Introduction
Traumatic brain injury (TBI) is the leading cause of
injury-related mortality and disability among young people
worldwide (1). Although more
individuals survive from TBI now than in the past, survivors often
suffer residual physical, emotional, behavioral and cognitive
impairments, resulting from TBI-induced pathological lesions in the
brain. Although substantial efforts have been made to develop a new
treatment for TBI, the treatment has been hampered by a lack of
understanding of the molecular and cellular mechanisms underlying
TBI.
Recently, several studies have demonstrated the
neuroprotective effect of progesterone in the CNS following TBI
(2,3). However, the molecular mechanisms
underlying the neuroprotective effect of progesterone following TBI
remains unclear. It has been reported that progesterone improves
cognition and movement function, decreases cerebral edema, inhibits
inflammation and reduces apoptosis (2–6).
However, the effect of progesterone on neuronal regeneration and
plasticity following TBI has not yet been well studied.
It is well known that damage to the central nervous
system (CNS) following TBI is detrimental due to the inability of
central neurons to regenerate their axons and dendrites (7). The failure of the CNS to regenerate
is not due to the intrinsic deficit of regenerative capabilities,
but is caused by the damaged environment that either does not
support or inhibits neuronal regeneration (7). Several inhibitors of axonal growth
have been identified in myelin, including Nogo-A (8), myelin-associated glycoprotein
(9) and oligodendrocyte myelin
glycoprotein (10). Nogo-A, which
is highly expressed in oligodendrocytes, is considered to be the
most important inhibitor of axonal growth in models of CNS injury
(7). In addition, the failure of
axonal regeneration may also result from the presence of a glial
scar, which prevents neuronal regrowth (11,12).
Glial fibrillary acidic protein (GFAP), specifically expressed in
astrocytes, forms a main component of the glial scar (12). Increased Nogo-A and GFAP expression
has been reported in experimental injury to the peripheral nervous
system and CNS in rodents (13–15).
However, the effect of progesterone on the expression of Nogo-A and
GFAP following TBI has yet to be examined.
Growth-associated protein-43 (GAP-43), expressed in
the neuronal growth cone, is involved in the transduction of
intracellular and extracellular signals that regulate neuron
growth, synaptic formation and synaptic plasticity (16–18).
GAP-43 is greatly upregulated during brain development, at the time
of neurite outgrowth and during synaptogenesis (16,18).
Several lines of evidence demonstrated that GAP-43 is upregulated
in regeneration and plasticity following TBI in rat models
(19–22). Therefore, it is noteworthy to
examine whether progesterone increases GAP-43 expression following
TBI.
Although progesterone has been reported to promote
remyelination and axonal regeneration in experimental models of
spinal neurodegeneration and peripheral nerve injury (23–25),
the roles of progesterone in axonal regeneration in the brain
following injury remain unknown. In the present study, the
expression of Nogo-A, GFAP and GAP-43 in TBI rats were investigated
and the effects of progesterone on their expression at 1, 3, 7, 14
and 28 days post-injury was examined. The present study revealed
that progesterone significantly decreased the expression of Nogo-A
and GFAP, and upregulated the GAP-43 protein, suggesting that
progesterone promoted neuronal regeneration and plasticity in TBI
rats.
Materials and methods
Animals and the TBI model
Animal experimental protocols were approved by the
Committee for Animal Experiments at The Third Xiangya Hospital of
Central South University (Changsha, China). In total, 75 adult male
Sprague Dawley rats (weighing 250–300 g) were housed at a constant
temperature (23°C) and humidity (7%) with a 12 h light/dark cycle,
and maintained on standard pelleted rat chow and water ad
libitum. They were habituated to the housing conditions for at
least a week prior to the surgical procedure. The TBI model was
produced by the weight drop method as previously described
(26). Briefly, rats were
intraperitoneally anesthetized with 10% chloral hydrate (3 mg/kg)
and were placed in a stereotaxic frame. During the surgical
procedure, body temperature was kept at 37°C. After the skull was
exposed, a 5 mm craniotomy was performed over the right
frontoparietal cortex to expose the dura, with the center of the
opening located 2 mm lateral to the midline and 2.2 mm posterior to
the bregma. A 20 g weight was dropped from a height of 30 cm onto a
piston resting on the exposed dura, resulting in a moderate injury
in the right brain. The rats that experienced brief convulsion and
apnea immediately following the TBI were included in the present
study and the rats that died or did not exhibit consciousness loss
and neurological dysfunction following recovery were excluded. The
locomotor behavior of rats following TBI was assessed, including
balancing (5 points), righting (1 point), head-holding (1 point),
walking (4 points), escaping behavior in response to tail clamping
(2 points) and paw withdrawal in response to tail clamping (2
points). Rats with a total of 10 or above (n=2) were excluded from
the present study. In the sham rats, the same surgical procedure
was performed, however, no weight was dropped on the dura.
The rats were assigned to three groups: a sham group
with no progesterone treatment (n=25), a TBI group with vehicle
treatment (n=25) and a TBI group with progesterone treatment
(n=25). In each group, the rats were sacrificed at 1 (n=5), 3
(n=5), 7 (n=5), 14 (n=5) and 28 days (n=5) following surgery.
Progesterone (10 mg/kg, qd) or 2-hydroxypropyl-β-cyclodextrin (as
the vehicle control) was injected intraperitoneally 6 h after the
surgery and continued until the rats were sacrificed. The dose and
duration of progesterone used in the present study was similar to
that used in previous studies (27,28).
