Introduction
Glioblastoma is one of the most aggressive human
tumors with a median survival time of 15 months. Despite
considerable advances in cancer treatment in the past decades,
glioblastoma remains a highly aggressive disease. Current standard
treatment includes a tripartite therapy of surgical resection
followed by radiation therapy combined with concurrent
administration of the adjuvant DNA alkylating agent temozolomide
(TMZ), which represents the most important chemotherapeutic option
for glioblastoma treatment (1–3). Novel
chemotherapeutic approaches utilizing loco-regional delivery or
concurrent chemoradiotherapy have been shown to improve the
survival of glioma patients. For instance, the combination of TMZ
and radiotherapy has conferred a significant survival advantage
compared with radiotherapy alone. In particular, survival benefits
of TMZ have been observed in treatments for high-grade gliomas
(4,5). Nevertheless, these treatment
modalities provide only limited survival benefits, largely due to
the frequent development of therapeutic resistance (6,7).
Increasing efforts have been made to understand the
molecular basis underlying the occurrence of TMZ-resistance in
glioblastoma and to identify combinatorial options to improve the
therapeutic efficacy of TMZ. Although the mechanism for resistance
development remains unclear, multiple combined modality treatments
have been suggested to sensitize TMZ. For example, glioblastoma
harboring epidermal growth factor receptor (EGFR) amplification or
mutation, EGFR vIII in particular, has been found to be more
sensitive to TMZ in combination with cetuximab. In addition, a
signal transducer and activator of transcription 3 (STAT3)
inhibitor can overcome TMZ resistance in glioblastoma by
downregulating methylguanine methyltransferase (MGMT) expression
(8). It has also been found that a
glutathione synthesis inhibitor potentiates the impact of TMZ in
glioblastoma (9). Regardless of
these advances, an adjuvant therapy for TMZ treatment that benefits
a broad range of glioblastoma patients is still needed.
Curcumin (CUM) is a bioactive food component that
exhibits a broad spectrum of pharmacological activities including
anti-inflammatory, antioxidative and anticancer properties
(10,11). While the antioxidative effect of CUM
has been well-documented, its anticancer activity has been
increasingly studied. CUM is believed to exert anticancer activity
in various cancer types by affecting cell proliferation,
angiogenesis, invasion, and metastasis via intervention of multiple
signaling molecules such as NF-κB, PPARγ, AMPK and AKT (12–16).
Additionally, CUM has also been reported to overcome
anti-multidrug-resistance activity (17). Despite its various activities, the
molecular basis behind the pharmacological activities of CUM
remains largely unknown.
Two previous studies reported that CUM is able to
sensitize TMZ in glioblastoma (18,19),
suggesting that CUM exhibits the potential to overcome resistance
in glioblastoma. In the present study, we validated this finding
both in vitro and in vivo and we also investigated
the underlying molecular mechanisms of the sensitization of TMZ
treatment by CUM. We showed that reactive oxygen species (ROS)
generation and AKT-mTOR signaling may be involved in the
sensitization of TMZ by CUM in glioblastoma.
Materials and methods
Cell culture and reagents
Human glioblastoma U87MG cells were obtained from
KeyGen Biotech (Nanjing, China). Cells were grown in RPMI-1640
medium supplemented with 10% fetal bovine serum (both from Gibco,
Grand Island, NY, USA) and 1% penicillin/streptomycin at 37°C in a
humidified atmosphere with 5% CO2. TMZ was provided by
Schering-Plough (Madison, NJ, USA). PI3K inhibitor LY294002
(purity, 99%) was obtained from Gibco. Antibodies to mouse PI3K,
AKT, phospho-AKT (Thr308), phospho-mTOR (Ser2448), Bad, phospho-Bad
(Ser112) and β-actin were purchased from Cell Signaling (Danvers,
MA, USA).
Cell viability assay
Cell viability was measured by a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
colorimetric assay (Millipore, Billerica, MA, USA) according to the
manufacturer’s instructions. In brief, 5×103 cells/well
were plated in 96-well tissue culture plates and grown for 24 h.
Cells were then treated with TMZ, CUM alone or their combination at
various concentrations. After incubation at 37°C for 72 h, 10 μl of
MTT [5 mg/ml dissolved in phosphate-buffered saline (PBS)] was
added to each well, and the plates were incubated at 37°C for
another 4 h. Then, 100 μl of isopropanol, 0.1 ml of 0.4 N HCl, and
50 μl of dimethyl sulfoxide were added to solubilize the formazan
crystals at room temperature. Within 1 h, the absorbance was
measured on a plate reader at a test wavelength of 490 nm. The
growth inhibitory rate was determined using the following formula:
(Inhibition rate, %) = (1 −
Atreated/Acontrol) × 100%. The inhibition
rate was plotted and the concentration that caused 50% inhibition
(IC50) was determined.
ROS detection
Accumulation of intracellular ROS was detected with
2′-7′-dichlorodihydrofluorescein diacetate (DCFH-DA), which crosses
cell membranes and is hydrolyzed by intracellular non-specific
esterases to non-fluorescent DCFH. In the presence of ROS, DCFH is
oxidized to highly fluorescent DCF, which is readily detected by
flow cytometry. Cells were seeded into 6-well plates at a density
of 5×105 cells/well, cultured overnight, and then
incubated with different concentrations of CUM and/or TMZ at 37°C
for the indicated times. Before harvesting, the cells were
incubated with DCFH-DA at a final concentration of 10 μM for 20
min. Cells were washed with ice-cold PBS and collected for
immediate detection using flow cytometry with excitation at 188 nm
and emission at 525 nm.
