Introduction
Despite advancements in tumor screening and
detection as well as development of new treatments, breast cancer
remains the leading cause of cancer-related mortality for women
worldwide (1). In solid tumors,
certain regions may become hypoxic (2); however, tumor cells overcome this
condition through increased angiogenesis, glycolysis, growth factor
expression as well as inhibition of apoptosis (3). In some cases, hypoxia can induce
resistance to radiotherapy and chemotherapy and increase metastasis
(4) due, in part, to the
downregulation of adhesion molecules (5).
Certain genes are altered in the presence of
hypoxia, including hypoxia-inducible factor 1 (HIF-1)
(4), a heterodimer consisting of
HIF-1α and HIF-1β transcription factors (6). In contrast to the constitutively
expressed nuclear HIF-1β (ARNT) (7), HIF-1α is a cytoplasmic protein that is
upregulated in response to hypoxia. In normoxia, HIF-1α is
hydroxylated via O2-dependent enzyme activity, resulting
in ubiquitin-proteasome-mediated degradation (8). Hypoxia-induced radioresistance of some
tumor cells is mediated by HIF-1 (9). Moreover, a role for HIF in
epithelial-mesenchymal transition (EMT) and prostate cancer cell
migration has been reported (10).
Tanshinone IIA (Tan IIA), a major lipophilic
component found in Salvia miltiorrhiza Bunge root extract,
has been used to treat myocardial infarction, angina pectoris,
stroke, diabetes, and sepsis (11).
In addition, Tan IIA alleviated residual tumor hypoxia and
inhibited EMT in vivo without altering HIF-1α expression
(12). Thus, the present study
examined the hypothesis that Tan IIA downregulates HIF-1α and
blocks EMT, thereby reversing hypoxia-induced chemoresistance in
breast cancer cells.
Materials and methods
Cell culture, induction of hypoxia and
Tan IIA treatment
MCF-7 cells were maintained in Dulbecco’s modified
Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS) and
1% penicillin/streptomycin at 37°C in an environment with 5%
CO2. All culture reagents were purchased from Life
Technologies (Carlsbad, CA, USA). HCC1937 cells were maintained in
RPMI-1640 medium containing 10% FBS and 1% penicillin/streptomycin
at 37°C in an environment with 5% CO2. To induce
hypoxia, cells were treated with 100 μM deferoxamine (Novartis,
Basel, Switzerland) for 24 h as previously reported (13). Cells in the Tan IIA groups received
10 μM Tan IIA (Nanjing Zelang Medical Technology Co., China).
CCK-8 assay
MCF-7 cells were maintained in MEM containing 10%
FBS and seeded onto 96-well plates at a density of 1×104
cells/well. HCC1937 cells were grown in RPMI-1640 containing 10%
FBS and seeded onto 96-well plates at a density of 5×103
cells/well. On the next day, cells were cultured in serum
containing antibiotic-free medium with 100 μM deferoxamine with and
without 10 μM Tan IIA for 48 h at 37°C after which 100 μl of CCK-8
solution (Dojindo, Kumamoto, Japan) was added per well for an
additional 3 h at 37°C. The optical density (OD) was measured at
450 nm with an MRX II microplate reader (Dynex, Chantilly, VA,
USA).
Transfection of HIF-1α and TWIST
siRNA
Scrambled, HIF-1α and TWIST siRNA were purchased
from Santa Cruz Biotechnology (Santa Cruz, CA, USA). siRNAs (100
nM) were transfected into cells in the presence of Lipofectamine
2000 transfection reagent (Invitrogen, Carlsbad, CA, USA) following
the manufacturer’s instructions. After 6–8 h, the medium was
removed and cells were maintained in normal medium for an
additional 24 h.
Western blot assay
Cells were washed with cold PBS and treated with
lysis buffer (Cell Signaling, Danvers, MA, USA) at 4°C or on ice
for 2 h. After the protein concentration was determined with BCA
kit (Thermo Fisher Scientific, Rockford, IL, USA), proteins (40 μg)
were separated by SDS-PAGE and transferred onto a PVDF membrane
(Millipore, Billerica, MA, USA). After the membranes were blocked
with 5% bovine serum albumin (BSA) in 0.1% Tween-20 (TBS/T) on ice
for 2 h, they were incubated with the following primary antibodies
(1:1,000; all from Abcam, Cambridge, MA, USA) at 4°C overnight:
HIF-1α, E-cadherin, vimentin and β-actin. The membranes were then
incubated with the appropriate secondary antibody (1:2,000; Abcam)
at room temperature for 2 h. Bands were visualized by
chemiluminescence (GE Healthcare, Piscataway, NJ, USA), and the
membranes were exposed to film (Kodak, Rochester, NY, USA).
Immunofluorescence staining
After the cells were washed with cold PBS and fixed
in 4% paraformaldehyde for 15 min, they were blocked with 5% BSA at
room temperature for 30 min. The cells were next incubated with
anti-E-cadherin or anti-vimentin antibodies (1:200; Abcam) at 4°C
overnight. After washing with PBS, cells were treated with FITC- or
CY3-conjugated secondary antibodies (1:200; Abcam) at room
temperature for 2 h. Nuclear staining was performed with DAPI
(Sigma) at room temperature for 2 min. Following washing in PBS
twice, observation was performed under an inverted fluorescence
microscope (Olympus, Tokyo, Japan).
