Introduction
Chondrocytes in cartilage are differentiated from
mesenchymal cells during embryonic development (1,2).
They are the only cell type located in normal mature cartilage and
they function to maintain extracellular matrix (ECM) integrity by
synthesizing cartilage-specific ECM in sufficient quantities. This
homeostasis is destroyed in degenerative diseases, including
osteoarthritis (OA) and rheumatoid arthritis (RA) (3). The biochemical and structural changes
in chondrocytes and cartilage that characterize arthritis include
the degradation of the cartilage matrix and insufficient ECM
synthesis due to a loss of chondrocyte phenotype. OA is the most
common form of joint disease that evolves from a local inflammatory
disease into a chronic process with a variable degree of
degeneration of the articular cartilage and inflammation. This
ultimately exposes the underlying bone and results in pain and
disability (4). The second type of
arthritis, RA, is a destructive and inflammatory, polyarticular
joint disease, with an etiology that remains to be clarified. RA is
characterized massive synovial proliferation and subintimal
infiltration of the inflammatory cells followed by the destruction
of cartilage and bone (5).
Tannins are water-soluble polyphenols that are
widely distributed in the plant kingdom, including in food grains
and fruits (6). Based on their
structural characteristics, tannins may be separated into four
major groups: gallotannins (GTs), ellagitannins, complex tannins
and condensed tannins. These tannins are thought to have notable
biological and pharmacological activities (7,8). The
hydrolysable tannin, gallotannin, is a compound of the polygalloyl
esters of glucose. Gallotannin is a type of tannic acid derived
from plant polyphenols. Tannins have two types of structure;
condensed tannins are a polymer of flavonoid units, while
hydrolysable tannins are carbohydrates. The hydroxyl groups of the
carbohydrate are partially or completely esterified with phenolic
groups, including gallic acid. Hydrolysable tannins are hydrolyzed
by weak acids or weak bases to produce carbohydrates and phenolic
acids. These hydrolysable tannins are used in medical agents for
their useful properties, including their anti-viral, anti-bacterial
and anti-parasitic effects. GTs have been shown to exhibit diverse
biological abilities, ranging from anti-inflammation to
anti-oxidant effects (9,10).
Cyclooxygenases (COXs) are known to exist in two
isoforms, COX-1 and COX-2. In addition, the two COX isoforms were
identified with a similar sequence (11). COX-1 is a constitutive enzyme
located in the majority of mammalian cells (12). COX-2, however, is undetectable in
the majority of normal tissues (13). COX-2 is an inducible enzyme that
becomes abundant in activated macrophages and other cells at sites
of inflammation as a result of various stimuli, including
cytokines. Expression of COX-2 was demonstrated to increase
prostaglandin E2 (PGE2) production (14,15)
and induce various inflammatory reactions (16). Mice that lack COX-2, but possess
COX-1 expression, exhibit reduced bone resorption in response to
parathyroid hormone (PTH) or 1, 25-hydroxyl vitamin
D3(17). COX-2 may also
have a role in bone formation as local or systemic injections of
PGE2 stimulate bone formation (18,19).
COX-2 mediates the increase in lamellar bone formation that occurs
as a response to mechanical strain (20,21).
The mitogen-activated protein kinase (MAPK) cascades
make up one of the major signaling systems by which cells transduce
and integrate diverse intracellular signals. MAPKs are
serine/threonine kinases that regulate a variety of processes,
including cell growth, proliferation, apoptosis and extracellular
matrix accumulation. The three MAPK subfamilies consist of
extracellular signal-regulated kinases (ERKs), p38 kinases and
c-Jun NH2-terminal kinases (JNKs) (22). Previous studies in articular
chondrocytes indicated that NO caused apoptosis and
dedifferentiation, which were mediated by the MAPK subtypes, ERK
and p38 kinase (23). These MAP
kinases play opposing roles; activated ERK-1/-2 induces
dedifferentiation, COX-2 expression and NO-induced apoptosis
inhibition, whereas p38 kinase signaling triggers apoptosis, COX-2
expression and maintains the differentiated states (24).
In the present study, ERK-1/-2 and p38 kinase are
demonstrated to regulate GT-induced differentiation and
inflammation of chondrocytes, respectively.
Materials and methods
Cell culture
Rabbit articular chondrocytes were isolated from
cartilage slices obtained from 2-week-old New Zealand white rabbits
using enzymatic digestion, as described previously (25). The cartilage slices were
dissociated enzymatically for 6 h in 0.2% collagenase type II (381
U/mg solid; Sigma Aldrich, Louis, MO, USA), in Dulbecco’s modified
Eagle’s medium (DMEM; Gibco-BRL, Gaithersburg, MD, USA). Individual
cells were suspended in DMEM supplemented with 10% (v/v) fetal
bovine-calf serum, 50 g/ml streptomycin and 50 U/ml penicillin,
after which they were plated on culture dishes at a density of
5×104 cells/cm2. The medium was changed every
2 days following seeding and the cells reached confluence in ~5
days. At 3.5 days the cell cultures were treated with GT purchased
from Sigma Aldrich and a stock solution (MW: 1701.23 mM) in DMSO
was prepared and stored at 4°C. The following pharmacological
agents were added 1 h prior to the GT: SB203580 (Calbiochem, San
Diego, CA, USA) to inhibit the p38 kinase and PD98059 (Calbiochem)
to inhibit the MEK-1/-2. The treated cells, which were cultured in
complete medium for 24 h, were used for further analysis, as
indicated in each experiment. The study was approved by the ethics
committee of Kongju National University, Gongju, Republic of
Korea.
