Introduction
Aspirin (acetylsalicylic acid) has been used as a
treatment for high body temperatures, headaches and muscle pain for
more than a century. Accumulating evidence from large-scale
epidemiological studies, animal models and in vitro
experiments suggest that regular use of aspirin may reduce the risk
of cancer by inducing cancer cell apoptosis (1–3).
Findings of recent studies have indicated that aspirin is able to
block NF-κB activation (4,5), induce iNOS to produce NO (6,7),
inhibit COX-2 (8), ErbB2 (9) and Bcl-2 (10) and acetylate or phosphorylate p53
(3,11), inducing cell apoptosis or
proliferation inhibition. However, the cell pathways through which
aspirin exerts its anticancer effects are not well understood and
require further elucidation.
Apoptosis in anticancer strategies is a multi-step
process involving the activation of caspases, a family of cysteine
proteases. There are two types of apoptotic caspases: initiators
(caspases-2, -8, -9 and -10) and effectors (caspases-3, -6 and -7).
Initiator caspases cleave inactive pro-forms of effector caspases
to activate them and then effector caspases catalyze the specific
cleavage of other key cellular proteins to trigger the apoptotic
process. In aspirin-induced cancer cell apoptosis, caspase-3 and
other caspase family members were previously demonstrated to be
crucial upregulated factors (4,10,12,13).
The transcription factor AP-2 family, which consists
of AP-2α, β, γ, δ and ɛ, regulates the transcription of numerous
genes involved in mammalian development, the cell cycle, cell
proliferation, apoptosis and carcinogenesis by binding to the
genes’ promoter regions (14,15).
Several reports have suggested that AP-2α and AP-2γ are marked
functional activators for ErbB2 overexpression in mammary carcinoma
(16,17), while ErbB2 overexpression is
associated with increased tumorigenicity, enhanced metastasis, poor
prognosis and decreased chemosensitivity (18).
The aim of the present study was to demonstrate
whether aspirin induces apoptosis in MDA-MB-453 cells by
upregulating caspase-3 expression and activity. Caspase-3 was found
to be directly and negatively regulated by AP-2α, which is degraded
following aspirin treatment. The caspase-3 pathway appeared to
participate in aspirin-induced apoptosis of MDA-MB-453 cells
through the downregulation of AP-2α.
Materials and methods
Plasmids, siRNA and reagents
The full-length coding region of AP-2α was cloned
into a pCMV-Myc plasmid (Clontech, Mountain View, CA, USA). The
promoter region (nt −799 to nt +93 from the transcription start
codon) of caspase-3 was generated by PCR (19) and cloned into the luciferase
reporter plasmid pTAL-luc (Clontech), denoted pTAL-luc-caspase-3.
The primer sequences used were: 5′-CGGCTAGCC TTTTTCCTCATGATGTT-3′
(forward) and 5′-GAAGATC TGCCTCCTCATACCTTCTAC-3′ (reverse). Single
or double AP-2α binding site mutants were created by site-directed
mutation and denoted as pTAL-luc-M1, -M2 and -M3. Two specific
small interfering RNAs (siRNAs) against AP-2α were purchased from
GenePharma Co. (Shanghai, China). The two siRNA sense sequences are
5′-UUUCUCAACCGACAA CAUUtt-3′ and 5′-CGAAGUCUUCUGUUCAGUUtt-3′.
Aspirin was purchased from Sigma-Aldrich (St. Louis, MO, USA) and
solubilized in water.
Cell culture, transfection and
treatment
MDA-MB-453 cells were cultured in DMEM (Gibco-BRL,
Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS;
Gibco-BRL), 2 mM L-glutamine, 100 U/ml penicillin and 100 μg/ml
streptomycin at 37°C in a 5% CO2 incubator. Cells were
cultured to 80% confluence and transiently transfected using
Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the
manufacturer’s instructions. Aspirin treatment proceeded at 90%
confluence continuously in low-serum (0.5% FBS) medium for 24
h.
Cell proliferation assay
Inhibition of cell proliferation was evaluated by
the MTT assay (Sigma-Aldrich) according to the manufacturer’s
instructions. Briefly, MDA-MB-453 cells were placed in 24-well
plates at a density of 1×105 cells/well in 450-μl medium
and 24 h after attachment, the cells were treated with 0–20 mM
aspirin for a further 24 h. Then, 50 μl of MTT (5 mg/ml in PBS)
solution were added to each well and the cells were incubated for a
further 4 h, followed by the addition of 150 μl of dimethyl
sulfoxide (DMSO; Sigma-Aldrich)/well. The cells were left for 30
min at room temperature to allow color development. Absorbance
values were determined using an enzyme-linked immunosorbent assay
(ELISA) reader (Model 680; Bio-Rad, Hercules, CA, USA) at 570
nm.
