Introduction
Cervical cancer is one of the most common malignant
cancers in gynecology (1–3). Recently, the incidence rate of the
disease has increased and has demonstrated an increasing trend in
younger females. However, the developmental process of the tumor is
complex and involves numerous factors at various stages (4,5).
Previous studies have demonstrated that angiogenesis was the
foundation for tumor growth (6,7) and
that NO is one of the most secondary messengers and is capable of
promoting the process (1).
Vascular endothelial growth factor (VEGF) is
considered to be one of the most significant of the 30 cytokines
whose function it is to promote vascular production (8). VEGF increases cancer cell growth by
promoting the cleavage and proliferation of tumor cells by binding
to the tyrosinase receptor (9–11).
Inducible nitric oxide synthase (iNOS) is a key
enzyme in NO synthesis and has a high expression in numerous types
of malignant tumors. Previous studies have demonstrated that iNOS
promotes the production of tumor blood vessels by catalyzing the
synthesis of additional NO (9,12).
Experimental evidence has suggested that iNOS may emerge as a VEGF
enhancer, due to the fact that the VEGF level is capable of being
induced by iNOS in endothelial cell migration and angiogenesis
(13). However, the correlation
between iNOS and VEGF in cervical cancer remains unclear (11,14).
Materials and methods
Cell culture
Two human cervical carcinoma cell lines, Hela and
SiHa, which exhibit relatively low metastatic capability, were
obtained from the China Center for Type Culture Collection (Wuhan,
China). Cells were all cultured in Dulbecco’s modified Eagle’s
medium supplemented with 10% fetal bovine serum (FBS; Gibco, Grand
Island, NY, USA) in an atmosphere of 5% CO2 and 95% air.
SiHa and HeLa cells were plated in 6-well plates.
Quantitative polymerase chain reaction
(qPCR)
Total RNA was extracted from 1×106 human
SiHa and HeLa cells using TRIzol (Invitrogen Life Technologies,
Carlsbad, CA, USA). Aliquots (1 g) of RNA were reverse transcribed
to cDNA and aliquots (4 μl) of cDNA were used as a template for
qPCR using an ABI7900HT PCR system (Applied Biosystems, Carlsbad,
CA, USA) according to the manufacturer’s instructions. The primers
were as follows: Forward: 5′-TGGAGAGAAACTGAAGAAATCG-3′ and reverse
5′-GTACCTGAATTTGTTGTTGAGC-3′ for iNOS; forward:
5′-GCTGTGAAGCCAGACAGC-3′ and reverse: 5′-GGCAAGTCACCCTGCTGA-3′ for
VEGF; and forward: 5′-CTCCATCCTGGCCTCGCTGT-3′ and reverse:
5′-GCTGTCACCTTCACCGTTCC-3′ for β-actin (ACTB). All experiments were
performed in triplicate, with each reading taken three times.
Average and standard deviations were subsequently calculated.
Western blotting
A nuclear extraction kit (Chemicon International,
Temecula, CA, USA) was used to isolate cytoplasmic proteins.
Proteins, 50 μg, from various groups were boiled for 5 min in
sample buffer and were subsequently separated in sodium dodecyl
sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) (10–15%) and
transferred onto a polyvinylidene fluoride (PVDF) membrane (Bio-Rad
Laboratories, Hercules, CA, USA). Nonspecific reactivity was
blocked using 5% bovine serum albumin in a Tris-buffered saline
(TBS) with Tween-20 buffer (100 ml 10% TBS, 10 ml 10% Tween-20
final 0.1% v/v, dissolved with 890 ml double
distilled-H2O) and subsequently incubated with primary
antibody mouse anti-VEGF (sc-1836, 1:200), rabbit anti-iNOS
(sc-650, 1:200) or rabbit anti-actin (sc-130656, 1:500) antibodies
(all from Santa Cruz Biotechnology, Santa Cruz, CA, USA) overnight
at 4°C and incubated with horseradish peroxidase (HRP)-conjugated
secondary antibodies (Santa Cruz Biotechnology). Bands were
detected using the Supersignal West Dura Extended Duration
Substrate (Pierce, Rockford, IL, USA), and quantified using the
Chemi-doc gel quantification system (Bio-Rad). All data were
normalized to β-actin. Western blotting experiments were repeated
at least twice in order to confirm the results.
Enzyme-linked immunosorbent assay (ELISA)
and NO detect assay (Griess method)
The quantity of VEGF released was measured by a
sandwich ELISA. ELISA plates (Cat. No: 655001; Greiner, Bio-One,
Frickenhausen, Germany) were coated with 100 μl of 2 μg/ml
anti-VEGF165 (R&D Systems, Minneapolis, MN, USA) antibody in
phosphate-buffered saline (PBS) for 12 h at 4°C. The plates were
washed with PBS containing 0.1% Tween 20 (TPBS) and incubated for 1
h at 25°C with 200 μl/well of 1% bovine serum albumin
(Sigma-Aldrich, St. Louis, MO, USA) in PBS. The conditioned medium,
or various concentrations of recombinant human VEGF, were incubated
for 2 h at 25°C and washed four times with TPBS. Following
incubation for 2 h at 25°C with 100 μl of 0.2 μg/ml biotinylated
anti-VEGF antibody, the plates were washed and incubated for a
further 45 min with 100 μl HRP-conjugated streptavidin (Vector
Laboratories, Burlingame, CA, USA). After washing, the plates were
developed by adding 50 μl tetramethylbenzidine (Sigma-Aldrich) and
the reaction was stopped by adding 50 μl
2NH2SO4. The absorbance at 450 nm was
measured with a Tecan 96-well plate reader (Tecan,
Untersbergstrasse, Austria). The NO level was detected by
supernatant absorbance at 540 nm, according to the Griess method
(11).