Flow cytometry
The BU-Annexin V-FITC apoptosis detection kit
(Biouniquer Technology Co., Nanjing, China) was used to evaluate
the effects of DOX and Tan IIA on apoptosis. Cells were digested
with an EDTA-free trypsin solution (0.25%; Life Technologies), and
a single-cell suspension (1–5×106) was prepared. After
washing in PBS twice, 100 μl of Binding Buffer and 5 μl of
FITC-conjugated Annexin-V (20 μg/ml) were added and incubated at
room temperature in the dark for 30 min. Following addition of 5 μl
of propidium iodide (PI) at 50 μg/ml for 5 min in the dark, 400 μl
of Binding Buffer was added. Flow cytometry was performed
immediately (within 1 h) with a FACScan flow cytometer (BD
Biosciences, San Jose, CA, USA). In the negative control groups,
Annexin V-FITC and/or PI were not added.
5-Ethynyl-2′-deoxyuridine (EdU)
assay
MCF-7 and HCC1937 were seeded onto 96-well plates at
a density of 3×103 cells/well in their respective growth
media. The medium was replaced with the corresponding serum-free
medium to synchronize the cells. After 24 h, the serum-free medium
was replaced with growth media containing 100 μM deferoxamine to
induce hypoxia as well as 0.2 μg/ml DOX without and with 10 μM Tan
IIA for 48 h. Cell proliferation was assessed using an EdU assay
using the Click-iTEdU imaging kit (Invitrogen) according to the
manufacturer’s instructions.
Statistical analysis
Data are expressed by the mean and standard
deviation (SD). Comparisons among two independent groups were
performed by the two independent samples t-test; comparisons among
three or more independent groups were performed by the one-way
ANOVA with the Bonferroni post-hoc test. The relative cell
viabilities between various treatment groups and various DOX
dosages were evaluated by ANCOVA with one covariate of DOX dosage.
The two-tailed P-values <0.05 were considered to indicate
statistically significant differences. Statistical analyses were
performed using SPSS 15.0 (SPSS, Chicago, IL, USA).
Results
Hypoxia induces resistance to DOX, which
is reversed with Tan IIA
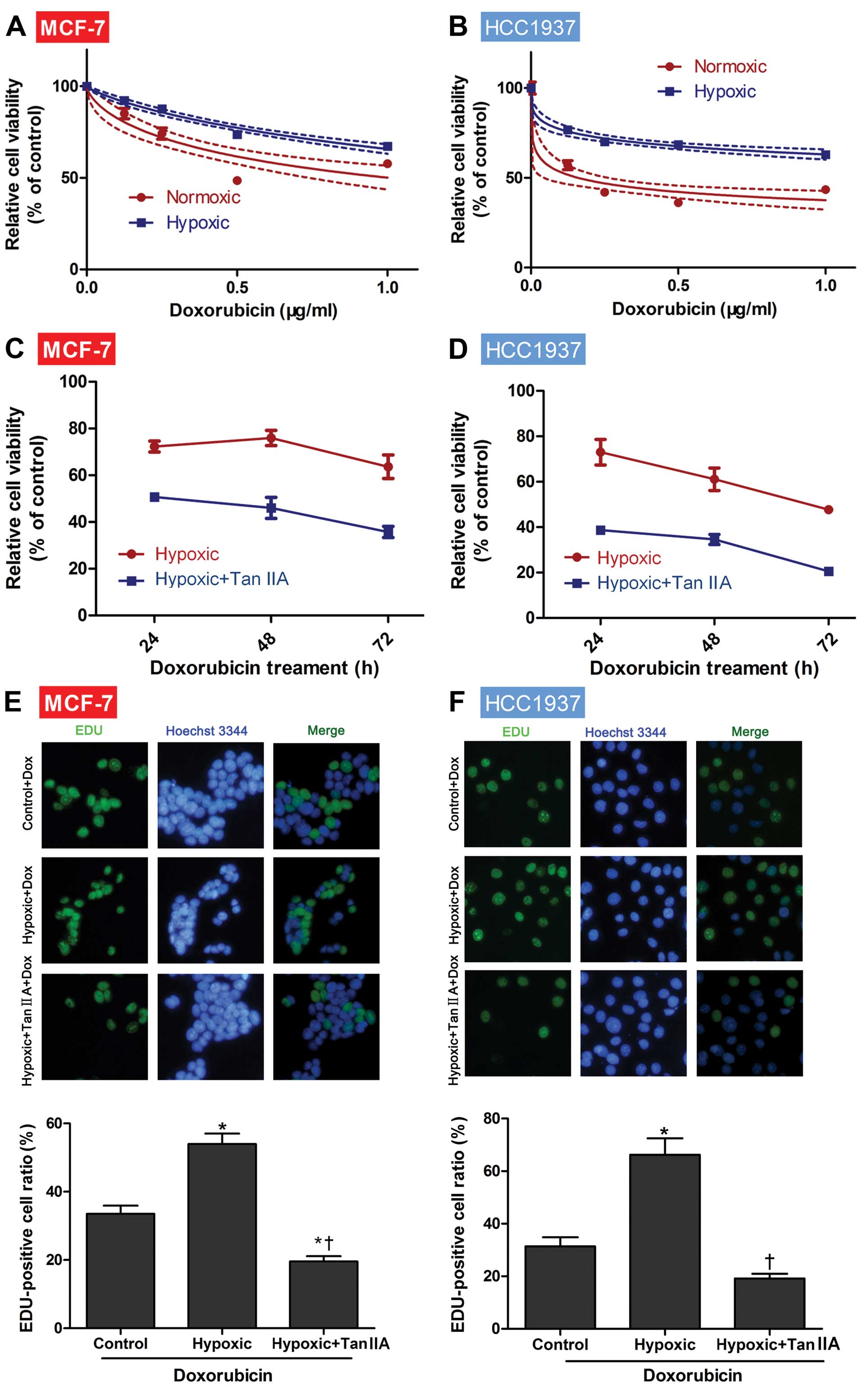
To investigate the sensitivity of MCF-7 and HCC1937
cells to DOX in normoxia and hypoxia, a CCK-8 assay was employed.
