Increased expression levels of vitronectin in the maternal‑fetal interface of placenta in early-onset severe preeclampsia
- Authors:
- Published online on: October 22, 2012 https://doi.org/10.3892/mmr.2012.1141
- Pages: 53-58
Abstract
Introduction
Preeclampsia (PE) is a pregnancy-specific disease which is characterized by serious hypertension and proteinuria and is associated with other complications, including edema and fetal growth restriction (1). PE is one of the main causes of maternal and perinatal morbidities and mortalities that complicate approximately 5–8% of all pregnancies (2–4). Early onset severe PE (EOSP) refers to severe PE before 34 weeks and must be observed closely for maternal and perinatal complications. EOSP leads to multi-organ dysfunction and child mortality (5). Several pathological studies associated with EOSP, including genetic, immunological, behavioral and environmental effects, reveal that placental ischemia/hypoxia is widely regarded as a key factor in EOSP (6,7).
Vitronectin (VN) is an adhesion protein, distributed in various tissues of the extracellular matrix (ECM). VN is mostly produced in the liver, however, its mRNA has been detected in various tissues, including the brain and heart, suggesting that VN is produced by local cells other than liver (8). As a multifunctional human glycoprotein, VN plays a significant role in cell migration, tissue repair, regulation of membrane attack complex (MAC) formation and enhances inflammatory process during infection (9). VN has physiological functions, including cell adhesion, regulation of fibrinolysis, coagulation and immune defense, which may be crucial in the occurrence and progression of severe PE, particularly EOSP (5,6).
Few studies have addressed the correlation between VN and PE thus far. The present study is the first to analyze VN expression in maternal-fetal interface placental tissues combined with determination of coagulation parameter changes in EOSP patients. We utilized immunohistochemistry, immunofluorescence and western blot analysis to identify maternal-fetal interface placental tissues of patients with EOSP with increased VN expression. Increased expression is associated with infarctions. Furthermore, reverse transcription polymerase chain reaction (RT-PCR) was used to identify whether VN is secreted locally in the maternal-fetal interface of the placenta. Coagulation parameter determination demonstrated that prothrombin time (PT) was significantly shorter in the EOSP group. Our results provide evidence of specific VN expression in the maternal-fetal interface and coagulation alterations in plasma of patients with EOSP. Moreover, the present study explored the role and significance of VN in EOSP, thus providing a theoretical basis for research and treatment of EOSP pathogenesis.
Materials and methods
Patients, collection of placenta and serum samples
The present study was approved by the Second Hospital of Jilin University and all patients provided written informed consent. A total of 63 individuals, 30 normal pregnant females (15 cases as early control group delivered for social reasons and fetal malformations before 34 weeks, 15 cases of normal females who delivered under Cesarean section after 37 weeks as late control group) and 33 PE females [17 cases of EOSP, 16 cases of late-onset severe PE (LOSP)] were included in this study. All females delivered by Cesarean section prior to the onset of labour, with the exception of the early control group. PE is characterized by new-onset hypertension, proteinuria and edema after 20 weeks of gestation and is complicated by renal failure, pulmonary edema and coagulopathy (10). Females were considered normal if their blood pressure remained <130/80 mmHg. Patients were excluded from the study if complicated by: chronic hypertension, diabetes, renal disease, premature rupture of membranes and other pregnancy complications, including fetal anomalies or chromosomal abnormalities. Human placental samples were obtained immediately following delivery or Cesarean section as follows: samples of maternal-fetal interface in placental tissues (infarct center, infarct edge, near the infarct tissue and away from the infarct tissues) and plasma of EOSP, LOSP and two groups corresponding to the control group were collected. The size of the samples was ~1.0×1.0×1.0 cm3. Fresh tissue samples were stored at −80°C for RT-PCR and western blot analysis.
