Introduction
Despite significant improvements in the treatment
methods for breast cancer over recent years, including surgical
operation, chemotherapy and radiotherapy, breast cancer remains the
second leading cause of cancer-induced mortality in women in North
America (1). To date, several
drugs have been isolated from plants, with the aim of controlling
breast cancer. For instance, taxanes and vinca alkaloids isolated
from yew tree and rosy periwinkle, respectively, are employed as
antimitotic and antimicrotubule agents (2,3).
Epipodophyllotoxins isolated from the American mayapple plant exert
an anticancer activity by inhibiting topoisomerase II (4). Therefore, it is important to identify
natural sources possessing tumor suppressor properties and to
investigate the molecular mechanisms underlying these effects, in
order to develop anticancer drugs.
Alpinia officinarum (A. officinarum),
also known as lesser galangal, is a plant of the ginger family
which is commonly found in Southeast Asia. The rhizome of A.
officinarum is used in traditional medicine for the treatment
of stomach ache, cold and swelling (5). Two major compounds, diarylheptanoids
and galangin, have been isolated. Previous studies have
demonstrated that the A. officinarum extract and its major
components exert an anticancer effect in numerous cancer cell
lines, including liver, lung, breast and neuroblastoma (5–8).
However, the molecular mechanisms underlying the anticancer
activity of this extract in the breast cancer cell line MCF-7 have
not been elucidated.
The results of the present study showed that a
methanolic extract of A. officinarum rhizome significantly
reduces MCF-7 cell proliferation in a dose- and time-dependent
manner. The effects of the extract on cell cycle progression and
apoptosis in MCF-7 cells were also assessed.
Materials and methods
A. officinarum extract preparation
Rhizomes of A. officinarum were purchased
from the Kyungdong oriental medicine market, Seoul, Korea.
Materials (100 μg) were extracted with 99.8% methanol (1 liter) for
72 h at room temperature. Extracts were evaporated to dryness using
a rotary evaporator and dissolved in dimethylsulfoxide (DMSO;
Sigma-Aldrich, St. Louis, MO, USA).
Cell culture
The MCF-7 human breast cancer cell line was
cultivated in Dulbecco's modified Eagle's medium (DMEM) containing
10% fetal bovine serum (FBS), 100 U/ml penicillin and 100 μg/ml
streptomycin (HyClone Laboratories, Inc., South Logan, UT, USA) at
37°C in a humidified atmosphere of 5% CO2.
Cell proliferation assay
The effect of the A. officinarum extract on
MCF-7 cell proliferation was measured using the
3-(4,5-dimethylthiazol-z-yl)-2,5-diphenyl tetrazolium bromide (MTT)
assay, based on the ability of live cells to cleave the tetrazolium
ring to a molecule that absorbs at 570 nm (9,10).
Briefly, cells (3×103/well) were seeded onto 96-well
microplates and incubated for 24 h. Next, cells were treated with
various concentrations of A. officinarum extract (0–100
μg/ml). At different time points (12, 24, 48 and 72 h), 10 μl MTT
solution (5 mg/ml; Sigma-Aldrich) was added to each well, followed
by further incubation at 37°C for 4 h. At the end of the incubation
period, 100 μl of isopropyl alcohol dissolved in 5% of 1 M HCl was
added to solubilize formazan crystals. Absorbance was measured
using a SpectraMax® Plus384 microplate reader (Molecular
Devices, Sunnyvale, CA, USA). All the measurements were performed
in quadruplicate. The cells were never exposed to a DMSO
concentration >0.5%.
Cell cycle analysis using flow
cytometry
MCF-7 cells (2×105 cells/dish) were
plated on 100-mm tissue culture dishes, and treated with the
indicated concentrations of A. officinarum extract for 48 h.
Cells were harvested via trypsinization, washed twice with ice-cold
phosphate-buffered saline (PBS), and fixed with 70% ethanol at
−20°C for 20 min. Fixed cells were washed with ice-cold PBS and
stained with propidium iodide (PI) solution (50 μg/ml of PI, 10
μg/ml of RNase A and 3.8 mM sodium citrate in PBS) at 4°C for 20
min. Cell cycle distribution was assessed using FACSVantage™ SE
(Becton-Dickinson, San Jose, CA, USA). Data from 10,000
cells/sample were collected and analyzed.
Western blot analysis
MCF-7 cells (2×105 cells/dish) were
plated on a 100-mm tissue culture dish, and treated with the
indicated concentrations of A. officinarum extract for 48 h.