For both MCF-1 and HCC1937 cells, the tumor cell viabilities were
significantly decreased with the increasing DOX concentrations
(P<0.001; Fig. 1A and B). In the
presence of 0.25, 0.5, and 1 μg/ml a significantly higher cell
viability was observed when cells were cultured in hypoxic
conditions as compared to normoxia (P=0.001; Fig. 1A and B). Thus, we confirmed that
hypoxia induced DOX resistance in MCF-1 and HCC1937 cancer cell
lines.
To determine if Tan IIA could reverse the
hypoxia-induced DOX resistance, cells were next cultured in hypoxic
conditions without and with Tan IIA. As shown in Fig. 1C, the relative cell viability of
MCF-7 cells cultured in hypoxia and treated with DOX+Tan IIA was
significantly decreased as compared to the cells treated with DOX
for 24 h (50.7 vs. 72.3%, respectively, P<0.001), 48 h (46.1 vs.
75.9%, respectively, P<0.001), and 72 h (35.7 vs. 63.7%,
respectively, P<0.001). Similar results were also observed in
HCC1937 cells (all time points, P<0.001; Fig. 1D), suggesting that Tan IIA may
reduce the resistance to DOX induced by hypoxia.
Effects of hypoxia, DOX and Tan IIA on
cell proliferation
The effects of hypoxia and Tan IIA on cell
proliferation were next assessed in the presence of 0.2 μg/ml DOX
using the EdU assay. As shown in Fig.
1E and F, culturing either MCF-7 or HCC1937 cells in hypoxia
significantly increased the proportion of EdU-positive cells as
compared to the control (normoxia, both P=0.001). Furthermore, cell
proliferation was significantly reduced in the presence of hypoxia
with the addition of Tan IIA (both P<0.001).
Effects of hypoxia, DOX and Tan IIA on
cell apoptosis
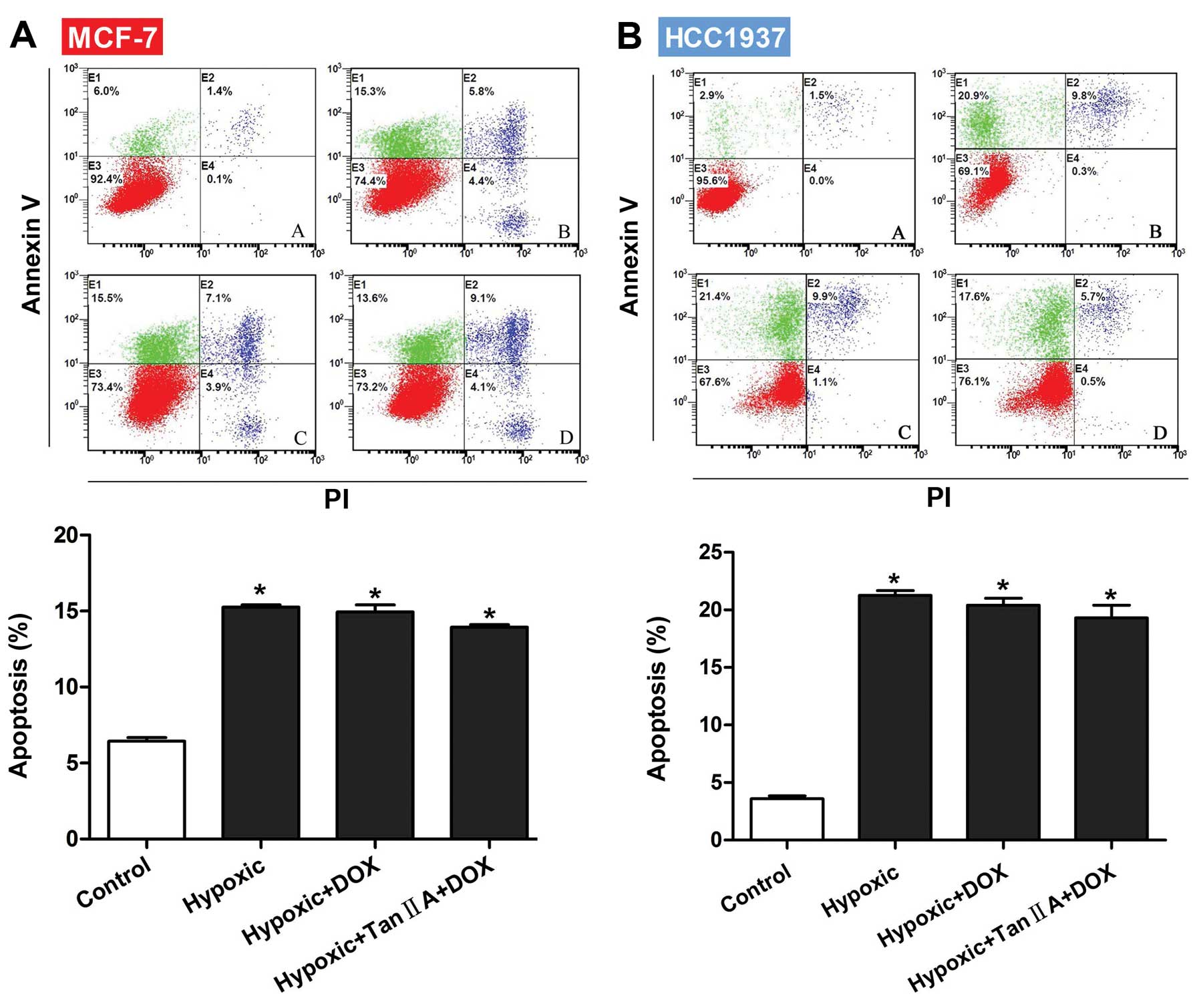
To determine if Tan IIA increased the sensitivity of
MCF-7 or HCC1937 cells to DOX by inducing apoptosis, flow cytometry
was performed to measure apoptosis rates in the presence of
hypoxia, DOX, and Tan IIA. As shown in Fig. 2A and B, the apoptosis rates of both
MCF-1 and HCC1937 cells were significantly higher than control when
cultured in the presence of hypoxia, hypoxia+DOX, or
hypoxia+DOX+Tan IIA (all P<0.001). However, no significant
differences were observed between the three groups, indicating that
the reduced viability observed with Tan IIA was not due to
apoptosis induction.
