Introduction
Colorectal cancer (CRC) is one of the most serious
malignancies with its incidence and mortality increasing annually
(1). Although surgical resection
offers the greatest prognosis, a substantial portion of CRC
patients already present with metastatic disease at the time of
diagnosis (2,3). Additionally, surgery is not always
able to extirpate the recurrence of advanced CRC (4). Therefore, chemotherapy remains one of
the major non-surgical therapeutic approaches for patients with
advanced CRC. However, the efficacy and safety of the
chemotherapies which are currently used remains a challenge due to
severe toxicity, multidrug resistance and other side effects
(5–7). Faced with these major problems, the
development of novel anticancer agents is urgently required.
Traditional Chinese medicine (TCM), dating back thousands of years,
is important in the treatment of various diseases, including cancer
(8–10). Clinical practice has also
demonstrated that numerous TCMs are effective for the treatment of
CRC (11).
Scutellaria barbata D. Don (SB) is a
medicinal herb widely distributed in northeast Asia. As a well
known traditional Chinese folk medicine, it has long been used to
clinically treat various types of cancer (12–16).
In particular, our previous studies demonstrated that the extracts
of SB were able to suppress colon cancer growth in vivo and
in vitro, possibly by inducing cancer cell apoptosis and
inhibiting cell proliferation and tumor angiogenesis (16–18).
However, the anticancer mechanisms of its bioactive ingredients are
largely unclear. In the present study, using three human colon
cancer cell lines SW620, HT-29 and HCT-8, the antitumor effect of
different polar fractions of SB were evaluated and the potential
underlying molecular mechanisms were investigated. It was revealed
that the chloroform fraction of SB (ECSB) exhibited the most potent
inhibitory effect on the growth of all three colon cancer cell
lines and SW620 cells exhibited the most sensitive response to ECSB
treatment. In addition, ECSB promoted apoptosis via the
upregulation of the pro-apoptotic Bax/Bcl-2 ratio and inhibited
proliferation by suppressing the expression of the
pro-proliferative cyclin D1 and cyclin-dependent kinase 4 (CDK4) in
SW620 cells.
Materials and methods
Materials and reagents
RPMI-1640 medium, KGML-15 SY medium, fetal bovine
serum (FBS), penicillin-streptomycin and trypsin-EDTA were obtained
from Hyclone (Carlsbad, CA, USA). TRIzol reagent and SuperScript II
reverse transcriptase were purchased from Invitrogen Life
Technologies (Grand Island, NY, USA). Anti-Bcl-2, Bax, Cyclin D1,
CDK4 and β-actin antibodies, and horseradish peroxidase
(HRP)-conjugated secondary antibodies were obtained from Cell
Signaling Technology (Beverly, MA, USA). A fluorescein
isothiocyanate (FITC)-conjugated Annexin V apoptosis detection kit
was provided by Becton-Dickinson (San Jose, CA, USA). All the other
chemicals, unless otherwise stated, were obtained from
Sigma-Aldrich (St. Louis, MO, USA).
Preparation of the SB extract
The herb was obtained from the Guo Yi Tang Chinese
Herbal medicine store (Fujian, China). SB (500 g) was extracted
three times with 5,000 ml of 85% ethanol using a refluxing method
and filtered. The solvent was fractionated by a series of solvents,
including petroleum ether, chloroform, ethyl acetate and N-butanol,
to obtain the petroleum ether fraction of SB (EPESB), ECSB, ethyl
acetate fraction of SB (EEASB) and the N-butanol fraction of SB
(ENBSB). These fractions were then evaporated on a rotary
evaporator. They were all dissolved in 100% dimethylsulfoxide
(DMSO) to a stock concentration of 200 mg/ml and stored at −20°C.
The final concentration of DMSO in the medium for all the
experiments was ≤0.25%.
Cell culture
Human carcinoma SW620, HT-29 and HCT-8 cells were
obtained from the Cell Bank of the Chinese Academy of Sciences
(Shanghai, China). SW620 cells were grown in L-15 medium and HT-29
and HCT-8 cells were cultured in RPMI-1640 medium, supplemented
with 10% fetal bovine serum, 100 U/ml penicillin and 100 mg/ml
streptomycin at 37°C in 5% CO2 humidified air.
Cell viability using an 3-(4,
5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
Cell viability was assessed by an MTT colorimetric
assay. SW620, HT-29 and HCT-8 cells were seeded into 96-well plates
at a density of 5×105, 3×105 and
1×105 cells/ml in 100 μl of medium, respectively. The
cells were treated with serial concentrations of fractions of SB
for 24 h. Following treatment, 100 μl of MTT (0.5 mg/ml in PBS) was
added to each well and the cells were incubated at 37°C for 4 h.