Animal studies
Three- to five-week old nu/nu athymic BALB/c mice
were purchased from the Shanghai SLAC Laboratory Animal Facility
(Shanghai, China). All studies were carried out in compliance with
the Institutional Animal Care and Use Committee Guidelines of
Nanjing Medical University. Tumors were generated by transplanting
1×106 U87MG cells resuspended in PBS (100 μl/mouse) into
the right flank. When the tumor volume reached 50–75
mm3, the mice were randomized into 4 groups (6
mice/group): control, CUM-treated group (CUM), TMZ-treated group
(TMZ) and CUM plus TMZ-treated group (CUM+TMZ). Mice in the control
group were administered vehicle alone, and mice in the treatment
groups were administered CUM and/or TMZ at the indicated
concentration once daily intraperitoneally (i.p.) for 14 days.
Tumor volumes and body weights of the mice were monitored every
other day. The sizes of the tumors were measured using
microcalipers. The tumor volume (V) was calculated as follows: V =
(length × width2)/2. The individual relative tumor
volume (RTV) was calculated as follows: RTV =
Vt/V0, where Vt is the volume on
each day and V0 is the volume at the beginning of the
treatment. The therapeutic effect of the compounds was expressed as
the volume ratio of treatment to control (T/C; ref. 31): T/C (%) =
(mean RTV of the treated group/mean RTV of the control group) ×
100%. On the last day of the experiment, mice were sacrificed and
tumor tissues were resected and homogenized in cold RIPA lysis
buffer supplemented with protease and phosphatase inhibitors and
then processed for immunoblotting.
Apoptosis assay
Cells were treated with TMZ, CUM alone or their
combination at various concentrations. After treatment, apoptotic
cells were detected by co-staining with Annexin V and propidium
iodide (PI), followed by flow cytometry analysis. Cells positive
for Annexin V staining are considered to undergo apoptosis.
Intratumoral apoptosis was examined by a dUTP nick-end labeling
(TUNEL) assay with in situ fluorescein in the sections
derived from xenograft tumor tissues using the commercially
available fluorescence and colorimetric TUNEL apoptosis assay kit
(KeyGen Biotech) following the manufacturer’s instructions.
Western blot analysis
Cells or tumor tissues after the indicated treatment
were collected and lysed. The supernatant was collected after
centrifugation and cell lysates were matched for protein
