Introduction
Acute promyelocytic leukemia (APL) is characterized
by a balanced reciprocal translocation between chromosomes 15 and
17, resulting in the fusion of promyelocytic leukemia (PML) and
retinoic acid receptor α (RARα) (1–3).
Administration of arsenic trioxide (arsenite, AsIII), an
arsenic derivative, has demonstrated marked therapeutic efficacy in
the treatment of relapsed and refractory APL patients. In order to
understand the mode of action of AsIII and provide an
effective treatment protocol for individual APL patients, studies
have been conducted on the pharmacokinetics of AsIII in
APL patients using biological samples such as urine, blood and
cerebrospinal fluid (4–7). In this regard, we have clarified the
distribution of arsenic metabolites in, not only peripheral blood
and cerebrospinal fluid, but also bone marrow from APL patients who
had received the consecutive administration of AsIII
(5,7,8). The
findings on the pharmacokinetics of AsIII in APL
patients provide novel insight into the clinical applications of
AsIII, and may contribute to improved therapeutic
protocols (9).
Although the clinical efficacy of
AsIII-based regimens against APL has been reported
(6,10), side-effects of AsIII
remain a serious concern and limit its clinical applications.
Application of new AsIII-based therapies may require the
generation of sensitizing strategies for improving the efficacy of
AsIII as well as minimizing its side-effects. In this
regard, there is emerging interest in the chemotherapeutic
application of natural substances, such as tea polyphenols and
resveratrol, for cancer treatment (11). Specifically, flavonoids such as
quercetin and genistein have been reported to potentiate the
apoptotic action of AsIII in leukemic cell lines, such
as HL-60, U937 and THP-1 (12,13).
Delphinidin (Fig.
1), a major anthocyanidin known to be present in pigmented
fruits and vegetables such as pomegranates, berries, dark grapes,
eggplants and red onions, is a diphenylpropane-based polyphenolic
ring structure that carries a positive charge in its central ring
(14). Delphinidin has gained
considerable attention as it appears to possess strong
antioxidant/oxidant properties as well as other potentially
beneficial characteristics, such as anti-inflammatory,
antimutagenic, antiangiogenic, and anti-adipocyte differentiation
activities (15–20). Treatment with delphinidin resulted
in a reduction of cells in G1 phase and an accumulation
in G2/M phase in human HeLa uterine carcinoma cell line,
and human CaCo-2 colorectal carcinoma cell line, accompanied by
apoptosis induction (18). Yun
et al (17) also
demonstrated that delphinidin suppresses the NF-κB pathway,
resulting in G2/M phase arrest and apoptosis induction
in human HCT116 colon cancer cell line and human PC3 prostate
cancer cell line (15). Despite
investigations into the antitumor activity of delphinidin against
various types of cancer cells derived from solid tumors (15–19,21,22),
to the best of our knowledge, few studies have been conducted to
investigate the effects of delphinidin on leukemic cells (23,24).
Although delphinidin and its glycosides have been shown to trigger
apoptosis in non-APL HL-60 (PML-RARα negative) cells through a
ROS/JNK-mediated mitochondrial death pathway (23,25),
the effects of delphinidin on the human APL cell line harboring
PML-RARα remain largely unclear.
In the present study, the effects of delphinidin
were investigated by focusing on growth inhibition, cell-cycle
arrest and apoptosis induction in the APL NB4 cell line (PML-RARα
positive). Furthermore, the cytocidal effects of delphinidin in
combination with AsIII were assessed to explore a
potential application of delphinidin as an effective
chemopreventive and/or chemotherapeutic agent for
AsIII-based therapy in patients with hematologic
malignancies.
Materials and methods
Materials
Sodium arsenite (AsIII) was purchased
from Tri Chemical Laboratories (Yamanashi, Japan). Delphinidin,
RPMI-1640 medium, propidium iodide (PI), ribonuclease A (RNase A)
and
2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium
hydroxide (XTT) were purchased from Sigma-Aldrich (St. Louis, MO,
USA). Fetal bovine serum (FBS) and phenazine methosulfate (PMS)
were obtained from Nichirei Biosciences (Tokyo, Japan) and Wako
Pure Chemical Industries (Osaka, Japan), respectively.
Cell lines and culture conditions
NB4, a human APL cell line with t(15;17), was
obtained from the Deutsche Sammalung von Mikroorganismen und
Zellkulturen GmbH (Braunschweig, Germany). Peripheral blood
mononuclear cells (PBMNCs) were isolated from three healthy
volunteers using Histopaque-1077 (Sigma-Aldrich) according to the
method previously described (26).
Briefly, 3 ml of heparinized blood was mixed with 5 ml of
phosphate-buffered saline (PBS), and loaded on 3 ml of
Histopaque-1077. After centrifugation at 400 × g for 30 min at room
temperature, the opaque interface containing PBMNCs was transferred
to a clean centrifuge tube and washed three times with PBS. The two
types of cells were cultured in RPMI-1640 medium supplemented with
10% heat-inactivated FBS and 100 U/ml of penicillin and 100
μg/ml of streptomycin (Wako Pure Chemical Industries) at
37°C in a humidified atmosphere at 5% CO2 in air. For
experiments, the cell density of NB4 and PBMNCs was adjusted to
1×105 ml and 5×105 cells/ml, respectively,
prior to the treatments. The present study was approved by the IRB
Committee of Tokyo University of Pharmacy and Life Sciences.
Informed consent was obtained from all the healthy volunteers.
XTT assay
Cell viability was determined by an XTT
dye-reduction assay according to the method previously described
(27). Briefly, after treatment
with various concentrations of delphinidin and AsIII,
alone or in combination, for a designated time, the cells were
washed with PBS twice and resuspended in an appropriate volume of
PBS. An aliquot (0.2 ml) of cell suspension was inoculated into
96-well plates (Iwaki, Tokyo, Japan) followed by the addition of 50
μl XTT/PMS mixed solution (1.5 mM XTT and 0.025 mM PMS).
After incubation at 37°C for 4 h, the plates were mixed on a
mechanical plate shaker, and absorbance at 450 nm was measured
using a microplate reader (Safire; Tecan, Männedorf, Switzerland).
The relative cell viability was expressed as the ratio of the
absorbance of each treatment group against that of the
corresponding untreated control group. The IC50 value of
delphinidin was calculated from the cell proliferation inhibition
curve. Data are shown as means ± SD from three independent
experiments.
Cell cycle analysis
After treatment with the IC50 value of
delphinidin at 14.0 μM for 24 h, a cell cycle analysis was
performed using a FACSCanto flow cytometer (Becton-Dickinson, San
Jose, CA, USA) according to a method previously described, with
modifications (26). To stain
cellular DNA, the cells were washed twice with PBS, fixed with 1%
paraformaldehyde/PBS for 30 min, washed twice again with PBS,
permeabilized in 70% (v/v) cold ethanol and kept at −20°C for at
least 4 h. The cell pellets were washed twice with PBS after
centrifugation and incubated with 0.25% Triton-X 100 for 5 min on
ice. After washing with PBS, the cells were centrifuged and
resuspended in 500 μl of PI/RNase A/PBS (5 μg/ml PI
and 0.1% RNase A in PBS) and incubated for 30 min in the dark at
room temperature. A total of 10,000 events were obtained and Diva
software (Becton-Dickinson) and ModFit LT™ ver.3.0 (Verity Software
House, Inc., Topsham, ME, USA) were used to calculate the number of
cells at each sub-G1, G0/G1, S and
G2/M phase fraction.
Annexin V/PI analysis
The TACS™ Annexin V-FITC apoptosis detection kit
(Trevigen, Gaithersburg, MD, USA) was used for the detection of
early apoptotic and late apoptotic/necrotic cells according to the
method previously described (26).