The medium was removed and the purple-blue MTT formazan precipitate
was dissolved in 100 μl of DMSO. The absorbance was measured at 570
nm using an ELISA reader (model ELX800; BioTek, Winooski, VT, USA).
The cell viability was determined using the formula: Cell viability
(%) = sample optical density (OD)/control OD × 100.
Detection of apoptosis by flow cytometric
analysis with Annexin V/propidium iodide (PI) staining
Following starvation for 12 h, SW620 cells were
incubated for 24 h in various concentrations of ECSB. Apoptosis of
SW620 cells was determined by flow cytometric analysis using a
fluorescence-activated cell sorting (FACS) caliber
(Becton-Dickinson) and an Annexin V-FITC/PI kit (Becton-Dickinson).
The procedure was performed according to the manufacturer’s
instructions. Annexin V-positivity and PI-negativity indicated the
presence of early apoptotic cells, while Annexin V-positivity and
PI-positivity indicated the presence of late apoptotic cells.
Observation of ultrastructural
characteristics by transmission electron microscopy (TEM)
Cells were fixed with 1.5% paraformaldehyde and 3%
glutaraldehyde in 0.1 mol/l sodium cacodylate buffer (pH 7.2–7.4)
at 4°C for 24 h. The cell suspensions were then rinsed twice with
phosphate-buffered saline (PBS) and post-fixed with 1% osmic acid
in 0.1 mol/l sodium cacodylate buffer for 2 h. The cells were
dehydrated in a graded series of alcohol and embedded with epoxy
resin 618. Ultrathin sections (80 nm) were mounted on the copper
wire mesh grids, air-dried, stained with 2.0% uranyl acetate for 15
min and counterstained with lead citrate for 15 min. The sections
were examined and images were captured using a Hitachi 7650
electron microscope (Tokyo, Japan).
Colony formation
SW620 cells were seeded into 6-well plates at a
density of 5×105 cells/ml in 2 ml medium. Following
treatment with various concentrations of ECSB for 48 h, cells were
harvested and diluted in 2 ml fresh medium without ECSB, and then
each well was reseeded at a density of 1,000 cells per well. The
medium was replaced with fresh medium every three days. Following
14 days, cells were fixed with 10% formaldehyde, stained with 0.01%
crystal violet and counted. The cell survival rate was calculated
by normalizing the survival rate of the control cells to 100%.
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis
SW620 cells were exposed to various concentrations
of ECSB for 48 h and then total RNA was isolated with TRIzol
reagent (Invitrogen Life Technologies). First-strand cDNA was
generated via reverse transcription of 2 μg total RNA using
Oligo(dT) primer and SuperScript II reverse transcriptase according
to the manufacturer’s instructions. The obtained cDNA was used to
determine the mRNA levels of Bax, Bcl-2, Cyclin D1 and CDK4 by PCR
with TaqDNA polymerase (Fermentas, Vilnius, Lithuania). GAPDH was
used as an internal control.
Western blot analysis
SW620 cells were treated with various concentrations
of ECSB for 48 h. Adherent and floating cells were harvested and
rinsed three times with PBS buffer. The cells were lysed with
radioimmunoprecipitation assay lysis buffer (Pierce Chemical Co.,
Rockford, Illinois, USA) containing phenylmethanesulfonyl fluoride
and extracts were quantified using the bicinchoninic acid protein
assay (Pierce Chemical Co.) The proteins (30 μg) were separated by
12% SDS-PAGE gels and transferred onto polyvinylidene fluoride
membranes (Millipore Corporation, Billerica, MA, USA). The
membranes were inhibited with 5% skimmed milk and probed with
primary antibodies against Bax, Bcl-2, cyclin D1, CDK4 and β-actin
(1:1,000) overnight at 4°C. The membranes were rinsed three times
with Tris-buffered saline with Tween-20 (TBST) and then the
appropriate HRP-conjugated secondary antibodies were diluted at
1:5,000 in blocking solutions for 1 h at room temperature.
Following washing again in TBST, the membranes were detected by
enhanced chemiluminescence. β-actin was used as an internal
control.
Statistical analysis
Data were analyzed using the SPSS package for
Windows (version 13.0; SPSS Inc., Chicago, IL, USA). The
quantitative data are expressed as the mean ± standard deviation.
Statistical analysis of the data was performed using Student’s
t-test. P<0.05 was considered to indicate a statistically
significant difference.
Results and Discussion
ECSB exhibits the most potent inhibitory
effect on the growth of colon cancer cells
By performing an MTT assay in three human colon
cancer cell lines, SW620, HT-29 and HCT-8, the in vitro
anticancer effect of different fractions of SB, including EPESB,
ECSB, EEASB and ENSB, was compared. As shown in Fig. 1, all the fractions inhibited the
viability of three cancer cells in a dose-dependent manner. In
particular, ECSB exhibited the most potent antitumor activity in
SW620 cells, with an IC50 of 65 μg/ml. To further
examine the mode of action of ECSB, SW620 cells were selected for
the following study.
