Introduction
The pathogenesis of the majority of chronic kidney
diseases (CKDs) involves a complex mechanism of hemodynamic and
inflammatory processes that leads to renal fibrosis and
tubulointerstitial scarring, with subsequent progression towards
end-stage renal disease (ESRD) (1). Tubulointerstitial fibrosis is the
final common pathway in late-stage renal disease. The pathogenesis
of kidney fibrosis is characterized by the overproduction and
deposition of extracellular matrix (ECM), which ultimately leads to
fibrotic lesions and tissue scarring (2,3).
Renal interstitial fibroblasts are the principal effector cells
responsible for ECM overproduction in the fibrotic kidney and their
activation is regarded as a key event in the pathogenesis of
chronic renal fibrosis (4). It is
well-known that the upregulation of transforming growth factor
(TGF)-β1 signaling is considered to be a convergent pathway
following renal injury, irrespective of the initial etiologies
(5). An increase in the production
of TGF-β is one of the most important mechanisms in the
pathogenesis of renal fibrogenesis (6). TGF-β1 induces renal fibrosis by
activating interstitial fibroblasts, causing them to produce large
amounts of matrix components. These actions lead to
glomerulosclerosis and tubulointerstitial fibrosis and ultimately
to ESRD (7).
Studies have shown that therapeutic interventions,
including the blockade of the renin-angiotensin-aldosterone system
and the use of immunosuppressive drugs retard the progression of
renal disease in experimental models (8) and human CKD clinical trials (9,10).
Although these strategies promote renoprotective effects, they fail
to arrest the progression of renal fibrosis and scarring.
Considering the fact that interstitial fibrosis represents the
final common pathway of CKD, a therapeutic intervention with drugs
that exhibit antifibrotic properties may be an attractive choice of
therapy for arresting the autonomous fibrogenic process in chronic
progressive nephropathies.
Amygdalin (vitamin B17; previously known as
Laetrile) is one of a number of nitrilosides, the natural
cyanide-containing substances abundant in the seeds of the prunasin
family, including apricots, almonds, peaches, apples and other
rosaceous plants. Among the prunasins, armeniacae semen has been
used for the treatment of asthma, bronchitis, emphysema, leprosy,
colorectal cancer, leucoderma and pain (11). Amygdalin is composed of two
molecules of glucose, one of which is benzaldehyde, which induces
an anti-neoplastic compound. Amygdalin has also been used to treat
cancers and relieve pain (12).
In the present study, the effect of amygdalin on the
kidney fibroblast (KFB) activation in normally cultured rat KFBs
and its therapeutic potential on renal fibrosis in animal models of
unilateral ureteral obstruction (UUO) was examined. Furthermore,
the mechanism by which amygdalin inhibits renal fibroblast
activation and fibrogenesis was investigated. The results suggested
that amygdalin is a potent drug that can be used to reduce renal
fibrosis during CKD progression and that its therapeutic mechanism,
at least in part, blocks interstitial fibroblast cell
activation.
Materials and methods
Separation and culture of the KFBs
A cell culture experiment was performed using renal
fibroblasts obtained from kidney explants. For the primary culture,
kidneys from rats were surgically and aseptically removed. Kidney
sections (1 mm3) were seeded in 25-cm2
bottles and cultured in Dulbecco’s modified Eagle’s medium (DMEM;
Gibco Corp., Carlsbad, CA, USA), supplemented with 20% fetal calf
serum (FCS; Cultilab, Campinas, Brazil) and antibiotics
(amphotericin, 2.5 mg/ml; ampicillin, 100 mg/ml; and streptomycin,
100 mg/ml; Gibco Corp.), at 37°C in a humidified atmosphere of 5%
CO2. When the cell outgrowth from the explants was
initiated, the remaining tissue was removed. Once the cells reached
confluence, they were harvested and split in a ratio of 1:3.
Subsequent to 4–6 passages, the cells exhibited a typical
fibroblast morphology. The cells were phenotypically characterized
on the basis of their immunocytochemistry. The following antibodies
were used: mouse anti-vimentin, mouse anti-desmin and mouse
anti-keratin (Sigma Aldrich, St. Louis, MO, USA).
Immunocytochemical staining analysis
Cells were grown on glass coverslips. Following
treatment, the cells were fixed with 4% paraformaldehyde for 20 min
and permeablilized with 1% Triton X-100 for 10 min at room
temperature. Subsequent to further washing, the cells were blocked
with 10% goat serum for 30 min at room temperature. The cells were
then incubated with the primary antibodies at room temperature for
2 h followed by incubation with the secondary antibody for an
additional 1 h. The slides were stained with
3,37prime;-diaminobenzidine (DAB) and the nuclei were
counterstained with hematoxylin for 10 min. The coverslips were
mounted on glass slides with anti-fade mounting media (Invitrogen,
Carlsbad, CA, USA) and the images were visualized using a Zeiss LSM
710 laser confocal fluorescence microscope (Zeiss, Oberkochen,
Germany).
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
The cells were grown in a final volume of 100 μl
medium per well in 96-well plates. To determine the
antiproliferative effect of amygdalin, the cells were treated with
amygdalin at concentrations of 25, 50, 80, 100 and 200 μg/ml for 24
h. The cells of the control group were left untreated. Subsequent
to adding 10 μl MTT labeling reagent, containing 5 mg/ml MTT in
phosphate-buffered saline (PBS), to each well, the plates were
incubated for 4 h. The optical density (OD) was calculated as the
difference between the absorbance at the reference wavelength and
that observed at the test wavelength. Inhibition ratio was
calculated as: (1 − OD value in medication group/OD value in
control group) × 100.
