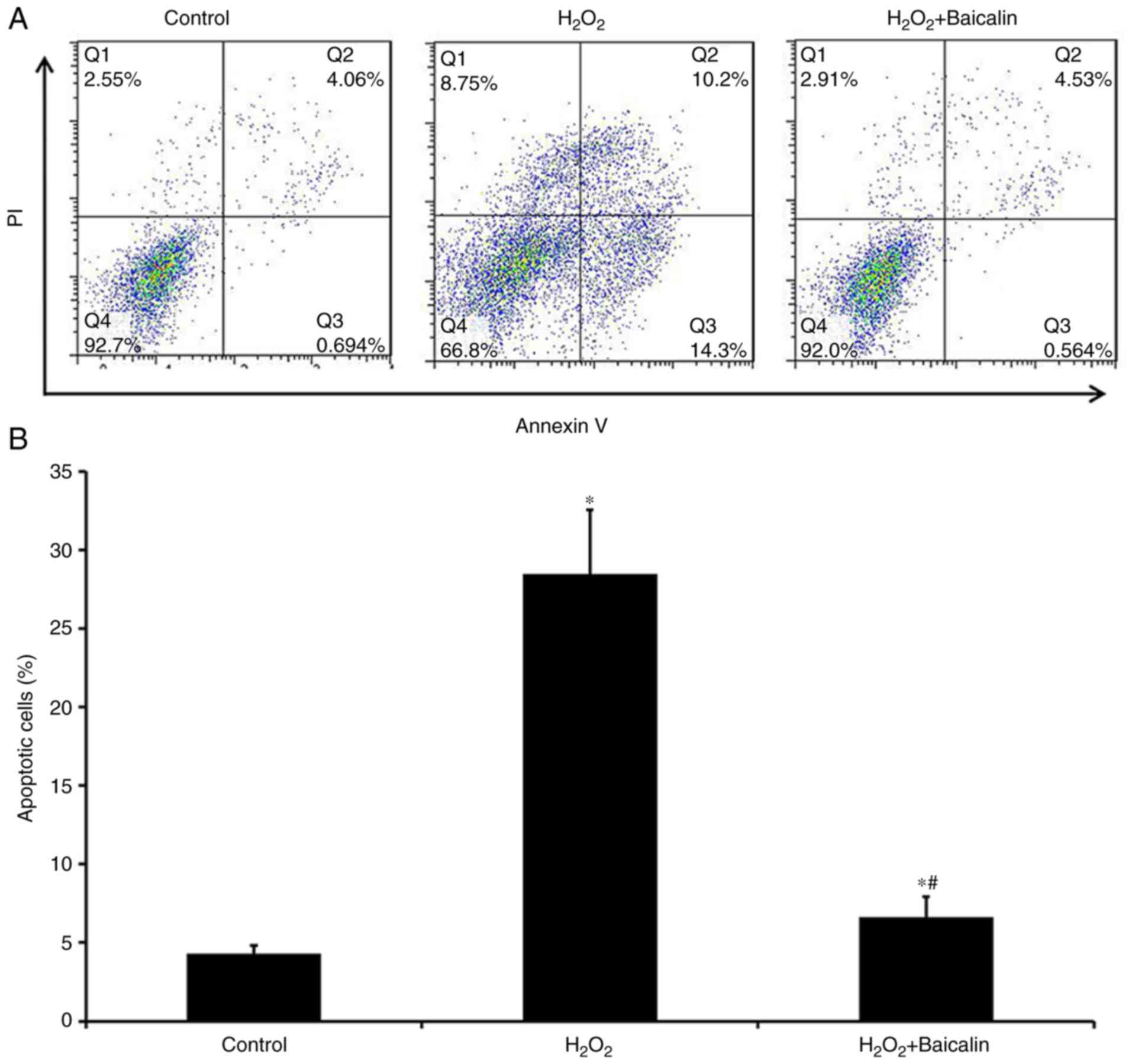
The Annexin V/PI staining analysis revealed that,
compared with the group treated with H2O2
alone for 4 h, the apoptosis of C2C12 myoblasts was significantly
decreased in the group that had been pre-treated with baicalin for
1 h at the 4-h time point (Fig. 2A and
B), which suggested that baicalin significantly suppressed the
apoptosis of C2C12 myoblasts induced by
H2O2.