concentration using the Bradford assay. Samples were loaded on
SDS-PAGE, transferred to nitrocellulose membranes, and blocked in
5% non-fat milk overnight, followed by an overnight incubation at
4°C with primary polyclonal antibodies to mouse PI3K, AKT,
phospho-AKT (Thr308), phospho-mTOR (Ser2448), Bad, phospho-Bad
(Ser112) and β-actin. All antibodies were used at a dilution of
1:1,000. Blots were subsequently washed three times with
Tris-buffered saline Tween-20 (TBST) and then incubated with the
appropriate horseradish peroxidase-conjugated secondary antibodies
for 1 h at room temperature. After three additional TBST washes,
the immunoreactive bands were visualized by enhanced
chemiluminescence (Amersham Biosciences, Buckinghamshire, UK)
according to the manufacturer’s instructions. The levels of β-actin
were estimated to check for equal sample loading. Films were
scanned and band densities were quantified with densitometric
analysis using Scion Image (Epson GT-X700, Tokyo, Japan).
Statistical analysis
All statistical analyses were performed using the
Statistical Product and Service Solutions software, version 17.0
(SPSS, Chicago, IL, USA). Data are expressed as means ± standard
deviation (SD). The independent samples were compared using the
Student’s t-test. A two-sided P-value of <0.05 was considered to
indicate a statistically significant difference.
Results
CUM sensitizes glioblastoma to TMZ
treatment
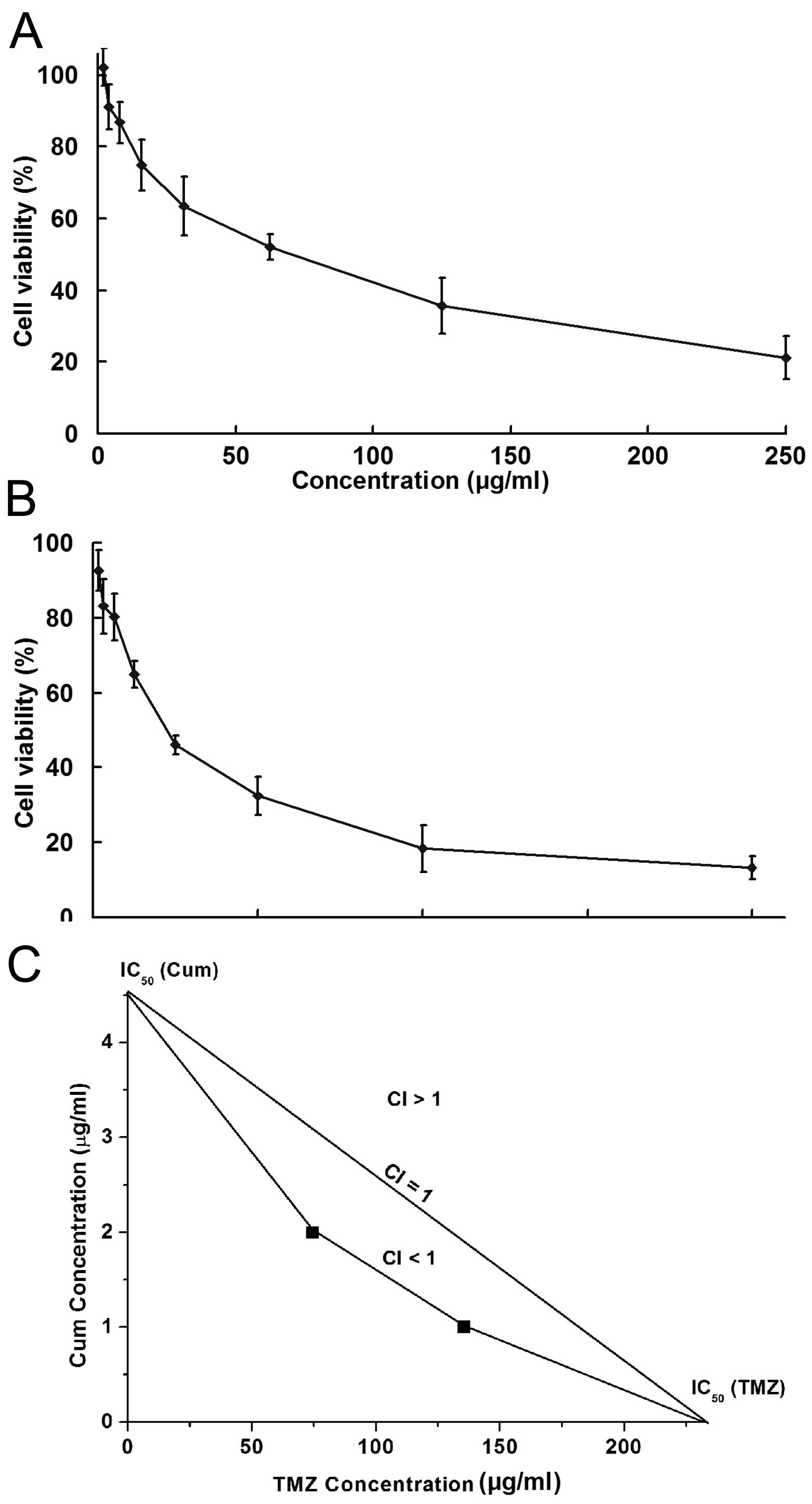
Consistent with previous studies, U87MG cells barely
responded to TMZ treatment with an IC50 value of ~390
μg/ml (Fig. 1A). A concentration of
15.63 μg/ml, which caused 25.11% inhibition, was used in the
following studies to test the synergistic effect of TMZ in
combination with CUM. Similarly, a subtoxic dose (1.25 μg/ml) of
CUM was used, which showed an inhibition rate against cell growth
of 19.75% (Fig. 1B). Combination
therapy with CUM and TMZ significantly enhanced the effect of TMZ
in U87MG cells. A synergistic antitumor effect was clearly observed
between CUM and TMZ through the analysis of their IC50
values (Fig. 1C).
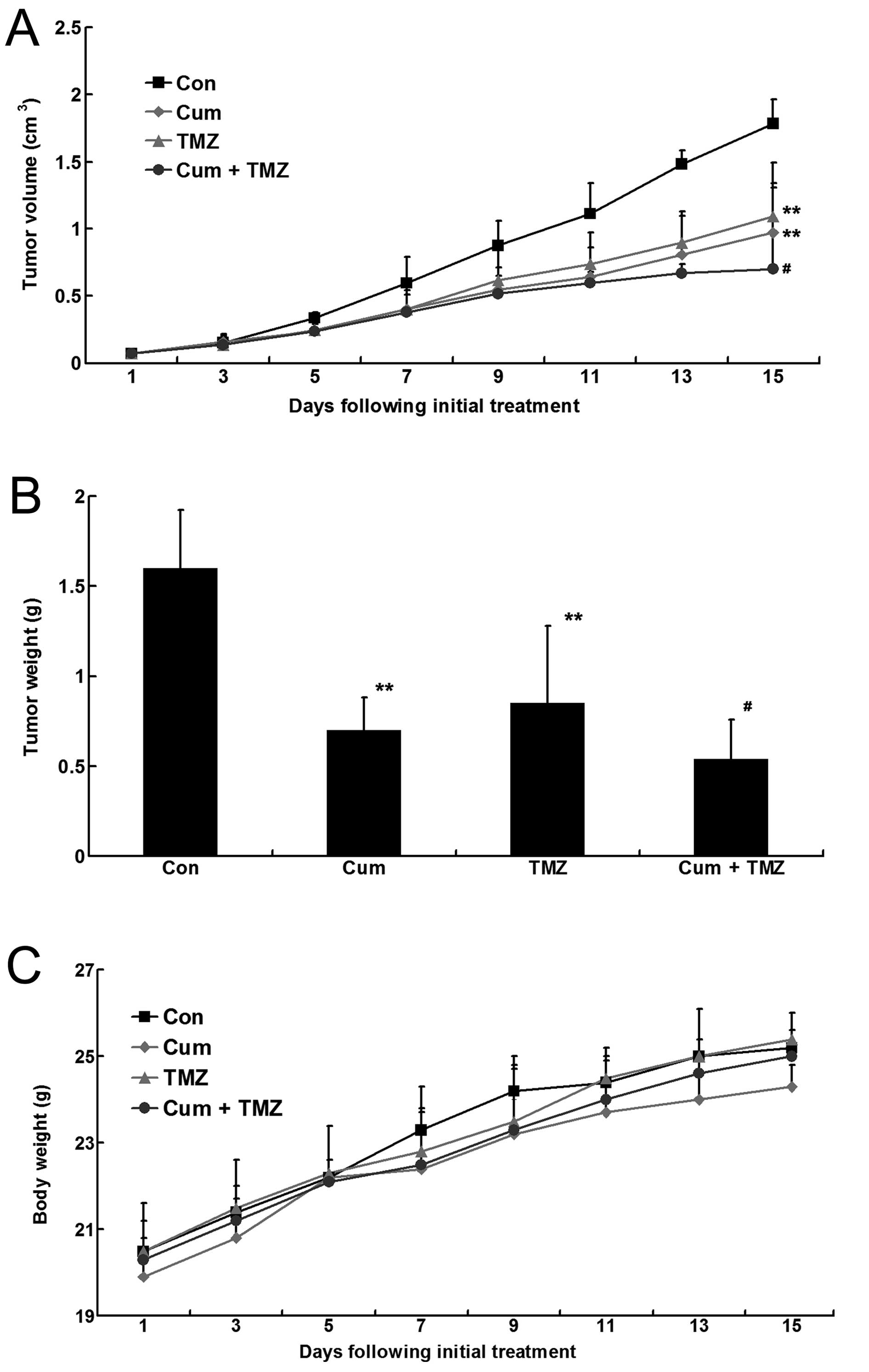
In the U87MG xenograft mouse model, CUM and TMZ
alone significantly decreased the tumor volume, as well as tumor
weight, compared to that in the untreated control group
(P<0.05). The combination treatment with CUM and TMZ showed a
significantly enhanced effect at inhibiting tumor growth compared
with the single treatment (Fig.