Western blot analysis
Whole cell lysates were prepared by extracting
proteins, using a buffer containing 50 mM Tris-HCl, 150 mM NaCl, 1%
Nonidet P-40 and 0.1% sodium dodecylsulfate (SDS) at pH 7.4,
supplemented with protease inhibitors [10 g/ml leupeptin, 10 g/ml
pepstatin A, 10 g/ml aprotinin and 1 mM of
4-(2-aminoethyl)-benzenesulfonyl fluoride] and phosphatase
inhibitors (1 mM NaF and 1 mM Na3VO4). The
proteins were size-fractionated by SDS-polyacrylamide gel
electrophoresis and transferred to a nitrocellulose membrane. The
nitrocellulose sheet was then blocked with 3% skimmed dry milk in
Tris-buffered saline. The following antibodies were used:
anti-COX-2 (Cayman Chemical, Ann Arbor, MI, USA), pp38 (Cell
Signaling, Beverly, MA, USA), pERK, ERK-2 and p38 (Santa Cruz
Biotechnology Inc., Santa Cruz, CA, USA) and type II collagen
(Chemicon, Temecula, CA, USA). The western blot samples were
developed using a peroxidase-conjugated secondary antibody on a
chemiluminescence system.
Alcian blue staining assay
The cells were fixed with 95% methanol at -20°C for
2 min and stained with 0.1% alcian blue in 0.1 M HCl overnight. The
chondrocytes were washed three times with PBS buffer and 6 M
guanidine HCl was added for 6 h. The production level of sulfated
proteoglycan was measured at 620 nm by enzyme-linked immunosorbent
assay (ELISA).
PGE2 assay
PGE2 production was determined by
measuring the levels of cellular and secreted PGE2 using
an assay kit (Amersham Pharmacia Biotech, Piscataway, NJ, USA).
Briefly, chondrocytes were seeded in standard 96-well microtiter
plates at 2×104 cells/well. Following addition of the
indicated pharmacological reagents, the supernatant was used to
quantify the amount of PGE2, according to the
manufacturer’s protocol. The PGE2 levels were calculated
using a PGE2 standard curve.
Immunohistochemistry
Rabbit joint cartilage explants were fixed in 4%
paraformaldehyde in PBS for 24 h at 4°C, washed with PBS,
dehydrated in ethanol, embedded in paraffin and sliced into 4-μm
sections, as described previously (26). The sections were stained by
standard procedures, using antibodies against type II collagen and
COX-2, then alcian blue staining followed by visualization by
development with a kit purchased from Dako (Carpinteria, CA, USA),
following the manufacturer’s instructions.
Immunofluorescence staining
Expression and distribution of type II collagen and
COX-2 in rabbit articular chondrocytes were determined by indirect
immunofluorescence microscopy, as described previously (26). Briefly, chondrocytes were fixed
with 3.5% paraformaldehyde in PBS for 10 min at room temperature.
The cells were permeabilized and blocked with 0.1% Triton X-100 and
5% fetal calf serum in PBS for 30 min. The fixed cells were washed
and incubated for 1 h with antibodies (10 g/ml) against type II
collagen and COX-2. The cells were washed, incubated with
rhodamine- or fluorescein-conjugated secondary antibodies for 30
min and observed under a fluorescence microscope.
Reverse transcription (RT)-PCR
Primary cultured chondrocytes were treated with GT.
Total RNA was isolated from the cells and reverse transcribed with
a Maxime RT-PCR PreMix kit (Intron Biotechnology, Seoul, Korea).
The following primers (based on the sequences of the rabbit type II
collagen and COX-2) and conditions were used for the PCR: Type II
collagen (370-bp product, annealing temperature 45°C, 30 cycles)
sense, 5′-GAC CCC ATG CAG TAC ATG CG-3′ and antisense, 5′-AGC CGC
CAT TGA TGG TCT CC-3′; COX-2 (282 bp product, annealing temperature
45°C, 30 cycles) sense, 5′-TCA GCC ACG CAG CAA ATC CT-3′ and
antisense, 5′-AGC CGC CAT TGA TGG TCT CC-3′. GAPDH was amplified
for control and normalization purposes using the following primers
and conditions: (299-bp product, annealing temperature 45°C, 30
cycle) sense, 5′-TCA CCA TCT TCC AGG AGC GA-3′ and antisense,
5′-CAC AAT GCC GAA GTG GTC GT-3′.
Data analysis and statistics
All results were expressed as the mean ± SE,
calculated from the specified number of determinations. A Student’s
t-test was used to compare individual treatments with their
respective control values. P<0.05 was considered to indicate a
statistically significant difference.
Results
GT induces differentiation in rabbit
articular chondrocytes
GT has known biological properties, including
anti-cancer, anti-inflammation and anti-oxidant effects (27,28).
First, the effects of GT on differentiation in articular
chondrocytes were examined. Various concentrations of the GT
treatment (10–150 μM) increased type II collagen expression and
activation of ERK-1/-2 and p38 kinase in a dose-dependent manner,
as determined by western blotting (Fig. 1A). When cells were treated with 100
μM GT, type II collagen expression was increased in a
time-dependent manner, while ERK-1/-2 phosphorylation was
transiently increased, as determined by the phosphorylation status
of the protein (Fig. 1B). Levels
of ERK-1/-2 phosphorylation began to increase at 10 min, reached
peak levels at 1 h and 24 h and decreased thereafter.
Phosphorylation of p38 was increased in a time-dependent manner
(Fig. 1B). As expected,
transcription levels of type II collagen were increased, as
determined by RT-PCR (Fig. 1C).
Consistent with the expression pattern of type II collagen, GT
treatment led to a dose-dependent decrease in the accumulation of
sulfated proteoglycan (Fig. 1D).
Overall, GT significantly increased the expression of type II
collagen and the phosphorylation of ERK-1/-2 and p38 kinase in the
chondrocytes, in a dose- and time- dependent manner.
