Introduction
Acute lung injury (ALI) and its more severe
manifestation, acute respiratory distress syndrome (ARDS), are
major complications in critically ill patients. The mortality rate
of ARDS/ALI remains high due to the lack of effective treatments
(1). ALI is a syndrome of
widespread lung inflammation and increased pulmonary vascular
permeability resulting in pulmonary edema and hypoxia, and may
contribute to multiple organ failure and death. ALI is most
commonly caused by sepsis, pneumonia, trauma or aspiration of the
gastric contents. Infection is also the most common cause of ALI
and is associated with a higher mortality rate than non-infectious
ALI (2,3). The inflammatory process is important
in the pathogenesis of infectious and non-infectious ALI, and the
degree of acute inflammation is strongly correlated with outcome
(4).
Recently, transplantation of various stem or
progenitor cells including bone marrow-derived mesenchymal stem
cells (MSCs) or endothelial progentior cells, has been reported to
reduce mortality and attenuate ALI induced by endotoxins or sepsis
in a rodent model (5–7). Bone marrow-derived MSCs retain the
ability to differentiate into a distinct variety of cell lineages
(8), including chondrocytes,
myoblasts, endothelial cells, epithelial cells and neurocytes, and
have self-renewal capacities. There is an increased interest in
understanding the biology of MSCs for potential clinical use as
cell-based therapies (9,10). Recent in vivo and in
vitro studies indicate that multi-potent mesenchymal stromal or
stem cells may also have therapeutic effects on ALI (9,11–14).
Del-1 is a 52 kDa glycoprotein secreted by
endothelial cells, which associates with the endothelial cell
surface and extracellular matrix (15–17).
Human Del-1 shares >97% amino acid identity with the mouse
counterpart; the expression of Del-1 was initially observed in
embryonic cells including in the endothelium and thymus, and
subsequent subsets of macrophages and hypertrophic chondrocytes.
However, it was recently reported that Del-1 is expressed in adult
mice in a tissue-specific manner, including high expression in the
lung, brain and eye, weakly in the kidney and absent in the
intestine, liver, heart, spleen, whole blood and bone marrow cells
(18). It was reported that Del-1
is a ligand for LFA-1 and that Del-1 actually inhibits the function
of LFA-1. Under physiological flow conditions, addition of Del-1 to
the system where leukocytes adhere onto immobilized ICAM-1, via
interactions with active LFA-1, represses the adhesion of
leukocytes (18). Del-1-mediated
inhibition of the interaction between LFA-1 and ICAM-1 further
implies that Del-1 may be used as a therapeutic tool for
inflammatory diseases.
The aim of the present study was to examine the
effect of Del-1 overexpressed-MSCs treatment on the ALI mouse model
that was induced by lipopolysaccharide (LPS) injection. MSCs were
isolated from the bone marrow of C57/B6 mice and expanded in
vitro prior to administration through the injection of the tail
vein. The cDNA of Del-1 were cloned from mouse lung tissue. The
data suggested that when over-expressed by retroviral transduction,
the treatment of Del-1 over-expressed MSCs have beneficial effects
on LPS-induced ALI, including lower neutrophils and proinflammatory
cytokines concentration in mice bronchoalveolar lavage (BAL). Our
data demonstrate a potential new approach to the treatment of
ALI.
Materials and methods
Mice
Thirty-six C57/B6 mice were purchased from the SLRC
laboratory (Shanghai, China). The mice were randomized into control
group (n=10, phosphate-buffered saline (PBS) treated), ALI group
(n=10, LPS treated only), MSCs control group (n=10, LPS+MSCs-GFP
treated) and MSCs-Del-1 treated group (n=10). Mice were sacrificed
at 24 h following LPS and MSC administration. The study was
approved by the Ethics Committee of Third Military Medical
University (Chongqing, China).
Establishment of the ACI model of
mice
Female C57/B6 mice (8–10 weeks old) were treated
with either 20 mg/kg LPS (Sigma-Aldrich, St. Louis, MO, USA) from
Escherichia coli (serotype 0111:B4) in 100 μl PBS or an
equal volume of PBS, as a vehicle control, by intraperitoneal
injection.
MSC isolation and culture
Fresh bone marrow cells were harvested from the
femurs of six-weeks-old male mice by flushing with DMEM (Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 15%
fetal bovine serum (FBS; Beijing Solarbio Science & Technology
Co., Ltd., Beijing, China). The bone marrow mononuclear cells were
isolated by density gradient centrifugation (Ficoll-Paque PLUS;
Pharmacia & Upjohn Company, Peapack, NJ, USA) and cultured in
low-glucose DMEM containing 15% FBS and 1% penicillin/streptomycin
(Beijing Solarbio Science & Technology Co., Ltd.) at 37°C in a
humidified incubator with 5% CO2. The non-adherent cells
were removed by medium changes. The cells were passaged every 3–4
days by trypsinization and the fourth passage cells were used for
in vivo experiments.