TGF-β1 enzyme-linked immunosorbent assay
(ELISA)
Human peripheral blood mononuclear cells (PBMC) were
obtained through common separation at a concentration of
1×106 cells/ml. Besides the control group, the cells in
the treatment groups were treated with amygdalin at concentrations
of 25, 50, 100, 200, 400 and 800 μg/ml. The cells were cultured for
48 h and the total TGF-β1 was measured in the cell culture
supernatant using a commercial sandwich ELISA kit according to the
manufacturer’s instructions.
UUO rat kidney model and amygdalin
treatment
Construction of the UUO model
The UUO model was established in male, 180–200 g
Wistar rats as described in a previous study, with minor
modifications (13). Briefly, the
abdominal cavity was exposed via a midline incision and the left
ureter was isolated and ligated. The contralateral kidney was used
as a control. To examine the efficacy of amygdalin in renal
fibrosis subsequent to UUO injury, various concentrations of
amygdalin (3 and 5 mg/kg/day) were intraperitoneally injected
immediately following ureteral ligation. The animals were
sacrificed and the kidneys removed at days 7, 14 and 21.
Pathological examination
Following anesthetization, the kidneys of the
animals were removed for pathological examination using Masson’s
trichrome staining to identify the interstitial collagen with a
blue coloration. The evaluation scope of each parameter was 0–3 and
the evaluation score of each tubulointerstitial sample was 0–9.
Masson’s staining of each kidney tissue sample was evaluated by
three observers using a blind method to obtain a mean value.
Statistical analysis
Values were expressed as the mean ± SD for each
group. Statistical differences between two groups were analyzed by
the unpaired Student’s t-test and differences between multiple
groups of data were analyzed by a one-way ANOVA with a Bonferroni
correction. P<0.05 was considered to indicate a statistically
significant difference.
Results
Impact of amygdalin on the proliferation
of the KFBs
Cell characterization
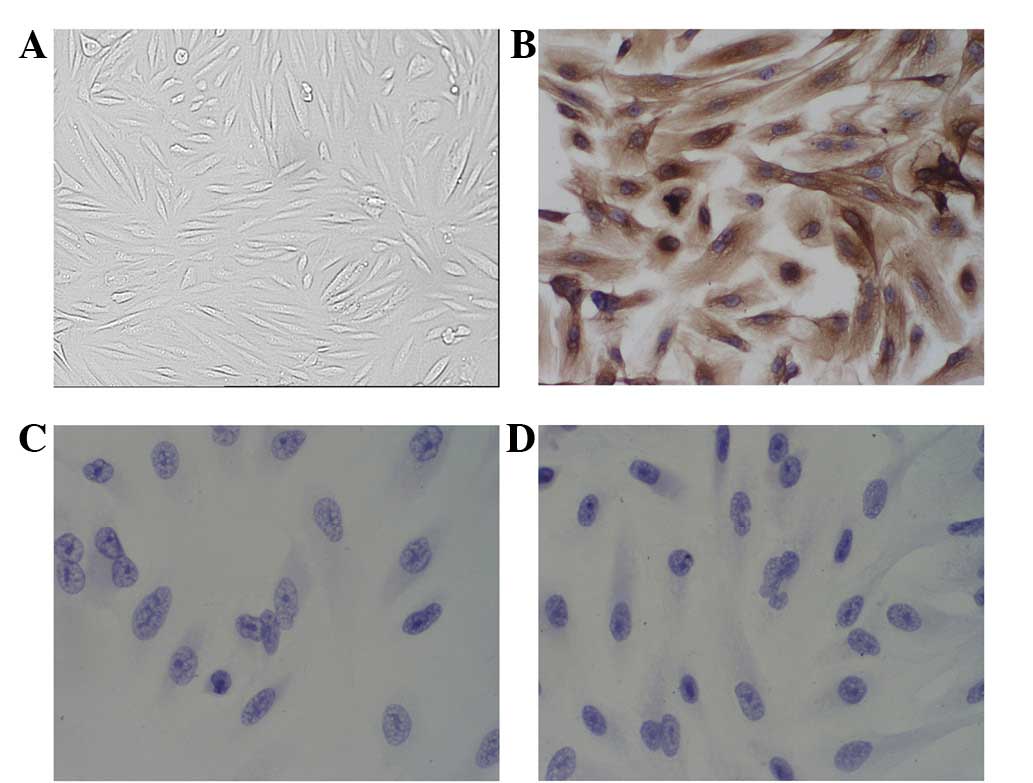
The cells were cultured and reached confluence after
3–4 days (Fig. 1). The cells were
characterized by their morphological appearance under phase
contrast microscopy and by the positive staining for vimentin and
the negative staining for keramin and desmin. This demonstrated
that the cells were KFBs.