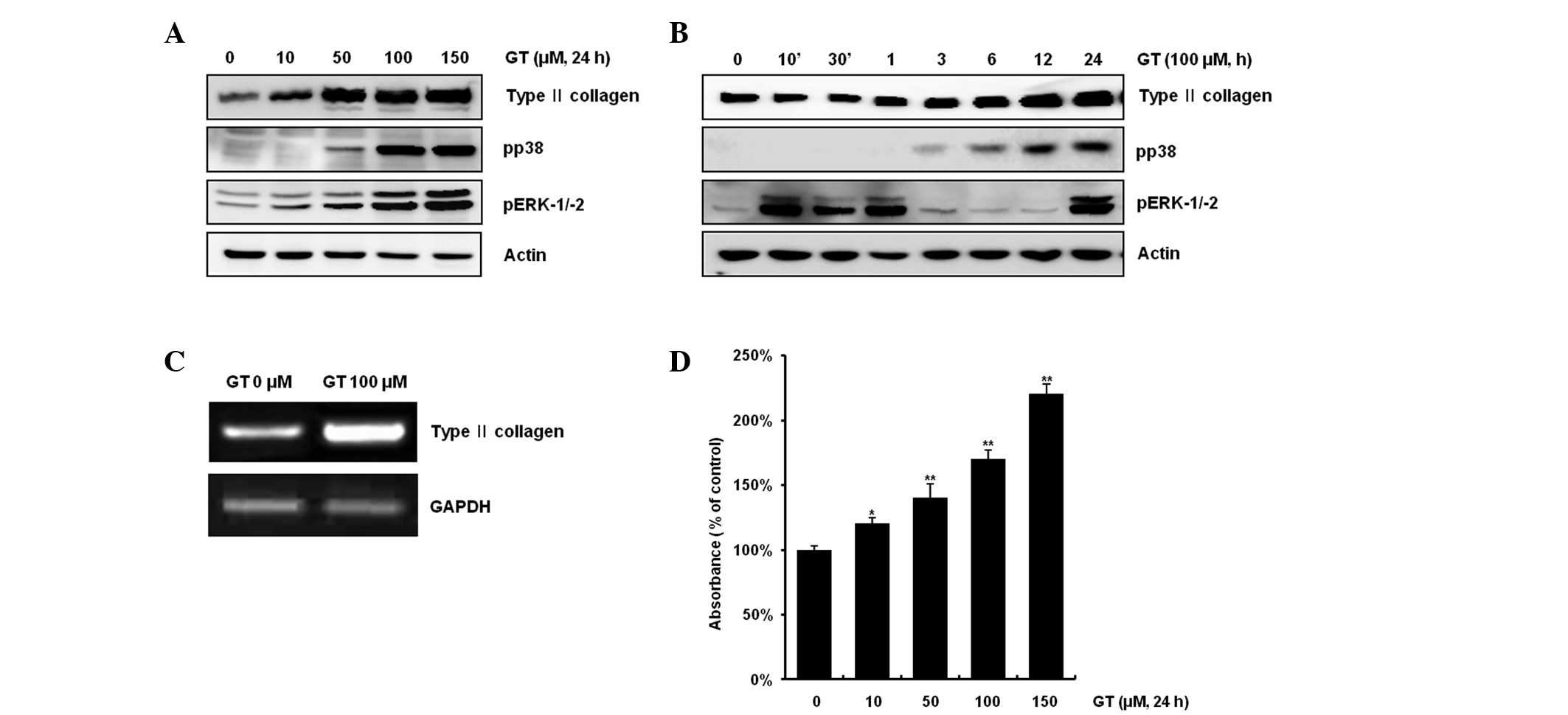 | Figure 1Gallotannin (GT) causes
differentiation in rabbit articular chondrocytes. (A) Rabbit
articular chondrocytes were treated with 10, 50, 100 or 150 μM GT
for 24 h and subjected to western blot analysis with antibodies for
type II collagen, pERK, pp38 and actin. (B) Chondrocytes were
treated with 100 μM GT for the indicated time periods. Western blot
analysis of the expression of type II collagen, pERK, pp38 and
actin was performed. Expression of β-actin was used as a loading
control. (C) Articular chondrocytes were treated with 100 μM GT for
24 h or left untreated. Expression of type II collagen and GAPDH
was detected by RT-PCR. Expression of GAPDH was used as a loading
control. (D) Rabbit articular chondrocytes were treated with 10,
50, 100 or 150 μM GT for 24 h. Accumulation of sulgated
proteoglycan was determined by alcian blue staining. The data
represent a typical experiment, whereby similar results were
obtained from three experiments. *p<0.05 and
**p<0.001, compared with untreated cells. RT-PCR,
reverse transcription PCR. |
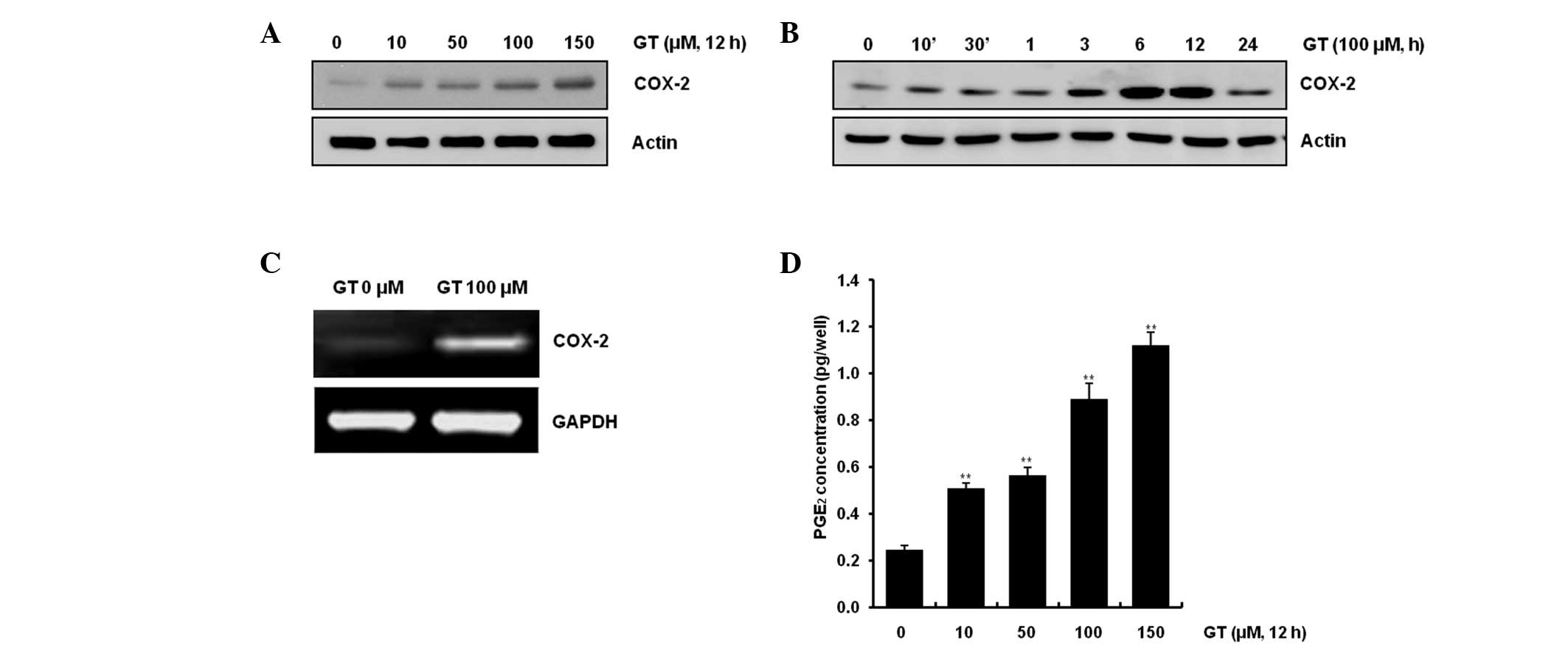
GT causes inflammation in
chondrocytes
Previous studies showed that expression of COX-2
