Introduction
Ectopic pregnancy is a complication of pregnancy in
which the gestational sac attaches outside the uterus (1). The reported rate of ectopic pregnancy
is approximately 2% and has significantly increased over the past
few decades (2–4). Tubal pregnancy is the most common
form of ectopic pregnancy. Vaginal bleeding and abdominal pain are
classic signs and symptoms of ectopic pregnancy, which may cause
maternal morbidity and accidental mortality in early pregnancy
(5–7). Although the spontaneous shedding of
ectopic pregnancy may occur, patients are still at risk of
catastrophic hemorrhage and tubal rupture (8). In addition, women with ectopic
pregnancy have a higher risk for another ectopic pregnancy or
subfertility (9). Although the
frequency of this severe situation is relatively high, the early
detection of ectopic pregnancy remains challenging (10). More than half of all women with
ectopic pregnancy who present at the emergency department are not
identified at the initial medical assessment (5,11).
Thus, during the early gestational period, obtaining a correct
diagnosis is rather difficult.
Pregnancy is diagnosed by measuring the serum or
urine concentration of β human chorionic gonadotropin (β-hCG) in
the emergency department (12). As
early as 1 week following conception, β-hCG can be detected in the
blood and urine (13). However,
the detection of the β-hCG concentration alone cannot identify the
exact location of the gestational sac (14). It has been observed that the serum
concentration of progesterone may be a potentially useful co-factor
for the measurement of serum β-hCG levels since progesterone levels
are stable and independent of gestational age in the first 3 months
(15). Cancer antigen-125 (CA-125)
is generally considered a biomarker of ovarian cancer (16). However, in benign conditions, such
as endometriosis, where inflammation or peritoneal irritation are
present, CA-125 levels are also increased (17). The serum CA-125 level has been
reported to be increased in women with viable intrauterine
pregnancies (18). In addition,
women with ectopic pregnancies have a wide range of CA-125
concentrations, although overall, the concentrations are lower
compared to those in women with intrauterine pregnancies (18).
As opposed to medical surgery, the use of
methotrexate (MTX), an anti-folate antagonist, has been
progressively administered for the treatment of ectopic pregnancy
(19). Methotrexate acts as a
competitive inhibitor of folinic acid and blocks DNA and RNA
synthesis by inhibiting folinic acid incorporation into thymidine
and purines (20). Notably,
trophoblast tissues, which possess the ability to rapidly divide,
are exceedingly sensitive to MTX treatment (21).
In this study, we investigated the early diagnosis
and treatment of patients with ectopic pregnancy. The pathological
changes in patients with ectopic pregnancy were analyzed by
detecting abnormal indexes, such as those of progesterone, β-hCG
and CA-125 along with the changes in immune cells. To explore the
efficacy of MTX, we treated patients with MTX to terminate
embryonic development and detected the gestational size by
transvaginal ultrasound. The early diagnosis of ectopic pregnancy
and the selection of appropriate treatment methods are crucial for
reducing the mortality of pregnant women and for improving future
pregnancy rates of patients with ectopic pregnancy.
Materials and methods
Patients
From September, 2014 to April, 2017, 100 women with
ectopic pregnancy and 100 women with a normal, early pregnancy were
recruited in this study. Women in both groups were in the early
stage of gestation, and no significant differences were observed as
regards the gestational age. The gestational age was determined by
the first day of the last menstrual period. Of the 100 patients
with ectopic pregnancy, 52 patients underwent surgery and 48
patients received medication. Patients with ectopic pregnancy who
underwent surgery were examined for pathological damage of the
fallopian tubes and villus tissue during the surgery. Another 48
patients received medication and were monitored by vaginal
ultrasound. None of the women with ectopic pregnancy who were
involved in this study had any abnormalities such as those in the
ovary and cornea, or a caesarean section scar. Written consents
were obtained from all the participants. The Ethics Committee of
The Second Hospital of Hebei Medical University approved all the
experiments in this study.
Chemiluminescent enzyme
immunoassays
The serum levels of progesterone, β-HCG and CA125
were examined by solid-phase, competitive binding chemiluminescent
enzyme immunoassays (Siemens Immulite 2000) as previously described
(22).
Flow cytometry
Peripheral blood was collected from the patients,
and peripheral blood mononuclear cells (PBMCs) were isolated using
Ficoll reagent (Sigma-Aldrich; Merck KGaA). PBMCs were carefully
washed in PBS and were subsequently stained with a fixable
viability dye. After washing, the cells were labeled with an
anti-CD3 antibody conjugated to a fluorescent dye (1:1,000; cat.
no. 48-0032-82; eBioscience; Thermo Fisher Scientific, Inc.) for 30
min at 4°C. A BD FACSVerse flow cytometer was used to quantify the
labeled cells, and FlowJo Software (BD Biosciences) was used to
analyze the collected data.
Histopathological examination
For patients with ectopic pregnancy undergoing
salpingectomy, we collected oviduct tissue for hematoxylin and
eosin (H&E; Sigma-Aldrich; Merck KGaA) staining during surgery.
H&E staining was performed according to standard protocols and
was used for the pathological diagnosis of ectopic pregnancy
(original magnification, ×100) (23).
Statistical analysis
All data are presented as the means ± SD.