Immunohistochemistry and immunofluorescence
Immunohistochemical staining was performed as previously described (11,12). Briefly, placental tissues were fixed with 4% paraformaldehyde, embedded in paraffin and then deparaffinized. Endogenous peroxidase activity of the tissue was blocked by incubation in 0.3% H2O2 for 10 min. Tissue sections were kept in 0.1 mol/l citrate buffer (pH 6.0) at 750 W for 20 min to unmask VN antigen and cool down at room temperature. Specimens were then incubated with primary antibody, rabbit monoclonal anti-human VN (ab46808, diluted 1:200; Abcam, Cambridge, MA, USA) for 1 h, washed with phosphate-buffered solution (PBS) twice and incubated with the PV-9000 secondary antibody, using an Immunohistological Staining kit (Zhongshan Goldenbridge Biotechnology Co., Beijing, China). The specimens were stained with diaminobenzidine (DAB) as a chromogen. A PBS stained sample was used as a blank control. VN protein expression levels of placentas in the experimental and control groups were analyzed with Image-Pro Plus 6.0 software. Immunofluorescence was performed in a similar manner with modifications. The secondary antibody was fluorescein isothiocyanate (FITC)-labeled goat anti-rabbit IgG antibody (Zhongshan Goldenbridge Biotechnology Co.).
RT-PCR
Total RNA was isolated from the fresh tissue samples using TRIzol reagent (Invitrogen, Paisley, UK) according to the manufacturer’s instructions and subjected to RT-PCR. For each sample, 25 μl of reaction mixture was transferred into a tube for PCR amplification. Primers (Sangong, Shanghai, China) used to detect VN expression levels were: forward: 5′-CCT TCA CCG ACC TCA AGA AC-3′, reverse: 5′-GAA GCC GTC AGA GAT ATT TCG-3′; GAPDH: forward: 5′-ATG ACA TCA AGA AGG TGG TG-3′, reverse: 5′-CATACCAGGAAATGAGCTTG-3′. PCR amplification of VN was performed under the following conditions: 94°C for 3 min, 30 cycles of 30 sec of denaturing at 94°C and 30 sec of annealing at 50°C, followed by a 1 min of extension at 72°C. PCR products were analyzed by electrophoresis of 10 μl of each PCR reaction mixture in a 1.5% agarose gel and bands were visualized by ethidium bromide staining.
Western blot analysis
Tissue lysates were prepared and protein levels were analyzed by western blot analysis, performed as previously described with modifications (13,14). Briefly, tissues were ground and lysates were collected and centrifuged. Tissue pellets were resuspended in lysis buffer and lysed on ice. Following centrifugation for 15 min, supernatant fluids were collected and the protein content of the supernatant was measured by a NanoDrop 1000 spectrophotometer (Thermo Scientific, Waltham, MA, USA). The protein lysates were separated by electrophoresis on a 12% SDS-polyacrylamide gel and transferred to a PVDF membrane (Amersham Biosciences, Piscataway, NJ, USA). Membranes were blocked with 5% skimmed milk for 2 h. Membranes were then probed with rabbit monoclonal anti-human VN (ab46808, diluted 1:20,000; Abcam) or mouse anti-human β-actin monoclonal antibody (TA-09, Zhongshan Goldenbridge Biotechnology Co.) and incubated at 37°C for 2 h or overnight. Blots were washed three times with TBS, then incubated with HRP-conjugated goat polyclonal anti-rabbit secondary antibodies (ZB-2301, 1:1,000; Zhongshan Goldenbridge Biotechnology Co.) for 2 h at room temperature. Signals were detected using enhanced chemiluminescence (Super Signal Dura Kit, Thermo Scientific) and chemiluminescence kit on X-ray film.
Coagulation assay
PT, partial thromboplastin time (APTT)and fibrinogen (FIB) coagulation assays were carried out with an ACL Top coagulation analyzer (Beckman Coulter, Brea, CA, USA).
Statistical analysis
For statistical analysis, we used the SPSS 17.0 software system (SPSS Inc., Chicago, IL, USA). To determine VN protein expression levels of placentas in the experimental and control groups, data were analyzed by independent sample t-tests. For all other comparisons, analysis was performed using a one-way ANOVA (using LSD correction to discriminate between the means). Homogeneity of data was assessed by Bartlett’s test and when necessary, data were logarithmically transformed prior to further analysis. Spearman’s rank correlation test was used to calculate the correlation coefficient. P<0.05 was considered to indicate a statistically significant difference. Data were expressed as the mean ± SEM.