Tissue preparation
At specific time points (1, 3, 7, 14 and 28 days
after surgery), rats were intraperitoneally anesthetized with 10%
chloral hydrate (3 mg/kg). The rats were then perfused via the left
ventricle with cold phosphate-buffered saline, followed by 4%
paraformaldehyde. The brains were dissected and post-fixed with 4%
formaldehyde at 4°C overnight. The tissues were then dehydrated
with ethanol, cleared in xylene, filtrated in paraffin wax and
embedded in paraffin wax. Serial sections (4 μm thick) were
obtained using a microtome (Leica, Bensheim, Germany).
Immunocytochemistry
Tissue sections (4 μm thick) were obtained from
paraffin-embedded tissue blocks. The tissue sections were
immunocytochemically stained for Nogo-A, GFAP and GAP-43. Briefly,
sections were washed in xylene to remove the paraffin, rehydrated
with serial dilutions of alcohol, followed by washing in a PBS
solution. The samples were then incubated in primary antibodies
against Nogo-A (1:200 dilution), GFAP (1:200 dilution) and GAP-43
(1:200 dilution), or non-immune rabbit IgG at 4°C overnight.
Non-immune rabbit IgG was used as the negative control to rule out
the non-specific staining (Fig.
1). All primary antibodies and the non-immune rabbit IgG were
rabbit anti-rat antibodies, purchased from Beijing Biosythesis
Biotechnology Co., Ltd. (Beijing, China). Following primary
antibodies being washed off, sections were incubated with goat
anti-rabbit biotin-conjugated secondary antibodies (1:1,000
dilution; Beijing Biosynthesis Biotechnology Co., Ltd) for 20 min
at 37°C. The tissue sections were then incubated with streptavidin
horseradish peroxidase for 20 min at 37°C. The DAB substrate was
applied to the section for 2 min and then the sections were
examined under a light microscope.
Coronal brain sections were selected between 3 and 4
mm posterior to the bregma (starting ~1 mm posterior to the lesion)
at the level of the cortex and the hippocampus. Images were
captured at the same magnification (×200) by a digital camera
(Nikon, Tokyo, Japan). Four sections at the same location from each
rat were selected to compare and five random fields without
overlaps in each section were evaluated. The average numbers of
positive cells were used for analysis.
Statistical analysis
Analyses were performed using SPSS 13.0. All values
are presented as the mean ± standard deviation. One-way analysis of
variance (ANOVA) was used to compare the differences among the
control, the TBI group with vehicle treatment and the TBI group