Next, cells were rinsed twice with ice-cold PBS and scraped using
lysis buffer (PBS containing 5 mM MgCl2, 1 mM EDTA, 0.1%
Triton X-100 and protease inhibitors). Cell lysates were
centrifuged at 12,000 rpm for 10 min at 4°C. Protein samples (60
μg) were mixed with SDS sample buffer, boiled for 5 min and
subjected to 10 and 12% SDS-PAGE before electrotransfer to PVDF
membrane (Westran S; Whatman, Florham Park, NJ, USA). The membrane
was blocked with 5% non-fat dry milk in TBST for 2 h at room
temperature and incubated with antibodies against E2F1,
cyclin-dependent protein kinase 2 (cdk2), cyclin A, caspase-7, poly
(ADP-ribose) polymerase (PARP), p53, Bcl-2, Bax (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) or tubulin (Upstate
Biotechnology, Temecula, CA, USA), followed by incubation with the
corresponding secondary antibodies, goat anti-mouse IgG
HRP-conjugate or goat anti-rabbit IgG HRP-conjugate (Zymed,
Carlsbad, CA, USA). Protein visualization was achieved using an
enhanced chemiluminescence detection kit (West-Zol; Intron
Biotechnology, Sungnam, Korea).
Analysis of nuclear morphology
The apoptotic effects of the A. officinarum
extract on MCF-7 cells were analyzed via nuclear DNA staining.
MCF-7 cells were plated on coverslips at a density of
1×103 cells/coverslip and treated with 50 μg/ml of A.
officinarum extract. After a 48-h incubation, the cells were
fixed with 4% paraformaldehyde for 10 min, washed twice with PBS
and stained with 1 μg/ml of Hoechst 33258 (Sigma-Aldrich) for 20
min. Nuclear morphology was observed under a fluorescence
microscope (BX-50; Olympus, Tokyo, Japan).
Annexin V/PI flow cytometric
analysis
MCF-7 cells (2×105 cells/dish) were
plated on a 100-mm tissue culture dish and treated with the
indicated amounts of A. officinarum extract for 48 h. The
cells were fixed with 70% ethanol for 20 min, and apoptosis was
assessed with an Annexin V FITC Apoptosis Detection kit I (BD
Biosciences, Palo Alto, CA, USA), according to the manufacturer's
protocol. Flow cytometry analysis was performed using FACSVantage™
SE. Data from 10,000 cells/sample were collected and analyzed.
Results
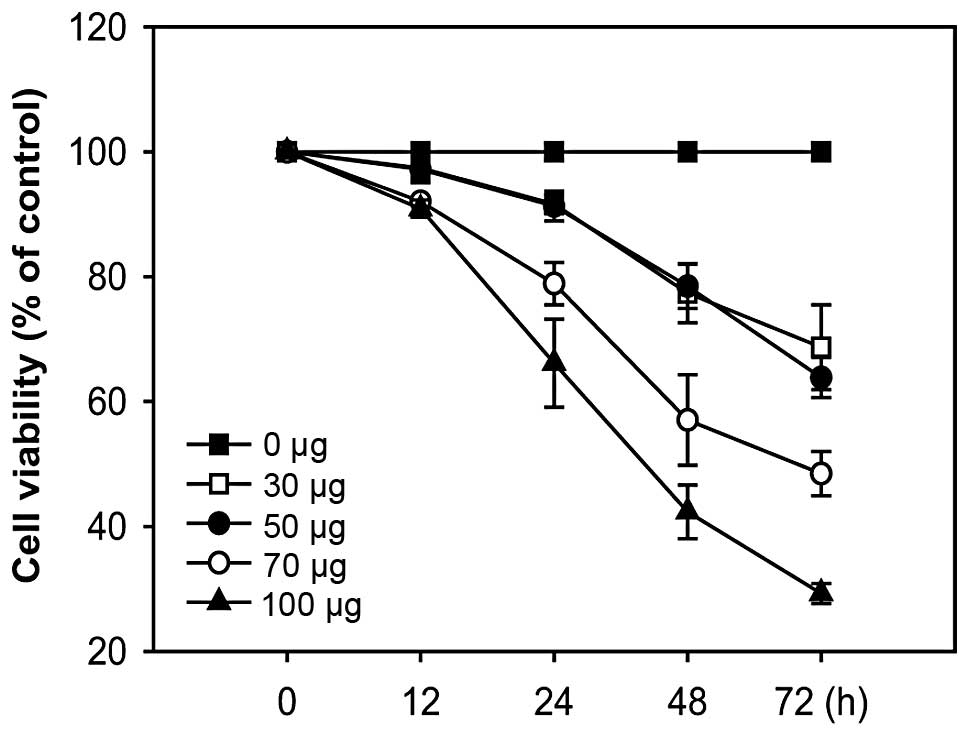
Effects of A. officinarum extract on the
proliferation of the human breast cancer cell line MCF-7
To determine whether A. officinarum extract
exerts an antiproliferative effect, MCF-7 cells were treated with
various concentrations of the extract for the indicated times, and
cell proliferation was determined using the MTT-based colorimetric
assay. In cells treated with the extract, proliferation was
significantly decreased in a time- and dose-dependent manner,
clearly demonstrating an antiproliferative effect (Fig. 1).