2A). A similar trend was observed in the measurement of tumor
weight at the end of the experiment (Fig. 2B). However, the mouse body weights
remained constant and very similar among the three groups (Fig. 2C).
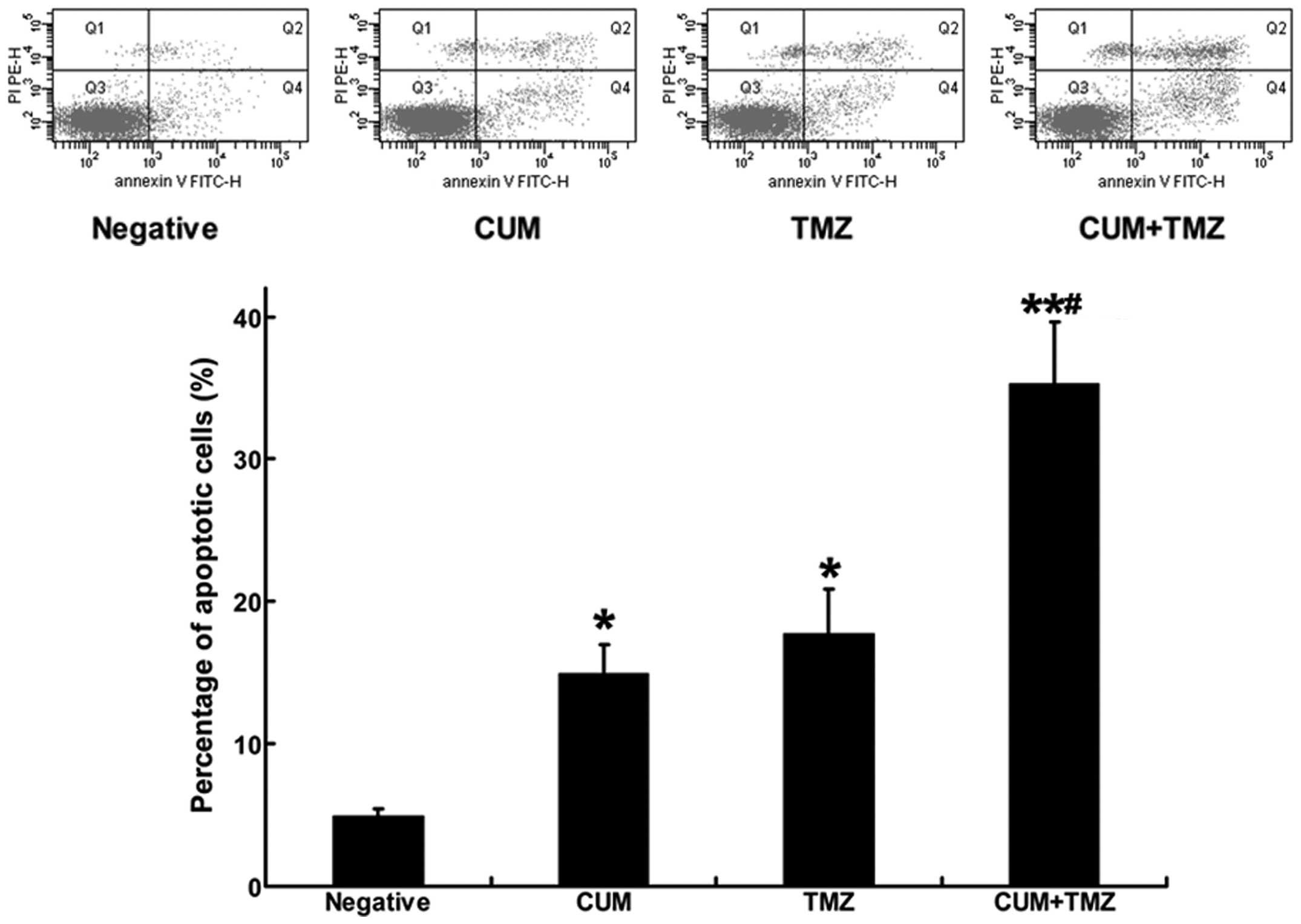
CUM enhances TMZ-induced apoptosis in
glioblastoma
TMZ, as a DNA alkylating agent, has been shown to
cause cell growth inhibition primarily via inducing apoptosis in
tumor cells (20). We tested
whether the increased apoptosis induction mainly accounted for the
CUM-enhanced therapeutic efficacy of TMZ. As shown in the upper
(early apoptosis) and lower (late apoptosis) right quadrants of
Fig. 3, the combination treatment
with CUM and TMZ significantly increased the ratio of apoptotic
cells compared with either CUM or TMZ treatment alone, suggesting
that CUM potentiated the effects of TMZ by sensitizing cells to
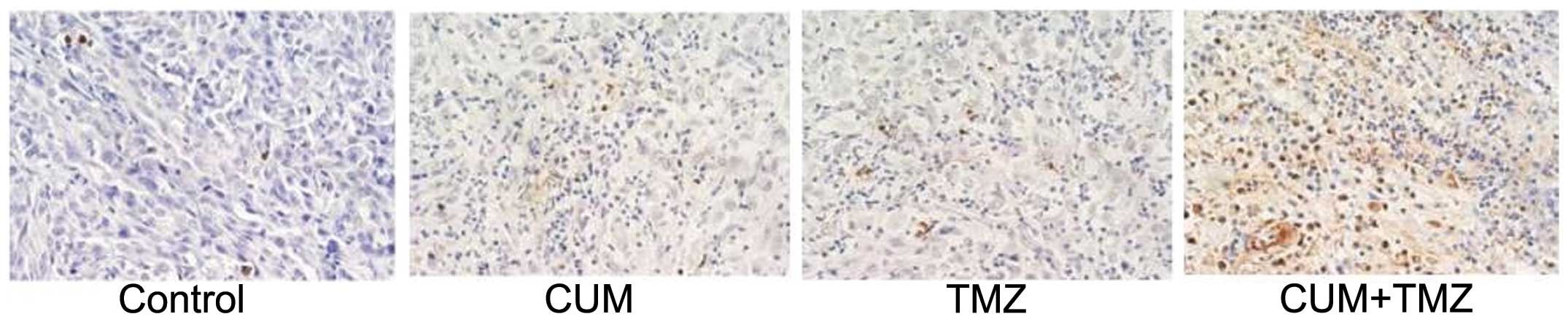
apoptosis induction. In vivo, intratumoral apoptosis was
assessed using a TUNEL assay on the sections derived from xenograft