Differences between samples were analyzed using a two-tailed
Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
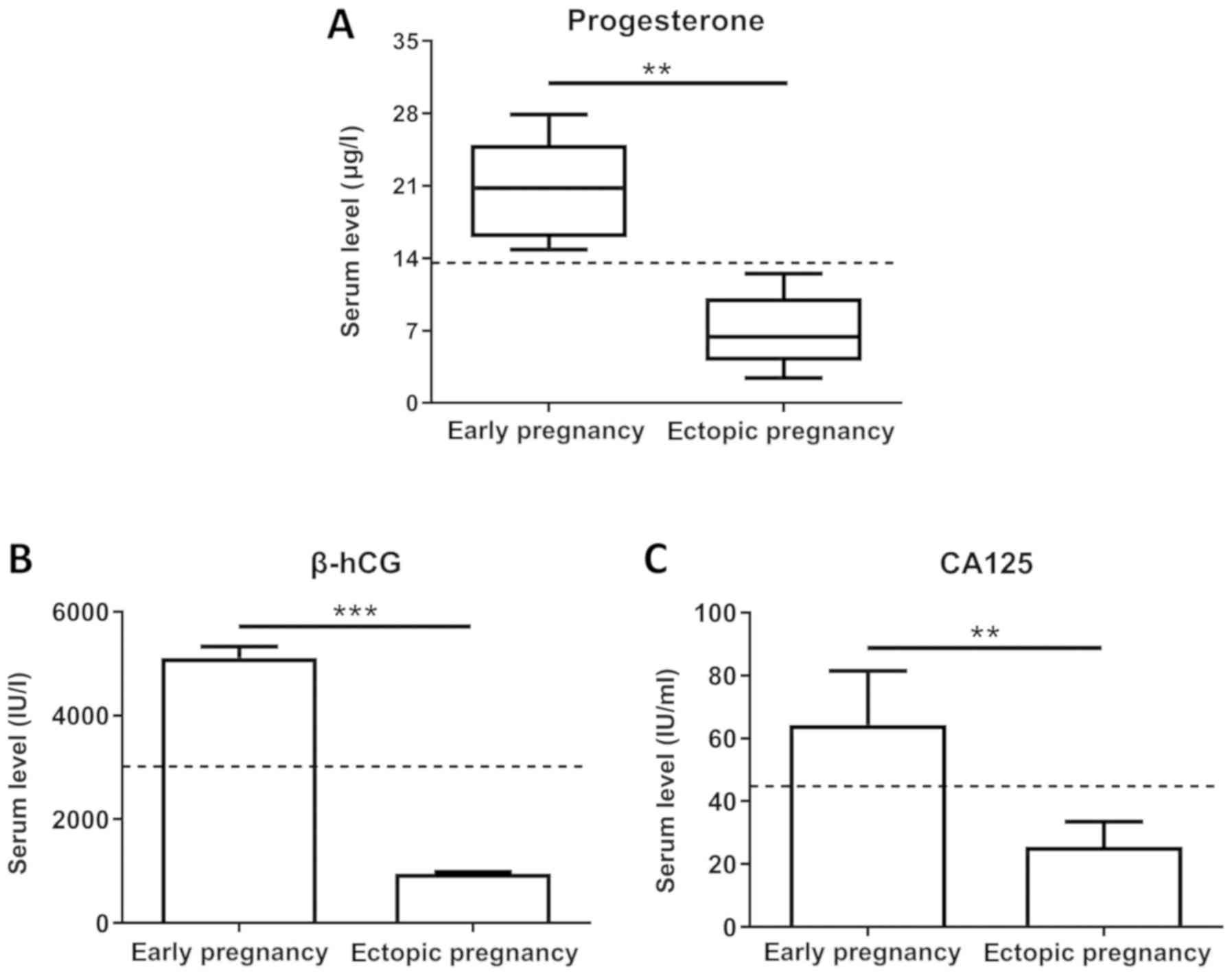
Serum levels of progesterone, β-HCG
and CA125 are significantly decreased in women with ectopic
pregnancy
For this study, we recruited 100 women with ectopic
pregnancy along with 100 women with normal early pregnancy as
controls from September, 2014 through April, 2017. Women in both
groups were in the early stage of gestation, and no significant
differences were observed as regards the gestational age, which was
determined by the first day of the last menstrual period. The serum
concentrations of progesterone, β-hCG and CA125 were assessed using
solid-phase, competitive binding chemiluminescent enzyme
immunoassays. As shown in Fig. 1A and
C, women with ectopic pregnancies expressed lower progesterone
and CA125 levels than those with normal early pregnancies
(P<0.01). In addition, the serum β-hCG levels were significantly
decreased in women with ectopic pregnancy (P<0.001) compared
with women with normal early pregnancy, as depicted in Fig. 1B.