Baicalin decreases oxidative activity
in C2C12 myoblasts induced by H2O2
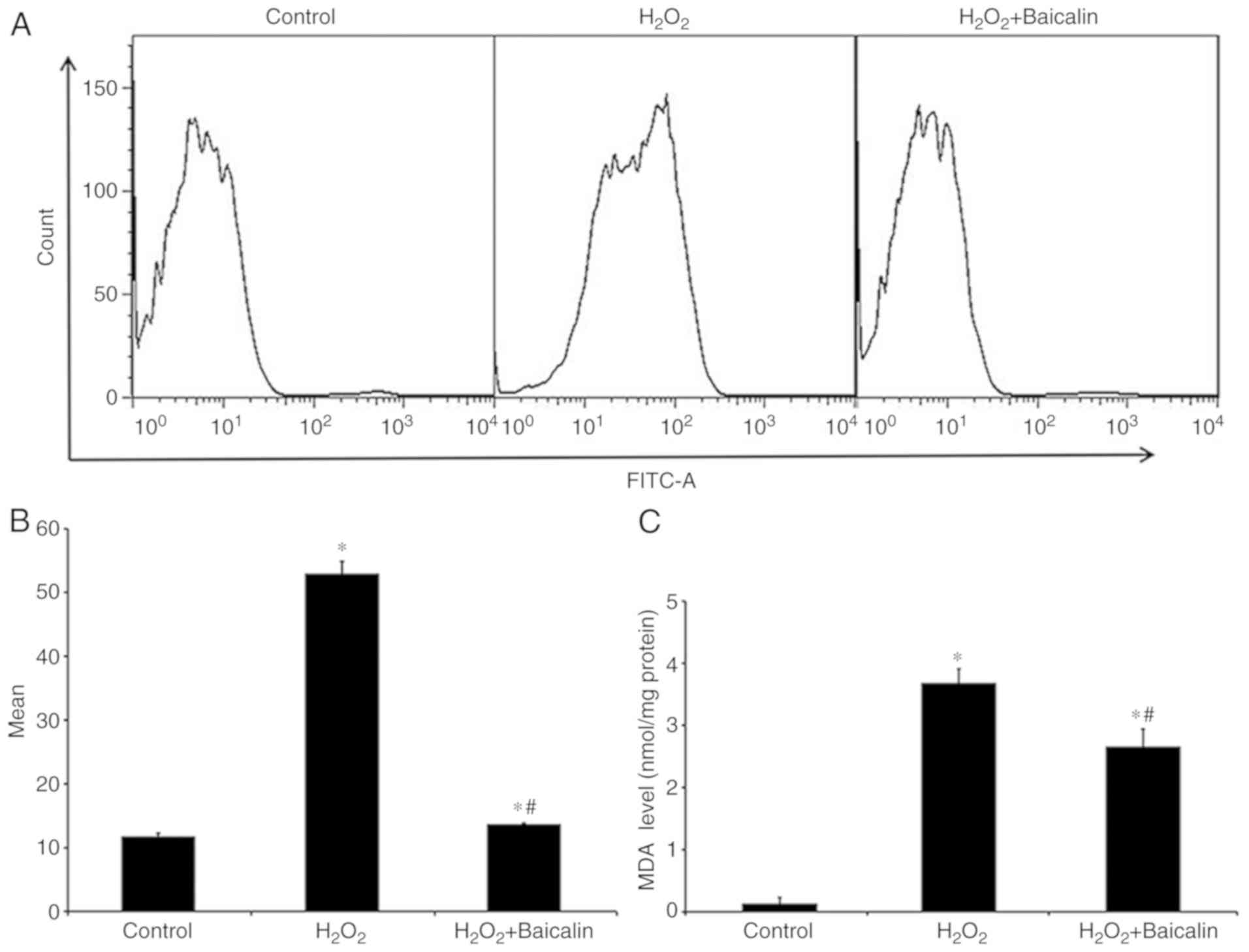
As a biomarker of oxidative stress, the ROS levels
of C2C12 myoblasts were increased following treatment with
H2O2 for 4 h, which were markedly reversed by
pre-treatment with baicalin for 1 h (Fig. 3A and B). Similarly, pre-treatment
with baicalin for 1 h effectively reversed the abnormal MDA levels
in C2C12 myoblasts exposed to H2O2 for 4 h
(Fig. 3C).
Baicalin reverses mitochondrial
dysfunction in C2C12 myoblasts induced by
H2O2
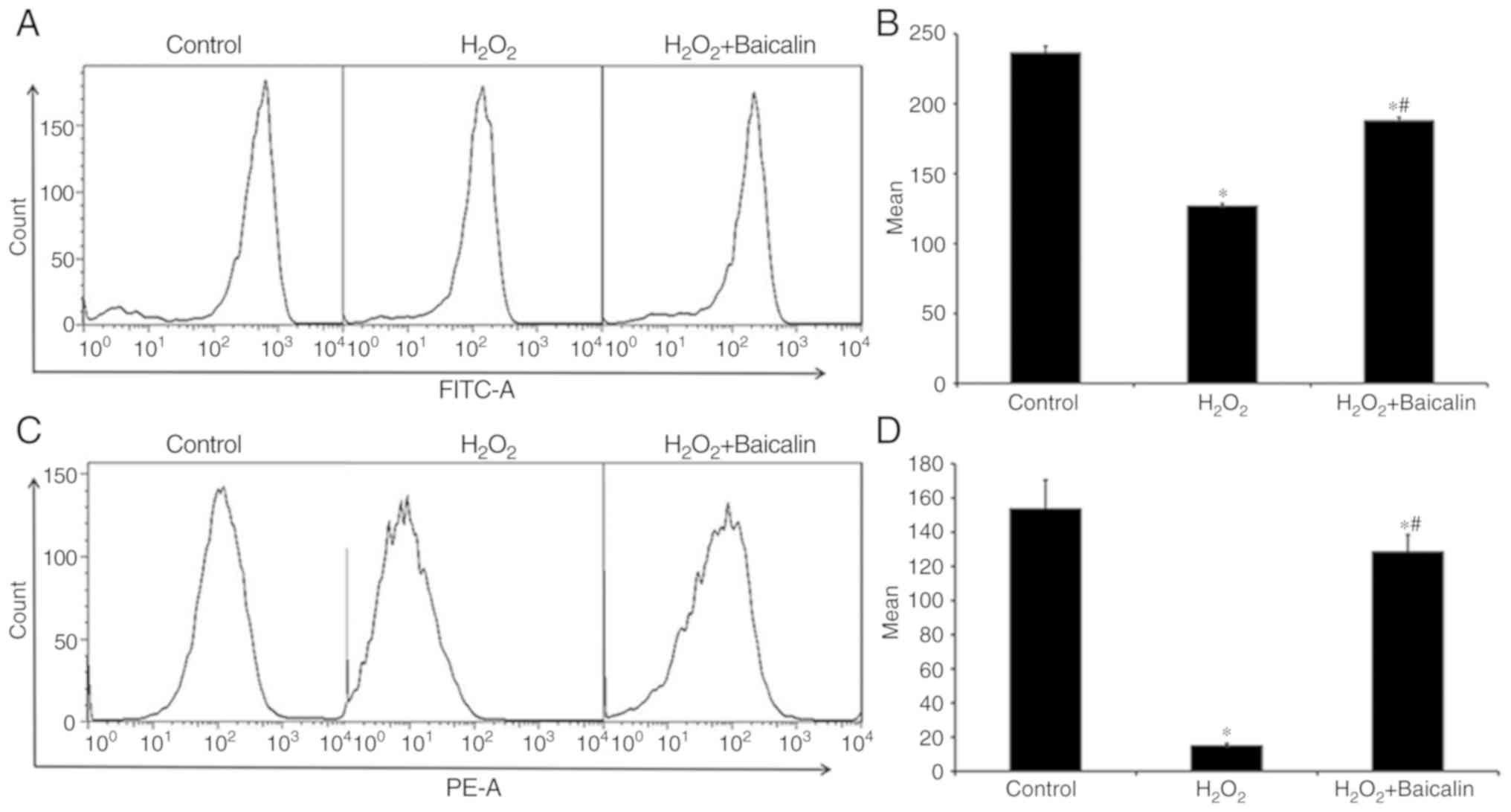
Mitochondrial dysfunction was also observed in C2C12
myoblasts following exposure to H2O2 for 4 h,
which was reflected by the loss of mitochondrial activity,
determined by Rhodamine 123 staining (Fig. 4A and B), and the decrease in
mitochondrial membrane potential (ΔΨm) determined via JC-1 staining
(Fig. 4C and D). However, as shown
in Fig. 4, pre-treatment with
baicalin for 1 h noticeably reversed the aforementioned effects and