Effects of hypoxia, DOX and Tan IIA on
EMT
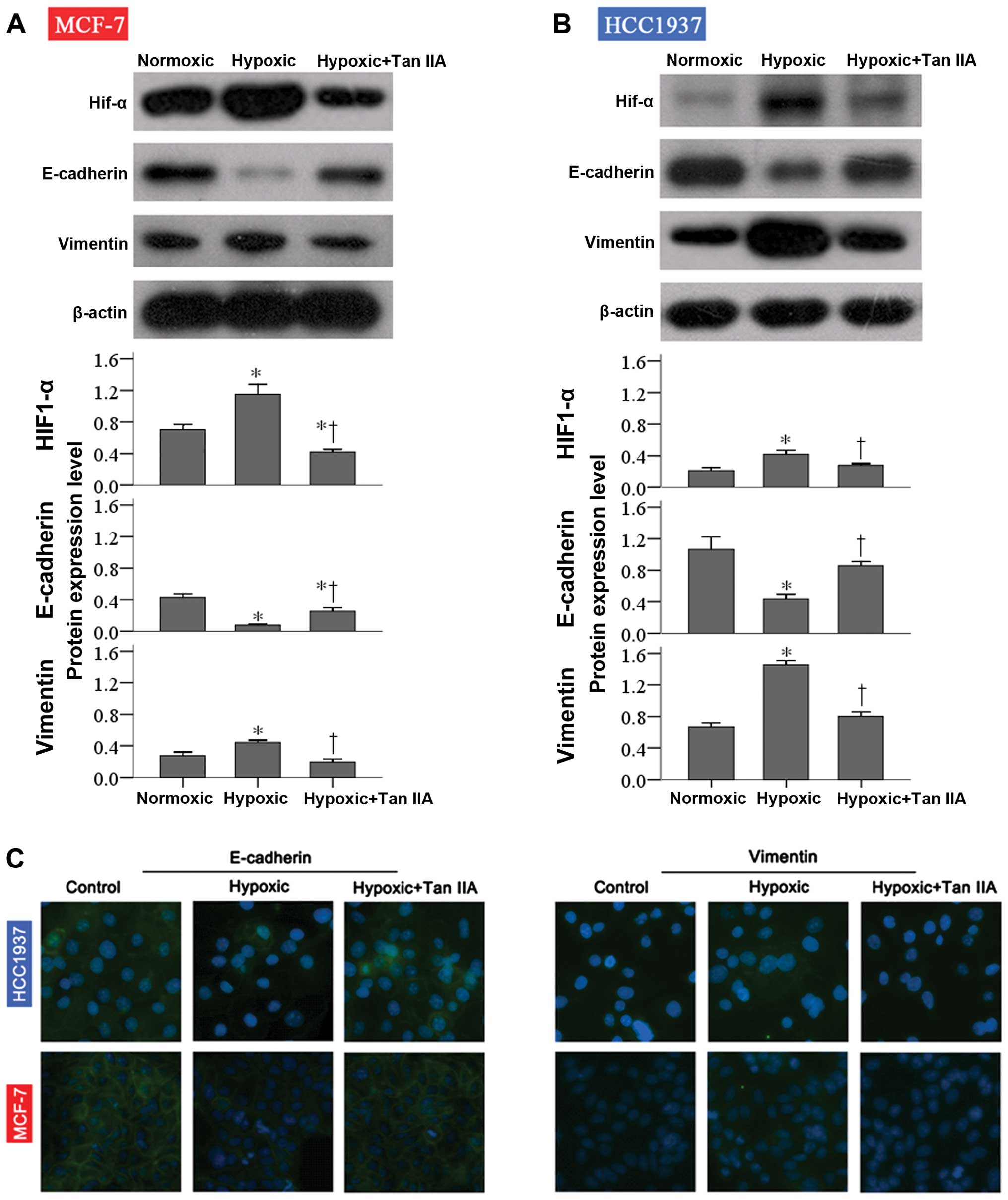
To determine if the effects of hypoxia and Tan IIA
were mediated by changes in EMT, E-cadherin and vimentin protein
expression was determined by western blot analysis. As shown in
Fig. 3A and B, E-cadherin protein
expression was significantly decreased in response to hypoxia (both
P<0.001). Treatment with Tan IIA significantly increased it, but
not to control levels in MCF-7 cells (both P=0.002). In contrast,
vimentin expression levels were significantly increased in the
hypoxia group compared to control in both cell lines (P≤0.002).