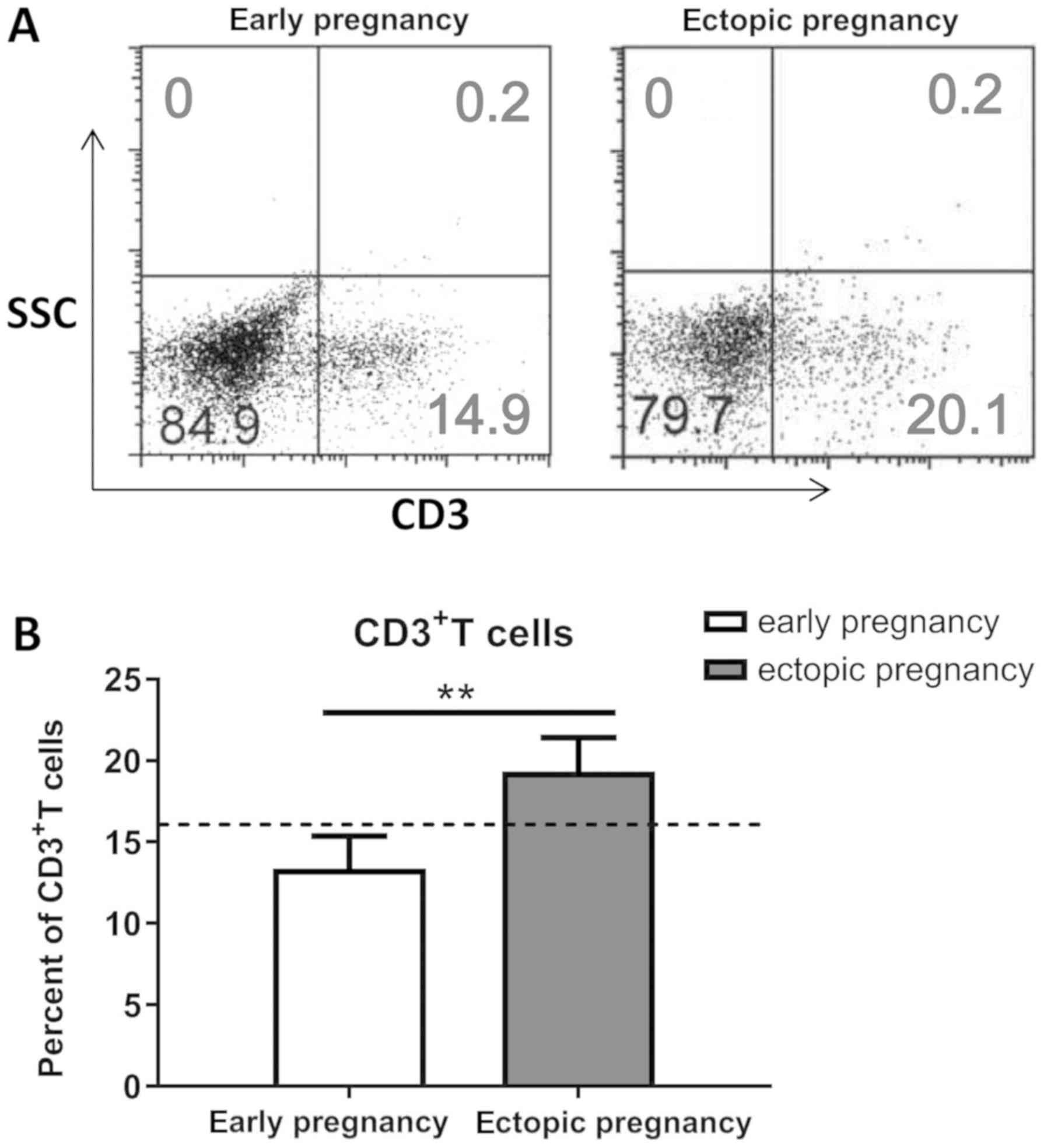
The percentage of CD3+ T
cells is increased in women with ectopic pregnancy
The expression of CD3 throughout all stages of T
cell development indicates that it is a useful marker for T cells.
Thus, in this study, we performed flow cytometry to isolate
CD3+ T cells from the peripheral blood of women with
ectopic pregnancy and women with a normal early pregnancy (Fig. 2A). As shown in Fig. 1B, the percentage of CD3+
T cells was significantly higher in women with ectopic pregnancy
than in women with normal early pregnancy (P<0.01).
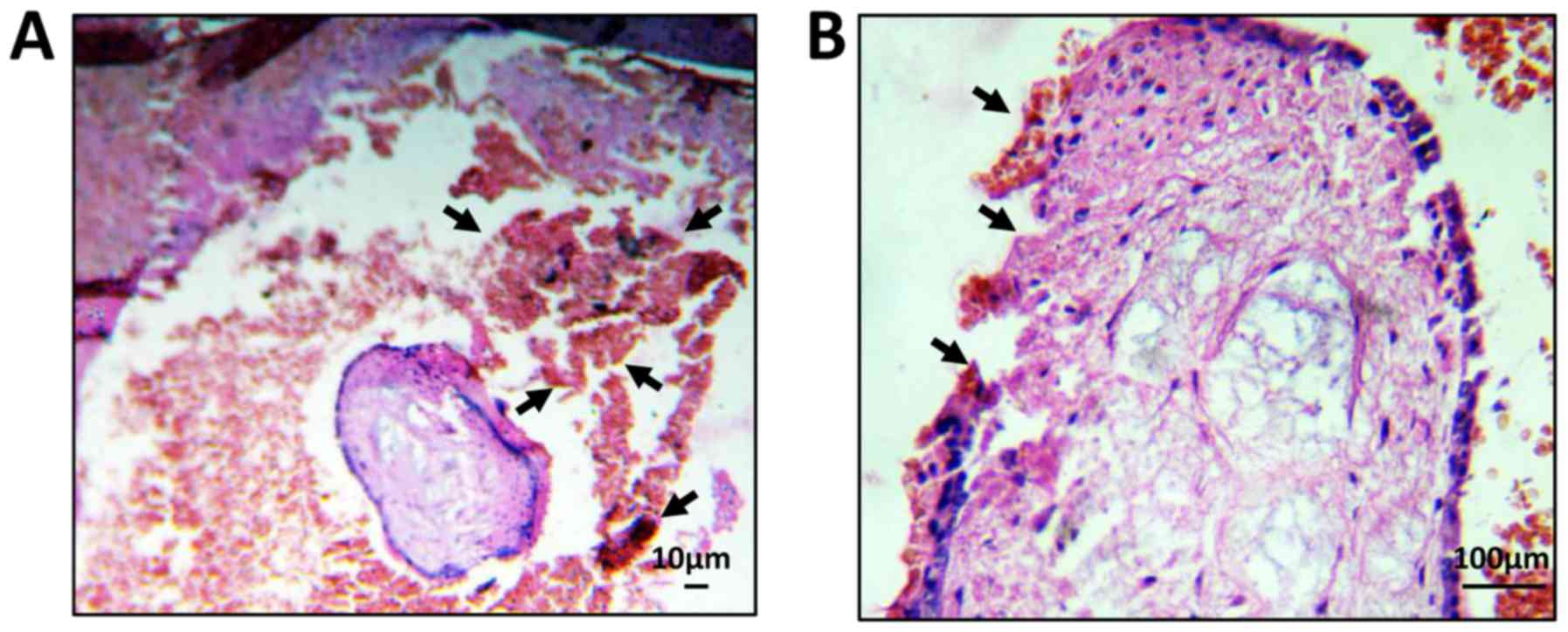
Histopathological examination of
ectopic pregnancy
The degree of pathological damage to the fallopian
tubes and villi in patients with ectopic pregnancy was determined
by H&E staining. As shown in Fig.