Results
Localization of VN in the maternal-fetal interface of placental tissues
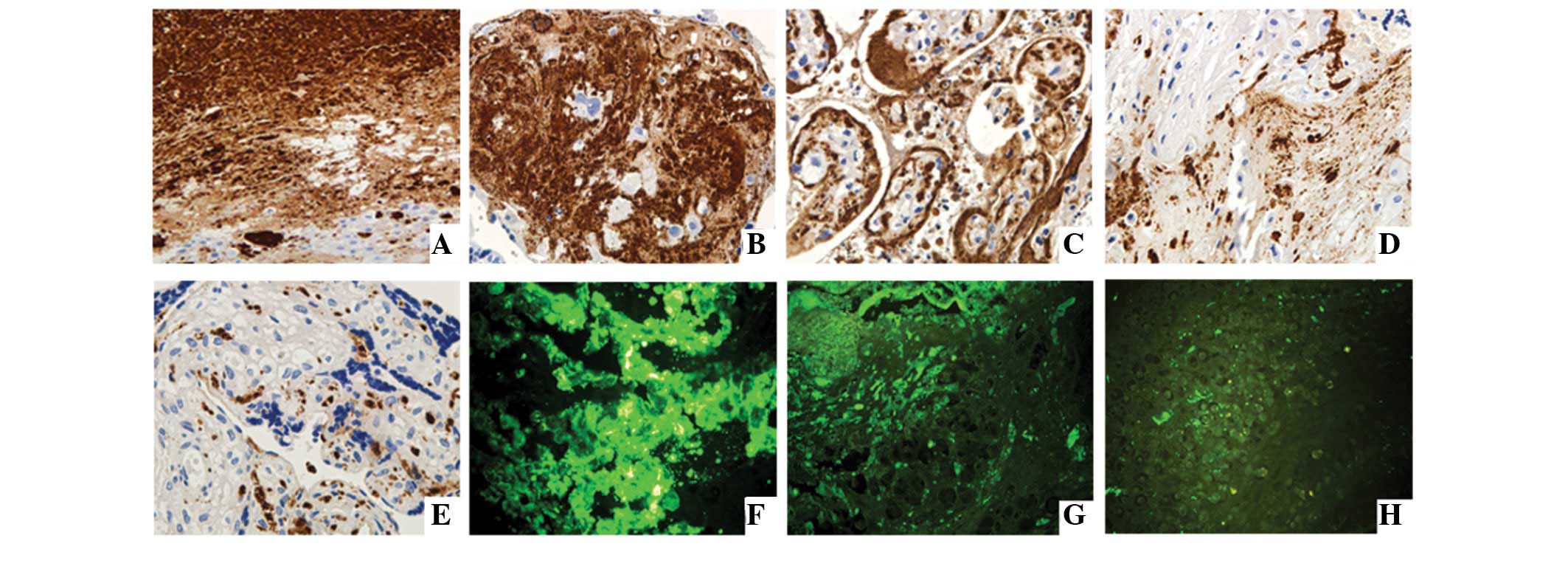
To determine the location of VN in sections from infarct center, infarct edge, near the infarct tissue and away from the infarct tissues of the maternal-fetal interface of placental tissues, we stained VN in each section of tissues using immunohistochemistry and immunofluorescence microscopy. Results indicate that VN was located in each group of placental tissue, particularly in necrotic and fibrous regions of the placental infarct area. In addition, it was observed that VN was largely expressed in the cytoplasm of extravillous trophoblasts (EVTs) of the maternal-fetal interface, as well as the cell membrane and the nuclei of EVTs (Fig. 1).
Expression levels of VN protein in placenta
To determine VN protein expression, we utilized immunohistochemistry. We selected five regions randomly and calculated expression using Image-pro Plus 6.0 software. Expression of VN in placentas of the EOSP group was found to be highest, followed by LOSP, late control and early control groups. Each group difference was considered statistically significant (P<0.001; Table I).
VN protein expression in placenta sections
To analyze whether the VN protein expression alters in different regions of the placenta of each group, immunohistochemistry was performed. We randomly selected the five regions of each section of placenta and calculated expression using Image-pro Plus 6.0 software. Our results demonstrate that VN protein expression level was altered in descending order, infarct center > infarct edge > near the infarct tissues > away from the infarct tissues. The difference among each section was considered statistically significant (P<0.001). Differences of the VN protein expression in the same section among the groups were not considered statistically significant (P>0.05; Table II).