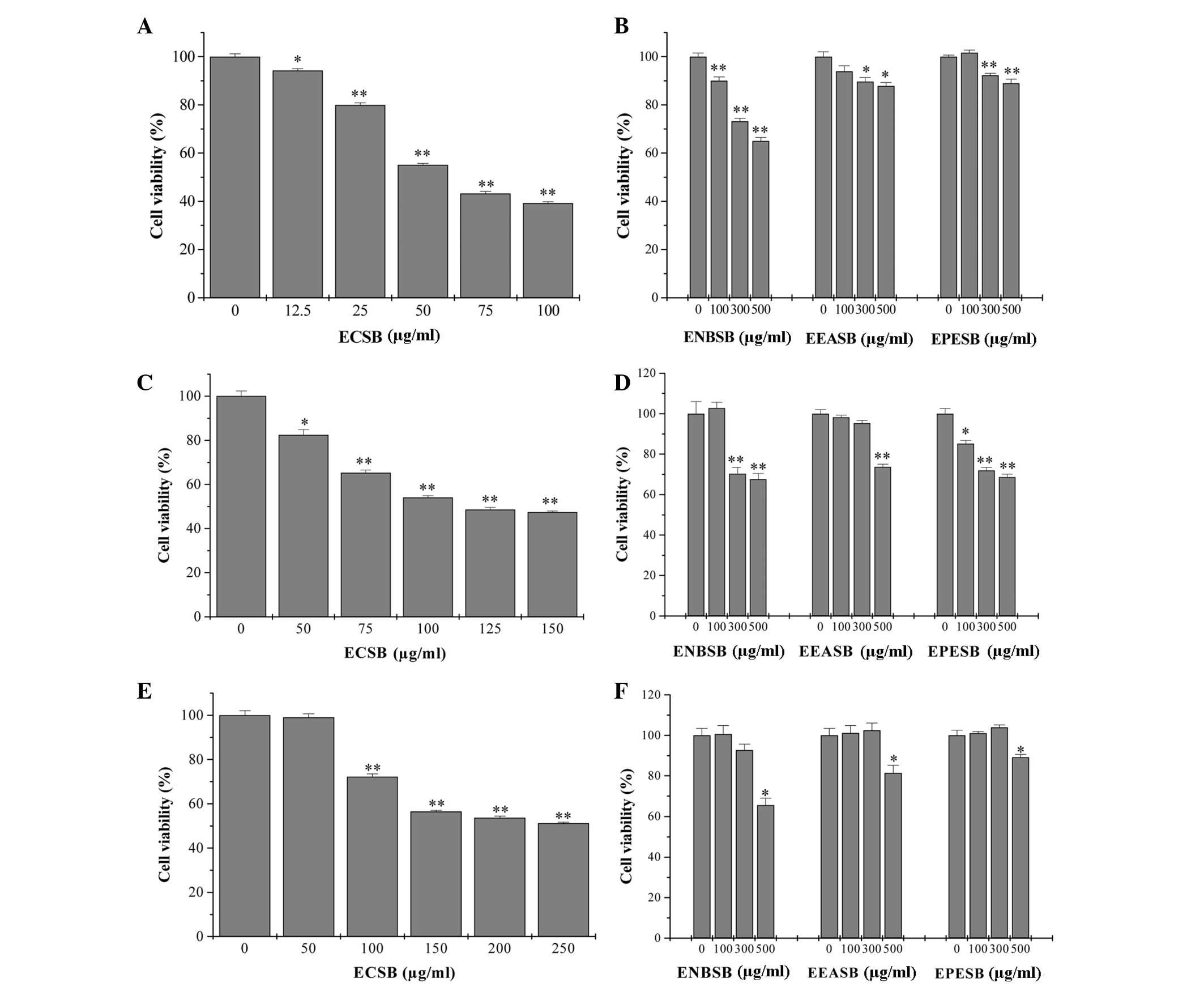 | Figure 1Effect of different polar fractions of
SB on the viability of human colon cancer cells. (A and B) SW620,
(C and D) HT-29 and (E and F) HCT-8 cells were treated with ECSB,
ENSB, EEASB or EPESB for 24 h, respectively. Cell viability was
determined by the MTT assay. The data were normalized to the
viability of the untreated control cells (100%). Data are expressed
as the mean ± standard deviation (error bars) from at least three
independent experiments. *P<0.05;
**P<0.01, versus untreated control cells. SB,
Scutellaria barbata D. Don; ECSB, chloroform fraction of SB;
EPESB, petroleum ether fraction of SB; EEASB, ethyl acetate
fraction of SB; ENSB, N-butanol fraction of SB. |
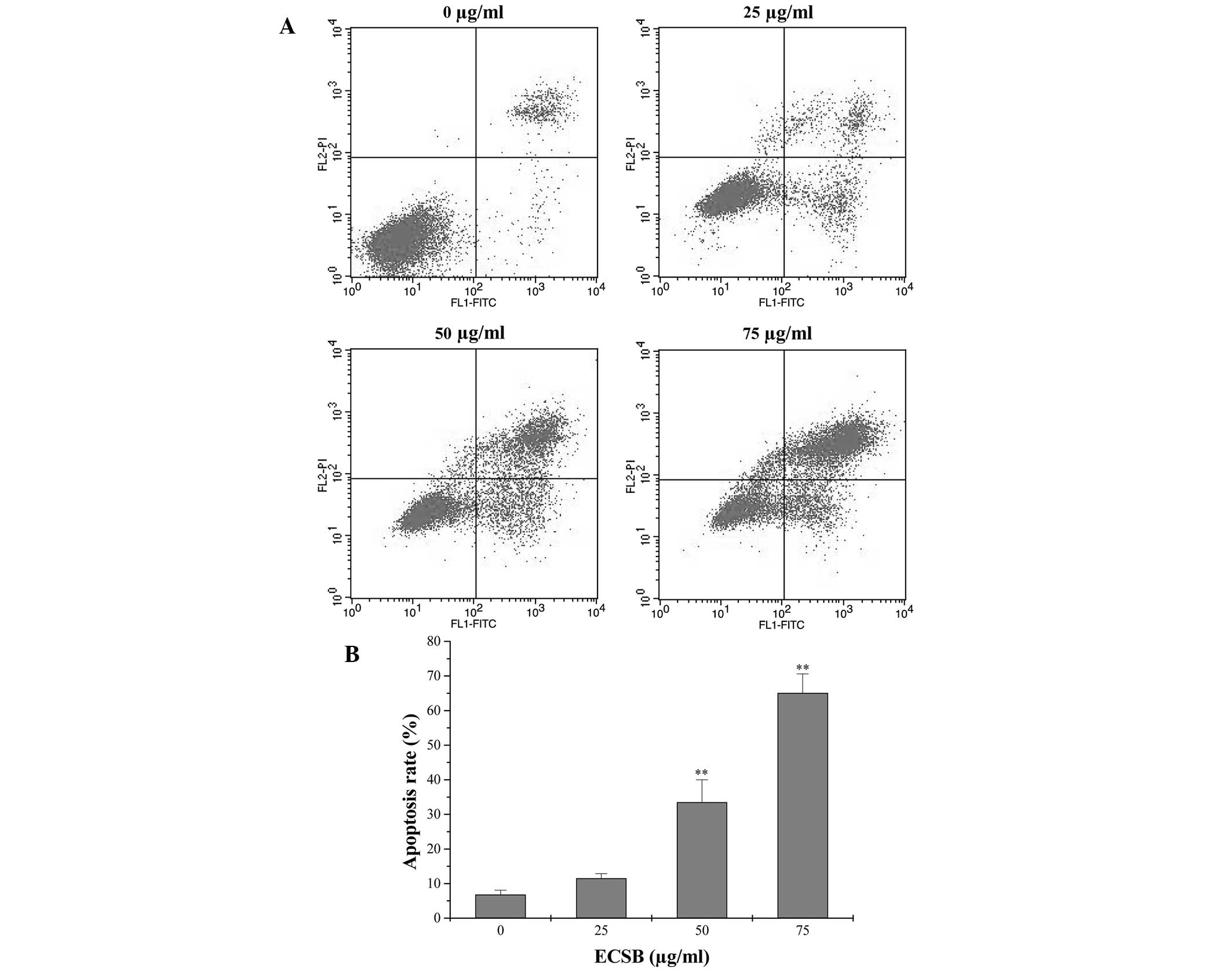
ECSB promotes apoptosis and inhibits the
proliferation of SW620 cells
Apoptosis eliminates excess, redundant, abnormal
cells in animals and thus is crucial for animal development and
tissue homeostasis. Disturbed regulation of this vital process
represents a major causative factor in tumorigenesis (19). Therefore, in order to determine the
mechanism of the growth suppressive activity of ECSB, its effect on
apoptosis in SW620 cells was examined. The in vitro cell
apoptosis was assessed via Annexin V/PI staining followed by FACS
analysis. As shown in Fig. 2, the
percentage of cells undergoing either early apoptosis or late
apoptosis following treatment with 0, 25, 50 and 75 μg/ml ECSB was
6.80, 11.54, 33.53 and 65.12%, respectively (P<0.05), suggesting
that ECSB treatment significantly induced apoptosis in SW620 cells
in a dose-dependent manner.