Cell proliferation and cell apoptosis
assays
Cell proliferation assays were performed with cell
counting kit-8 (CCK-8; Dojindo, Kumamoto, Japan). SiHa and HeLa
cells treated with nonsense sequence (SCR) or lentiviruses
expressing iNOS-targeted shRNA (iNOS-SH) were seeded into 96-well
plates at 5×103 per well in a 100 μl cell culture medium
and maintained at 37°C in a humidified incubator containing 5%
CO2 for 24 h. One hundred microlitres of 2% FBS medium
was used as a control. After 96 cultures, 10 μl CCK-8 solution was
added to the triplicate wells and incubated for 3 h. Subsequently,
the absorbance at 450 nm was measured in order to calculate the
number of vital cells in each well. Cell proliferation is
represented as the mean percentage ± standard deviation of
absorbance. Cell apoptosis assays were performed by flow cytometry
with propidium iodide (PI) and Annexin V-fluorescein isothiocyanate
(FITC) once the cells had been digested with trypsin, washed and
harvested.
Short hairpin RNA (shRNA) interference,
lentivirus production and infection
iNOS-SH shRNA was generated from two different
annealed oligonucleotides (target 1: 5′-CCGGCCAGAAGCAGAATGTGACATCTC
GAGTGGTCACATTCTGCTTCTGGTTTTTG-3′ and
5′-AATTCAAAAACCAGAAGCAGAATGTGACATCTCGA GATGGTCACATTCTGCTTCTGG-3′;
target 2: 5′-CCGGGAAGCGGTAACAAAGGAGATACTCGAGTATC
TCCTTTGTTACCGCTTCTTTTTG-3′ and
5′-AATTCAAAAAGAAGCGGTAACAAAGGAGATACTCGAG TATCTCCTTTGTTACCGCTTC-3′;
and target 3: 5′-CCGGCGAGGACTATTTCTTTCAGCTCTCGAGAGCT
GAAAGAAATAGTCCTCGTTTTTG-3′ and 5′-AA
TTCAAAAACGAGGACTATTTCTTTCAGCTCTCGAGAGCT GAAAGAAATAGTCCTCG-3′) that
were inserted into the AgeI and EcoRI sites of SCR
vector purchased from Addgene; the human iNOS target sequence is
underlined. The control shRNA was purchased from Addgene. All
target sequences were designed and verified as specific for iNOS by
Blast searching against the human genome. The resulting plasmids,
iNOS-SH1, iNOS-SH2 and iNOS-SH3, were sequence-verified.
To obtain a recombinant lentivirus, HEK-293T cells
were cotransfected with the package, envelope and iNOS-SH
constructs. The virus-containing supernatant was harvested
following 60 h cotransfection and concentrated by
ultracentrifugation at 100,000 × g for 2 h with an Avanti J-30I
(Beckman Coulter, Miami, FL, USA). For infection, the viral stock
was supplemented with 8 μg/ml polybrene.
Tumorigenicity assays in nude mice
All studies involving animals were approved by the
Ethics Committee of Obstetrics and Gynecology Hospital of Fudan
University (Shanghai, China). Five- to six-week-old female athymic
BALB/c nude mice were purchased from Sino-British Sippr/BK Lab
Animal Ltd, Co. (Shanghai, China). iNOS-SH and SCR-infected HeLa
cells (1×106) were suspended in 100 μl PBS and were
subcutaneously injected into the posterior flank of 4–5 week-old
female nude mice. Tumor size measurement was performed every three
days over the course of 4 weeks and the tumor volume was estimated
using the formula: Volume = length × width2 × 0.5. The
comparison of tumor volumes between the iNOS-SH and vehicle control
groups was performed using paired t-tests.
Immunohistochemisty (IHC)
Tissues were fixed in 10% buffered formalin fixative
at room temperature overnight. Samples were subsequently
deparaffinized in xylene, rehydrated through graded ethanol,
quenched for endogenous peroxidase activity in 0.3% hydrogen
peroxide and processed for antigen retrieval by microwave heating
in 10 mM citrate buffer (pH 6.0) using antibodies against iNOS,
VEGF, and myeloperoxidase (MPO) (Dako, Carpinteria, CA, USA).
Immunostaining was performed according to the manufacturer’s
instructions using the ChemMate Dako EnVision Detection kit,
Peroxidase/DAB, Rabbit/Mouse (DakoCytomation, Glostrup, Denmark),
which resulted in a brown precipitate at the antigen site.
Statistical analysis
All results are expressed as the mean ± standard
error of the mean. Differences were analyzed by Student’s t-test
with SPSS 15.0 software, (SPSS, Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Inhibition of iNOS expression in SiHa and
HeLa cells
In order to determine the function of iNOS in the
development of cervical cancer, a number of SiHa and HeLa cells
were not infected or infected with lentiviruses expressing a 20
base fragment of human shRNA and control scramble, respectively.