Flow cytometric analysis of
apoptosis
Apoptotic and total dead cells were stained by the
Annexin V-FITC/propidium iodide (PI) detection kit (Bender
MedSystems, Vienna, Austria) according to the manufacturer’s
instructions. In brief, MDA-MB-453 cells (1×105
cells/well) were washed with 1X Annexin V binding buffer and
stained with Annexin V (5 μl) and PI (10 μl) for 15 min at room
temperature in the dark. Following the addition of 400 μl of
binding buffer, cells were analyzed by flow cytometry (FACSCalibur;
BD Biosciences, San Jose, CA, USA).
Caspase-3 activity assay
The activity of caspase-3 was determined using the
Caspase-3 Activity Detection kit (Beyotime Institute of
Biotechnology, Haimen, China). To evaluate the activity of
caspase-3, cell lysates were prepared following the designated
treatment. Assays were performed in 96-well plates by incubating 10
μl protein of cell lysate/sample with 10 μl caspase-3 substrate
(Ac-DEVD-pNA, 2 mM) in 80 μl of reaction buffer [1% NP-40, 20 mM
Tris-HCl (pH 7.5), 137 mM NaCl and 10% glycerol] at 37°C for 5 h.
Absorbance values were determined using an enzyme-linked
immunosorbent assay (ELISA) reader (Model 680; Bio-Rad) at 405
nm.
The detailed procedure including the standard curve
preparation was described in the manufacturer’s instructions. All
the experiments were performed in triplicate.
RNA extraction, semi-quantitative RT-PCR
and real-time PCR
Total RNA extraction, RT- and real-time PCR were
performed as described previously (9). The real-time PCR primers used were:
AP2α 5′-CTCAACCGACAACATTCC-3′ (forward) and
5′-CGGTGAACTCTTTGCATATC-3′ (reverse) (20); caspase-3 5′-CAGTGGAGGCCGACTTC
TTG-3′ (forward) and 5′-TGGCACAAAGCGACTGGAT-3′ (reverse) (21). For semi-quantitative RT-PCR, DNA
was amplified under the following conditions: denaturation at 94°C
for 30 sec, annealing at 55°C for 30 sec and extension at 72°C for
30 sec and the number of cycles for amplification was 21–25. PCR
products were separated by electrophoresis on a 2% agarose gel and
visualized by ethidium bromide staining.
Western blotting
Cells were lysed in RIPA buffer with protease
inhibitors (Sigma-Aldrich). Equal amounts of protein were separated
on a 10% SDS-polyacrylamide gel and transferred onto a PVDF
membrane (Millipore, Billerica, MA, USA). After blocking, the PVDF
membranes were washed three times for 10 min with TBST at room
temperature and incubated for 1–2 h at room temperature with
TBST-diluted primary antibodies, anti-AP2α (1:500) and
anti-caspase-3 (1:500; Santa Cruz Biotechnology, Inc., Santa Cruz,
CA, USA). Following extensive washing, the membranes were further
incubated with the secondary peroxidase-labeled antibodies
(1:2,000) in 5% non-fat dry milk/TBST for 1 h. The membranes were
again washed three times for 10 min with TBST at room temperature,
immunoreactivity was visualized using the enhanced
chemiluminescence ECL kit (Pierce Biotechnology, Rockford, IL, USA)
and the membranes were exposed to Kodak film. The membranes were
then stripped and reprobed with anti-GAPDH antibody (1:1,000; Santa
Cruz Biotechnology, Inc.) as a loading control.
Chromatin immunoprecipitation (ChIP)
assay
The ChIP assay was performed using the EZ ChIP™ kit
(Millipore) according to the manufacturer’s instructions, as
previously described (22). The
antibodies used included anti-AP2α antibody (Santa Cruz
Biotechnology, Inc.) and normal rabbit IgG (Millipore). The
caspase-3 promoter ChIP primers used in the present study were:
5′-AACACAGCATGCGTGGACCT-3′ (forward) and 5′-GCCTCCTCATACCTTCTAC-3′
(reverse).
Luciferase reporter assays
MDA-MB-453 cells were seeded at 5×105
cells/well in 12-well plates and transfected at 80% confluence
using Lipofectamine 2000 reagent according to the manufacturer’s
instructions. After 24 h of transfection, the luciferase activity
was measured using the luciferase reporter assay system (Promega,
Madison, WI, USA).
Statistical analysis
Results of bar graphs were expressed as the mean ±
SD obtained from three independent experiments. Statistical
differences were evaluated using the Student’s t-test. P<0.05
was considered to indicate statistically significant
differences.