protected mitochondrial function.
Baicalin inhibits the release of
mitochondrial apoptogenic factors induced by
H2O2
The levels of cyto-C and AIF from cytosolic
fractions were identified following the treatment of C2C12
myoblasts with H2O2 for 24 h, and these were
marginally lower than those in the normal control (Fig. 5A). Pre-treatment with baicalin was
observed to marginally upregulate the expression of cyto-C and AIF
(Fig. 5A). This effect was
corroborated by the quantification of the western blotting results,
which are illustrated in Fig. 5B.
The levels of cyto-C and AIF from mitochondrial fractions were also
identified, and these were markedly lower than those in the normal
control group (Fig. 5C). In
addition, pre-treatment with baicalin significantly upregulated the
levels of cyto-C and AIF (Fig.
5C). The quantification results of western blotting are
presented in Fig. 5D.
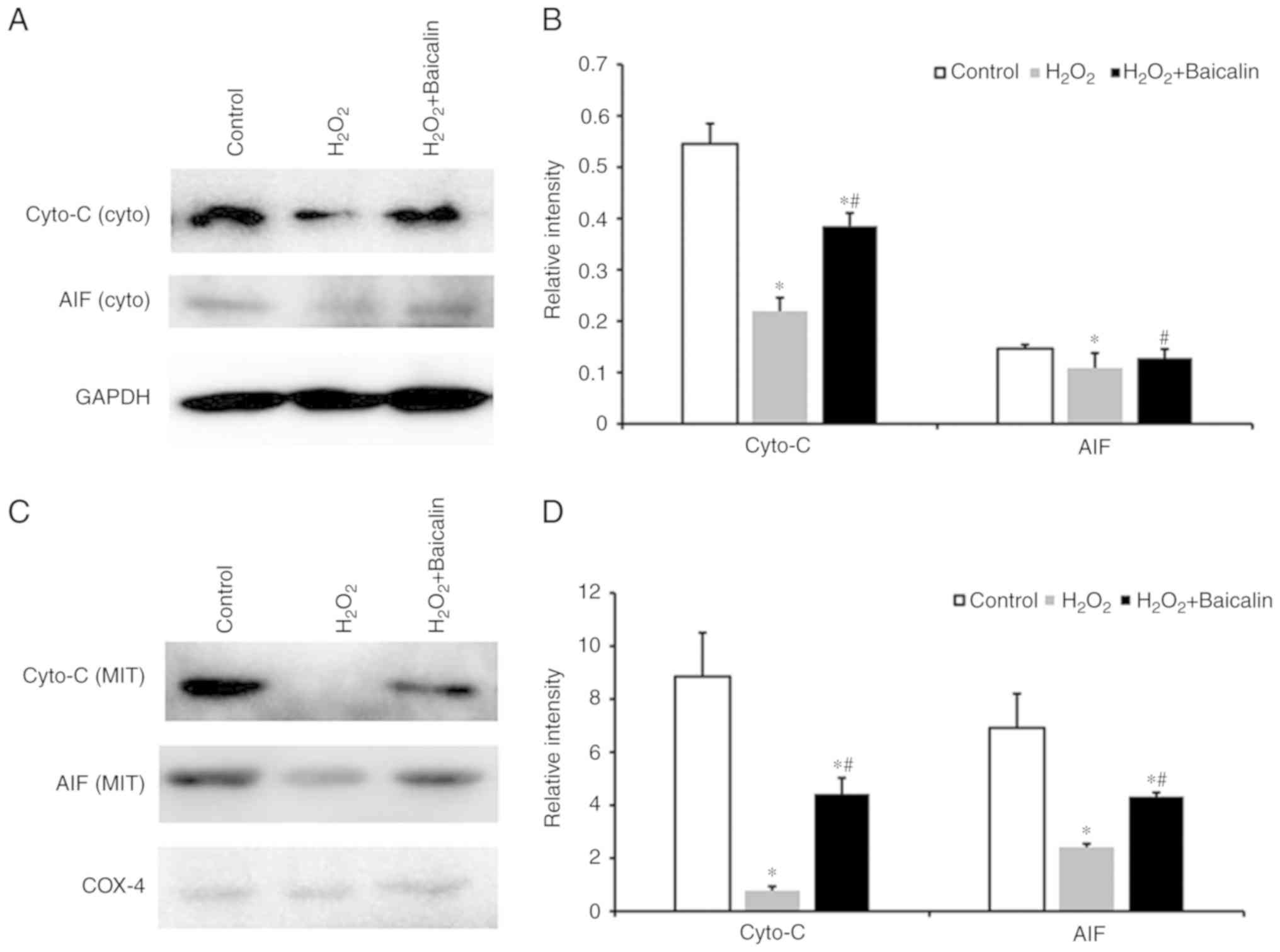 | Figure 5.Levels of cyto-C and AIF. (A) Western