Briefly, after treatment with 14 μM of delphinidin for 12,
24 and 48 h, respectively, the cells were washed twice with PBS.
Cells (1×106) were then resuspended in 100 μl
Annexin V incubation reagent (10 mM HEPES pH 7.4, 150 mM NaCl, 5 mM
kCl, 1 mM MgCl2, 1.8 mM CaCl2, 5 μg/ml
PI, and Annexin V-FITC). The cells were incubated in the dark for
15 min at room temperature, followed by the addition of 400
μl binding buffer. Fluorescence intensities of FITC and PI
were measured by a FACSCanto flow cytometer (Becton-Dickinson). A
total of 30,000 events were obtained and data were analyzed by Diva
software. Annexin V(−)PI(−), annexin V(+)PI(−), and Annexin
V(+)PI(+) cells were defined as viable, early apoptotic, and late
apoptotic/necrotic cells, respectively.
Western blot analysis
Western blot analysis was carried out according to
the methods previously described (28). Briefly, after separation of the
proteins on an SDS polyacrylamide gel electrophoresis, followed by
transferring to a nitrocellulose membrane, the protein bands were
detected using the following primary antibodies and dilution
ratios: rabbit antihuman caspase-3 (Enzo Life Sciences, New York,
NY, USA) at 1:1,000; rabbit anti-human caspase-8 (BD Biosciences,
Franklin Lakes, NJ, USA) at 1:4,000; rabbit anti-human caspase-9
(Cell Signaling Technology, Danvers, MA, USA) at 1:1,000; mouse
anti-human Bid (BD Biosciences) at 1:1,000; and mouse anti-human
β-actin (Sigma) at 1:5,000. Blotted protein bands were detected
with respective horseradish peroxidase-conjugated secondary
antibodies and an enhanced chemiluminescence (ECL) western blot
analysis system (GE Healthcare, Buckinghamshire, UK).
Measurement of caspase-3, -8 and -9
activities
Activity of caspase-3, -8 or -9 was measured using
the caspase fluorometric assay kit (BioVision, Inc., Milpitas, CA,
USA) according to the methods previously described (29). Protein (50 μg/50 μl)
was plated on a 96-well plate, followed by the addition of 50
μl of 2X reaction buffer containing 10 mM DTT to each
sample, and then 5 µl of 1 mM caspase substrate (final
concentration of 50 μM). After incubation at 37°C for 1 h,
fluorescent intensity was measured with a 400 nm excitation filter
and 505 nm emission filter using a microplate reader (Safire).
Determination of loss of mitochondrial
membrane potential (ΔΨm)
ΔΨ m was determined by flow cytometry after cell
loading with Rhodamine 123 as previously described with
modifications (26). After
treatment with 14 μM of delphinidin for 3, 6, 12 and 24 h,
respectively, the cells were washed with PBS, followed by
incubation with 10 μM Rhodamine 123 in PBS for 15 min in the
dark at room temperature. The fluorescence intensities of Rhodamine
123 were measured by a FACSCanto flow cytometer (Becton-Dickinson).
A total of 30,000 events were obtained and data were analyzed by
Diva software.
Analysis of intracellular arsenic
accumulation (As[i])
After exposure of NB4 cells to 2 μM
AsIII alone or in combination with 8 μM
delphinidin for 0, 1, 3 or 6 h, the cells were washed three times
with PBS and harvested in 2% SDS solution. Protein concentrations
were determined by Bradford’s method using the protein assay dye
reagent (Bio-Rad Laboratories, Hercules, CA, USA) according to the
manufacturer’s instructions, and using BSA as the standard. The
As[i] was normalized by the amount of proteins and given as parts
per billion (ppb) of arsenic per mg of proteins. The analysis of
total arsenic was performed by inductively coupled plasma-mass
spectrometry (ICP-MS) (Perkin-Elmer Sciex, Thornhill, ON, Canada)
according to the methods previously reported (7,27,30).
Statistical analysis
Experiments were independently repeated three times,
and the results were shown as the mean ± standard deviation (SD) of
three assays. The Student’s t-test was used to compare sample means
from two groups, and one-way ANOVA followed by the Tukey’s post
test was used to compare sample means from more than three groups.
P<0.05 was considered to indicate a significant result.
Results
Cytotoxic effect of delphinidin against
NB4 cells
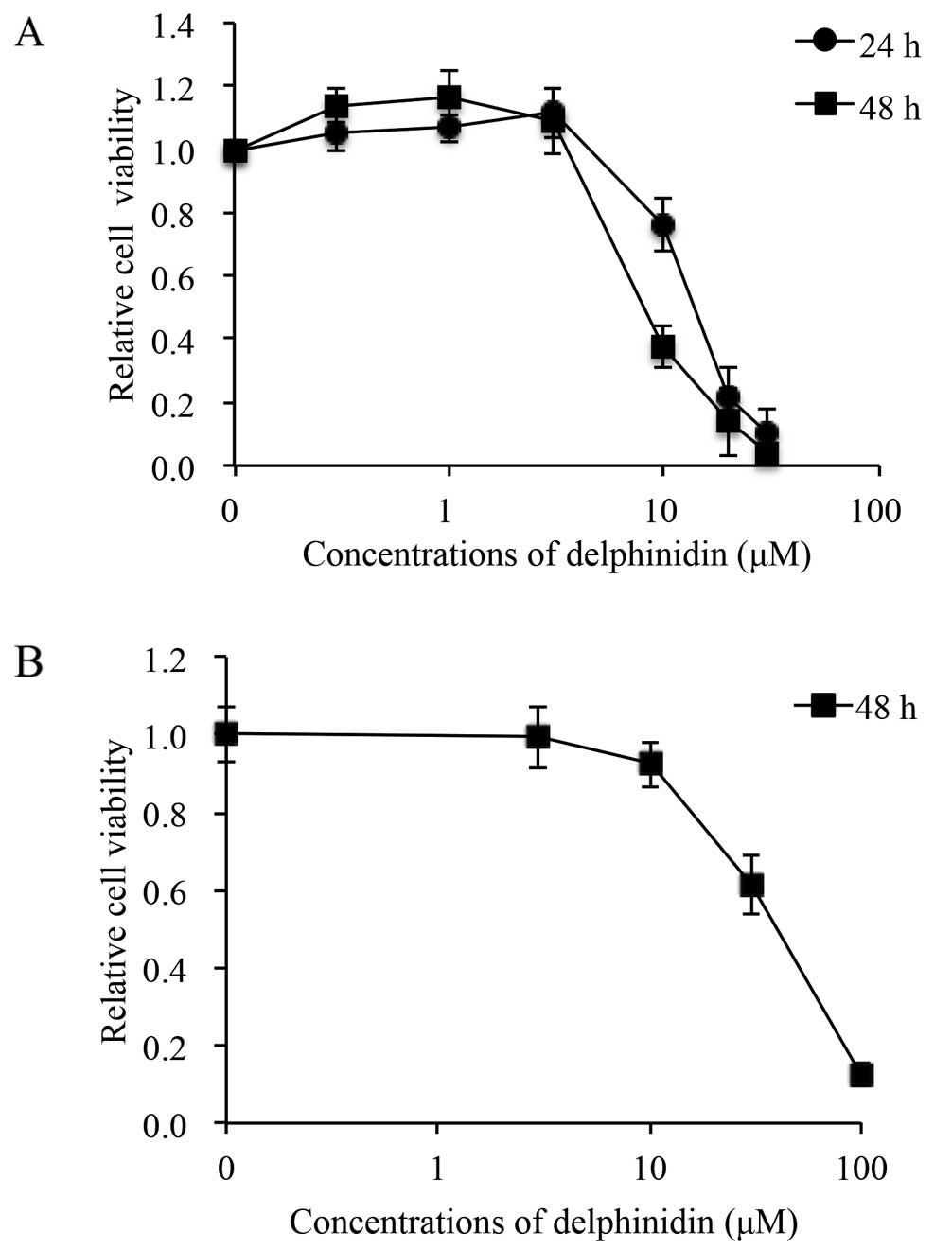
Delphinidin exhibited dose- and time-dependent
cytotoxic effects on NB4 cells after treatment with various
concentrations of delphinidin (0.3, 1, 3, 10, 20 and 30 μM)
for 24 and 48 h (Fig. 2A). when the
concentration of delphinidin was increased to 10 μM,
statistically significant differences were observed between the
delphinidin-exposed and control groups (Fig. 2A). Furthermore, the IC50
values were 14.0 and 8.1 μM for 24- and 48-h treatment,
respectively, calculated from the respective cell proliferation
inhibition curve. On the other hand, after treatment with various
concentrations of delphinidin (3, 10, 30 and 100 μM) for 48
h, the apparent cytotoxicity of delphinidin was observed in the
PBMNCs only when the concentration was >10 μM, and the
IC50 value of delphinidin was 39.7 μM (Fig. 2B). These results showed that
delphinidin exerted more potent cytotoxicity against NB4 cells than
normal PBMNCs. Therefore, subsequent experiments were conducted to
clarify the details underlying delphinidin-induced cytotoxicity in
NB4 cells following treatment with the IC50 value of
delphinidin at 14.0 μM for the indicated time-point.