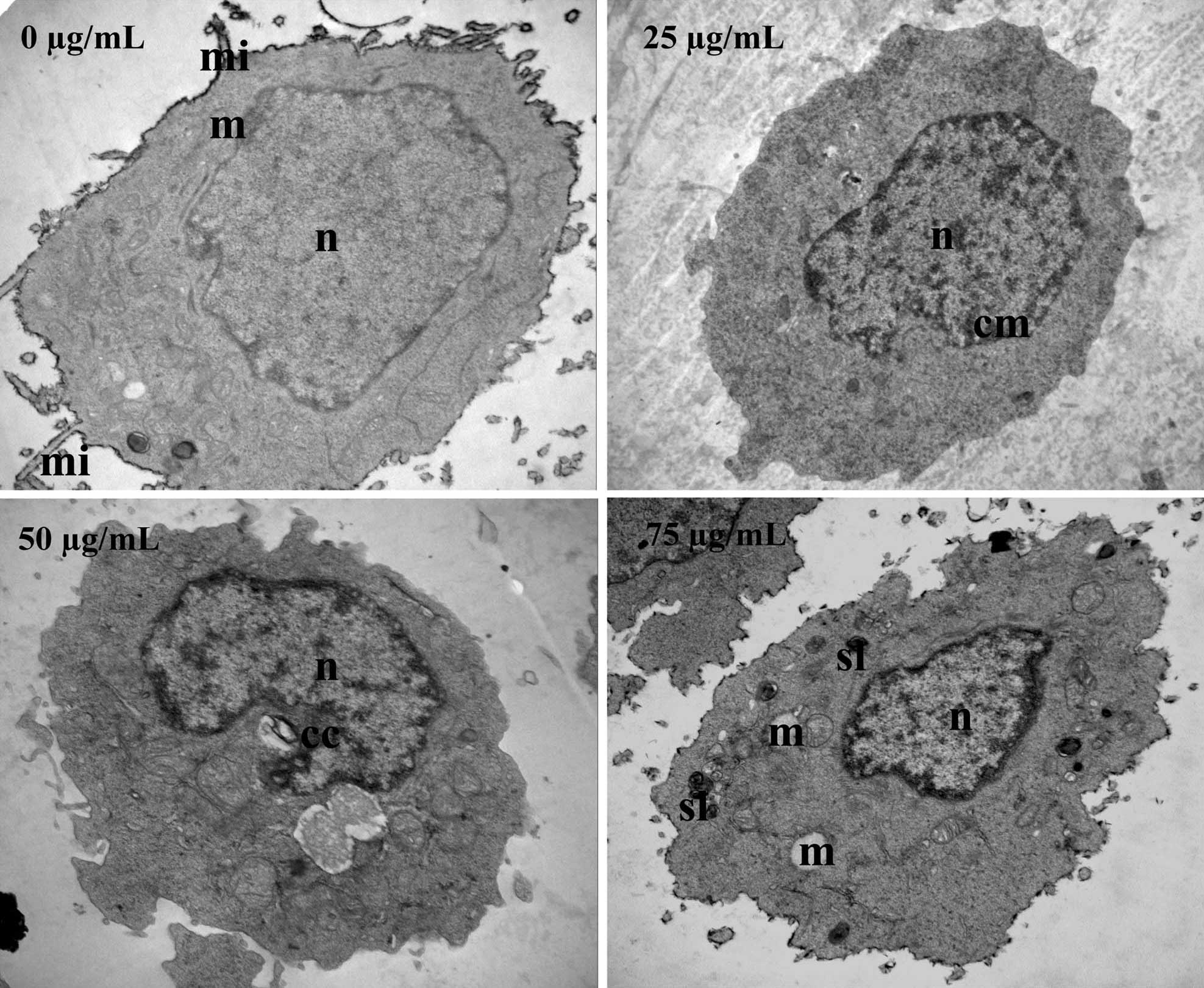
In order to verify these results, TEM was used to
observe ultrastructural changes in SW620 cells following ECSB
treatment. As shown in Fig. 3,
untreated cells exhibited a normal ultrastructure, including
numerous microvilli on the cell surface, evenly distributed
chromatin, a large nucleolus in the nucleus and numerous large
mitochondria in the cytoplasm. By contrast, following treatment
with ECSB, SW620 cells underwent significant ultrastructural
changes that represented the typical morphological features of
apoptosis, including a low nucleus/cytoplasm ratio, chromatin
margination and condensation, formation of vacuoles in the
mitochondria, formation of secondary lysosomes, loss of cellular
microvilli and mitochondrial cristae. These data further
demonstrated the pro-apoptotic activity of ECSB.