blot of cyto-C and AIF from cytosolic fractions of C2C12 myoblasts
in the control group, the group treated with 500 µM
H2O2, and the group incubated with 500 µM
H2O2 that had been pre-treated with 100 µM
baicalin for 1 h. (B) Quantification of the western blotting
results of cyto-C and AIF levels from cytosolic fractions.
*P<0.05, compared with the control group; #P<0.05,
compared with the H2O2 group. (C) Western
blot of cyto-C and AIF from mitochondrial fractions of C2C12
myoblasts in the three aforementioned groups. (D) Quantification of
western blotting results of cyto-C and AIF levels from
mitochondrial fractions. *P<0.05, compared with the control
group; #P<0.05, compared with the
H2O2 group. cyto-C, cytochrome c
oxidase; AIF, apoptosis-inducing factor; GAPDH,
glyceraldehyde-3-phosphate dehydrogenase; cyto, cytosolic; MIT,
mitochondrial. |
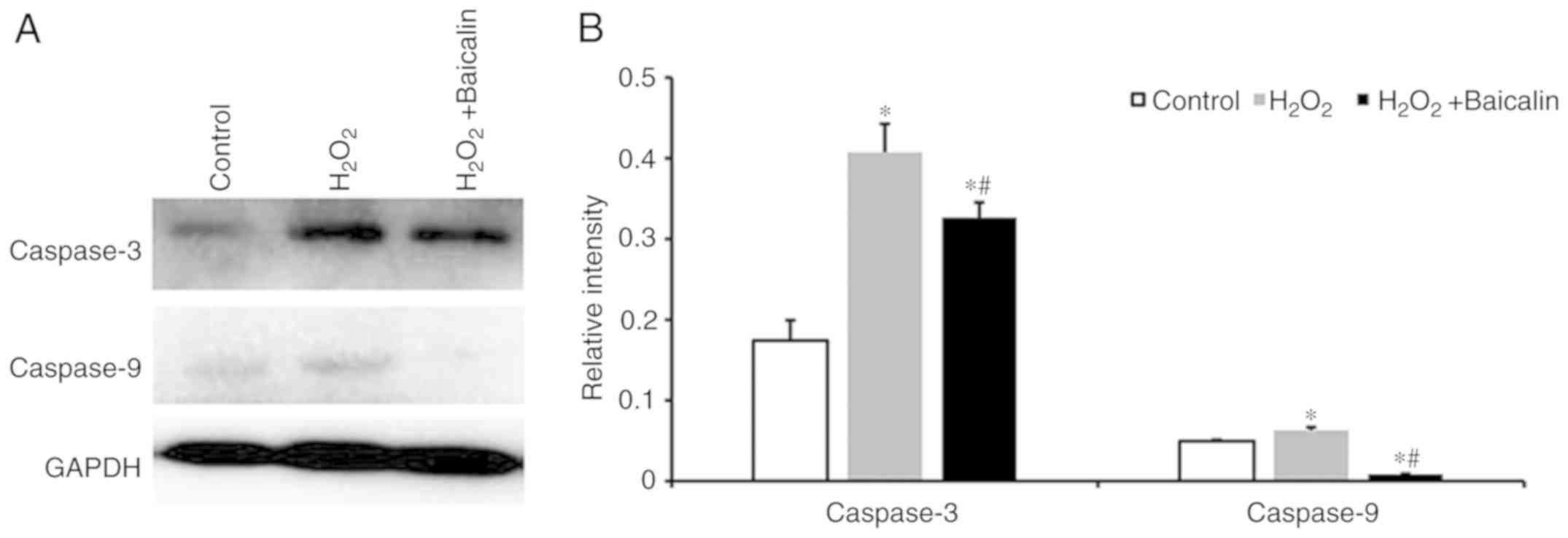
Baicalin inhibits the activation of
caspases in C2C12 myoblasts induced by
H2O2
As presented in Fig.