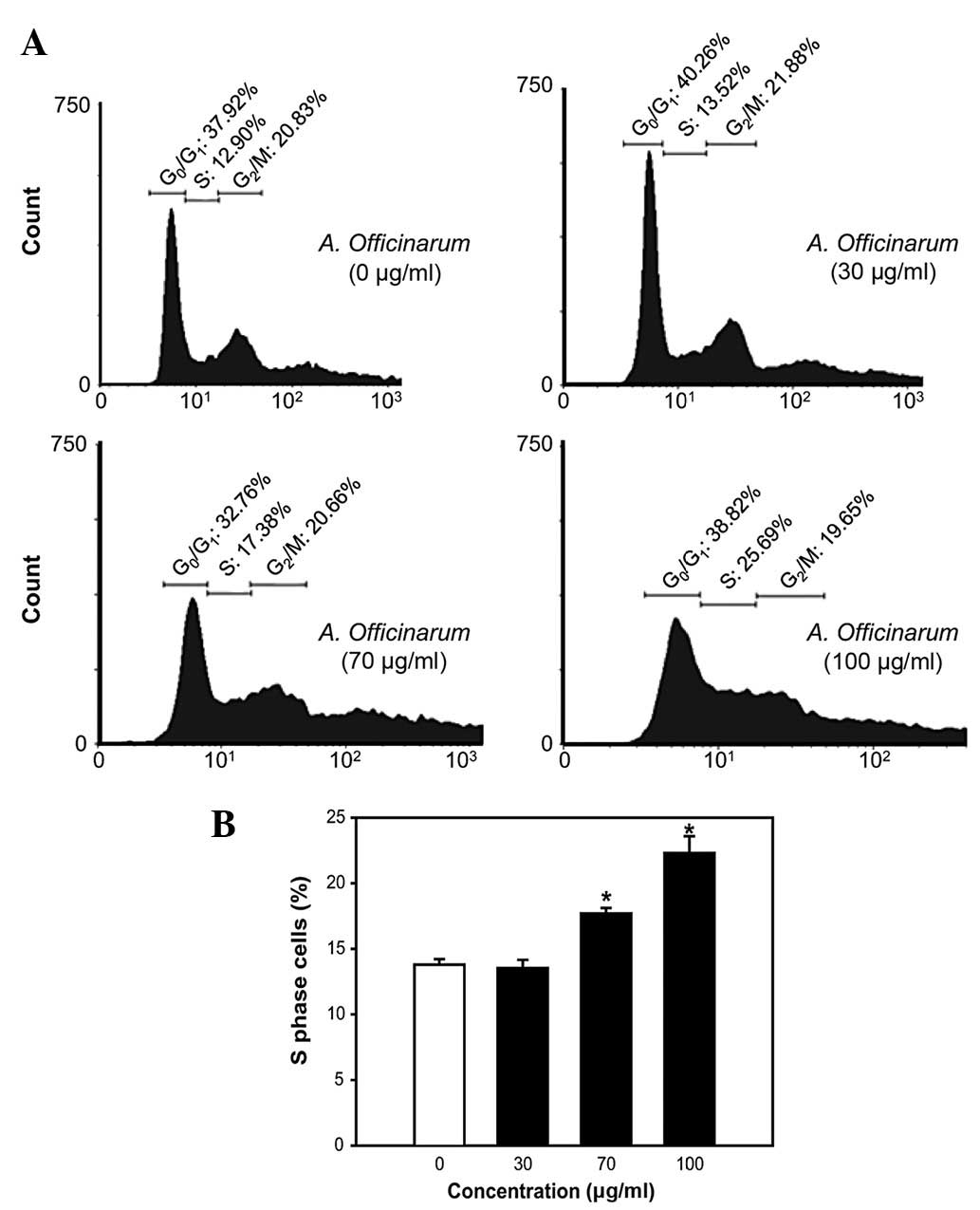
A. officinarum extract induces S-phase
cell cycle arrest in MCF-7 cells
Next, we investigated the mechanisms underlying the
antiproliferative activity of the extract. MCF-7 cells treated with
the indicated amounts of extract for 48 h were stained with PI and
flow cytometric analysis was performed. The A. officinarum
extract induced an increase in the proportion of cells in the
S-phase in a dose-dependent manner (Fig. 2). Particularly, the cell population
in the S-phase was 12.90% in the untreated control group. After 48
h of incubation with 100 μg/ml extract, the S-phase population was
significantly enhanced to 25.69% (Fig.
2A).