3A, blood clots were observed around the tubal mass, and
epithelial tissue fragments had been shed from the fallopian tube.
In addition, the structure of the fallopian tube was not complete
in women with ectopic pregnancy (Fig.
3B). These data suggested that the increase in peripheral
CD3+ T cells in patients with ectopic pregnancy promoted
the occurrence of inflammation, which may cause pathological damage
to fallopian tubes and villi.
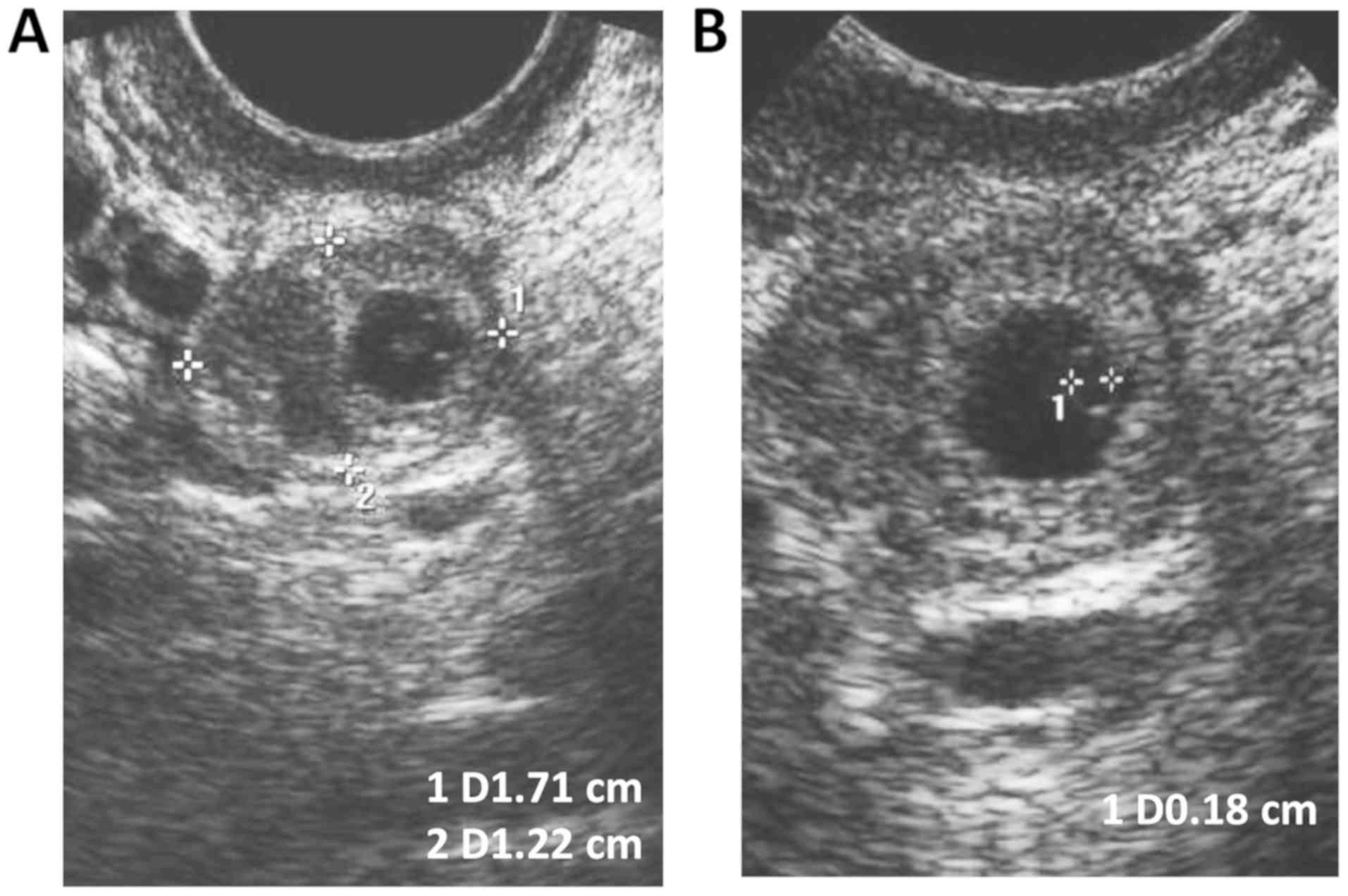
Transvaginal ultrasound images after
MTX injection
To terminate the abnormal pregnancy, the patients
were subjected to a single intramuscular injection of MTX (50
mg/m2). Transvaginal ultrasound revealed that the size
of the echogenic mass was 1.7×1.2×1.6 cm and included an inner
gestational sac-like structure and a 0.2-cm-sized yolk sac
(Fig. 4). These data further
confirmed that MTX administration was an efficacious approach for
the treatment of patients with ectopic pregnancy.