Results
Aspirin induced apoptosis and upregulated
the expression and activity of caspase-3 in MDA-MB-453 cells
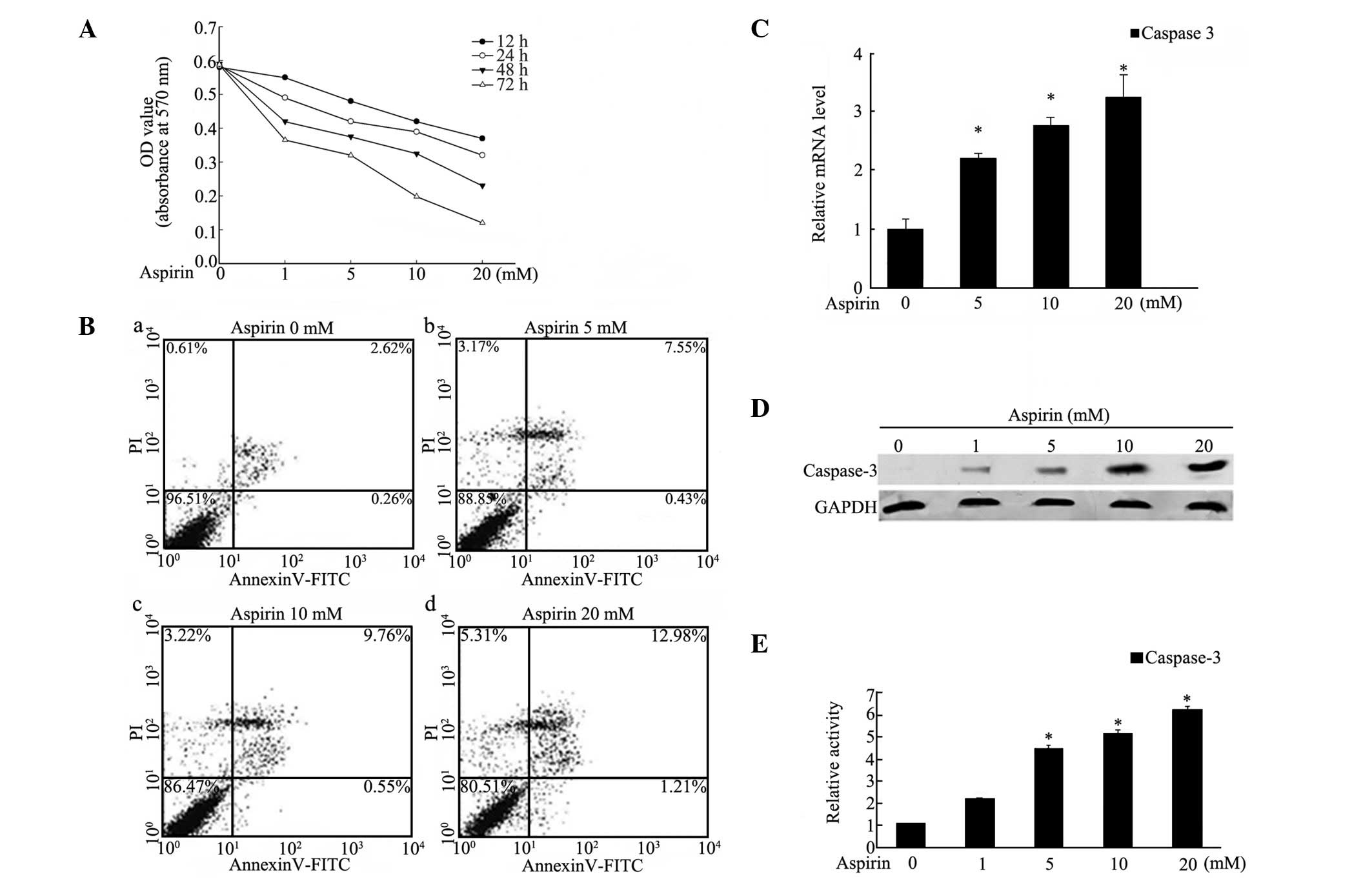
To evaluate the effect of aspirin on the
proliferation of human MDA-MB-453 breast cancer cells, the MTT
assay was used and the results indicated that aspirin decreased
cell proliferation in a dose- and time-dependent manner (Fig. 1A). A significant antiproliferative
effect of aspirin appeared following treatment with 20 mM for
various time periods. The percentage of viable cells decreased to
62.38% under treatment with 20 mM of aspirin for 24 h and then to
18.97% after 72 h treatment, compared with the controls. To
investigate whether aspirin induced cell apoptosis and death, cells
were treated with various concentrations (0–20 mM) of aspirin for
24 h and then subjected to Annexin V/PI-based flow cytometry.
Exposure to aspirin significantly affected apoptosis in the
MDA-MB-453 cells (Fig. 1B). When
exposed to 20 mM aspirin for 24 h, the percentage of apoptotic
cells reached 12.98%, notably higher than the untreated cells. As
other studies have described upregulation of the expression and
activity of caspase-3 in gastric, cervical and prostate cancer cell
apoptosis (12,13,23,24),
the expression of caspase-3 was investigated by real-time PCR and
western blotting. As shown in Fig. 1C
and D, the mRNA and protein levels of caspase-3 were increased
in a dose-dependent manner when exposed to 0–20 mM aspirin for 24
h. Moreover, to investigate the expression of activated caspase-3,
the activity of caspase-3 was evaluated and the results showed that
activity was increased in a similar dose-dependent manner in
response to aspirin treatment for 24 h (Fig. 1E). These data suggested that
aspirin induced apoptosis in MDA-MB-453 cells via caspase-3
upregulation.