Effect of amygdalin on the cell
proliferation in the KFBs
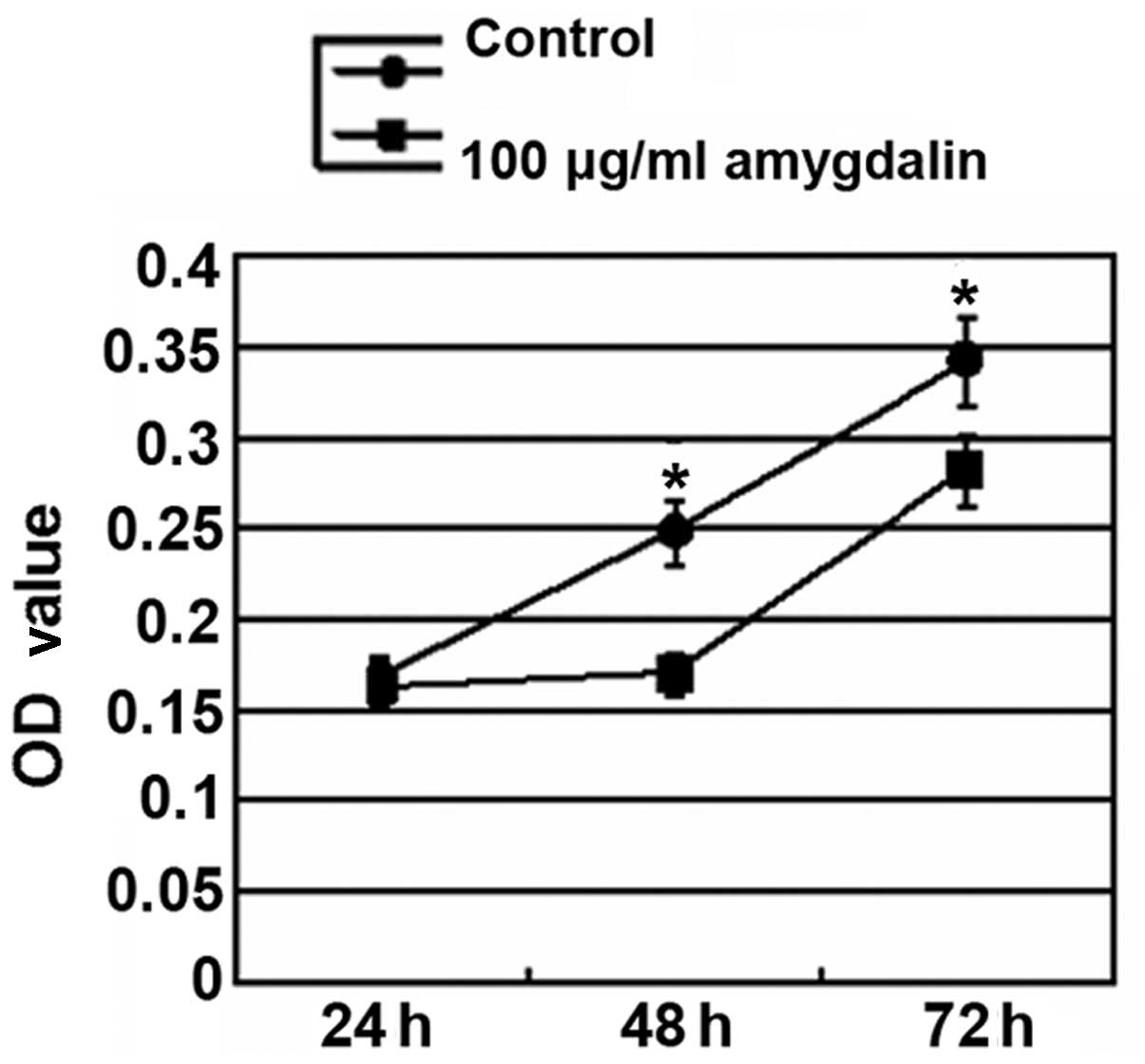
As is evident in Fig.
2, with the extension of the culture period, the cell
proliferation was significantly enhanced in the control group. Cell
proliferation was significantly decreased in the amygdalin group
compared with the control group following 48 h and 72 h of
treatment (P<0.05). However, there was no significant difference
between the control and amygdalin groups subsequent to 24 h of
treatment.