Table IIVN protein expression levels of placenta in regions around the infarct organizations (IOD, ×10−2). |
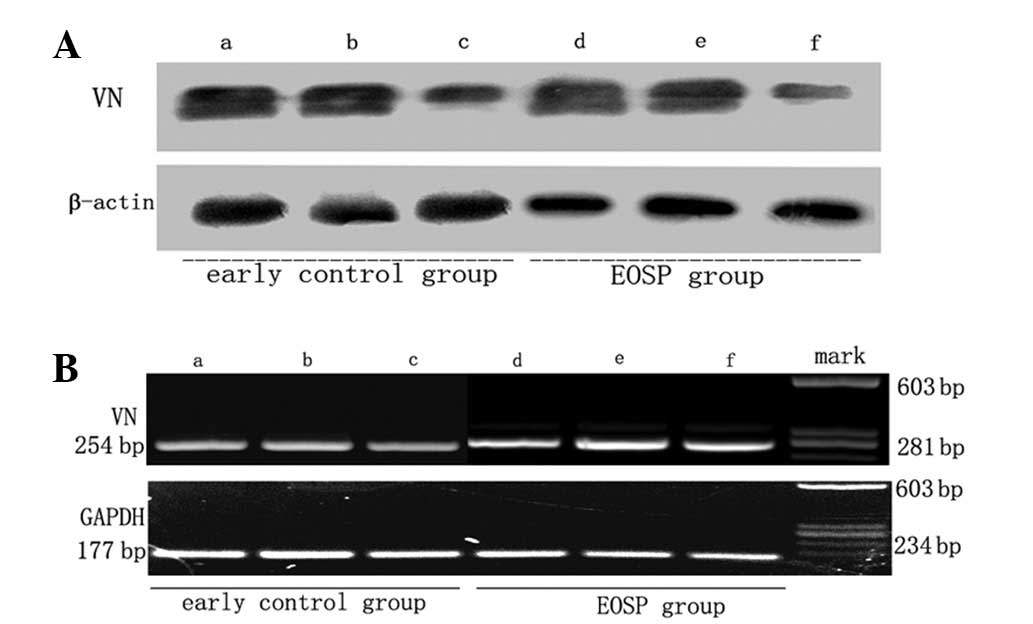
Immunoblot analysis of VN protein expression in sections of EOSP placentas
To validate the immunohistochemistry observations, western blot analysis was performed to analyze VN protein expression in sections of EOSP placentas. These data demonstrate that VN protein expression is higher in the infarct center than near infarct organizations and away from infarct organizations, while expression in near infarct organizations is higher than that away from infarct organizations. Results of the western blot analysis in the present study are consistent with immunohistochemical and immunofluorescence analysis (Fig. 2A).
VN mRNA expression levels in placentas of EOSP and the early control group
Results obtained in the present study confirm that VN protein levels change significantly between groups. To this end, we hypothesized that VN mRNA expression may alter in the placentas of the EOSP and early control groups. RT-PCR was used to detect VN mRNA expression in the infarct center, infarct edge, near the infarct tissue and away from the infarct tissue of the two groups. The trend of mRNA levels was consistent with immunohistochemistry and western blot analysis results (Fig. 2B). These effects may be due to necrosis in the infarct region. To address this question in detail further studies are required.
Coagulation parameters determination in the maternal plasma of each group
The effects on coagulation parameters in the maternal plasma of each group was examined. Coagulation assays were performed to elucidate the effects on coagulation parameters, including PT, APTT, FIB and international normalized ratio (INR). Our results reveal that the PT was significantly shorter in the EOSP group compared with the early control group and the difference was considered statistically significant (P<0.05). When the PT of the LOSP group was compared with the late control group, no significant difference was observed (P>0.05). In contrast to PT, no statistically significant difference was found in the APTT and INR in each group (P>0.05; Table III).