Treatment with Tan IIA ameliorated the effects of hypoxia on
vimentin expression (both P≤0.005; Fig.
3A and B). Similar results were observed with
immunofluorescence analysis (Fig.
3C).
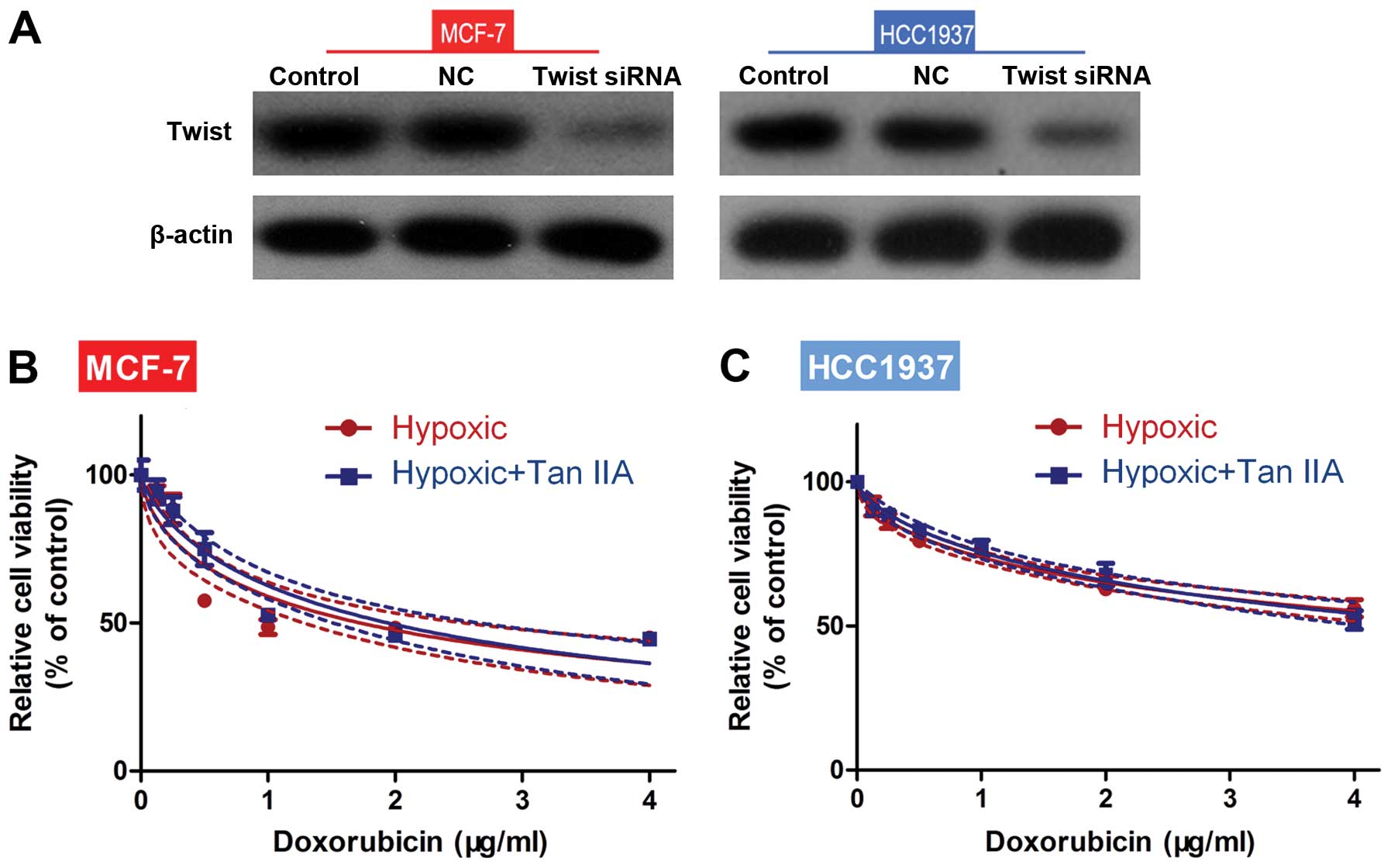
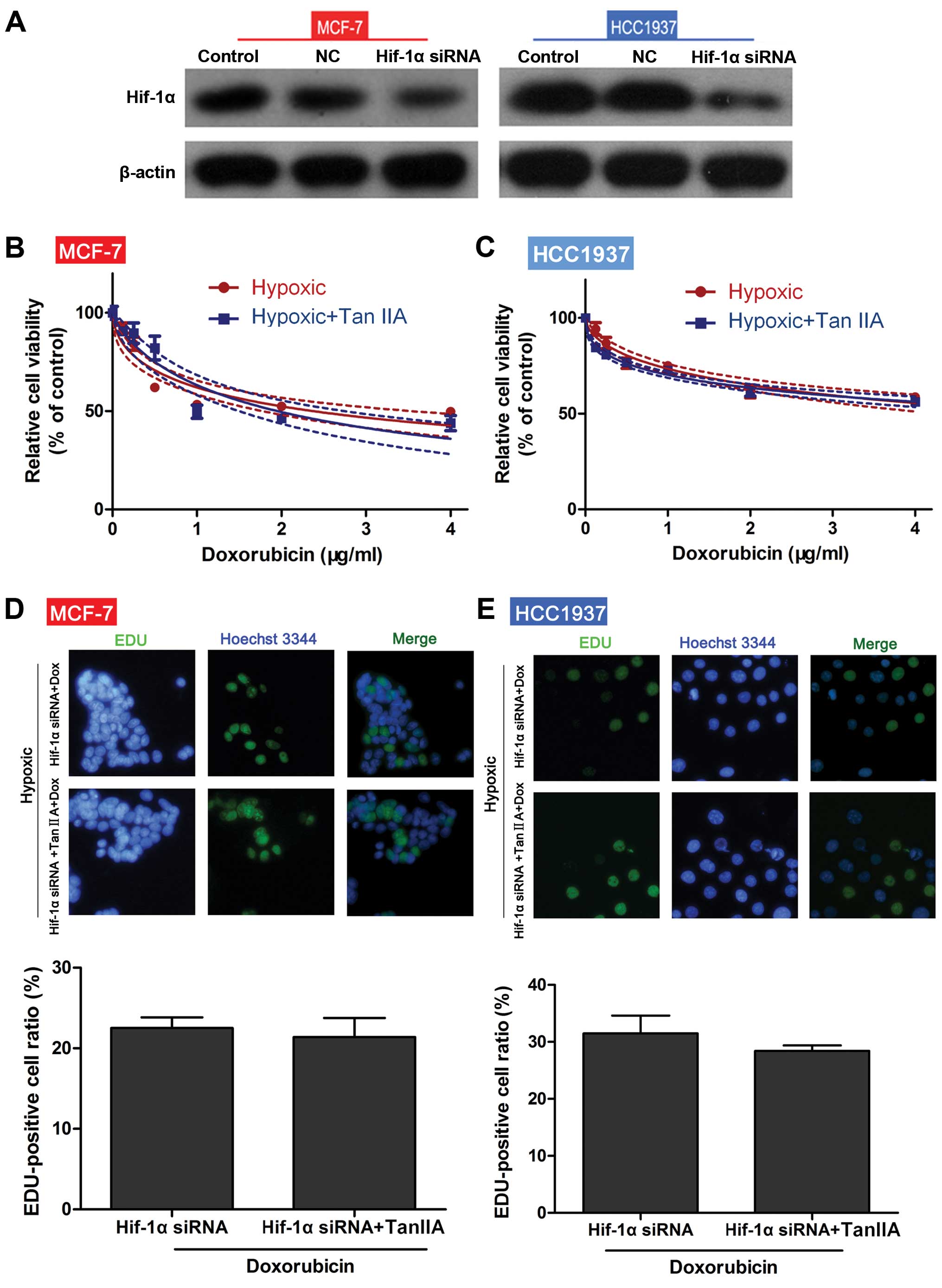
Given the importance of TWIST regulation by HIF-1α
in EMT (14), the effects of its
knockdown were next assessed in cells cultured in the presence of
hypoxia and Tan IIA. As shown in Fig.
4A, TWIST siRNA reduced TWIST protein expression in both MCF-1
and HCC1937 cells. After TWIST knockdown, no significant difference
in tumor cell viability was observed between the hypoxia and
hypoxia+Tan IIA groups in response to DOX (Fig. 4B and C). These results suggest that
Tan IIA may inhibit hypoxia-induced EMT.
Effects of Tan IIA on cell viability and
proliferation are mediated by HIF-1α expression
As shown in Fig. 3A and
B, HIF-1α expression levels were significantly increased in the
hypoxia group compared to control in both cell lines (both
P<0.001), and treatment with Tan IIA ameliorated the effects of
hypoxia on HIF-1α expression (both P≤0.005).
To determine if the effects of Tan IIA were mediated
by HIF-1α, both MCF-1 and HCC1937 cells were transfected with
HIF-1α siRNA. As shown in Fig. 5A,
transfection of both MCF-1 and HCC1937 cells with HIF-1α siRNA
reduced HIF-1α protein expression levels. In HIF-1α
siRNA-transfected cells, no significant differences in cell
viability (Fig. 5B and C) and
proliferation (Fig. 5D and E) in
response to DOX were observed between the hypoxia and hypoxia+Tan
IIA groups. These results suggest that HIF-1α mediates the
biological effects of Tan IIA.
Discussion
Considering the in vitro and in vivo
growth inhibitory effects of Tan IIA on leukemia cells (15), prostate cancer cells (16), colon cancer cells (17), pancreatic cancer cells (18), hepatocellular carcinoma (19), gastric cancer cells (20), cervical cancer cells (21), and breast cancer cells (22), the effects of Tan IIA on
hypoxia-induced DOX resistance were analyzed in two breast cancer
cell lines. Tan IIA increased the sensitivity of both MCF-1 and
HCC1937 cells cultured in hypoxia to DOX in part through HIF-1α.
Tan IIA also reduced the expression of EMT markers, suggesting that
it may play a role in reducing metastasis.