AP2α directly binds to the promoter
region of caspase-3 in MDA-MB-453 cells
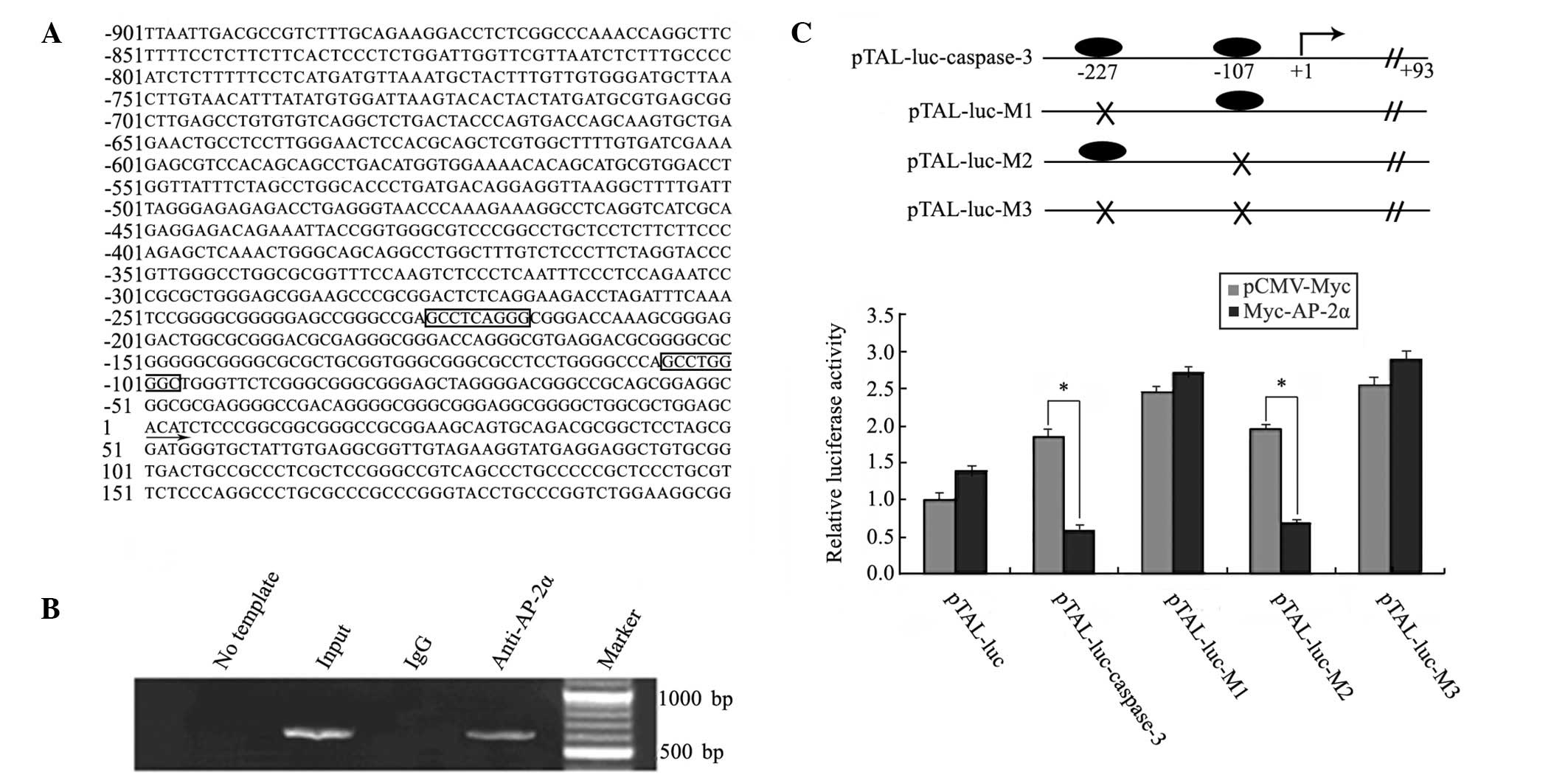
Since the transcription level of caspase-3 is
upregulated in aspirin-treated MDA-MB-453 cells, the promoter
region of caspase-3 was analyzed by the JASPAR and MatInspector
programs. As shown in Fig. 2A, two
AP-2α consensus DNA-binding sites in the promoter region of
caspase-3 (nt −901 to nt +200 from transcription start codon) were
observed, nt −227 and nt −107. As AP-2α is important in mammary
carcinoma and highly expressed in MDA-MB-453 cells (16,17,25),
the direct binding of AP-2α to the caspase-3 promoter was
demonstrated by ChIP experiments (Fig.
2B). The predicted band was detected in the input and
AP-2α-ChIP-derived DNA samples, but not in the control
IgG-ChIP-derived DNA samples. To confirm the precise AP-2α binding
sites in the caspase-3 promoter, the promoter region (nt −799 to nt
+93) of caspase-3 was used for a luciferase reporter assay.