The efficacy of this fragment in inhibiting the expression of iNOS
in these cell lines was determined by western blotting, and SH3 was
selected as the iNOS shRNA of the three shRNAs for its high
knockdown efficiency (Fig. 1A).
According to the results of western blotting and qPCR, the iNOS
protein product from the SiHa and HeLa cells was significantly
different between the experimental and control groups following
iNOS stable knockdown (P<0.05). In cells expressing iNOS-shRNA,
there was significant inhibition of iNOS expression (60% mRNA
knockdown in SiHa cells, and 75% mRNA knockdown in HeLa cells;
>70% protein knockdown efficacy in the two cell lines) (Fig. 1).
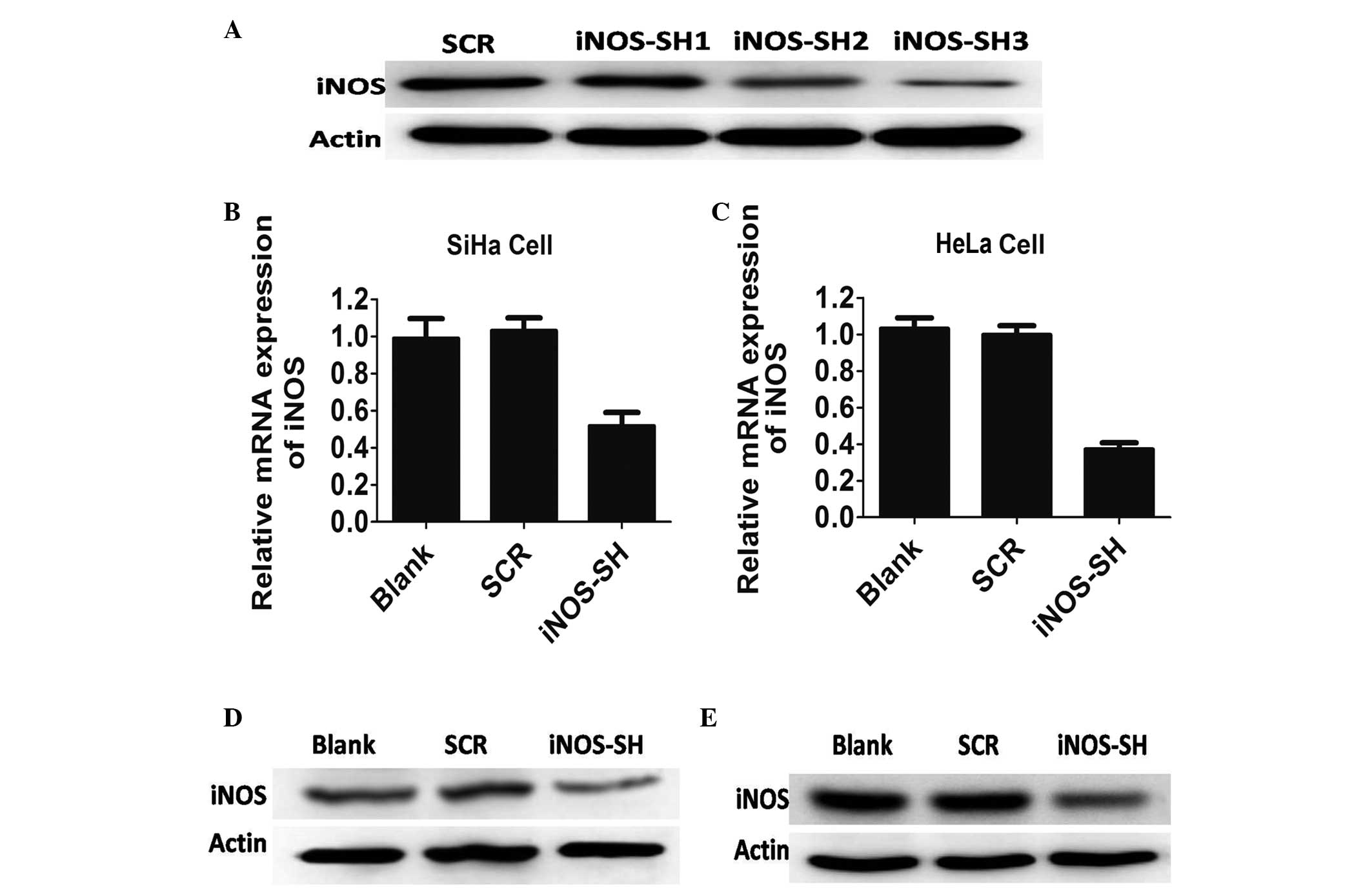 | Figure 1Characterisation of antisense iNOS
cell lines in vitro. (A) HeLa cells that were stably
transfected with control or iNOS short hairpin RNA and were
analyzed by western blotting with antibodies against iNOS and
actin. (B) iNOS expression in SiHa cell lines treated with blank,
scramble (SCR) and iNOS-SH, determined by quantitative polymerase
chain reaction. Results are provided as the mean ± standard error
of the mean (SEM) of triplicates of three separate experiments,
*P<0.05, Student’s t-test. (C) iNOS expression in
HeLa cell lines treated with blank, SCR and iNOS-SH, determined by
qPCR. Results are provided as the means ± SEM of triplicate of
three separate experiments, *P<0.05, Student’s
t-test. (D) iNOS expression in SiHa cell lines treated with blank,
SCR and iNOS-SH, determined by western blotting. (E) iNOS
expression in HeLa cell lines treated with blank, SCR and iNOS-SH,
determined by western blotting. Decreased iNOS expression occurred
in the iNOS-SH-SiHa and iNOS-SH-HeLa cell lines.