Lentivirus preparation and infection
Vectors for lentivirus packaing including pMDL, pRev
pVSVG and lentivirus transfer vector were purchased from Clontech
Laboratories (Mountain View, CA, USA). Lentiviral vectors were
transfected into 293T cells using Lipofectamine (Invitrogen Life
Technologies, Carlsbad, CA, USA). Following 24 h, the medium was
replaced with DMEM containing 15% FBS. A total of 48 h
post-transfection, the lentiviral supernatants were harvested,
supplemented with 6 μg/ml polybrene and used to infect MSC
cells.
MSC administration
A total of 1 h following LPS injection, the mice
were given either 5×106 MSCs in 100 μl PBS (in the
MSCs-treated ALI group) or 100 μl PBS (in the PBS-treated ALI and
control groups) via tail vein injection. At 24 h following the
injection, samples were collected from each mouse for assessment of
lung injury.
Histopathological analysis and lung
injury scores
Following sacrifice, at each time point the whole
left lower lobe of the lung was fixed in a 4% formaldehyde neutral
buffer solution for 24 h, dehydrated in a graded ethanol series,
embedded in paraffin and sliced at 5 μm. Paraffin sections were
stained with hematoxylin and eosin (H&E) for histopathological
analysis.
In order to assess the severity of the lung damage,
a semi-quantitative histological index of quantitative assessment
(IQA) of lung injury was utilized. Eight sections were randomly
selected from each group of rats and ten fields from each section
were examined by microscopy (magnification, ×40). A pathologist
evaluated all of the sections in a blinded manner. The average
values of the lung injury obtained were considered a
semi-quantitative histological IQA of lung injury.
Quantitative (q)PCR analysis
Total RNA was extracted from pulmonary tissues using
TRIzol reagent (Invitrogen Life Technologies) according to the
manufacturer’s instructions. RT-PCR kits (Takara Healthcare, Inc.,
Kyoto, Japan) were used for the qPCR experiment. Total RNA template
(4 μg) were used to construct the cDNA by using AMV reverse
transcriptase and random 9-mers as the first-strand primer.
Synthesized cDNA was used in qPCR experiments. qPCR was performed
using a 40-cycle two-step PCR with sequence-specific primer pairs
using the ABI7900 fast real-time detection system (Invitrogen Life
Technologies). Primers were designed using the Primer Express 3.0
software. The level of the mRNA expression was evaluated as a ratio
based on qPCR results for lung tissue GAPDH mRNA.
ELISA
Detection of TNF-α and IL-6 was conducted using
ELISA according to the manufacturer’s instructions. The experiment
was repeated three times and the results expressed with the mean
value.
Lung wet/dry weight ratio
The superior lobe of the right lung was cleansed and
weighed to obtain the wet weight, and was then place in an oven at
80°C for 48 h for measurement of the dry weight. The ratio of the
wet weight to dry weight was calculated to assess the tissue
edema.
Bronchoalveolar lavage (BAL)
examination
The trachea was exposed and cannulated with a
catheter. The left lung was lavaged three times with sterile PBS in
a volume of 0.5 ml/wash. The fluid recovered following lavage was
>90% on average. The BAL fluid (BALF) was centrifuged at 700 × g
for 10 min at 4°C and the supernatant was stored at −80°C for
cytokine and protein analysis, while the cell pellet was
resuspended in PBS for counting the neutrophils.
Myeloperoxidase (MPO) activity assay
MPO activity in the homogenized lung tissue was
measured as previously described by Gray et al (19). The MPO concentration was detected
using a MPO ELISA kit (Blue Gene Biotech, Shanghai, China).
Briefly, the lung tissues were homogenized and centrifuged at
15,000 × g for 20 min at 4°C. The supernatants and standard sample
were added into a microtiter plate (100 μl/well) precoated with a
murine anti-MPO mAb. Following incubation for 1 h at 37°C, the
plate was washed six times followed by the addition of the
substrate and stop solution, and the optical density (OD) at 450 nm
was measured using a microplate reader. All the samples were
assayed in triplicate.
Measurement of protein concentration in
lung BALF
The concentration of protein in the BALF was
measured using Bradford reagent and a protein assay kit (Bio-Rad,
Hercules, CA, USA). Briefly, 160 μl of each standard and sample
solution was pipetted into separate microtiter plate wells and 40
μl of the dye reagent was added to each well and mixed thoroughly.
The mixture was incubated at room temperature for at least 5 min
prior to the measurement of the OD at 595 nm. Comparison to a
standard curve provided a relative measurement of the protein
concentration.
Statistical analysis
All the data were analyzed using SPSS 13.0 software
(SPSS, Inc., Chicago, IL, USA) and expressed as the mean ± SD.