Discussion
Ectopic pregnancy is a serious obstetrical disease
that can be fatal in the first trimester of pregnancy (24). The most common complication is
hypovolemic shock, which is caused by tubal rupture and subsequent
internal bleeding (25). Death
from tubal rupture still ranks as the leading cause of mortality
among women in their first trimester of pregnancy (26). Therefore, early diagnosis and
timely treatment are essential for women with ectopic pregnancy
(27,28). Previous studies have revealed that
blood-based biomarkers can be used to identify an ectopic pregnancy
when ultrasound cannot determine the exact location of the embryo,
which would contribute to an early diagnosis (29). In this study, we explored several
biomarkers that can be used to diagnose ectopic pregnancy; these
biomarkers may be crucial for early definitive treatment and
management.
Pregnancy is initially diagnosed based on the serum
or urine β-hCG concentration (30). However, the measured β-hCG
concentration alone cannot identify the exact location of the
gestational sac (31,32). Although the β-hCG concentration in
women with ectopic pregnancy tends to be lower compared to that in
women with an intrauterine pregnancy, a considerable overlap has
been found (33). Thus, a low
level of serum β-hCG (<1,000 IU/l) is commonly associated with a
higher probability of ectopic pregnancy, whereas a single very low
serum β-hCG level (<100 IU/l) cannot predict a benign clinical
condition (34). Although a single
very low level (<100 IU/l) may not indicate an adverse outcome,
in a review of 716 confirmed cases of ectopic pregnancy, 208 of
those with a very low level experienced tubal rupture according to
laparoscopy (35). In addition,
the risk of tubal rupture does not seem to be associated with the
β-hCG level (36). Another study
found that the serum β-hCG levels ranged from 10 to 189,720 IU/l in
38 women with tubal rupture (37).
Thus, the concentration of serum β-hCG alone cannot eliminate the
possibility of ectopic pregnancy or predict the risk of fallopian
tube rupture.
Progesterone has been long considered an effective
marker that distinguishes a normal early pregnancy (higher levels)
from an ectopic pregnancy or miscarriage (lower levels in both)
(38). Since the serum
concentrations of progesterone are stable and unrelated to
gestational age in the first trimester, progesterone has been
considered an effective adjunct to serum β-hCG in early pregnancy
(39). Actually, any possible
method (if any) used to diagnose ectopic pregnancy may be used in
conjunction with other biomarkers (40).
CA-125 is a typical biomarker indicative of ovarian
cancer (41). Its levels are also
frequently elevated in non-malignant conditions, including pelvic
inflammatory disease, leiomyoma, endometriosis and pregnancy
(42). In one study, the CA-125
levels were found to be increased in the first trimester of
pregnancy and immediately postpartum (43). It has also been reported that
CA-125 can be used as an adjunct to β-hCG to distinguish between
fallopian tube abortion and viable ectopic pregnancy (44). Others have found that women with
normal early pregnancy, hydatidiform mole and spontaneous abortion
expressed high levels of serum CA-125; however, lower levels were
observed in women with tubal pregnancy, particularly in the absence
of uterine bleeding (45). The
peak CA-125 level is typically observed in the maternal serum in
early pregnancy and immediately postpartum, and this transient
increase in CA-125 may be caused by the destruction of decidual
tissues (46). Brumsted et
al (17), reported that CA-125
levels were relatively lower in women with abnormal pregnancies
compared with those with normal intrauterine pregnancies and that
this led to a greater risk of bleeding. Thus, CA-125 may be
clinically relevant in early pregnancy surveillance.
MTX has been used as a medical alternative to
surgery for women with ectopic pregnancy since the 1990s (47). Previous studies demonstrated that
MTX had a success rate of approximately 94% when administered to
the appropriate patients (48).
The success rate of MTX treatment for ectopic pregnancy is largely
dependent on the β-hCG concentration (49). A meta-analysis revealed that MTX
treatment decreased the serum β-hCG level, which indicates that
increasing levels of β-hCG are significantly associated with
treatment failure (50).
Furthermore, MTX is more effective when administered to small
ectopic pregnancies compared with large ectopic pregnancies
(51).
Multiple-dose MTX treatment has an overall success
rate of 93%, whereas single-dose MTX has a lower rate of 88%
(52). However, multiple-dose
therapy is more expensive, has a higher rate of side effects, and
requires more intensive and continuous monitoring and rescue with
folinic acid (53). Moreover,
single-dose therapy is actually efficacious in most cases of
ectopic pregnancy. However, multiple-dose MTX treatment should be
considered in cases of relative contraindications, including the
presence of fetal cardiac activity and high serum β-hCG
concentration (≥5,000 IU/l) (54).