Correlation between VN protein in maternal-fetal interface placental tissues of EOSP and coagulation parameters
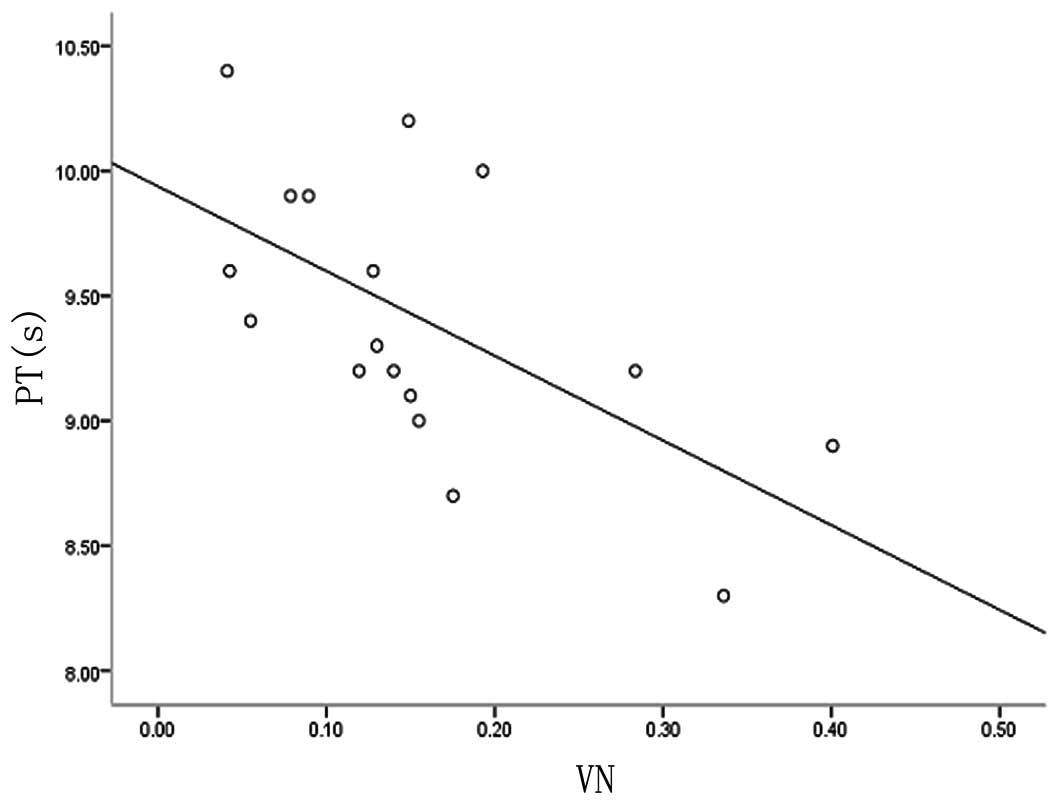
In the present study, we have demonstrated that VN protein is expressed in the maternal-fetal interface of placental tissues of EOSP and that PT is significantly shorter in the EOSP group. We also analyzed whether VN protein is correlated with PT in the EOSP group. Spearman’s rank correlation test was used to calculate the correlation coefficient. A significant negative correlation was observed between plasma PT and the expression levels of VN in maternal-fetal interface placental tissues of EOSP [y(PT)=9.938–3.39×(VN), r=0.612, P<0.05; Fig. 3]. This finding indicates that overexpressed VN protein in maternal-fetal interface placental tissues of EOSP may affect maternal coagulation.
Discussion
Although the etiology of PE remains enigmatic, it is widely considered that the pathophysiology of placenta is the driving force. The current protocol for PE is childbirth and subsequent removal of the placenta (15). Additional evidence has demonstrated that EOSP has unique pathophysiological changes and its severity appears to have a close correlation with placental lesions, including infarct, fibrosis, necrosis and atherosclerosis, as well as differences in gene expression (16,17). Placental lesions of EOSP aggravate oxidative stress levels in the placenta, resulting in changes in expression profiles of vascular endothelial growth factors, placental growth factor and soluble vascular endothelial growth factor receptor (18–21). Alterations in the expression of these molecules are responsible for the induction of maternal endothelial dysfunction and clinical manifestitions associated with severe PE, including hypertension and proteinuria.
VN is also known as the S protein of complement, epibolin and serum spreading factor and plays a significant role in cell migration, tissue repair and regulation of MAC formation, enhancing the inflammatory process during infection (9). As a human multifunctional glycoprotein, VN is associated with numerous macromolecular reactions with cell surface integrin receptors, ECM, anti-thrombin and thrombin. VN is not only involved in adhesion between cells and cells and stromal cells, but is also involved in the regulation of coagulation and fibrinolysis and immune defense. The circulating VN concentration is 200–400 mg/ml, constituting 0.2–0.5% of total plasma proteins. The concentration of VN in plasma has been reported to decrease in patients with liver disease and patients with paroxysmal nocturnal hemoglobinuria (22). Elevated expression of VN protein has been found in breast cancer (23). Previous studies (12) have shown that VN is expressed in the placenta, but few have analyzed VN expression levels in placental lesions, particularly in patients with EOSP.
