Introduction
In 2013, the prevalence of diabetes in Chinese
adults was 11.6% (1). In patients
with type 2 diabetes, approximately one-half to two-thirds of the
cases are associated with a decrease in bone density, and one-third
of the patients are diagnosed with osteoporosis (OP) (2). OP is a systemic bone disease
characterized by bone density loss and bone microstructural
changes, which results in increased bone fragility, decreased bone
strength and increased risk of fracture (3,4).
Estradiol (E2) is a key factor in the development of OP, with
evidence of significant bone loss in postmenopausal women (5,6).
Since the effect of estrogen on diabetes-related OP is not well
understood, studies are necessary to provide new therapeutic
strategies for clinical treatment.
E2 is one of the most potent estrogens (7), which serve important roles in human
bone development and bone balance. Estrogen deficiency changes the
activity of osteoclasts; as osteoclast function becomes relatively
active, osteoblast function weakens (8). In addition, reduced apoptotic rate of
osteoclasts indicates that bone formation rate is lower compared
with bone resorption rate, which results in a decrease in bone
minerals (9,10).
Osteoblast genes are the main factors that promote
the secretion and mineralization of the bone matrix (11). Runt-related transcription factor 2
(Runx2), which is a core binding factor, is one of the most
important osteoblast-specific transcription factors. It is
associated with extracellular osteogenic induction factors and
intracellular factors, which also affect the signaling pathway of
osteoblast functional differentiation (12). Osteocalcin is a bone-building
compound expressed by osteoblasts that reflects the rate of bone
turnover (13). Serum osteocalcin
levels in patients with OP are significantly increased (14). Therefore, osteocalcin is clinically
used as a specific index in the diagnosis of OP (15).
Previous research has demonstrated the important
role of nuclear factor erythrocyte 2-related factor 2/heme
oxygenase-1 (Nrf2/HO1) signaling in high glucose-induced osteoblast
injury (16). Nrf2 is a regulatory
and transcription factor that produces cellular resistance to
oxygen-derived free radicals, binds to antioxidant response
elements and acts as a sensor for oxidative and electrophilic
stress. In addition, Nrf2 has a protective function against
oxidative damage in combination with other antioxidant enzymes,
such as HO-1 (17–19).
The aim of the present study was to elucidate the
role of E2 in the development of HG-induced OP and provide a
theoretical foundation for the treatment of OP.
Materials and methods
Cells and drugs
Mouse pre-osteoblast cell line MC3T3-E1 was
purchased from American Type Culture Collection. E2 was obtained
from Sigma-Aldrich (cat. no. E4260; HPLC>98%; Merck KGaA).
Transfection reagent Lipofectamine® 3000 was purchased
from Invitrogen (Thermo Fisher Scientific, Inc.). Cell Counting
Kit-8 (CCK-8) was obtained from Nanjing Jiancheng Bioengineering
Institute.
Cell culture and transfection
MC3T3-E1 cells (1×106 cells/ml, 24-well
plates) were cultured in a-minimum essential medium (α-MEM; Gibco;
Thermo Fisher Scientific, Inc.) containing 10% FBS (Gibco; Thermo
Fisher Scientific, Inc.), 1% antibiotics (100 µg/ml streptomycin
and 100 U/ml penicillin) at 37°C in a humidified atmosphere with 5%
CO2. Lipofectamine® 3000 was used following
the manufacturer's protocol. Briefly, 2 µl
Lipofectamine® 3000 and 40 pmol of small interfering
(si)RNA targeting Nrf2 (5′-GAGUAUGAGCUGGAAAAACUU-3′) or negative
control siRNA (siNC; 5′-GACGAGCGGCACGUGCACAUU-3′) (Shanghai
GenePharma Co., Ltd.) were mixed in 50 µl serum-free medium and
incubated at room temperature for 15 min. The lipid compounds were
diluted in 300 µl serum-free medium and 600 µl medium containing
FBS, added to the cells and incubated at 37°C with 5%
CO2 for 24 h. Following transfection for 24 h, cells
were harvested and used for subsequent experiments.
Cell treatments and groups
Different concentrations (0, 10, 50, 100, 200 and
300 mg/dl) of glucose were added to the medium and used to treat
MC3T3-E1 cells to test viability and to determine the optimal
glucose concentration for further experiments. The cells were
incubated at 37°C with 5% CO2 for 96 h. Then, the cell
viability of the cells treated with the optimal glucose
concentration for 1, 3, 5 and 7 days was determined. MC3T3-E1 cells
were treated with 1, 10 nM, 0.1 and 1 µM E2 to test cell viability
and to select an appropriate dose for further experiments (20). In subsequent experiments, cells
were divided into the following groups: i) Control, cells were
added to normal medium; ii) HG, cells treated with 200 mg/dl HG;
iii) E2, cells treated with 0.1 µM E2; iv) siNrf2, cells
transfected with siNrf2; v) NC, cells transfected with siNC; vi) HG
+ E2, cells co-treated with 200 mg/dl HG and 0.1 µM E2; vii) E2 +
siNrf2, cells were co-treated with 0.1 µM E2 and siNrf2; viii) HG +
E2 + siNC, cells co-treated with 200 mg/dl HG, 0.1 µM E2 and siNC;
ix) HG + E2 + siNrf2, cells co-treated with 200 mg/dl HG, 0.1 µM E2
and siNrf2.
Cell viability assay
Cell viability was detected by CCK-8 assay according
to the manufacturer's protocol. A total of 10 µl of CCK-8 solution
was added to MC3T3-E1 cells (3×103 cells/well) and the
cells were incubated at 37°C for 2 h in the dark with 5%
CO2. Subsequently, the optical density of each well at
450 nm was determined by a microplate reader (Bio-Rad Laboratories,
Inc.).