A recent systematic review and meta-analysis revealed that the
incidence of side effects in the two-dose regimen was similar to
that of the single-dose regimen (54). However, side-effects are more
common in multiple-dose regimens (54). This meta-analysis revealed that the
incidence of side-effects due to the multi-dose regimen was
significantly higher than that due to the single-dose regimen; both
regimens had a similar success rate (54). Therefore, the dual-dose regimen is
an effective and safe alternative to the single-dose regimen
(54). Recently, new research has
been conducted to try to establish a safer solution with similar
efficacy and minimal side effects. To this end, some researchers
have developed clinical trials of combining MTX with other drugs
(55).
Notably, patients treated with MTX should be paid
close attention, and serum β-hCG levels should be examined once a
week. Three or four days after MTX administration, the serum β-hCG
level should be decreased, and thus an increased level is abnormal
(56). A second dose of MTX should
be administered if the serum β-hCG level has not decreased by at
least twenty-five percent one week after the first MTX treatment
(57). Overall, a second dose is
required in 15 to 20% of patients, and only 1% of patients require
more than two doses (58). MTX
treatment may cause hematoma formation, which may result in
abdominal pain from tubal distention or abortion in patients
(59,60). However, severe abdominal pain may
be necessary for fallopian tube rupture that is imminent or that
has already begun. A rupture can occur even when the β-hCG levels
continue to decrease after MTX treatment (55). The predictors of MTX treatment
failure include the embryonic cardiac activity, the size and volume
of the gestational mass (>4 cm), the presence of free peritoneal
blood, the rapidly increasing β-hCG titers before methotrexate
(>50%/48 h), and the high initial β-hCG level (>5,000 mUI/ml)
(55,61).
In conclusion, in this study, we investigated the
early diagnosis and treatment of patients with ectopic pregnancy.
Through joint detection of serum progesterone, β-hCG, and CA125
levels, as well as the changes in the proportion of immune cells,
ectopic pregnancy could be diagnosed early. We then applied a MTX
intervention in patients with ectopic pregnancy to terminate their
embryonic development, and transvaginal ultrasound determination of
gestational size demonstrated that MTX exhibited a viable clinical
efficacy in patients with ectopic pregnancy. This article provides
evidence for the early diagnosis of ectopic pregnancy and the
selection of appropriate treatment methods, which may be of
clinical significance for reducing the mortality of pregnant women
and improving the future pregnancy rate of patients with ectopic
pregnancy.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HL, YL, SW and HJ conducted the experiments. HL and
YL analyzed the data. HL and YD wrote the paper, Y.D conceived the
study.
Ethics approval and consent to
participate
Written consents were obtained from all the
participants. The Ethics Committee of The Second Hospital of Hebei
Medical University approved all the experiments in this study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
β-hCG
|
β human chorionic gonadotropin
|
|
CA-125
|
cancer antigen-125
|
|
MTX
|
methotrexate
|
References
|
1
|
Alkatout I, Honemeyer U, Strauss A,
Tinelli A, Malvasi A, Jonat W, Mettler L and Schollmeyer T:
Clinical diagnosis and treatment of ectopic pregnancy. Obstet
Gynecol Surv. 68:571–581. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bronson R: Ectopic pregnancy-still a
challenge. Fertil Steril. 110:1265–1266. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Buster JE and Carson SA: Ectopic
pregnancy: New advances in diagnosis and treatment. Curr Opin
Obstet Gynecol. 7:168–176. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ma S, Xiang Y and Yang X: Diagnosis and
treatment of ectopic molar pregnancy. Zhonghua Fu Chan Ke Za Zhi.
36:618–620. 2001.(In Chinese). PubMed/NCBI
|
|
5
|
Taran FA, Kagan KO, Hubner M, Hoopmann M,
Wallwiener D and Brucker S: The diagnosis and treatment of ectopic
pregnancy. Dtsch Arztebl Int. 112:693–705. 2015.PubMed/NCBI
|
|
6
|
Dai Z: Diagnosis and treatment of ectopic
pregnancy should be strengthened. Zhonghua Yi Xue Za Zhi.
77:403–404. 1997.(In Chinese). PubMed/NCBI
|
|
7
|
Murray H, Baakdah H, Bardell T and Tulandi
T: Diagnosis and treatment of ectopic pregnancy. CMAJ. 173:905–912.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Epee-Bekima M and Overton C: Diagnosis and
treatment of ectopic pregnancy. Practitioner. 257:15–17, 22.
2013.PubMed/NCBI
|
|
9
|
Walid MS and Heaton RL: Diagnosis and
laparoscopic treatment of cornual ectopic pregnancy. Ger Med Sci.
8(pii): Doc162010.PubMed/NCBI
|
|
10
|
Jiang W, Lv S, Sun L, Singer G, Xu C and
Lu X: Diagnosis and treatment of retroperitoneal ectopic pregnancy:
Review of the literature. Gynecol Obstet Invest. 77:205–210. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kirk E, Bottomley C and Bourne T:
Diagnosing ectopic pregnancy and current concepts in the management
of pregnancy of unknown location. Hum Reprod Update. 20:250–261.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kaplan BC, Dart RG, Moskos M, Kuligowska
E, Chun B, Adel Hamid M, Northern K, Schmidt J and Kharwadkar A:
Ectopic pregnancy: Prospective study with improved diagnostic
accuracy. Ann Emerg Med. 28:10–17. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mol BW and Van der Veen F: A study of
ruptured tubal ectopic pregnancy. Obstet Gynecol. 90:866–867. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kohn MA, Kerr K, Malkevich D, O'Neil N,
Kerr MJ and Kaplan BC: Beta-human chorionic gonadotropin levels and
the likelihood of ectopic pregnancy in emergency department
patients with abdominal pain or vaginal bleeding. Acad Emerg Med.