with progesterone treatment, followed by a post-hoc LSD test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Progesterone decreases Nogo-A expression
in TBI rats
The protein expression of Nogo-A was detected in the
cortex 1 mm posterior to the lesions from the sham rats, TBI rats
treated with the vehicle control and TBI rats treated with
progesterone using immunocytochemistry (Fig. 2). Positive Nogo-A immunoreactivity
was observed in the oligodendrocytes. The number of positive
immunoreactive cells at 1, 3, 7, 14 and 28 days after TBI was
analyzed. In the sham group, only a few positive Nogo-A
immunoreactive cells were identified (Fig. 2K). However, compared with the sham
rats, TBI rats significantly increased the Nogo-A expression at 1,
3, 7, 14 and 28 days post-injury (P<0.05; Fig. 2A, C, E, G, I and L). The time
course of Nogo-A expression is shown in Fig. 3L. The Nogo-A expression increased
on day 1, reached a peak on day 14 and declined to a level above
the control 28 days post-injury. Compared with the vehicle control,
progesterone significantly decreased the expression of Nogo-A in
TBI rats 1, 3, 7, 14 and 28 days post-injury (P<0.05; Fig. 2B, D, F, H, J and L).
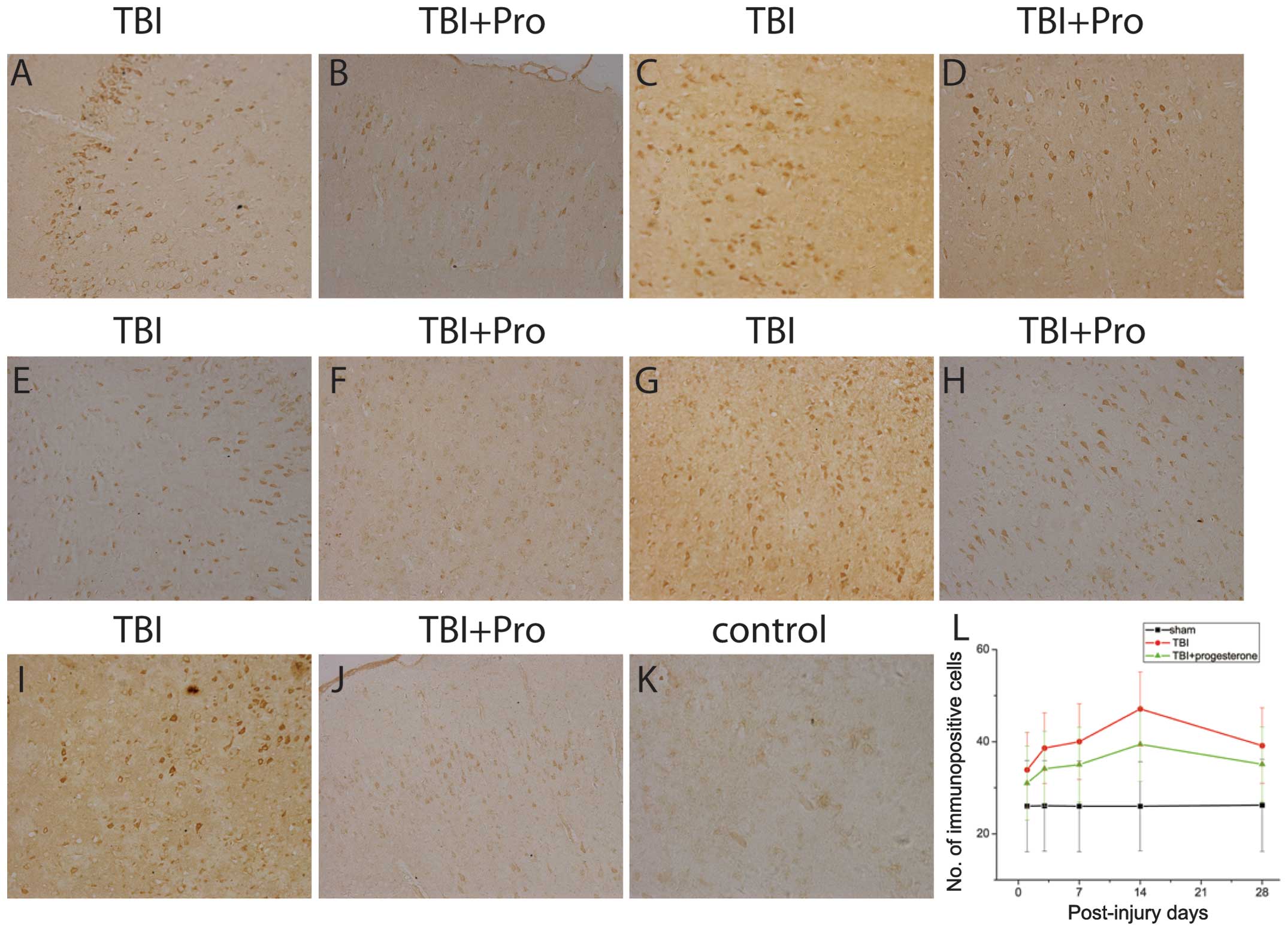 | Figure 2Photomicrographs of Nogo-A
immunostaining in the cortex 1 mm posterior to the lesions from TBI
rats treated with (A, C, E, G, I) the vehicle control and (B, D, F,
H, J) progesterone at (A and B) 1, (C and D) 3, (E and F) 7, (G and
H) 14 and (I and J) 28 days post-injury; (K) sham rats. (L) Number
of immunopositive cells in the sham rats, TBI rats treated with the
vehicle control and TBI rats treated with progesterone at 1, 3, 7,
14 and 28 days post-injury. TBI, traumatic brain injury. |
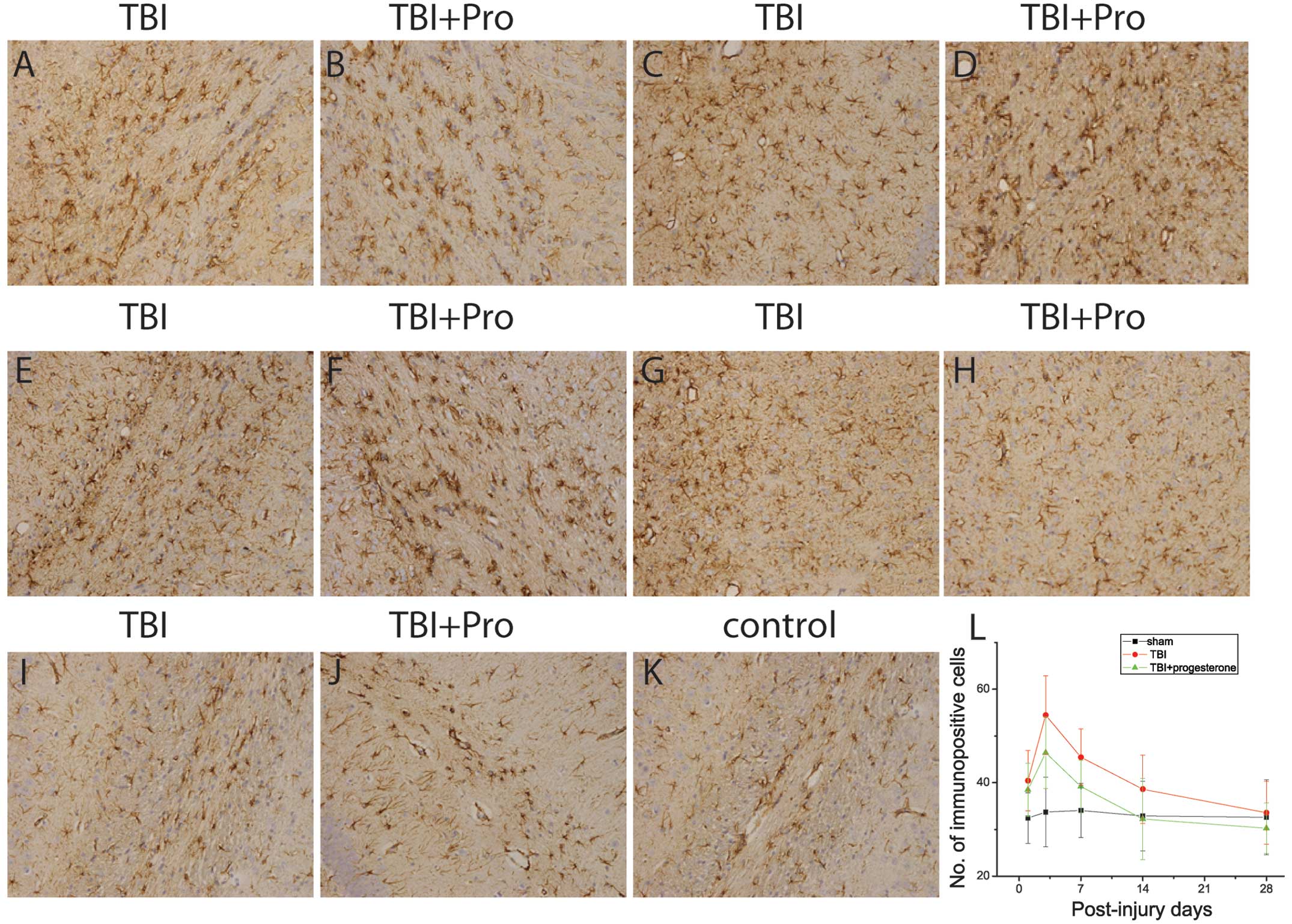 | Figure 3Photomicrographs of GFAP
immunostaining in hippocampal CA1 regions from TBI rats treated
with (A, C, E, G, I) the vehicle control and (B, D, F, H, J)
progesterone at (A and B) 1, (C and D) 3, (E and F) 7, (G and H) 14
and (I and J) 28 days post-injury; (K) sham rats. (L) Number of
immunopositive cells in the sham rats, TBI rats treated with the
vehicle control and TBI rats treated with progesterone at 1, 3, 7,
14 and 28 days post-injury. TBI, traumatic brain injury; GFAP,
glial fibrillary acidic protein. |
Progesterone downregulates GFAP
expression in TBI rats
The protein expression of GFAP was then examined in
the hippocampal CA1 region from sham-injured rats, TBI rats treated
with the vehicle control and TBI rats treated with progesterone,
using immunocytochemistry (Fig.
3). Positive GFAP immunoreactivity was observed in the