Significant differences were assessed by one-way analysis of
variance (ANOVA) followed by Fisher protected least significant
difference test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Increased expression of Del-1 in
LPS-induced ACI
Previous studies have demonstrated that, as an
inhibitor of cell migration, Del-1 inhibits the migration of a
variety of different cells, particularly the process of immune cell
migration through the blood vessel wall to the lesion site. In
order to study the function of Del-1 in ACI, an ACI in vivo
mouse model was established by intravenous endotoxin injection and
the lung tissue of the different groups of mice were obtained for
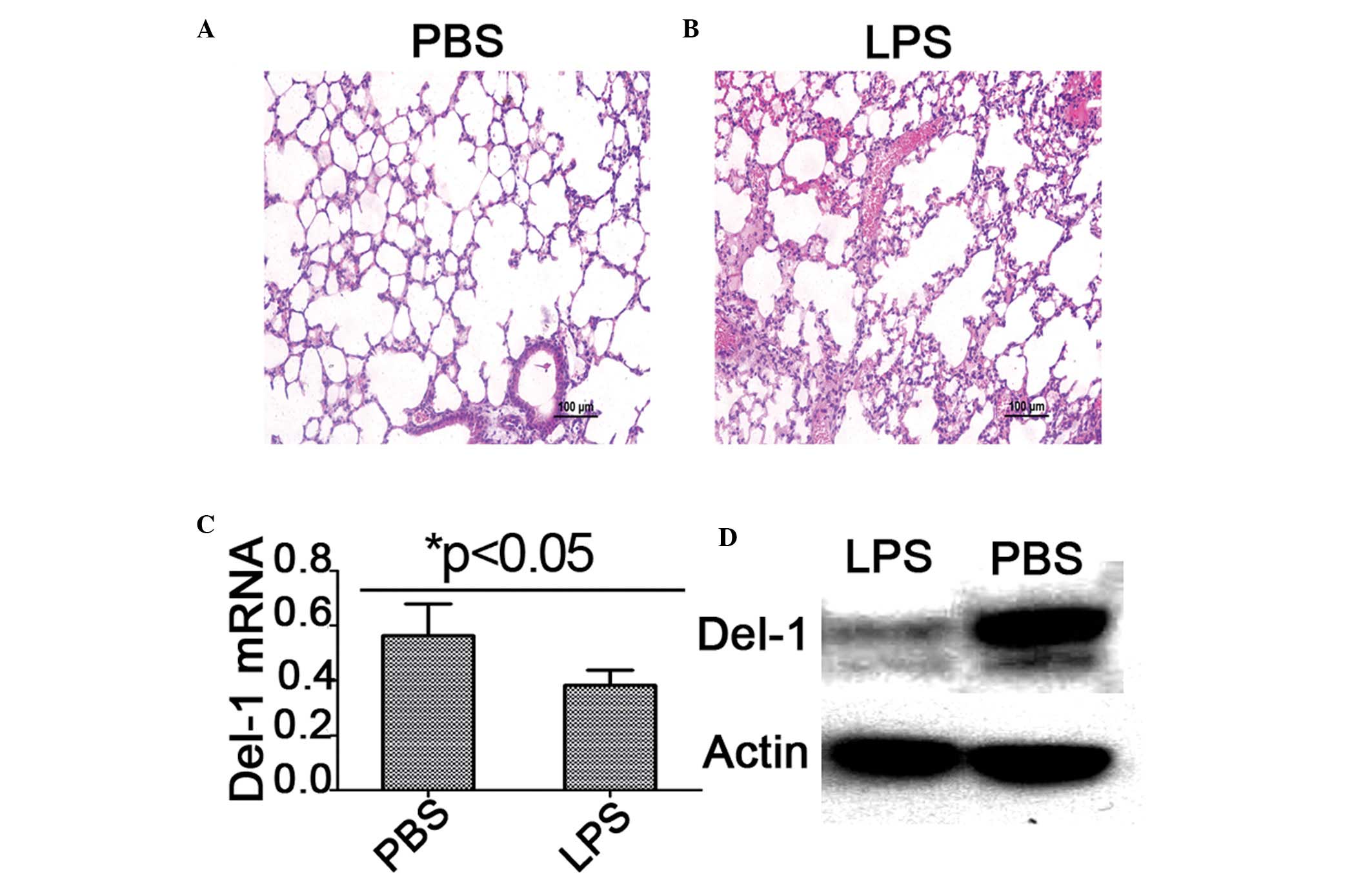
paraffin section. As demonstrated in Fig. 1, microscopic examination of the
lung following H&E staining revealed exudative changes, patchy
hemorrhage, a thickened alveolar interstitium and heavy
infiltration of inflammatory neutrophils and lymphocytes into the
intra-alveolar and interstitial spaces in the lungs of mice with
LPS-induced ALI. To further assess the level of Del-1 in the
injured lung tissue, the expression of Del-1 in different mice lung
tissue samples was detected by qPCR and western blot analysis. It
was identified that the level of Del-1 mRNA and protein was
decreased compared with the health control group (Fig. 1B).