tumor tissues. Apoptotic cells showed brown staining (Fig. 4). Consistent with the results from
the in vitro experiment using U87MG cells, intratumoral
apoptotic cells were significantly increased in tumor issues from
mice treated with the combination of CUM and TMZ compared to TMZ or
CUM alone.
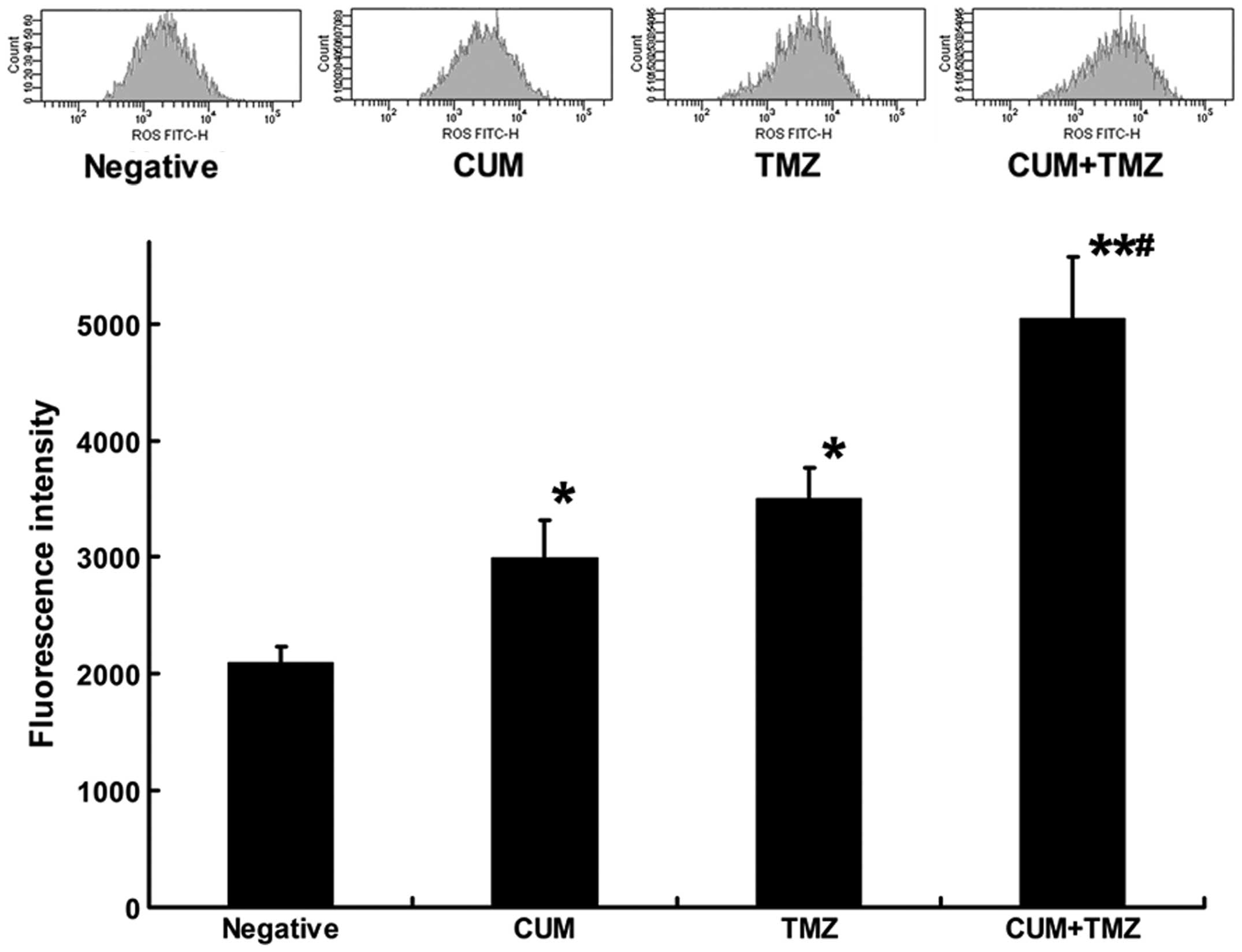
CUM and TMZ cause a synergistic effect on
ROS generation
Previous studies suggested that TMZ induces
apoptosis through a ROS burst (21). Moreover, CUM has been reported to
stimulate ROS production during apoptosis, which is induced by
anticancer drugs (22). To test
whether there is a synergistic effect of CUM and TMZ on ROS
production, we measured the ROS amount in U87MG cells after
exposure to CUM, TMZ alone or their combination. The single
treatment with TMZ significantly generated ROS in U87MG cells,
whereas CUM alone only had a non-significant effect. However, ROS
production in U87MG cells was markedly increased after the
combination treatment with CUM and TMZ (Fig. 5), suggesting a synergistic effect of
the combination of CUM and TMZ to generate ROS in U87MG cells.