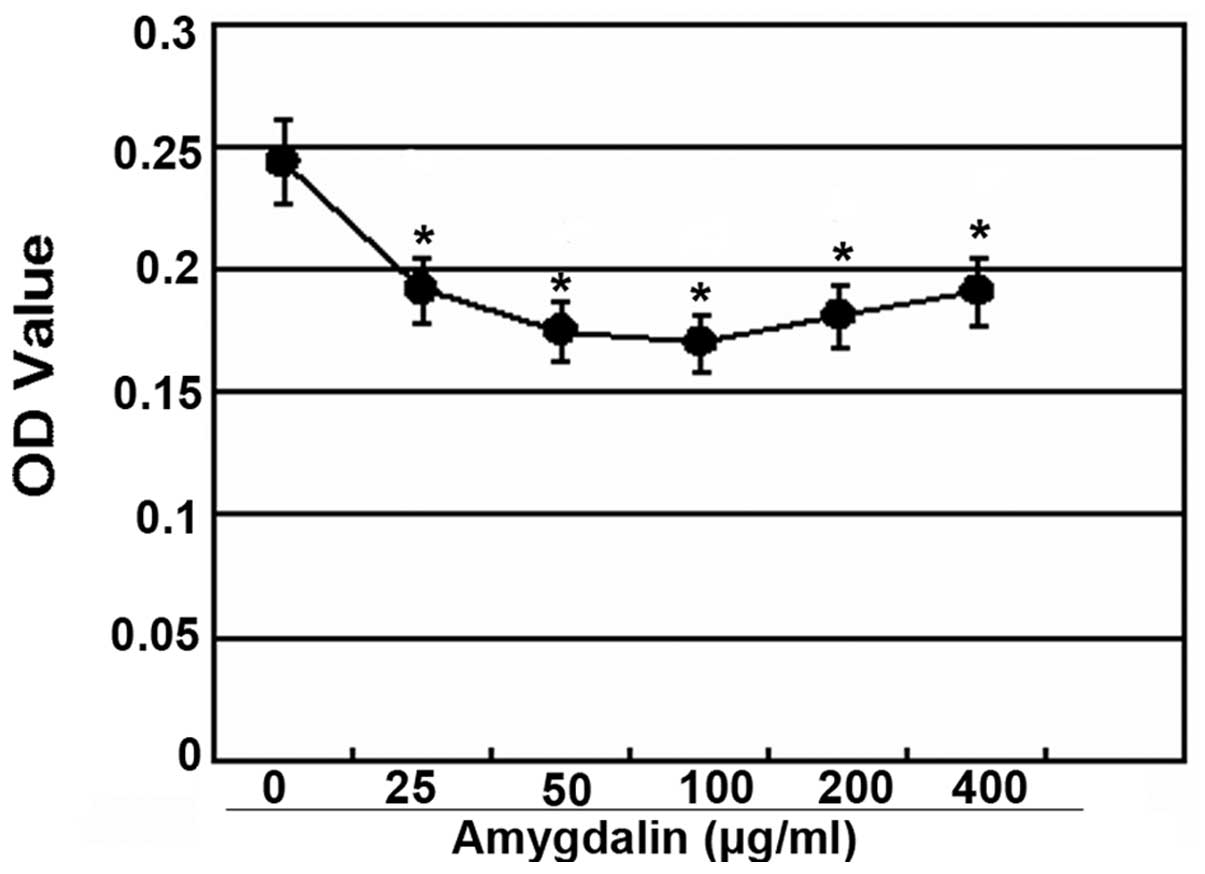
In addition, the antiproliferative effect of
amygdalin was observed at a concentration of 25–400 μg/ml and the
most potent inhibition was observed at a concentration of 100
μg/ml, with an inhibition rate of 31.53% (Fig. 3).
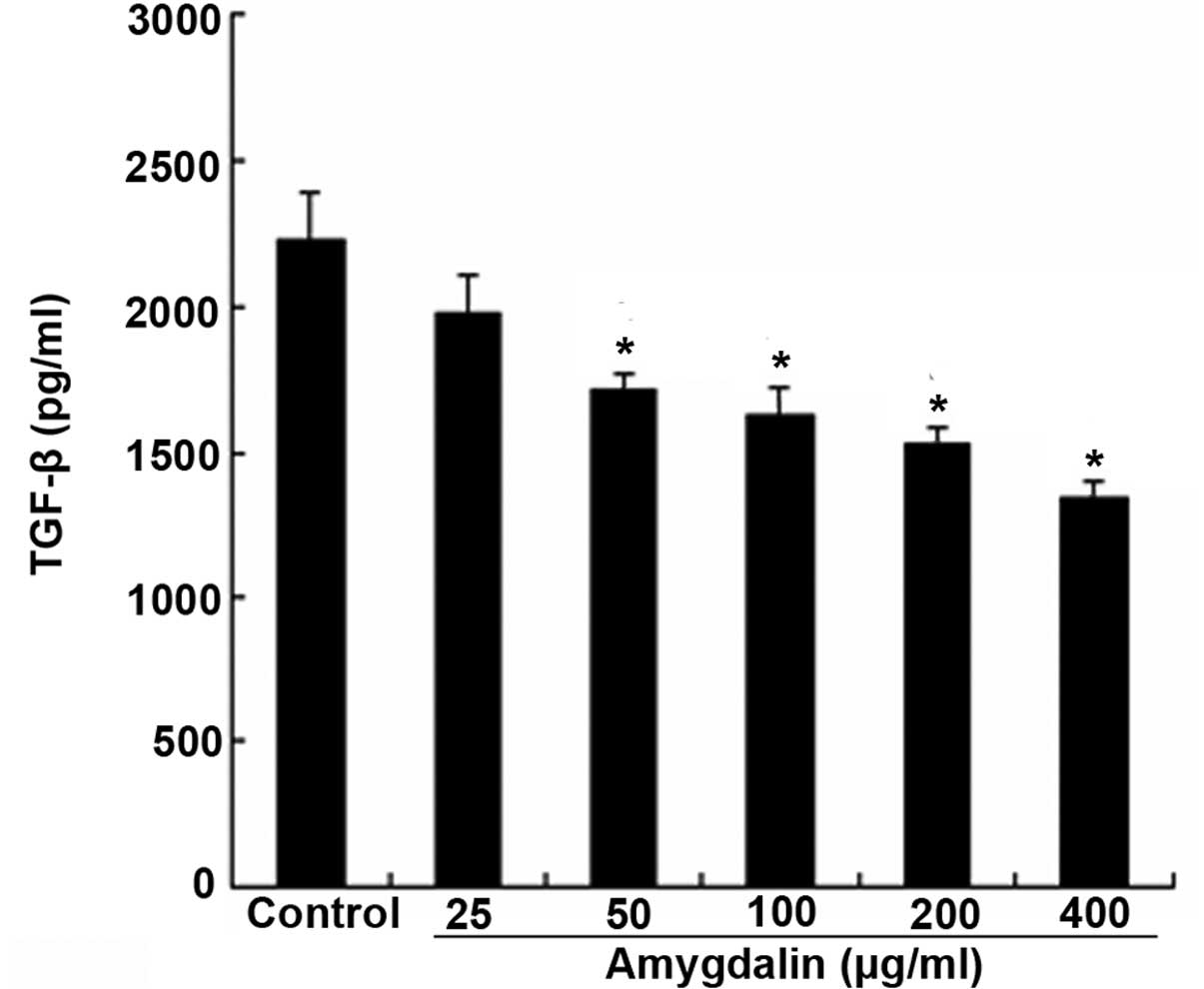
Effect of amygdalin on the expression
of TGF-β1 produced by human lymphocytes
As TGF-β1 is a major cytokine that is able to induce
the transformation of quiescent renal fibroblasts to
myofibroblasts, the present study first examined the effect of
amygdalin on TGF-β1 production. Fig.