10:119–126. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Barnhart KT, Sammel MD, Rinaudo PF, Zhou
L, Hummel AC and Guo W: Symptomatic patients with an early viable
intrauterine pregnancy: HCG curves redefined. Obstet Gynecol.
104:50–55. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Iwanari O, Miyako J, Date Y, Nakayama S,
Kijima S, Moriyama M, Takahashi K, Yoshino N, Karino K, Endoh J, et
al: Differential diagnosis of ovarian cancer, benign ovarian tumor
and endometriosis by a combination assay of serum sialyl SSEA-1
antigen and CA125 levels. Gynecol Obstet Invest. 29:71–74. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brumsted JR, Nakajima ST, Badger G,
Riddick DH and Gibson M: Serum concentration of CA-125 during the
first trimester of normal and abnormal pregnancies. J Reprod Med.
35:499–502. 1990.PubMed/NCBI
|
|
18
|
Sadovsky Y, Pineda J and Collins JL: Serum
CA-125 levels in women with ectopic and intrauterine pregnancies. J
Reprod Med. 36:875–878. 1991.PubMed/NCBI
|
|
19
|
Yao M and Tulandi T: Current status of
surgical and nonsurgical management of ectopic pregnancy. Fertil
Steril. 67:421–433. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Barnhart KT, Gosman G, Ashby R and Sammel
M: The medical management of ectopic pregnancy: A meta-analysis
comparing ‘single dose’ and ‘multidose’ regimens. Obstet Gynecol.
101:778–784. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lipscomb GH, Bran D, McCord ML, Portera JC
and Ling FW: Analysis of three hundred fifteen ectopic pregnancies
treated with single-dose methotrexate. Am J Obstet Gynecol.
178:1354–1358. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fu X, Meng M, Zhang Y, Yin Y, Zhang X and
Xi R: Chemiluminescence enzyme immunoassay using magnetic
nanoparticles for detection of neuron specific enolase in human
serum. Anal Chim Acta. 722:114–118. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Feldman AT and Wolfe D: Tissue processing
and hematoxylin and eosin staining. Methods Mol Biol. 1180:31–43.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Escobar-Padilla B, Perez-López CA and
Martinez-Puon H: Risk factors and clinical features of ectopic
pregnancy. Rev Med Inst Mex Seguro Soc. 55:278–285. 2017.(In
Spanish). PubMed/NCBI
|
|
25
|
Diagnosi s and management of ectopic
pregnancy: Green-top Guideline no. 21. BJOG. 123:e15–e55. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Alsunaidi M: Incidence of ectopic
pregnancy after assisted reproduction treatment. Saudi Med J.
28:590–592. 2007.PubMed/NCBI
|
|
27
|
Strandell A, Thorburn J and Hamberger L:
Risk factors for ectopic pregnancy in assisted reproduction. Fertil
Steril. 71:282–286. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bird S: Failure to diagnose: Ectopic
pregnancy. Aust Fam Physician. 34:175–176. 2005.PubMed/NCBI
|
|
29
|
Horne AW, Duncan WC and Critchley HO: The
need for serum biomarker development for diagnosing and excluding
tubal ectopic pregnancy. Acta Obstet Gynecol Scand. 89:299–301.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Surampudi K and Gundabattula SR: The role
of serum Beta hCG in early diagnosis and management strategy of
ectopic pregnancy. J Clin Diagn Res. 10:QC08–QC10. 2016.PubMed/NCBI
|
|
31
|
Jacobson ED: Ectopic pregnancy and hCG. S
Afr Med J. 57:887–888. 1980.(In Afrikaans). PubMed/NCBI
|
|
32
|
Abdul-Hussein MM, Abdul-Rasheed OF and
Al-Moayed HA: The values of CA-125, Progesterone, β-HCG and
estradiol in the early prediction of ectopic pregnancy. Oman Med J.