*P<0.05, compared with SCR. iNOS, inducible nitric
oxide synthase; SCR, scramble; iNOS-SH, iNOS-targeted shRNA; SEM,
standard error of the mean. |
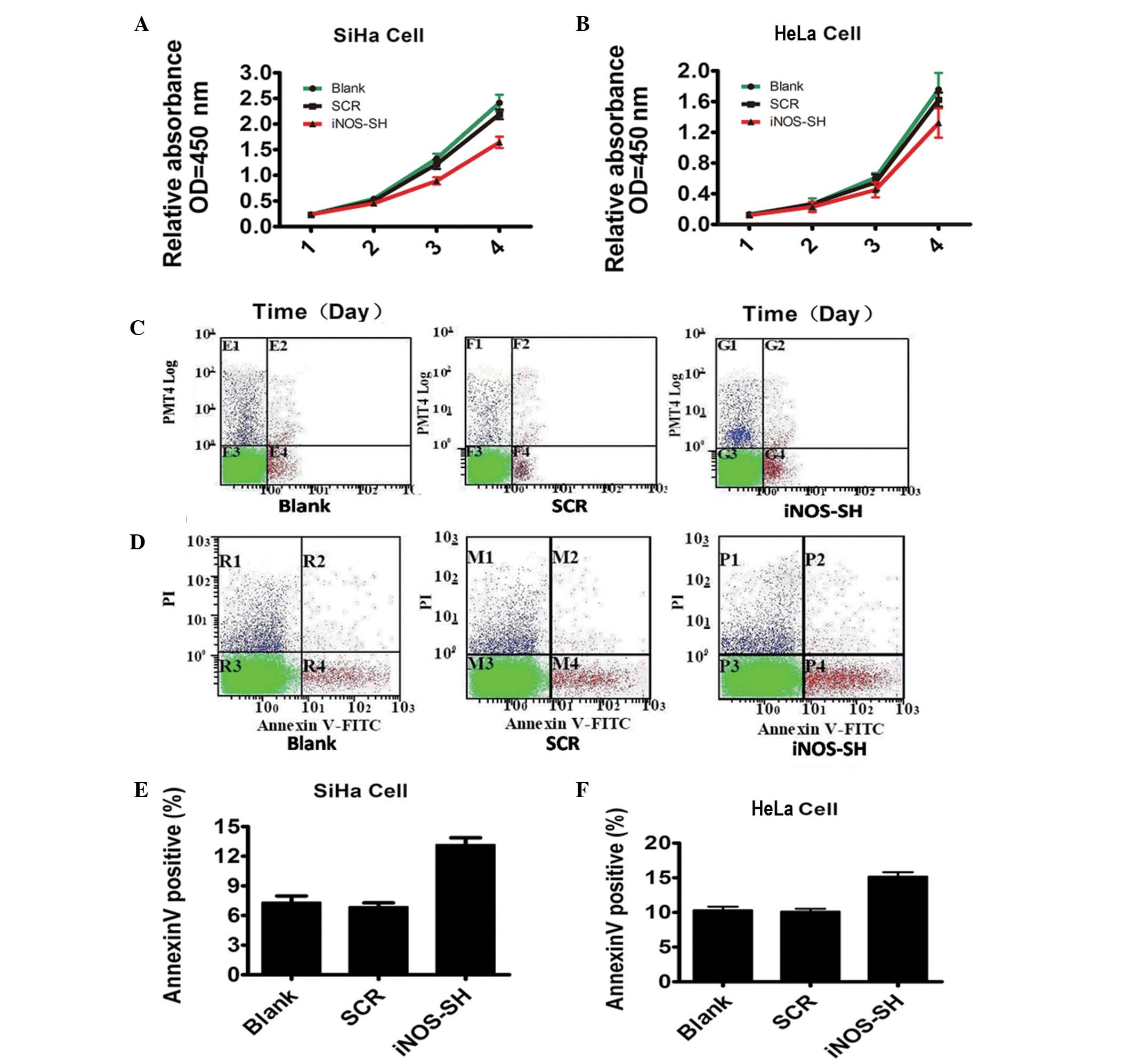
Effect of inhibiting iNOS expression on
tumor growth in vitro and in vivo
To test the function of iNOS in cervical cancer
growth, cell proliferation was investigated following iNOS
knockdown. As expected, the number of SiHa and HeLa cells decreased
markedly under iNOS-SH downregulation, compared with the blank and
scramble groups (Fig. 2A and B).