Effect of delphinidin on the cell cycle
profiling of NB4 cells
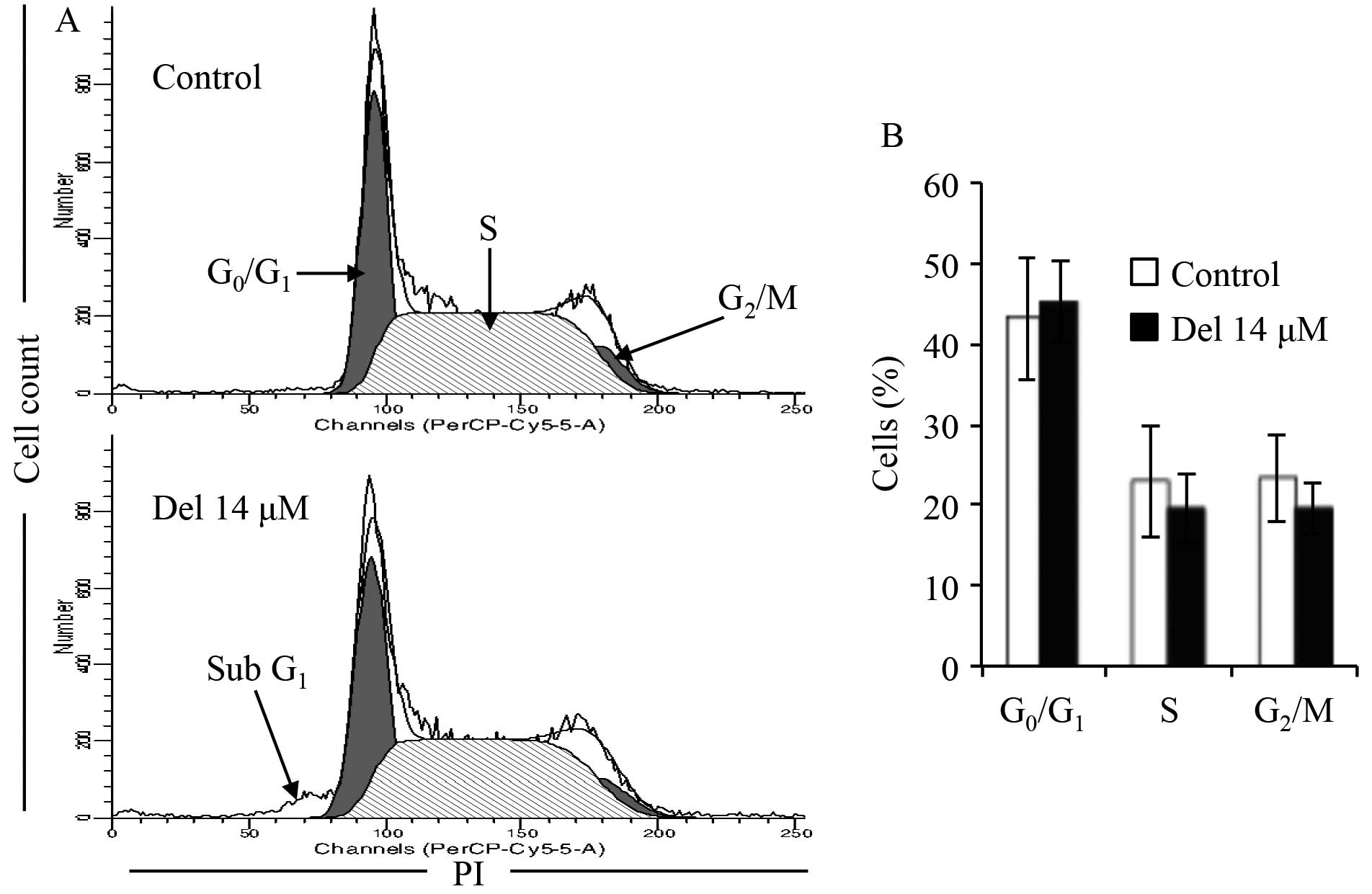
The flow cytometric analysis showed that almost no
cell arrest was observed in NB4 cells, although there was a
decrease in the number of cells in S and G2/M phases
following treatment with 14.0 μM delphinidin for 24 h
(Fig. 3). An apparent increase in
the number of cells in sub-G1 phase was observed, indicating
apoptosis induction in NB4 cells treated with delphinidin (Fig. 3A).
Apoptosis induction in NB4 cells treated
with delphinidin
After treatment with 14.0 μM of delphinidin
for 12, 24 and 48 h, apoptosis was measured with FACS analysis.
Consistent with cell-cycle results (Fig. 3A), the cells treated with 14.0
μM delphinidin underwent early and late stage apoptosis in a
time-dependent manner as compared with the control group, as
demonstrated by the transition from Annexin V(−)PI(−) to Annexin
V(+)PI(−), and then to Annexin V(+)PI(+) (Fig. 4). The transition of cells through
these three stages clearly indicated the induction of apoptosis in
NB4 cells treated with delphinidin.