6A, the protein expression levels of the caspase-3 and
caspase-9 were identified following the incubation of C2C12
myoblasts with 500 µM H2O2 for 24 h, which
were markedly higher than those in the normal control group.
Pre-treatment with baicalin significantly downregulated the
expression of caspase-3 and caspase-9, as shown from the
quantification of western blotting results in Fig. 6B (P<0.05).
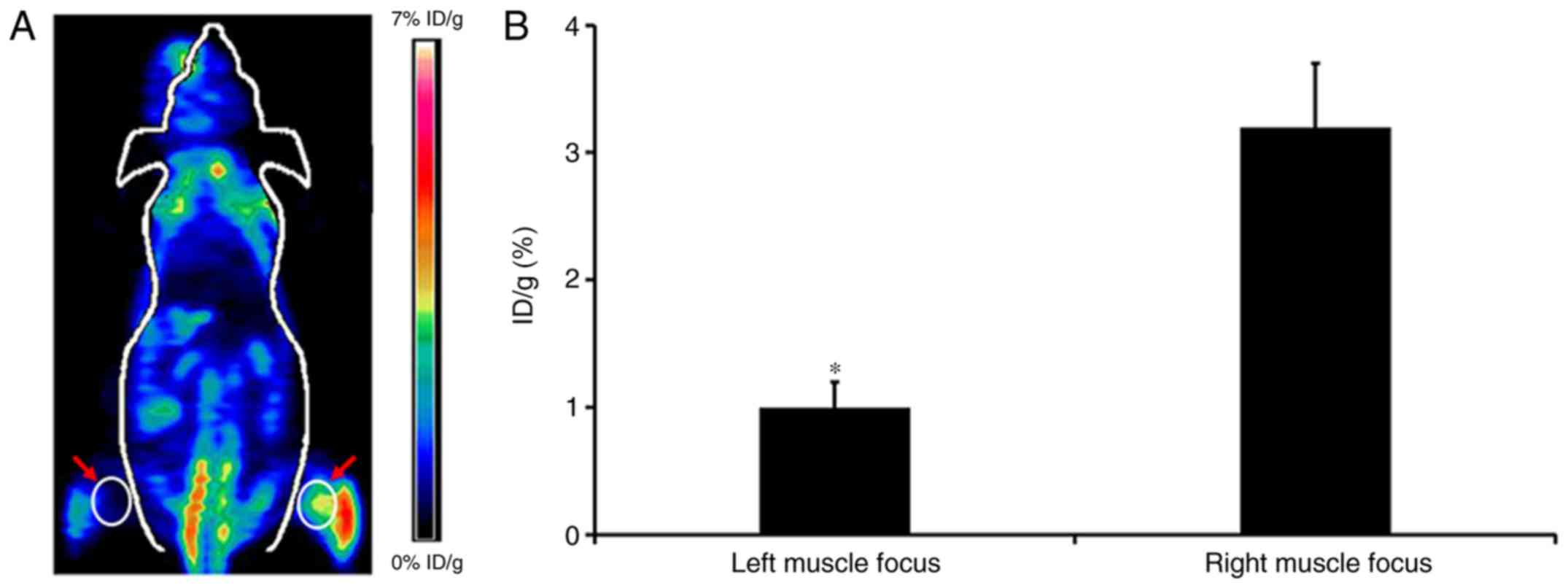
Small animal PET imaging of injured
skeletal muscle
As illustrated in Fig.
7A, a representative coronal small animal 18F-FDG
PET image of an animal model of skeletal muscle injury induced by
H2O2 is presented. The radioactivity uptake
in the muscle lesion of the right leg was observed at 1 h p.i.
However, there was no obvious FDG accumulation in the left leg
lesion. Further quantification analysis demonstrated that the
radioactivity uptake by the right and left muscle lesions were
3.20±0.52% ID/g and 1.09±0.22% ID/g, respectively, and the ratio of
right-to-left lesion was 2.94±0.25 (Fig. 7B). In addition, the physiological
radioactivity accumulation of normal muscle tissues was observed.
In the other examined normal organs and tissues, including the
brain, lung, heart, liver and kidneys, relatively low radioactivity
accumulation was also observed, and the majority were <2%
ID/g.