4 shows that when within the concentration range of 50–400
μg/ml, amygdalin suppressed the TGF-β1 secretion in the peripheral
blood lymphocytes, which was stimulated by PHA, in a
concentration-dependent manner (P<0.05). These data collectively
indicate that amygdalin is a potent agent for blocking the
activation of cultured renal interstitial fibroblasts.
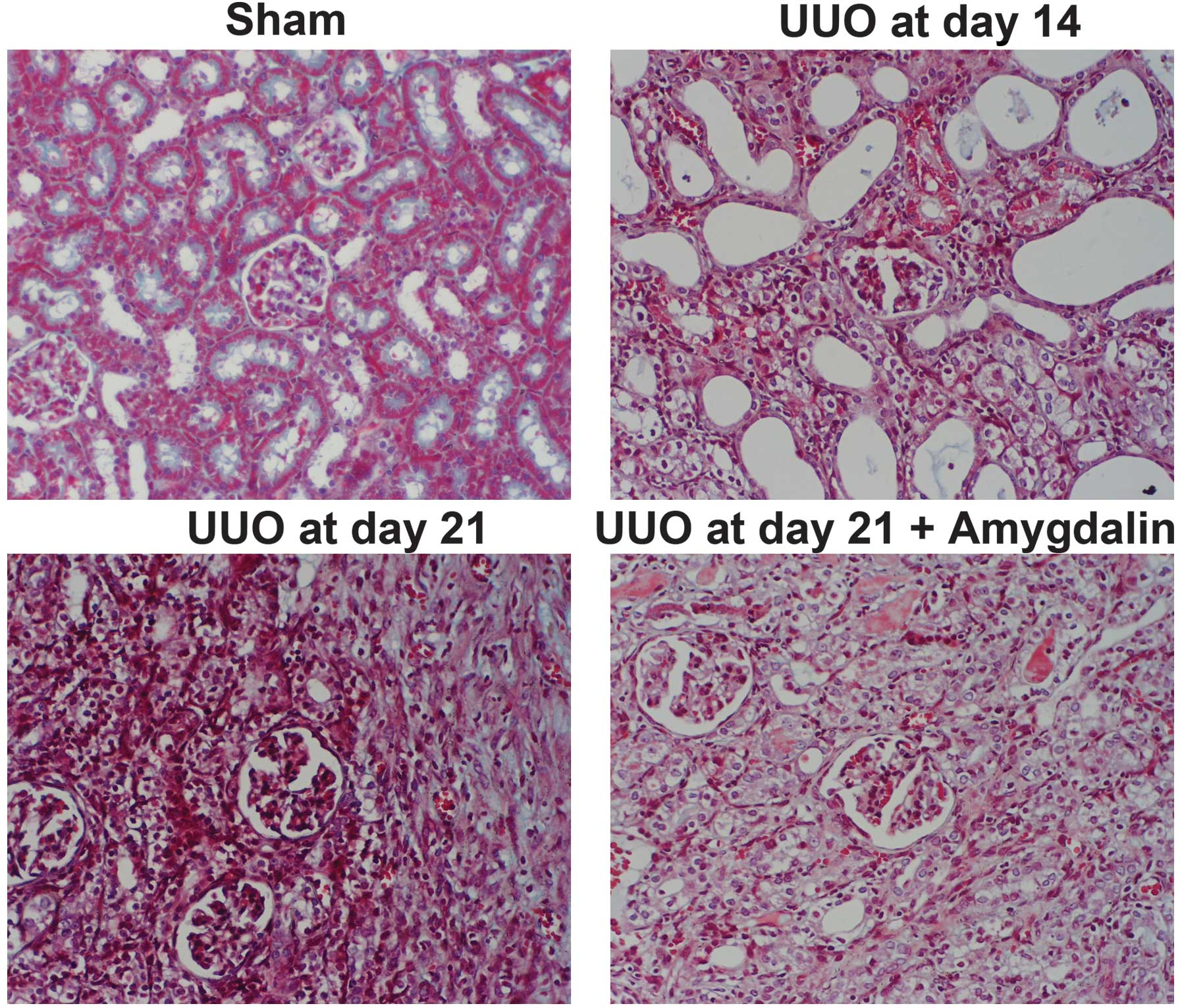
Amygdalin ameliorated renal fibrosis
in the obstructed kidney
As the major feature of renal fibrosis is the
increased levels of ECM, the present study examined the effect of
amygdalin on the expression of interstitial collagen fibrils, using
Masson trichrome staining to investigate the ability of amygdalin
in suppressing myofibroblast activation in vivo. As shown in
Fig. 5, the kidneys with ureteral
obstructions for 7 days exhibited severe morphological lesions
characterized by tubular dilation with epithelial atrophy and
interstitial expansion with collagen accumulation and deposition,
as evidenced by an increase in the trichrome-positive areas within
the tubulointerstitium subsequent to UUO injury. By contrast, the
kidneys from the rats administered with amygdalin exhibited a
marked attenuation of these morphological lesions, with less
fibrosis in the interstitium. These data showed an efficacy of
amygdalin for inhibiting the accumulation of the ECM proteins
following obstructive injury.