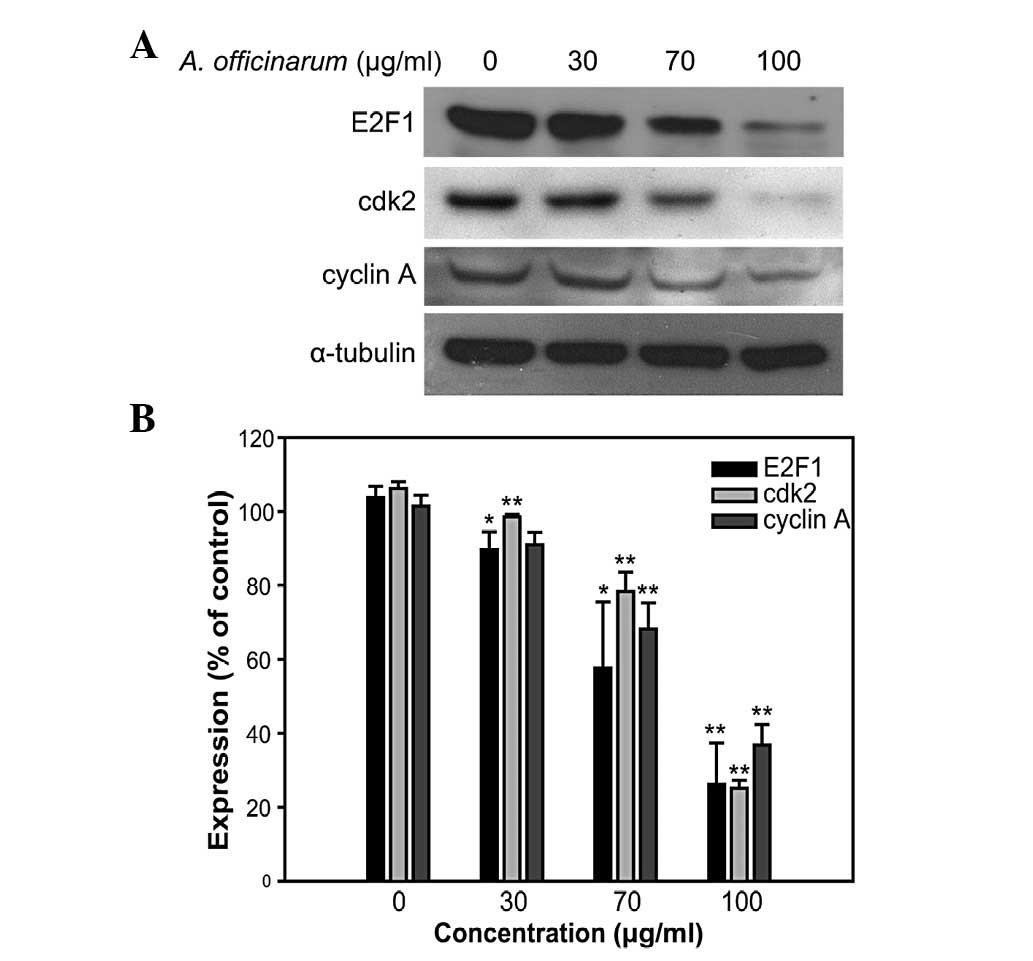
Western blot analysis was performed to determine the
expression levels of S-phase cell cycle regulatory proteins,
including E2F1, cdk2 and cyclin A. These proteins are essential for
the progression of the S-phase of the cell cycle. The levels of all
the proteins examined were significantly suppressed in groups
treated with the A. officinarum extract in a dose-dependent
manner (Fig. 3). These results
suggest that the extract inhibits MCF-7 cell proliferation by
inducing S-phase cell cycle arrest.