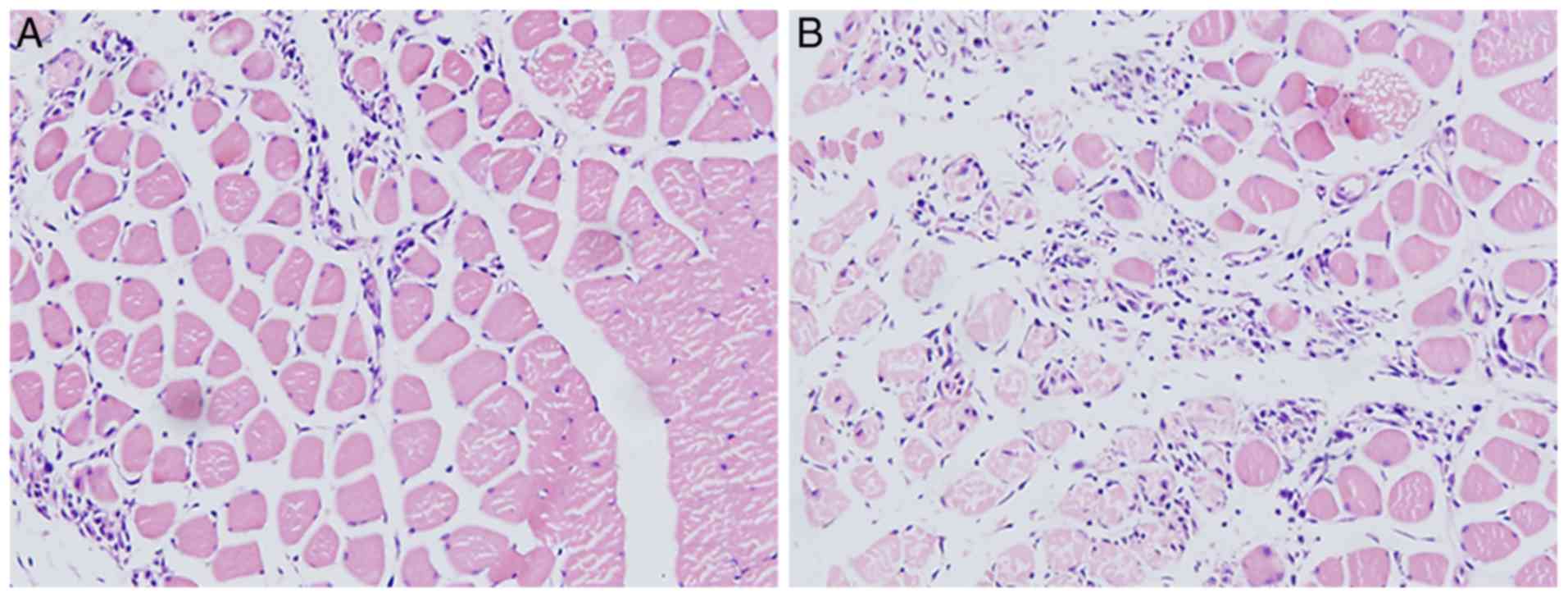
Pathological results
The H&E staining revealed that the muscle
tissues of the right leg were injured and infiltrated with masses
of inflammatory cells following the induction of
H2O2 (Fig.
8A, ×100 magnification). The muscle tissues of the left leg
exhibited a lesser degree of injury and less inflammatory cell
infiltration following pre-treatment with baicalin for 1 h
(Fig. 8B, ×100 magnification).
Discussion
As a flavonoid glycoside extracted from
Scutellaria baicalensis, a type of traditional Chinese
medicine, baicalin has been reported to possess significant
anti-inflammatory and anti-apoptotic properties, and is widely used
in the treatment of injuries and inflammatory diseases (7–12).
In addition, the results of our previous study confirmed the
protective effects of treatment with baicalin on
H2O2-induced apoptosis in endplate
chondrocytes (13). Therefore, the
aim of the present study was focused on the protective effects of
baicalin on H2O2-stimulated C2C12 myoblasts
in vitro and animal models with skeletal muscle injury in
vivo.
Initially, the present study confirmed the
protective effects of baicalin on the viability of C2C12 myoblasts
using a CCK-8 assay. H2O2 was observed to
decrease the viability of the C2C12 myoblasts, and the effects of
H2O2 on C2C12 myoblasts were dose-dependent.
With increased H2O2 dose, the viability of
the C2C12 myoblasts was significantly decreased. The potential
protective effects of baicalin against H2O2,
with an obvious increase in cell viability, were observed using the
CCK-8 assay, with baicalin pre-treatment for 1 h exhibiting an
optimal protective effect against the
H2O2-induced loss of C2C12 myoblasts. In
addition, the results under different concentrations of baicalin
pre-treatment were analyzed to identify an appropriate
concentration with the most effective protective effect; the
results revealed that 100 µM baicalin had the highest efficacy, and
was selected as a model dose for further in vitro and in
vivo experiments.
The present study subsequently observed that
pre-treatment with baicalin suppressed the activation of the
apoptotic C2C12 cell death pathway triggered by
H2O2, which was similar to the results
obtained in previous studies (7,8,13).
Decreased apoptosis was found to be associated with the suppression
of H2O2-stimulated oxidative activity in
C2C12 myoblasts by baicalin, via effectively reducing the levels of
ROS and MDA, which demonstrated that the protective effects of
baicalin on C2C12 myoblasts were associated with reduced oxidative
stress (15–17). Mitochondrial dysfunction was also
observed in C2C12 myoblasts following exposure to
H2O2, which was reflected by the loss of
mitochondrial activity (Rhodamine 123 staining) and ΔΨm (JC-1
staining). Baicalin also reversed the effect of
H2O2 and preserved mitochondrial function.
One of the important functions of the mitochondria is the
regulation of apoptosis (18). The
present study demonstrated that pre-treatment with baicalin
significantly upregulated the expression levels of cyto-C and AIF
from the cytosolic and mitochondrial fractions of C2C12 myoblasts
exposed to H2O2. In addition, caspase-3 and
caspase-9 are commonly accepted indicators of apoptosis (15,19),
and pre-treatment with baicalin in the present study downregulated
the activities of caspase-3 and caspase-9 in the C2C12 myoblasts
induced by H2O2 stimulation. In summary,
these pathophysiological processes demonstrated the protective
effects of baicalin on C2C12 myoblasts via inhibiting activation of
the intrinsic apoptotic pathway, which was consistent with
previously reported findings (13,18,20).