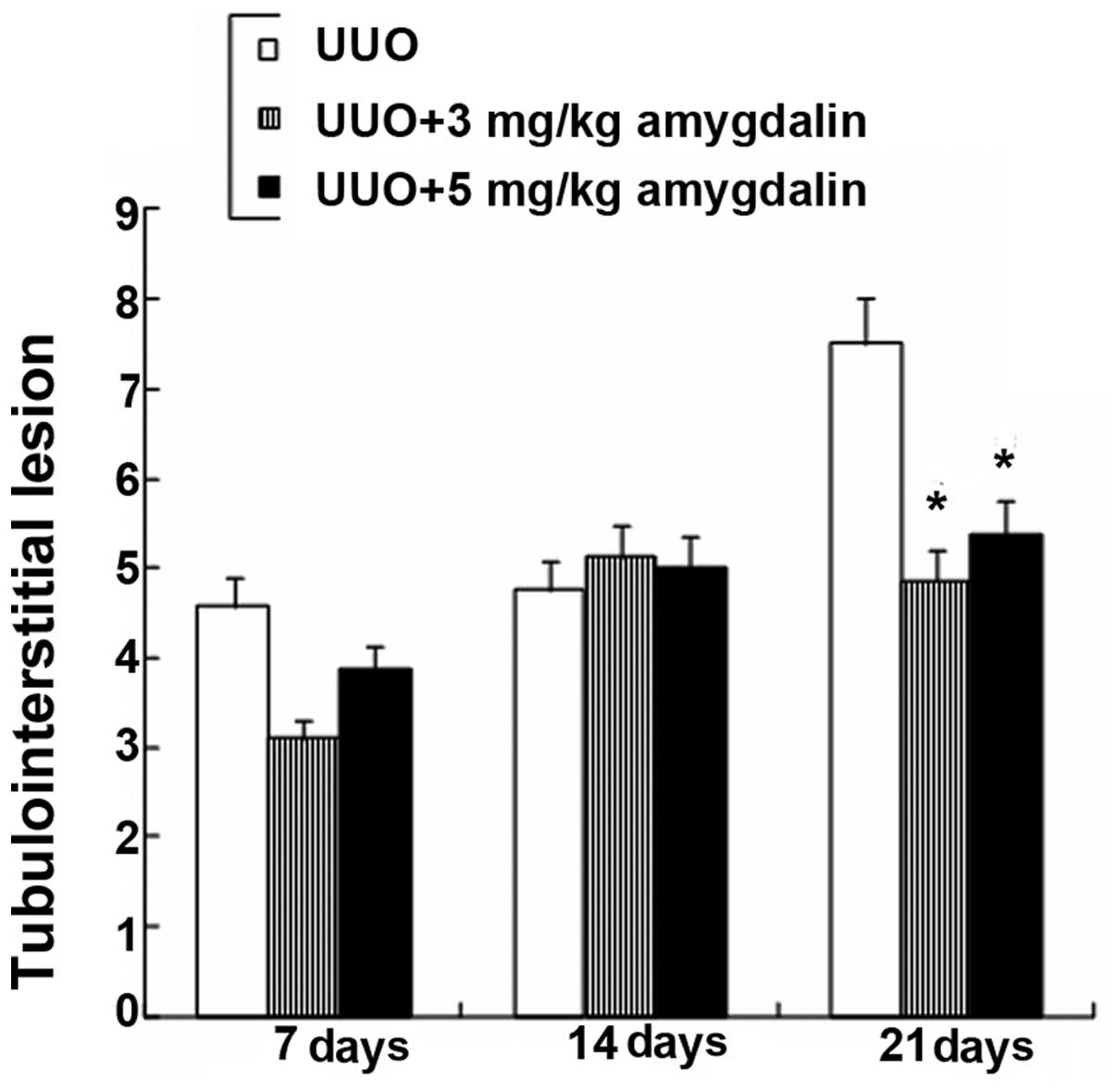
Fig. 6 shows the
tubulointerstitial lesions (TILs) in the experimental rats were
aggravated on day 21. However, the extent of the rat TILs in the 3
mg and 5 mg amygdalin treatment groups was significantly reduced on
day 21 (P<0.05). In addition, there was no significant
difference in the TILs among the three groups on the 7th and 14th
days (P>0.05). Amygdalin is therefore effective in preventing
renal fibrosis progression in rats following UUO injury.