27:124–128. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Alhamdan D, Bignardi T, Casikar I, Riemke
J and Condous G: Pre-treatment human chorionic gonadotrophin (hCG)
ratio in the management of non-tubal ectopic pregnancy. Ceylon Med
J. 56:70–71. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ahlgren M, Friberg B and Sjöholm A:
Diagnosis of suspected ectopic pregnancy using an immunoenzyme
method for urinary HCG. Lakartidningen. 84:760–763. 1987.(In
Swedish). PubMed/NCBI
|
|
35
|
Buck RH, Pather N, Moodley J, Joubert SM
and Norman RJ: Bedside application of an ultrasensitive urine test
for HCG in patients with suspected ectopic pregnancy. Ann Clin
Biochem. 24:268–272. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cacciatore B, Stenman UH and Ylöstalo P:
Diagnosis of ectopic pregnancy by vaginal ultrasonography in
combination with a discriminatory serum hCG level of 1000 IU/l
(IRP). Br J Obstet Gynaecol. 97:904–908. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nyberg DA, Filly RA, Laing FC, Mack LA and
Zarutskie PW: Ectopic pregnancy: Diagnosis by sonography correlated
with quantitative HCG levels. J Ultrasound Med. 6:145–150. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bustillo M, Stern JJ, King D and Coulam
CB: Serum progesterone and estradiol concentrations in the early
diagnosis of ectopic pregnancy after in vitro fertilization-embryo
transfer. Fertil Steril. 59:668–670. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Buck RH, Joubert SM and Norman RJ: Serum
progesterone in the diagnosis of ectopic pregnancy: A valuable
diagnostic test? Fertil Steril. 50:752–755. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gelder MS, Boots LR and Younger JB: Use of
a single random serum progesterone value as a diagnostic aid for
ectopic pregnancy. Fertil Steril. 55:497–500. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Schmidt T, Rein DT, Foth D, Eibach HW,
Kurbacher CM, Mallmann P and Römer T: Prognostic value of repeated
serum CA 125 measurements in first trimester pregnancy. Eur J
Obstet Gynecol Reprod Biol. 97:168–173. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Predanic M: Differentiating tubal abortion
from viable ectopic pregnancy with serum CA-125 and beta-human
chorionic gonadotropin determinations. Fertil Steril. 73:522–525.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sauer MV, Vasilev SA, Campeau J and
Vermesh M: Serum cancer antigen 125 in ectopic pregnancy. Gynecol
Obstet Invest. 27:164–165. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Canney PA, Moore M, Wilkinson PM and James
RD: Ovarian cancer antigen CA125: A prospective clinical assessment
of its role as a tumour marker. Br J Cancer. 50:765–769. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Jacobs IJ, Fay TN, Stabile I, Bridges JE,
Oram DH and Grudzinskas JG: The distribution of CA 125 in the
reproductive tract of pregnant and non-pregnant women. Br J Obstet
Gynaecol. 95:1190–1194. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kobayashi F, Sagawa N, Nakamura K,
Nonogaki M, Ban C, Fujii S and Mori T: Mechanism and clinical
significance of elevated CA 125 levels in the sera of pregnant
women. Am J Obstet Gynecol. 160:563–566. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Svirsky R, Ben-Ami I, Berkovitch M,
Halperin R and Rozovski U: Outcomes of conception subsequent to
methotrexate treatment for an unruptured ectopic pregnancy. Int J
Gynaecol Obstet. 139:170–173. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tas EE, Akcay GF and Avsar AF: Single-dose
methotrexate for the treatment of ectopic pregnancy: Our experience
from 2010 to 2015. Pak J Med Sci. 33:13–17. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Thoen LD and Creinin MD: Medical treatment
of ectopic pregnancy with methotrexate. Fertil Steril. 68:727–730.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Tulandi T, Bret PM, Atri M and Senterman
M: Treatment of ectopic pregnancy by transvaginal intratubal
methotrexate administration. Obstet Gynecol. 77:627–630.
1991.PubMed/NCBI
|
|
51
|
Usta IM, Nassar AH, Yunis KA and Abu-Musa
AA: Methotrexate embryopathy after therapy for misdiagnosed ectopic
pregnancy. Int J Gynaecol Obstet. 99:253–255. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
van Mello NM, Mol F, Verhoeve HR, van Wely
M, Adriaanse AH, Boss EA, Dijkman AB, Bayram N, Emanuel MH,
Friederich J, et al: Methotrexate or expectant management in women
with an ectopic pregnancy or pregnancy of unknown location and low
serum hCG concentrations? A randomized comparison. Hum Reprod.
28:60–67. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Weinman SA: Nonsurgical treatment of an
ectopic pregnancy with methotrexate. J Emerg Nurs. 22:597–599.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yang C, Cai J, Geng Y and Gao Y:
Multiple-dose and double-dose versus single-dose administration of
methotrexate for the treatment of ectopic pregnancy: A systematic
review and meta-analysis. Reprod Biomed Online. 34:383–391. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Cecchino GN, Araujo Júnior E and Elito
Júnior J: Methotrexate for ectopic pregnancy: When and how. Arch
Gynecol Obstet. 290:417–423. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Yao M, Tulandi T and Falcone T: Treatment
of ectopic pregnancy by systemic methotrexate, transvaginal
methotrexate, and operative laparoscopy. Int J Fertil Menopausal
Stud. 41:470–475. 1996.PubMed/NCBI
|
|
57
|
Ander DS and Ward KR: Medical management
of ectopic pregnancy-the role of methotrexate. J Emerg Med.
15:177–182. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Atkinson M, Gupta S and McGee T: βhCG
monitoring after single-dose methotrexate treatment of tubal
ectopic pregnancy: Is the Day 4 βhCG necessary? A retrospective
cohort study. Aust N Z J Obstet Gynaecol. 54:475–479. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Helmy S, Koch M, Kolbl H, Grohmann-Izay B,
Solomayer E and Bader Y: Correlation of the volume of ectopic
pregnancy and MTX therapy outcome: A retrospective cohort study.
Eur J Obstet Gynecol Reprod Biol. 184:108–111. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Balasch J, Ballesca JL, Fábregues F,
Puerto B, Casamitjana R and Vanrell JA: Transvaginal intratubal
insemination, ectopic pregnancy and treatment by single-dose
parenteral methotrexate. Hum Reprod. 7:1457–1460. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Sagiv R, Debby A, Feit H, Cohen-Sacher B,
Keidar R and Golan A: The optimal cutoff serum level of human
chorionic gonadotropin for efficacy of methotrexate treatment in
women with extrauterine pregnancy. Int J Gynaecol Obstet.
116:101–104. 2012. View Article : Google Scholar : PubMed/NCBI
|