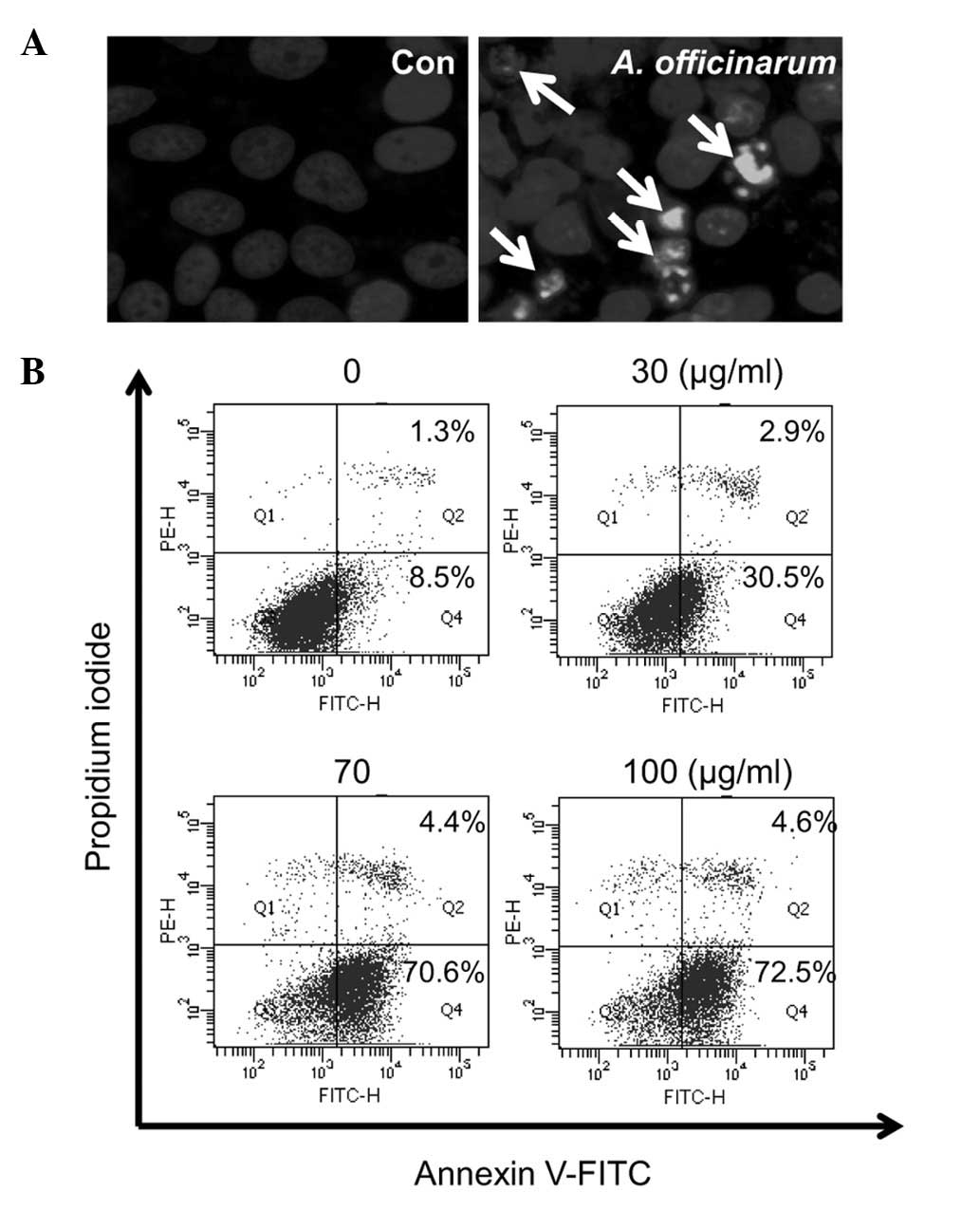
Apoptotic activity of A. officinarum
extract in MCF-7 cells
To further determine whether the extract triggers
apoptosis in MCF-7 cells, the cells were treated with or without 50
μg extract for 48 h and stained with Hoechst 33258. Morphological
changes in the nucleus were observed under a fluorescence
microscope. Nuclear condensation was evident in the presence of
A. officinarum extract, while not control cells (Fig. 4A). The apoptosis-promoting
potential of the extract was subsequently examined using flow
cytometric analysis after Annexin V/PI dual staining (Fig. 4B). The dot plots show non-apoptotic
live cells in the lower left quadrant (Annexin
V−/PI−), apoptotic cells in the lower right
quadrant (Annexin V+/PI−) and dead cells in
the upper right quadrant (Annexin V+/PI+).
Following treatment with the indicated amounts of A.
officinarum extract for 48 h, the proportion of apoptotic cells
(Annexin V+/PI−) was significantly increased
in a dose-dependent manner. These data collectively indicate that
the A. officinarum extract induces apoptosis in MCF-7
cells.