MSC characterization and retrovirals
induces Del-1 overexpression in MSCs
Previous studies have demonstrated that the MSCs
have therapeutic effects on LPS-induced ALI mice, in addition, the
Del-1 may inhibit the migration of pro-inflammatory cells to the
lesion. Therefore, whether the Del-1 overexpressed MSCs have an
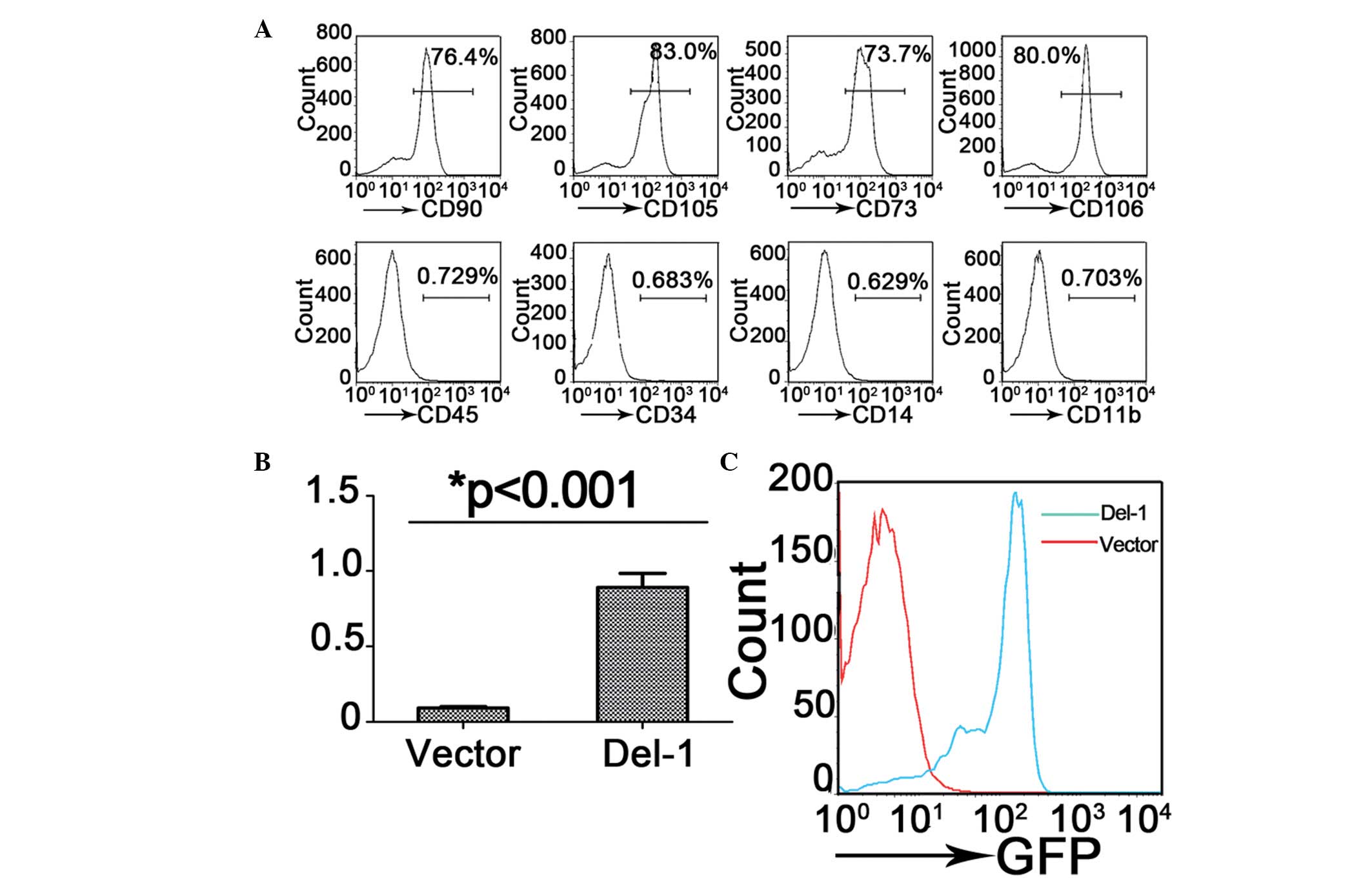
optimal effect on ALI was investigated. Bone marrow-derived MSCs
were isolated from the mice and flow cytometric immunophenotype
analysis revealed that almost all of these cells expressed the
typical mesenchymal stem cell surface markers CD105, CD90, CD73 and
CD106, but not the hematopoietic lineage markers CD45, CD34, CD14
and CD11b (Fig. 2A).
To investigate the therapeutic effect of Del-1
overexpressed MSCs on ALI, a Del-1 expressing lentiviral vector was
generated and MSCs cells were infected. Overexpression of Del-1
mediated by lentiviral transduction was confirmed in GFP cells by
FACS sorting (Fig. 2C), the level
of Del-1 mRNA in MSC cell was detected by qPCR (Fig. 2B). The data suggested that the
Del-1 expressing lentiviral vector increased the expression level
of Del-1 in bone marrow-derived MSCs.