Wild-type and mutant promoter luciferase plasmids, including
pTAL-luc-caspase-3, pTAL-luc-M1 (nt −227), -M2 (nt −107) and -M3
(nt −227 and nt −107), were constructed and co-transfected with
pCMV-Myc or pCMV-Myc-AP-2α plasmids, independently. As shown in
Fig. 2C, compared with the
control, overexpression of AP-2α depressed the transcriptional
activities of the constructs pTAL-luc-caspase-3 and pTAL-luc-M2 by
~3-fold. However, pTAL-luc-M1 and -M3 eliminated the effect of
AP-2α, suggesting that AP-2α directly bound to the caspase-3
promoter at the nt −227 site.
AP2α negatively regulated caspase-3 in
MDA-MB-453 cells
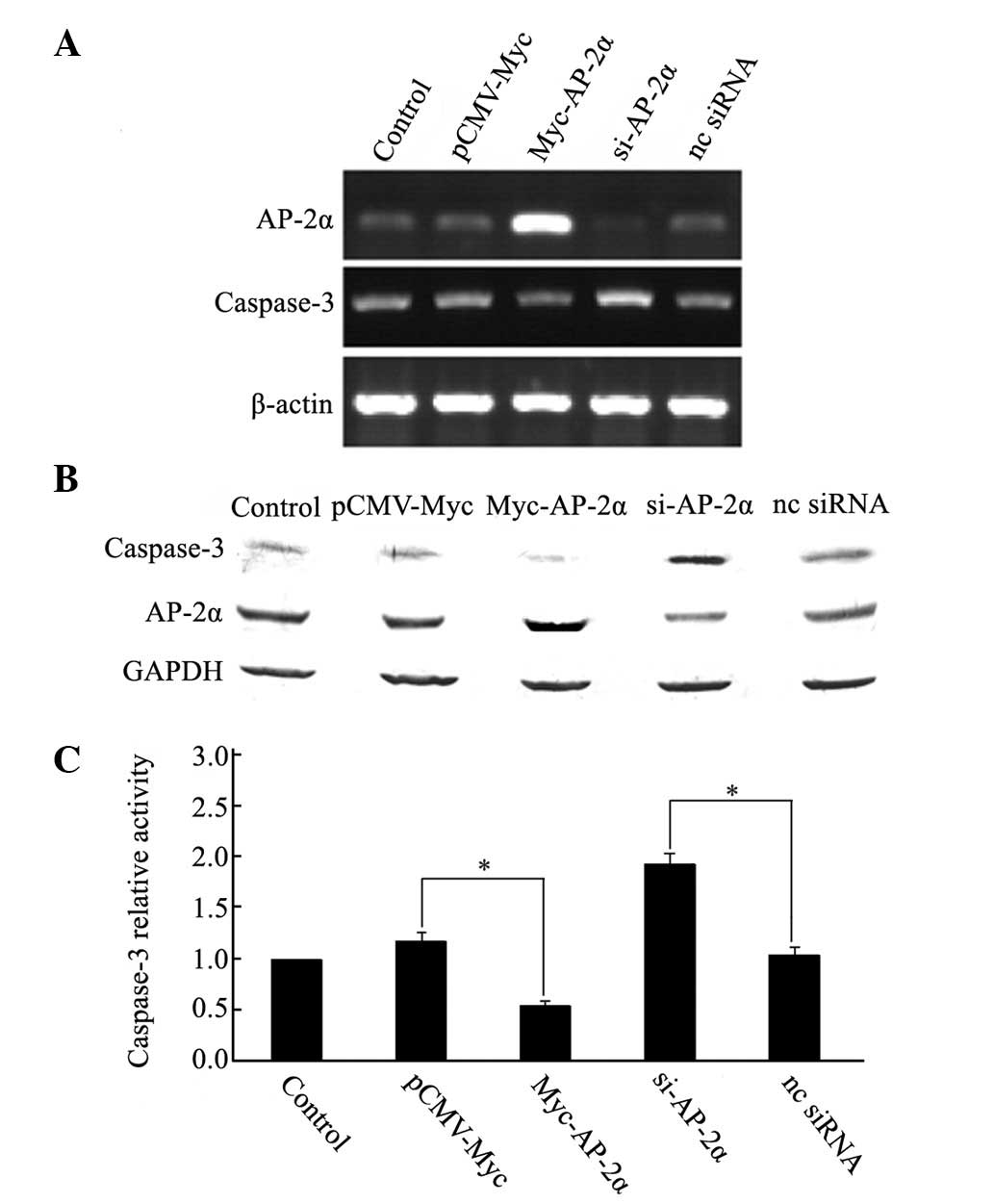
To determine whether this binding conferred positive
or negative regulation on caspase-3, the expression of AP-2α was
knocked down in MDA-MB-453 cells by siRNA and the expression of
caspase-3 was detected using semi-quantitative RT-PCR and western
blotting. Cells transfected with siRNAs against AP-2α showed clear
increases in caspase-3 mRNA (Fig.
3A) and protein levels (Fig.