To determine the molecular mechanisms underlying
apoptosis induction, cells were treated with the indicated amounts
of extract for 48 h, and western blot analysis was performed using
specific antibodies against apoptosis-related proteins. The
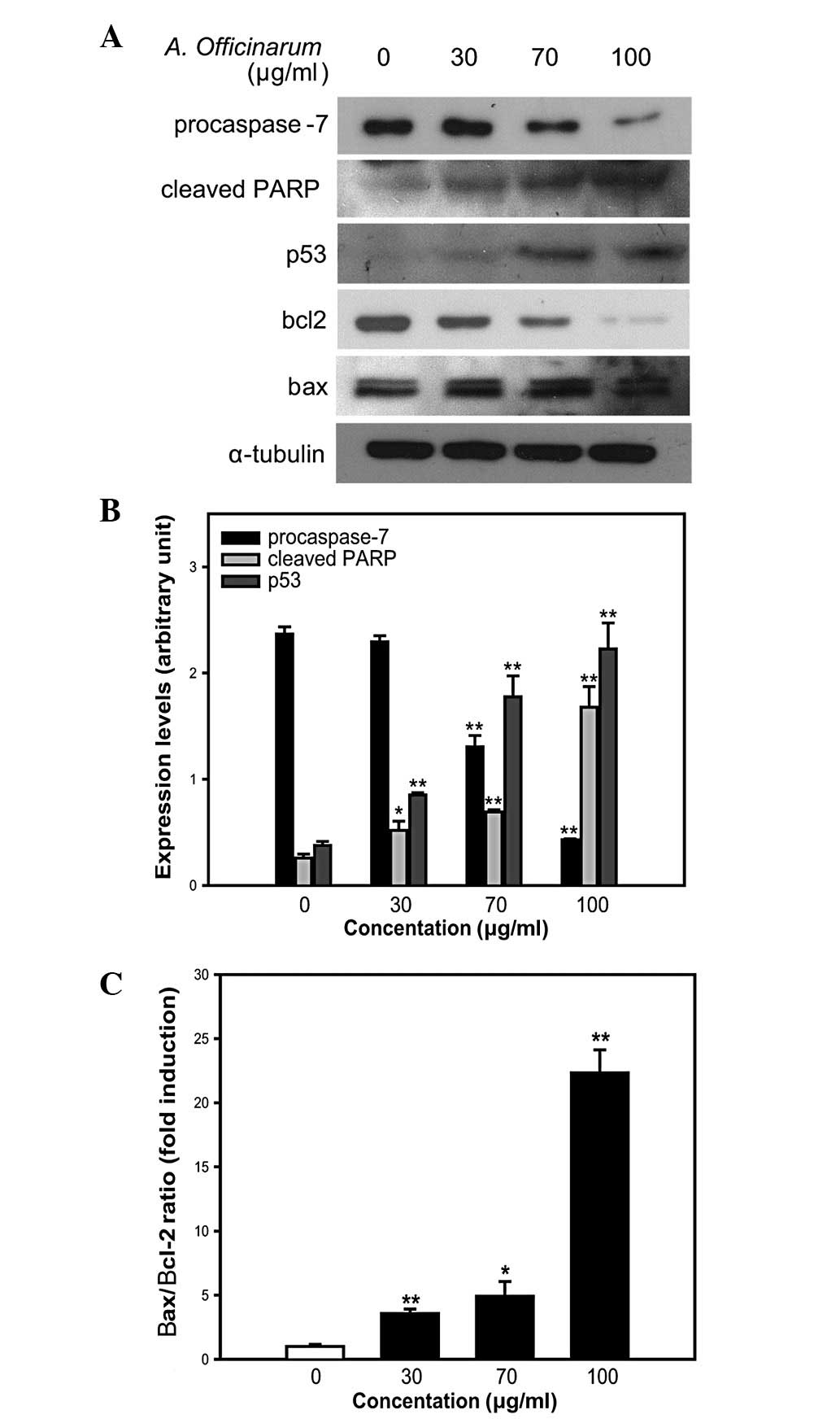
expression level of procaspase-7 was significantly decreased by the
extract in a dose-dependent manner (Fig. 5A and B). Procaspase-7, the
precursor form of caspase-7, is cleaved into caspase-7. This
process is mediated by active caspases or other proteases in
response to apoptosis signaling (11). Caspases, a family of cysteine
proteases, are the final executors of apoptosis, and are activated
via both intrinsic and extrinsic pathways (12,13).
Additional evidence of caspase activation was provided by examining
the cleavage of the caspase substrate, PARP. Upon introduction of
the A. officinarum extract into MCF-7 cells, the presence of
an 85-kDa protein fragment, representing the cleaved form of PARP,
was increased in a dose-dependent manner (Fig. 5A and B), suggesting the induction
of caspase-mediated apoptosis in MCF-7 cells.
We also examined the expression of p53, a tumor
suppressor, which mediates apoptosis in response to DNA damage. p53
level was significantly increased by the A. officinarum
extract in a dose-dependent manner (Fig. 5A and B). Based on the finding that
an increase in the Bax/Bcl-2 ratio indicates mitochondrial
dysfunction (14,15), expression levels of Bax and Bcl-2
proteins were further examined. The A. officinarum extract
induced a slight increase in Bax and significant decrease in Bcl-2
expression in a dose-dependent manner (Fig. 5A). Subsequent estimation of band
intensity further confirmed this dose-dependent increase in the
Bax/Bcl-2 ratio (Fig. 5C). These
results collectively suggest that apoptosis induced by the A.
officinarum extract is mediated via caspase- and
mitochondrial-dependent pathways.
Discussion
In the present study, we investigated the effects of
the A. officinarum extract on MCF-7 cell proliferation, and
determined the molecular mechanisms underlying its activity. As
shown in Fig. 1, the A.
officinarum extract inhibited cell proliferation in a dose- and
time-dependent manner. Additionally, S-phase cell cycle progression
was inhibited in a dose-dependent manner by the extract (Fig. 2). Inhibition of cancer cell
proliferation is usually affected by cell cycle arrest. Cell cycle
progression is regulated by a complex of cyclin and cdk (16). In particular, the key regulators
for S-phase cell cycle progression are cyclin A and cdk2 (17). The cyclin A-cdk2 complex modulates
the function of the E2F1 transcription factor, subsequently
activating several target genes required for cell cycle progression
and DNA synthesis in the S-phase (18). As shown in Fig. 3, the expression levels of cyclin A,
cdk2 and E2F1 were downregulated by the A. officinarum
extract. Based on these data, it is suggested that the extract
suppresses S-phase cell cycle progression by inhibiting regulatory
protein expression.