Discussion
Renal interstitial fibrosis is the final result of
TILs, which have various causes, and also one of the major reasons
behind final-stage renal failure (14). Modern pharmacology indicates that
Traditional Chinese Medicine shows promising application prospects
for the prevention and treatment of renal fibrosis and has
consequently drawn the attention of investigators. Amygdalin is an
aromatic cyanogenic glycoside that exists in the seeds of rosaceous
plants, including armeniaca, wild apricot, peach, mountain peach
and plum. The structural formula of amydalin is
(6-O-β-D-glucopyranosyl-β-D-glucopyranosyl)oxy] (phenyl)
acetonitrile (15). In the present
study, fibroblasts were successfully separated from the rat kidneys
by a digestive method and then identified by a immunocytochemical
method. Notably, with the extension of the culture period,
significant cell proliferation was observed in the control group;
the proliferative levels in the amygdalin groups at the varying
concentrations were significantly lower than those of the control
group. Amygdalin was able to suppress the proliferative activity of
the fibroblasts in a concentration-dependent manner. Furthermore,
the suppressive effect of amygdalin reached a peak subsequent to 48
h of incubation at a concentration of 100 μg/ml. However, the
detailed mechanism by which amygdalin suppresses KFB proliferation
has yet to be fully investigated. Fibroblasts play a significant
role in the physiological and pathological processes of renal
fibrosis, and the treatment countermeasures that have used
fibroblasts as the target cells have shown the corresponding
effects (16).
In the present study, amygdalin was able to suppress
TGF-β1 secretion in the lymphocytes at concentrations of 25–400
μg/ml. As an important profibrogenic cytokine, TGF-β1 plays a
pivotal role in the occurrence, development and other links of
renal interstitial fibrosis (7).
Furthermore, it has been demonstrated that the induction and
activation of TGF-β1/Smad is essential for eliciting the fiber
formation reaction; equally important is the loss of the Smad
antagonist, which may cause the fiber formation signal to become
out of control (17,18). In the pathogenesis of renal
interstitial fibrosis, fibroblasts and myofibroblasts are the major
source of TGF-β1 production. The cytological basis for renal
interstitial fibrosis is the activation of myofibroblasts, which
may come from various resources. With the exception of the
fibroblasts inherent in the kidney, a fairly large number of
myofibroblasts are from the renal tubular epithelial cells.
Specifically, renal tubular epithelial cells may transform into
myofibroblasts through the mechanism of epithelial-myofibroblast
transdifferentiation (EMT) (19,20),
resulting in a large quantity of ECM and promoting the occurrence
of fibrosis. TGF-β1 plays a significant role in the EMT process
(21). The present study also
demonstrated that amygdalin exerted an antifibrotic effect through
the inhibition of TGF-β1 secretion in the lymphocytes.
Based on in vivo results that showed that
amygdalin was able to suppress KFB proliferation, the present study
further investigated the antifibrotic effect of amygdalin on renal
interstitial fibrosis by means of UUOs. The pathological changes of
the kidney tissues in the obstruction process and the effect of
amygdalin treatment on the damage in obstructed kidneys were
examined. Renal interstitial fibrosis is characterized by the
accumulation and increase of renal interstitial cells and collagen
components, in combination with renal tubular fibrosis or expansion
and deformation (22). At seven
days post-UUO surgery, inflammatory cell infiltration, cell
proliferation, renal tubular expansion and other pathological
changes in the kidney tissues were observed. Thereafter the kidney
tissues showed progressive renal tubular fibrosis and renal
interstitial fibrosis. Following UUO surgery, early-stage renal
interstitial fibrosis damage had already occurred in the obstructed
side of the kidney on the 7th day. Although renal interstitial
fibrosis had become fairly severe on the 21st day, there were no
significant pathological changes to the glomerulus, as in
accordance with previous studies (14,23).
This confirmed the characteristics for the pathological change in
the injured kidney. Furthermore, on the 21st day, in comparison
with the rats in the obstruction group, the extent of the rat
tubulointerstitial lesions in the amygdalin treatment groups was
significantly reduced. Consequently, amygdalin treatment may
significantly alleviate the extent of the pathological damage to
the kidney and postpone the process of renal interstitial
fibrosis.
In summary, the present study has demonstrated that
amygdalin was able to suppress KFB proliferation and TGF-β1
secretion in the lymphocytes and thus was able to significantly
alleviate the extent of the UUO pathological damage to the kidney
and postpone the process of renal interstitial fibrosis, which
further accounts for the anti-fibrotic effect of amygdalin.
Although the detailed mechanisms behind the action of amygdalin
remain undefined, we hypothesize that the mechanisms may be
involved in increasing the secretion of type I collagenase,
inhibiting KFB proliferation, accelerating apoptosis and
suppressing type I collagen synthesis. However, future studies are
required to investigate the mechanisms by which amygdalin protects
against renal interstitial fibrosis.
Acknowledgements
This study is supported by grants from the Key
Laboratory of Fujian Province (No. 2008J1006) and the Nanjing
Medical Technology Innovation Project, China (No. 2009MA093).