3B). Expression of the activated caspase-3 was also enhanced,
as determined by a caspase-3 activity assay (Fig. 3C). However, overexpression of AP-2α
decreased the expression levels (Fig.
3A and B) and activity (Fig.
3C) of caspase-3, whereas the controls did not change.
Aspirin induced the degradation of AP-2α
through the proteasome pathway in MDA-MB-453 cells
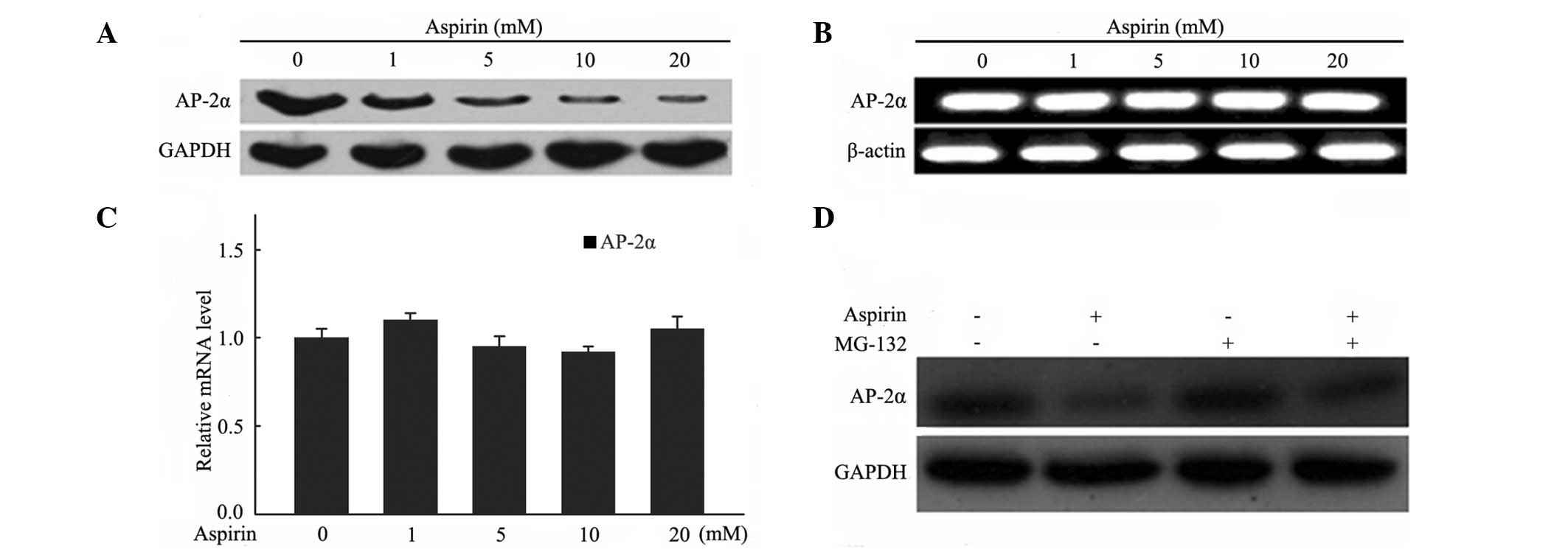
To investigate whether AP-2α responds to aspirin
treatment in MDA-MB-453 cells, the AP-2α expression levels of mRNA
and protein were evaluated. The results showed that the protein
level of AP-2α was downregulated in a dose-dependent manner in
MDA-MB-453 cells following exposure to various concentrations of
aspirin for 24 h (Fig. 4A).
However, no significant changes were observed in the mRNA levels in
the semi-quantitative and real-time PCR experiments (Fig. 4B and C). It appeared that aspirin
did not affect AP-2α expression at the transcriptional level.
A previous study reported that aspirin treatment
affects protein proteasomal degradation in breast cancer cells
(26). MG132, a proteasome
inhibitor, was used to evaluate whether it was able to block the
degradation of AP-2α in MDA-MB-453 cells following aspirin
treatment. Cells were pre-incubated with MG132 (20 μM) for 5 h
prior to aspirin (10 mM) treatment for 24 h and cell extracts were
used for western blotting to detect AP-2α expression. As shown in
Fig. 4D, the degradation of AP-2α
protein was blocked in the presence of MG132, suggesting that
aspirin induced AP-2α degradation via a proteasome-mediated
pathway.
Discussion
Aspirin has multiple effects and is involved in
various cellular processes. In the last few years, several studies
have shown that aspirin inhibits cancer cell proliferation and
induces apoptosis (3,11,24),
which is consistent with the present results that aspirin exhibits
the same activity in MDA-MB-453 breast cancer cells in a dose- and
time-dependent manner. Although the inhibition of certain proteins,
such as COX and NF-κB, may contribute to the anticancer effects of
aspirin (8,27), at present the pathways leading to
these effects remain to be determined.