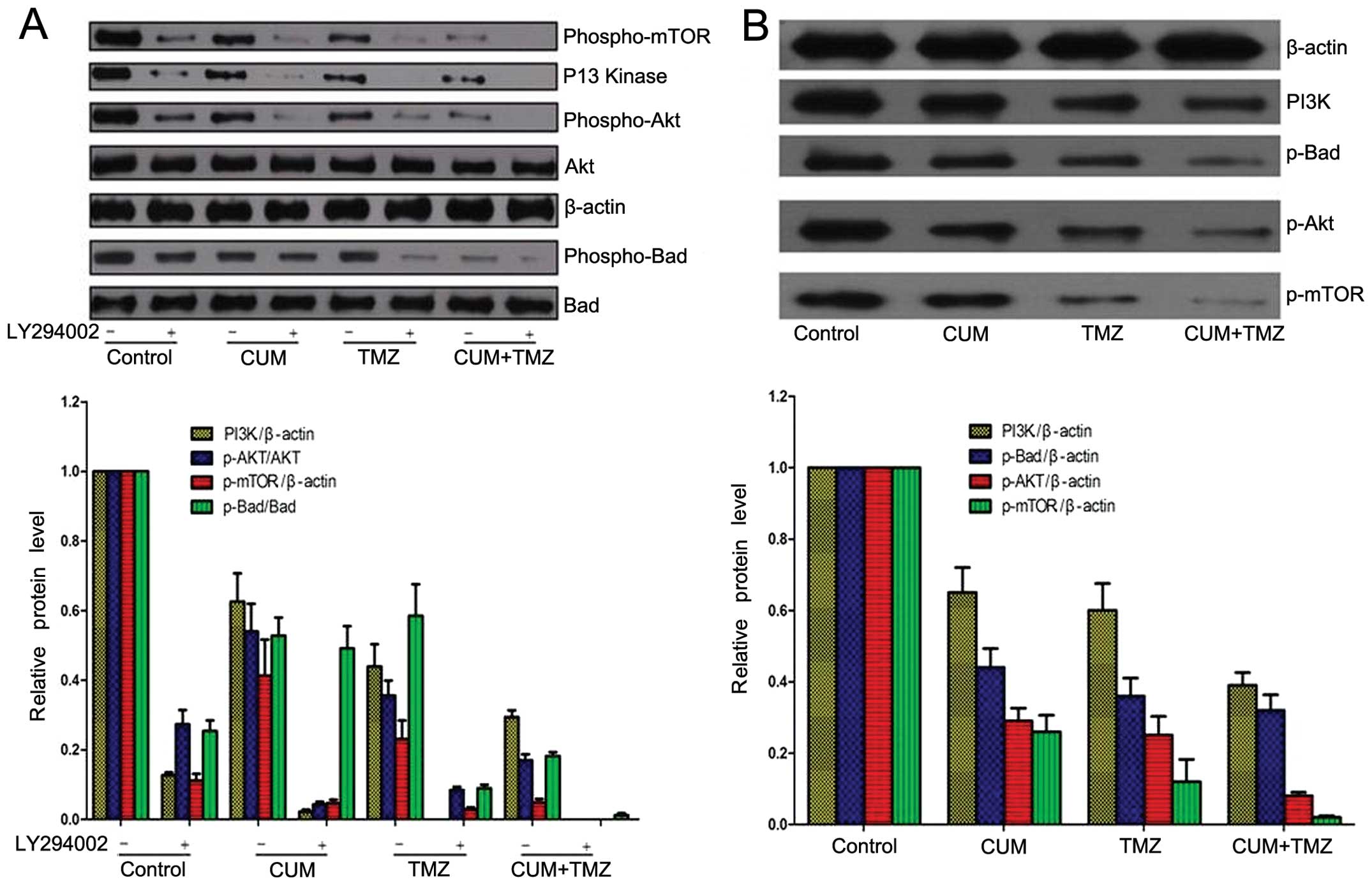
CUM enhances TMZ-disrupted AKT/mTOR
signaling
Previous studies have shown that activated AKT
signaling overrides TMZ-induced cytotoxicity, leading to a
compromised therapeutic effect of TMZ (23). Inhibition of the PI3K/AKT/mTOR
pathway enhances TMZ-induced cytotoxicity (24). In addition, CUM is known to exhibit
profound inhibitory effects on the AKT/mTOR signaling network,
which accounts for CUM-induced apoptosis in cancer cells (15,25).
We examined the AKT/mTOR signaling pathway after CUM and TMZ
treatments in U87MG cells. Both CUM and TMZ treatment alone
significantly suppressed phosphorylated AKT and mTOR, but their
combination achieved a more pronounced inhibitory effect (Fig. 6A). AKT is known to promote cell
survival via phosphorylating BAD, a pro-apoptotic member of the
Bcl-2 protein family (26). We also
observed that the combination treatment of CUM and TMZ
significantly decreased the phosphorylation of BAD, compared to the
untreated control and treatment with the agents alone (Fig. 6A). Furthermore, using the PI3K
inhibitor LY294002, we observed a similar trend of the alterations
of phosphorylation of AKT and BAD following CUM and TMZ treatment
(Fig. 6A).
These findings were also confirmed in vivo.