Western blotting
Treated MC3T3-E1 cells (2×104 cells/well
in 6-well plates) were washed twice with PBS, lysed with RIPA
buffer (Cell Signaling Technology, Inc.) for 2 h on ice, and
centrifuged at 12,000 × g for 30 min at 4°C. Subsequently,
supernatant was collected. The nuclear and cytoplasmic extracts
were prepared using an NE-PER Nuclear and Cytoplasmic Extraction
Reagents kit (Pierce; Thermo Fisher Scientific, Inc.) according to
the manufacturer's directions. The protein concentration was
determined using the BCA protein kit (Bio-Rad Laboratories, Inc.)
and adjusted to a concentration of 5 µg/µl using 1X loading buffer
and diethyl pyrocarbonate (DEPC)-treated water. Proteins (at least
30 µg/lane) were separated by 10% SDS-PAGE, transferred to a PVDF
membrane (Bio-Rad Laboratories, Inc.), blocked with 5% non-fat milk
at room temperature for 2 h and washed with PBS three times for 5
min. The membranes were then incubated overnight at 4°C with the
corresponding primary antibody, washed with PBS three times for 15
min, incubated with horseradish peroxidase-conjugated goat
anti-mouse or goat anti-rabbit immunoglobulin G secondary antibody
(1:2,000; cat. nos. sc-2005 and sc-2004; Santa Cruz Biotechnology,
Inc.) for 2 h at room temperature, washed with PBS three times for
15 min, and washed with PBS + 0.1% Tween-20 for 15 min. Development
was carried out using the EZ-ECL developer kit (Biological
Industries), densitometric analysis was performed using ImageJ 5.0
(National Institutes of Health). The antibodies used in the present
study were as follows: Rabbit anti-β-actin (1:1,000; cat. no.
LS-B1625; LifeSpan BioSciences, Inc.), mouse anti-Lamin B1
(1:1,000; cat. no. LS-B6062; LifeSpan BioSciences, Inc.), rabbit
anti-Nrf2 (1:1,000; cat. no. ab62352; Abcam) and mouse anti-HO1
(1:1,000; cat. no. ab13248; Abcam). The detection method for
nuclear Nrf2 protein was the same as for the other genes mentioned
above, and the Lamin B1 was used an internal reference.
RNA isolation and
reverse-transcription quantitative PCR (RT-qPCR)
Total RNA was extracted from treated MC3T3-E1 cells
(2×105 cells) using TRIzol reagent (1 ml/well;
Invitrogen; Thermo Fisher Scientific, Inc.). The lysate was
transferred to 1.5 ml Eppendorf tubes and kept at room temperature
for 5 min; subsequently 200 µl chloroform was added to each tube
and inverted for 15 sec. Following emulsification, the tubes were
left in room temperature for 5 min, centrifuged at 12,000 × g at
4°C for 15 mins, and the upper aqueous phase was pipetted into new
1.5 ml tubes with an equal volume of isopropanol (~400 µl); the
tubes were kept at room temperature for 10 min. Following another
centrifugation at 12,000 × g at 4°C for 15 min, the supernatant was
discarded and 1 ml of pre-cooled 75% ice ethanol was added. The
tubes were centrifuged at 7,500 × g at 4°C for 10 min, and the
supernatant was discarded. DEPC-treated water (20 µl) was added to
the tubes to dissolve the RNA. The purity and concentration of RNA
was tested using the NanoDrop ND-1000 spectrophotometer (NanoDrop
Technologies; Thermo Fisher Scientific, Inc.) at 260/280 nm.
RevertAid First Strand cDNA Synthesis kit (Thermo Fisher
Scientific, Inc.) was used according to the manufacturer's protocol
to reverse transcribe 1 µg total RNA to cDNA (42°C for 60 min
followed by 70°C for 5 min). SYBR Green PCR Master Mix (Roche
Diagnostics) was used to perform qPCR using ABI 7500 Real-Time PCR
Detection System (Thermo Fisher Scientific, Inc.). The PCR cycle
was as follows: Initial denaturation at 95°C for 10 min, followed
by 40 cycles of 94°C for 15 sec and 60°C for 1 min; final extension
at 60°C for 1 min. Relative mRNA quantity was determined using the
2−ΔΔCq method (21) and
normalized to the internal reference gene GAPDH. The primer
sequences used for RT-qPCR analysis are presented in Table I.
 | Table I.Primers for reverse
transcription-quantitative PCR. |
Table I.
Primers for reverse
transcription-quantitative PCR.
| Gene | Primer sequence
(5′→3′) |
|---|
| Runx2 | F:
AACGATCTGAGATTTGTGGGC |
|
| R:
CCTGCGTGGGATTTCTTGGTT |
| Osteocalcin | F:
CTGACCTCACAGATCCCAAGC |
|
| R:
TGGTCTGATAGCTCGTCACAAG |
| Nrf2 | F:
AAACCAGTGGATCTGCCAAC |
|
| R:
ACGTAGCCGAAGAAACCTCA |
| HO1 | F:
TCTCCGATGGGTCCTTACACTC |
|
| R:
GGCATAAAGCCCTACAGCAACT |
| GAPDH | F:
ACTTTGGTATCGTGGAAGGACTCAT |
|
| R:
GTTTTTCTAGACGGCAGGTCAGG |
Apoptosis assay
Treated MC3T3-E1 cells (1.3×105
cells/well) were seeded in six-well plates. Supernatant was
collected in a 15 ml centrifuge tube, and the culture flask was
washed once with 2 ml PBS. The cells were digested with 1 ml
trypsin without EDTA and gently shaken. The mixture was kept at
room temperature for 1 min, and α-MEM containing 10% FBS was added
to terminate the digestion. The cells were centrifuged at 1,000 × g
for 3 min and the supernatant was removed. The cells were washed
twice with pre-cooled PBS and resuspended in 1X Annexin V binding
buffer. Annexin-V-FITC Cell Apoptosis Detection kit (cat. no.
K201-100; BioVision, Inc.) was used according to the manufacturer's
protocol. Briefly, cells were collected and stained with Annexin
V-FITC and propidium iodide (PI) at room temperature for 15 min and
counted by flow cytometry using BD FACSCalibur™ with FlowJo
software (version 10.0; BD Biosciences). Flow cytometry scatter
diagrams demonstrate living cells in the lower left quadrant,
necrotic cells in the upper left quadrant, advanced apoptotic cells
in the upper right quadrant and early apoptotic cells in the lower
right quadrant. The apoptosis rate of total apoptotic cells (early
+ advanced) was calculated.
Statistical analysis
All data were analyzed using GraphPad Prism 6
software (GraphPad Software, Inc.). All data are expressed as mean
± SEM. Comparisons of multiple groups were performed by analysis of
variance between groups followed by Dunnett's test. P<0.05 was
considered to indicate a statistically significant difference.