In the present study, Tan IIA reduced MCF-1 and
HCC1937 cell viability and proliferation, suggesting that Tan IIA
targets the cell cycle. Cell cycle arrest at the G0/G1 phase in
response to Tan IIA has previously been reported in LNCaP prostate
cancer cells (16) through p53
activation (23). Similar cell
cycle arrest was observed in pancreatic (18), gastric (20) and cervical (21) cancer cells. However, Chiu et
al (16) reported that these
effects were mediated through endoplasmic reticulum (ER) stress.
Further studies will assess the effects of Tan IIA on the cell
cycle progression of both MCF-1 and HCC1937 cells.
Induction of apoptosis by Tan IIA in human leukemia
cell lines through caspase-3 activation, downregulation of bcl-2
and bcl-xl and upregulation of bax has been reported (15). Similar results were reported for
H146 small cell lung cancer cells (24), hepatocellular carcinoma (19), chronic myeloid leukemia cells
(25) as well as BxPC-3 pancreatic
cancer cells (18). However, no
changes in apoptosis were observed in the present study. These
differences may be due to cell-type specific effects of Tan IIA.
Alternatively, Tan IIA may only induce apoptosis in normoxic
conditions.
In the present study, Tan IIA increased the
sensitivity of breast cancer cell lines to DOX, which is similar to
that reported for SGC7901 gastric cancer cells in response to
adriamycin and 5-fluorouracil (20). The mechanisms of cell growth
inhibition by Tan IIA were also explored in vitro. The
increased chemosensitivity observed with Tan IIA in hypoxia was in
part mediated through HIF-1α. This is consistent with Xu et
al (26) who reported that Tan
IIA reduced LPS-induced sepsis syndrome through targeting HIF-1α.
However, in hepatocellular carcinoma cells, HIF-1α levels were not
altered with Tan IIA in hypoxic conditions in vitro
(12).
Differences in oxygen levels, presence of immune
cells, growth factor expression as well as EMT between the center
and periphery of solid tumors may result in resistance of some of
these tumors to chemotherapies (27). Given the role of EMT in cancer
progression and metastasis, the effects of Tan IIA on EMT marker
expression were assessed. Hypoxia altered the expression of
E-cadherin and vimentin EMT markers, which returned to control
levels with Tan IIA in vitro. These results suggest that Tan
IIA may inhibit metastasis (28)
possibly through inhibition of HIF-1α/TWIST-induced EMT (14). These results are partially
consistent with Wang et al (12) who reported reduced EMT with Tan IIA
in an in vivo model of hepatocellular carcinoma. However,
similar results were not observed in hypoxic conditions in
vitro (12). These
inconsistencies may be due to differences in establishing the
hypoxic conditions; they may also indicate cell-type differences in
response to Tan IIA.
The present study is limited in that the pathway
mediating changes in HIF-1α expression in response to Tan IIA
treatment was not investigated. Tan IIA activated the c-Jun
N-terminal protein kinase (JNK) pathway in chronic myeloid leukemia
cells (25) as well as the
IL-6/STAT3/NF-κB signaling pathways in breast cancer cells
(22); therefore, these pathways
will be assessed in breast cancer cells in future studies. In
addition, although the effect of Tan IIA on EMT markers is
suggestive of inhibition of migration, further analyses will
specifically assess the effects of Tan IIA on cell migration in
vitro and metastasis in vivo. Furthermore, the results
were not confirmed using in vivo studies, which will be
undertaken in further analyses.
In conclusion, Tan IIA ameliorated hypoxia-induced
chemotherapy resistance to DOX and EMT in breast cancer cell lines,
which may be attributed to the downregulation of HIF-1α expression.
Further in vivo studies are required to fully elucidate the
therapeutic potential of Tan IIA in increasing the sensitivity of
breast cancer cells to chemotherapy.
Acknowledgements
The authors thank Zheng Xiaoxiao and Cai Ying for
their assistance with the experimental techniques and data
processing. This study was supported by a grant from the
Traditional Chinese Medicine Research Foundation of Zhejiang
Province (2013ZA076).
References
|
1
|
Redig A and McAllister S: Breast cancer as
a systemic disease: a view of metastasis. J of Intern Med.
274:113–126. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brown JM: Tumor hypoxia in cancer therapy.
Methods Enzymol. 435:297–321. 2007.PubMed/NCBI
|
|
3
|
Ryan HE, Poloni M, McNulty W, et al:
Hypoxia-inducible factor-1alpha is a positive factor in solid tumor
growth. Cancer Res. 60:4010–4015. 2000.PubMed/NCBI
|
|
4
|
Vaupel P: The role of hypoxia-induced
factors in tumor progression. Oncologist. 9(Suppl 5): 10–17. 2004.
View Article : Google Scholar
|
|
5
|
Czekay RP, Aertgeerts K, Curriden SA and
Loskutoff DJ: Plasminogen activator inhibitor-1 detaches cells from
extracellular matrices by inactivating integrins. J Cell Biol.
160:781–791. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Giordano FJ and Johnson RS: Angiogenesis:
the role of the microenvironment in flipping the switch. Curr Opin
Genet Dev. 11:35–40. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Maxwell PH, Pugh CW and Ratcliffe PJ:
Activation of the HIF pathway in cancer. Curr Opin Genet Dev.
11:293–299. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Semenza GL: Hypoxia-inducible factor 1:
control of oxygen homeostasis in health and disease. Pediatr Res.