In the present study, several novel observations
were reported, including a mechanism by which aspirin may exert its
anticancer effects. The present results showed that the expression
and activity of caspase-3 were upregulated following aspirin
treatment, suggesting that the induction of apoptosis by aspirin in
MDA-MB-453 cells may depend on the caspase pathway and caspase-3
was regulated at its transcriptional level. Promoter analysis then
revealed that AP-2α may be a regulator of caspase-3. Considering
the important roles of AP-2α in breast cancer and its high
expression levels in several breast cancer cell types including
MDA-MB-453 (16,25), further experiments were performed
to study the association between AP-2α and caspase-3. The data
showed that AP-2α negatively regulates caspase-3 transcription via
binding to the promoter region of caspase-3, thus downregulating
the expression of caspase-3 and decreasing its activity. The
expression of AP-2α in cells treated with aspirin was then
evaluated. The present results have shown that AP-2α was
downregulated following aspirin treatment at the protein but not
mRNA level. Aspirin has been reported to affect protein degradation
through the proteasome pathway (26). To assess whether this
downregulation of AP-2α depends on the proteasome pathway, MG132
was selected to block the proteasome pathway. MG132 abrogated the
aspirin-induced reduction of AP-2α.
From the present study, a working model may be
proposed. In this model, AP-2α negatively regulates caspase-3 by
binding to its proximal promoter region in AP-2α-positive cancer
cells. Aspirin promotes the proteasome pathway-dependent
degradation of AP-2α, which increases the expression of apoptotic
effector caspase-3, leading to an increase in activated caspase-3
and finally causing cell apoptosis. This suggests a novel mechanism
for aspirin in breast cancer treatment.
AP-2α acts as an oncogene, which is consistent with
previous reports (16,17,25,28).
However, there are also several lines of evidence indicating that
AP-2α may act as a tumor suppressor gene. Overexpression of AP-2α
induces apoptosis in a number of cancer cells (29–31).
AP-2α has been shown to act as both a tumor suppressor and an
oncogene in various cancer types that may depend on the expression
level and signaling pathways AP-2α is involved in. The controversy
concerning the role of AP-2α reflects the complexity of the cell
signaling pathways. However, a number of critical questions remain
to be answered in order to understand the mechanisms of
aspirin-induced apoptosis.
In summary, the present study demonstrated for the
first time that aspirin upregulates caspase-3 activity through the
downregulation of AP-2α gene expression, leading to the apoptosis
of human breast cancer cells. The present findings also suggest
that aspirin is a potentially powerful therapeutic agent for
various AP-2α-dependent human cancers.
Acknowledgements
The present study was supported in part by the
National Natural Science Foundation of China (Nos. 30972624 and
81172112), New Century Excellent Talents in University
(NCET-10-0145), Science and Technology Department of Hunan Province
(2011FJ3140, 2011TT2008), Scientific Research Fund of Hunan
Provincial Education Department (11A072) and China Postdoctoral
Science Foundation (2012M511380).
References
|
1
|
Qiao L, Hanif R, Sphicas E, Shiff SJ and
Rigas B: Effect of aspirin on induction of apoptosis in HT-29 human
colon adenocarcinoma cells. Biochem Pharmacol. 55:53–64. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kutuk O and Basaga H: Aspirin inhibits
TNFalpha- and IL-1-induced NF-kappaB activation and sensitizes HeLa
cells to apoptosis. Cytokine. 25:229–237. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Alfonso LF, Srivenugopal KS, Arumugam TV,
Abbruscato TJ, Weidanz JA and Bhat GJ: Aspirin inhibits
camptothecin-induced p21CIP1 levels and potentiates
apoptosis in human breast cancer cells. Int J Oncol. 34:597–608.
2009.PubMed/NCBI
|
|
4
|
Chattopadhyay M, Goswami S, Rodes DB, et
al: NO-releasing NSAIDs suppress NF-κB signaling in vitro and in
vivo through S-nitrosylation. Cancer Lett. 298:204–211.
2010.PubMed/NCBI
|
|
5
|
Zhang Z, Huang L, Zhao W and Rigas B:
Annexin 1 induced by anti-inflammatory drugs binds to NF-kappaB and
inhibits its activation: anticancer effects in vitro and in vivo.
Cancer Res. 70:2379–2388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou H, Huang L, Sun Y and Rigas B: Nitric
oxide-donating aspirin inhibits the growth of pancreatic cancer
cells through redox-dependent signaling. Cancer Lett. 273:292–299.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rigas B and Williams JL: NO-donating
NSAIDs and cancer: an overview with a note on whether NO is
required for their action. Nitric Oxide. 19:199–204. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Harris RE, Beebe-Donk J, Doss H and Burr
Doss D: Aspirin, ibuprofen, and other non-steroidal
anti-inflammatory drugs in cancer prevention: a critical review of
non-selective COX-2 blockade (Review). Oncol Rep. 13:559–583.
2005.
|
|
9
|
Xiang S, Sun Z, He Q, Yan F, Wang Y and
Zhang J: Aspirin inhibits ErbB2 to induce apoptosis in cervical
cancer cells. Med Oncol. 27:379–387. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim KM, Song JJ, An JY, Kwon YT and Lee
YJ: Pretreatment of acetylsalicylic acid promotes tumor necrosis
factor-related apoptosis-inducing ligand-induced apoptosis by
down-regulating BCL-2 gene expression. J Biol Chem.