Effect of different treated MSC
transplantation on lung histopathology and lung injury scores
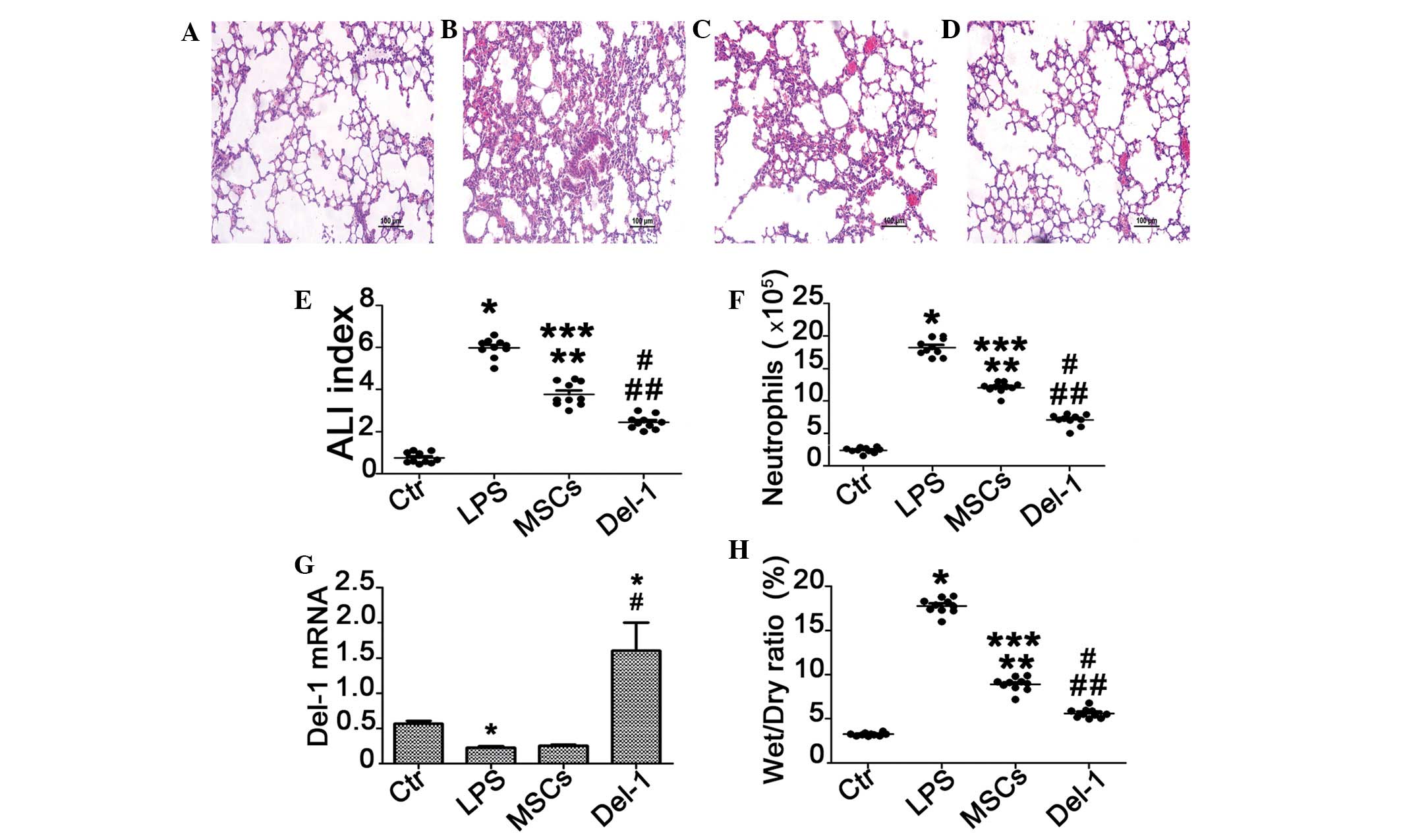
As demonstrated in Fig.
3, the transplantation of MSCs markedly reduced the
infiltration of inflammatory cells and improved the histological
features of the lung, 24 h following injection via the tail vein
(Fig. 3C). The transplantation of
a Del-1-MSCs had a distinct therapeutic effect on LPS-induced ALI
compared with the MSCs treated group (Fig. 3D).
To further assess the degree of pulmonary damage,
injury in the left lung was scored in each group (Fig. 3E). The lung injury index reached
the highest value 24 h following transplantation and the scores of
the MSCs and Del-1-MSCs-treated groups were significantly lower
than those of the PBS-treated control group (P<0.001).
The neutrophil count in BALF and the lung wet/dry
ratio are revealed in Fig. 3F and
H. The level of lung wet/dry ratio and neutrophil count
decreased significantly in the MSC-treated group and
Del-1-expressed MSC-treated group mice compared with the controls.