The present study further investigated the potential
protective role of baicalin in vivo on the basis of animal
models of skeletal muscle injury. Baicalin was pre-administered
intra-muscularly 1 h prior to H2O2 injection
at the same position, with the contralateral muscle receiving
H2O2 injection only. At present, numerous
imaging agents have been used for inflammation PET imaging,
including 18F-FDG, 68Ga-Citrate and
64CuCl2 (21–27).
18F-FDG is produced by the cyclotron, which is easy and
convenient to access, and has been widely used in a clinical
setting (27). Therefore, in the
present study, the animal models of skeletal muscle injury
underwent small animal 18F-FDG PET imaging, which
revealed that baicalin decreased ~65.9% of the FDG accumulation in
the skeletal muscle injury induced by H2O2.
In addition, baicalin inhibited and prevented the physiological
uptake in normal leg muscle. These pathological findings confirmed
the protective effect of baicalin on skeletal muscle injury.
Therefore, baicalin was confirmed to effectively protect against
the skeletal muscle injury induced by H2O2
in vivo.
However, the primary limitation of the present study
was the mechanism of baicalin-mediated protection against apoptosis
and inflammation, which was not fully elucidated and may be
associated with molecular signaling, including nuclear factor
erythroid 2-related factor 2 signaling, AMP-activated protein
kinase, peroxisome proliferator-activated receptor-γ, proteasome,
macrophages and notch signaling. These possibilities were not
examined in the present study, and warrant further investigation
(28–32).
In conclusion, the results of the present study
suggested that pre-treatment with baicalin has the potential to
protect myoblasts from apoptosis via decreasing the production of
ROS and MDA, preserving mitochondrial function and reversing
caspase protein expression, which was further verified by small
animal PET imaging and pathological analysis. These results
indicate the potential benefits of baicalin for patients with
skeletal muscle injury.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Science
Foundation for Young Scholars of China (grant no. 81601675 to
YP).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZL, YP and DS designed the study. YP, DS, WZ, XL and
HW carried out the experiments and collected the data. ZL, YP and
DS wrote and edited the manuscript.
Ethics approval and consent to
participate
All procedures were approved by the Animal Ethics
Committee at Shanghai East Hospital, Tongji University School of
Medicine.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Urso ML: Anti-inflammatory interventions
and skeletal muscle injury: Benefit or detriment. J Appl Physiol
(1985). 115:920–928. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bardouille C, Vullhorst D and Jockusch H:
Expression of chloride channel 1 mRNA in cultured myogenic cells: A
marker of myotube maturation. FEBS Lett. 396:177–180. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Smith C, Kruger MJ, Smith RM and Myburgh
KH: The inflammatory response to skeletal muscle injury:
Illuminating complexities. Sports Med. 38:947–969. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Arnold L, Henry A, Poron F, Baba-Amer Y,
van Rooijen N, Plonquet A, Gherardi RK and Chazaud B: Inflammatory
monocytes recruited after skeletal muscle injury switch into
antiinflammatory macrophages to support myogenesis. J Exp Med.
204:1057–1069. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Joulia D, Bernardi H, Garandel V,
Rabenoelina F, Vernus B and Cabello G: Mechanisms involved in the
inhibition of myoblast proliferation and differentiation by
myostatin. Exp Cell Res. 286:263–275. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Steffens AA, Hong GM and Bain LJ: Sodium
arsenite delays the differentiation of C2C12 mouse myoblast cells
and alters methylation patterns on the transcription factor
myogenin. Toxicol Appl Pharmacol. 250:154–161. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lin M, Li L, Zhang Y, Zheng L, Xu M, Rong
R and Zhu T: Baicalin ameliorates H2O2 induced cytotoxicity in HK-2
cells through the inhibition of ER stress and the activation of
Nrf2 signaling. Int J Mol Sci. 15:12507–12522. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lin M, Li L, Li L, Pokhrel G, Qi G, Rong R
and Zhu T: The protective effect of baicalin against renal
ischemia-reperfusion injury through inhibition of inflammation and
apoptosis. BMC Complement Alternat Med. 14:192014. View Article : Google Scholar
|
|
9
|
Cao Y, Mao X, Sun C, Zheng P, Gao J, Wang
X, Min D, Sun H, Xie N and Cai J: Baicalin attenuates global
cerebral ischemia/reperfusion injury in gerbils via anti-oxidative
and anti-apoptotic pathways. Brain Res Bull. 85:396–402. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhu J, Wang J, Sheng Y, Zou Y, Bo L, Wang
F, Lou J, Fan X, Bao R, Wu Y, et al: Baicalin improves survival in
a murine model of polymicrobial sepsis via suppressing inflammatory
response and lymphocyte apoptosis. PLoS One. 7:e355232012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xiping Z, Guanghua F, Jinxian H, Weihong
W, Rujun X, Wei Z, Jing Y, Qijun Y, Meijuan Y, Qing W and Lini F:
Baicalin protects thymus of rats with severe acute pancreatitis.