increased PGE2(15) and
that PGE2 induced various inflammation reactions
(16). In the present study,
chondrocytes were treated with varying concentrations of GT
(Fig. 2A) or with 100 μM GT for
the indicated period (Fig. 2B). As
shown in Fig. 2A and B, GT caused
COX-2 expression in a dose- and time-dependent manner, as
determined by western blotting. Transcription levels of COX-2 were
increased with the 100 μM GT treatment, as determined by RT-PCR
(Fig. 2C). As expected, similar
results were observed when PGE2 production was
quantified by PGE2 assay (Fig. 2D). GT exhibited production of
PGE2 that was ~5.6-, 6.3-, 10.2- and 16.7-fold more than
the control in the rabbit articular chondrocytes. These results
demonstrated that GT caused inflammation of articular chondrocytes
in primary cultured cells.
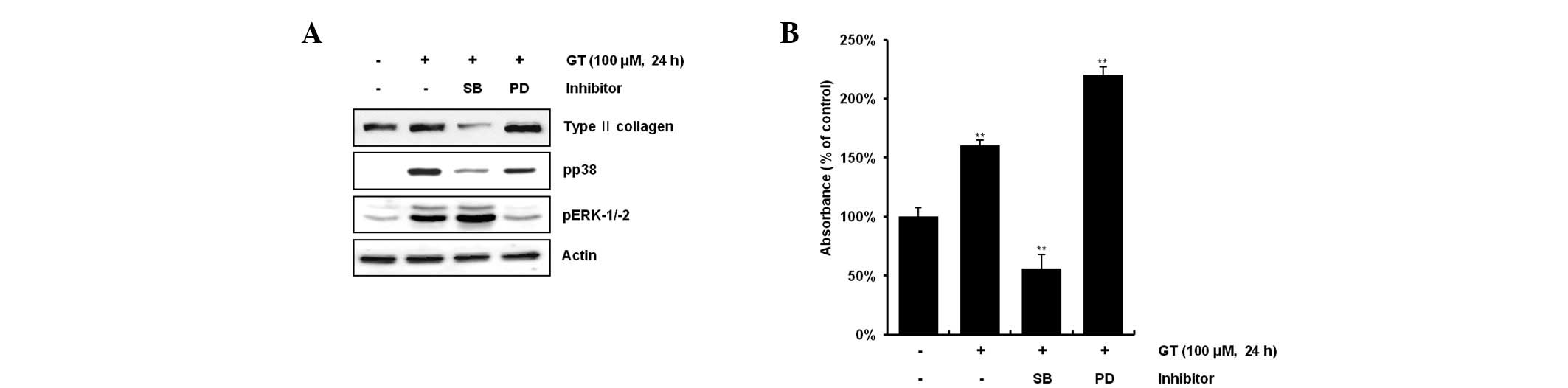
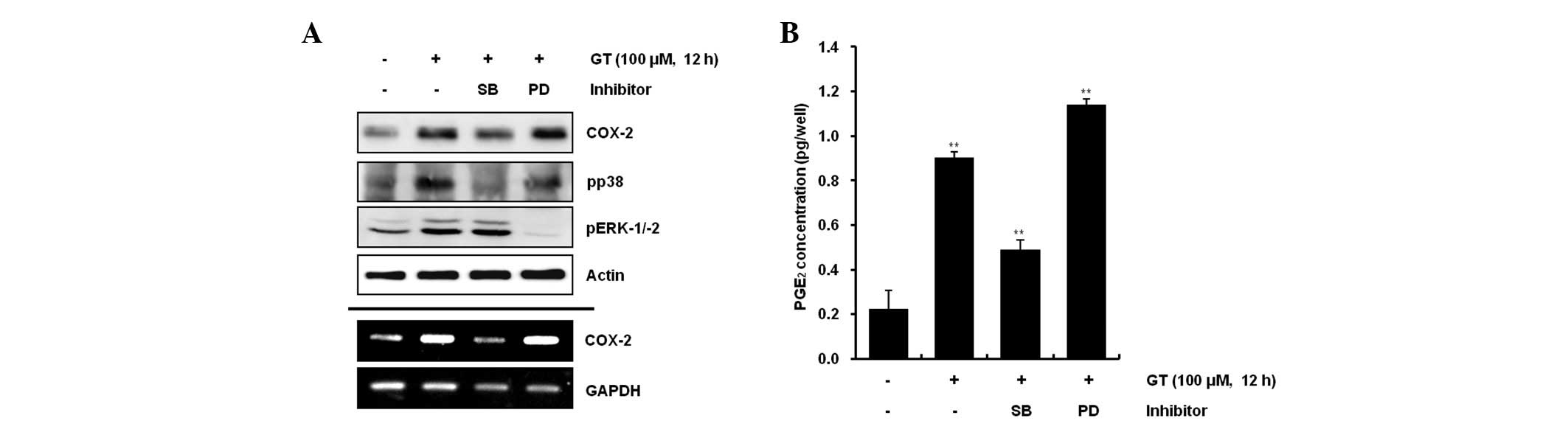
GT regulates differentiation and
inflammation via the ERK-1/-2 and p38 kinase pathways in rabbit
articular chondrocytes
The present study examined the possible modulation
of GT-induced activation of the MAP kinase subtypes ERK-1/-2 and
p38 kinase on GT-induced differentiation and inflammation.