Results
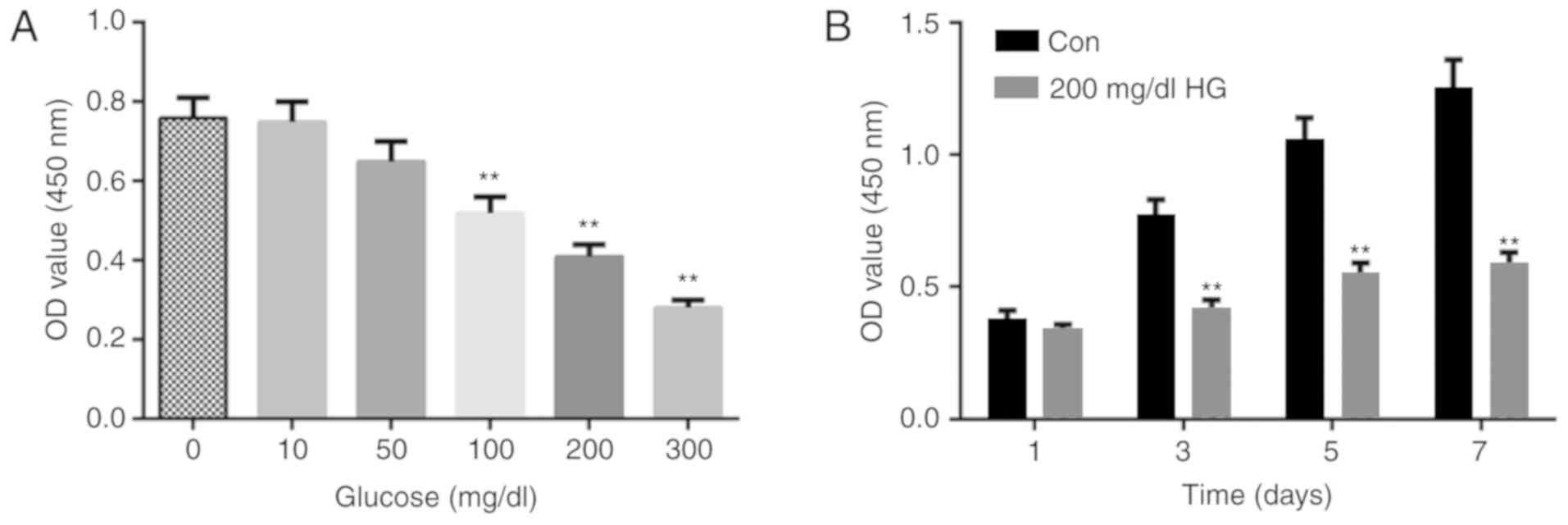
Cell viability is decreased by HG
CCK-8 assay was used to detect cell viability of
MC3T3-E1 cells treated with various concentrations of glucose for
96 h, which revealed that high concentrations of glucose decreased
cell viability compared with cells cultured in medium with normal
glucose levels (Fig. 1A).
Therefore, 200 mg/dl glucose was selected for subsequent
experiments. In addition, cell viability was inhibited by 200 mg/dl
glucose on treatment days 3, 5 and 7 compared with control (0
mg/dl) (Fig. 1B).
E2 relieves HG-induced osteoblast
injury
Cell viability was tested in MC3T3-E1 cells by CCK-8
assay using a range of concentrations of E2. Cell viability was
significantly increased by E2 at 0.1 µM (Fig. 2A); thus, 0.1 µM E2 was used for
subsequent experiments. To investigate the effect of E2 on
HG-induced osteoblast injury, cell viability, osteoblast
differentiation markers osteocalcin and Runx2, and Nrf2 and HO1
expression levels were determined following co-incubation with E2
and HG. The results demonstrated that compared with the HG-only
group, cell viability was increased (Fig. 2B), osteocalcin and Runx2 mRNA
expression levels were increased (Fig.
2C) in the HG + E2 group. The mRNA expression level of HO1 in
the HG + E2 group was significantly higher than that in HG-only
group, and there was no significant difference in Nrf2 mRNA
expression in control group, HG group, HG+E2 group and E2 group
(Fig. 2D). The protein levels of
nuclear Nrf2 and downstream target effector HO1 were increased
(Fig. 2E-G) in the HG + E2 group
compared with the HG-only group.
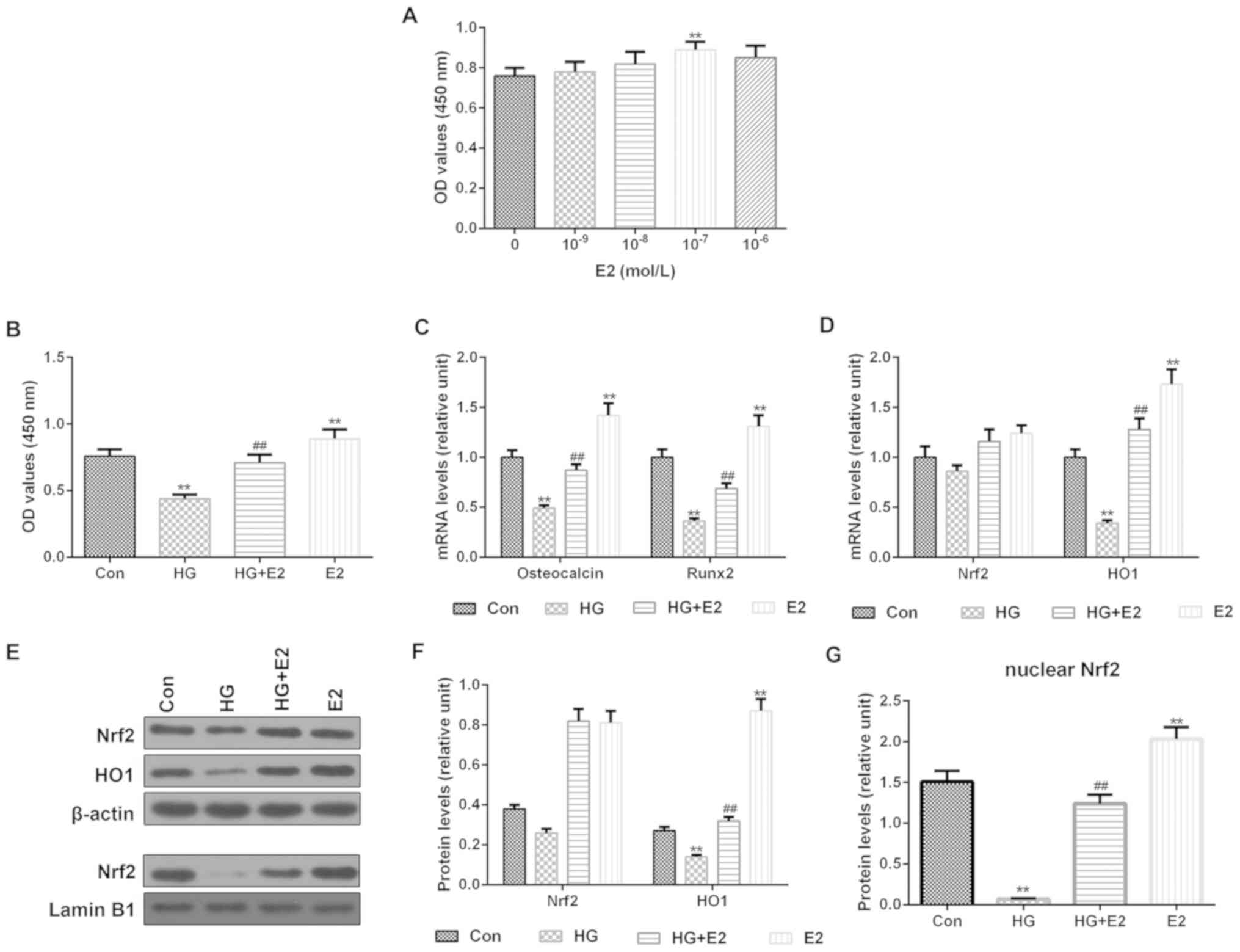 | Figure 2.HG-induced osteoblast injury is
reversed by E2. (A) CCK-8 was used to detect cell viability in
MC3T3-E1 cells treated with different concentrations of E2. (B)
Viability of MC3T3-E1 cells treated with HG, E2 or HG + E2 was
detected by CCK-8 assay. (C) RT-qPCR was used to determine the mRNA
expression levels of osteocalcin and Runx2. (D-G) Nrf2, nuclear
Nrf2 and downstream target effector HO1 protein expression levels
were determined by western blotting. The relative levels of
proteins were calculated using β-actin or Lamin B1 for
normalization. **P<0.01 vs. control; ##P<0.01 vs.