However, data from flow cytometry revealed a statistically
significant increase in the levels of SiHa and HeLa apoptosis
(Fig. 2C and D). The experimental
evidence reveals the critical role of iNOS in SiHa and HeLa growth
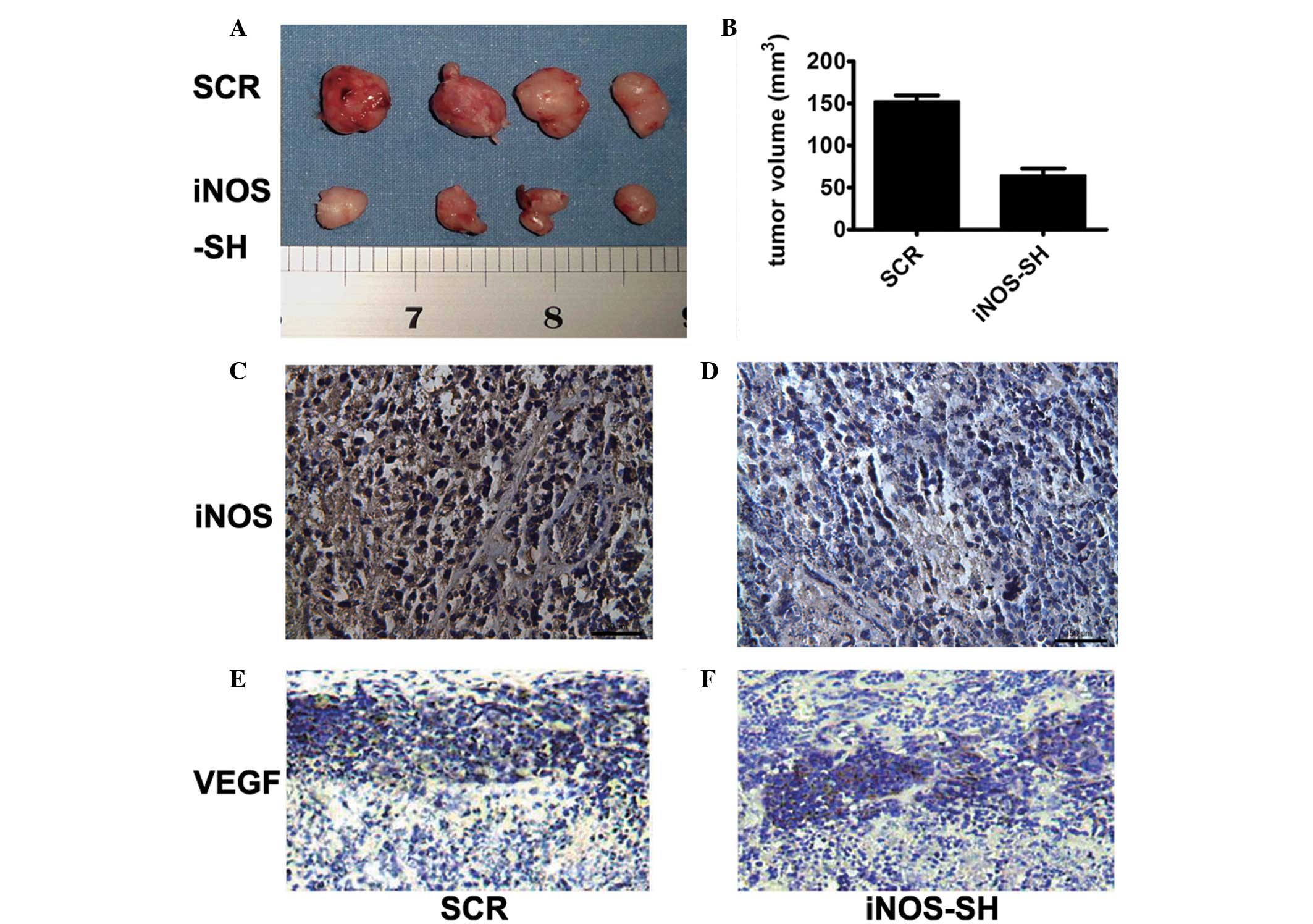
in vitro. In contrast to their growth in vitro, when
SiHa and HeLa cells were inoculated into the flanks of nude mice,
the iNOS-SH HeLa cells growth rate in vivo was significantly
slower compared with that of the control cells (Fig. 3A and B). Tumors derived from
iNOS-SH SiHa cells exhibited a growth rate similar to that of
iNOS-SH HeLa tumors. HeLa tumors became palpable and measurable 10
days after cell inoculation compared with SiHa tumors, which could
be measured 13 days following inoculation. Following 20 days of
growth, the mean tumor size of HeLa tumors was half that of the
control tumors. Inhibition of iNOS expression in HeLa tumors was
confirmed by IHC analysis iNOS (Fig.
3C and D) and VEGF (Fig. 3E and
F) of tumors with or without iNOS knockdown.