In addition to promoting MCF-7 cell cycle arrest,
the A. officinarum extract showed an ability to trigger
apoptosis. The cell population displaying nuclear condensation and
Annexin V+/PI− staining was increased
following treatment with the extract (Fig. 4). Annexin V binds to
phosphatidylserine with high affinity. Under normal conditions,
phosphatidylserine is located in the intracellular portion of
plasma membrane lipid bilayer. During early apoptosis,
phosphatidylserines are redistributed from the intracellular to
extracellular portion of the lipid bilayer (19,20).
Thus, Annexin V+ cells are representative of progressive
apoptosis. However, dead cells are also stained with Annexin V,
owing to disruption of the cell membrane. In contrast to dead
cells, those undergoing progressive apoptosis are undetectable by
staining with PI. Dead cells are stained with both Annexin V and
PI, whereas viable cells cannot be stained with either dye. The
A. officinarum extract induced a marked increase in the
Annexin V+/PI− cell population from 8.5 to
72.5% (Fig. 4B), indicating the
progression of apoptosis.
Apoptosis is induced by the death receptor-mediated
extrinsic and mitochondrial cytochrome c-mediated intrinsic
pathways. In the extrinsic pathway, extracellular signals,
including toxins, hormones, growth factors and cytokines, promote
the formation of the death-inducing signaling complex, subsequently
activating caspases-8 and −10. In the intrinsic pathway,
intracellular apoptotic signals, including heat, radiation,
nutrient deprivation and viral infection, trigger the release of
cytochrome c from mitochondria. Released cytochrome c activates
caspases-9 and −2 by forming apoptosomes (11). Caspases are broadly classified into
two groups; initiator (caspase-2, −8, −9 and −10) and executioner
(caspases-3, −6 and −7) caspases. Executioner caspases are
stimulated by active initiator caspases through proteolytic
cleavage. Active executioner caspases subsequently cleave various
intracellular substrates, including PARP, to perform the cell death
program (11). As shown in
Fig. 5A and B, the A.
officinarum extract induced a dose-dependent decrease and
increase in the expression levels of procaspase-7 and cleaved PARP,
respectively, supporting a role in the promotion of
caspase-mediated apoptosis in MCF-7 cells.
The levels of p53 and the Bax/Bcl-2 expression ratio
are important biochemical markers of apoptosis. p53 mediates either
cell cycle arrest or apoptosis in response to DNA damage, and
prevents the replication of damaged DNA via apoptosis (12,21).
During apoptosis, p53 induces the transcriptional repression of
Bcl-2 and the activation of Bax (22). Therefore, p53 level and Bax/Bcl-2
ratio are generally increased during apoptosis. Furthermore, the
increase in Bax/Bcl-2 ratio mediates mitochondria-dependent
apoptosis by inducing cytochrome c release from the mitochondria
into the cytosol (23). In our
experiments, both expression levels of p53 and the Bax/Bcl-2 ratio
were significantly increased in the presence of A.
officinarum extract in a dose-dependent manner, suggesting an
induction of mitochondrial-dependent apoptosis (Fig. 5).
Recently, several compounds have been isolated from
A. officinarum extract, including diarylheptanoids and
galangin. These compounds exert anticancer effects on numerous
cancer cell lines (5–8), and may be responsible for the
antiproliferative activity of the extract in MCF-7 cells. Thus,
further studies focusing on the molecular mechanisms underlying the
anticancer activities of these compounds are essential. Following
confirmation of these findings in vivo, the extract or its
isolates may be effectively employed to mediate chemotherapeutic
and cytostatic activities in human breast cancer.
Acknowledgements
This study was supported by Kyonggi University
Research Grant 2010.
References
|
1
|
DeSantis C, Siegel R, Bandi P and Jemal A:
Breast cancer statistics. CA Cancer J Clin. 61:409–418. 2011.
View Article : Google Scholar
|
|
2
|
Tsavaris N, Kosmas C, Vadiaka M,
Kanelopoulos P and Boulamatsis D: Immune changes in patients with
advanced breast cancer undergoing chemotherapy with taxanes. Br J
Cancer. 87:21–27. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jordan MA, Horwitz SB, Lobert S and
Correia JJ: Exploring the mechanisms of action of the novel
microtubule inhibitor vinflunine. Semin Oncol. 35:S6–S12. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Baldwin EL and Osheroff N: Etoposide,
topoisomerase II and cancer. Curr Med Chem Anticancer Agents.