We observed that the combination treatment of CUM and TMZ
significantly decreased phosphorylated AKT expression in the
extracts derived from U87MG xenograft tumor tissues, compared to
the untreated control and the individual treatments (Fig. 6B). These data suggest that AKT
signaling may be implicated in the therapeutic effects of CUM and
TMZ in glioblastoma.
Discussion
In the present study, we showed that CUM is able to
sensitize glioblastoma to TMZ treatment both in vitro and
in vivo. We showed that CUM potentiated apoptosis caused by
TMZ, and ROS generation may contribute to the impact of CUM when
combined with TMZ in glioblastoma cells. We also showed that CUM
and TMZ treatment significantly suppressed phosphorylated mTOR, AKT
and BAD expression, and their combination achieved a more
pronounced inhibitory effect. These data suggest a synergistic
effect of the combination of CUM and TMZ in generating ROS and
inhibiting AKT/mTOR signaling in glioblastoma cells.
The mechanism of the development of TMZ resistance
has been widely studied. Current insights have mainly focused on
the upregulation of DNA repair capacity in human tumors, since TMZ
is an alkylating agent prodrug that delivers a methyl group to
purine bases of DNA (O6-guanine, N7-guanine and N3-adenine).
TMZ-caused DNA lesions are considered to be the key reason to
trigger apoptosis and consequently inhibit tumor growth. The
primary cytotoxic lesion, O6-methylguanine (O6-MeG), can be removed
by MGMT in tumors expressing this protein or tolerated in mismatch
repair-deficient tumors (27). Base
excision repair (BER), which removes potentially lethal N7- and
N3-purine lesions, has also been suggested to contribute
significantly to repair or toleration of TMZ treatment. Recently,
several small molecule inhibitors of poly(ADP-ribose) polymerase-1
(PARP-1), a critical BER protein, have yielded promising results in
combination with TMZ in the clinic.
In the present study, we showed that TMZ combined
with CUM significantly increased ROS production and inhibited
AKT/mTOR signaling by decreasing the phosphorylation of AKT, BAD
and mTOR in glioblastoma cells. Previous studies have shown that
activated AKT signaling overrides TMZ-induced cytotoxicity, leading
to a compromised therapeutic effect of TMZ (23). Inhibition of the PI3K/AKT/mTOR
pathway has been shown to enhance TMZ-induced cytotoxicity
(24). In addition, CUM is known to
exhibit profound inhibitory effects on the AKT/mTOR signaling
network, which accounts for CUM-induced apoptosis in cancer cells
(15,25). Our results are directly or
indirectly supported by these previous studies and provide new
insight into the mechanisms (such as the AKT/mTOR signaling
pathway) of the development of TMZ resistance.
In addition to understanding the mechanism
underlying the resistance of TMZ, considerable efforts have been
made to identify approaches to overcome this resistance. Although a
broad range of pharmacologically active agents, such as inhibitors
against PI3K and STAT3, have been suggested to exhibit potential as
sensitizers to TMZ, most of these therapeutic options, as an
adjuvant therapy to TMZ, are associated with largely enhanced
toxicity. In particular, the current standard treatment already
includes a tripartite therapy, where both TMZ and radiotherapy are
known to cause severe adverse effects in general. The inclusion of
extra therapy, particularly one with a considerable impact on vital
signaling pathways, will foreseeably be challenged by drug
intolerance in patients.
In this respect, CUM, an active ingredient of the
dietary spice turmeric, has been consumed for medicinal purposes
for a long time (28). CUM
modulates various signaling molecules, including inflammatory
molecules, transcription factors, enzymes, protein kinases, protein
reductases, adhesion molecules, growth factors, receptors,
chemokines and even DNA, RNA and metal ions. As a consequence, in
the past half century, CUM has been shown to have increasing
pharmacological properties including anti-inflammatory,
antioxidant, pro-apoptotic, chemopreventive, chemotherapeutic,
antiproliferative and wound healing, as well as antinociceptive,
antiparasitic and antimalarial properties. However, the current
mechanistic insights into the molecular basis underlying the
bioactive properties of CUM remain uncertain.
Our findings suggest the important implications of
both ROS and AKT-BAD signaling in the therapeutic effect of CUM
when combined with TMZ. We acknowledged that the involvement of
other pathways is not excluded. For example, our preliminary data
suggested that the expression level of the DNA repair gene MGMT,
which has been suggested to play an important role in the
resistance development of TMZ (29), was modulated by CUM treatment in the
xenograft mouse models. To fully understand the biological impact
of CUM, more systematic and comprehensive studies are urgently
required to elucidate the mechanism of CUM in different
scenarios.
In summary, our data indicated the important
implications of apoptosis and AKT-BAD signaling in maintaining the
survival of glioblastoma. In addition, ROS generation and the
efficient disruption of the AKT-BAD cascade may, at least
partially, explain the CUM-sensitized TMZ efficacy in glioblastoma.
Although CUM has not yet been approved for the treatment of any
human disease, it features an inexpensive, apparently
well-tolerated and potentially active adjuvant for TMZ in the
treatment of glioblastoma. Our findings provide insights into the
molecular basis and will potentially benefit its eventual
application in the treatment of glioblastoma.
Acknowledgements
This study was supported by a grant from the Nanjing
Medical Technology Development Project (no. YKK10072), and the
Jiangsu Province Health Research Fund (no. H201235).
References
|
1
|
Stupp R, Mason WP, van den Bent MJ, et al:
Radiotherapy plus concomitant and adjuvant temozolomide for
glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
DeAngelis LM: Brain tumors. N Engl J Med.
344:114–123. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Laws ER, Parney IF, Huang W, et al:
Survival following surgery and prognostic factors for recently
diagnosed malignant glioma: data from the Glioma Outcomes Project.