Inhibition of GT-induced p38 kinase activation with 20 μM SB203580
reduced differentiation and inflammation, whereas inhibition of
GT-induced ERK-1/-2 with 20 μM PD98059 resulted in the potentiation
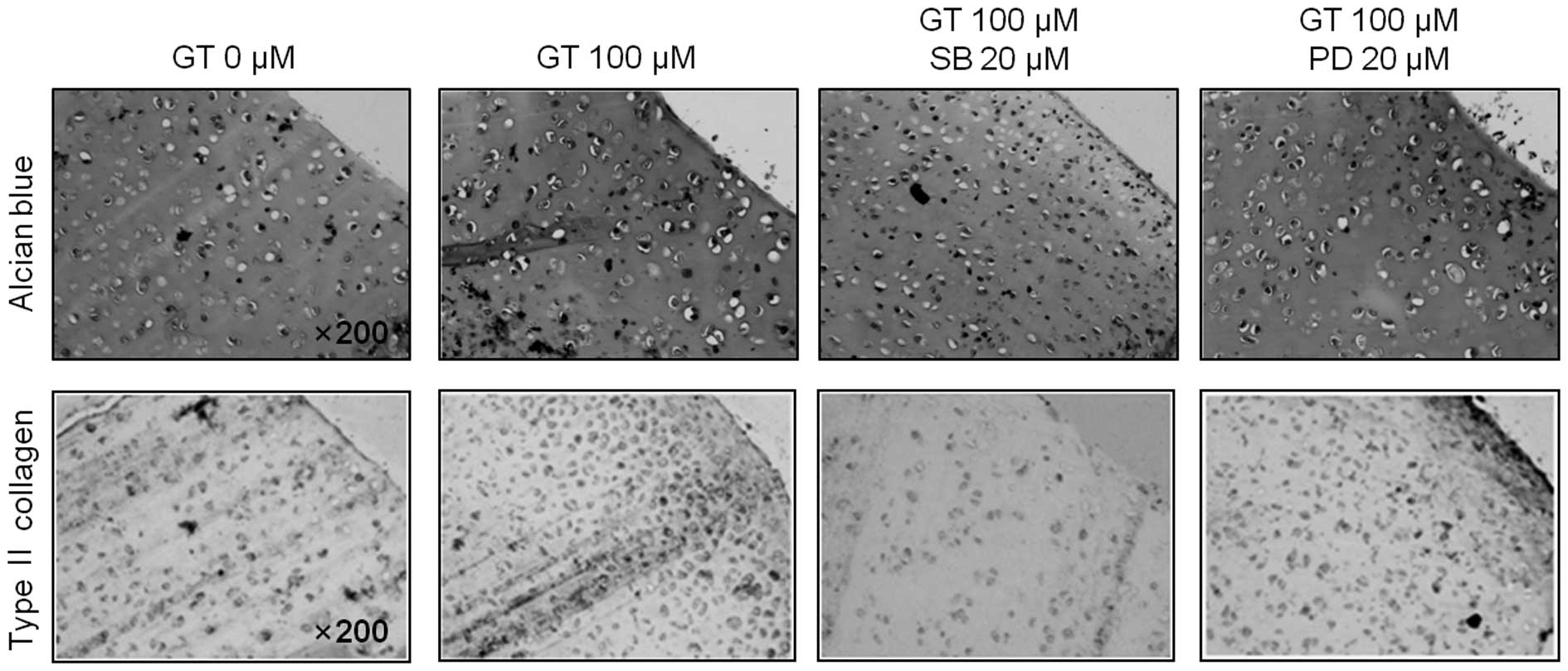
of differentiation and inflammation (Figs. 3A and 4A). Consistent with the expression
patterns of type II collagen and COX-2, inhibition of GT-induced
p38 kinase activation led to a decrease in the accumulation of
sulfated proteoglycan and PGE2 production, whereas
inhibition of GT-induced ERK-1/-2 promoted the accumulation of
sulfated proteoglycan and PGE2 production, as determined
by alcian blue staining and PGE2 assays, respectively
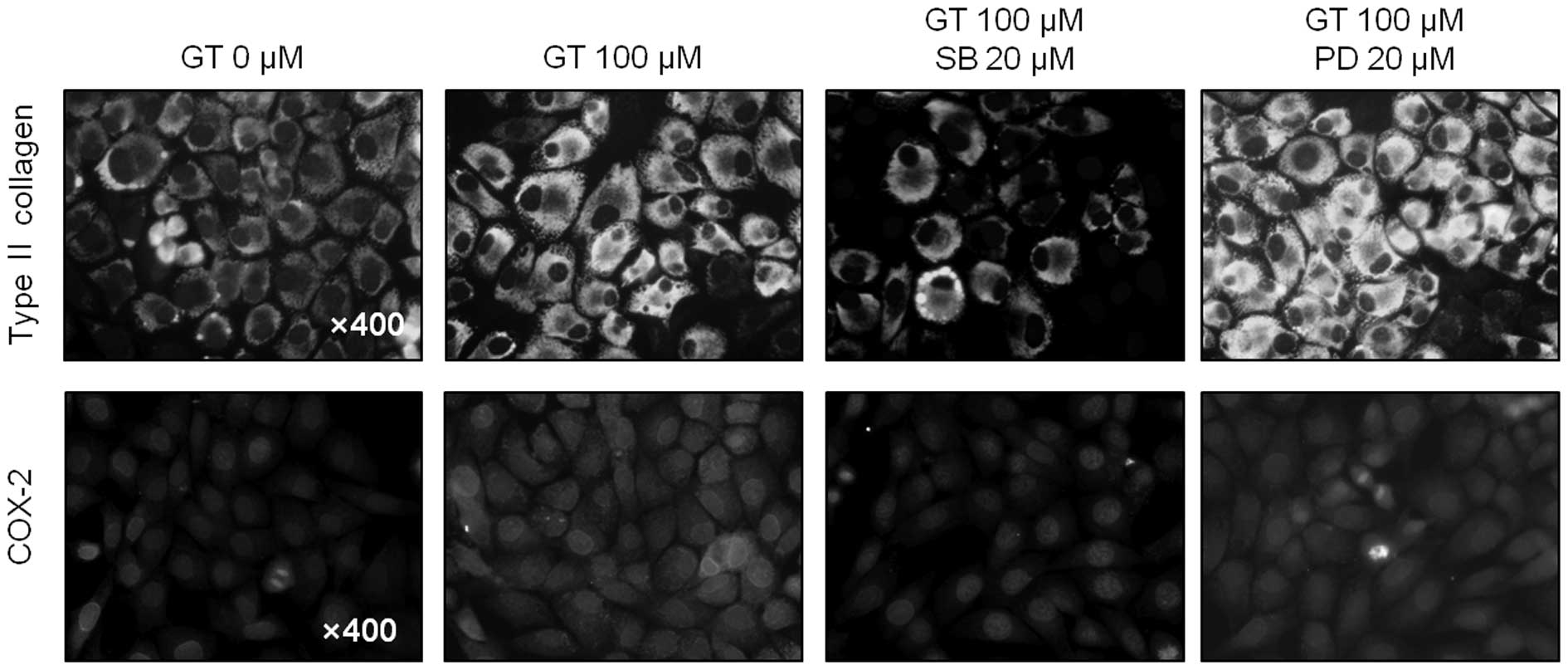
(Figs. 3B and 4B). Consistent with the western blot
data, the immunostaining results also showed that inhibition of p38
kinase markedly blocked type II collagen and COX-2 expression
levels, whereas ERK-1/-2 inhibition caused a significant increase
in type II collagen and COX-2 expression (Fig. 5). Similar results were observed
when inhibition of GT caused ERK-1/-2 and when p38 kinase had the
opposite effect on GT-induced differentiation, as determined by
immunohistochemical staining (Fig.
6). The results collectively indicate that GT-induced ERK-1/-2
and p38 kinase had opposite effects on differentiation and
inflammation in rabbit articular chondrocytes.
Discussion
GT is a type of tannic acid derived from plant
polyphenols, that is usually an agonist of plant defense
mechanisms. The tannin GT is used in medical agents for its
anti-viral, anti-bacterial and anti-parasitic properties (27–29).
GT has been shown to exhibit diverse biological effects, including
the inhibition of chemokine and inflammatory cytokine expression in
A549 cells (30).
Generic cells develop into specific cell types by
cell differentiation as a response to specific triggers from the
body or the cells themselves. This process allows multicellular
adult organisms, containing hundreds of varying types of cells, to
develop from a single-celled zygote. Cell differentiation also has
roles in the functions of numerous organisms, particularly complex
mammals, throughout their lives. A sequential process of
differentiation is commonly known to show indications of
chondrogenic differentiation within fibrous, mesenchymal tissue,
marked by the onset of type II collagen. The next stage of the
process is characterized by the appearance of transitory,
fibrocartilaginous cells expressing collagen types II and III.
Chondrocyte phenotypes are classically categorized, mainly by the
subtyping of collagen gene expression. Thus, the expression of the
alternative splice variant of type II collagen characterizes
chondroprogenitor cells. Mature chondrocytes express the typical
cartilage collagen types II, IX and XI, as well as aggrecan and
link protein. Chick chondrocytes are able to undergo