Inflammation. 33:157–165. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen X, Nishida H and Konishi T: Baicalin
promoted the repair of DNA single strand breakage caused by
H2O2 in cultured NIH3T3 fibroblasts. Biol
Pharm Bull. 26:282–284. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pan Y, Chen D, Lu Q, Liu L, Li X and Li Z:
Baicalin prevents the apoptosis of endplate chondrocytes by
inhibiting the oxidative stress induced by H2O2. Mol Med Rep.
16:2985–2991. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiang L, Kimura RH, Miao Z, Silverman AP,
Ren G, Liu H, Li P, Gambhir SS, Cochran JR and Cheng Z: Evaluation
of a (64)Cu-labeled cystine-knot peptide based on agouti-related
protein for PET of tumors expressing alphavbeta3 integrin. J Nucl
Med. 51:251–258. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mao CY, Lu HB, Kong N, Li JY, Liu M, Yang
CY and Yang P: Levocarnitine protects H9c2 rat cardiomyocytes from
H2O2-induced mitochondrial dysfunction and
apoptosis. Int J Med Sci. 11:1107–1115. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen S, Tang Y, Qian Y, Chen R, Zhang L,
Wo L and Chai H: Allicin prevents
H2O2-induced apoptosis of HUVECs by
inhibiting an oxidative stress pathway. BMC Complement Altern Med.
14:3212014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hsu PC and Guo YL: Antioxidant nutrients
and lead toxicity. Toxicology. 180:33–44. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu CY, Chiang RL, Chang TH, Liao CL and
Lin YL: The interferon stimulator mitochondrial antiviral signaling
protein facilitates cell death by disrupting the mitochondrial
membrane potential and by activating caspases. J Virol.
84:2421–2431. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shen J, Zhu Y, Huang K, Jiang H, Shi C,
Xiong X, Zhan R and Pan J: Buyang huanwu decoction attenuates
H2O2-induced apoptosis by inhibiting reactive oxygen
species-mediated mitochondrial dysfunction pathway in human
umbilical vein endothelial cells. BMC Complement Alternat Med.
16:1542016. View Article : Google Scholar
|
|
20
|
Xue HY, Niu DY, Gao GZ, Lin QY, Jin LJ and
Xu YP: Aucubin modulates Bcl-2 family proteins expression and
inhibits caspases cascade in H2O2-induced
PC12 cells. Mol Biol Rep. 38:3561–3567. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jiang L, Song D, Chen H, Zhang A, Wang H
and Cheng Z: Pilot Study of 64CuCl2 for PET
Imaging of Inflammation. Molecules. 23(pii): E5022018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xie F, Cai H and Peng F:
64CuCl2 PET/CT imaging of mouse muscular
injury induced by electroporation. Am J Nucl Med Mol Imaging.
7:33–39. 2017.PubMed/NCBI
|
|
23
|
Kumar V and Boddeti DK:
(68)Ga-radiopharmaceuticals for PET imaging of infection and
inflammation. Recent Results Cancer Res. 194:189–219. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rudd JH, Myers KS, Bansilal S, Machac J,
Pinto CA, Tong C, Rafique A, Hargeaves R, Farkouh M, Fuster V and
Fayad ZA: Atherosclerosis inflammation imaging with 18F-FDG PET:
Carotid, iliac, and femoral uptake reproducibility, quantification
methods, and recommendations. J Nucl Med. 49:871–878. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pellegrino D, Bonab AA, Dragotakes SC,
Pitman JT, Mariani G and Carter EA: Inflammation and infection:
Imaging properties of 18F-FDG-labeled white blood cells versus
18F-FDG. J Nucl Med. 46:1522–1530. 2005.PubMed/NCBI
|
|
26
|
de Prost N, Tucci MR and Melo MF:
Assessment of lung inflammation with 18F-FDG PET during acute lung
injury. AJR Am J Roentgenol. 195:292–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kobayashi Y, Ishii K, Oda K, Nariai T,
Tanaka Y, Ishiwata K and Numano F: Aortic wall inflammation due to
Takayasu arteritis imaged with 18F-FDG PET coregistered with
enhanced CT. J Nucl Med. 46:917–922. 2005.PubMed/NCBI
|
|
28
|
Ma Y, Yang F, Wang Y, Du Z, Liu D, Guo H,
Shen J and Peng H: CaMKKβ is involved in AMP-activated protein
kinase activation by baicalin in LKB1 deficient cell lines. PLoS
One. 7:e479002012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lim HA, Lee EK, Kim JM, Park MH, Kim DH,
Choi YJ, Ha YM, Yoon JH, Choi JS, Yu BP and Chung HY: PPARγ
activation by baicalin suppresses NF-κB-mediated inflammation in
aged rat kidney. Biogerontology. 13:133–145. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu YX, Sato E, Kimura W and Miura N:
Baicalin and scutellarin are proteasome inhibitors that
specifically target chymotrypsin-like catalytic activity. Phytother
Res. 27:1362–1367. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu LL, Gong LK, Wang H, Xiao Y, Wu XF,
Zhang YH, Xue X, Qi XM and Ren J: Baicalin inhibits macrophage
activation by lipopolysaccharide and protects mice from endotoxin
shock. Biochem Pharmacol. 75:914–922. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang AM, Ku HH, Liang YC, Chen YC, Hwu YM
and Yeh TS: The autonomous notch signal pathway is activated by
baicalin and baicalein but is suppressed by niclosamide in K562
cells. J Cell Biochem. 106:682–692. 2009. View Article : Google Scholar : PubMed/NCBI
|