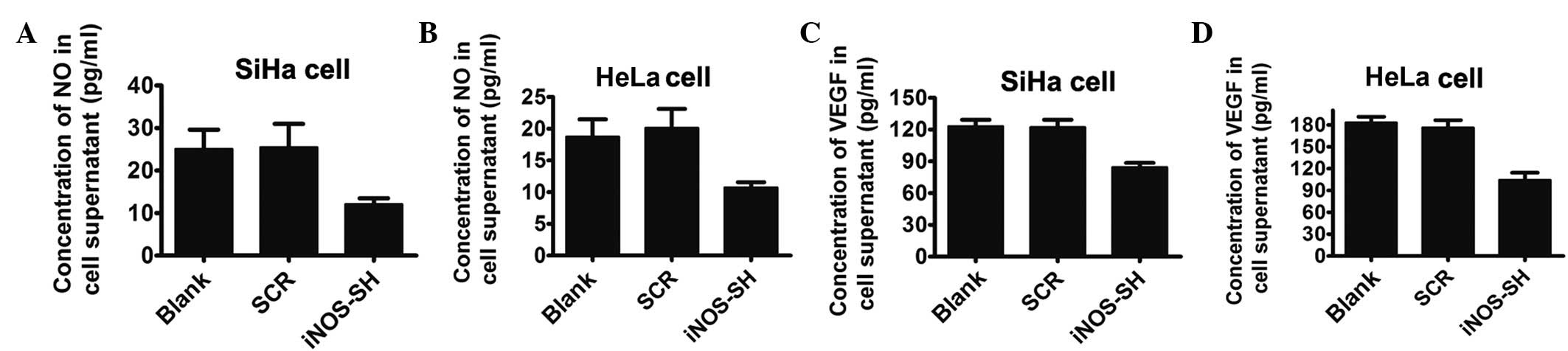
Decreased NO and VEGF level in
supernatant and cells in iNOS-knockdown SiHa and HeLa culture
NO has been demonstrated to stimulate and inhibit
VEGF expression in a cell-dependent manner and is a potent
modulator of vascular development and tumor progression. In in
vitro studies, the iNOS-shRNA group demonstrated that the two
cell lines produced lower levels of VEGF compared with the control
group. The inhibition of iNOS with lentivirus-based-RNAi for 24 h
resulted in a significant 1.5-fold downregulation of NO in the
supernatant of SiHa and HeLa cell lines (Fig. 4A and B). Furthermore, the
concentration of VEGF in the same supernatant was markedly reduced
in the iNOS-shRNA group, compared with the control group, as
determined by western blotting (Fig.
4C and D).
iNOS increases VEGF levels in SiHa and
HeLa cell cultures
To assess whether NO is a mediator for the reduction
of VEGF in the supernatant and iNOS-SH treated cells, sodium
nitroprusside (SN), an exogenous NO donor, was applied to stimulate
cells. The mRNA level of iNOS and VEGF in cells incubated in media
with or without SN (DMSO, 0.25, 0.5 and 1 mM) were assessed
(Fig. 5A and B). In all groups,
the VEGF mRNA level (Fig. 5B)
demonstrated a significant increase with treatment with a high
concentration of SN (0.5 mM), without changing the iNOS level,
which may mimic the effect of iNOS. However, in SiHa and HeLa
cells, SN increased the supernatant concentration of NO in a
dose-dependent manner. The fact that SN increased the supernatant
concentration of NO in iNOS-SH SiHa and HeLa cells reveals that SN
induced NO production in an iNOS-independent way (Fig. 5C and D). Furthermore, in SiHa and
HeLa cells the stable knockdown of iNOS-SH, the protein levels of
VEGF and iNOS peaked at 0.5 mM SN treatment, but exhibited a
smaller increase with 1 mM SN (Fig. 5E
and F).
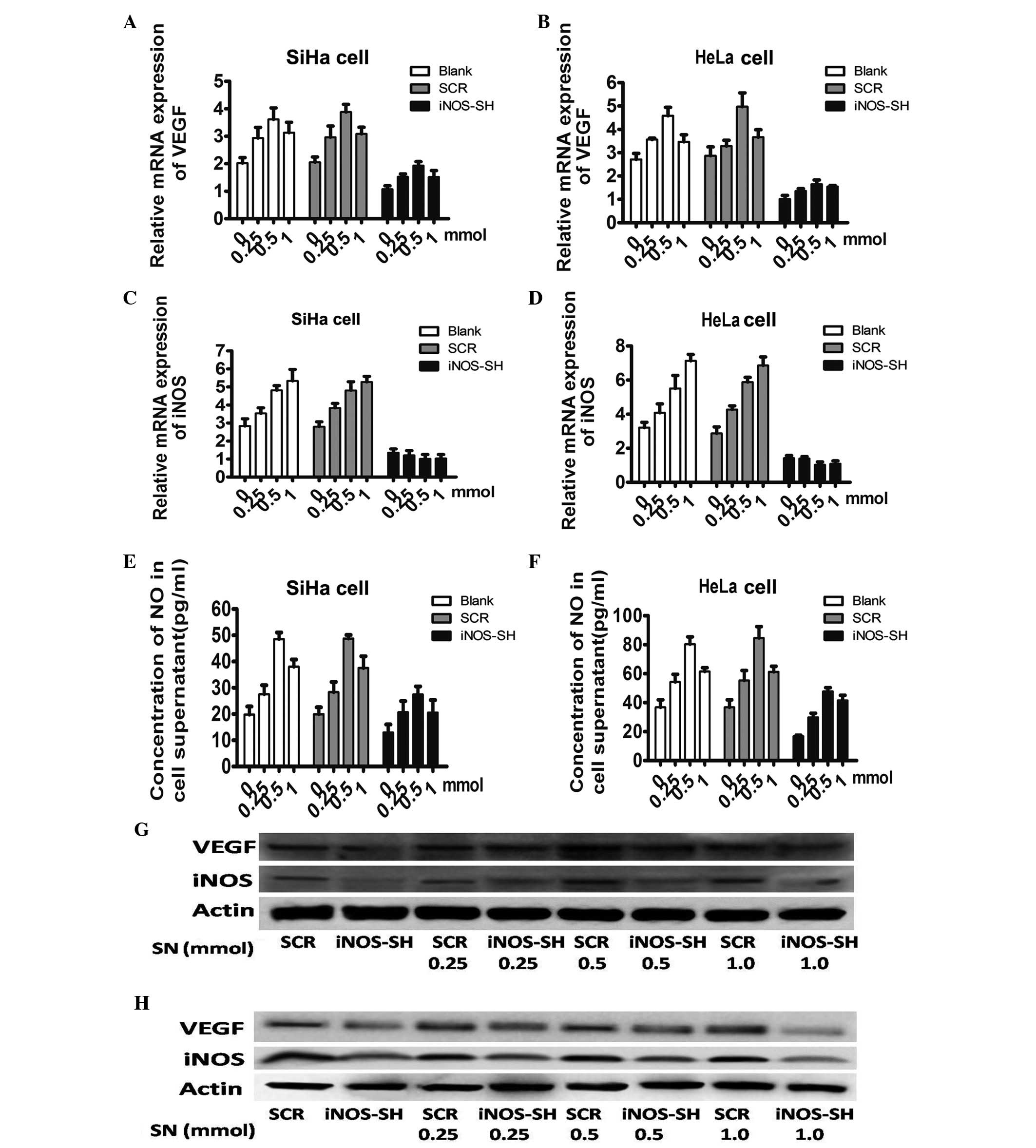 | Figure 5Effect of extrogenous NO on VEGF in
SiHa and HeLa cells. (A and B) Quantitation of iNOS mRNA level
obtained from SiHa and HeLa treated with SN among each experiment
group. Cells harvested following stimulation for 24 h with 0.25,
0.5 or 1 mM SN, DMSO was applied as the control. (C and D)
Quantitation of VEGF mRNA level obtained from SiHa and HeLa treated
with SN among each experiment group. Cells harvested after
stimulation for 24 h with 0.25, 0.5 or 1 mM SN, DMSO was applied as
the control. (E and F) The supernatant concentration of NO treated
with or without SN (DMSO, 0.25, 0.5 and 1 mM) (G and H) Protein
levels of VEGF and iNOS in cells treated with SN. Results are
provided as the means ± SEM. *P<0.05, Student’s
t-test. NO, nitric oxide; VEGF, vascular endothelial growth factor;