49:614–617. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Harada H: How can we overcome tumor
hypoxia in radiation therapy? J Radiat Res. 52:545–556. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Luo Y, Lan L, Jiang YG, et al:
Epithelial-mesenchymal transition and migration of prostate cancer
stem cells is driven by cancer-associated fibroblasts in an
HIF-1α/β-catenin-dependent pathway. Mol Cells. 36:138–144.
2013.PubMed/NCBI
|
|
11
|
Shang Q, Xu H and Huang L: Tanshinone IIA:
A promising natural cardioprotective agent. Evid Based Complement
Alternat Med. 2012:7164592012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang WQ, Liu L, Sun HC, et al: Tanshinone
IIA inhibits metastasis after palliative resection of
hepatocellular carcinoma and prolongs survival in part via vascular
normalization. J Hematol Oncol. 5:692012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen Z, Zhang D, Yue F, Zheng M, Kovacevic
Z and Richardson DR: The iron chelators Dp44mT and DFO inhibit
TGF-β-induced epithelial-mesenchymal transition via up-regulation
of N-Myc downstream-regulated gene 1 (NDRG1). J Biol Chem.
287:17016–17028. 2012.PubMed/NCBI
|
|
14
|
Yang MH, Wu MZ, Chiou SH, et al: Direct
regulation of TWIST by HIF-1alpha promotes metastasis. Nat Cell
Biol. 10:295–305. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu JJ, Lin DJ, Liu PQ, Huang M, Li XD and
Huang RW: Induction of apoptosis and inhibition of cell adhesive
and invasive effects by tanshinone IIA in acute promyelocytic
leukemia cells in vitro. J Biomed Sci. 13:813–823. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chiu SC, Huang SY, Chen SP, Su CC, Chiu TL
and Pang CY: Tanshinone IIA inhibits human prostate cancer cells
growth by induction of endoplasmic reticulum stress in vitro and in
vivo. Prostate Cancer Prostatic Dis. 16:315–322. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Su CC, Chen GW and Lin JG: Growth
inhibition and apoptosis induction by tanshinone I in human colon
cancer Colo 205 cells. Int J Mol Med. 22:613–618. 2008.PubMed/NCBI
|
|
18
|
Huang CY, Chiu TL, Kuo SJ, Chien SY, Chen
DR and Su CC: Tanshinone IIA inhibits the growth of pancreatic
cancer BxPC-3 cells by decreasing protein expression of TCTP, MCL-1
and Bcl-xL. Mol Med Rep. 7:1045–1049. 2013.PubMed/NCBI
|
|
19
|
Yuan SL, Wei YQ, Wang XJ, Xiao F, Li SF
and Zhang J: Growth inhibition and apoptosis induction of
tanshinone II-A on human hepatocellular carcinoma cells. World J
Gastroenterol. 10:2024–2028. 2004.PubMed/NCBI
|
|
20
|
Xu M, Cao FL, Li NY, Liu YQ, Li YP and Lv
CL: Tanshinone IIA reverses the malignant phenotype of SGC7901
gastric cancer cells. Asian Pac J Cancer Prev. 14:173–177. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pan TL, Hung YC, Wang PW, et al:
Functional proteomic and structural insights into molecular targets
related to the growth inhibitory effect of tanshinone IIA on HeLa
cells. Proteomics. 10:914–929. 2010.PubMed/NCBI
|
|
22
|
Lin C, Wang L, Wang H, Yang L, Guo H and
Wang X: Tanshinone IIA inhibits breast cancer stem cells growth in
vitro and in vivo through attenuation of IL-6/STAT3/NF-κB signaling
pathways. J Cell Biochem. 114:2061–2070. 2013.PubMed/NCBI
|
|
23
|
Won SH, Lee HJ, Jeong SJ, Lü J and Kim SH:
Activation of p53 signaling and inhibition of androgen receptor
mediate tanshinone IIA induced G1 arrest in LNCaP prostate cancer
cells. Phytother Res. 26:669–674. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cheng CY and Su CC: Tanshinone IIA may
inhibit the growth of small cell lung cancer H146 cells by
up-regulating the Bax/Bcl-2 ratio and decreasing mitochondrial
membrane potential. Mol Med Rep. 3:645–650. 2010.PubMed/NCBI
|
|
25
|
Yun SM, Jeong SJ, Kim JH, et al:
Activation of c-Jun N-terminal kinase mediates tanshinone
IIA-induced apoptosis in KBM-5 chronic myeloid leukemia cells. Biol
Pharm Bull. 36:208–214. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu M, Cao F, Liu L, et al: Tanshinone
IIA-induced attenuation of lung injury in endotoxemic mice is
associated with reduction of hypoxia-inducible factor 1α
expression. Am J Respir Cell Mol Biol. 45:1028–1035.
2011.PubMed/NCBI
|
|
27
|
Nguyen L, Fifis T, Malcontenti-Wilson C,
et al: Spatial morphological and molecular differences within solid
tumors may contribute to the failure of vascular disruptive agent
treatments. BMC Cancer. 12:5222012. View Article : Google Scholar
|
|
28
|
Hu Y, Wang S, Wu X, et al: Chinese herbal
medicine-derived compounds for cancer therapy: a focus on
hepatocellular carcinoma. J Ethnopharmacol. 149:601–612. 2013.
View Article : Google Scholar : PubMed/NCBI
|