post-hypertrophic differentiation into osteoblast-like cells,
expressing type I collagen. The present study has shown that GT
significantly induced type II collagen expression in rabbit
articular chondrocytes following 24 h of treatment in a
dose-dependent manner, as examined by western blot analysis and
RT-PCR assays. Type II collagen is a known marker of
differentiation in chondrocytes.
The metabolites of COX activity have long been
suspected to be important in skeletal reparative processes. The
administration of PGE2 has increased the rate of
fracture healing in several animal models (31,32),
indicating that the metabolites of COXs may be necessary for
efficient bone healing. The effect that PGE2 has on
chondrocytes is dependent on physiological conditions, the
microenvironment and the culture system (33,34).
PGE2 exerts anabolic effects, including proteoglycan and
type II collagen synthesis, as well as catabolic effects, including
the enhancement of matrix degradation (35–37).
For example, PGE2 has been shown to promote chondrocyte
differentiation and increase type II collagen expression in
inflammation (19,21,34,37).
In the results of our previous study, it was observed that the
addition of exogenous PGE2 did not affect chondrocyte
dedifferentiation (38). GT
decreased nitric oxide (NO) production, through inhibition of
nuclear factor (NF)-κB in macrophages (39). Generally, an increase in COX-2
expression and PGE2 products induces activation of
NF-κB. However, the present study demonstrated that GT increased
COX-2 expression and PGE2 production in articular
chondrocytes (Fig. 2). These
results suggested that GT induced the inflammation in the
chondrocytes.
Mitogen-activated protein kinase (MAPK) cascades
have been shown to play key roles in the transduction of
extracellular signals to cell responses. The MAPK families that
have been clearly characterized in mammalian cells are classical
MAPK (also known as ERK), C-Jun N-terminal kinse/stress-activated
protein kinase (JNK/SAPK) and p38 kinase (40). The MAPK pathways relay, amplify and
integrate signals from a wide range of stimuli prior to eliciting
an appropriate physiological response that may include cell
proliferation, differentiation, development, inflammatory responses
and apoptosis in mammalian cells (41). Cellular stresses, including UV
irradiation, heat shock, high osmotic stress, lipopolysaccharide,
protein synthesis inhibitors, proinflammatory cytokines and certain
mitogens activate the p38 MAPK families. p38 MAPK appears to play a
major role in apoptosis, differentiation, survival, proliferation,
development and inflammation. Previously it was reported that p38
was involved in the differentiation processes of various vertebrate
cells, including adipocytes, cardiomyocytes, chondroblasts,
erythroblasts, myoblasts and neurons (42). The ERK family (p42/44 MAPK) is
known to be an intracellular checkpoint for cellular mitogenesis.