astrocytes at 1, 3, 7, 14 and 28 days in the sham rats (Fig. 3K and L). In TBI rats, increased
GFAP expression was observed in the CA1 region. In TBI rats, GFAP
expression was significantly increased at 1, 3, 7, 14 and 28 days
post-injury compared with that in the sham rats (P<0.05;
Fig. 3A, C, E, G, I and L). The
GFAP protein expression increased on day 1, reached a peak on day 3
and gradually declined to a control level 28 days post-injury.
Compared with the vehicle control, progesterone significantly
decreased the expression of GFAP in TBI rats 3, 7 and 14 days
post-injury (P<0.05; Fig. 3B, D, F,
H, J and L).
Progesterone increases GAP-43 expression
in TBI rats
The present study also investigated the GAP-43
expression in the cortex 1 mm posterior to the lesions from sham
rats, TBI rats treated with the vehicle control and TBI rats
treated with progesterone using immunocytochemistry (Fig. 4). In the sham rats, GAP-43 positive
immunoreactivity was identified in the perinuclear cytoplasm of
neurons in the cortex. In TBI rats, GAP-43 expression was
significantly increased at 1, 3, 7 and 14 days post-injury compared
with that in the sham rats (P<0.05; Fig. 4A, C, E, G, I and L). The GAP-43
protein expression increased on day 1, reached a peak on day 7 and
gradually declined to a level above the control 28 days
post-injury. Progesterone significantly increased the expression of
GAP-43 in TBI rats at 1, 3, 7, 14 and 28 days post-injury, compared
with the vehicle control (P<0.05; Fig. 4B, D, F, H, J and L).
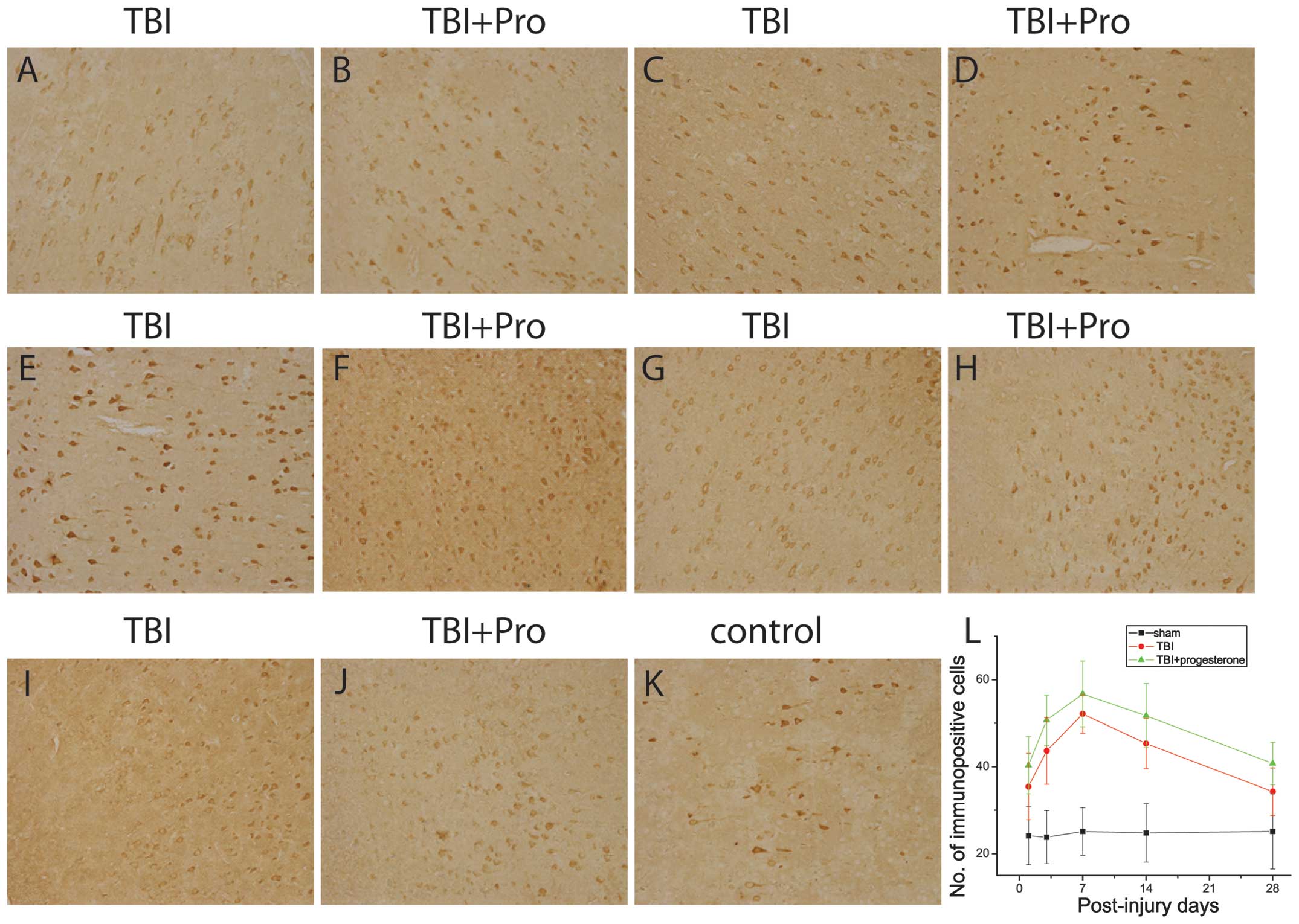 | Figure 4Photomicrographs of GAP-43
immunostaining in the cortex from TBI rats treated with (A, C, E,
G, I) the vehicle control and (B, D, F, H, J) progesterone at (A
and B) 1, (C and D) 3, (E and F) 7, (G and H) 14 and (I and J) 28
days post-injury; (K) sham rats. (L) Number of immunopositive cells
in the sham rats, TBI rats treated with the vehicle control and TBI
rats treated with progesterone at 1, 3, 7, 14 and 28 post-injury.
TBI, traumatic brain injury; GAP-43, growth-associated
protein-43. |
Discussion
The neuroprotective role of progesterone has been
established in the experimental models of TBI (2,3).
However, the mechanisms underlying these neuroprotective effects
have not been well characterized. Numerous cellular mechanisms have
been reported to be important in the neuroprotective effects of
progesterone, including the reduction of edema, inflammation and
apoptosis, and the inhibition of oxidative stress (2–6,29,30).
However, it is unclear whether progesterone can improve neuronal