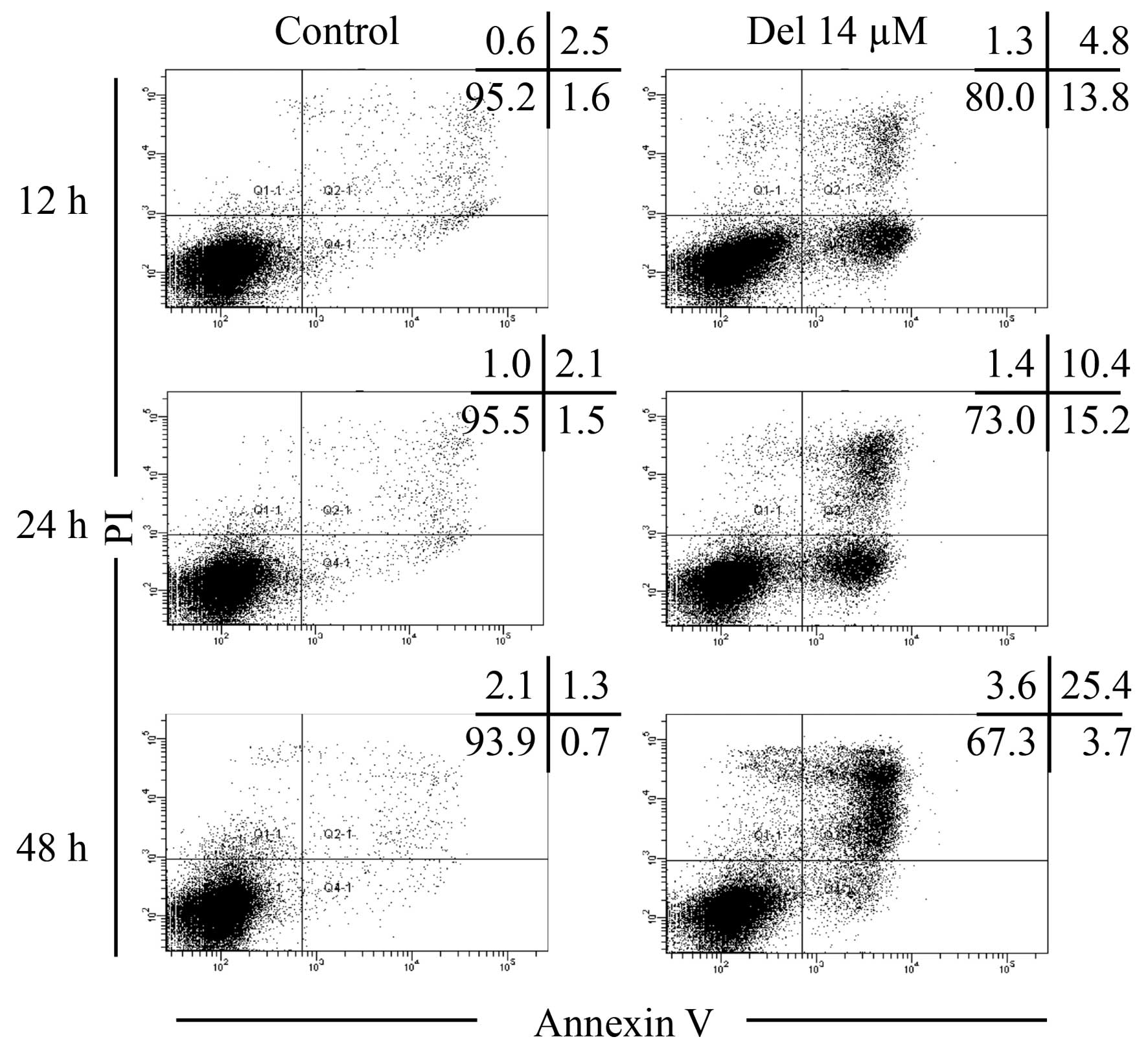 | Figure 4Apoptosis induction in NB4 cells
treated with delphinidin. After treatment with 14.0 μM
delphinidin for 12, 24 and 48 h, the cells were stained with
Annexin V-FITC and PI, and measured with a FACSCanto flow cytometer
as described in Materials and methods. A total of 30,000 events
were obtained and data were analyzed by Diva software. Annexin
V(−)PI(−), Annexin V(+)PI(−), and Annexin V(+)PI(+) cells were
defined as viable, early apoptotic, and late apoptotic/necrotic
cells, respectively. The numbers in the upper-right corner of each
dot plot show the percentage of Annexin V(−)PI(−), Annexin
V(+)PI(−), Annexin V(+)PI(+) and Annexin V(−)PI(+) cells.
Representative FACS dot plots from three independent experiments
are shown. Del, delphinidin. |
Delphinidin-mediated caspase activation
and Bid truncation in NB4 cells
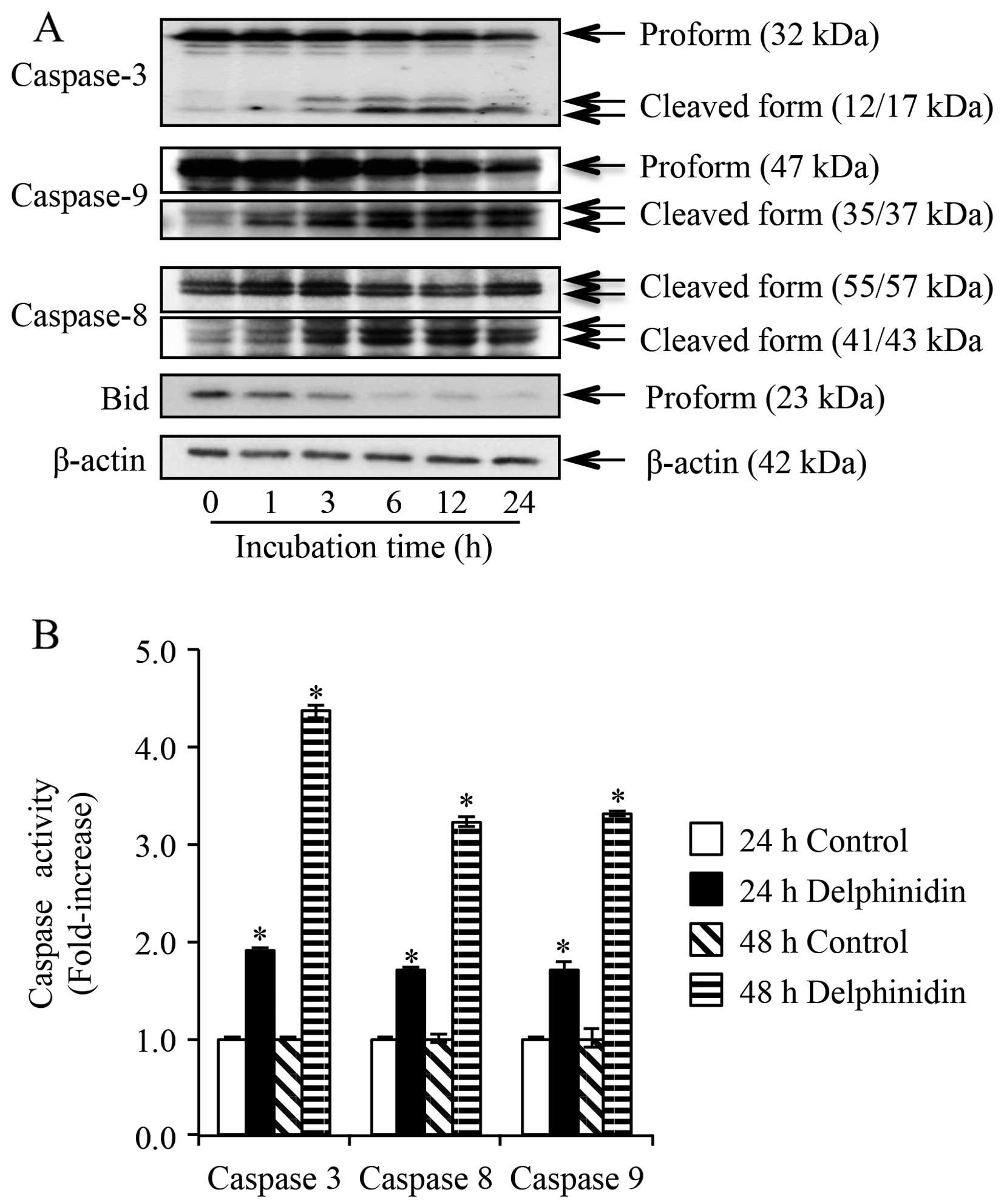
After treatment of NB4 cells with 14.0 μM of
delphinidin for 1, 3, 6, 12 and 24 h, western blot analysis was
conducted to determine the activation of caspase-3, -8, and -9, as
well as Bid truncation. As shown in Fig. 5A, the cleaved forms of caspase-8 and
-9 were observed as early as 1-h postexposure to delphinidin and
continued up to 24 h, indicating the activation of caspase-8 and -9
in the cells. Activation of caspase-3 was also confirmed based on
the appearance of its cleaved form from 3-h post-exposure and
continued up to 24 h (Fig. 5A).