HG-only. CCK-8, Cell Counting Kit-8; Con, control; E2, estradiol;
HG, high glucose; HO1, heme oxygenase-1; Nrf2, nuclear factor
E2-related factor 2; OD, optical density; RT-qPCR, reverse
transcription-quantitative PCR; Runx2, Runt-related transcription
factor 2. |
Protective effect of E2 on HG-induced
osteoblast injury is Nrf2-dependent
Markers of osteoblast differentiation were examined
to detect changes in cell differentiation following HG, E2 and
siNrf2 treatment. Nrf2 was effectively downregulated by siRNA
transfection (Fig. 3A). Following
Nrf2 silencing, mRNA expression levels of osteocalcin, Runx2, Nrf2
and HO1 were significantly reduced, which was reversed by E2; HG
treatment significantly reduced the mRNA expression of osteocalcin,
Runx2 and HO1; E2 treatment alleviated the inhibitory effect of HG
on the mRNA expression of osteocalcin, Runx2, Nrf2 and HO1, while
Nrf2 silencing reversed this protective effect of E2, excepting the
expression of Runx2 (Fig.
3B-E).
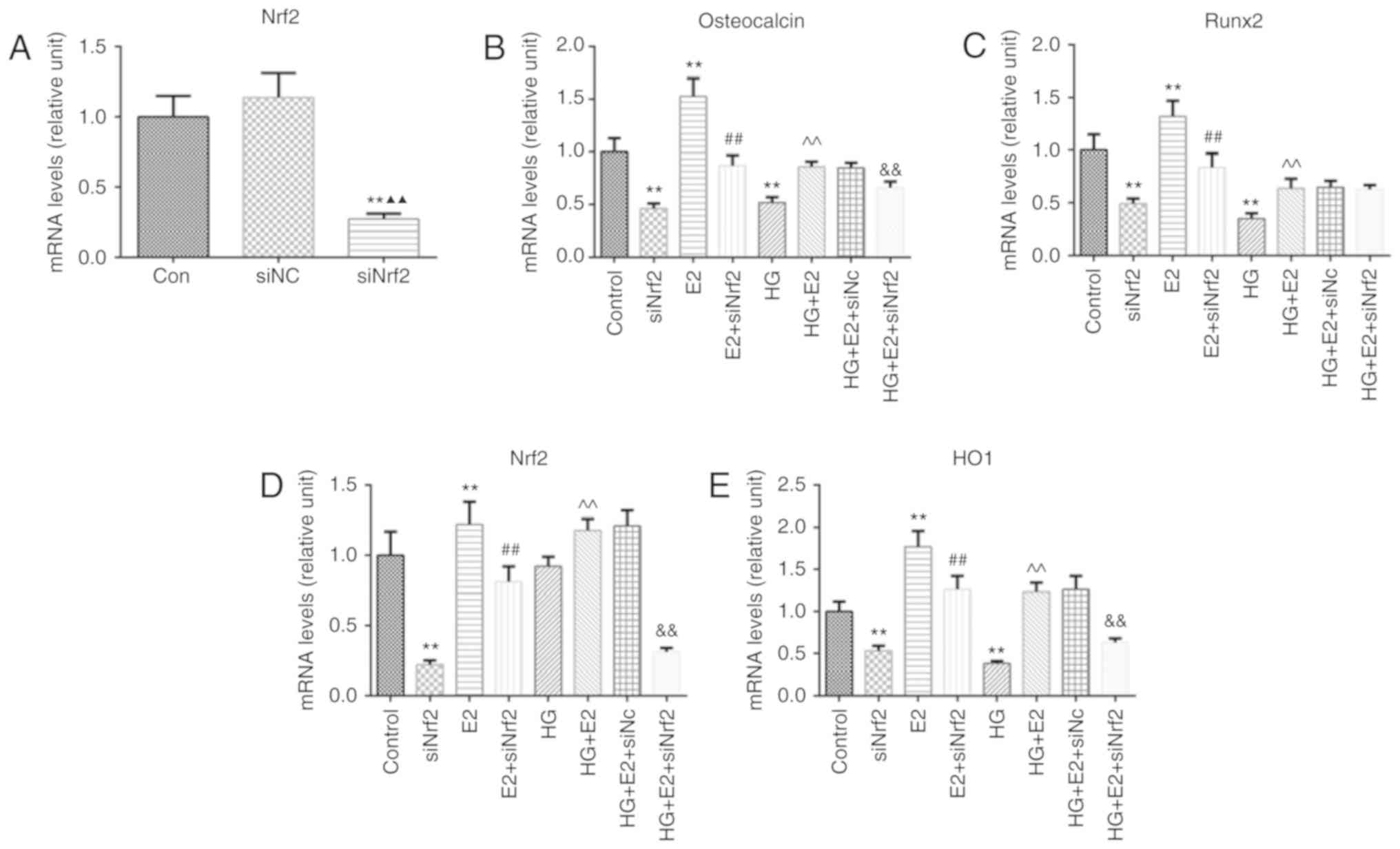 | Figure 3.Protective effect of E2 on HG-induced
osteoblast injury is Nrf2-dependent. (A) RT-qPCR was used to