regeneration following TBI. One of the therapeutic goals in TBI is
to identify ways of repairing and regenerating the damaged or lost
neural circuit. The present study revealed that progesterone
decreases the protein expression of Nogo-A, an inhibitor of axonal
growth, downregulates the expression of GFAP, the main component of
the glial scar and enhances the expression of GAP-43, a signaling
molecule involved in neuronal growth and synaptic formation. Our
findings demonstrated that progesterone is able to improve the
regeneration of damaged neurons following TBI. Since Nogo-A, GFAP
and GAP-43 are known to be involved in neuroregeneration (7,11,12,18,20,21),
our data suggest that progesterone may increase neuroprotection
following TBI by inhibiting the expression of Nogo-A and GFAP and
increasing the expression of GAP-43.
The dose-response study of progesterone on the
behavioral performance of rats following cortical injury revealed
that 8 and 16 mg/kg doses of progesterone was better than 32 mg/kg
administered at 1, 6 and 24 h with repeated administration every 24
h (27), suggesting that 8–16
mg/kg is an optimal dose of progesterone in the treatment of TBI.
In the present study, a 10 mg/kg dose of progesterone was
intraperitoneally administered 6 h after TBI until the rats were
sacrificed. This protocol is similar to a study by Anderson et
al whereby progesterone was administered intraperitoneally
every 12 h until rats were sacrificed at 72 h after cortical injury
(28). Thus, the dose and duration
of progesterone used in the present study is able to produce
neuroprotection to prevent the death of neurons.
Nogo-A, highly expressed in the myelin, has been
regarded as the strongest inhibitor of axonal growth (7). Nogo-A has been extensively studied
for its roles in inhibiting axonal regeneration in the injured CNS
(31–33). The present study revealed that
Nogo-A expression is increased at 1 day post-injury and is
maintained at a high level for ~4 weeks, suggesting that the
long-lasting presence of Nogo-A may contribute to the failure of
neuronal regeneration following brain injury. It has been reported
that the neutralization of Nogo-A with the anti-Nogo-A antibodies
induces neuronal growth following brain injury (33,34),
suggesting that the inhibition of Nogo-A may be useful for the
treatment of TBI. In agreement with this theory, the present study
revealed that progesterone inhibits Nogo-A expression in TBI rats
from 1–28 days post-injury, suggesting that progesterone can
decrease the inhibitory effect of Nogo-A on neuronal regeneration.