280:41047–41056. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Luciani MG, Campregher C and Gasche C:
Aspirin blocks proliferation in colon cells by inducing a G1 arrest
and apoptosis through activation of the checkpoint kinase ATM.
Carcinogenesis. 28:2207–2217. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Royle JS, Ross JA, Ansell I, Bollina P,
Tulloch DN and Habib FK: Nitric oxide donating nonsteroidal
anti-inflammatory drugs induce apoptosis in human prostate cancer
cell systems and human prostatic stroma via caspase-3. J Urol.
172:338–344. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gu Q, Wang JD, Xia HH, et al: Activation
of the caspase-8/Bid and Bax pathways in aspirin-induced apoptosis
in gastric cancer. Carcinogenesis. 26:541–546. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hilger-Eversheim K, Moser M, Schorle H and
Buettner R: Regulatory roles of AP-2 transcription factors in
vertebrate development, apoptosis and cell-cycle control. Gene.
260:1–12. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Turner BC, Zhang J, Gumbs AA, et al:
Expression of AP-2 transcription factors in human breast cancer
correlates with the regulation of multiple growth factor signalling
pathways. Cancer Res. 58:5466–5472. 1998.PubMed/NCBI
|
|
16
|
Bosher JM, Williams T and Hurst HC: The
developmentally regulated transcription factor AP-2 is involved in
c-erbB-2 overexpression in human mammary carcinoma. Proc Natl Acad
Sci USA. 92:744–747. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bosher JM, Totty NF, Hsuan JJ, Williams T
and Hurst HC: A family of AP-2 proteins regulates c-erbB-2
expression in mammary carcinoma. Oncogene. 13:1701–1707.
1996.PubMed/NCBI
|
|
18
|
Agus DB, Akita RW, Fox WD, et al:
Targeting ligand-activated ErbB2 signaling inhibits breast and
prostate tumor growth. Cancer Cell. 2:127–137. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sudhakar C, Jain N and Swarup G: Sp1-like
sequences mediate human caspase-3 promoter activation by p73 and
cisplatin. FEBS J. 275:2200–2213. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hubert MA, Sherritt SL, Bachurski CJ and
Handwerger S: Involvement of transcription factor NR2F2 in human
trophoblast differentiation. PLoS One. 5:e94172010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ji BC, Hsu WH, Yang JS, et al: Gallic acid
induces apoptosis via caspase-3 and mitochondrion-dependent
pathways in vitro and suppresses lung xenograft tumor growth in
vivo. J Agric Food Chem. 57:7596–7604. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ailan H, Xiangwen X, Daolong R, et al:
Identification of target genes of transcription factor activator
protein 2 gamma in breast cancer cells. BMC Cancer. 9:2792009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Power JJ, Dennis MS, Redlak MJ and Miller
TA: Aspirin-induced mucosal cell death in human gastric cells:
evidence supporting an apoptotic mechanism. Dig Dis Sci.
49:1518–1525. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee SK, Park MS and Nam MJ: Aspirin has
antitumor effects via expression of calpain gene in cervical cancer
cells. J Oncol. 2008:2853742008.PubMed/NCBI
|
|
25
|
Begon DY, Delacroix L, Vernimmen D,
Jackers P and Winkler R: Yin Yang 1 cooperates with activator
protein 2 to stimulate ERBB2 gene expression in mammary cancer
cells. J Biol Chem. 280:24428–24434. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lu M, Strohecker A, Chen F, et al: Aspirin
sensitizes cancer cells to TRAIL-induced apoptosis by reducing
survivin levels. Clin Cancer Res. 14:3168–3176. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
McCarty MF and Block KI: Preadministration
of high-dose salicylates, suppressors of NF-kappaB activation, may
increase the chemosensitivity of many cancers: an example of
proapoptotic signal modulation therapy. Integr Cancer Ther.
5:252–268. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhu CH and Domann FE: Dominant negative
interference of transcription factor AP-2 causes inhibition of
ErbB-3 expression and suppresses malignant cell growth. Breast
Cancer Res Treat. 71:47–57. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wajapeyee N and Somasundaram K: Cell cycle
arrest and apoptosis induction by activator protein 2alpha
(AP-2alpha) and the role of p53 and p21WAF1/CIP1 in
AP-2alpha-mediated growth inhibition. J Biol Chem. 278:52093–52101.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Müller FU, Loser K, Kleideiter U, et al:
Transcription factor AP-2alpha triggers apoptosis in cardiac
myocytes. Cell Death Differ. 11:485–493. 2004.PubMed/NCBI
|
|
31
|
Wajapeyee N, Britto R, Ravishankar HM and
Somasundaram K: Apoptosis induction by activator protein 2alpha
involves transcriptional repression of Bcl-2. J Biol Chem.
281:16207–16219. 2006. View Article : Google Scholar : PubMed/NCBI
|