Furthermore, an approximate 2- and 3- to 4-fold increase in the
activity of caspase-3, -8 and -9, respectively, was observed in NB4
cells after treatment for 24 and 48 h, respectively (Fig. 5B). Moreover, it was confirmed that
no alteration in caspase activation was observed in the control
group cells between 24- and 48-h treatment. Similar to the
activation pattern of caspase-8 and -9, a substantial decrease in
the expression level of Bid was observed as early as 1-h
post-exposure, indicating its truncation in NB4 cells treated with
delphinidin (Fig. 5A).
Delphinidin-induced loss of mitochondrial
membrane potential (ΔΨm) in NB4 cells
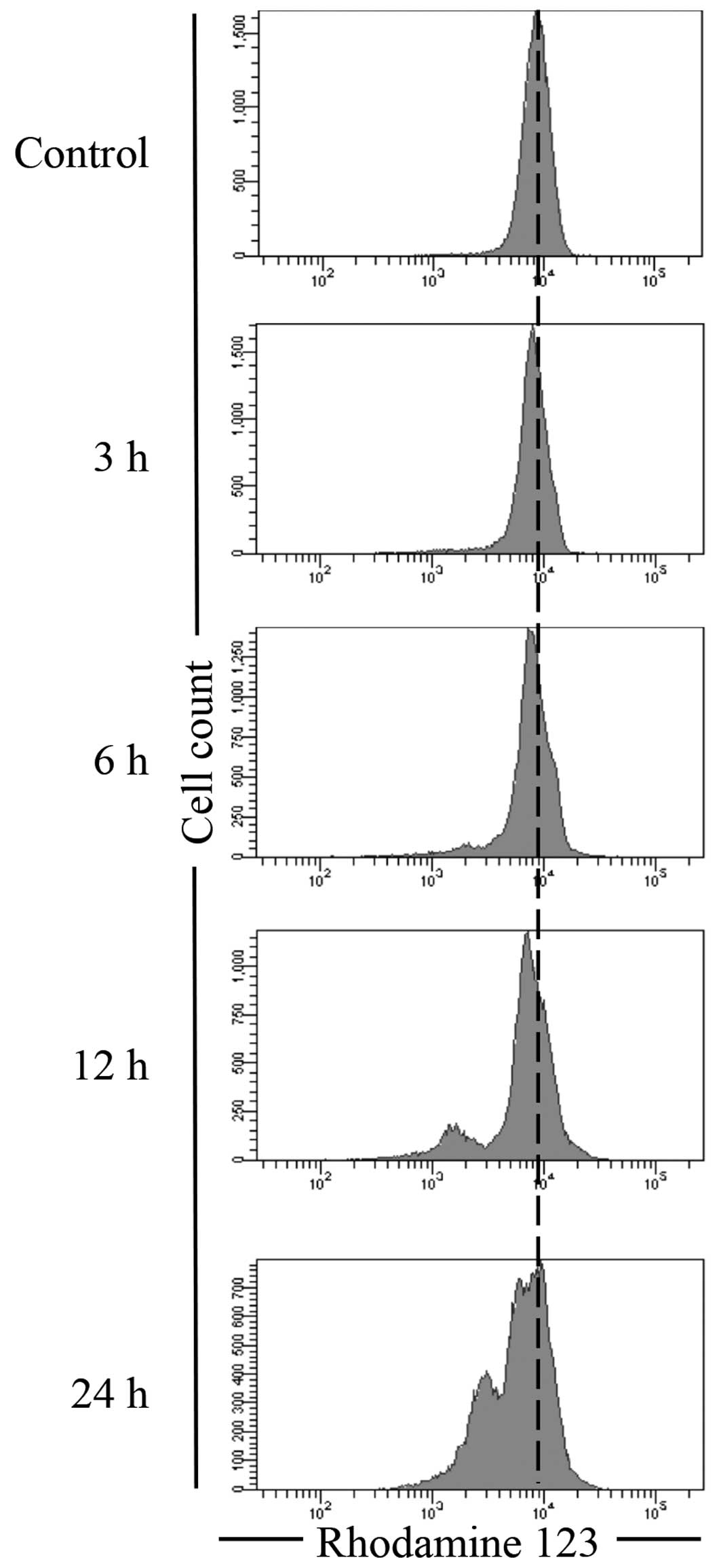
After treatment with 14.0 μM of delphinidin
for 3, 6, 12 and 24 h, Rhodamine 123, a cell-permeant cationic
fluorescent dye, was used to assay ΔΨm in NB4 cells. As shown in
Fig. 6, only a modest decrease in
ΔΨm was observed at 3 h post-exposure, followed by a substantial
time-dependent decrease in ΔΨm in NB4 cells treated with
delphinidin.
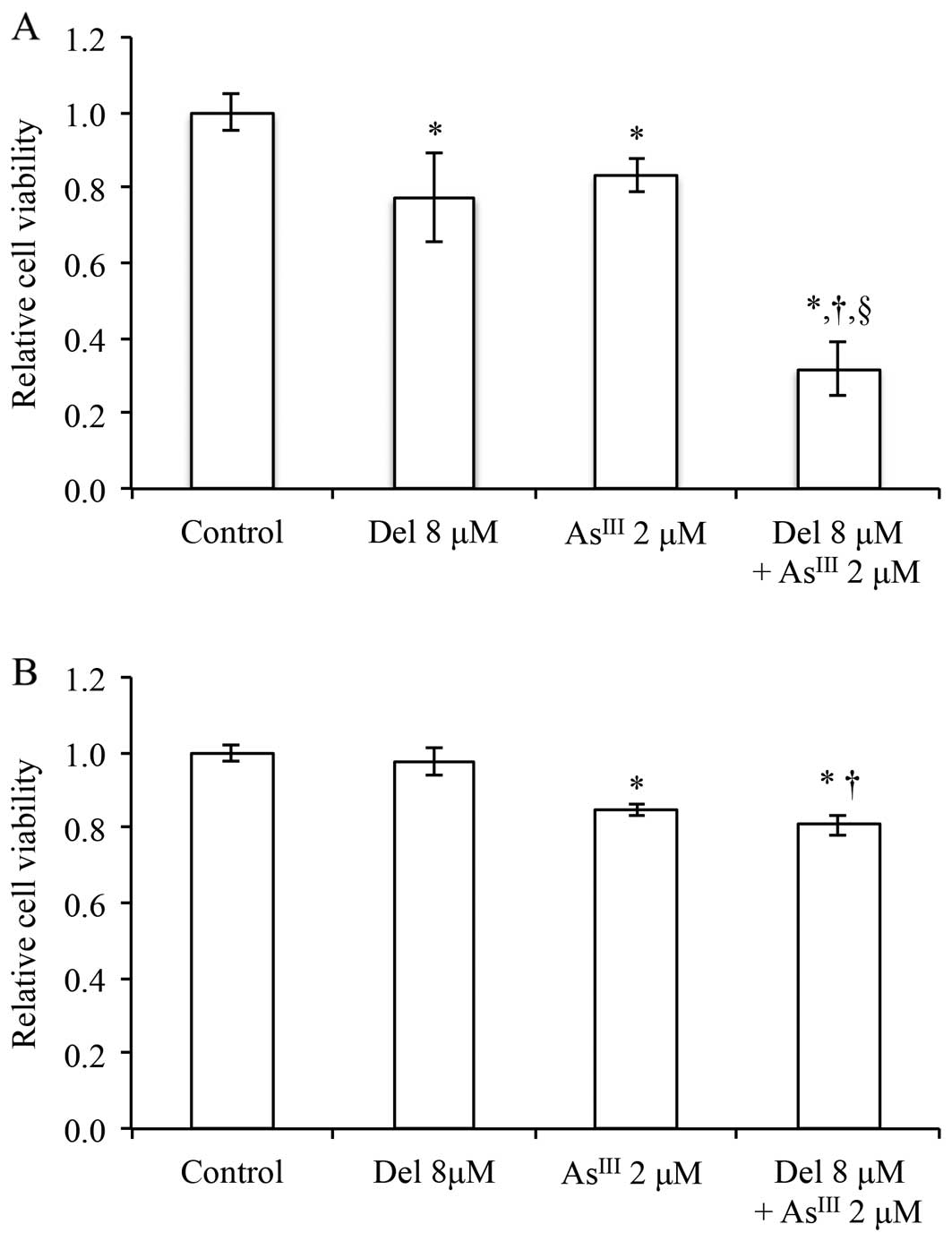
Enhanced cytotoxic effect of delphinidin
in combination with AsIII in NB4 cells
Since delphinidin exerted more potent cytotoxicity
against NB4 cells than normal PBMNCs (Fig. 2), we examined the potential for the
application of the combination of delphinidin and AsIII,
which has demonstrated notable clinical efficacy in the treatment
of relapsed and refractory APL patients (9,31). To
clarify whether enhanced cytotoxicity was induced by the
combination treatment, 8 μM of delphinidin, which was
estimated to inhibit ~20% of cell proliferation in NB4 cells by the
cell proliferation inhibition curve for 24 h, was combined with
AsIII. After treatment with delphinidin (8 μM)
and AsIII [2 μM, concentrations achieved
clinically (9)], alone or in
combination, respectively, for 24 h, cell viability was determined
by XTT assay. As shown in Fig. 7A,
cell viability significantly decreased to 77.5, 83.3 and 31.9% of
the control when treated with delphinidin and AsIII
either alone or in combination, respectively. In agreement with the
results in Fig. 2B, 8 μM of delphinidin did not exhibit
cytotoxicity against PBMNCs. Although treatment with 2 μM of
AsIII alone reduced cell viability to 84.8% of the
control in PBMNCs, a similar enhanced cytotoxic effect of
delphinidin in combination with AsIII was not observed,
indicating that the reduction in cell viability was primarily
attributed to AsIII (Fig.
7B).
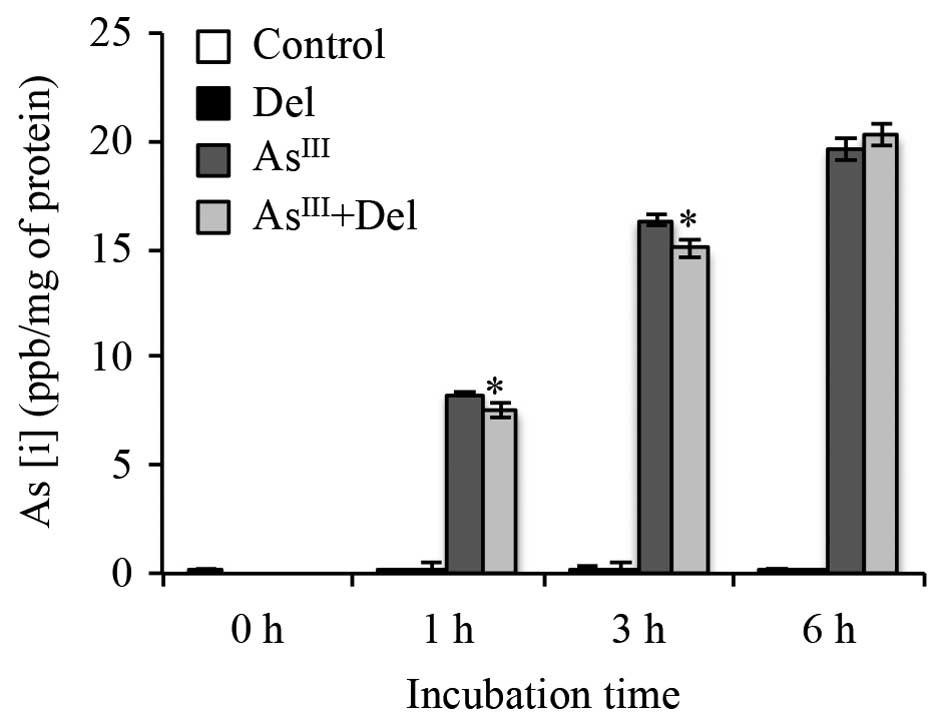
As[i] in NB4 cells treated with
AsIII alone or in combination with delphinidin