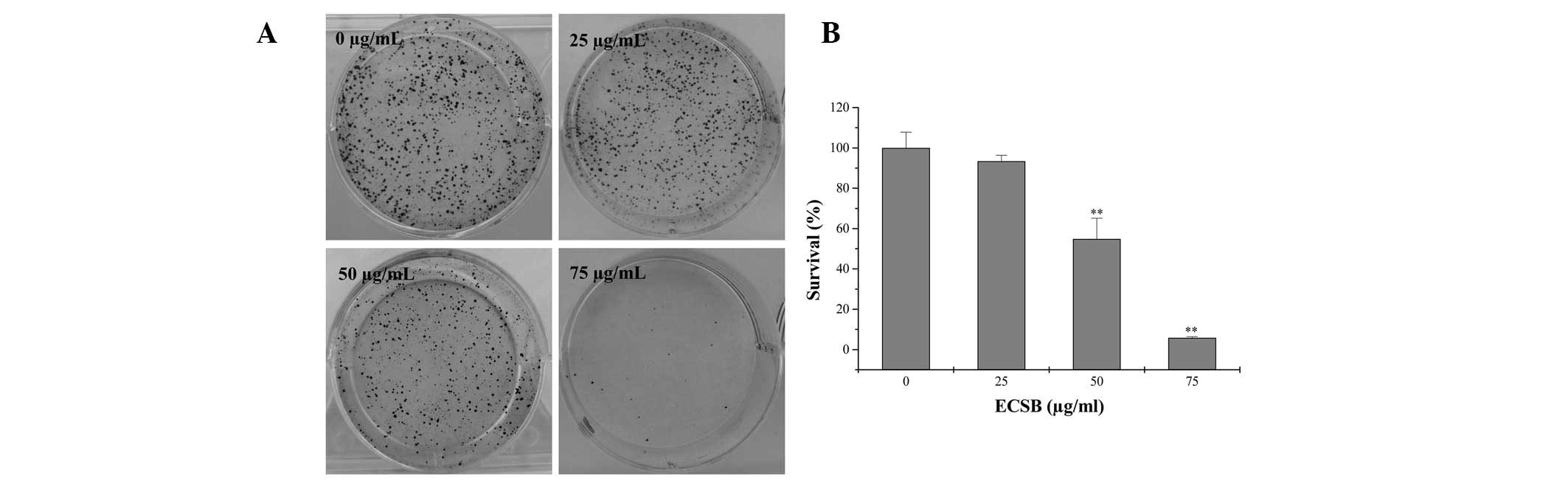
Cancer cells are also characterized by uncontrolled
proliferation, therefore, inhibiting the excessive proliferation of
tumor cells is one of the key approaches for the development of
anticancer drugs. In order to determine the effect of ECSB on the
proliferation of cancer cells, the survival rate in ECSB-treated
SW620 cells was evaluated using a colony formation assay. As shown
in Fig. 4, treatment with 25, 50
and 75 μg/ml ECSB for 48 h, respectively, reduced the survival rate
of SW620 cells to 93.42, 54.82 and 5.85%, as compared with the
untreated control cells (P<0.05), suggesting that ECSB
suppressed the proliferation of colon cancer cells in a
dose-dependent manner.
ECSB regulated the expression of Bax,
Bcl-2, cyclin D1 and CDK4
Bcl-2 family proteins are key regulators of
apoptosis, functioning as either suppressors, including Bcl-2, or
promoters, including Bax (20).
Tissue homeostasis is maintained by controlling the ratio of active
anti- and pro-apoptotic Bcl-2 family proteins. A higher Bcl-2/Bax
ratio caused by aberrant expression of the proteins is commonly
found in various types of cancer, which not only confers a survival
advantage to the cancer cells, but also results in drug resistance.
Eukaryotic cell proliferation is primarily regulated by the cell
cycle. G1/S transition is one of the main checkpoints of the cell
cycle and is responsible for the initiation and completion of DNA
replication. G1/S progression is regulated by cyclin D1, which
exerts its function via forming an active complex with its CDK
major catalytic partners (CDK4/6) (21,22).
An unchecked or hyperactivated cyclin D1/CDK4 complex often leads
to uncontrolled cell division and malignancy (23–25).
In order to further examine the mechanisms of the
pro-apoptotic and antiproliferative activities of ECSB, RT-PCR and
western blot analysis were performed to examine the expression of
Bax, Bcl-2, cyclin D1 and CDK4. As shown in Fig. 5, ECSB significantly reduced the
mRNA and protein expression of anti-apoptotic Bcl-2 as well as the
pro-proliferative cyclin D1 and CDK4, whereas that of pro-apoptotic
Bax was markedly increased following ECSB treatment.