iNOS, inducible nitric oxide synthase; SN, sodium nitroprusside;
DMSO, dimethylsulfoxide ; SCR, scramble; iNOS-SH, iNOS-targeted
shRNA. |
In conclusion, the data indicated that the knockdown
of iNOS based on depressed lentivirus proliferation of SiHa and
HeLa, may be mimicked by applying an extrogenous NO donor.
Furthermore, iNOS is capable of regulating the proliferation of
cervical cancer cells and the expression of VEGF by regulating the
concentration of NO.
Discussion
In recent years, the incidence rate of cervical
cancer has increased markedly, with an increasing trend in young
females. However, the mechanisms underlying the disease remain
unknown. Several factors have been implicated in tumor development.
One of the most prominent cytokines is VEGF, which induces
sprouting angiogenesis, increases blood vessel permeability and
maintains tumor vessel integrity (15). VEGF expression has been shown to be
a survival factor for blood vessels in SiHa (14).
iNOS and VEGF are important in tumor growth. The
level of iNOS is correlated with that of VEGF. One of the most
important mechanisms is that angiogenesis is induced by iNOS, which
is capable of synthesizing more NO, regulating VEGF levels in
tumors. NO is capable of inducing VEGF expression and mediating the
angiogenic effects of VEGF (16).
Therefore it was hypothesized that the decline in growth and vessel
perfusion of SiHa and HeLa tumor cells may correlate with decreased
VEGF expression. Notably, analyses of VEGF expression and
production in tumor cells and explants following RNAi of iNOS
revealed a correlation between VEGF and NO levels. This, coupled
with previously published data, suggests that NO produced from iNOS
activity is important for the maintenance of tumor blood vessel
tone and function and further highlights the different roles of NOS
isotypes and their tissue/cell type of origin in vivo
(17).
NO production is induced by numerous angiogenic
stimuli and is an important extracellular and intracellular
signaling molecule synthesized from arginine by NOS. Of the three
NOS isoforms, including iNOS, endothelial (eNOS) and neuronal NOS,
iNOS specifically produces the greatest quantities of NO and is
associated with a number of pathologies (18). Although their precise roles remain
unclear, the activites of iNOS and eNOS have been implicated in
tumor progression and angiogenesis, and their effectiveness appears
to be dependent on their activity and distribution, the
concentration and duration of exposure to NO and the intrinsic
sensitivity of cells to NO (19).
The effect of altered NO levels on tumor growth and angiogenesis
has also been exploited through pharmacological manipulation with
either NO donors or NOS inhibitors.