Arsenic uptake was measured to examine whether
delphinidin affected As[i] in NB4 cells when combined with
AsIII. After exposure of NB4 cells to 2 μM
AsIII alone or in combination with 8 μM
delphinidin for 0, 1, 3 or 6 h, As[i] was measured by ICP-MS. As
shown in Fig. 8, the levels of
As[i] increased with time in NB4 cells following treatment with
AsIII alone. In comparison to treatment with
AsIII alone, the levels of As[i] decreased slightly, but
significantly, in the cells after treatment with AsIII
in combination with delphinidin for 1 and 3 h, and were restored at
6 h to the level of the group receiving AsIII alone.
Discussion
In the present study, we have demonstrated that
delphinidin exhibited dose- and time-dependent cytotoxic effects on
NB4 cells, and exerted more potent cytotoxicity against NB4 cells
than normal PBMNCs. Similarly, delphinidin has been demonstrated to
inhibit the cell growth of AU-565 and MCF-10A breast cancer cell
lines, and to have only minimal effects on normal mammary
epithelial 184A1 cells (16).
Hafeez et al (15) have
demonstrated that delphinidin induces a dose-dependent inhibition
of cell growth in LNCaP, C4-2, 22Rv1 and PC3 human prostate cancer
cell lines, without having any substantial effects on normal human
prostate epithelial cells. Collectively, delphinidin seems to
possess a selective cytotoxic activity against tumor cells rather
than normal cells.
Cell-cycle arrest is involved in the
anti-proliferative effects of a large number of plant-derived
agents including delphinidin in various solid tumor cells (17,18).
In the case of leukemic cells, it has been demonstrated that
quercetin induces cell cycle arrest at G2/M phase in NB4
cells, but not in other leukemic cells such as U937 and HL-60
(12). Moreover, genistein has been
demonstrated to provoke a dose-and time-dependent accumulation of
cells at G2/M phase in U937 cells (13). These findings suggest that the
induction of cell cycle arrest is strongly dependent on the inducer
and/or cell types. In the present study, we have demonstrated that
almost no cell-cycle arrest, but a time-dependent apoptosis
induction, was observed in NB4 cells treated with delphinidin,
suggesting that the delphinidin-mediated cytotoxic effect was
attributed to apoptosis induction rather than cell cycle arrest. It
is well known that the aim of anticancer therapy is generally
focused on apoptosis induction in premalignant and malignant cells
(32). Our results thus raise a
possibility that delphinidin may be developed as an effective
chemopreventive and/or chemotherapeutic agent.