In this study, we demonstrated that expression levels of VN protein were significantly increased in the maternal-fetal interface of EOSP in comparison with other groups. VN expression gradually decreased and mRNA was detected in the infarct center, infarct edge, near the infarct and away from the infarct of each group by immunohistochemistry, western blot analysis and RT-PCR. These results indicate that VN may be involved in the development of EOSP. A large number of cases of placental infarction and atherosclerosis in EOSP lead to placental ischemia, hypoxia and failure in local perfusion of placenta. Increased VN may stabilize the ECM and inhibit cell membrane damage (24), through its adhesive properties and its capacity to combine with MAC (25). This may protect blood vessel integrity, preventing the shedding of necrotic placental tissues and the toxic substances into maternal circulation. As a result, these processes prevent occurrence and development of PE. Moreover, local termination of bleeding during the necrotic period of placental infarct may be attributed to VN coagulation function.
Blumenstein et al(4) demonstrated that the 75-kDa single-chain VN molecule increases 1.6- to 1.9-fold in plasma of patients with PE and hypothesized that the profile of VN in plasma may be associated with PE. Immunohistochemical analysis in the present study revealed numerous free granules or fragments with VN antibody stain in the infarct center of the placental maternal-fetal interface, which may be released by necrotic trophoblasts and secreted by EVTs near the infarct center. Results were consistent with those of RT-PCR and western blot analysis. Therefore, we hypothesized that the free granules or fragments and EVTs near the infarct center may be the source of elevated VN levels in plasma of PE.
Through analysis of the coagulation parameter changes in maternal plasma of each group, we found that PT was shorter in the EOSP group. These data indicate that VN may be involved in the regulation of coagulation and fibrinolysis. When maternal vascular endothelial cell damage occurs in PE patients, VN may protect vascular integrity and promote local thrombosis and coagulation to prevent bleeding through combination with platelets, antithrombin III and thrombin and plasminogen activator inhibitor-1 (26). With PT shortened in EOSP patients, the extrinsic coagulation system may be activated. In addition, a negative correlation (r=0.612, P<0.05) between plasma PT and VN expression levels in maternal-fetal interface of placenta of EOSP was observed. Free VN in maternal circulation may be a cause of imbalance between the coagulation and fibrinolytic systems. This hypothesis needs to be confirmed by futher studies on changes in maternal plasma levels of VN.
In summary, increased expression levels of VN protein and mRNA in placental maternal-fetal interface of EOSP may be involved in adhesion and repair of placental infarct lesions. Utilization of an antibody against VN-stained free granules or fragments within and near placental infarct and EVTs near the infarct center and may be the source of plasma VN of PE. Negative correlation between plasma PT and expression levels of VN in maternal-fetal interface of EOSP placenta suggests that VN has a crucial impact on the activation of the extrinsic coagulation system.
Acknowledgements
This study was supported by a grant from the Science and Technology Department of Jilin Province (no. 20090464) and the Science and Technology Agency of Changchun, China (no. 08SF44). The author thanks Hongliang Sha and Yang Xia from the Department of Pathology of Jilin University Bethune Second Hospital for their kind cooperation during experiments.
References
|
Marzioni D, Todros T, Cardaropoli S, et al: Activating protein-1 family of transcription factors in the human placenta complicated by preeclampsia with and without fetal growth restriction. Placenta. 31:919–927. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Lindheimer MD, Taler SJ and Cunningham FG: Hypertension in pregnancy. J Am Soc Hypertens. 2:484–494. 2008. View Article : Google Scholar | |
|
World Health Organization (WHO). Attending to 136 million births, every year. The World Health Report 2005: Make Every Mother and Child Count. 1st edition. WHO Press; Geneva, Switzerland: pp. 642005 | |
|
Blumenstein M, Prakash R, Cooper GJ and North RA: Aberrant processing of plasma vitronectin and high-molecular-weight kininogen precedes the onset of preeclampsia. Reprod Sci. 16:1144–1152. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Chanprapaph P: Update in pre-eclampsia. J Med Assoc Thai. 87(Suppl 3): S104–S112. 2004.PubMed/NCBI | |
|