evaluate the transfection efficiency of siNrf2. (B-E) mRNA
expression levels of (B) osteocalcin, (C) Runx2, (D) Nrf2 and (E)
HO1 were identified by RT-qPCR in untransfected MC3T3-E1 cells and
MC3T3-E1 cells transfected with siNrf2 or siNC and co-treated with
HG and/or E2. **P<0.01 vs. control; ▲▲P<0.01 vs. siNC;
##P<0.01 vs. E2; ^^P<0.01 vs. HG;
&&P<0.01 vs. HG + E2. Con, control; E2,
estradiol; HG, high glucose; HO1, heme oxygenase-1; NC, negative
control; Nrf2, nuclear factor E2-related factor 2; OD, optical
density; RT-qPCR, reverse transcription-quantitative PCR; Runx2,
Runt-related transcription factor 2; si, small interfering RNA. |
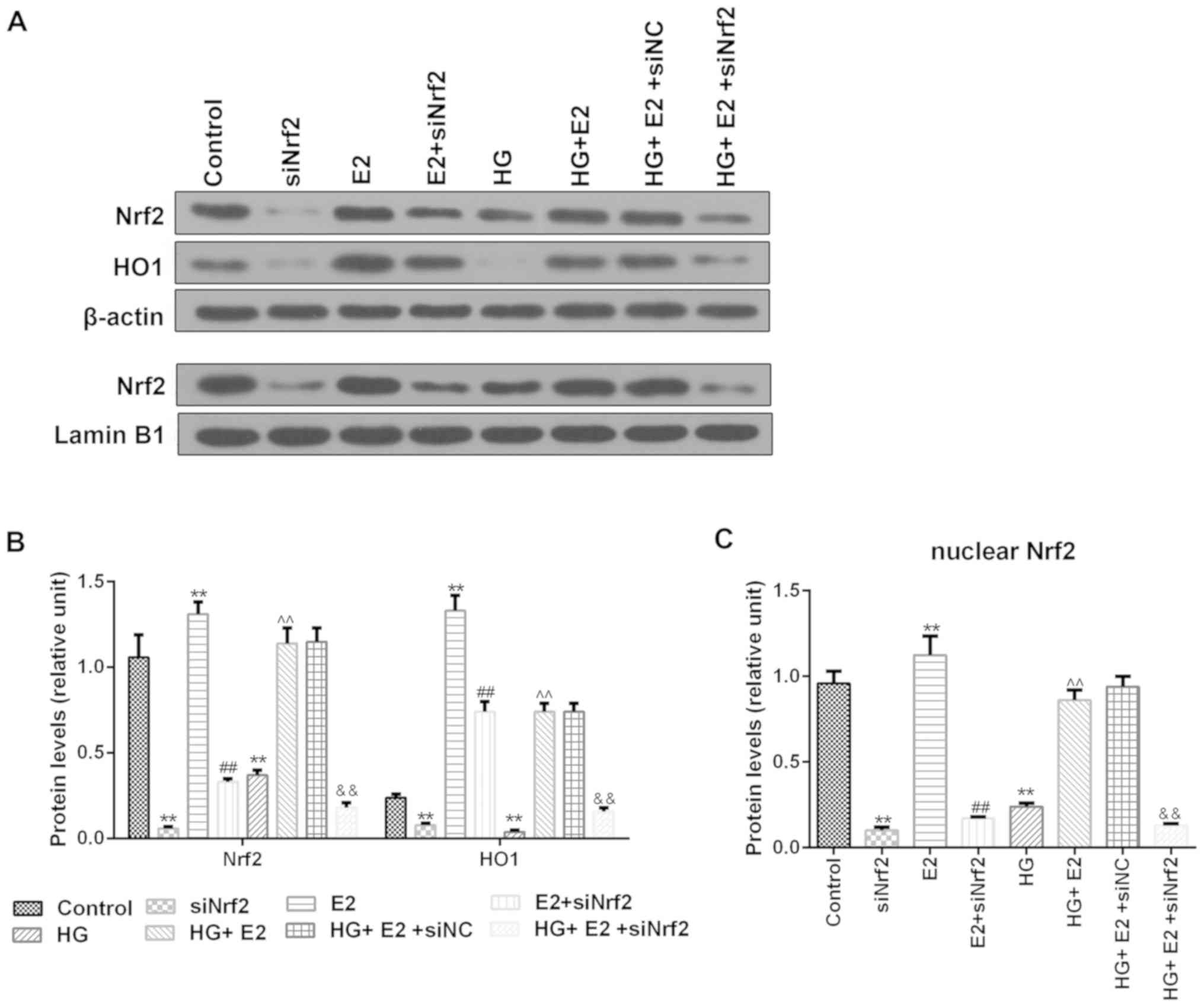
Protein expression levels of Nrf2 and
HO1 are decreased by siNrf2
Western blotting was used to identify the protein
expression levels of Nrf2 and HO1. siNrf2 reduced the protein
expression of Nrf2 and HO1 and the Nrf2 and HO1 protein levels in
E2 + siNrf2 group were significantly lower than those in E2 group;
E2 treatment alleviated the inhibitory effect of HG on the protein
expression of Nrf2 and HO1, while Nrf2 silencing reversed this
protective effect of E2 (Fig.
4).