To clarify the molecular details of the apoptosis
pathway, we focused on the activation of caspases including
caspase-3, -8 and -9, which are key players in two principal
signaling pathways of apoptosis induction, known as the intrinsic
and extrinsic pathways (9,33). The intrinsic mechanism of apoptosis
involves a disruption of the mitochondrial cell membrane, resulting
in the loss of ΔΨm associated with cytochrome c release,
followed by the activation of caspase-3 and -9 (9,32,33).
By contrast, the extrinsic pathway induced by death receptors, such
as Fas and tumor necrosis factor receptor, is responsible for the
activation of caspase-8 accompanied by the activation of caspase-3
(9,32). In the present study, the activation
of caspase-8 and -9 was observed as early as 1-h post-exposure to
delphinidin, followed by the activation of caspase-3 from 3-h
post-exposure, suggesting the contribution of the intrinsic and
extrinsic pathways to the delphinidin-triggered apoptosis induction
in NB4 cells. we also demonstrated that a substantial decrease in
the expression levels of Bid was observed as early as 1-h
post-exposure, the same time-point as the activation of caspase-8.
Only a modest decrease in ΔΨm was observed at 3-h post-exposure,
followed by a substantial time-dependent decrease in ΔΨm in
delphinidin-treated NB4 cells. It is noteworthy that the
caspase-8-mediated cleavage of Bid into a pro-apoptotic active
truncated form provides the connection between the intrinsic and
extrinsic pathway (34). It has
become clear that truncated Bid translocates to the mitochondria
and then leads to a decrease of ΔΨm, following the release of
cytochrome c from mitochondria to cytosol (35,36).
Taking the previous results and our observations into account, we
suggest that truncated Bid, as a result of activation of caspase-8,
contributes to the loss of ΔΨm, and further amplifies the apoptotic
signal triggered by delphinidin in NB4 cells.
More importantly, we also demonstrated that
delphinidin in combination with AsIII achieved an
enhanced cytocidal effect against NB4 cells, but lesser on PBMNCs.
Although the marked clinical efficacy of AsIII-based
regimens against APL has been reported (6,10), its
side effects remain a serious concern and limit its clinical
applications. The successful application of new
AsIII-based therapies may require the generation of
sensitizing strategies to improve the efficacy of AsIII,
and consequently reduce the drug dose to clinically tolerable
concentrations. Flavonoids such as quercetin and genistein have
been reported to selectively potentiate AsIII-induced
apoptosis via ROS generation resulting from intracellular GSH
depletion, and activation of the intrinsic and extrinsic apoptotic
pathway in human leukemia cell lines such as HL-60, U937 and THP-1,
but not in phytohemagglutininstimulated non-tumor peripheral blood
lymphocytes (12,13). Collectively, the present results
suggest that delphinidin is a promising cancer chemopreventive
agent candidate for enhancing the clinical efficacy of
AsIII.
Based on an evaluation of the intracellular
accumulation of Rhodamine 123 and/or daunorubicin in
multidrug-resistant cells expressing P-glycoprotein (Pgp) or
non-Pgp multidrug drug resistance protein, flavonoids such as
genistein have been demonstrated to inhibit these drug
transporters, resulting in enhanced accumulation of these
substrates (37,38). However, in comparison to treatment
with AsIII alone, the level of As[i] was decreased by
the addition of delphinidin in the first 3 h of treatment, and
returned at 6 h to the level of the group receiving
AsIII alone. These results suggested that the enhanced
cytotoxicity effect of delphinidin in combination with
AsIII in NB4 cells may not be attributed to the
alterations of As[i]. However, detailed experimental studies
focusing on the mechanisms underlying the enhanced cytotoxic effect
are needed.
In conclusion, to the best of our knowledge, we have
demonstrated for the first time that delphinidin showed selective
cytotoxic effects against NB4 cells, but minimal effects on PBMNCs.
we have also shown that the intrinsic and extrinsic pathways linked
by Bid contributed to the cytotoxicity. More importantly,
delphinidin selectively sensitized the cells to AsIII,
resulting in the enhancement of AsIII cytotoxicity by
strengthening intrinsic/extrinsic pathway-mediated apoptosis
induction, rather than affecting the levels of As[i]. These
observations may offer a rationale for the use of delphinidin to
improve the clinical efficacy of AsIII.
Acknowledgments
We thank Professor Steven R. Kayser of UCSF School
of Pharmacy for the critical reading of this manuscript. The
present study was supported in part by grants from the Japan China
Medical Association to Bo Yuan.
References
|
1
|
de Thé H, Chomienne C, Lanotte M, Degos L
and Dejean A: The t(15;17) translocation of acute promyelocytic
leukaemia fuses the retinoic acid receptor alpha gene to a novel
transcribed locus. Nature. 347:558–561. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Goddard AD, Borrow J, Freemont PS and
Solomon E: Characterization of a zinc finger gene disrupted by the
t(15;17) in acute promyelocytic leukemia. Science. 254:1371–1374.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tong JH, Dong S, Geng JP, Huang W, Wang
ZY, Sun GL, Chen SJ, Chen Z, Larsen CJ and Berger R: Molecular
rearrangements of the MYL gene in acute promyelocytic leukemia
(APL, M3) define a breakpoint cluster region as well as some
molecular variants. Oncogene. 7:311–316. 1992.PubMed/NCBI
|
|
4
|
Fujisawa S, Ohno R, Shigeno K, Sahara N,
Nakamura S, Naito K, Kobayashi M, Shinjo K, Takeshita A, Suzuki Y,
et al: Pharmacokinetics of arsenic species in Japanese patients
with relapsed or refractory acute promyelocytic leukemia treated
with arsenic trioxide. Cancer Chemother Pharmacol. 59:485–493.
2007. View Article : Google Scholar
|
|
5
|
Kiguchi T, Yoshino Y, Yuan B, Yoshizawa S,
Kitahara T, Akahane D, Gotoh M, Kaise T, Toyoda H and Ohyashiki K:
Speciation of arsenic trioxide penetrates into cerebrospinal fluid
in patients with acute promyelocytic leukemia. Leuk Res.
34:403–405. 2010. View Article : Google Scholar
|
|
6
|
Shen Zx, Chen GQ, Ni JH, Li XS, Xiong SM,
Qiu QY, Zhu J, Tang W, Sun GL, Yang KQ, et al: Use of arsenic
trioxide (As2O3) in the treatment of acute
promyelocytic leukemia (APL): II. Clinical efficacy and
pharmacokinetics in relapsed patients. Blood. 89:3354–3360.
1997.PubMed/NCBI
|
|
7
|
Yoshino Y, Yuan B, Miyashita SI, Iriyama
N, Horikoshi A, Shikino O, Toyoda H and Kaise T: Speciation of
arsenic trioxide metabolites in blood cells and plasma of a patient
with acute promyelocytic leukemia. Anal Bioanal Chem. 393:689–697.
2009. View Article : Google Scholar
|
|
8
|
Iriyama N, Yoshino Y, Yuan B, Horikoshi A,
Hirabayashi Y, Hatta Y, Toyoda H and Takeuchi J: Speciation of
arsenic trioxide metabolites in peripheral blood and bone marrow
from an acute promyelocytic leukemia patient. J Hematol Oncol.
5:12012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yuan B, Yoshino Y, Kaise T and Toyoda H:
Application of arsenic trioxide therapy for patients with leukemia.
Biological Chemistry of Arsenic, Antimony and Bismuth. Sun H: John
Wiley and Sons, Ltd; Chichester: pp. 263–292. 2011
|
|
10
|
Soignet SL, Maslak P, Wang ZG, Jhanwar S,
Calleja E, Dardashti LJ, Corso D, DeBlasio A, Gabrilove J,
Scheinberg DA, et al: Complete remission after treatment of acute
promyelocytic leukemia with arsenic trioxide. N Engl J Med.