In addition, the administration of the Del-1-expressed MSCs also
reduced the neutrophil counts in BALF and the lung wet/dry ratio,
and the difference in cell count and wet/dry ratio between MSCs
treated group and Del-1 treated group were significant
(P<0.05).
Additionally, we detected the mRNA level of Del-1 in
the lung tissue of the MSC-treated groups of mice. We demonstrated
that the expression of Del-1 was increased in the Del-1-expressed
MSCs treated mice and this data was consistent with the result of
the therapeutic effect.
IL-6, TNF-α and protein concentration in
BALF and MPO in lung homogenates
The expression levels of TNF-α and IL-6 in BALF were
significantly increased in PBS-treated ALI group compared with
those in the healthy control group. The MSCs or Del-1-expressed MSC
treatment significantly reduced the elevated TNF-α and IL-6 levels.
Furthermore, the level of TNF-α in BALF was significantly decreased
in Del-1 expressed MSC-treated group compared with those in the
MSC-treated group, however there was no change in the concentration
of IL-6 between MSC-treated group and Del-1 expressed MSC-treated
group. In addition, the protein concentration in the BALF and the
level of MPO in the lung homogenates were consistent with the
results of the cytokine level in BALF (Fig. 4C and D).
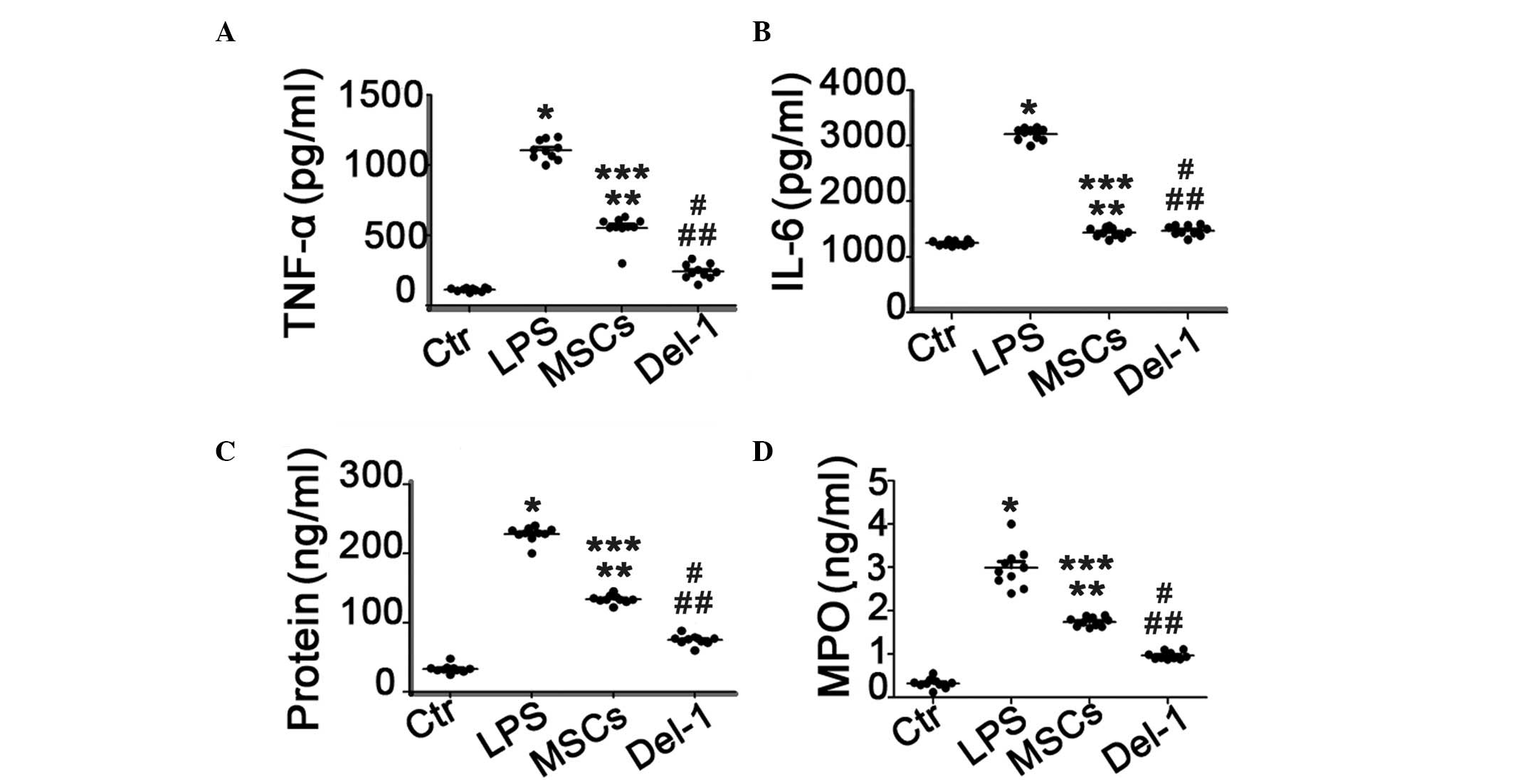 | Figure 4Proinflammatory cytokines, protein and
MPO concentration in BALF of ACI mice lung homogenates. (A) TNF-α;
(B) IL-6; (C) protein and (D) MPO concentration in BALF of ALI mice
following the different types of treatment.*P<0.05
compared with the Ctr group mice; **P<0.05 compared
with the LPS group mice; ***P<0.05 compared with the
Ctr group mice; #P<0.05 compared with the LPS group
mice; ##P<0.05 compared with the MSCs group mice.