Alanis MC, Robinson CJ, Hulsey TC, Ebeling M and Johnson DD: Early-onset severe preeclampsia: induction of labor vs elective cesarean delivery and neonatal outcomes. Am J Obstet Gynecol. 199:262.e1–262.e6. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Aris A, Benali S, Ouellet A, Moutquin JM and Leblanc S: Potential biomarkers of preeclampsia: inverse correlation between hydrogen peroxide and nitric oxide early in maternal circulation and at term in placenta of women with preeclampsia. Placenta. 30:342–347. 2009. View Article : Google Scholar | |
|
Wang AG, Yen MY, Hsu WM and Fann MJ: Induction of vitronectin and integrin alphav in the retina after optic nerve injury. Mol Vis. 12:76–84. 2006.PubMed/NCBI | |
|
Singh B, Su YC and Riesbeck K: Vitronectin in bacterial pathogenesis: a host protein used in complement escape and cellular invasion. Mol Microbiol. 78:545–560. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Lam C, Lim KH and Karumanchi SA: Circulating angiogenic factors in the pathogenesis and prediction of preeclampsia. Hypertension. 46:1077–1085. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Keeton M, Eguchi Y, Sawdey M, Ahn C and Loskutoff DJ: Cellular localization of type 1 plasminogen activator inhibitor messenger RNA and protein in murine renal tissue. Am J Pathol. 142:59–70. 1993.PubMed/NCBI | |
|
Tedesco F, Radillo O, Candussi G, Nazzaro A, Mollnes TE and Pecorari D: Immunohistochemical detection of terminal complement complex and S protein in normal and pre-eclamptic placentae. Clin Exp Immunol. 80:236–240. 1990. View Article : Google Scholar : PubMed/NCBI | |
|
Rasul A, Yu B, Khan M, et al: Magnolol, a natural compound, induces apoptosis of SGC-7901 human gastric adenocarcinoma cells via the mitochondrial and PI3K/Akt signaling pathways. Int J Oncol. 40:1153–1161. 2012.PubMed/NCBI | |
|
Rasul A, Yu B, Zhong L, Khan M, Yang H and Ma T: Cytotoxic effect of evodiamine in SGC-7901 human gastric adenocarcinoma cells via simultaneous induction of apoptosis and autophagy. Oncol Rep. 27:1481–1487. 2012.PubMed/NCBI | |
|
James JL, Whitley GS and Cartwright JE: Pre-eclampsia: fitting together the placental, immune and cardiovascular pieces. J Pathol. 221:363–378. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Redman CW and Sargent IL: Latest advances in understanding preeclampsia. Science. 308:1592–1594. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Vedin JA, Wilhelmsson CE and Werkö L: Comparative study of alprenolol and methyldopa in previously untreated essential hypertension. Br Heart J. 35:1285–1292. 1973. View Article : Google Scholar : PubMed/NCBI | |
|
Egbor M, Ansari T, Morris N, Green CJ and Sibbons PD: Morphometric placental villous and vascular abnormalities in early- and late-onset pre-eclampsia with and without fetal growth restriction. BJOG. 113:580–589. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Meler E, Figueras F, Bennasar M, Gomez O, Crispi F and Gratacos E: The prognostic role of uterine artery Doppler investigation in patients with severe early-onset preeclampsia. Am J Obstet Gynecol. 202:559.e1–559.e4. 2010.PubMed/NCBI | |
|
Ohkuchi A, Hirashima C, Matsubara S, et al: Alterations in placental growth factor levels before and after the onset of preeclampsia are more pronounced in women with early onset severe preeclampsia. Hypertens Res. 30:151–159. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Wikström AK, Nash P, Eriksson UJ and Olovsson MH: Evidence of increased oxidative stress and a change in the plasminogen activator inhibitor (PAI)-1 to PAI-2 ratio in early-onset but not late-onset preeclampsia. Am J Obstet Gynecol. 201:597.e1–597.e8. 2009.PubMed/NCBI | |
|
Kobayashi J, Yamada S and Kawasaki H: Distribution of vitronectin in plasma and liver tissue: relationship to chronic liver disease. Hepatology. 20:1412–1417. 1994. View Article : Google Scholar : PubMed/NCBI | |
|
Aaboe M, Offersen BV, Christensen A and Andreasen PA: Vitronectin in human breast carcinomas. Biochim Biophys Acta. 1638:72–82. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Chauhan AK and Moore TL: Presence of plasma complement regulatory proteins clusterin (Apo J) and vitronectin (S40) on circulating immune complexes (CIC). Clin Exp Immunol. 145:398–406. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Li DQ, Lundberg F and Ljungh A: Binding of vitronectin and clusterin by coagulase-negative staphylococci interfering with complement function. J Mater Sci Mater Med. 12:979–982. 2001. View Article : Google Scholar : PubMed/NCBI | |
|
Preissner KT: Structure and biological role of vitronectin. Annu Rev Cell Biol. 7:275–310. 1991. View Article : Google Scholar : PubMed/NCBI |