In the present study, it was observed that iNOS
knockdown results in the decreased proliferation of SiHa and HeLa
cells and the concentration of VEGF in the supernatant, which means
that iNOS regulates the growth of cervical cancer cells in a
VEGF-dependent process. Further studies are required in order to
confirm that SN is capable of rescuing the iNOS-induced
decrease.
In conclusion, it was demonstrated i) Using a
lentivirus-delivered RNAi approach that iNOS is a key modulator of
tumor growth and angiogenesis; ii) that the level of VEGF
correlates with the expression of iNOS in SiHa and HeLa cells,
which may be mediated by the level of NO; and iii) that the level
of NO generated in the tumor is lower than the concentration at
which apoptosis occurs, which promotes growth and angiogenesis.
Acknowledgements
This work was supported by The National Natural
Science Foundation of China (81272877) and Cancer Foundation of
China.
References
|
1
|
Walboomers JM, Jacobs MV, Manos MM, et al:
Human papillomavirus is a necessary cause of invasive cervical
cancer worldwide. J Pathol. 189:12–19. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bosch FX, Manos MM, Munoz N, et al:
Prevalence of human papillomavirus in cervical cancer: a worldwide
perspective. International biological study on cervical cancer
(IBSCC) Study Group. J Natl Cancer Inst. 87:796–802. 1995.
View Article : Google Scholar
|
|
3
|
Dürst M, Gissmann L, Ikenberg H and zur
Hausen H: A papillomavirus DNA from a cervical carcinoma and its
prevalence in cancer biopsy samples from different geographic
regions. Proc Natl Acad Sci USA. 80:3812–3815. 1983.PubMed/NCBI
|
|
4
|
Bosch FX, Lorincz A, Muñoz N, Meijer CJ
and Shah KV: The causal relation between human papillomavirus and
cervical cancer. J Clin Pathol. 55:244–265. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Morris M, Eifel PJ, Lu J, et al: Pelvic
radiation with concurrent chemotherapy compared with pelvic and
para-aortic radiation for high-risk cervical cancer. N Engl J Med.
340:1137–1143. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Isacson C, Kessis TD, Hedrick L and Cho
KR: Both cell proliferation and apoptosis increase with lesion
grade in cervical neoplasia but do not correlate with human
papillomavirus type. Cancer Res. 56:669–674. 1996.PubMed/NCBI
|
|
7
|
Tsang RW, Fyles AW, Li Y, et al: Tumor
proliferation and apoptosis in human uterine cervix carcinoma I:
correlations between variables. Radiother Oncol. 50:85–92. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lo HW, Hsu SC, Ali-Seyed M, et al: Nuclear
interaction of EGFR and STAT3 in the activation of the iNOS/NO
pathway. Cancer Cell. 7:575–589. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kroll J and Waltenberger J: VEGF-A induces
expression of eNOS and iNOS in endothelial cells via VEGF
receptor-2 (KDR). Biochem Biophys Res Commun. 252:743–746. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ichinoe M, Mikami T, Shiraishi H and
Okayasu I: High microvascular density is correlated with high VEGF,
iNOS and COX-2 expression in penetrating growth-type early gastric
carcinomas. Histopathology. 45:612–618. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang J and Peng B: NF-kappaB promotes
iNOS and VEGF expression in salivary gland adenoid cystic carcinoma
cells and enhances endothelial cell motility in vitro. Cell
Prolif. 42:150–161. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Saura M, Zaragoza C, McMillan A, et al: An
antiviral mechanism of nitric oxide: inhibition of a viral
protease. Immunity. 10:21–28. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kostourou V, Cartwright JE, Johnstone AP,
et al: The role of tumour-derived iNOS in tumour progression and
angiogenesis. Br J Cancer. 104:83–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Benjamin LE, Hemo I and Keshet E: A
plasticity window for blood vessel remodelling is defined by
pericyte coverage of the preformed endothelial network and is
regulated by PDGF-B and VEGF. Development. 125:1591–1598. 1998.
|
|
15
|
Holash J, Davis S, Papadopoulos N, et al:
VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl
Acad Sci USA. 99:11393–11398. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Feng CW, Wang LD, Jiao LH, Liu B, Zheng S
and Xie XJ: Expression of p53, inducible nitric oxide synthase and
vascular endothelial growth factor in gastric precancerous and
cancerous lesions: correlation with clinical features. BMC Cancer.
2:82002. View Article : Google Scholar
|
|
17
|
Chiarugi V, Magnelli L and Gallo O: Cox-2,
iNOS and p53 as play-makers of tumor angiogenesis (review). Int J
Mol Med. 2:715–724. 1998.PubMed/NCBI
|
|
18
|
Martínez-Estrada OM, Rodríguez-Millán E,
González-De Vicente E, Reina M, Vilaró S and Fabre M:
Erythropoietin protects the in vitro blood-brain barrier
against VEGF-induced permeability. Eur J Neurosci. 18:2538–2544.
2003.
|
|
19
|
Fukumura D and Jain RK: Tumor
microvasculature and microenvironment: targets for
anti-angiogenesis and normalization. Microvasc Res. 74:72–84. 2007.
View Article : Google Scholar : PubMed/NCBI
|