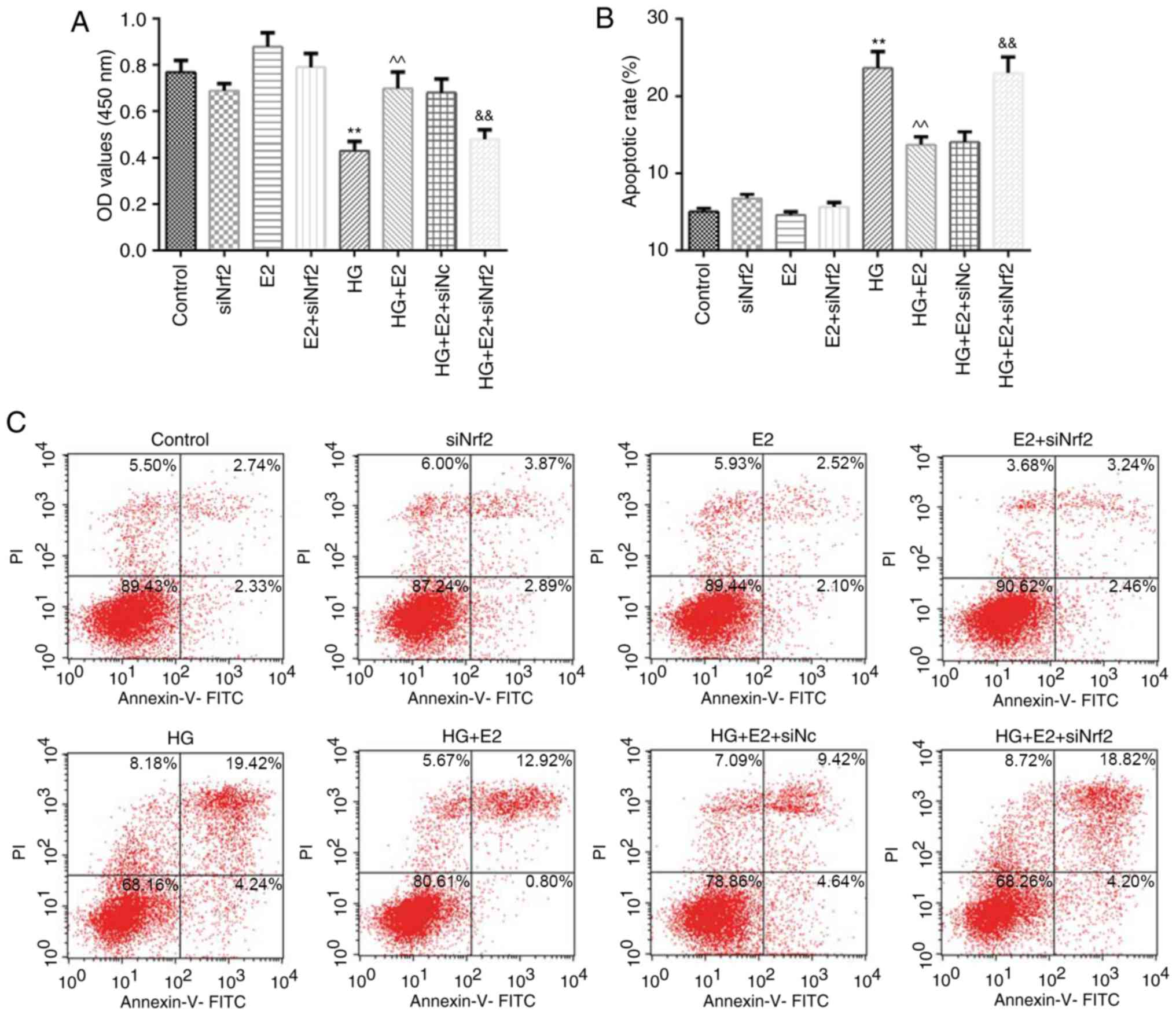
Effects of E2 on HG-induced cell
viability and apoptosis were reversed by siNrf2
MC3T3-E1 cell viability and apoptotic rates were
examined by CCK-8 assay and flow cytometry, respectively. SiNrf2
treatment alone, E2 treatment alone, and siNrf2 and E2 co-treatment
had no significant effect on cell viability, although the cell
viability was inhibited in the HG group; E2 treatment effectively
enhanced cell viability in HG-treated cells, which was inhibited by
cotreatment with siNrf2 (Fig. 5A).
Additionally, SiNrf2 treatment alone, E2 treatment alone, and
siNrf2 and E2 co-treatment had no significant effect on cell
apoptosis, although the apoptosis rate was promoted in the HG
group. The apoptotic rate was significantly lower in the HG + E2
group compared with the HG-only group, whereas cells in the HG + E2
+ siNrf2 group exhibited a higher apoptotic rate compared with the
HG + E2 + siNC group (Fig. 5B and
C).
Discussion
Hyperglycemia is one of the possible causes for OP
and fracture in diabetes (22). OP
is characterized by reduced bone mass, alterations in the
microarchitecture of bone tissue, reduced bone strength, and an
increased risk of fracture (23).
A previous study has demonstrated that bone mass in diabetic
patients is significantly decreased, and the risk of fracture is
increased (24). Estrogen
reduction significantly increases the risk of OP (25,26).
E2 the most potent estrogen; however, its role in diabetes-related
OP is poorly understood.
To explore the mechanism of diabetes-related OP,
mouse pre-osteoblast MC3T3-E1 cells were treated with high
concentrations of glucose to simulate diabetes-induced OP. The
results of CCK-8 experiments demonstrated that the cell viability
was significantly reduced by HG on treatment days 3, 5 and 7. These
data suggested that HG may contribute to bone damage.
Previous studies have demonstrated that
postmenopausal bone turnover rate is reduced, and E2 serves an
important role in the development of OP (27–29).
Gopalakrishnan et al revealed that insulin and E2 increased
the number of mineralized nodules, the area stained for collagen
and mineralization, as well as the proliferation of rat bone marrow
stromal cells under HG (30).
Estrogen-induced transforming growth factor β receptor 1 and bone
morphogenetic protein receptor type 1A expression levels were
suppressed by estrogen receptor β activation in MC3T3-E1 cells
(31). The present study
demonstrated that E2 may serve an important protective role in
HG-induced osteogenic injury. Different concentrations of E2 were
added to mouse osteoblasts, and the results revealed that 0.1 µM E2
significantly increased MC3T3-E1 cell viability. This result was
consistent with the study of He et al (31) that high concentration of E2 can
improve the cell viability of MC3T3-E1.
Runx2 and osteocalcin are essential for promoting
osteoblast differentiation in the early stage of induction
(32). A previous study
demonstrated that bone cements exhibit osteoinductive activity,
based on the results of elevated expression levels of genes
encoding for osteocalcin and Runx2 in both undifferentiated and
differentiated MC3T3-E1 cells (33). Low expression of Nrf2 and HO-1 in
osteoblast injury has also been demonstrated (34). HO-1 inhibits osteoclastogenesis and
bone destruction in an arthritis model (35) and upregulates osteogenic
differentiation in human periodontal ligament cells (36). Recent studies have indicated that
HO-1 expression is mediated by Nrf2 activation in MC3T3-E1 cells
(37,38). In the present study, HG treatment
decreased cell viability and reduced osteocalcin, Runx2, HO1 and
nuclear Nrf2 expression levels; these changes were reversed by E2.
To verify whether the protective effect of E2 was related to Nrf2,
siRNA silencing was used to downregulate Nrf2. Expression levels of
osteocalcin, HO1 and nuclearNrf2 were upregulated by E2 compared
with control cells under HG-induced osteogenic injury; however,
these effects were reversed by siNrf2, with the exception of Runx2.