ALI, acute lung injury, Del-1, developmental endothelial locus-1;
PBS, phosphate-buffered saline; LPS, lipopolysaccharide; H&E,
hematoxylin and eosin; BALF, bronchoalveolar lavage fluid; MPO,
myeloperoxidase; MSC, mesencyhmal stem cells; Ctr, control. |
Discussion
As demonstrated in previous animal studies, bone
marrow-derived MSCs are able to alleviate LPS-induced ACI by
restoring lung function and increasing survival rate via its
anti-inflammatory, anti-apoptotic and immune regulatory properties
and may therefore provide a novel therapy for ALI. Recently, it was
demonstrated that Del-1 functions as an endogenous inhibitor of a
major leukocyte adhesion receptor, LFA-1, to prevent leukocyte
adhesion to the endothelium (18).
Unlike many adhesion molecules, including selectins and Ig
superfamily members promoting leukocyte adhesion on the
endothelium, Del-1 inhibits the process of leukocyte binding to the
endothelium, thereby suppressing entrance of leukocytes to inflamed
tissues.
Our data indicate that the administration of Del-1
modified MSCs after LPS induction protects lung tissue from injury
by significantly reducing the extent of inflammation, although only
a limited effect was observed following the administration of MSCs
alone. Additionally, our in vivo data suggested that the
histopathology of lung injury was greatly improved following the
administration of MSCs carrying the Del-1 gene compared with the
treatment with MSCs alone.
A number of studies have suggested that
bone-marrow-derived MSCs (9,20–22)
or Del-1 (18,23,24)
have therapeutic applications in acute lung injury. To further
define the therapeutic potential of MSCs, we used a lentivirus
vector containing the Del-1 gene to infect the MSCs, then treated
ALI mice with these Del-1 expressed MSCs. Our results suggested
that the MSCs were able to have a therapeutic effect, as well as
act as gene delivery vehicles.
In the present study, the cells that were utilized
for ALI therapy were isolated from the bone marrow. These cells
exhibited several typical characteristics of MSCs. As
plastic-adherent cells, these bone marrow-derived MSCs express
surface markers typically associated with MSCs but that are not
hematopoietic stem cell markers. The MSCs differentiated from bone
marrow were analyzed by flow cytometric immunophenotype, and it was
identified that almost all these cells expressed the cell surface
markers CD105, CD90, CD73 and CD106, but not the hematopoietic
lineage markers CD45, CD34, CD14 and CD11b (Fig. 2).
Lentiviral vectors were selected to modify MSCs with
the Del-1 gene, due to their high efficiency in gene delivery and
ability to integrate the gene into the MSCs during cell division
(18). The results demonstrated
that high gene transduction efficiency was achieved at day 5
following exposure to lentiviral vectors, suggesting gene
integration was successful (Fig.
2).
It has been reported that Del-1 is a ligand for
LFA-1, and that Del-1 is able to inhibit the function of LFA-1.
Under physiological flow conditions, the addition of Del-1 to the
system where leukocytes adhere onto immobilized ICAM-1 via
interaction with active LFA-1, represses the adhesion of leukocytes
(24). In the present study, the
over-expression of Del-1 in the recipient lung was achieved by
MSC-based gene delivery (Fig. 2B).
It was demonstrated that MSC-based gene therapy attenuates the
inflammatory reaction and vascular leakage in the lung following
LPS exposure. Furthermore, it was also able to improve lung
histopathological changes. These beneficial effects may be mediated
by the downregulation of pro-inflammatory gene expression,
including TNF-α and IL-6 (Fig.
4).
In summary, our data indicate that Del-1-carrying
MSC therapy may ameliorate the injurious effects of LPS-induced
acute lung injury. As an effective cellular vehicle for the
treatment of lung disease, MSCs and Del-1 may provide a platform
for designing novel attractive therapeutic modalities to reduce
tissue injury in lung injury disease.
Acknowledgements
The authors thank all subjects enrolled in this
study. The present study was supported by grants from the Pudong
New Area health bureau Foundation of Shanghai (grant no. PWRd
2012-07 to Yun-feng Zhao) and the National Natural Science
Foundation of China (grant no. 81070056 and 81270130 to Xue-ling
Wu).