Therefore, our results suggest that progesterone may improve
neuronal regeneration through the inhibition of Nogo-A. However, it
remains unclear how progesterone inhibits Nogo-A expression in
TBI.
GFAP, a specific marker for mature astrocytes, is
important in maintaining the normal morphology and function of
astrocytes in the CNS. Following brain injury, astrocytes begin to
proliferate and increase the expression of GFAP (12,15).
Increased GFAP expression is a hallmark of reactive astrocytes,
which is a major cellular component of the glial scar. The glial
scar has long been implicated as a major barrier for axonal
regeneration (11,12). Inhibition of the glial scar is
considered to be a therapeutic strategy for TBI (35). Therefore, the optimum time window
for inhibiting glial scar formation is critical for therapy. The
present study demonstrated that GFAP expression reaches a peak at 3
days post-injury and declines quickly to normal control levels at
28 days post-injury, suggesting that increased GFAP expression does
not last long and glial scar formation starts early (1–3 days)
following brain injury. In addition, our data demonstrated that
progesterone inhibits GFAP expression at early time points (1–3
days) post-injury, suggesting that progesterone is a good drug to
prevent glial scar formation. However, the exact mechanism
underlying the effects of progesterone on glial scar formation
remains to be determined.
GAP-43, the intracellular growth associated protein,
is important in neuron growth during brain development and
neuroregeneration following TBI (16–18).
Following brain injury, GAP-43 protein synthesis greatly increases
and is shuttled along the axon by fast axonal transport to the site
of injury to promote axonal regeneration (17,19,21,22).
Consistent with these studies, the present study found that GAP-43
protein expression is increased following TBI. Progesterone
treatment further increases the expression of GAP-43, suggesting
that progesterone may promote axonal regeneration. However,
progesterone has been reported to inhibit the high expression of
GAP-43 mRNA in Wobbler mice, which exhibit motor neuron
degeneration (36). Although
increased expression of GAP-43 has been identified in a TBI rat
model in the present study and in Wobbler mice, the mechanisms
underlying the overexpression of GAP-43 remain unclear in these
diseases. It has been reported that it is not the injury per
se, but the disconnection of axons from peripheral target
tissues regulating the expression of GAP-43 (37). It is also suggested that the
disruption of the neuronal membrane due to the impact and shearing
forces caused by the TBI may result in TBI-induced increases in the
expression of GAP-43 (38). The
neuronal degeneration in Wobbler mice caused by an autosomal
recessive mutation is characterized by perikayal vascular
degeneration and abnormalities of mitochondrial function in motor
neurons (25,39). The inhibition of GAP-43 expression
by progesterone in Wobbler mice is proposed to be associated with
the antioxidative effect of progesterone (40), while numerous cellular mechanisms
have been reported to be involved in the neuroprotective effect of
progesterone following TBI, including the reduction of edema,
inflammation and apoptosis (2–6,29,30).
The difference in GAP-43 expression in response to progesterone
treatment may largely result from the different pathogenesis of the
diseases and the multiple mechanisms of action of progesterone.
In addition, the present study revealed that GAP-43
upregulation lasts ~1 week and its protein level gradually declines
to a normal control level within 4 weeks post-injury, suggesting
that GAP-43 promotes axonal regeneration at the early stage of
brain injury, and its neuroregenerative effects are gradually
reduced. Progesterone increases GAP-43 expression within 28 days
post-injury, suggesting that progesterone is able to promote axonal
regeneration following TBI through the upregulation of GAP-43.
However, the time course of GAP-43 expression in the process of
neuroregeneration following TBI has not been well established. It
has been reported that the expression of GAP-43 is differentially
regulated in CNS development and regeneration (41). The mechanisms underlying
neuroregeneration following TBI and the beneficial effect of
progesterone on the upregulation of GAP-43 need to be further
determined.
The function of GAP-43 is dependent on PKC-mediated
phosphorylation at S41. The regulation of GAP-43 at S41 has been
reported to be associated with its distribution to different
membrane domains, its regulation of actin filaments, its
interaction with Ca/calmodulin and its binding to PIP2 (17,42).
In the present study, immunocytochemistry was performed to study
the protein expression of GAP-43 following TBI, however, not the
phosphorylation of GAP-43. It remains to be elucidated whether
progesterone regulates the phosphorylation of GAP-43 at S41.
Preclinical studies have demonstrated that
progesterone administered during the first few hours to days
following injury significantly improved behavioral recovery in rats
(27,43,44).
Two Phase II clinical trials have demonstrated that progesterone
treatment has an improved survival and functional outcome than the
placebo control in the treatment of TBI patients, indicating that
progesterone is a safe and efficacious treatment for TBI (45,46).
A study using a rat model of TBI demonstrated that a continuous
infusion of progesterone produces more behavioral recovery in TBI
rats than bolus injections of progesterone (43). These results suggest that a
continuous mode of progesterone administration may be more
beneficial in the clinical testing than bolus injection in the
treatment of TBI. In the present study, a bolus injection of
progesterone was used to treat TBI and found that progesterone
significantly inhibited the expression of Nogo-A and GFAP, and
increased the GAP-43 expression. Although a continuous mode of
progesterone administration was not used in the present, it is
expected that a continuous mode of progesterone administration may
produce more apparent effects on the expression of Nogo-A, GFAP and
GAP-43.