It is possible that not all tested osteogenic genes were involved
in the effect of Nrf2 on osteoblast injury. The results of the
present study suggested that E2 may effectively protect osteoblasts
from damage, and this protection may rely on the expression of
Nrf2.
Severe apoptosis can be observed in certain disease
conditions, including diabetes (39). By regulating the balance between
cell growth and death, apoptosis serves a key role in maintaining
homeostasis (40,41). Therefore, targeting apoptosis has
become a common therapeutic method for diabetes-induced bone damage
(42). In the present study, the
results of the apoptosis assay by flow cytometry demonstrated that
silencing Nrf2 effectively reversed the inhibitory effect of E2 on
HG-induced apoptosis. Therefore, E2 may protect osteoblasts against
injury through a Nrf2-dependent mechanism. However, the potential
role of E2 in diabetes-related OP remains unclear, and the
involvement of other signaling pathways involved in the regulation
of diabetes-related OP by E2 should be further investigated.
There are certain limitations to the present study.
Firstly, the functions of E2 on HG-treated MC3T3-E1 cells were only
supported by in vitro experiments. Secondly, alkaline
phosphatase is a typical marker in the early stage of osteogenic
differentiation and its level requires further examination.
Additionally, the expression levels of estrogen receptor in
differentiated-MC3T3-E1 need to be quantified.
In the present study, mouse pre-osteoblast MC3T3-E1
cell was used to set up a model of diabetes-induced OP, which was
induced by HG. E2 treatment resulted in increases in cell
viability, expression levels of osteoblast genes osteocalcin and
Runx2, as well as increased expressions of HO1 and nuclear Nrf2.
These changes were reversed by Nrf2 silencing, with the exception
of Runx2. Additionally, following Nrf2 silencing, cell apoptosis
was significantly increased in HG-treated cells. Thus, E2 may
prevent HG-induced osteoblast injury by activating Nrf2/HO1
signaling pathways, E2 may be a potential drug for the treatment of
diabetes-related OP.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets generated and analyzed during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
Substantial contributions to conception and design:
GL, XJ. Data acquisition, data analysis and interpretation: LL, XL,
HL, ZZ. Drafting the article or critically revising it for
important intellectual content: GL, XJ. All authors read and
approved the manuscript and agree to be accountable for all aspects
of the research in ensuring that the accuracy or integrity of any
part of the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Xu Y, Wang L, He J, Bi Y, Li M, Wang T,
Wang L, Jiang Y, Dai M, Lu J, et al: Prevalence and control of
diabetes in Chinese adults. JAMA. 310:948–959. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yan P, Zhang Z, Wan Q, Zhu J, Li H, Gao C,
Ma H and Xu Y: Association of serum uric acid with bone mineral
density and clinical fractures in Chinese type 2 diabetes mellitus
patients: A cross-sectional study. Clin Chim Acta. 486:76–85. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen JS, Yang A and Murrell DF: Prevalence
and pathogenesis of osteopenia and osteoporosis in epidermolysis
bullosa: An evidence based review. Exp Dermatol. Aug 17–2018.(Epub
ahead of print). doi: 10.1111/exd.13771. View Article : Google Scholar
|
|
4
|
Schousboe JT: Vertebral fracture
identification as part of a comprehensive risk assessment in
patients with osteoporosis. Curr Osteoporos Rep. 16:573–583. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Crandall CJ: Risk assessment tools for
osteoporosis screening in postmenopausal women: A systematic
review. Curr Osteoporos Rep. 13:287–301. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Eastell R, O'Neill TW, Hofbauer LC,
Langdahl B, Reid IR, Gold DT and Cummings SR: Postmenopausal
osteoporosis. Nat Rev Dis Primers. 2:160692016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Baker ME and Lathe R: The promiscuous
estrogen receptor: Evolution of physiological estrogens and
response to phytochemicals and endocrine disruptors. J Steroid
Biochem Mol Biol. 184:29–37. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mathis KM, Sturgeon KM, Winkels RM,
Wiskemann J, De Souza MJ and Schmitz KH: Bone resorption and bone
metastasis risk. Med Hypotheses. 118:36–41. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang X, Lv Y, He P, Wang Z, Xiong F, He
L, Zheng X, Zhang D, Cao Q and Tang C: HDL impairs
osteoclastogenesis and induces osteoclast apoptosis via
upregulation of ABCG1 expression. Acta Biochim Biophys Sin
(Shanghai). 50:853–861. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang Y, Luo TB, Liu L and Cui ZQ: LncRNA
LINC00311 promotes the proliferation and differentiation of
osteoclasts in osteoporotic rats through the notch signaling
pathway by targeting DLL3. Cell Physiol Biochem. 47:2291–2306.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tantikanlayaporn D, Tourkova IL,
Larrouture Q, Luo J, Piyachaturawat P, Witt MR, Blair HC and
Robinson LJ: Sphingosine-1-phosphate modulates the effect of
estrogen in human osteoblasts. JBMR Plus. 2:217–226. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Valenti MT, Dalle Carbonare L and Mottes
M: Ectopic expression of the osteogenic master gene RUNX2 in
melanoma. World J Stem Cells. 10:78–81. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kanazawa I, Tanaka S and Sugimoto T: The
association between osteocalcin and chronic inflammation in
patients with type 2 diabetes mellitus. Calcif Tissue Int.
103:599–605. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu CC, Wang CC, Lu DH, Hsu LH, Yang KC and
Lin FH: Calcium phosphate cement delivering zoledronate decreases
bone turnover rate and restores bone architecture in ovariectomized
rats. Biomed Mater. 7:0350092012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tanaka S, Miyazaki T, Uemura Y, Kuroda T,
Miyakawa N, Nakamura T, Fukunaga M, Ohashi Y, Ohta H, Mori S, et
al: Design of a randomized clinical trial of concurrent treatment
with vitamin K2 and risedronate compared to risedronate alone in
osteoporotic patients: Japanese Osteoporosis Intervention Trial-03
(JOINT-03). J Bone Miner Metab. 32:298–304. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin H, Wei B, Li G, Zheng J, Sun J, Chu J,
Zeng R and Niu Y: Sulforaphane reverses glucocorticoid-induced
apoptosis in osteoblastic cells through regulation of the Nrf2
pathway. Drug Des Devel Ther. 8:973–982. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vargas MR and Johnson JA: The Nrf2-ARE
cytoprotective pathway in astrocytes. Expert Rev Mol Med.