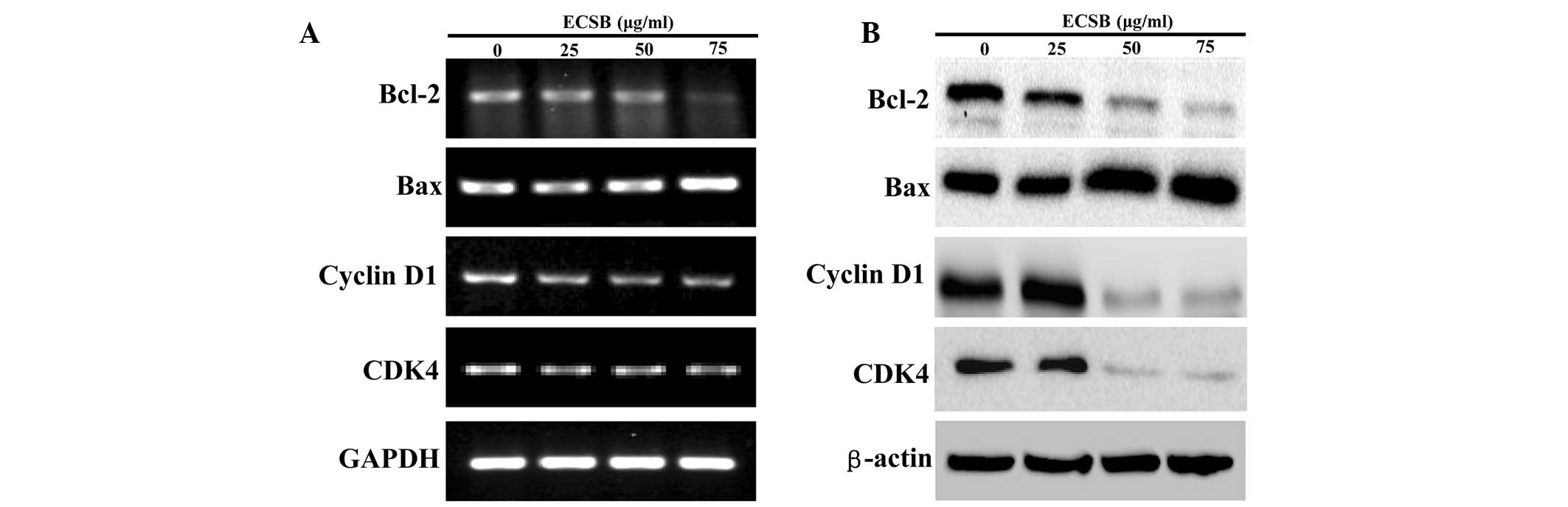 | Figure 5Effect of ECSB on the expression of
Bcl-2, Bax, cyclin D1 and CDK4 in SW620 cells. Cells were treated
with the indicated concentrations of ECSB for 48 h. (A) mRNA
expression of Bcl-2, Bax, Cyclin D1 and CDK4 were evaluated by
RT-PCR. (B) Protein expression levels of Bcl-2, Bax, cyclin D1 and
CDK4 were determined by western blotting. GAPDH and β-actin were
used as the internal controls for the RT-PCR or western blotting,
respectively. Images are representative of three independent
experiments. ECSB, chloroform fraction of Scutellaria
barbata D. Don; CDK4, cyclin dependent kinase 4; RT-PCR,
reverse transcription-polymerase chain reaction. |
In conclusion, the present study demonstrated that
ECSB exhibited a potent inhibitory effect on colon cancer cell
growth, which was mediated by its pro-apoptotic and
antiproliferative activity. These results provide a strong
scientific foundation for the development of novel anticancer
agents from the bioactive ingredients in ECSB.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81073097).
Abbreviations:
|
CRC
|
colorectal cancer
|
|
SB
|
Scutellaria barbata D. Don
|
|
ECSB
|
chloroform fraction of Scutellaria
barbata D. Don
|
|
EPESB
|
petroleum ether fraction of
Scutellaria barbata D. Don
|
|
EEASB
|
ethyl acetate fraction of
Scutellaria barbata D. Don
|
|
ENSB
|
N-butanol fraction of Scutellaria
barbata D. Don
|
|
TCM
|
traditional Chinese medicine
|
|
TEM
|
transmission electron microscopy
|
|
MTT
|
3-(4,
5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
|
References
|
1
|
Spiro S: Lung cancer: principles and
practice. Br J Cancer. 85:16092001. View Article : Google Scholar
|
|
2
|
Bosset JF, Calais G, Mineur L, et al:
Enhanced tumorocidal effect of chemotherapy with preoperative
radiotherapy for rectal cancer: preliminary results-EORTC 22921. J
Clin Oncol. 23:5620–5627. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rougier P, Bugat R, Douillard JY, et al:
Phase II study of irinotecan in the treatment of advanced
colorectal cancer in chemotherapy-naive patients and patients
pretreated with fluorouracil-based chemotherapy. J Clin Oncol.