Abbreviations:
|
CKD
|
chronic kidney disease
|
|
UUO
|
unilateral ureteral obstruction
|
|
ECM
|
extracellular matrix
|
|
TGF-β
|
transforming growth factor-β
|
|
KFB
|
kidney fibroblast
|
References
|
1
|
Noronha IL, Fujihara CK and Zatz R: The
inflammatory component in progressive renal disease - are
interventions possible? Nephrol Dial Transplant. 17:363–368. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wynn TA: Cellular and molecular mechanisms
of fibrosis. J Pathol. 214:199–210. 2008. View Article : Google Scholar
|
|
3
|
Neilson EG: Mechanisms of disease:
Fibroblasts - a new look at an old problem. Nat Clin Pract Nephrol.
2:101–108. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Boor P, Ostendorf T and Floege J: Renal
fibrosis: novel insights into mechanisms and therapeutic targets.
Nat Rev Nephrol. 6:643–656. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu Y: Renal fibrosis: new insights into
the pathogenesis and therapeutics. Kidney Int. 69:213–217. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Eddy AA: Molecular basis of renal
fibrosis. Pediatr Nephrol. 15:290–301. 2000. View Article : Google Scholar
|
|
7
|
Böttinger EP and Bitzer M: TGF-beta
signaling in renal disease. J Am Soc Nephrol. 13:2600–2610.
2002.
|
|
8
|
Fujihara CK, Malheiros DM, Zatz R and
Noronha IL: Mycophenolate mofetil attenuates renal injury in the
rat remnant kidney. Kidney Int. 54:1510–1519. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lewis EJ, Hunsicker LG, Clarke WR, et al;
Collaborative Study Group. Renoprotective effect of the
angiotensin-receptor antagonist irbesartan in patients with
nephropathy due to type 2 diabetes. N Engl J Med. 345:851–860.
2001. View Article : Google Scholar
|
|
10
|
Brenner BM, Cooper ME, de Zeeuw D, et al;
RENAAL Study Investigators. Effects of losartan on renal and
cardiovascular outcomes in patients with type 2 diabetes and
nephropathy. N Engl J Med. 345:861–869. 2001. View Article : Google Scholar
|
|
11
|
Chang HK, Yang HY, Lee TH, et al:
Armeniacae semen extract suppresses lipopolysaccharide-induced
expressions of cyclooxygenase (correction of cycloosygenase)-2 and
inducible nitric oxide synthase in mouse BV2 microglial cells. Biol
Pharm Bull. 28:449–454. 2005. View Article : Google Scholar
|
|
12
|
Fukuda T, Ito H, Mukainaka T, Tokuda H,
Nishino H and Yoshida T: Anti-tumor promoting effect of glycosides
from Prunus persica seeds. Biol Pharm Bull. 26:271–273.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu N, Tolbert E, Pang M, Ponnusamy M, Yan
H and Zhuang S: Suramin inhibits renal fibrosis in chronic kidney
disease. J Am Soc Nephrol. 22:1064–1075. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Robertson H, Ali S, McDonnell BJ, Burt AD
and Kirby JA: Chronic renal allograft dysfunction: the role of T
cell-mediated tubular epithelial to mesenchymal cell transition. J
Am Soc Nephrol. 15:390–397. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chang HK, Shin MS, Yang HY, et al:
Amygdalin induces apoptosis through regulation of Bax and Bcl-2
expressions in human DU145 and LNCaP prostate cancer cells. Biol
Pharm Bull. 29:1597–1602. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vongwiwatana A, Tasanarong A, Rayner DC,
Melk A and Halloran PF: Epithelial to mesenchymal transition during
late deterioration of human kidney transplants: the role of tubular
cells in fibrogenesis. Am J Transplant. 5:1367–1374. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zeisberg M, Hanai J, Sugimoto H, et al:
BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal
transition and reverses chronic renal injury. Nat Med. 9:964–968.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang W, Huang XR, Li AG, et al: Signaling
mechanism of TGF-beta1 in prevention of renal inflammation: role of
Smad7. J Am Soc Nephrol. 16:1371–1383. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shihab FS, Bennett WM, Yi H and Andoh TF:
Pirfenidone treatment decreases transforming growth factor-beta1
and matrix proteins and ameliorates fibrosis in chronic
cyclosporine nephrotoxicity. Am J Transplant. 2:111–119. 2002.
View Article : Google Scholar
|
|
20
|
Liu Y: Epithelial to mesenchymal
transition in renal fibrogenesis: pathologic significance,
molecular mechanism, and therapeutic intervention. J Am Soc
Nephrol. 15:1–12. 2004. View Article : Google Scholar
|
|
21
|
Heeg MH, Koziolek MJ, Vasko R, et al: The
antifibrotic effects of relaxin in human renal fibroblasts are
mediated in part by inhibition of the Smad2 pathway. Kidney Int.
68:96–109. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Satoh S, Yamaguchi T, Hitomi A, et al:
Fasudil attenuates interstitial fibrosis in rat kidneys with
unilateral ureteral obstruction. Eur J Pharmacol. 455:169–174.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Strutz F and Müller GA: Renal fibrosis and
the origin of the renal fibroblast. Nephrol Dial Transplant.
21:3368–3370. 2006. View Article : Google Scholar : PubMed/NCBI
|