References
|
1
|
Rubenfeld GD, Caldwell E, Peabody E,
Weaver J, et al: Incidence and outcomes of acute lung injury. N
Engl J Med. 353:1685–1693. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sheu CC, Gong MN, Zhai R, et al: Clinical
characteristics and outcomes of sepsis-related vs
non-sepsis-related ARDS. Chest. 138:559–567. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hu X, Qian S, Xu F, et al: Incidence,
management and mortality of acute hypoxemic respiratory failure and
acute respiratory distress syndrome from a prospective study of
Chinese paediatric intensive care network. Acta Paediatr.
99:715–721. 2010.
|
|
4
|
Ware LB, Koyama T, Billheimer DD, et al:
Prognostic and pathogenetic value of combining clinical and
biochemical indices in patients with acute lung injury. Chest.
137:288–296. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cribbs SK, Matthay MA and Martin GS: Stem
cells in sepsis and acute lung injury. Crit Care Med. 38:2379–2385.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee JW, Gupta N, Serikov V and Matthay MA:
Potential application of mesenchymal stem cells in acute lung
injury. Expert Opin Biol Ther. 9:1259–1270. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Matthay MA, Goolaerts A, Howard JP and Lee
JW: Mesenchymal stem cells for acute lung injury: preclinical
evidence. Crit Care Med. 38(Suppl): S569–S573. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Das R, Jahr H, van Osch GJ and Farrell E:
The role of hypoxia in bone marrow-derived mesenchymal stem cells:
considerations for regenerative medicine approaches. Tissue Eng
Part B Rev. 16:159–168. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rojas M, Xu J, Woods CR, et al: Bone
marrow-derived mesenchymal stem cells in repair of the injured
lung. Am J Respir Cell Mol Biol. 33:145–152. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Krause DS, Theise ND, Collector MI, et al:
Multi-organ, multi-lineage engraftment by a single bone
marrow-derived stem cell. Cell. 105:369–377. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Krause DS: Bone marrow-derived cells and
stem cells in lung repair. Proc Am Thorac Soc. 5:323–327. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gupta N, Su X, Popov B, Lee JW, Serikov V
and Matthay MA: Intrapulmonary delivery of bone marrow-derived
mesenchymal stem cells improves survival and attenuates
endotoxin-induced acute lung injury in mice. J Immunol.
179:1855–1863. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu J, Woods CR, Mora AL, et al: Prevention
of endotoxin-induced systemic response by bone marrow-derived
mesenchymal stem cells in mice. Am J Physiol Lung Cell Mol Physiol.
293:L131–L141. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu J, Qu J, Cao L, et al: Mesenchymal stem
cell-based angiopoietin-1 gene therapy for acute lung injury
induced by lipopolysaccharide in mice. J Pathol. 214:472–481. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Phillipson M, Heit B, Colarusso P, Liu L,
Ballantyne CM and Kubes P: Intraluminal crawling of neutrophils to
emigration sites: a molecularly distinct process from adhesion in
the recruitment cascade. J Exp Med. 203:2569–2575. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schenkel AR, Mamdouh Z and Muller WA:
Locomotion of monocytes on endothelium is a critical step during
extravasation. Nat Immunol. 5:393–400. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Barreiro O, Yanez-Mo M, Serrador JM, et
al: Dynamic interaction of VCAM-1 and ICAM-1 with moesin and ezrin
in a novel endothelial docking structure for adherent leukocytes. J
Cell Biol. 157:1233–1245. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Choi EY, Chavakis E, Czabanka MA, et al:
Del-1, an endogenous leukocyte-endothelial adhesion inhibitor,
limits inflammatory cell recruitment. Science. 322:1101–1104. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gray KD, Simovic MO, Chapman WC, et al:
Endotoxin potentiates lung injury in cerulein-induced pancreatitis.
Am J Surg. 186:526–530. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Aslam M, Baveja R, Liang OD, et al: Bone
marrow stromal cells attenuate lung injury in a murine model of
neonatal chronic lung disease. Am J Respir Crit Care Med.
180:1122–1130. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ortiz LA, Gambelli F, McBride C, et al:
Mesenchymal stem cell engraftment in lung is enhanced in response
to bleomycin exposure and ameliorates its fibrotic effects. Proc
Natl Acad Sci USA. 100:8407–8411. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
van Haaften T, Byrne R, Bonnet S, et al:
Airway delivery of mesenchymal stem cells prevents arrested
alveolar growth in neonatal lung injury in rats. Am J Respir Crit
Care Med. 180:1131–1142. 2009.PubMed/NCBI
|
|
23
|
Basit A, Reutershan J, Morris MA, Solga M,
Rose CE Jr and Ley K: ICAM-1 and LFA-1 play critical roles in
LPS-induced neutrophil recruitment into the alveolar space. Am J
Physiol Lung Cell Mol Physiol. 291:L200–L207. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Choi EY: Inhibition of leukocyte adhesion
by developmental endothelial locus-1 (del-1). Immune Netw.
9:153–157. 2009. View Article : Google Scholar : PubMed/NCBI
|