15:251–260. 1997.PubMed/NCBI
|
|
4
|
Jiang WQ, Fu FF, Li YX, et al: Molecular
biomarkers of colorectal cancer: prognostic and predictive tools
for clinical practice. J Zhejiang Univ Sci B. 13:663–675. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar
|
|
6
|
Lippman SM: The dilemma and promise of
cancer chemoprevention. Nat Clin Pract Oncol. 3:5232006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Longley DB, Allen WL and Johnston PG: Drug
resistance, predictive markers and pharmacogenomics in colorectal
cancer. Biochim Biophys Acta. 1766:184–196. 2006.PubMed/NCBI
|
|
8
|
Gordaliza M: Natural products as leads to
anticancer drugs. Clin Transl Oncol. 9:767–776. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jia L: Cancer complementary and
alternative medicine research at the US National Cancer Institute.
Chin J Integr Med. 18:325–332. 2012. View Article : Google Scholar
|
|
10
|
Carmady B and Smith CA: Use of Chinese
medicine by cancer patients: a review of surveys. Chin Med. 6:1–8.
2011. View Article : Google Scholar
|
|
11
|
Tan KY, Liu CB, Chen AH, et al: The role
of traditional Chinese medicine in colorectal cancer treatment.
Tech Coloproctol. 12:1–6. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chinese Pharmacopoeia Commission.
Pharmacopoeia of the People’s Republic of China. 1. Chinese Medical
Science and Technology Press; pp. 109–110. 2010
|
|
13
|
Kim KW, Jin UH, Kim DI, et al:
Antiproliferative effect of Scutellaria barbata D. Don. on
cultured human uterine leiomyoma cells by down-regulation of the
expression of Bcl-2 protein. Phytother Res. 22:583–590. 2008.
|
|
14
|
Dai ZJ, Wang XJ, Xue Q, et al: Effects of
Scutellaria Barbata drug-containing serum on apoptosis and
mitochondrial transmembrane potential of hepatoma H22 cells. Zhong
Xi Yi Jie He Xue Bao. 6:821–826. 2008.(In Chinese).
|
|
15
|
Wei PY, Pu HQ, Wei X, Li CG and Nong S:
Apoptosis-inducing effect of Scutellaria barbata extract on
human lung cancer SPC-A-1 cells and the expression of apoptosis
associated genes. Zhong Yao Cai. 30:1270–1273. 2007.(In
Chinese).
|
|
16
|
Fong S, Shoemaker M, Cadaoas J, et al:
Molecular mechanisms underlying selective cytotoxic activity of
BZL101, an extract of Scutellaria barbata, towards breast
cancer cells. Cancer Biol Ther. 7:577–586. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wei L, Lin J, Wu G, et al: Scutellaria
barbata D. Don induces G1/S arrest via modulation of p53 and
Akt pathways in human colon carcinoma cells. Oncol Rep.
29:1623–1628. 2013.
|
|
18
|
Wei L, Lin J, Xu W, et al: Scutellaria
barbata D. Don inhibits tumor angiogenesis via suppression of
Hedgehog pathway in a mouse model of colorectal cancer. Int J Mol
Sci. 13:9419–9430. 2012. View Article : Google Scholar
|
|
19
|
Adams J and Cory S: The Bcl-2 apoptotic
switch in cancer development and therapy. Oncogene. 26:1324–1337.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Adams JM and Cory S: Bcl-2-regulated
apoptosis: mechanism and therapeutic potential. Curr Opin Immunol.
19:488–496. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Serrano M, Hannon GJ and Beach D: A new
regulatory motif in cell-cycle control causing specific inhibition
of cyclin D/CDK4. Nature. 366:704–707. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Resnitzky D, Gossen M, Bujard H and Reed
S: Acceleration of the G1/S phase transition by expression of
cyclins D1 and E with an inducible system. Mol Cell Biol.
14:1669–1679. 1994.PubMed/NCBI
|
|
23
|
Chung DC, Brown SB, Graeme-Cook F, et al:
Overexpression of cyclin D1 occurs frequently in human pancreatic
endocrine tumors. J Clin Endocrinol Metab. 85:4373–4378.
2000.PubMed/NCBI
|
|
24
|
Kim H, Ham EK, Kim YI, et al:
Overexpression of cyclin D1 and cdk4 in tumorigenesis of sporadic
hepatoblastomas. Cancer Lett. 131:177–183. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Keum J, Kong G, Yang S, et al: Cyclin D1
overexpression is an indicator of poor prognosis in resectable
non-small cell lung cancer. Br J Cancer. 81:127–132. 1999.
View Article : Google Scholar : PubMed/NCBI
|