In summary, the present study investigated the
temporal changes of Nogo-A, GFAP and GAP-43 protein expression in
TBI rats and the effects of progesterone on Nogo-A, GFAP and Gap-43
protein expression. Progesterone significantly inhibited the
expression of Nogo-A and GFAP expression and increased the GAP-43
expression, suggesting that progesterone is able to promote
neuroprotection following TBI.
References
|
1
|
Asemota AO, George BP, Bowman SM, Haider
AH and Schneider EB: Causes and trends in traumatic brain injury
for United States adolescents. J Neurotrauma. 30:67–75. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Feeser VR and Loria RM: Modulation of
traumatic brain injury using progesterone and the role of glial
cells on its neuroprotective actions. J Neuroimmunol. 237:4–12.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gibson CL, Gray LJ, Bath PM and Murphy SP:
Progesterone for the treatment of experimental brain injury; a
systematic review. Brain. 131:318–328. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Roof RL, Duvdevani R, Braswell L and Stein
DG: Progesterone facilitates cognitive recovery and reduces
secondary neuronal loss caused by cortical contusion injury in male
rats. Exp Neurol. 129:64–69. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Roof RL, Duvdevani R and Stein DG:
Progesterone treatment attenuates brain edema following contusion
injury in male and female rats. Restor Neurol Neurosci. 4:425–427.
1992.PubMed/NCBI
|
|
6
|
Gibson CL, Constantin D, Prior MJ, Bath PM
and Murphy SP: Progesterone suppresses the inflammatory response
and nitric oxide synthase-2 expression following cerebral ischemia.
Exp Neurol. 193:522–530. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Horner PJ and Gage FH: Regenerating the
damaged central nervous system. Nature. 407:963–970. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xiong NX, Zhao HY, Zhang FC and He ZQ:
Negative correlation of Nogo-A with the malignancy of
oligodendroglial tumor. Neurosci Bull. 23:41–45. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hsieh SH, Ferraro GB and Fournier AE:
Myelin-associated inhibitors regulate cofilin phosphorylation and
neuronal inhibition through LIM kinase and Slingshot phosphatase. J
Neurosci. 26:1006–1015. 2006. View Article : Google Scholar
|
|
10
|
Wang KC, Koprivica V, Kim JA, Sivasankaran
R, Guo Y, Neve RL and He Z: Oligodendrocyte-myelin glycoprotein is
a Nogo receptor ligand that inhibits neurite outgrowth. Nature.
417:941–944. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yiu G and He Z: Glial inhibition of CNS
axon regeneration. Nat Rev Neurosci. 7:617–627. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fitch MT and Silver J: CNS injury, glial
scars, and inflammation: Inhibitory extracellular matrices and
regeneration failure. Exp Neurol. 209:294–301. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brenneman MM, Wagner SJ, Cheatwood JL,
Heldt SA, Corwin JV, Reep RL, Kartje GL, Mir AK and Schwab ME:
Nogo-A inhibition induces recovery from neglect in rats. Behav
Brain Res. 187:262–272. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang H, Yao YJ and Chen DP: Expression of
Nogo-A mRNA and Nogo-A protein in brain tissue of neonatal mice
with ischemic-hypoxic brain damage. Zhonghua Er Ke Za Zhi.
44:792–793. 2006.(In Chinese).
|
|
15
|
Stichel CC and Müller HW: The CNS lesion
scar: new vistas on an old regeneration barrier. Cell Tissue Res.
294:1–9. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Clayton GH, Mahalik TJ and Finger TE:
Expression of GAP43 mRNA in normally developing and transplanted
neurons from the rat ventral mesencephalon. J Comp Neurol.
347:470–480. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Denny JB: Molecular mechanisms, biological
actions, and neuropharmacology of the growth-associated protein
GAP-43. Curr Neuropharmacol. 4:293–304. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Benowitz LI and Routtenberg A: GAP-43: an
intrinsic determinant of neuronal development and plasticity.
Trends Neurosci. 20:84–91. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Donovan SL and McCasland JS: GAP-43 is
critical for normal targeting of thalamocortical and
corticothalamic, but not trigeminothalamic axons in the whisker
barrel system. Somatosens Mot Res. 25:33–47. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hughes-Davis EJ, Cogen JP, Jakowec MW,
Cheng HW, Grenningloh G, Meshul CK and McNeill TH: Differential
regulation of the growth-associated proteins GAP-43 and superior
cervical ganglion 10 in response to lesions of the cortex and
substantia nigra in the adult rat. Neuroscience. 135:1231–1239.
2005. View Article : Google Scholar
|
|
21
|
Nagamoto-Combs K, Morecraft RJ, Darling WG
and Combs CK: Long-term gliosis and molecular changes in the
cervical spinal cord of the rhesus monkey after traumatic brain
injury. J Neurotrauma. 27:565–585. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schmidt-Kastner R, Bedard A and Hakim A:
Transient expression of GAP-43 within the hippocampus after global
brain ischemia in rat. Cell Tissue Res. 288:225–238. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Labombarda F, González Deniselle MC, De
Nicola AF and González SL: Progesterone and the spinal cord: good
friends in bad times. Neuroimmunomodulation. 17:146–149. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Garay L, Deniselle MC, Meyer M, Costa JJ,
Lima A, Roig P and De nicola AF: Protective effects of progesterone
administration on axonal pathology in mice with experimental
autoimmune encephalomyelitis. Brain Res. 1283:177–185. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
De Nicola AF, Labombarda F, Deniselle MC,
Gonzalez SL, Garay L, Meyer M, Gargiulo G, Guennoun R and
Schumacher M: Progesterone neuroprotection in traumatic CNS injury
and motoneuron degeneration. Front Neuroendocrinol. 30:173–187.