In cultured cell lines, mitogenic stimulation by growth factors
correlates with stimulation of p42/44 MAPK. When components of the
ERK signaling pathway are interfered with using dominant negative
mutants or antisense constructs for raf-1 or ERK1, significant
inhibition of cell proliferation is revealed. In another study,
MAPKs have been shown to play opposing roles, with activated
ERK-1/-2 inducing dedifferentiation, COX-2 expression and the
inhibition of NO-induced apoptosis, while p38 kinase signaling
triggers apoptosis, COX-2 expression and maintains the
differentiated states (24). In
the present study, GT caused an increase in differentiation of the
chondrocyte phenotype, as demonstrated by the increase in type II
collagen expression and sulfated proteoglycan synthesis in a time-
and dose-dependent manner. Moreover, GT induced COX-2 expression
and PGE2 production. Inhibition of ERK-1/-2 with PD98059
potentiated GT-induced type II collagen and COX-2 expression,
whereas inhibition of p38 kinase with SB203580 decreased type II
collagen and COX-2 expression (Figs.
3 and 4). GT-induced type II
collagen and COX-2 expression and PGE2 production are
modulated by ERK-1/-2 and p38 kinase signaling (43,44).
In summary, the data from the present study have
demonstrated that ERK-1/-2 and p38 kinase oppositely regulate
GT-induced differentiation and inflammation in rabbit articular
chondrocytes.
Acknowledgements
This study was supported by a National Research
Foundation of Korea (NRF) grant, funded by the Korean Government
(MEST; 2011-0027473 and 2012-0004359).
References
|
1
|
DeLise AM, Fischer L and Tuan RS: Cellular
interactions and signaling in cartilage development. Osteoarthritis
Cartilage. 8:309–334. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sandell LJ and Adler P: Developmental
patterns of cartilage. Front Biosci. 4:D731–D742. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sandell LJ and Aigner T: Articular
cartilage and changes in arthritis. An introduction: cell biology
of osteoarthritis. Arthritis Res. 3:107–113. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Feldmann M: Pathogenesis of arthritis:
recent research progress. Nat Immunol. 2:771–773. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Feldmann M, Brennan FM and Maini RN:
Rheumatoid arthritis. Cell. 85:307–310. 1996. View Article : Google Scholar
|
|
6
|
Erdèlyi K, Kiss A, Bakondi E, Bai P, Szabó
C, Gergely P, Erdödi F and Virag L: Gallotannin inhibits the
expression of chemokines and inflammatory cytokines in A549 cells.
Mol Pharmacol. 68:895–904. 2005.PubMed/NCBI
|
|
7
|
Li W, Zhang J, Flechner L, Hyun T, Yam A,
Franke TF and Pierce JH: Protein kinase C-alpha overexpression
stimulates Akt activity and suppresses apoptosis induced by
interleukin 3 withdrawal. Oncogene. 18:6564–6572. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rapizzi E, Fossati S, Moroni F and
Chiarugi A: Inhibition of poly(ADP-ribose) glycohydrolase by
gallotannin selectively up-regulates expression of proinflammatory
genes. Mol Pharmacol. 66:890–898. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Feldman KS, Sahasrabudhe K, Lawlor MD,
Wilson SL, Lang CH and Scheuchenzuber WJ: In vitro and in vivo
inhibition of LPS-stimulated tumor necrosis factor-alpha secretion
by the gallotannin beta-D-pentagalloylglucose. Bioorg Med Chem
Lett. 11:1813–1815. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hagerman AE, Riedl KM and Rice RE: Tannins
as biological antioxidants. Basic Life Sci. 66:495–505.
1999.PubMed/NCBI
|
|
11
|
Smith WL, DeWitt DL and Garavito RM:
Cyclooxygenases: structural, cellular, and molecular biology. Annu
Rev Biochem. 69:145–182. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dubois RN, Abramson SB, Crofford L, Gupta
RA, Simon LS, Van De Putte LB and Lipsky PE: Cyclooxygenase in
biology and disease. FASEB J. 12:1063–1073. 1998.PubMed/NCBI
|
|
13
|
Wu KK: Inducible cyclooxygenase and nitric
oxide synthase. Adv Pharmacol. 33:179–207. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Goldring MB and Berenbaum F: Human
chondrocyte culture models for studying cyclooxygenase expression
and prostaglandin regulation of collagen gene expression.
Osteoarthritis Cartilage. 7:386–388. 1999. View Article : Google Scholar
|
|
15
|
Namkoong S, Lee SJ, Kim CK and Kim YM,
Chung HT, Lee H, Han JA, Ha KS, Kwon YG and Kim YM: Prostaglandin
E2 stimulates angiogenesis by activating the nitric oxide/cGMP
pathway in human umbilical vein endothelial cells. Exp Mol Med.
37:588–600. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Smith WL, Garavito RM and DeWitt DL:
Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and -2.
J Biol Chem. 271:33157–33160. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Okada Y, Lorenzo JA, Freeman AM, Tomita M,
Morham SG, Raisz LG and Pilbeam CC: Prostaglandin G/H synthase-2 is
required for maximal formation of osteoclast-like cells in culture.
J Clin Invest. 105:823–832. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Suponitzky I and Weinreb M: Differential
effects of systemic prostaglandin E2 on bone mass in rat long bones
and calvariae. J Endocrinol. 156:51–57. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Weinreb M, Suponitzky I and Keila S:
Systemic administration of an anabolic dose of PGE2 in
young rats increases the osteogenic capacity of bone marrow. Bone.