339:1341–1348. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Surh YJ: Cancer chemoprevention with
dietary phytochemicals. Nat Rev Cancer. 3:768–780. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ramos AM and Aller P: Quercetin decreases
intracellular GSH content and potentiates the apoptotic action of
the antileukemic drug arsenic trioxide in human leukemia cell
lines. Biochem Pharmacol. 75:1912–1923. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sánchez Y, Amrán D, Fernández C, de Blas E
and Aller P: Genistein selectively potentiates arsenic
trioxide-induced apoptosis in human leukemia cells via reactive
oxygen species generation and activation of reactive oxygen
species-inducible protein kinases (p38-MAPk, AMPK). Int J Cancer.
123:1205–1214. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hou DX, Fujii M, Terahara N and Yoshimoto
M: Molecular mechanisms behind the chemopreventive effects of
anthocyanidins. J Biomed Biotechnol. 2004:321–325. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hafeez BB, Siddiqui IA, Asim M, Malik A,
Afaq F, Adhami VM, Saleem M, Din M and Mukhtar H: A dietary
anthocyanidin delphinidin induces apoptosis of human prostate
cancer PC3 cells in vitro and in vivo: Involvement of nuclear
factor-kappaB signaling. Cancer Res. 68:8564–8572. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Afaq F, Zaman N, Khan N, Syed DN, Sarfaraz
S, Zaid MA and Mukhtar H: Inhibition of epidermal growth factor
receptor signaling pathway by delphinidin, an anthocyanidin in
pigmented fruits and vegetables. Int J Cancer. 123:1508–1515. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yun JM, Afaq F, Khan N and Mukhtar H:
Delphinidin, an anthocyanidin in pigmented fruits and vegetables,
induces apoptosis and cell cycle arrest in human colon cancer
HCT116 cells. Mol Carcinog. 48:260–270. 2009. View Article : Google Scholar
|
|
18
|
Lazzè MC, Savio M, Pizzala R, Cazzalini O,
Perucca P, Scovassi AI, Stivala LA and Bianchi L: Anthocyanins
induce cell cycle perturbations and apoptosis in different human
cell lines. Carcinogenesis. 25:1427–1433. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cvorovic J, Tramer F, Granzotto M,
Candussio L, Decorti G and Passamonti S: Oxidative stress-based
cytotoxicity of delphinidin and cyanidin in colon cancer cells.
Arch Biochem Biophys. 501:151–157. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Suzuki R, Tanaka M, Takanashi M, Hussain
A, Yuan B, Toyoda H and Kuroda M: Anthocyanidins-enriched bilberry
extracts inhibit 3T3-L1 adipocyte differentiation via the insulin
pathway. Nutr Metab (Lond). 8:142011. View Article : Google Scholar
|
|
21
|
Kausar H, Jeyabalan J, Aqil F, Chabba D,
Sidana J, Singh IP and Gupta RC: Berry anthocyanidins
synergistically suppress growth and invasive potential of human
non-small-cell lung cancer cells. Cancer Lett. 325:54–62. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Aiyer HS, Warri AM, Woode DR,
Hilakivi-Clarke L and Clarke R: Influence of berry polyphenols on
receptor signaling and cell-death pathways: Implications for breast
cancer prevention. J Agric Food Chem. 60:5693–5708. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hou DX, Ose T, Lin S, Harazoro K, Imamura
I, Kubo M, Uto T, Terahara N, Yoshimoto M and Fujii M:
Anthocyanidins induce apoptosis in human promyelocytic leukemia
cells: Structure-activity relationship and mechanisms involved. Int
J Oncol. 23:705–712. 2003.PubMed/NCBI
|
|
24
|
Katsube N, Iwashita K, Tsushida T, Yamaki
K and Kobori M: Induction of apoptosis in cancer cells by Bilberry
(Vaccinium myrtillus) and the anthocyanins. J Agricultural and Food
Chem. 51:68–75. 2003. View Article : Google Scholar
|
|
25
|
Hou DX, Tong X, Terahara N, Luo D and
Fujii M: Delphinidin 3-sambubioside, a Hibiscus anthocyanin,
induces apoptosis in human leukemia cells through reactive oxygen
species-mediated mitochondrial pathway. Arch Biochem Biophys.
440:101–109. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kon A, Yuan B, Hanazawa T, Kikuchi H, Sato
M, Furutani R, Takagi N and Toyoda H: Contribution of membrane
progesterone receptor α to the induction of progesterone-mediated
apoptosis associated with mitochondrial membrane disruption and
caspase cascade activation in Jurkat cell lines. Oncol Rep.
30:1965–1970. 2013.PubMed/NCBI
|
|
27
|
Yoshino Y, Yuan B, Kaise T, Takeichi M,
Tanaka S, Hirano T, Kroetz DL and Toyoda H: Contribution of
aquaporin 9 and multidrug resistance-associated protein 2 to
differential sensitivity to arsenite between primary cultured
chorion and amnion cells prepared from human fetal membranes.
Toxicol Appl Pharmacol. 257:198–208. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yuan B, Ohyama K, Bessho T and Toyoda H:
Contribution of inducible nitric oxide synthase and
cyclooxygenase-2 to apoptosis induction in smooth chorion
trophoblast cells of human fetal membrane tissues. Biochem Biophys
Res Commun. 341:822–827. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Imai M, Yuan B, Kikuchi H, Saito M, Ohyama
K, Hirobe C, T Oshima T, Hosoya T, Morita H and Toyoda H: Growth
inhibition of a human colon carcinoma cell, COLO 201, by a natural
product, Vitex agnus-castus fruits extract, in vivo and in vitro.
Adv Biol Chem. 2:20–28. 2012. View Article : Google Scholar
|
|
30
|
Iriyama N, Yuan B, Yoshino Y, Hatta Y,
Horikoshi A, Aizawa S, Takeuchi J and Toyoda H: Aquaporin 9, a
promising predictor for the cytocidal effects of arsenic trioxide
in acute promyelocytic leukemia cell lines and primary blasts.
Oncol Rep. 29:2362–2368. 2013.PubMed/NCBI
|
|
31
|
Wang ZY and Chen Z: Acute promyelocytic
leukemia: From highly fatal to highly curable. Blood.
111:2505–2515. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bremer E, van Dam G, Kroesen BJ, de Leij L
and Helfrich W: Targeted induction of apoptosis for cancer therapy:
Current progress and prospects. Trends Mol Med. 12:382–393. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ryter SW, Kim HP, Hoetzel A, Park JW,
Nakahira K, Wang X and Choi AM: Mechanisms of cell death in
oxidative stress. Antioxid Redox Signal. 9:49–89. 2007. View Article : Google Scholar
|
|
34
|
Kantari C and Walczak H: Caspase-8 and
bid: Caught in the act between death receptors and mitochondria.
Biochim Biophys Acta. 1813:558–563. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li H, Zhu H, Xu CJ and Yuan J: Cleavage of
BID by caspase 8 mediates the mitochondrial damage in the Fas
pathway of apoptosis. Cell. 94:491–501. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Luo X, Budihardjo I, Zou H, Slaughter C
and Wang X: Bid, a Bcl2 interacting protein, mediates cytochrome c
release from mitochondria in response to activation of cell surface
death receptors. Cell. 94:481–490. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Castro AF and Altenberg GA: Inhibition of
drug transport by genistein in multidrug-resistant cells expressing
P-glycoprotein. Biochem Pharmacol. 53:89–93. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kitagawa S: Inhibitory effects of
polyphenols on P-glycoprotein-mediated transport. Biol Pharm Bull.
29:1–6. 2006. View Article : Google Scholar : PubMed/NCBI
|