2009.PubMed/NCBI
|
|
26
|
Feeney DM, Boyeson MG, Linn RT, Murray HM
and Dail WG: Responses to cortical injury: I. Methodology and local
effects of contusions in the rat. Brain Res. 211:67–77. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Goss CW, Hoffman SW and Stein DG:
Behavioral effects and anatomic correlates after brain injury: a
progesterone dose-response study. Pharmacol Biochem Behav.
76:231–242. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Anderson GD, Farin FM, Bammler TK, Beyer
RP, Swan AA, Wilkerson HW, Kantor ED and Hoane MR: The effect of
progesterone dose on gene expression after traumatic brain injury.
J Neurotrauma. 28:1827–1843. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pajović SB, Saicić ZS, Spasić MB, Petrović
VM and Martinović JV: Effects of progesterone and estradiol
benzoate on glutathione dependent antioxidant enzyme activities in
the brain of female rats. Gen Physiol Biophys. 18:35–44.
1999.PubMed/NCBI
|
|
30
|
Pierson RC, Lyons AM and Greenfield LJ Jr:
Gonadal steroids regulate GABAA receptor subunit mRNA expression in
NT2-N neurons. Brain Res Mol Brain Res. 138:105–115. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
GrandPré T, Nakamura F, Vartanian T and
Strittmatter SM: Identification of the Nogo inhibitor of axon
regeneration as a Reticulon protein. Nature. 403:439–444.
2000.PubMed/NCBI
|
|
32
|
Oertle T, van der Haar ME, Bandtlow CE, et
al: Nogo-A inhibits neurite outgrowth and cell spreading with three
discrete regions. J Neurosci. 23:5393–5406. 2003.PubMed/NCBI
|
|
33
|
Marklund N, Bareyre FM, Royo NC, Thompson
HJ, Mir AK, Grady MS, Schwab ME and McIntosh TK: Cognitive outcome
following brain injury and treatment with an inhibitor of Nogo-A in
association with an attenuated downregulation of hippocampal
growth-associated protein-43 expression. J Neurosurg. 107:844–853.
2007. View Article : Google Scholar
|
|
34
|
Lenzlinger PM, Shimizu S, Marklund N, et
al: Delayed inhibition of Nogo-A does not alter injury-induced
axonal sprouting but enhances recovery of cognitive function
following experimental traumatic brain injury in rats.
Neuroscience. 134:1047–1056. 2005. View Article : Google Scholar
|
|
35
|
Mueller BK, Mueller R and Schoemaker H:
Stimulating neuroregeneration as a therapeutic drug approach for
traumatic brain injury. Br J Pharmacol. 157:675–685. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gonzalez Deniselle MC, Lopez Costa JJ,
Gonzalez SL, Labombarda F, Garay L, Guennoun R, Schumacher M and De
Nicola AF: Basis of progesterone protection in spinal cord
neurodegeneration. J Steroid Biochem Mol Biol. 83:199–209.
2002.PubMed/NCBI
|
|
37
|
Schreyer DJ and Skene JH:
Injury-associated induction of GAP-43 expression displays axon
branch specificity in rat dorsal root ganglion neurons. J
Neurobiol. 24:959–970. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hulsebosch CE, DeWitt DS, Jenkins LW and
Prough DS: Traumatic brain injury in rats results in increased
expression of Gap-43 that correlates with behavioral recovery.
Neurosci Lett. 255:83–86. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Santoro B, Bigini P, Levandis G, Nobile V,
Biggiogera M, Botti F, Mennini T and Curti D: Evidence for chronic
mitochondrial impairment in the cervical spinal cord of a murine
model of motor neuron disease. Neurobiol Dis. 17:349–357. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
González Deniselle MC, González SL and De
Nicola AF: Cellular basis of steroid neuroprotection in the wobbler
mouse, a genetic model of motoneuron disease. Cell Mol Neurobiol.
21:237–254. 2001.PubMed/NCBI
|
|
41
|
Udvadia AJ, Köster RW and Skene JH: GAP-43
promoter elements in transgenic zebrafish reveal a difference in
signals for axon growth during CNS development and regeneration.
Development. 128:1175–1182. 2001.PubMed/NCBI
|
|
42
|
Nguyen L, He Q and Meiri KF: Regulation of
GAP-43 at serine 41 acts as a switch to modulate both intrinsic and
extrinsic behaviors of growing neurons, via altered membrane
distribution. Mol Cell Neurosci. 41:62–73. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cutler SM, VanLandingham JW, Murphy AZ and
Stein DG: Slow-release and injected progesterone treatments enhance
acute recovery after traumatic brain injury. Pharmacol Biochem
Behav. 84:420–428. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wali B, Sayeed I and Stein DG: Improved
behavioral outcomes after progesterone administration in aged male
rats with traumatic brain injury. Restor Neurol Neurosci. 29:61–71.
2011.PubMed/NCBI
|
|
45
|
Wright DW, Kellermann AL, Hertzberg VS, et
al: ProTECT: a randomized clinical trial of progesterone for acute
traumatic brain injury. Ann Emerg Med. 49:391–402. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Xiao G, Wei J, Yan W, Wang W and Lu Z:
Improved outcomes from the administration of progesterone for
patients with acute severe traumatic brain injury: a randomized
controlled trial. Crit Care. 12:R612008. View Article : Google Scholar : PubMed/NCBI
|