20:521–526. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Duncan RL and Turner CH:
Mechanotransduction and the functional response of bone to
mechanical strain. Calcif Tissue Int. 57:344–358. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Forwood MR: Inducible cyclo-oxygenase
(COX-2) mediates the induction of bone formation by mechanical
loading in vivo. J Bone Miner Res. 11:1688–1693. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Park EH, Kang SS, Lee YS, Kim SJ, Jin EJ,
Tak EN and Sonn JK: Integrity of the cortical actin ring is
required for activation of the PI3K/Akt and p38 MAPK signaling
pathways in redifferentiation of chondrocytes on chitosan. Cell
Biol Int. 32:1272–1278. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim SJ, Kim HG, Oh CD, Hwang SG, Song WK,
Yoo YJ, Kang SS and Chun JS: p38 kinase-dependent and -independent
Inhibition of protein kinase C zeta and -alpha regulates nitric
oxide-induced apoptosis and dedifferentiation of articular
chondrocytes. J Biol Chem. 277:30375–30381. 2002. View Article : Google Scholar
|
|
24
|
Kim SJ, Ju JW, Oh CD, Yoon YM, Song WK,
Kim JH, Yoo YJ, Bang OS, Kang SS and Chun JS: ERK-1/2 and p38
kinase oppositely regulate nitric oxide-induced apoptosis of
chondrocytes in association with p53, caspase-3, and
differentiation status. J Biol Chem. 277:1332–1339. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yoon YM, Kim SJ, Oh CD, Ju JW, Song WK,
Yoo YJ, Huh TL and Chun JS: Maintenance of differentiated phenotype
of articular chondrocytes by protein kinase C and extracellular
signal-regulated protein kinase. J Biol Chem. 277:8412–8420. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ryu JH, Kim SJ, Kim SH, Oh CD, Hwang SG,
Chun CH, Oh SH, Seong JK, Huh TL and Chun JS: Regulation of the
chondrocyte phenotype by beta-catenin. Development. 129:5541–5550.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lü L, Liu SW, Jiang SB and Wu SG: Tannin
inhibits HIV-1 entry by targeting gp41. Acta Pharmacol Sin.
25:213–218. 2004.PubMed/NCBI
|
|
28
|
Lu Y, Jiang F, Jiang H, Wu K, Zheng X, Cai
Y, Katakowski M, Chopp M and To SS: Gallic acid suppresses cell
viability, proliferation, invasion and angiogenesis in human glioma
cells. Eur J Pharmacol. 641:102–107. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Akiyama H, Fujii K, Yamasaki O, Oono T and
Iwatsuki K: Antibacterial action of several tannins against
Staphylococcus aureus. J Antimicrob Chemother. 48:487–491.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yu SM, Gweon EJ, Chung KW, Kim KH, Cho HS
and Kim SJ: Gallotannin regulates apoptosis and COX-2 expression
via Akt and p38kinase pathway in human lung cancer cell line, A549.
Animal Cells and Systems. 16:366–375. 2012. View Article : Google Scholar
|
|
31
|
Keller J: Effects of indomethacin and
local prostaglandin E2 on fracture healing in rabbits. Dan Med
Bull. 43:317–329. 1996.PubMed/NCBI
|
|
32
|
Norrdin RW and Shih MS: Systemic effects
of prostaglandin E2 on vertebral trabecular remodeling in beagles
used in a healing study. Calcif Tissue Int. 42:363–368. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Amin AR, Dave M, Attur M and Abramson SB:
COX-2, NO, and cartilage damage and repair. Curr Rheumatol Rep.
2:447–453. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Schwartz Z, Gilley RM, Sylvia VL, Dean DD
and Boyan BD: The effect of prostaglandin E2 on costochondral
chondrocyte differentiation is mediated by cyclic adenosine
3′,5′-monophosphate and protein kinase C. Endocrinology.
139:1825–1834. 1998.PubMed/NCBI
|
|
35
|
Abramson SB: The role of COX-2 produced by
cartilage in arthritis. Osteoarthritis Cartilage. 7:380–381. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Goldring MB, Birkhead JR, Suen LF, Yamin
R, Mizuno S, Glowacki J, Arbiser JL and Apperley JF: Interleukin-1
beta-modulated gene expression in immortalized human chondrocytes.
J Clin Invest. 94:2307–2316. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Goldring MB, Suen LF, Yamin R and Lai WF:
Regulation of collagen gene expression by prostaglandins and
interleukin-1beta in cultured chondrocytes and fibroblasts. Am J
Ther. 3:9–16. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lee WK, Yu SM, Cheong SW, Sonn JK and Kim
SJ: Ectopic expression of cyclooxygenase-2-induced
dedifferentiation in articular chondrocytes. Exp Mol Med.
40:721–727. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kim MS, Park SB, Suk K, Kim IK, Kim SY,
Kim JA, Lee SH and Kim SH: Gallotannin isolated from Euphorbia
species, 1,2,6-tri-O-galloyl-beta-D-allose, decreases nitric oxide
production through inhibition of nuclear factor-kappa B and
downstream inducible nitric oxide synthase expression in
macrophages. Biol Pharm Bull. 32:1053–1056. 2009. View Article : Google Scholar
|
|
40
|
Widmann C, Gibson S, Jarpe MB and Johnson
GL: Mitogen- activated protein kinase: conservation of a
three-kinase module from yeast to human. Physiol Rev. 79:143–180.
1999.PubMed/NCBI
|
|
41
|
Zhang W and Liu HT: MAPK signal pathways
in the regulation of cell proliferation in mammalian cells. Cell
Res. 12:9–18. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Houde M, Laprise P, Jean D, Blais M,
Asselin C and Rivard N: Intestinal epithelial cell differentiation
involves activation of p38 mitogen-activated protein kinase that
regulates the homeobox transcription factor CDX2. J Biol chem.
276:21885–21894. 2001. View Article : Google Scholar
|
|
43
|
Guan Z, Buckman SY, Miller BW, Springer LD
and Morrison AR: Interleukin-1beta-induced cyclooxygenase-2
expression requires activation of both c-Jun NH2-terminal kinase
and p38 MAPK signal pathways in rat renal mesangial cells. J Biol
Chem. 273:28670–28676. 1998. View Article : Google Scholar
|
|
44
|
Matsuura H, Sakaue M, Subbaramaiah K,
Kamitani H, Eling TE, Dannenberg AJ, Tanabe T, Inoue H, Arata J and
Jetten AM: Regulation of cyclooxygenase-2 by interferon gamma and
transforming growth factor alpha in normal human epidermal
keratinocytes and squamous carcinoma cells. Role of
mitogen-activated protein kinases. J Biol Chem. 274:29138–29148.
1999. View Article : Google Scholar
|