5:363–372. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
An N, Zou ZM, Tian Z, Luo XZ, Yang SL and
Xu LZ: Diarylheptanoids from the rhizomes of Alpinia
officinarum and their anticancer activity. Fitoterapia.
79:27–31. 2008.
|
|
6
|
Lee CC and Houghton P: Cytotoxicity of
plants from Malaysia and Thailand used traditionally to treat
cancer. J Ethnopharmacol. 100:237–243. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tabata K, Yamazaki Y, Okada M, Fukumura K,
Shimada A, Sun Y, Yasukawa K and Suzuki T: Diarylheptanoids derived
from Alpinia officinarum induce apoptosis, S-phase arrest
and differentiation in human neuroblastoma cells. Anticancer Res.
29:4981–4988. 2009.
|
|
8
|
Zhang HT, Wu J, Wen M, Su LJ and Luo H:
Galangin induces apoptosis in hepatocellular carcinoma cells
through the caspase 8/t-Bid mitochondrial pathway. J Asian Nat Prod
Res. 14:626–633. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim DY, Kang SH and Ghil SH: Cirsium
japonicum extract induces apoptosis and anti-proliferation in
the human breast cancer cell line MCF-7. Mol Med Rep. 3:427–432.
2010.
|
|
10
|
Jung HW and Ghil SH: A Torilis
japonica extract exerts anti-proliferative activities on the
U87MG human glioblastoma cell line. Mol Med Rep. 3:1041–1045.
2010.
|
|
11
|
Boatright KM and Salvesen GS: Mechanisms
of caspase activation. Curr Opin Cell Biol. 15:725–731. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Das A, Tang KS, Gopalakrishnan S, Waring
MJ and Tomasz M: Reactivity of guanine at m5CpG steps in DNA:
evidence for electronic effects transmitted through the base pairs.
Chem Biol. 6:461–471. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Budihardjo I, Oliver H, Lutter M, Luo X
and Wang X: Biochemical pathways of caspase activation during
apoptosis. Annu Rev Cell Dev Biol. 15:269–290. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang HL, Chen CS, Chang WH, Lu FJ, Lai YC,
Chen CC, Hseu TH, Kuo CT and Hseu YC: Growth inhibition and
induction of apoptosis in MCF-7 breast cancer cells by Antrodia
camphorata. Cancer Lett. 231:215–227. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shim HY, Park JH, Paik HD, Nah SY, Kim DS
and Han YS: Acacetin-induced apoptosis of human breast cancer MCF-7
cells involves caspase cascade, mitochondria-mediated death
signaling and SAPK/JNK1/2-c-Jun activation. Mol Cells. 24:95–104.
2007.
|
|
16
|
Chibazakura T, McGrew SG, Cooper JA,
Yoshikawa H and Roberts JM: Regulation of cyclin-dependent kinase
activity during mitotic exit and maintenance of genome stability by
p21, p27, and p107. Proc Natl Acad Sci USA. 101:4465–4470. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen T and Wong YS: Selenocystine induces
S-phase arrest and apoptosis in human breast adenocarcinoma MCF-7
cells by modulating ERK and Akt phosphorylation. J Agric Food Chem.
56:10574–10581. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu M, Sheppard KA, Peng CY, Yee AS and
Piwnica-Worms H: Cyclin A/CDK2 binds directly to E2F-1 and inhibits
the DNA-binding activity of E2F-1/DP-1 by phosphorylation. Mol Cell
Biol. 14:8420–8431. 1994.PubMed/NCBI
|
|
19
|
Koopman G, Reutelingsperger CP, Kuijten
GA, Keehnen RM, Pals ST and van Oers MH: Annexin V for flow
cytometric detection of phosphatidylserine expression on B cells
undergoing apoptosis. Blood. 84:1415–1420. 1994.PubMed/NCBI
|
|
20
|
Martin SJ, Reutelingsperger CP, McGahon
AJ, Rader JA, van Schie RC, LaFace DM and Green DR: Early
redistribution of plasma membrane phosphatidylserine is a general
feature of apoptosis regardless of the initiating stimulus:
inhibition by overexpression of Bcl-2 and Abl. J Exp Med.
182:1545–1556. 1995. View Article : Google Scholar
|
|
21
|
Zhang XP, Liu F and Wang W: Two-phase
dynamics of p53 in the DNA damage response. Proc Natl Acad Sci USA.
108:8990–8995. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Basu A and Haldar S: The relationship
between BcI2, Bax and p53: consequences for cell cycle progression
and cell death. Mol Hum Reprod. 4:1099–1109. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim R, Emi M and Tanabe K:
Caspase-dependent and -independent cell death pathways after DNA
damage. Oncol Rep. 14:595–599. 2005.PubMed/NCBI
|