J Neurosurg. 99:467–473. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kapoor R, Revesz T and Powell M: Solitary
cervical lymphoma presenting as a neurofibroma. Br J Neurosurg.
6:583–586. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Perry JR, Bélanger K, Mason WP, et al:
Phase II trial of continuous dose-intense temozolomide in recurrent
malignant glioma: RESCUE study. J Clin Oncol. 28:2051–2057. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mirimanoff RO, Gorlia T, Mason W, et al:
Radiotherapy and temozolomide for newly diagnosed glioblastoma:
recursive partitioning analysis of the EORTC 26981/22981-NCIC CE3
phase III randomized trial. J Clin Oncol. 24:2563–2569. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mrugala MM and Chamberlain MC: Mechanisms
of disease: temozolomide and glioblastoma - look to the future. Nat
Clin Pract Oncol. 5:476–486. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kohsaka S, Wang L, Yachi K, et al: STAT3
inhibition overcomes temozolomide resistance in glioblastoma by
downregulating MGMT expression. Mol Cancer Ther. 11:1289–1299.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kohsaka S, Takahashi K, Wang L, et al:
Inhibition of GSH synthesis potentiates temozolomide-induced
bystander effect in glioblastoma. Cancer Lett. 331:68–75. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Joe B, Vijaykumar M and Lokesh BR:
Biological properties of curcumin-cellular and molecular mechanisms
of action. Crit Rev Food Sci Nutr. 44:97–111. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Maheshwari RK, Singh AK, Gaddipati J and
Srimal RC: Multiple biological activities of curcumin: a short
review. Life Sci. 78:2081–2087. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kunnumakkara AB, Anand P and Aggarwal BB:
Curcumin inhibits proliferation, invasion, angiogenesis and
metastasis of different cancers through interaction with multiple
cell signaling proteins. Cancer Lett. 269:199–225. 2008. View Article : Google Scholar
|
|
13
|
Das T, Sa G, Saha B and Das K: Multifocal
signal modulation therapy of cancer: ancient weapon, modern
targets. Mol Cell Biochem. 336:85–95. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Basile V, Ferrari E, Lazzari S, Belluti S,
Pignedoli F and Imbriano C: Curcumin derivatives: molecular basis
of their anticancer activity. Biochem Pharmacol. 78:1305–1315.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hussain AR, Al-Rasheed M, Manogaran PS, et
al: Curcumin induces apoptosis via inhibition of PI3′-kinase/AKT
pathway in acute T cell leukemias. Apoptosis. 11:245–254.
2006.PubMed/NCBI
|
|
16
|
Zheng S and Chen A: Activation of
PPARgamma is required for curcumin to induce apoptosis and to
inhibit the expression of extracellular matrix genes in hepatic
stellate cells in vitro. Biochem J. 384:149–157. 2004. View Article : Google Scholar
|
|
17
|
Lu JJ, Cai YJ and Ding J: The short-time
treatment with curcumin sufficiently decreases cell viability,
induces apoptosis and copper enhances these effects in
multidrug-resistant K562/A02 cells. Mol Cell Biochem. 360:253–260.
2012. View Article : Google Scholar
|
|
18
|
Ramachandran C, Nair SM, Escalon E and
Melnick SJ: Potentiation of etoposide and temozolomide cytotoxicity
by curcumin and turmeric force in brain tumor cell lines. J
Complement Integr Med. 9:Article 20. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen CC, Taniguchi T and D’Andrea A: The
Fanconi anemia (FA) pathway confers glioma resistance to DNA
alkylating agents. J Mol Med. 85:497–509. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Roos WP, Batista LF, Naumann SC, et al:
Apoptosis in malignant glioma cells triggered by the
temozolomide-induced DNA lesion O6-methylguanine.
Oncogene. 26:186–197. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin CJ, Lee CC, Shih YL, et al:
Resveratrol enhances the therapeutic effect of temozolomide against
malignant glioma in vitro and in vivo by inhibiting autophagy. Free
Radic Biol Med. 52:377–391. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sánchez Y, Simón GP, Calviño E, de Blas E
and Aller P: Curcumin stimulates reactive oxygen species production
and potentiates apoptosis induction by the antitumor drugs arsenic
trioxide and lonidamine in human myeloid leukemia cell lines. J
Pharmacol Exp Ther. 335:114–123. 2010.
|
|
23
|
Hirose Y, Katayama M, Mirzoeva OK, Berger
MS and Pieper RO: Akt activation suppresses Chk2-mediated,
methylating agent-induced G2 arrest and protects from
temozolomide-induced mitotic catastrophe and cellular senescence.
Cancer Res. 65:4861–4869. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen L, Han L, Shi Z, et al: LY294002
enhances cytotoxicity of temozolomide in glioma by down-regulation
of the PI3K/Akt pathway. Mol Med Rep. 5:575–579. 2012.PubMed/NCBI
|
|
25
|
Yu S, Shen G, Khor TO, Kim JH and Kong AN:
Curcumin inhibits Akt/mammalian target of rapamycin signaling
through protein phosphatase-dependent mechanism. Mol Cancer Ther.
7:2609–2620. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Datta SR, Dudek H, Tao X, et al: Akt
phosphorylation of BAD couples survival signals to the
cell-intrinsic death machinery. Cell. 91:231–241. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang J, Stevens MF and Bradshaw TD:
Temozolomide: mechanisms of action, repair and resistance. Curr Mol
Pharmacol. 5:102–114. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gupta SC, Patchva S, Koh W and Aggarwal
BB: Discovery of curcumin, a component of golden spice, and its
miraculous biological activities. Clin Exp Pharmacol Physiol.
39:283–299. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hegi ME, Diserens AC, Gorlia T, et al:
MGMT gene silencing and benefit from temozolomide in
glioblastoma. N Engl J Med. 352:997–1003. 2005. View Article : Google Scholar
|