11:e172009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ma Q: Role of nrf2 in oxidative stress and
toxicity. Annu Rev Pharmacol Toxicol. 53:401–426. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dong F, Wang S, Wang Y, Yang X, Jiang J,
Wu D, Qu X, Fan H and Yao R: Quercetin ameliorates learning and
memory via the Nrf2-ARE signaling pathway in d-galactose-induced
neurotoxicity in mice. Biochem Biophys Res Commun. 491:636–641.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shang ZZ, Li X, Sun HQ, Xiao GN, Wang CW
and Gong Q: Differentially expressed genes and signalling pathways
are involved in mouse osteoblast-like MC3T3-E1 cells exposed to
17-β estradiol. Int J Oral Sci. 6:142–149. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
You L, Gu W, Chen L, Pan L, Chen J and
Peng Y: MiR-378 overexpression attenuates high glucose-suppressed
osteogenic differentiation through targeting CASP3 and activating
PI3K/Akt signaling pathway. Int J Clin Exp Pathol. 7:7249–7261.
2014.PubMed/NCBI
|
|
23
|
Ralston SH and de Crombrugghe B: Genetic
regulation of bone mass and susceptibility to osteoporosis. Genes
Dev. 20:2492–2506. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hofbauer LC, Brueck CC, Singh SK and
Dobnig H: Osteoporosis in patients with diabetes mellitus. J Bone
Miner Res. 22:1317–1328. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cheng YZ, Yang SL, Wang JY, Ye M, Zhuo XY,
Wang LT, Chen H, Zhang H and Yang L: Irbesartan attenuates advanced
glycation end products-mediated damage in diabetes-associated
osteoporosis through the AGEs/RAGE pathway. Life Sci. 205:184–192.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mederle OA, Balas M, Ioanoviciu SD, Gurban
CV, Tudor A and Borza C: Correlations between bone turnover
markers, serum magnesium and bone mass density in postmenopausal
osteoporosis. Clin Interv Aging. 13:1383–1389. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Prior JC: Progesterone for the prevention
and treatment of osteoporosis in women. Climacteric. 21:366–374.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pardhe BD, Pathak S, Bhetwal A, Ghimire S,
Shakya S, Khanal PR and Marahatta SB: Effect of age and estrogen on
biochemical markers of bone turnover in postmenopausal women: A
population-based study from Nepal. Int J Womens Health. 9:781–788.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tian L, Yang R, Wei L, Liu J, Yang Y, Shao
F, Ma W, Li T, Wang Y and Guo T: Prevalence of osteoporosis and
related lifestyle and metabolic factors of postmenopausal women and
elderly men: A cross-sectional study in Gansu province,
Northwestern of China. Medicine (Baltimore). 96:e82942017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gopalakrishnan V, Vignesh RC, Arunakaran
J, Aruldhas MM and Srinivasan N: Effects of glucose and its
modulation by insulin and estradiol on BMSC differentiation into
osteoblastic lineages. Biochem Cell Biol. 84:93–101. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
He HL, Liu C, Li BX, Wang CQ, Li HT and Gu
L: Estrogen-induced Tgfbr1 and Bmpr1a expression
repressed via estrogen receptor beta in MC3T3-E1 cells. Chin Med J
(Engl). 131:2558–2565. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fan S, Gao X, Chen P and Li X: Myricetin
ameliorates glucocorticoid-induced osteoporosis through the ERK
signaling pathway. Life Sci. 207:205–211. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Uskoković V, Graziani V, Wu VM, Fadeeva
IV, Fomin AS, Presniakov IA, Fosca M, Ortenzi M, Caminiti R and Rau
JV: Gold is for the mistress, silver for the maid: Enhanced
mechanical properties, osteoinduction and antibacterial activity
due to iron doping of tricalcium phosphate bone cements. Mater Sci
Eng C Mater Biol Appl. 94:798–810. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kook SH, Kim KA, Ji H, Lee D and Lee JC:
Irradiation inhibits the maturation and mineralization of
osteoblasts via the activation of Nrf2/HO-1 pathway. Mol Cell
Biochem. 410:255–266. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zwerina J, Tzima S, Hayer S, Redlich K,
Hoffmann O, Hanslik-Schnabel B, Smolen JS, Kollias G and Schett G:
Heme oxygenase 1 (HO-1) regulates osteoclastogenesis and bone
resorption. FASEB J. 19:2011–2013. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kook YA, Lee SK, Son DH, Kim Y, Kang KH,
Cho JH, Kim SC, Kim YS, Lee HJ, Lee SK and Kim EC: Effects of
substance P on osteoblastic differentiation and heme oxygenase-1 in
human periodontal ligament cells. Cell Biol Int. 33:424–428. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Choi EM, Suh KS, Kim YJ, Hong SM, Park SY
and Chon S: Glabridin alleviates the toxic effects of methylglyoxal
on osteoblastic MC3T3-E1 cells by increasing expression of the
glyoxalase system and Nrf2/HO-1 signaling and protecting
mitochondrial function. J Agric Food Chem. 64:226–235. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Han D, Gao J, Gu X, Hengstler JG, Zhang L,
Shahid M, Ali T and Han B: P21Waf1/Cip1 depletion
promotes dexamethasone-induced apoptosis in osteoblastic MC3T3-E1
cells by inhibiting the Nrf2/HO-1 pathway. Arch Toxicol.
92:679–692. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tekula S, Khurana A, Anchi P and Godugu C:
Withaferin-A attenuates multiple low doses of Streptozotocin
(MLD-STZ) induced type 1 diabetes. Biomed Pharmacother.
106:1428–1440. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ghiasi SM, Dahllof MS, Osmai Y, Osmai M,
Jakobsen KK, Aivazidis A, Tyrberg B, Perruzza L, Prause MCB,
Christensen DP, et al: Regulation of the β-cell inflammasome and
contribution to stress-induced cellular dysfunction and apoptosis.
Mol Cell Endocrinol. 478:106–114. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zheng G, Li H, Zhang T, Yang L, Yao S,
Chen S, Zheng M, Zhao Q and Tian H: Irisin protects macrophages
from oxidized low density lipoprotein-induced apoptosis by
inhibiting the endoplasmic reticulum stress pathway. Saudi J Biol
Sci. 25:849–857. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhao W, Zhang WL, Yang B, Sun J and Yang
MW: NIPA2 regulates osteoblast function via its effect on apoptosis
pathways in type 2 diabetes osteoporosis. Biochem Biophys Res
Commun. 513:883–890. 2019. View Article : Google Scholar : PubMed/NCBI
|